Abstract
Great efforts in clinical and basic research have shown progress in understanding the neurobiological mechanisms of neurodevelopmental disorders, such as autism, schizophrenia, and attention-deficit hyperactive disorders. Literature on this field have suggested that these disorders are affected by the complex interaction of genetic, biological, psychosocial and environmental risk factors. However, this complexity of interplaying risk factors during neurodevelopment has prevented a complete understanding of the causes of those neuropsychiatric symptoms. Recently, with advances in modern high-resolution neuroscience methods, the neural circuitry analysis approach has provided new solutions for understanding the causal relationship between dysfunction of a neural circuit and behavioral alteration in neurodevelopmental disorders. In this review we will discuss recent progress in developing novel optogenetic and chemogenetic strategies to investigate neurodevelopmental disorders.
Introduction
The development of the nervous system is orchestrated by genetically-programed processes and is tightly regulated by environmental factors (Sahin & Sur, 2015). Defective timing and asynchrony of developmental processes (which can be induced by genetic mutations, epigenetic factors such as teratogenic neurotoxins, or socio-environmental factors) may result in abnormal growth of the central nervous system. This can lead to neurodevelopmental disorders such as autism spectrum disorders (ASD), schizophrenia spectrum disorders (SSD), obsessive-compulsive disorder (OCD) and attention-deficit hyperactive disorders (ADHD) (Sontheimer, 2015; Sahin & Sur, 2015; Lein, 2015; van Loo & Martens, 2007). The consequences of these psychiatric disorders are characterized by impairments in learning, memory, emotional regulation, sociality, and self-control, and can cause academic, social, and occupational dysfunction (American Psychiatric Association, 2013). Not only do these conditions require expensive and time-consuming intervention; they also have an enormous economic and emotional impact on society. The incidence of these disorders is increasing, and many research teams have been dedicated to finding the ultimate cure for many of them that inflict the population. However, in many cases the underlying mechanism by which such neurodevelopmental disorders develop is still unclear (Szpir, 2006).
Multiple hypotheses have been put forth over the year in an effort to better define the cause of neurodevelopmental disorders. Many early studies focused on genetic anomalies in an attempt to determine the etiology of neurodevelopmental disorders. As this research progressed, accumulating evidence strongly supported what is now widely accepted: that gene expression is also regulated by environmental factors in either an inheritable or non-inheritable manner (Lein, 2015; Bale et al., 2010). This has led to a shift from earlier psycho-pathological theories of neurodevelopmental disorders to a more contemporary understanding that complex interactions between genetic anomalies and environmental factors, such as early life stress or insufficient parenting, can generate abnormal behavior during development or in adulthood (van Loo & Martens, 2007). However, given that multiple etiological factors underlie most neurodevelopmental disorders, it is difficult to identify a fundamental root cause of abnormal behaviors in real-time adult psychopathology.
Literature in this field has described the heterogeneity of neurodevelopmental disorders and created a system of categorization based on the particular symptoms of each psychiatric disorder (American Psychiatric Association, 2013; World Health Organization, 2015). Although considerable research using genetic analyses and human brain imaging has improved our understanding of the pathology of neurodevelopmental disorders, many studies have relied on correlational research, making it difficult to clearly identify a cause and effect relationship between the variables involved (Gallo & Posner, 2016; Jazayeri & Afraz, 2017; Owen, O’Donovan, Thapar, & Craddock, 2011; Markram & Markram, 2010). On the other hand, similar concerns also arise when developing mutant animal models for neurodevelopmental disorders. Genetically manipulated rodent models have been widely studied to gain insights into neurobiological mechanisms, specifically if genes are associated with a core symptom of these disorders. With this strategy of combining genetic manipulation with behavioral phenotyping, several transgenic rodent models have been validated, recapitulating the core symptoms of these disorders (van Loo & Martens, 2007). However, since the manifestation of disease symptoms occurs through a chronic process during development, this approach still limits the ability to draw firm conclusions as to whether the genetic manipulation causes the behavioral dysfunctions through distortion in the developmental trajectory or in real-time neural circuit dysfunction (e.g. change of physiological responsivity) in juvenile or adult. Moreover, in the field of clinical neuroscience, debates arise over use of traditional clinical diagnostic tools, which are also used in validating animal models, such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) and the International Classification of Diseases (ICD). Although these diagnostic tools have contributed to the classification of mental disease and provided a manual to manage mental disorders efficiently, the reliability of psychiatric diagnosis is reported to be relatively low in clinical practice, and many symptoms are common across different mental disorders, making it difficult to chisel out the fundamental neurobiological mechanism of a single mental illness. (Woodbury-Smith, 2010; Insel et al., 2010).
This ambiguity in differentiating mental illnesses with overlapping symptoms is not uncommon in research on neurodevelopmental disorders. In clinical settings, it has been reported that one type of neurodevelopmental disorder appears to be highly comorbid with many other such disorders (Goldstein, Minshew, Allen, & Seaton, 2002; Woodbury-Smith, 2010). For example, the phenotype of persistent deficits in social interaction in ASD is also observed in SSD (Yizhar, 2012; Yizhar et al., 2011). This heterogeneity of clinical manifestations, differential responses to treatment, and varied prognoses have long suggested myriad underlying causes. To overcome the limitation of traditional diagnostic tools lacking linkage with biomarkers, NIMH recently initiated a new strategy for classification, the Research Domain Criteria (RDoC) framework. Under RDoC, mental disorders are viewed as a malfunction of brain circuitry (Insel et al., 2010; Stein, Lund, & Nesse, 2013; Colibazzi, 2014). With this strategy, animal models are utilized to determine the neural circuit mechanism of specific behavioral dysfunctions relevant to a mental disorder, rather than evaluating all of the clinical diagnostic criteria of that disorder. Such strategies have the potential to unlock, breaking down the specific roles of neurochemistry and neural circuits that contribute to dysfunction in psychiatric disorders, overcoming a gap between clinical neuroscience and clinical phenomenology (Figure 1) (Nestler & Hyman, 2010; Sahin & Sur, 2015).
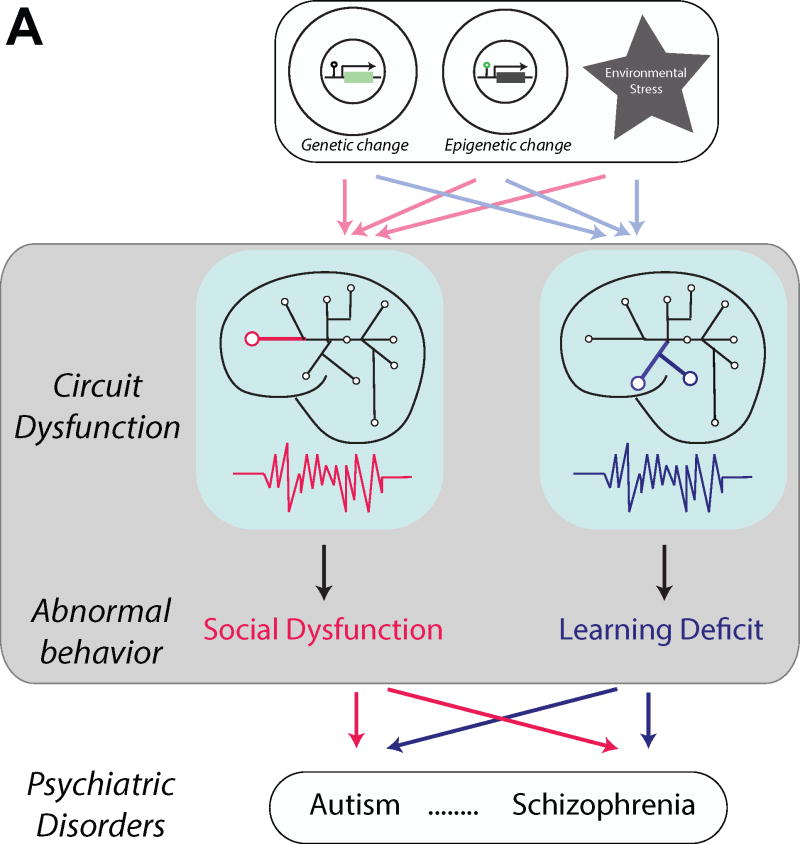
Figure 1. Neural circuitry approach to understand neurodevelopmental disorders.
Multiple risk factors, such as genetic anomalies and environmental factors (Top box; e.g. epigenetic changes, cellular stress, systemic hormonal changes) lead to abnormal neurophysiological alteration at the circuit level in the brain (Gray colored middle box) and generate abnormal behavior (e.g. social dysfunction or learning deficit), which can be a phenotype of multiple different psychiatric disorders.
This recent transition in diagnosis and research strategy has been possible due to the availability of advanced techniques for identifying and quantifying the connections between neural circuits in vivo, including electrophysiology and calcium imaging of specific cell populations in behaving animals (Alivisatos et al., 2013). In addition to these advanced monitoring techniques, the recent emergence of optogenetic and chemogenetic approaches has fostered a new revolution in neuroscience by enabling the direct identification and selective control of specific populations of brain cells and neural circuits with high temporal and spatial precision (Zemelman, Lee, Ng, & Miesenböck, 2002; Rein & Deussing, 2012; Spangler & Bruchas, 2017; Bruchas & Roth, 2016; Kim, Adhikari, & Deisseroth, 2017). Here, we will summarize the practical considerations for the use of optogenetics and chemogenetics and review the current state of the neural circuitry analysis approaches in research on neurodevelopmental disorders.
Optogenetic and chemogenetic tools for studying neurodevelopmental disorders
Over the last decade, neural circuitry studies relevant to a variety of different behaviors have been rapidly progressing, and many recent discoveries have demonstrated how particular neural circuits are involved in generating behavior. These achievements have been enabled by bidirectional, cell-type specific light-based (optogenetics) and chemical-based (chemogenetics) manipulation of neural activity. While these studies have been “built on the shoulders of giants” in neuroscience, who pioneered tracing, electrophysiology, and anatomical work, there was a remaining gap in knowledge, and that gap centered around a lack of understanding of how selected cell types are engaged and recruited to orchestrate behavioral responses. Cell type selective tools were in need, and optogenetic and chemogenetic approaches have begun to fill these gaps, and open new doors into our understanding the neural circuit basis of behavior, including neurodevelopmental disorders.
Optogenetics
Optogenetics is biotechnology in which genetically modified light-sensitive proteins activate ion-conductance regulators or cellular signaling proteins, which allows us to control the activity of a confined neural population (Rein & Deussing, 2012; Spangler & Bruchas, 2017; Kim et al., 2017). As new technology has developed, the research discipline named “optogenetics” has expanded to include optical recording techniques applying genetic engineering encoding biochemical sensing proteins, such as GCaMP (Ca2+ sensor), GluSnFRs (glutamate releasing sensor), or ASAP1 (membrane voltage sensor). In this review, we will focus on the use of optogenetics as an “actuator” reagent to control neural activity (with light).
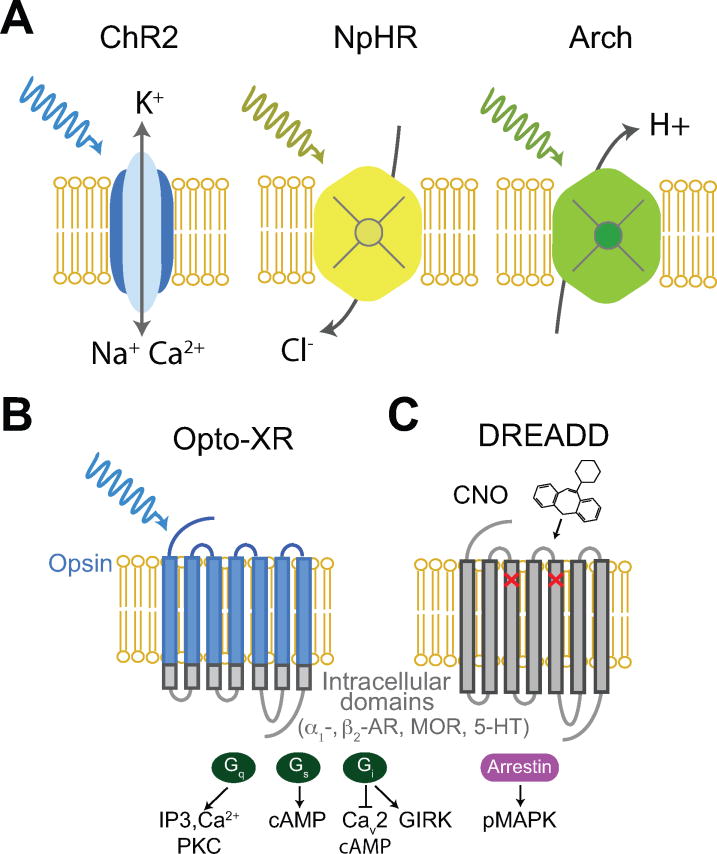
Various types of bioengineered microbial light-sensitive protein (e.g. opsins) for optogenetics have developed to achieve efficient excitatory or inhibitory effects on neural activity (Figure 2) (Deisseroth, 2015). The two most commonly used effectors are channelrhodopsins (ChRs) and halorhodopsin (NpHR). Channelrhodopsins (ChRs) are light-sensitive nonspecific cation channels (Nagel et al., 2002; Bamann, Kirsch, Nagel, & Bamberg, 2008; Boyden, Zhang, Bamberg, Nagel, & Deisseroth, 2005). The absorption of blue light (ChR2 peak effect at ~480 nm) induces conformational changes in the opsin and opens the pore (rise time > 200 μs) to more than 6 Å in size, which allows the passive movement of cations (Na+, H+, Ca2+, and K+) and results in depolarization of the neuron. When the light switches off, the opened pore closes immediately with a short deactivation time (~10-12 ms in neurons) as the channel returns to its original structure (Bamann et al., 2008; Deisseroth & Hegemann, 2017; Boyden et al., 2005). Therefore, this rapid action of the opsin in response to the blue light allows us to excite neurons on a millisecond timescale. On the other hand, halorhodopsin (NpHR) is a light-sensitive inward Cl− pump (Matsuno-Yagi & Mukohata, 1977). When exposed to yellow or green light (peak effect at 570 nm), the microbial opsin Cl- pumps increase influx of Cl− inside the cell membrane and results in hyperpolarization and subsequent inhibition of action potentials in the targeted neurons (Han & Boyden, 2007; Zhang et al., 2007). In addition to ChRs and NpHR, new opsins have been discovered and updated to improve the efficiency of controlling neural activity and minimize side effects that will be discussed further later in this review. Archaerhodopsin-3 (Arch) is a light-driven outward proton pump that responds to yellow or green light, similar to the NpHR activation spectra, and results in very efficient (near 100% in vitro) silencing of the targeted neurons (Clair, Ogren, Mamaev, Kralj, & Rothschild, 2012; Chow et al., 2010; Han et al., 2011).
Figure 2. Basic properties of optogenetic and chemogenetic tools.
A left, light-sensitive activating tool channelrhodopsin-2 (ChR2). A range of blue light drives depolarization of target cells through the opening of nonselective cation channels. Middle and right, light-sensitive silencing ion pumps driven by yellow and green light. Halorhodopsin from Natronomonas (NpHR) works as a chloride pump (middle). Archaerhodopsin-3 (Arch) from Halorubrum sodomense works as a proton pump (right). These light-sensitive ion pumps lead to hyperpolarization of the target cell. B and C. light- (B) and drug- (CNO; C) activated G-protein-coupled receptors (GPCR) control intracellular signaling of the target cells. Gq couples to phospholipase C to generate IP3 and DAG which regulates intracellular calcium stores. Gs facilitates cyclic AMP (cAMP) production. Gi activates G protein-coupled inwardly-rectifying potassium channels (GIRK) to hyperpolarize the cell. Gi also inhibits voltage-gated calcium channels (CaV2) to inhibit synaptic vesicle release. Arrestin signaling is mediated through phosphorylation of MAP kinases (pMAPK).
New variations of opsins have been developed and are expected to be continuously updated so as to meet the needs of various research projects (e.g. efficiency improvement, multi-plexed control of neurons, various prolonged time of an effect). ChR2+H134R (Also called ChR2-TC) and ChIEF/ChEF allows induction of light-driven action potentials in low light with high frequency (Berndt et al., 2011; Lin, Lin, Steinbach, & Tsien, 2009). Step-function opsins (SFO) can modulate neurons in an active state for up to 30 minutes with a short pulse of light induction (Berndt, Yizhar, Gunaydin, Hegemann, & Deisseroth, 2009; Yizhar et al., 2011). Recent studies that identify naturally occurring light-gated chloride-conducting channelrhodopsins provide new optogenetic silencing tools with higher chloride selectivity and conductivity and rapid kinetics (Berndt et al., 2016; Govorunova, Sineshchekov, Janz, Liu, & Spudich, 2015).
Beyond the precise excitation and inhibition being achieved by the engineering of microbial light-sensitive ion channels discussed above, several new approaches have been devised to modulate biochemical signaling in the form of chimeric G protein-coupled receptors (GPCR) (Spangler & Bruchas, 2017). Effort began by developing a chimeric protein combining the intracellular loops of GPCR, such as metabotropic glutamate receptor mGluR6 or serotonin receptors to target opsins with light-sensing domains of melanopsin (Masseck et al., 2014; McGregor, Bécamel, Marin, & Andrade, 2016). Further, utilizing the fact that vertebrate rhodopsins are GPCRs, the innovation was progressed by replacing the intracellular loops of these rhodopsins with those of peptide receptors, such as adrenergic receptors (AR) and opioid receptors, to create unique functional properties that initiate and terminate receptor-specific signaling events with high temporal precision enabled by pulses of light (Figure 2) (Airan, Thompson, Fenno, Bernstein, & Deisseroth, 2009; Siuda et al., 2015b). For example, the photo-activation of opto-α1 AR, which drives Gq signaling in the nucleus accumbens, a basal forebrain region that is relevant to drug addiction, was found to have a profound impact on reward-related behavior while the Gs-coupled opto-β2 AR had only a modest impact on reward behavior (Airan et al., 2009). On the other hand, photo-activation of opto-β2 in the basolateral amygdala induced anxiety-like behavioral states (Siuda, Al-Hasani, McCall, Bhatti, & Bruchas, 2016; Siuda et al., 2015b). This suggests that opto-XRs can be used as a tool to study circuit-related biochemical signaling in behavior.
Chemogenetics
An alternative method to control neuronal activity is chemogenetics, which utilizes exogenous compounds that are otherwise biologically inert to trigger specific biological processes (Figure 2) (Sternson, Atasoy, Betley, Henry, & Xu, 2016; Sternson & Roth, 2014; Whissell, Tohyama, & Martin, 2016; Bruchas & Roth, 2016). The most widely used chemogenetic technique involves ‘designer receptors exclusively activated by designer drugs’ (DREADD), which are synthetic variants of muscarinic acetylcholine receptors coupled to Gi/o, Gq/11, or Gs (Sternson & Roth, 2014). It has been shown that these mutant muscarinic receptors respond only to the biologically inert synthetic compound clozapine-N-oxide (CNO), without being significantly activated by the natural ligand acetylcholine in vitro and in vivo (Alexander et al., 2009; Armbruster, Li, Pausch, Herlitze, & Roth, 2007). Depending on the intracellular pathway activated, this tool provides spatiotemporal control of the activity of the targeted neuronal population, depending on the presence of downstream effectors in the targeted neurons (e.g. usually Gq –coupled DREADD hM3Dq increase and Gi – coupled DREADD hM4Di decrease cellular activities) (Garner et al., 2012; Kong et al., 2012; Kozorovitskiy, Saunders, Johnson, Lowell, & Sabatini, 2012; Ray et al., 2011). Recently, to effectively translate the DREADD technology, new non-CNO DREADD actuators have been developed and been tested for their potential in remote control of behavior (Roth, 2016; Chen et al., 2015; Wacker, Stevens, & Roth, 2017). For example, a new Gi-coupled DREADD was developed using the k-opioid receptor (KOR) as a template. This KOR-DREADD is activated by salvinorin B (SALB), instead of CNO that is used for the previous version of DREADD, and acutely attenuates neuronal activity. Because this non-CNO based DREADD system is activated by SALB, not CNO, this tool can be utilized for bidirectional chemogenetic manipulation of neural circuits combining with other CNO-sensitive DREADD (Vardy et al., 2015).
In contrast to optogenetics, which can switch neuronal activity on and off very rapidly within a millisecond time resolution, DREADD has a relatively slow onset and a prolonged modulation effect due to the activation of GPCR signaling cascades. An alternative chemogenetic approach with faster pharmacokinetics are pharmacologically selective actuator modules (PSAMs), a system that directly modulates ionic conductance through engineered ligand-gated ion channels (e.g. modified glycine receptor as a silencing tool), which are only activated by pharmacologically selective effector molecule (PSEM) agonists (Sternson et al., 2016; Magnus et al., 2011).
Anatomical targeting strategy of optogenetics and chemogenetics
The optogenetic and chemogenetic toolbox can target specific cell populations using site-specific recombinase technology combined with viral vectors, transgenic animals, or both. A common approach is to inject a single recombinant adeno-associated viruses (AAVs) or lentiviruses packaged with engineered opsin or designer receptor transgenes into the specific brain region of interest (Kim et al., 2017; Burnett & Krashes, 2016; Wiegert, Mahn, Prigge, Printz, & Yizhar, 2017). The opsin or designer receptor expression would be driven by cell-type specific promoters: vesicular glutamate transporters (vGLUT; expressed in excitatory neurons), vesicular GABA transporters (vGAT; expressed in inhibitory neurons), dopamine transporter (DAT; dopaminergic neurons), or glial fibrillary acidic protein (GFAP; astrocyte). Therefore, these tools can then be isolated for expression only in a selected cell type in a specific brain region. For example, injecting AAV viral vector expressing ChR2 driven by CaMKII promoter (AAV-CaMKII-ChR2) into the amygdala will express ChR2 opsins mostly in excitatory neurons in the amygdala.
However, this single viral vector approach to deliver genetic toolboxes has limitations if an experiment requires a package with a large transgene construct. Therefore, to overcome some of these viral limitations and target a wide-range of cell types, an alternative approach has recently been developed: combining transgenic animals (or secondary viral vectors) that express recombinase driven by a specific cell type with recombinase-dependent opsin or designer receptor-expressing viral vectors (Gompf, Budygin, Fuller, & Bass, 2015; Sohal, Zhang, Yizhar, & Deisseroth, 2009; Tsai et al., 2009). For example, injecting AAV viral vectors packaging cre-recombinase dependent ‘Double-floxed Inverted open Reading frame’ (DIO) floxed with ChR2 opsin into a brain region of a vGAT-cre mouse can provide strong targeted ChR2 opsin expression selectively in GABAergic cells in the injected brain region (Seo et al., 2016).
Both optogenetics and chemogenetics have advantages and disadvantages and therefore are well suited for different applications. Optogenetics has extremely accurate temporal precision and can control neural activity reversibly, and this feature makes optogenetics useful for generating spike patterns mimicking the endogenous neural firing pattern by adjusting the frequency and duration of the laser delivery. However, in optogenetics, the light is generally delivered via fiber optics that demand an invasive surgical implanting procedure (Figure 3). On the other hand, chemogenetics does not necessarily require the implantation of sometimes cumbersome hardware to deliver chemical actuators to the targeted area. Chemical agents can be delivered systemically via i.p. injections or put in the animal’s drinking water, unless the chemical actuators cannot cross the brain-blood barrier (Jain et al., 2013; Whissell et al., 2016). Also, chemogenetics has several advantages that it does not require numerous equipments such as optic cables and a laser diode/LED to produce light. The decreased spatio-temporal precision of chemogenetics (compared to optogenetics), could be well suited for prolonged manipulations of neural activity or manipulations of larger brain regions (Robinson et al., 2014). Application duration can easily be controlled in the range of minutes to days depending on the ligand delivery method (e.g. i.p., mini-osmotic pumps, mix in drinking water/food) and the pharmacokinetic interaction between the synthesized ligand and biochemical pathways.
Figure 3. Anatomical and cell-type specific targeting strategy of optogenetics and chemogenetics.
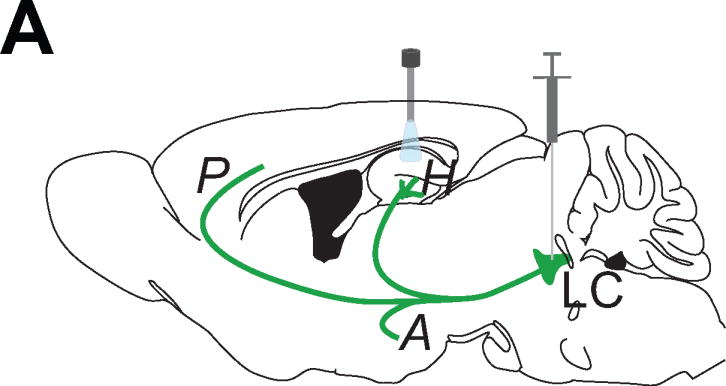
A Schematic representation of the method for expressing opsins in neurons and activation of opsins or designer receptors. A DNA vector encoding an opsin is injected into the brain region of interest (e.g. locus coeruleus, LC), inducing opsin expression in target neurons. Cell type specificity of opsin expression can be achieved by injecting the recombinase dependent (e.g. cre-) opsin virus into a recombinase driver animal (e.g. injecting cre-dependent opsins into the LC of TH-cre transgenic mice will specifically express opsins in the TH positive LC cells). Light or drugs can be delivered to a specific target area either over the cell bodies that project to multiple target areas or in a specific projection region (e.g. prefrontal cortex, P; amygdala, A; hippocampus, H). E.g. to activate the LC projections targeting hippocampus (H), fiber optic can be implanted above hippocampus in the illustration.
In addition, unlike optogenetics, chemogenetics does not require physical tethering to experimental animals, and this allows the testing of complex behaviors. As conventional optogenetics hardware cause restriction of mobility during dynamic interactions between animals, chemogenetics is ideally suited to study these phenomena, such as social behaviors (e.g. playing, mating, aggressions. Also, because tethering can generate a stress response in animals, the absence of fiber optic cables allows more naturalistic behavioral outcomes. Recently, to overcome this limitation in optogenetics, there have been efforts to create wireless technology using miniaturized, thin, flexible opto-electronic implants, which allow complete optical control in a variety of behavioral paradigms (Shin et al., 2017; Kim et al., 2013; Jeong et al., 2015).
The subcellular location of photostimulation or chemical actuator delivery is an important factor to functionally dissect neuronal circuitry. Conventional optogenetics or chemogenetics usually activate effectors in the cell body of the structure, but projection targeting enables some versatile experimental leverage. For example, the control of projecting terminals can provide selective control of projection between brain regions without compromising the activities of other synapses originating from the same neurons (Figure 3) (Kim et al., 2017; Burnett & Krashes, 2016).
Recent findings in neurodevelopmental disorder research using optogenetic and chemogenetic methods
Neurodevelopmental disorders are a group of psychiatric diseases marked by abnormal growth of the central nervous system, which often include symptoms of impaired cognitive and motor functions (van Loo & Martens, 2007; Sontheimer, 2015). As discussed earlier, although the large scale correlational studies in the field create implications for the underlying gene and molecular mechanisms of these abnormal behaviors and improves our understanding of neuro-psychiatric disorders, there has been a lack of research testing the hypothesis that alteration of genes and molecular pathways causes the observed abnormal behavior. The advent of cell-type specific perturbation tools, such as optogenetics and chemogenetics, opens up new opportunities for causal investigation of brain circuitry in a reversible manner with behavioral analysis being related to psychiatric symptoms. Recent progress in the field suggests that multiple unrelated genetic abnormalities and their related downstream molecular pathways feature unusual neurophysiology in certain neural circuits and can generate abnormal behavioral phenotypes (Sohal, Zhang, Yizhar, & Deisseroth, 2009; Yizhar et al., 2011; Walsh et al., 2008; Sahin & Sur, 2015).
In the following section, we highlight recent rodent studies where optogenetic and chemogenetic tools have been employed to dissect the neuronal basis of neurodevelopmental disorders, with particular focus on circuit-level concepts with several major neurodevelopmental disorders, including autism spectrum disorders (ASD), schizophrenia spectrum disorders (SSD), obsessive-compulsive disorder (OCD) and attention-deficit hyperactive disorders (ADHD).
Autism Spectrum Disorders
Autism spectrum disorder (ASD) is a complex developmental disability. Conditions that define ASD appear during early childhood and include deficits in social communications and interactions as well as restricted or repetitive behavior (Sahin & Sur, 2015; Peñagarikano et al., 2015). Social deficits are one of major signs of ASD, and are also common to schizophrenia (discussed below) (Woodbury-Smith, 2010). One emerging hypothesis to explain this behavioral characteristic is that the impairment of homeostatic balance between excitation and inhibition in cortical neural networks causes the social and cognitive deficits characterized in autism and schizophrenia (Rubenstein & Merzenich, 2003). Recently, Yizhar and colleagues (2011) tested this hypothesis employing optogenetic techniques to modulate the ratio of cellular excitation and inhibition (E/I ratio) in cortical neurons (Yizhar et al., 2011). The team utilized bistable step-function opsin (SFO), which can depolarize neurons for prolonged periods, to selectively activate a population of either excitatory (opsin expression driven by CaMKIIα) or inhibitory (opsin expression driven by parvalbumin) neurons. This manipulation was combined with several behavioral tests that are relevant to autism and schizophrenia, such as social interaction and episodic memory tests. They found that activation of excitatory neurons in the medial prefrontal cortex, which mimics elevation of the E/I ratio, reduced social interaction of juvenile mice with an unfamiliar mouse in their home cage. Activation of the excitatory neurons also resulted in impairment of episodic fear memory formation. Furthermore, the authors demonstrated that this behavioral impairment is associated with elevated high-frequency power in the range of gamma waves (30–80 Hz), which has been consistently reported in clinical settings in humans (Rojas, Maharajh, Teale, & Rogers, 2008). These results provide causal support for the cellular E/I hypothesis that the disturbance of information processing associated with E/I imbalance causes abnormal behavioral and physiological phenotypes, such as deficits in social interaction and cognition. Moreover, this study also directly demonstrates that such a physiological imbalance affects behavioral changes in real time as the phenotype manifested by the induced E/I imbalance distorts developmental trajectory.
Many studies have supported the idea that fast-spiking parvalbumin (PV) positive GABAergic interneurons are involved in generating cortical gamma oscillations (Sohal et al., 2009; Cho & Sohal, 2014; Cho et al., 2015). It is remarkable that Yizhar and colleagues (2011) also observed that an elevation of the E/I ratio increased high-frequency power. Prior studies from the same group have shown that the optogenetic inhibition of PV positive interneurons suppresses gamma oscillations in vivo, whereas driving these interneurons through activating excitatory input is sufficient to generate gamma wave in the prefrontal cortex acute slice setting, which implies that abnormal activity of PV interneurons in the PFC may drive E/I imbalance and cause dysfunctional behaviors and physiological patterns (Sohal et al., 2009).
A recent study focused on the intra-amygdala circuit, which is known to be widely engaged in a range of affective behaviors, for a role of social interaction (Felix-Ortiz & Tye, 2014; Siuda et al., 2016; Twining, Vantrease, Love, Padival, & Rosenkranz, 2017). Robert Twining and colleagues (2017) used a social fear conditioning paradigm, in which a ‘demonstrator’ rat is conditioned to pair a tone with foot-shocks while an ‘observer’ rat can interact with the ‘demonstrator’ through a mesh barrier. In the study, the ‘observer’ processes the conditioned stimuli through social interaction. This social transmission process was impaired with the inactivation of the lateral nucleus of amygdala (LA) to medial amygdala (MeA) pathway using the Gi-coupled DREADD. Furthermore, knockout rats lacking Nrxn1, an analog of autism-associated gene NRXN, showed similar LA–MeA impairment, and the behavioral deficit was rescued by Gs-coupled DREADD activation of CaMKII positive cells of the MeA. Another study using optogenetics showed that glutamatergic neurons in the ventral tegmental area (VTA) drive unconditioned sociability (Krishnan et al., 2017). Similarly observed in autism disorders, this study demonstrated that downregulation of Cbln1, which is a gene that drops in response to the excess UBE3A that encode ubiquitin ligase with transcriptional co-regulatory functions, resulted in sociability deficit. The deficit was rescued by activating glutamatergic VTA neurons using Gq-coupled DREADDs. Future studies will likely unravel which circuit is more relevant to specific behavioral tasks. For example, the amygdala is more important for the learning component of sociability whereas the motivational component of social interaction is more related to the VTA and nuclecus accumbens outputs.
Schizophrenia
Schizophrenia is a psychiatric disorder characterized by a variety of symptoms (Owen et al., 2011; Goldstein et al., 2002). Positive symptoms like hallucinations and delusions are often accompanied by negative symptoms such as anhedonia, apathy, and social withdrawal. Furthermore, schizophrenia is often associated with a decline in cognitive functions such as ability to focus and to retain information. These wide-ranging symptoms manifest in different extents across individuals, which suggests that environmental factors likely play a role in the development and progression of the disease (Van Assche, Morrens, Luyten, Van de Ven, & Vandenbulcke, 2017; American Psychiatric Association, 2013). Because of these seemingly disparate symptoms and unknown environmental contribution, it has been historically difficult to characterize the underlying neuropathology of schizophrenia. The marked impairment in interpersonal relations and cognitive dysfunction is a feature shared with ASD symptoms, implying that some circuit mechanisms related to social interaction and cognition can explain symptoms of schizophrenia, which was discussed above for ASD such as E/I imbalance of neural activity in PFC excitatory, intra-amygdala and VTA circuitry (Huang, Tang, & Jiang, 2013; Van Assche et al., 2017; Yizhar, 2012; Yizhar et al., 2011; Krishnan et al., 2017). Also, many executive functions which are impaired in schizophrenia have been shown to involve synchronous neuronal activity known as gamma oscillations, and these gamma oscillations are thought to be controlled by parvalbumin-expressing inhibitory interneurons (Cho & Sohal, 2014; Cho et al., 2015).
Unlike ASD, positive schizophrenic symptoms typically appear in adulthood (Van Assche et al., 2017; Goldstein et al., 2002). Dopamine has long been suspected as playing a major role in the progression of positive symptoms and as such has consistently been a target for therapeutic treatments (Moore, West, & Grace, 1999). Recent studies have found that the abnormal activity of the PFC-VTA/SNc circuit drive hyper-locomotor activity, which is a behavioral model for positive symptoms of psychosis (Kim et al., 2015). Kim and colleagues (2015) showed that the deletion of the actin-related protein 2/3 complex (Arp2/3) in excitatory neurons in the frontal cortex resulted in hyperexcitability of PFC neurons and drove hyper-locomotor activity. The behavioral effect was mimicked by optogenetic stimulation of the PFC-VTA/SNc circuit, which interestingly also elevated striatal dopamine levels. Further studies with more rigorous behavioral analysis that can dissociate between negative and positive symptoms will help reveal the neural circuit mechanisms relevant to psychiatric disorders.
OCD
Obsessive compulsive disorder (OCD) is marked by two kinds of maladaptive behavior: obsessions and compulsions. Obsessions are intrusive thoughts and preoccupations which occur unbidden, and which typically cause distress and/or dysfunction. Common obsessions include fixation on hygiene, fear of violating extreme cultural taboos, and a strong desire for symmetry or perfectness (Ahmari & Dougherty, 2015). Compulsions are any kind of repeated habit or ritual which may or may not be harmful in and of itself, but is often performed to an extent where it causes dysfunction. Moreover, not performing compulsions can also lead to psychological distress (American Psychiatric Association, 2013). While the causes of OCD are not yet well-understood, there are several emerging lines of research which have explored potential neurological factors underlying this condition. As with many diseases, environmental factors likely influence the course of the disorder, and as such the specificities and symptoms vary across individuals. Much of the existing body of research on OCD implicates glutamatergic, serotoninergic and dopaminergic circuits linked to cortico-striato-thalamo-cortical pathway in the progression of the disease (Ahmari & Dougherty, 2015; Ahmari et al., 2013). Ahmari and colleagues (2013) have recently provided causal evidence showing that optogenetic stimulation (5 min/day) of projections from the orbitofrontal cortex to the ventromedial striatum (OFC-VMS) led to an elevation of grooming behavior, which is considered a mouse behavioral model related to OCD, over the course of several days (Ahmari et al., 2013). Increased grooming behavior persisted for2 weeks after stimulation cessation. Also, the excessive grooming behavior induced by hyperactivity of the OFC-VMS circuit was normalized by fluoxetine treatment, a medication regimen used to treat OCD. Another study used a mutant mouse model with a deleted Sapap3 gene, which is involved in the molecular organization of synapses and neuronal cell signaling (Burguière, Monteiro, Feng, & Graybiel, 2013). Both mutant and control mice were conditioned to groom by dropping water on their forehead at the sound of a tone. After the training, the mutants began to groom to the tone even without a water drop. This excessive repetitive behavior was alleviated by optogenetic stimulation of lateral OFC. Such a rapid relief from symptoms in this study is a somewhat different pattern from the Ahmari et al. study (2013), which used chronic repeated hyperactivation of the OFC-VMS to model aspects of the symptom. This could be due to either methodological differences, such as in the mouse model or behavioral tasks utilized, or which particular subregion (medial versus lateral) of the OFC is engaged. Still, the exact mechanism by which this behavior is evoked remains to be elucidated.
A recent study proposed aberrant histaminergic function is engaged in excessive grooming behavior (Rapanelli et al., 2017; Rapanelli, Frick, Bito, & Pittenger, 2017). Rapanelli and colleages (2017) showed that chemogenetic silencing of histaminergic neurons in the tuberomammillary nucleus (TMN) of the hypothalamus leads to markedly elevated grooming using Gi-coupled DREADD system. Thus, the role of histamine in local neurocircuitry may present a fruitful line of study in further elucidating the development of compulsive behavior.
Attention-deficit hyperactivity disorders (ADHD)
ADHD is a type of neurodevelopmental disorder that is characterized by difficulty in focusing (Gallo & Posner, 2016). However, understanding the neurobiological mechanism of ADHD, as with other disorders, is complicated because the behavioral characteristics of ADHD are not necessarily unique to ADHD. For instance, deficits in cognitive flexibility and attention and emotional dysregulation can also be observed in schizophrenia (Egeland, 2007). Some recent studies used optogenetic and chemogenetic tools to map neural circuits involved in attention-related behavioral tasks and found that the anterior cingulate gyrus and locus coeruleus are implicated in the tasks (Janitzky et al., 2015; Koike et al., 2016). However, it is still too preliminary to conclude that this is the unique neurobiological basis of the ADHD. Future studies combining ADHD-specific mouse models characterizing core features of ADHD along with an appropriate control line will advance understanding of this complex disease.
Current limitation and future directions
As described above, the current approach on neuropsychiatric disorders has been moving from large-scale correlational studies using large data analysis to investigate gene and protein expression patterns in the brain and behavioral patterns, such as behavioral genomics, to a neural circuit analysis for understanding dysfunction in neurodevelopmental disorders (Jazayeri & Afraz, 2017). This transition has also been influenced by novel engineering approaches and unique biological tools necessary to better understand these neurobiological questions. This type of research requires a careful transition between new biological questions and developing new tools to overcome the limitation of existing tools, and mechanistic interpretations.
There are several limitations and factors that need to be carefully considered in experimental design before getting started using these new tools. For example, in optogenetics, laser intensity and duration of delivery to a target area via fiber optics should be carefully adjusted, considering that high intensity and/or long-lasting laser application may cause brain tissue damage or alter physiological properties through changing brain tissue temperature (Stujenske, Spellman, & Gordon, 2015). The intensity of the laser is also a factor that determines the possible range that can be studied in the target of interest (i.e. higher intensity light will spread to a wider area). This factor is more important in projection-targeting experiments compared to cell body-targeting experiments, because in some cases, collateral axonal projections are widely spread, and it is difficult to activate opsins in a small focal area with traditional fiber optics. Recently, to limit unexpected light diffusion – longer wavelength devices and opsins that can penetrate deeper into the brain have been developed. These can deliver light to a limited focal area, a new fiber optic has been developed (Pisanello et al., 2017; Shin et al., 2017; Al-Hasani et al., 2015). Generally, projection-targeting experiments present more obstacles. The opsins in the long-range axonal terminal will take longer to express fully (e.g. 6 weeks after virus injection). Also, axonal optogenetic stimulation (or non-specific en passant stimulation) may cause antidromic spiking to the cell body and eventually activate collateral axon-terminals that branch out from the cell body (Jennings et al., 2013). In this case, it is difficult to interpret behavioral changes as an outcome of specific terminal activation within a region. Systemic analysis combining electrophysiology and immediate early gene expression, or measured with electrophysiology and pharmacology presents a possible solution to rule out the possibility that an upstream brain region is affected by optogenetic stimulation. Also, a recent development of high efficient retrograde access to projection neurons from a certain terminal region allows the control of specific efferent projection by activating cell-body region (Tervo et al., 2016). This approach can be additional solution to the issues related to terminal stimulation that are discussed above. In addition, the many physiological characterizations of optogenetic tools have been tested in vitro. The characterization of those tools could be different in vivo depending on neural connectivity, the level of viral expression, and light delivery. Therefore, careful adjustment of the factors and in vivo re-characterization should be considered before embarking on using these tools for testing a particular hypothesis.
Further efforts are needed to improve inhibitory optogenetic tools (Wiegert et al., 2017; Kim et al., 2017). The current versions of inhibitory opsins (Cl− pump: NpHR or H+ pumps: Arch) are relatively less efficient compared to excitatory opsin channels. In addition, they present some consistency issues (Raimondo, Kay, Ellender, & Akerman, 2012; Mahn, Prigge, Ron, Levy, & Yizhar, 2016). For example, silencing the activity of neurons with NpHR can increase the probability of synaptically-evoked spiking following the termination of photoactivation (Raimondo et al., 2012), but this does not occur when using Arch. However, photoactivation in axons with Arch leads to increased spontaneous neurotransmitter release 2-3 minitues after photoactivation (Mahn et al., 2016). GPCR-based optogenetics (opto-XR) has not been assessed for these particular issues, but could also be a potential solution (Spangler & Bruchas, 2017; Siuda et al., 2015a; Siuda et al., 2015b) given that many GPCRs effectively inhibit release of transmitters from presynaptic terminals in vivo, within endogenous circuits.
When using chemogenetic DREADD-based systems, new concerns have arisen over CNO (DREADD actuator) usage (Gomez et al., 2017). Recently, Gomez and colleagues (2017) reported that upon systemic injection of CNO, the CNO first rapidly converts to clozapine, which is a chemical form of antipsychotic medication that binds serotonin and dopamine receptors, and then enters the central nervous system, which is followed by binding to CNS-expressed DREADDs. This finding demands careful interpretation of results from past DREADD studies. Authors suggest using subthreshold doses of clozapine instead of CNO as an actuator. However, because there could be potential off-target effects elicited by clozapine itself, proper DREADD null CNO-injected controls are necessary in order to draw reliable conclusions(e.g. low doses of clozapine in the absence of the designer receptors)(Mahler & Aston-Jones, 2018).
In this review, we focused on modern tools to manipulate neural circuits, but there are increasing attempts to integrate these neural-control tools with techniques for monitoring neural activity and tagging activated cells, such as Ca2+ imaging and neural tagging to reactivate specific populations of cells that were previously activated (Carrillo-Reid, Yang, Kang Miller, Peterka, & Yuste, 2017; Liu et al., 2012). For integrating optogenetics with Ca2+ imaging, opsins should be carefully selected, and fluorescent light intensity and wavelength sensitive of the opsin should be tightly titrated to avoid cross-stimulation of opsins by fluorescent light during Ca2+ imaging. For example, fluorescent light for Green Fluorescent-Calmodulin Protein (GCaMP) imaging can partially activate blue-shifted opsins and also red-shifted opsins. Integrating chemogenetics with Ca2+ imaging is an alternative option albeit with limited spatiotemporal advantages, but future generations of engineered opsins and Ca2+ sensors will likely resolve these issues. Recently engineered concurrent detection of elevated calcium and light in a living cell will lead to the development of new tools to “tag” specific populations of cells activated within a specific time window, which would allow the targeted neurons to be controlled by optogenetics or chemogenetics at a later time point (Wang et al., 2017). This tagging technique can be useful in developmental studies to track certain populations of cells tagged in an early stage of development and then investigate their function in later life. For instance, a certain population of neural ensembles that are activated by early life stress/traumatic event can be re-activated or inhibited to test if that particular ensemble is involved in shaping neurodevelopmental disorders (e.g. SSD, ASD, PTSD) (Kerns, Newschaffer, & Berkowitz, 2015; Schäfer et al., 2012).
As described above, the recent findings using these new tools provides evidence of the functional relevance of brain circuitry to disease, but some studies have even demonstrated functional rescue in mutant mice. These findings underpin the potential utility of optogenetics and chemogenetics as potentially useful for developing therapeutic applications in human patients (Gilbert, Harris, Neuroscience, & 2014,). However, there are some considerations and challenges which must be addressed before moving into human applications. First, these techniques are still highly invasive medical procedures. It would be challenging to express opsins or designer receptor genes into adult human neurons within a specific brain area, given the known limitations of gene therapy. In particular, for optogenetic application, effective large fiber optics would have to be designed on a human scale with a sufficient light source. The recent progress in developing wireless options using miniaturized, thin, flexible optoelectronic implants is a promising advance (Shin et al., 2017). The other issue regarding human application of these tools is that most current neural circuitry studies apply optogenetic and chemogenetic tools for acute treatment. These techniques are still poorly understood in terms of their stability and impact following chronic stimulation and/or long term expression in cells and neurons. For instance, there is a lack of information regarding cellular health with long-term virus expression or chronic repeated photo- or chemical activation of neurons. There will certainly be further technological advancement in optogenetics and chemogenetics as the National Institutes of Health and National Science Foundation BRAIN initiatives continue to invest in new tools for dissecting brain function; undoubtedly this will lend several new tools for exploring psychiatric dysfunction.
Lastly, in parallel with technological developments to control neural circuits, systematic evaluation of behavioral tests is of great importance in making careful interpretations and conclusions with results of optogenetic and chemogenetic circuit manipulations. Although the contemporary tools that we have discussed above offer a highly sophisticated approach to controlling neural circuits, the way the field translates the function of a circuit is ultimately based on outcomes of behavioral changes. Unfortunately, most behavioral tests have an inherent complexity (i.e. multiple cognitive / motivational factors could affect a single behavior test), and it can be difficult to draw a clear conclusion with a simple behavior test and measurement in rodents. For example, as we discussed above, most literature in this field uses rodent social interaction tests controlling a targeted-circuit, to claim that the targeted-circuit is involved in social dysfunction that is a common phenotype of many psychiatric disorders including ASD and SSD (Yizhar et al., 2011; Krishnan et al., 2017; Cho & Sohal, 2014). In the standard social interaction test, animals explore a chamber where conspecific animals are constrained under a mashed cup, and their exploration time with the constrained mice is measured. However, human social behavior is much more complex, and measuring exploration time, even without contacts between animals in the test, provides only limited insights into social behavior (Hånell & Marklund, 2014). That is, it is not clear if the circuit manipulation affected only the recognition/memory of a conspecific animal or changed their social interest/communication within the measurement. Measuring a complimentary rich suite of other social behaviors such as sniffing, playing, and ultrasonic vocalization would help make a stronger interpretations of the circuit manipulation and assist in finding adequate matches to known phenotypic traits of psychiatric disorders. Also, it is often important to test behaviors combining optogenetic and chemogenetic controls with etiologically relevant pharmacological treatment. For example, in the OCD study mentioned above, Ahmari and colleagues (2013) showed that cortico-striatal optogenetic stimulation increased self-grooming behavior. However, it may not necessarily implicate an emotion-related circuit mechanism of OCD (i.e. it could be just increment of motor function related to the pattern of grooming behavior). In the study, the authors showed that the excessive grooming behavior evoked by optogenetic stimulation was reversed by chronic fluoxetine treatment that is used as a first-line OCD treatment. Therefore, rigorous behavioral testis using parallel classical and contemporary neural control tools are demanded. Also, it is important to continue developing additional behavioral models that more realistically reflect clinical behavioral traits; and further developments in machine – learning to measure behavioral outcomes in non-biased ways are at the forefront of some of this research (Wiltschko et al., 2015).
Conclusions
Recent advances in the field of neural circuit manipulation have allowed for inference of causal relationships between neural circuitry dysfunction and neurodevelopmental disorders. Optogenetics and chemogenetics both provide a variety of means to regulate neural activity facilitating the ability to map various relationships between different cell types within specific neural circuits.
While these tools are powerful and have undeniably advanced our understanding of neural circuitry, the complex nature of mental illnesses still presents major obstacles in the search for the mechanisms and underlying causes. Due to the substantial overlap of symptoms between disorders, it is difficult to know to what extent some underlying mechanisms are separable from each other. An integrative approach including neuronal manipulations within a disease-related genetic animal models will be an ideal strategy for better determining the casual relationship between a neural circuit and a disease. Eventually, these systematic, step-by-step analyses from gene, to circuit, to behavior will be crucial for dissecting the fundamental origins of abnormal behavior in psychiatric disorders including neurodevelopmental disorders. These new discoveries will then eventually help to provide promising therapeutic solutions and interventions.
Highlights.
Brain circuitry approach to understand neurodevelopmental disorders.
Modern neuroscience tools for neural circuitry analysis: optogenetics and chemogenetics.
Causal relationship between a neural circuit dysfunction and behavioral alteration.
Acknowledgments
We thank Skylar M. Spangler for comments on the manuscript. This work was supported by National Institute of Health Grants: NIH R01MH112355, and BRAIN Initiative 1U01 MH10913301.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmari SE, Dougherty DD. DISSECTING OCD CIRCUITS: FROM ANIMAL MODELS TO TARGETED TREATMENTS. Depress Anxiety. 2015;32(8):550–562. doi: 10.1002/da.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, Hen R. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340(6137):1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458(7241):1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63(1):27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Bruchas MR. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron. 2015;87(5):1063–1077. doi: 10.1016/j.neuron.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alivisatos AP, Andrews AM, Boyden ES, Chun M, Church GM, Deisseroth K, Zhuang X. Nanotools for neuroscience and brain activity mapping. ACS Nano. 2013;7(3):1850–1866. doi: 10.1021/nn4012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) 2013 doi: 10.1590/s2317-17822013000200017. Retrieved from http://books.google.com/books?id=-JivBAAAQBAJ&hl=&source=gbs_api. [DOI] [PubMed]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104(12):5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nestler EJ. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68(4):314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamann C, Kirsch T, Nagel G, Bamberg E. Spectral characteristics of the photocycle of channelrhodopsin-2 and its implication for channel function. J Mol Biol. 2008;375(3):686–694. doi: 10.1016/j.jmb.2007.10.072. [DOI] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Wietek J, Ramakrishnan C, Steinberg EE, Rashid AJ, Deisseroth K. Structural foundations of optogenetics: Determinants of channelrhodopsin ion selectivity. Proc Natl Acad Sci U S A. 2016;113(4):822–829. doi: 10.1073/pnas.1523341113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Schoenenberger P, Mattis J, Tye KM, Deisseroth K, Hegemann P, Oertner TG. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc Natl Acad Sci U S A. 2011;108(18):7595–7600. doi: 10.1073/pnas.1017210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bistable neural state switches. Nat Neurosci. 2009;12(2):229–234. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Roth BL. New Technologies for Elucidating Opioid Receptor Function. Trends Pharmacol Sci. 2016;37(4):279–289. doi: 10.1016/j.tips.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguière E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340(6137):1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett CJ, Krashes MJ. Resolving Behavioral Output via Chemogenetic Designer Receptors Exclusively Activated by Designer Drugs. J Neurosci. 2016;36:9268–9282. doi: 10.1523/JNEUROSCI.1333-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reid L, Yang W, Kang Miller JE, Peterka DS, Yuste R. Imaging and Optically Manipulating Neuronal Ensembles. Annu Rev Biophys. 2017 doi: 10.1146/annurev-biophys-070816-033647. [DOI] [PubMed] [Google Scholar]
- Chen X, Choo H, Huang XP, Yang X, Stone O, Roth BL, Jin J. The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Neurosci. 2015;6(3):476–484. doi: 10.1021/cn500325v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KK, Sohal VS. Optogenetic approaches for investigating neural pathways implicated in schizophrenia and related disorders. Hum Mol Genet. 2014;23(R1):R64–8. doi: 10.1093/hmg/ddu225. [DOI] [PubMed] [Google Scholar]
- Cho KK, Hoch R, Lee AT, Patel T, Rubenstein JL, Sohal VS. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/-) mice. Neuron. 2015;85(6):1332–1343. doi: 10.1016/j.neuron.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463(7277):98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clair EC, Ogren JI, Mamaev S, Kralj JM, Rothschild KJ. Conformational changes in the archaerhodopsin-3 proton pump: detection of conserved strongly hydrogen bonded water networks. J Biol Phys. 2012;38(1):153–168. doi: 10.1007/s10867-011-9246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colibazzi T. Journal Watch review of Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. J Am Psychoanal Assoc. 2014;62(4):709–710. doi: 10.1177/0003065114543185. [DOI] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18(9):1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Hegemann P. The form and function of channelrhodopsin. Science. 2017;357(6356) doi: 10.1126/science.aan5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland J. Differentiating attention deficit in adult ADHD and schizophrenia. Arch Clin Neuropsychol. 2007;22(6):763–771. doi: 10.1016/j.acn.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 2014;34(2):586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo EF, Posner J. Moving towards causality in attention-deficit hyperactivity disorder: overview of neural and genetic mechanisms. Lancet Psychiatry. 2016;3(6):555–567. doi: 10.1016/S2215-0366(16)00096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. Generation of a synthetic memory trace. Science. 2012;335(6075):1513–1516. doi: 10.1126/science.1214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert F, Harris AR, Neuroscience, R. M. I. K.-A. J. O. B. Controlling brain cells with light: ethical considerations for optogenetic clinical trials. Taylor & Francis; 2014. Retrieved from http://www.tandfonline.com/doi/full/10.1080/21507740.2014.911213. [Google Scholar]
- Goldstein G, Minshew NJ, Allen DN, Seaton BE. High-functioning autism and schizophrenia: a comparison of an early and late onset neurodevelopmental disorder. Arch Clin Neuropsychol. 2002;17(5):461–475. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14592000. [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357(6350):503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompf HS, Budygin EA, Fuller PM, Bass CE. Targeted genetic manipulations of neuronal subtypes using promoter-specific combinatorial AAVs in wild-type animals. Front Behav Neurosci. 2015;9:152. doi: 10.3389/fnbeh.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Janz R, Liu X, Spudich JL. NEUROSCIENCE. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science. 2015;349(6248):647–650. doi: 10.1126/science.aaa7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One. 2007;2(3):e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Boyden ES. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hånell A, Marklund N. Structured evaluation of rodent behavioral tests used in drug discovery research. Front Behav Neurosci. 2014;8:252. doi: 10.3389/fnbeh.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Tang B, Jiang H. Optogenetic investigation of neuropsychiatric diseases. Int J Neurosci. 2013;123(1):7–16. doi: 10.3109/00207454.2012.728651. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jain S, Ruiz de Azua I, Lu H, White MF, Guettier JM, Wess J. Chronic activation of a designer G(q)-coupled receptor improves β cell function. J Clin Invest. 2013;123(4):1750–1762. doi: 10.1172/JCI66432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janitzky K, Lippert MT, Engelhorn A, Tegtmeier J, Goldschmidt J, Heinze HJ, Ohl FW. Optogenetic silencing of locus coeruleus activity in mice impairs cognitive flexibility in an attentional set-shifting task. Front Behav Neurosci. 2015;9:286. doi: 10.3389/fnbeh.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri M, Afraz A. Navigating the Neural Space in Search of the Neural Code. Neuron. 2017;93(5):1003–1014. doi: 10.1016/j.neuron.2017.02.019. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496(7444):224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, McCall J, Shin G, Zhang Y, Al-Hasani R, Kim M, Rogers J. Wireless Optofluidic Systems for Programmable In Vivo Pharmacology and Optogenetics. Cell. 2015;162(3):662–674. doi: 10.1016/j.cell.2015.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, Newschaffer CJ, Berkowitz SJ. Traumatic Childhood Events and Autism Spectrum Disorder. J Autism Dev Disord. 2015;45(11):3475–3486. doi: 10.1007/s10803-015-2392-y. [DOI] [PubMed] [Google Scholar]
- Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci. 2017;18(4):222–235. doi: 10.1038/nrn.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IH, Rossi MA, Aryal DK, Racz B, Kim N, Uezu A, Soderling SH. Spine pruning drives antipsychotic-sensitive locomotion via circuit control of striatal dopamine. Nat Neurosci. 2015;18(6):883–891. doi: 10.1038/nn.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TI, McCall JG, Jung YH, Huang X, Siuda ER, Li Y, Bruchas MR. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340(6129):211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Demars MP, Short JA, Nabel EM, Akbarian S, Baxter MG, Morishita H. Chemogenetic Inactivation of Dorsal Anterior Cingulate Cortex Neurons Disrupts Attentional Behavior in Mouse. Neuropsychopharmacology. 2016;41(4):1014–1023. doi: 10.1038/npp.2015.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151(3):645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Saunders A, Johnson CA, Lowell BB, Sabatini BL. Recurrent network activity drives striatal synaptogenesis. Nature. 2012;485(7400):646–650. doi: 10.1038/nature11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Stoppel DC, Nong Y, Johnson MA, Nadler MJ, Ozkaynak E, Anderson MP. Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1. Nature. 2017;543(7646):507–512. doi: 10.1038/nature21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein PJ. Environmental Factors in Neurodevelopmental and Neurodegenerative Disorders. Elsevier; 2015. Overview of the Role of Environmental Factors in Neurodevelopmental Disorders; pp. 3–20. [DOI] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96(5):1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484(7394):381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011;333(6047):1292–1296. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahn M, Prigge M, Ron S, Levy R, Yizhar O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat Neurosci. 2016;19(4):554–556. doi: 10.1038/nn.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones G. CNO Evil? Considerations for the Use of DREADDs in Behavioral Neuroscience. Neuropsychopharmacology. 2018 doi: 10.1038/npp.2017.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram K, Markram H. The intense world theory – a unifying theory of the neurobiology of autism. Front Hum Neurosci. 2010;4:224. doi: 10.3389/fnhum.2010.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masseck OA, Spoida K, Dalkara D, Maejima T, Rubelowski JM, Wallhorn L, Herlitze S. Vertebrate cone opsins enable sustained and highly sensitive rapid control of Gi/o signaling in anxiety circuitry. Neuron. 2014;81(6):1263–1273. doi: 10.1016/j.neuron.2014.01.041. [DOI] [PubMed] [Google Scholar]
- Matsuno-Yagi A, Mukohata Y. Two possible roles of bacteriorhodopsin; a comparative study of strains of Halobacterium halobium differing in pigmentation. Biochem Biophys Res Commun. 1977;78(1):237–243. doi: 10.1016/0006-291x(77)91245-1. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20882. [DOI] [PubMed] [Google Scholar]
- McGregor KM, Bécamel C, Marin P, Andrade R. Using melanopsin to study G protein signaling in cortical neurons. J Neurophysiol. 2016;116(3):1082–1092. doi: 10.1152/jn.00406.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, West AR, Grace AA. The regulation of forebrain dopamine transmission: relevance to the pathophysiology and psychopathology of schizophrenia. Biol Psychiatry. 1999;46(1):40–55. doi: 10.1016/s0006-3223(99)00078-5. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10394473. [DOI] [PubMed] [Google Scholar]
- Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296(5577):2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13(10):1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, O’Donovan MC, Thapar A, Craddock N. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198(3):173–175. doi: 10.1192/bjp.bp.110.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Lázaro MT, Lu XH, Gordon A, Dong H, Lam HA, Geschwind DH. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7(271):271ra8. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanello F, Mandelbaum G, Pisanello M, Oldenburg IA, Sileo L, Markowitz JE, Sabatini BL. Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber. Nat Neurosci. 2017;20(8):1180–1188. doi: 10.1038/nn.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo JV, Kay L, Ellender TJ, Akerman CJ. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat Neurosci. 2012;15(8):1102–1104. doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Frick L, Bito H, Pittenger C. Histamine modulation of the basal ganglia circuitry in the development of pathological grooming. Proc Natl Acad Sci U S A. 2017;114(25):6599–6604. doi: 10.1073/pnas.1704547114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Frick L, Pogorelov V, Ohtsu H, Bito H, Pittenger C. Histamine H3R receptor activation in the dorsal striatum triggers stereotypies in a mouse model of tic disorders. Transl Psychiatry. 2017;7(1):e1013. doi: 10.1038/tp.2016.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333(6042):637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein ML, Deussing JM. The optogenetic (r)evolution. Mol Genet Genomics. 2012;287(2):95–109. doi: 10.1007/s00438-011-0663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, Bucci DJ. Chemogenetic silencing of neurons in retrosplenial cortex disrupts sensory preconditioning. J Neurosci. 2014;34(33):10982–10988. doi: 10.1523/JNEUROSCI.1349-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale P, Rogers SJ. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry. 2008;8:66. doi: 10.1186/1471-244X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89(4):683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14606691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M, Sur M. Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science. 2015;350(6263) doi: 10.1126/science.aab3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer I, Fisher HL, Aderhold V, Huber B, Hoffmann-Langer L, Golks D, et al. Dissociative symptoms in patients with schizophrenia: relationships with childhood trauma and psychotic symptoms. Compr Psychiatry. 2012;53(4):364–371. doi: 10.1016/j.comppsych.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Seo D-O, Funderburk SC, Bhatti DL, Motard LE, Newbold D, Girven KS, Bruchas MR. A GABAergic Projection from the Centromedial Nuclei of the Amygdala to Ventromedial Prefrontal Cortex Modulates Reward Behavior. Journal of Neuroscience. 2016;36(42):10831–10842. doi: 10.1523/JNEUROSCI.1164-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin G, Gomez AM, Al-Hasani R, Jeong YR, Kim J, Xie Z, Rogers JA. Flexible Near-Field Wireless Optoelectronics as Subdermal Implants for Broad Applications in Optogenetics. Neuron. 2017 doi: 10.1016/j.neuron.2016.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuda ER, Al-Hasani R, McCall JG, Bhatti DL, Bruchas MR. Chemogenetic and Optogenetic Activation of Gαs Signaling in the Basolateral Amygdala Induces Acute and Social Anxiety-Like States. Neuropsychopharmacology. 2016;41(8):2011–2023. doi: 10.1038/npp.2015.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuda ER, Copits BA, Schmidt MJ, Baird MA, Al-Hasani R, Planer WJ, Bruchas MR. Spatiotemporal control of opioid signaling and behavior. Neuron. 2015a;86(4):923–935. doi: 10.1016/j.neuron.2015.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuda ER, McCall JG, Al-Hasani R, Shin G, Il Park S, Schmidt MJ, Bruchas MR. Optodynamic simulation of β-adrenergic receptor signalling. Nat Commun. 2015b;6:8480. doi: 10.1038/ncomms9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H. Diseases of the Nervous System. Elsevier; 2015. Neurodevelopmental Disorders; pp. 319–347. [DOI] [Google Scholar]
- Spangler SM, Bruchas MR. Optogenetic approaches for dissecting neuromodulation and GPCR signaling in neural circuits. Curr Opin Pharmacol. 2017;32:56–70. doi: 10.1016/j.coph.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Lund C, Nesse RM. Classification systems in psychiatry: diagnosis and global mental health in the era of DSM-5 and ICD-11. Curr Opin Psychiatry. 2013;26(5):493–497. doi: 10.1097/YCO.0b013e3283642dfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Atasoy D, Betley JN, Henry FE, Xu S. An Emerging Technology Framework for the Neurobiology of Appetite. Cell Metab. 2016;23(2):234–253. doi: 10.1016/j.cmet.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- Stujenske JM, Spellman T, Gordon JA. Modeling the Spatiotemporal Dynamics of Light and Heat Propagation for In Vivo Optogenetics. Cell Rep. 2015;12(3):525–534. doi: 10.1016/j.celrep.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpir M. New thinking on neurodevelopment. Environ Health Perspect. 2006;114(2):A100–7. doi: 10.1289/ehp.114-a100. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16451834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo D, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola K, Karpova A. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron. 2016 doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Vantrease JE, Love S, Padival M, Rosenkranz JA. An intra-amygdala circuit specifically regulates social fear learning. Nat Neurosci. 2017;20(3):459–469. doi: 10.1038/nn.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche L, Morrens M, Luyten P, Van de Ven L, Vandenbulcke M. The neuropsychology and neurobiology of late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: A critical review. Neurosci Biobehav Rev. 2017 doi: 10.1016/j.neubiorev.2017.08.024. [DOI] [PubMed] [Google Scholar]
- van Loo KM, Martens GJ. Genetic and environmental factors in complex neurodevelopmental disorders. Curr Genomics. 2007;8(7):429–444. doi: 10.2174/138920207783591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy E, Robinson JE, Li C, Olsen RHJ, DiBerto JF, Giguere PM, Roth BL. A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron. 2015;86(4):936–946. doi: 10.1016/j.neuron.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320(5875):539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wacker D, Stevens RC, Roth BL. How Ligands Illuminate GPCR Molecular Pharmacology. Cell. 2017;170(3):414–427. doi: 10.1016/j.cell.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wildes CP, Pattarabanjird T, Sanchez MI, Glober GF, Matthews GA, Ting AY. A light- and calcium-gated transcription factor for imaging and manipulating activated neurons. Nat Biotechnol. 2017 doi: 10.1038/nbt.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whissell PD, Tohyama S, Martin LJ. The Use of DREADDs to Deconstruct Behavior. Front Genet. 2016;7:70. doi: 10.3389/fgene.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert JS, Mahn M, Prigge M, Printz Y, Yizhar O. Silencing Neurons: Tools, Applications, and Experimental Constraints. Neuron. 2017;95(3):504–529. doi: 10.1016/j.neuron.2017.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, Datta SR. Mapping Sub-Second Structure in Mouse Behavior. Neuron. 2015;88(6):1121–1135. doi: 10.1016/j.neuron.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury-Smith M. Autism spectrum disorders, schizophrenia and diagnostic confusion. J Psychiatry Neurosci. 2010;35(5):360. doi: 10.1503/jpm.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 2015 Retrieved from http://books.google.com/books?id=CE_gsgEACAAJ&hl=&source=gbs_api.
- Yizhar O. Optogenetic insights into social behavior function. Biol Psychiatry. 2012;71(12):1075–1080. doi: 10.1016/j.biopsych.2011.12.029. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemelman BV, Lee GA, Ng M, Miesenböck G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33(1):15–22. doi: 10.1016/s0896-6273(01)00574-8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11779476. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446(7136):633–639. doi: 10.1038/nature0. [DOI] [PubMed] [Google Scholar]