Abstract
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation of the synovial tissue of the joints. Inadequately controlled disease may cause severe joint damage and deformity. Currently, the anti-arthritic drugs are given systemically, and therefore, they are widely distributed to other organs that are not the intended therapeutic target. Accordingly, using a particular dose/regimen of a drug to achieve an effective local concentration of the drug in arthritic joints may lead to expected adverse effects involving other organs. Thus, improved methods of drug delivery are needed for arthritis therapy. One attractive approach is the targeting of a systemically administered drug to the inflamed joints. We describe here a prototypic drug delivery system using a novel peptide ligand denoted as ART-1. We previously reported ART-1 (= ADK) as a peptide that preferentially homes to the inflamed joints of arthritic rats and binds to synovial endothelial cells. We tested the ART-1-coated liposomes encapsulating a fluorescent compound for binding to activated endothelial cells in vitro and homing to arthritic joints in vivo, compared to control liposomes lacking the ART-1 coating. Similar liposomes but encapsulating an immunomodulatory cytokine interleukin-27 (ART-1-IL-27 liposomes) were tested for their anti-arthritic activity compared with control liposomes. ART-1-displaying liposomes showed better binding to endothelial cells as well as in vivo homing to arthritic joints compared to control liposomes. Furthermore, ART-1-IL-27 liposomes, when intravenously injected to arthritic rats after the onset of arthritis, were more effective in suppressing disease progression than control-IL-27 liposomes lacking ART-1 or free IL-27 at an equivalent dose of IL-27. In addition, ART-1-directed liposomal IL-27 had a better safety profile than undirected liposomal IL-27 or free IL-27, thereby offering an improved therapeutic index for IL-27 therapy. These results provide a proof-of concept for the use of a novel joint-homing peptide for targeted delivery of drugs including biologics or small molecule compounds to arthritic joints with enhanced efficacy and reduced systemic exposure. This targeted therapy platform may be suitable for use in RA patients.
Keywords: Adjuvant arthritis, Endothelial cells, Inflammation, Interleukin-27, Liposomes, Rheumatoid arthritis, Targeted delivery
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disease of the joints, which may lead to joint damage, deformity, and disability, if not managed effectively [1, 2]. Many anti-arthritic drugs, including non-steroid anti-inflammatory drugs (NSAIDs) and disease-modifying anti-rheumatic drugs (DMARDs) are available [3–7], but frequently their prolonged use is accompanied by severe adverse effects. Furthermore, about 40% of patients fail to respond to biologics (e.g., anti-tumor necrosis factor-α (TNF-α) and anti-interleukin-6 receptor (IL-6R) antibodies) [8, 9], and patients treated with those drugs are at risk of severe infections [7, 10]. The abovementioned drugs are given to RA patients either orally or by systemic injection. Accordingly, in the process of achieving an effective local dose of the drug at the target site (the inflamed joints), several other organs are inadvertently exposed to these drugs, resulting in damage to the liver or kidneys, for example. Furthermore, many of these drugs are not meant for use by local intra-articular injection. Thus, efficient and safe delivery of drugs is a desired goal for the management of RA.
We reasoned that targeted delivery of systemically administered anti-arthritic drugs preferentially into the inflamed joints might help overcome some of the above-mentioned limitations of the anti-arthritic drugs and thereby, improve their therapeutic index. Drug delivery vehicles belong to two main categories, viral and non-viral. In general, the biosafety profiles of non-viral vectors such as liposomes, polymers and dendrimers are better than that of viral vectors. Liposomes are well-studied vehicles for encapsulating drugs and other biomolecules for therapeutic purposes [11, 12]. Most of the previous studies on liposomal drug delivery are based on plain liposomes that lack a specific ligand to direct them to the desired target site. However, a series of studies by Ruoslahti and colleagues [13–15] and others [16, 17] have described targeted delivery of the entrapped drugs to the tumor site using liposomes whose surface was modified with a tumor-homing peptide [13, 16–18]. In addition, these liposomes were conjugated with polyethylene glycol (PEG) to reduce their uptake by the reticuloendothelial system and thereby, preventing rapid elimination from systemic circulation. Similar attempts using a peptide or other ligands are being made for the targeted delivery of drugs in autoimmune diseases, including RA [19–22] and systemic lupus erythematosus (SLE, lupus) [23].
In this study, we set out to develop and test a peptide-directed liposomal drug delivery system in arthritic rats for inhibiting the progression of the disease. In a previous study, we have reported the identification of a 9-amino acid peptide ligand ADK ((henceforth denoted as ART-1) that preferentially homed to inflamed joints of arthritic rats [24]. This peptide was identified using ex vivo and in vivo screening of a phage peptide-display library. It showed binding to the vascular endothelium in sections of inflamed joint tissue as well as to joint-derived rat endothelial cells and cultured (activated) human endothelial cells in vitro. We selected peptide ART-1 for targeting a systemically administered immunomodulatory cytokine (IL-27) into the joints of arthritic rats. IL-27 is a new member of the IL-12 family and it consists of the Epstein-Barr virusinduced gene 3 (EBI3) and p28 subunits [25–27]. In an earlier study, we have shown that the treatment of arthritic rats with IL-27 inhibited the development and progression of arthritis [28]. The beneficial effect of IL-27 was attributable in part to the inhibition of IL-17 response. The immunomodulatory activity of IL-27 in arthritic rats was further validated by subsequent reports in a mouse model of arthritis by other investigators [29].
We hypothesized that surface conjugation of IL-27-encapsulating liposomes with peptide ART-1 would help route them preferentially into the inflamed arthritic joints, and thereby, enhance the efficacy but reduce any adverse effects of treatment compared with free IL-27. The results described below support this proposition. We believe that with appropriate modifications, this peptide-directed drug delivery approach can be adapted for RA therapy with IL-27 or another drug of interest.
MATERIALS AND METHODS
IL-27 was purchased from PeproTech, USA. DOPC (1, 2-Dioleoyl-sn-glycero-3-phosphocholine), DOPE (1, 2-Dioleoyl-sn-glycero-3-phosphoethanolamine), cholesterol and FITC (fluorescein isothiocyanate) were obtained from Sigma-Aldrich, USA. DSPE-PEG (2000) amine (1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-N- [amino (polyethylene glycol)-2000] (ammonium salt)) was purchased from Avanti Polar Lipids, USA. Cyanine 7 azide (Cy7) was purchased from Lumiprobe, USA. ART-1-lipopeptide (CRNADKFPC-NH-C18H37) was customsynthesized by GenScript/Lifetein, USA. The structure of ART-1-lipopeptide is shown in Fig. S1. The peptide in both the lipopeptide and liposomal formulation used in this study was found to be in cyclic form (C-C disulfide 1–9) as assessed by LC- MS spectra of ART-1-lipopeptide: m/z calculated 1301.74 for CRNADKFPC-NH-C18H37, found 651.88 ([M+H]+/2) and 434.92 ([M+H]+/3) (protonated molecular ions of the formula (M+H)+ in positive ionization mode).
Preparation of liposomes
FITC-/Cy7- containing liposomes
The lipids DOPC, DOPE, cholesterol, and DSPE-(PEG)45-NH2, and a fluorescent dye (FITC/Cy7) were dissolved in chloroform/methanol in a molar ratio (1: 0.5: 0.5: 0.01: 0.05) in a glass vial. This composition was based on a study by Barui et al [30] but further optimized for our preparation to obtain good stability and entrapment efficiency, but no aggregation of liposomes. The solvent was removed with a thin flow of moisture-free nitrogen gas. For preparing liposomes displaying the ART-1 peptide on their surface, the ART-1-lipopeptide was added (at 1 molar ratio relative to above-mentioned molar ratio for different lipids) to the lipids, but for control liposomes, only the lipids were used. One mL of sterile deionized water or phosphate-buffered saline (PBS) was added to the dried lipid film and the mixture was allowed to swell overnight at room temperature. The vial was then vortexed for 3 min at room temperature to produce multilamellar vesicles (MLVs). MLVs were then sonicated, initially using an Ultrasonic water bath (CPX series, Branson Ultrasonics, CT, USA) followed by probe sonifier (Fisher Scientific) at 100% duty cycle and 25 W output power, to produce small unilamellar vesicles (SUVs). These liposomes containing FITC/Cy7 were then centrifuged using Amicon ultra-4 centrifugal filter units (10 kDa) for 20 min at 5000 rpm to remove un-encapsulated FITC/Cy7. A similar procedure was followed for preparing ART-1-containing liposomes but without any fluorescent dye (ART-1 liposomes).
IL-27-containing liposomes
For liposomal entrapment of IL-27, the dried lipid film prepared using above-mentioned lipids in the indicated molar ratio was hydrated with 1 ml PBS containing IL-27 (10 μg), and the mixture was allowed to swell overnight at 4°C. The vial was then vortexed for 3 min at room temperature to produce MLVs and then SUVs as described above. The resulting liposomes were then centrifuged for 60 min at 60,000 rpm at 4°C using a Beckman L8–80M ultracentrifuge to remove un-encapsulated IL-27. The supernatant was removed and the pellet was hydrated with PBS [31]. Depending on the presence or absence of the ART-1-lipopeptide, the end product was ART-1-IL-27 liposome or control-IL-27 liposome, respectively. Above-mentioned ART-1 liposomes, which displayed ART-1 but lacked any entrapped test agent (in this case IL-27), served as an additional control for ART-1-IL-27 liposomes in the experiments described below.
Physical characteristics of liposomes
Measurement of zeta size and zeta potential
The zeta size and zeta potential of liposomes containing FITC, Cy7 or IL-27 were measured using a Zetasizer (Malvern Zetasizer Nano). The size measurement was done in deionized water (1 ml) with a sample (50 μl) refractive index of 1.59 and a viscosity of 0.89. Dynamic light scattering (DLS) was used to measure particle size of liposomes. Samples were diluted 1 in 5 with deionized water and zeta potential was measured using the following parameters: viscosity, 0.89 cP; dielectric constant, 79; and temperature, 25 °C. All the size measurements were done 10 times in triplicate with the zero field correction, and values represented as the average of triplicate measurements. The zeta potential was measured 10 times and represented as an average value, as calculated by using the Smoluchowski approximation [32].
Transmission electron microscope (TEM) images
Five microliters of IL-27-liposomes were placed on a 200-mesh copper grid and allowed to adsorb. The surplus sample was removed by a filter paper. Five μL of 2% (w/v) aqueous solution of uranyl acetate were added and left in contact with the sample for 5 min. The surplus water was removed and the sample was dried under room conditions. The liposomes were imaged with a Transmission electron microscope FEI tecnai T12 operating at an acceleration voltage of 80 KV [33].
Determination of liposomal loading efficiency for IL-27 by HPLC
The entrapment efficiency (EE) of IL-27 in the liposomes was quantified by the indirect method using RP-HPLC and UV detection. The analytical system consisted of a Waters Alliance 2695 HPLC and a 2996 photodiode array detector coupled to a Waters Symmetry C4 reversed phase column (2.1mm x 150mm). Absorbance was monitored at 215nm. The solvents consisted of 0.1% trifluoroacetic acid (TFA) in water (A) and 0.1% TFA in acetonitrile (B). The column was equilibrated before each run for 10min in 95% A, 5% B. The flow rate and temperature were maintained at 0.2ml/min and 25°C, respectively. IL-27 standards and un-encapsulated IL-27 were diluted into PBS, and 20μl of each were injected onto the column. The following gradient method was used to elute IL-27: 0 to 5 min, 5 to 20% B; 5 to 50 min, 20 to 65% B; 50 to 55 min, 65 to 95% B; 55 to 60 min, 90 to 5% B. The concentration range of the IL-27 standards and that from the un-encapsulated IL-27 was chosen such that the amounts analyzed were in the linear range of the detector response for these analytes. The amount of IL-27 in the aqueous phase was subtracted from the total amount of IL-27 added to the liposomal preparation. The entrapment efficiency was then calculated from the weight of IL-27 encapsulated in liposomes as a percentage of the total weight of IL-27 added.
Stability of liposomes
The stability of liposomes was assessed by measuring their size and polydispersity index (PDI) at different time points after preparation. Liposomes containing FITC were stored at room temperature, and their size and PDI were measured on d 0, d 10 and d 50. However, considering the fragility of entrapped IL-27, the stability testing of IL-27-liposomes was limited to 7 days. These liposomes were stored at 4 °C and the size and PDI measured on d 0 and d 7.
In-vitro binding of liposomes to endothelial cells
Human umbilical vein endothelial cell (HUVEC) line was obtained from the ATCC (American Type Culture Collection, Manassas, VA, USA). HUVEC were grown in endothelial cell basal medium (EGM-2 BulletKit (CC-3156 & CC-4176), Lonza, USA) containing 10% fetal bovine serum. Cells were incubated at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The binding of FITC-liposomes to HUVEC was determined qualitatively by using a flow cytometer (BD FACS CANTO II). HUVEC were seeded at a density of 2×105 cells per well in a 12-well plate. After 18–24 h, cells were stained with ART-1-FITC liposomes or control-FITC liposomes at different concentrations (0.25 μM, 0.5 μM and 1 μM) for two hours. After washing with PBS, cells were fixed with 1% paraformaldehyde for 15 min, and analyzed using a flow cytometer (BD FACS CANTO II). The same procedure was used for the time kinetic study except that HUVEC were stained with ART-1-FITC or control-FITC liposomes at 0.25 μM concentration for different time points (5 min, 30 min, 1h, 2h and 4h). The results were analyzed using FCS express 6 flow cytometry software (De Novo Software, CA, USA).
Animals
Lewis (LEW/SsNHsd) rats were purchased from Envigo (Indianapolis, IN). Five to 6 weeks old male rats were used in this study. The rats were housed in the vivarium of the University of Maryland School of Medicine. Animals had free access to water and food, and were maintained on a 12 h light- 12 h dark cycle at room temperature of 21–23°C. The handling of rats and experimental procedures performed on them in this study were done after due approval from the Institutional Animal Care and Use Committee (IACUC) for compliance with the National Institutes of Health for use of laboratory animals. Additional experimental details using these animals are provided below.
Induction and evaluation of adjuvant arthritis in rats
Lewis rats were immunized subcutaneously (s.c.) at the base of the tail with 1.5 mg/rat of heatkilled Mycobacterium tuberculosis H37Ra (Mtb) (Difco, Detroit, MI) suspended in mineral oil as described elsewhere [24, 28]. Thereafter, the rats were observed regularly and graded for the severity of arthritis on the basis of erythema and swelling of the paws on a scale of 0–4 per paw as follows: 0 = no erythema or swelling, 1 = slight erythema or swelling of the ankle or wrist, 2 = moderate erythema and swelling at the wrist or ankle, 3 = moderate erythema and swelling at the wrist/ metacarpals or ankle/metatarsals, 4 = severe erythema and swelling of the forepaw or hind paw. At the conclusion of experiments, the hind paws of rats were harvested. These paws were decalcified by incubation in a fixative-cum-decalcifier (Cal-Ex II) solution for 2 weeks at room temperature, followed by embedding in paraffin. Thin sections (5 μm) of the paws were cut and stained with hematoxylin-eosin (H&E) for the assessment of pannus formation, cartilage damage, and bone erosion [34–36].
In-vivo distribution of liposomes in naïve and arthritic rats
The bio-distribution of liposomes was studied using Cy7-labeled liposomes. Naïve or arthritic rats were injected intravenously with ART-1-Cy7 liposomes or control-Cy7 liposomes (200μM) of comparable fluorescence intensity, and then subjected to real-time fluorescence imaging at 0, 2, 4 and 6 h post-injection using the IVIS® Spectrum system (PerkinElmer). After 6 h time point, rats were euthanized and their organs including the liver, heart, lung, spleen, kidney and paws were collected and examined by fluorescence imaging using the same equipment [37].
Treatment of arthritic rats with liposomal/free IL-27
A cohort of Mtb-immunized Lewis rats was observed until the onset of arthritis. Thereafter, following randomization, these rats were separated into 5 subgroups (n= 5–7 per group). These groups of rats received intravenously (i.v.) 200 μl/rat on d 10 and 12 after disease induction one of the following: ART-1-IL-27 liposomes, control-IL-27 liposomes lacking ART-1, ART-1-displying liposomes lacking IL-27, or free IL-27. On the basis of entrapment efficiency of IL-27 in liposomes, the dose of IL-27 was 0.83 μg/injection/rat. The arthritic control group received equal volume of PBS (vehicle) i.v. All rats were then observed regularly and the severity of arthritis was assessed as described above. At the conclusion of the experiment, hind paws of rats were harvested and processed for histological examination.
Serum chemistry analysis to assess the systemic exposure profiles of various treatment modalities
The relative profiles of the effects on other organs of various treatment modalities were evaluated in the same therapeutic efficacy study described above. Blood samples were drawn from the 5 groups of rats (n= 4–5 per group) on d 25 after Mtb immunization and the sera prepared from them was analyzed for serum chemistry (Charles River, MA). Specifically, hepatic function was assessed by measuring serum levels of aspartate aminotransferase (AST), alanine transaminase (ALT), bilirubin (TBIL), whereas renal function was assessed by measuring blood urea nitrogen (BUN), creatinine, and BUN/Creatinine ratio. Furthermore, acute/chronic tissue damage was assessed by measuring lactate dehydrogenase (LDH).
Statistical analysis
Results were analyzed and graphed using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA) and reported as standard error of the mean (SEM). Comparisons among multiple groups were assessed for significance using analysis of variance (ANOVA) and Wilcoxon ranksum test, as applicable to the data. The threshold of significance was set at P < 0.05.
RESULTS
Physical characteristics and stability of liposomes
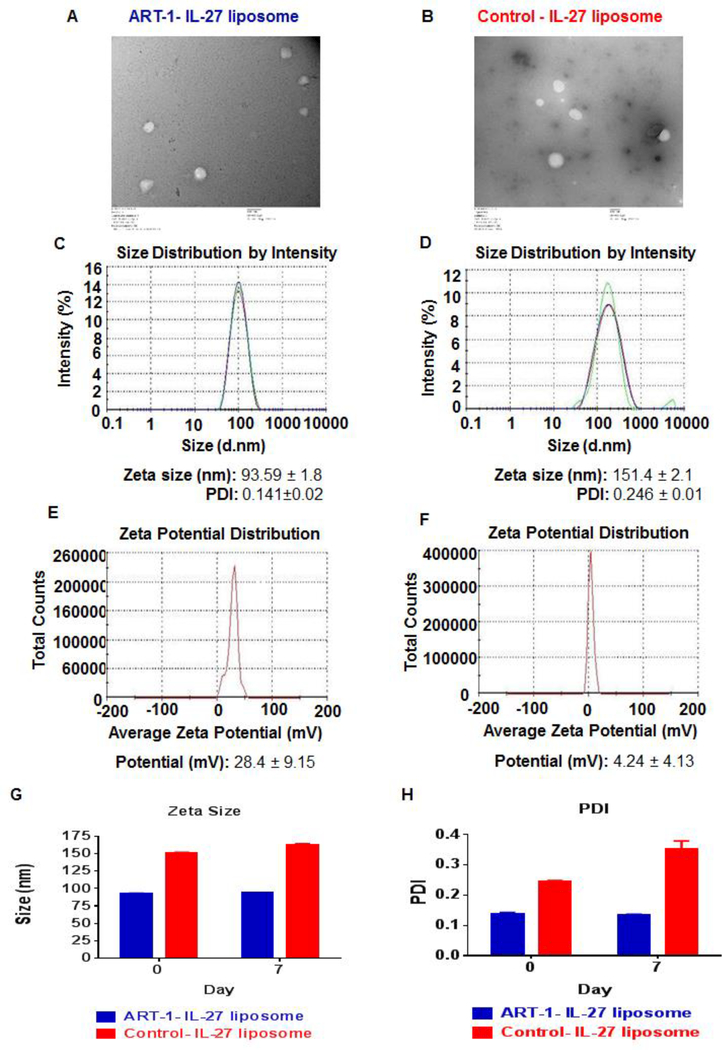
The molar ratio of ART-1-lipopeptide, DOPC, DOPE, cholesterol, and DSPE-(PEG)45-NH2 of 1: 1: 0.5: 0.5: 0.01 was found to be optimal for liposomal encapsulation of FITC/Cy7/IL-27 by ART-1-displaying liposomes; the control liposomes had a similar composition except for the ART-1 peptide. The size of these liposomes was within the range of 53–165 nm, with the liposomes containing FITC (53–101 nm) or Cy7 (66–135 nm) being slightly smaller (Fig. S2, S3) than the IL-27-containing liposomes (93–165 nm) (Fig. 1C, D). The morphology and size of the liposomes was further assessed by TEM imaging (Fig. 1A, B), whereas their charge was measured by zeta sizer (Fig. 1E, F). The entrapment efficiency for FITC/Cy7 was found to be almost 100% in both ART-1-displaying liposomes and control liposomes, whereas that for IL-27 ranged from 38–41% in these two types of liposomes. The liposomes containing FITC were stable at room temperature for 50 days (Fig. S2), whereas those containing IL-27 were stable at 4°C for at least one week of the testing period (Fig. 1G, H). The testing of IL-27 was done at 4°C and limited to one week owing to fragile nature of the cytokines in general.
Figure 1.
Characterization of ART-1-IL-27-liposomes (A, C, E) and control-IL-27 liposomes (B, D, F): TEM images (A, B) (- bar is 100nm), and measurements of zeta size (C, D) and zeta potential (E, F), are shown. Also tested was the stability of these liposomes by measuring and comparing zeta size (G) and PDI (H) day 0 and day 7.
ART-1-displying liposomes show higher binding to endothelial cells than control liposomes lacking ART-1
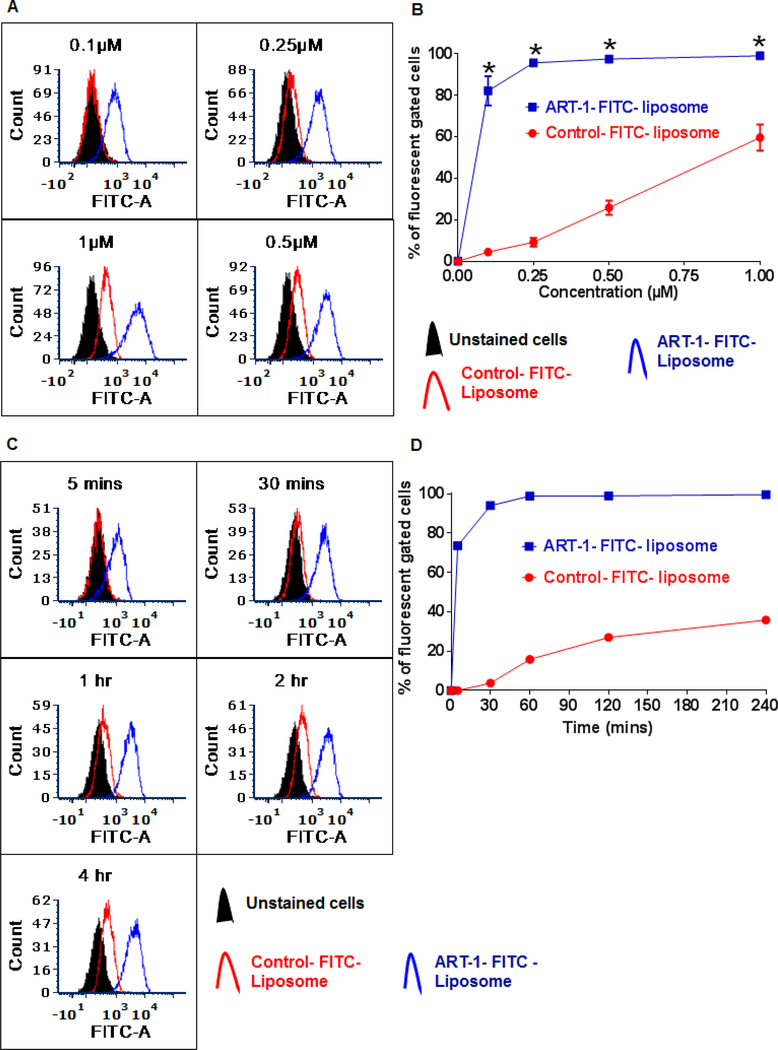
We previously reported [24] that peptide ART-1 (= ADK) showed binding to endothelial cells derived from the joints of arthritic rats as well as activated HUVEC; cultured HUVEC resemble angiogenic, activated endothelial cells, particularly when they are in active growth phase [38]. However, in this study, we used ART-1-lipopeptide for liposome preparation. To determine whether the endothelial cell-binding property of peptide ART-1 might have been affected following either its conjugation with a lipid (ART-1-lipopeptide) or its subsequent incorporation into liposomes, we tested and compared the endothelial cell binding of the ART-1-FITC liposomes and control- FITC liposomes at different concentrations as well as at each of the time points (5min - 4h) tested (Fig. 2).
Figure 2.
The binding of ART-1-/ control-FITC liposomes to HUVEC in vitro. (A, B) Cells were incubated with ART-1-/control-FITC liposomes at different concentrations (0.1 μM, 0.25 μM, 0.5 μM, or 1 μM). After two hours, the cells were washed with PBS, fixed with 1% paraformaldehyde solution for 15 min, and analyzed by flow cytometry. Representative histograms (A) and graph (B) are shown. (C, D) Cells were incubated with ART-1-/control-FITC liposomes at 0.25 μM concertation for 5 min, 30 min, 1 h, 2 h, or 4 h, washed with PBS, fixed with 1% paraformaldehyde for 15 min, and analyzed by flow cytometry. Representative histograms (C) and graph (D) are shown
Systemically administered ART-1-displaying liposomes preferentially home to arthritic joints
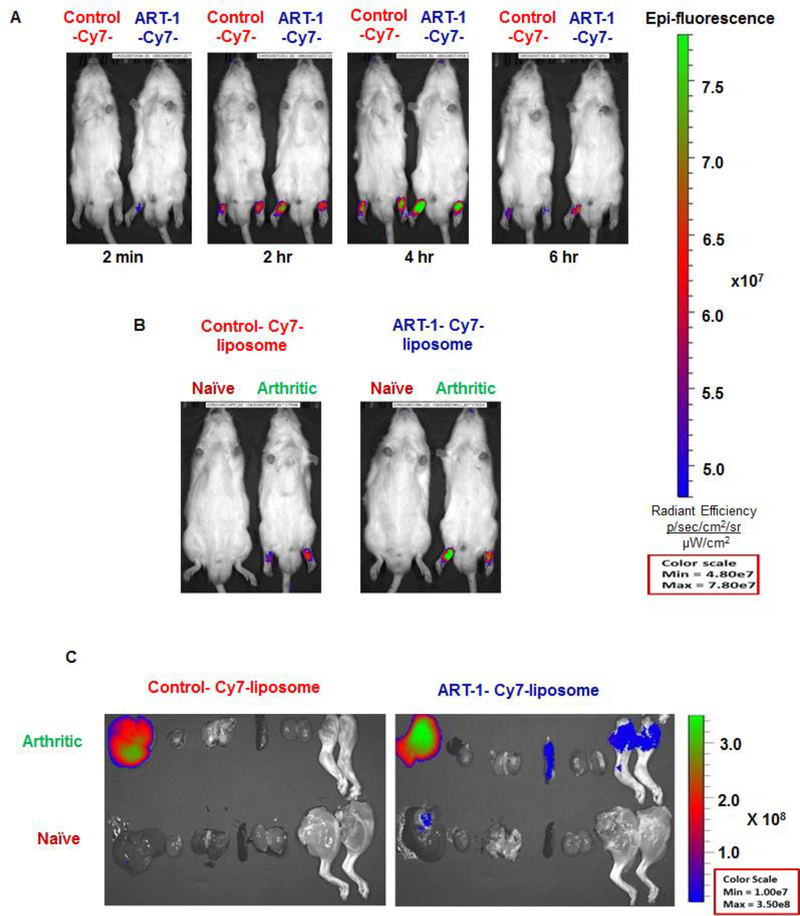
Following intravenous (i.v.) injection, ART-1-Cy7 liposomes showed significantly higher fluorescence intensity in the inflamed hind paw joints than control-Cy7 liposomes (Fig. 3A, 3B). With ART-1-Cy7 liposomes, a fluorescence signal was detected in inflamed joints as early as 2 min after i.v. injection. At 4 h time point, ART-1-Cy7 liposomes showed higher fluorescence intensity than control-Cy7 liposomes in arthritic joints. At 6 h, ex vivo imaging of different organs harvested from these rats showed fluorescence signal in the liver and inflamed joints (Fig. 3C). In contrast, no fluorescence signal was detectable in the hind paws of naïve rats at any of the time points tested under the same conditions. We suggest that signal from the liver might be owing to clearance of Cy7 by that organ. A similar finding of fluorescence in the liver has been reported by other investigators during in vivo imaging of tumors in mice [39]. Thus, we conclude that the ART-1-Cy7 liposomes preferentially homed to inflamed joints.
Figure 3.
In-vivo distribution of Cy7-labeled liposomes: (A) Real-time fluorescence imaging of arthritic rats at different time points (2 min, 2 h, 4 h, or 6 h) after i.v. injection of ART-1-Cy7 liposomes or control-Cy7 liposomes. (B) Real-time fluorescence imaging comparing arthritic and naive rats 4 h after i.v. injection of ART-1-Cy7 liposomes (Right) or control-Cy7 liposomes (Left). (C) Ex-vivo fluorescence images of different organs harvested from naïve or arthritic rats at 6 h after i.v. injection of ART-1-Cy7-liposomes (Right) and control-Cy7 liposomes (Left). The images were obtained using IVIS® Spectrum system (PerkinElmer).
Targeted delivery of liposomal IL-27 effectively suppressed the disease in arthritic rats
We observed that rats treated with IL-27 delivered via ART-1-IL-27 liposomes showed significantly reduced severity of AA compared to the vehicle-treated, control arthritic rats (Fig. 4A-C). The difference in arthritic scores of these two groups of rats was statistically significant. However, under similar conditions, the same dose of IL-27 but delivered via liposomes lacking any surface-conjugated ART-1 (control-IL-27 liposomes) failed to inhibit the progression of AA to the same extent as the ART-1-IL-27 liposomes; instead, the severity of arthritis in the control-IL-27 liposome group was comparable to that of the vehicle-treated group. The difference in arthritic scores in different groups of rats was further reinforced by histological evaluation of pannus formation, cartilage damage, and bone erosion (Fig. 4C). These results clearly demonstrate that the display of ART-1 on liposomal surface had a profound effect on the outcome of treatment with IL-27. We next compared the relative efficacy of IL-27 delivered via ART-1-IL-27 liposomes with that of free IL-27. In this case, the liposomal delivery of IL-27 had a much higher suppressive effect on AA than the free IL-27, and the difference in arthritic scores of these two groups of rats was statistically significant.
Figure 4.
Arthritis treatment with liposomal IL-27. Lewis rats were immunized s.c. at the base of the tail with Mtb (1.5 mg/rat). Beginning at the onset of arthritis, groups of rats (n= 5–7 per group) were treated i.v. on day 10 and 12 (arrows) after Mtb immunization with free IL-27, ART-1-IL-27 liposomes, control-IL-27 liposomes, or ART-1 liposomes lacking IL-27. A group of arthritic rats given PBS served as an untreated control group. (A) Arthritic scores (mean ± SEM) of rats are shown. P ˂0.05 was considered statistically significant. The differences in arthritic scores of each of the following 4 pairs of groups was significant: ART-1-IL-27 liposomes versus control rats; ART-1-IL-27 liposomes versus ART-1 liposomes lacking IL-27; ART-1-IL-27 liposomes versus control-IL-27 liposomes, and ART-1-IL-27 liposomes versus free IL-27. (B) Representative photographs of hind paws and (C) images of H&E-stained hind paw sections of the indicated groups of rats are shown. In ‘C’, histopathological features associated with arthritis are shown: B, bone; C, cartilage; JS, joint space; P, pannus.
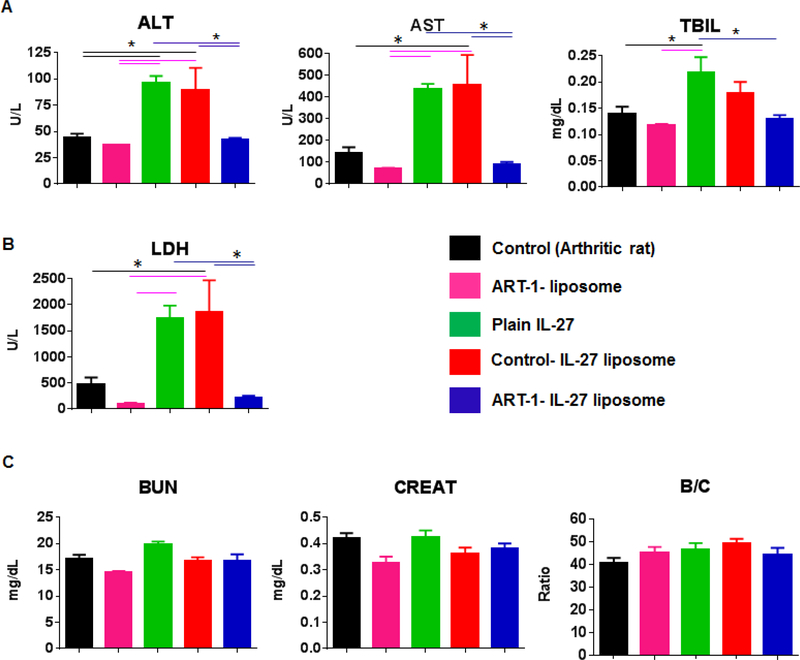
Targeted liposomal delivery of IL-27 is effective in reducing the systemic exposure to the cytokine
The sera of rats subjected to different treatment modalities described above were tested for various analytes (Fig. 5) indicative of hepatobiliary effects (ALT, AST, and TBIL), renal effects (BUN, creatinine, and their ratio), and acute/chronic tissue damage (LDH), as elaborated under Methods. Untreated arthritic rats served as a control for other treatment groups. The results are shown in Fig. 5. Sera of rats treated with free IL-27 or control-IL-27 liposomes showed comparable levels of enzymes tested for hepatobiliary (Fig. 5A) and tissue toxic effects (Fig. 5B), and these enzyme levels were significantly higher than those of the ART-1-IL-27 liposome group. The latter group was comparable to the untreated arthritic controls and rats treated with ART-1 liposomes lacking IL-27 (ART-1 liposomes) in this regard. However, for renal effects (Fig. 5C), serum chemistry profiles of various test groups were similar to that of control rats. These results demonstrate that the adverse effects of IL-27 therapy were significantly reduced when the same cytokine was delivered via targeted therapy using ART-1-IL-27 liposomes in comparison to free IL-27 or control-IL-27 liposomes.
Figure 5:
Serum chemistry analysis to assess the systemic exposure profiles of various treatment modalities. Blood samples were collected from rats (n= 4–5 per group) subjected to different treatment regimen (shown in Fig 4) on d 25 after Mtb immunization and serum was separated for testing. Serum levels of (A) aspartate aminotransferase (AST), alanine transaminase (ALT), and total bilirubin (TBIL) indicative of hepatobiliary effects; (B) lactate dehydrogenase (LDH) indicative of acute/chronic tissue damage; and (C) blood urea nitrogen (BUN), creatinine (CREAT), and BUN/Creatinine (B/C) ratio indicative of renal effects were measured. (*, p<0.05)
Taking together results of efficacy assessment of arthritis severity (Fig. 4) and serum chemistry for systemic exposure evaluation (Fig. 5), we conclude that ART-1-mediated targeted delivery significantly improved the therapeutic index of IL-27 compared with other modalities of treatment tested.
DISCUSSION
The therapeutic index of currently available drugs for RA is compromised by adverse reactions that accompany the prolonged use of these drugs and/or their mode of action. This is attributable in part to the unwanted exposure of other organs besides the joints to the drugs which are given orally or by systemic injection. We hypothesized that it might be possible to circumvent the side effect problem by targeting a systemically administered drug into inflamed joints, so that majority of the drug reaches the arthritic joints, and the dose administered could be reduced. Specifically, the goal of our targeted drug delivery approach was to enhance the therapeutic index of IL-27 compared to the conventional delivery approach. In this proof-ofconcept study, we exploited 3 components: a novel ligand, peptide ART-1; a relatively new immunomodulatory cytokine, IL-27; and a well-studied drug delivery vehicle, liposome. Overall, the size of different types of liposomes is within expected range. Although the net charge on ART-1-IL-27 liposomes was different from that of control liposomes. We attribute that primarily to the inclusion of the peptide in the liposomal membrane. However, for therapy, equivalent amount of drug (per Kg body weight) was given to rats, therefore, all rat groups received equal amount of IL-27. Our results show that the peptide-targeted liposomal delivery of IL-27 is more effective than free IL-27 in controlling arthritis.
Previous studies by other investigators have reported on the use of liposomal drug delivery (e.g., methotrexate, dexamethasone and prednisolone, etc.) for arthritis therapy in AA as well as in a collagen-induced arthritis model [40–43]. However, most of these studies were based on plain liposome formulations that lacked a surface ligand to direct the liposomes to the inflamed joints. Three studies using peptide ligands for drug delivery have been reported in the AA model [19–21]. Koning et al. entrapped dexamethasone in the PEGylated, RGD-conjugated liposomes for drug delivery into inflamed arthritic joints [19]. Vanniasinghe et al. conjugated a targeting peptide for fibroblast-like synoviocytes onto the surface of prednisolone-carrying liposomes [20]. In a recent study, Poh et al. used folate-conjugated, betamethasone-containing liposomes to image and treat arthritis [21]. Liposomal drug delivery systems have also been used in other autoimmune diseases. Liposomes were used for infliximab delivery to the eyes in the rat model of experimental autoimmune uveoretinitis [44] and methylprednisolone hemisuccinate in an experimental autoimmune encephalomyelitis (EAE) model of human multiple sclerosis [45]. Furthermore, liposomes displaying a peptide [46], an antibody (e.g., anti-α8 integrin or anti-CD4 antibody) [23, 47], or glutathione [48] have been employed for drug delivery in other autoimmune diseases including EAE, lupus, and other glomerular diseases. These studies, along with that mentioned below [49], reveal the rapid expansion of the targeted drug delivery approaches to the field of autoimmunity. The present study on arthritis describes a liposomal drug delivery system with a targeting ligand (ART-1) and therapeutic agent (IL-27) that have not been previously used in nanoparticle-based arthritis therapy.
Three main considerations in developing targeted liposomal delivery of IL-27 into inflamed joints were that- a) the ART-1 peptide displayed on the surface of liposomes retained its binding activity to activated endothelial cells; b) IL-27 retained its biological activity in the liposomal entrapment procedure; and c) the therapeutic index of IL-27 delivered via ART-1-displaying liposomes was superior to that of free IL-27, implying higher efficacy and reduced systemic adverse effects. Our results show that all these 3 conditions were met by our experimental system. For example, ART-1-FITC liposomes displayed much higher binding to HUVEC than control-FITC liposomes, showing the efficacy of the ART-1-coating of the liposomes. Similarly, liposomal IL-27 induced a comparable (when using control-IL-27 liposome) or higher (when using ART-1-IL-27 liposome) suppressive effect on AA compared to free IL-27, demonstrating that the bioactivity of IL-27 was maintained in the process of liposomal encapsulation. Furthermore, ART-1-directed liposomal IL-27 had higher efficacy than undirected (control) liposomal IL-27 or free IL-27. Finally, in regard to the systemic exposure profile, ART-1-IL-27 liposome group showed significantly reduced adverse effects compared with free IL-27, thus enhancing the therapeutic index of IL-27 therapy.
IL-27 has been shown to possess both pro- and anti-inflammatory properties depending on the type of disease (infectious, autoimmune) and local biological milieu at the site of inflammation, including the presence of other cytokines and mediators of inflammation. However, increasing evidence from studies in autoimmune disease models favors the immunoregulatory effects of IL-27 in arthritis, EAE, and some other autoimmune diseases [25, 27]. In our previous study, we showed that the treatment of arthritic rats with free IL-27, injected intraperitoneally, suppressed AA [28]. Similarly, other investigators have shown that the induction of IL-27 expression in the synovial tissue inhibits arthritis progression in mice with collagen-induced arthritis [29]. IL-27 inhibits Th17 differentiation and IL-17 response, but increases Th1 differentiation and IFN-ɣ response. Apparently, the increased IFN-ɣ also contributes to the inhibition of IL-17 response. In addition, we have previously shown that IFN-ɣ can induce IL-27 in vitro. Taken together, these observations suggest that IFN-ɣ and IL-27 may cooperate during the course of AA to downregulate the pathogenic Th17/IL-17 response. IL-27 also modulates other processes associated with inflammation and tissue damage, such as angiogenesis, osteoclastogenesis, and matrix degradation, and has also been found to be effective in the treatment of autoimmune diseases other than arthritis [25]. For example, s.c. injection of IL-27 has been shown to suppress EAE induction [50]. Similarly, oral delivery of genetically-engineered, IL-27-expressing Lactococcus lactis suppressed enterocolitis [51].
Significant effort has been invested over the past few decades to the development of anticytokine/ cytokine receptor agents (e.g., anti-TNFα or anti-IL-6R antibodies) for RA therapy, and these advances has revolutionized RA therapy [52]. This effort can be further complemented by employing certain immunomodulatory cytokines per se as drugs, as exemplified by the use of IL-27 for arthritis therapy in our previous study in AA [28] and targeted therapy with IL-4 using a conjugate of the cytokine with a synovial endothelium-targeting peptide CKSTHDRLC in the synovial xenograft model of RA [49]. The latter study elaborated the advantages of the IL-4-peptide conjugate compared with IL-4 alone. However, because of the inherent difference in the amount of cytokine that can potentially be delivered in the form of a peptide-cytokine conjugate versus cytokine entrapped in a peptide-directed nanoparticle, the relative comparison of these two approaches remains to be assessed for different peptide ligands mentioned above [19–21, 24, 49]. In the current study, we demonstrate the benefit of targeted liposomal IL-27 therapy over free IL-27. Here, IL-27 is presented as a proof-of-concept therapeutic. A similar liposomal approach may be suitable for targeted therapy of well-established biologics mentioned above, and other immunomodulatory cytokines such as IL-10 or IL-35 [53]. The platform technology described in this study can be adapted for any drug of interest for arthritic therapy. Also, if desired, more than one drug can be incorporated into the targeted liposomes for combination therapy.
Supplementary Material
Figure S1. Structure of ART-1-lipopeptide (CRNADKFPC-octadecylamine) in the liposomal formulation. The peptide structure is shown in blue color. The peptide both as a lipopeptide and as part of the liposome used in this study was found to be in cyclic form (C-C disulfide 1–9) as assessed by LC- MS spectra performed on the ART-1-lipopeptide used for liposome preparation: m/z calculated 1301.74 for CRNADKFPC-NH-C18H37, found 651.88 ([M+H]+/2) and 434.92 ([M+H]+/3) (protonated molecular ions of the formula (M+H)+ in positive ionization mode).
Figure S2. (A) Zeta size and (B) PDI of ART-1-FITC liposomes (Blue bar) and control-FITC liposomes (Red bar) on d 0, d 10 and d 50 after preparation of these liposomes.
Figure S3. Zeta size of (A) ART-1-Cy7 liposomes and (B) control-Cy7 liposomes.
Acknowledgements:
This work was supported in part by Merit Review Award # 5 I01 BX002424 from the United States (U.S.) Department of Veterans Affairs [Biomedical Laboratory Research and Development Service], by R01 AT004321 grant from the National Institutes of Health, Bethesda, MD, and the Rheumatology Research Foundation, Atlanta, GA. Our sincere thanks to Dr. Erkki Ruoslahti (Sanford Burnham Prebys Medical Discovery Institute) for his guidance and help at different stages of this study as well as insightful comments on the manuscript. We also thank Dr. Arabinda Chaudhury (CSIR-IICT, Hyderabad) for helpful discussions. We thank Dr. Peter Swaan and Joanna Pak for kindly providing Zetasizer facility. We also thank the staff of the VA Research Facilities, Baltimore VA Medical Center, Baltimore, MD, and that of various Core facilities of UMB (CIBR), particularly the Electron Microscopy Core Imaging Facility, the Translational Research in Imaging Core, and the Protein Analysis Laboratory.
Footnotes
Conflict of interest:
“The authors have declared that no competing interest exists”.
Declaration:
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature 2003. May 15;423(6937):356–361. [DOI] [PubMed] [Google Scholar]
- 2.Harris ED Jr., Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med 1990. May 03;322(18):1277–1289. [DOI] [PubMed] [Google Scholar]
- 3.Gibofsky A Current therapeutic agents and treatment paradigms for the management of rheumatoid arthritis. Am J Manag Care 2014. May;20(7 Suppl):S136–144. [PubMed] [Google Scholar]
- 4.Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther 2011. June;33(6):679–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim GW, Lee NR, Pi RH, Lim YS, Lee YM, Lee JM, et al. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res 2015;38(5):575–584. [DOI] [PubMed] [Google Scholar]
- 6.Bui VL, Brahn E. Cytokine targeting in rheumatoid arthritis. Clinical immunology 2018. April 3. [DOI] [PubMed] [Google Scholar]
- 7.Sfikakis PP, Tsokos GC. Towards the next generation of anti-TNF drugs. Clinical immunology 2011. December;141(3):231–235. [DOI] [PubMed] [Google Scholar]
- 8.Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011. February 16(2):CD008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampropoulos CE, Orfanos P, Bournia VK, Karatsourakis T, Mavragani C, Pikazis D, et al. Adverse events and infections in patients with rheumatoid arthritis treated with conventional drugs or biologic agents: a real world study. Clin Exp Rheumatol 2015. Mar-Apr;33(2):216–224. [PubMed] [Google Scholar]
- 10.Ramiro S, Sepriano A, Chatzidionysiou K, Nam JL, Smolen JS, van der Heijde D, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Annals of the rheumatic diseases 2017. June;76(6):1101–1136. [DOI] [PubMed] [Google Scholar]
- 11.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Advanced drug delivery reviews 2013. January;65(1):36–48. [DOI] [PubMed] [Google Scholar]
- 12.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and Challenges of Liposome Assisted Drug Delivery. Frontiers in pharmacology 2015;6:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruoslahti E Peptides as targeting elements and tissue penetration devices for nanoparticles. Advanced materials 2012. July 24;24(28):3747–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. The Journal of cell biology 2010. March 22;188(6):759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teesalu T, Sugahara KN, Ruoslahti E. Tumor-penetrating peptides. Frontiers in oncology 2013;3:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Herrero E, Fernandez-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV 2015. June;93:52–79. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Rodriguez BL. Molecular targeting of liposomal nanoparticles to tumor microenvironment. International journal of nanomedicine 2013;8:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruoslahti E Tumor penetrating peptides for improved drug delivery. Advanced drug delivery reviews 2017. February;110–111:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koning GA, Schiffelers RM, Wauben MH, Kok RJ, Mastrobattista E, Molema G, et al. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphatecontaining RGD peptide liposomes inhibits experimental arthritis. Arthritis and rheumatism 2006. April;54(4):1198–1208. [DOI] [PubMed] [Google Scholar]
- 20.Vanniasinghe AS, Manolios N, Schibeci S, Lakhiani C, Kamali-Sarvestani E, Sharma R, et al. Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis. Clinical immunology 2014. March;151(1):43–54. [DOI] [PubMed] [Google Scholar]
- 21.Poh S, Chelvam V, Kelderhouse LE, Ayala-Lopez W, Vaitilingam B, Putt KS, et al. Folate-conjugated liposomes target and deliver therapeutics to immune cells in a rat model of rheumatoid arthritis. Nanomedicine 2017. October;12(20):2441–2451. [DOI] [PubMed] [Google Scholar]
- 22.Nogueira E, Lager F, Le Roux D, Nogueira P, Freitas J, Charvet C, et al. Enhancing Methotrexate Tolerance with Folate Tagged Liposomes in Arthritic Mice. Journal of biomedical nanotechnology 2015. December;11(12):2243–2252. [DOI] [PubMed] [Google Scholar]
- 23.Look M, Stern E, Wang QA, DiPlacido LD, Kashgarian M, Craft J, et al. Nanogel-based delivery of mycophenolic acid ameliorates systemic lupus erythematosus in mice. The Journal of clinical investigation 2013. April;123(4):1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang YH, Rajaiah R, Ruoslahti E, Moudgil KD. Peptides targeting inflamed synovial vasculature attenuate autoimmune arthritis. Proceedings of the National Academy of Sciences of the United States of America 2011. August 02;108(31):12857–12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meka RR, Venkatesha SH, Dudics S, Acharya B, Moudgil KD. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmunity reviews 2015. December;14(12):1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002. June;16(6):779–790. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annual review of immunology 2015;33:417–443. [DOI] [PubMed] [Google Scholar]
- 28.Rajaiah R, Puttabyatappa M, Polumuri SK, Moudgil KD. Interleukin-27 and interferongamma are involved in regulation of autoimmune arthritis. The Journal of biological chemistry 2011. January 28;286(4):2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JS, Jung YO, Oh HJ, Park SJ, Heo YJ, Kang CM, et al. Interleukin-27 suppresses osteoclastogenesis via induction of interferon-gamma. Immunology 2012. December;137(4):326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barui S, Saha S, Mondal G, Haseena S, Chaudhuri A. Simultaneous delivery of doxorubicin and curcumin encapsulated in liposomes of pegylated RGDK-lipopeptide to tumor vasculature. Biomaterials 2014. February;35(5):1643–1656. [DOI] [PubMed] [Google Scholar]
- 31.Migliore MM, Vyas TK, Campbell RB, Amiji MM, Waszczak BL. Brain delivery of proteins by the intranasal route of administration: a comparison of cationic liposomes versus aqueous solution formulations. Journal of pharmaceutical sciences 2010. April;99(4):1745–1761. [DOI] [PubMed] [Google Scholar]
- 32.Rakeshchandra R Meka SG, Marepallyb Srujan Thorat Ketan, Reddy Rachamalla Hari Krishna, Dhayani Ashish, Hiwale Ankita, Banerjee Rajkumar, Chaudhuri Arabinda and Kumar Vemula Praveen. Asymmetric cationic lipid based non-viral vectors for an efficient nucleic acid delivery. RSC Advances 2016;6(81):77841–77848. [Google Scholar]
- 33.Helmy HS, El-Sahar AE, Sayed RH, Shamma RN, Salama AH, Elbaz EM. Therapeutic effects of lornoxicam-loaded nanomicellar formula in experimental models of rheumatoid arthritis. International journal of nanomedicine 2017;12:7015–7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Astry B, Venkatesha SH, Laurence A, Christensen-Quick A, Garzino-Demo A, Frieman MB, et al. Celastrol, a Chinese herbal compound, controls autoimmune inflammation by altering the balance of pathogenic and regulatory T cells in the target organ. Clinical immunology 2015. April;157(2):228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moudgil KD, Chang TT, Eradat H, Chen AM, Gupta RS, Brahn E, et al. Diversification of T cell responses to carboxy-terminal determinants within the 65-kD heat-shock protein is involved in regulation of autoimmune arthritis. The Journal of experimental medicine 1997. April 07;185(7):1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. The Journal of biological chemistry 2011. April 29;286(17):15138–15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heo R, You DG, Um W, Choi KY, Jeon S, Park JS, et al. Dextran sulfate nanoparticles as a theranostic nanomedicine for rheumatoid arthritis. Biomaterials 2017. July;131:15–26. [DOI] [PubMed] [Google Scholar]
- 38.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nature medicine 1999. September;5(9):1032–1038. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Li K, Dai Y, Xu X, Cao X, Zeng Q, et al. In vivo near infrared fluorescence imaging and dynamic quantification of pancreatic metastatic tumors using folic acid conjugated biodegradable mesoporous silica nanoparticles. Nanomedicine 2018. August;14(6):1867–1877. [DOI] [PubMed] [Google Scholar]
- 40.Anderson R, Franch A, Castell M, Perez-Cano FJ, Brauer R, Pohlers D, et al. Liposomal encapsulation enhances and prolongs the anti-inflammatory effects of water-soluble dexamethasone phosphate in experimental adjuvant arthritis. Arthritis research & therapy 2010;12(4):R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofkens W, Grevers LC, Walgreen B, de Vries TJ, Leenen PJ, Everts V, et al. Intravenously delivered glucocorticoid liposomes inhibit osteoclast activity and bone erosion in murine antigen-induced arthritis. Journal of controlled release : official journal of the Controlled Release Society 2011. June 30;152(3):363–369. [DOI] [PubMed] [Google Scholar]
- 42.Prabhu P, Shetty R, Koland M, Vijayanarayana K, Vijayalakshmi KK, Nairy MH, et al. Investigation of nano lipid vesicles of methotrexate for anti-rheumatoid activity. International journal of nanomedicine 2012;7:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Hoven JM, Hofkens W, Wauben MH, Wagenaar-Hilbers JP, Beijnen JH, Nuijen B, et al. Optimizing the therapeutic index of liposomal glucocorticoids in experimental arthritis. International journal of pharmaceutics 2011. September 20;416(2):471–477. [DOI] [PubMed] [Google Scholar]
- 44.Zhang R, Qian J, Li X, Yuan Y. Treatment of experimental autoimmune uveoretinitis with intravitreal injection of infliximab encapsulated in liposomes. The British journal of ophthalmology 2017. October 06. [DOI] [PubMed] [Google Scholar]
- 45.Turjeman K, Bavli Y, Kizelsztein P, Schilt Y, Allon N, Katzir TB, et al. Nano-Drugs Based on Nano Sterically Stabilized Liposomes for the Treatment of Inflammatory Neurodegenerative Diseases. PloS one 2015;10(7):e0130442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuhrmann T, Ghosh M, Otero A, Goss B, Dargaville TR, Pearse DD, et al. Peptidefunctionalized polymeric nanoparticles for active targeting of damaged tissue in animals with experimental autoimmune encephalomyelitis. Neuroscience letters 2015. August 18;602:126–132. [DOI] [PubMed] [Google Scholar]
- 47.Scindia Y, Deshmukh U, Thimmalapura PR, Bagavant H. Anti-alpha8 integrin immunoliposomes in glomeruli of lupus-susceptible mice: a novel system for delivery of therapeutic agents to the renal glomerulus in systemic lupus erythematosus. Arthritis and rheumatism 2008. December;58(12):3884–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaillard PJ, Appeldoorn CC, Rip J, Dorland R, van der Pol SM, Kooij G, et al. Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. Journal of controlled release : official journal of the Controlled Release Society 2012. December 28;164(3):364–369. [DOI] [PubMed] [Google Scholar]
- 49.Wythe SE, DiCara D, Taher TE, Finucane CM, Jones R, Bombardieri M, et al. Targeted delivery of cytokine therapy to rheumatoid tissue by a synovial targeting peptide. Annals of the rheumatic diseases 2013. January;72(1):129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. Journal of immunology 2007. September 01;179(5):32683275. [DOI] [PubMed] [Google Scholar]
- 51.Hanson ML, Hixon JA, Li W, Felber BK, Anver MR, Stewart CA, et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology 2014. January;146(1):210–221 e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nam JL, Takase-Minegishi K, Ramiro S, Chatzidionysiou K, Smolen JS, van der Heijde D, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Annals of the rheumatic diseases 2017. June;76(6):1113–1136. [DOI] [PubMed] [Google Scholar]
- 53.Astry B, Harberts E, Moudgil KD. A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J Interferon Cytokine Res 2011. December;31(12):927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Structure of ART-1-lipopeptide (CRNADKFPC-octadecylamine) in the liposomal formulation. The peptide structure is shown in blue color. The peptide both as a lipopeptide and as part of the liposome used in this study was found to be in cyclic form (C-C disulfide 1–9) as assessed by LC- MS spectra performed on the ART-1-lipopeptide used for liposome preparation: m/z calculated 1301.74 for CRNADKFPC-NH-C18H37, found 651.88 ([M+H]+/2) and 434.92 ([M+H]+/3) (protonated molecular ions of the formula (M+H)+ in positive ionization mode).
Figure S2. (A) Zeta size and (B) PDI of ART-1-FITC liposomes (Blue bar) and control-FITC liposomes (Red bar) on d 0, d 10 and d 50 after preparation of these liposomes.
Figure S3. Zeta size of (A) ART-1-Cy7 liposomes and (B) control-Cy7 liposomes.