Abstract
Background
Early detection and classification of chronic pancreatitis (CP) are both important and challenging.
Purpose
To investigate the diagnostic performance of MR elastography and T1 mapping of the pancreas for different stages of CP.
Study type
Retrospective.
Subjects
Clinical and imaging records of 81 patients (from 5/2015 to 7/2017) with suspected CP were analyzed. Patients were categorized into the normal control (n=35), mild CP (n=30) and moderate/severe CP groups (n=16) according to the Cambridge classification based on concordant endoscopic retrograde cholangiopancreatography or ultrasound endoscopy findings.
Field strength/sequence
3T pancreatic MRI, which included MR elastography and T1 mapping.
Assessment
T1 relaxation times, pancreatic stiffness values, the main pancreatic duct (MPD) diameter and pancreatic thickness were measured in all patients.
Statistical tests
Cut-off values of T1 relaxation times and pancreatic stiffness values for diagnosis of CP were calculated using receiver operating characteristic analysis. Associations of imaging parameters with different stages of CP were assessed using logistic regression analysis.
Results
Both T1 relaxation times (865±220 ms vs 1075±221 ms vs 1350±139 ms) and pancreatic stiffness (1.21±0.13 kPa vs 1.50±0.15 kPa vs 1.90±0.16 kPa) differed significantly (P < 0.001) among the control, mild CP and moderate/severe CP groups. Pancreatic stiffness (>1.34 kPa) achieved significantly higher area under the curve (AUC) than T1 relaxation time (>908.4 ms) for detection of mild CP (AUC: 0.928 vs 0.751, P = 0.011). Pancreatic stiffness values (>1.61 kPa) also achieved significantly higher AUC than T1 relaxation times (>1,131.6 ms) (AUC: 0.981 vs 0.910, P = 0.033) for diagnosing moderate/severe CP from the other two groups. Multiple regression analysis showed that T1 relaxation time and stiffness were the independent factors associated with mild CP (P = 0.025 and <0.001, respectively).
Data conclusion
Both MRE and T1 mapping are promising quantitative imaging methods for evaluation of CP; MRE slightly outperformed T1 mapping.
Keywords: MR elastography, Diagnosis, Chronic pancreatitis, T1 mapping
Introduction
Chronic pancreatitis (CP) is characterized by progressive inflammation, pancreatic fibrosis, and impairment of both exocrine and endocrine function [1]. The diagnosis of mild CP is usually inferred from the results of conventional imaging modalities [2, 3], such as ultrasound and CT, can detect the late findings of CP but have limited value for the diagnosis of early or mild CP. Although pancreatic fluid and bicarbonate output (i.e. pancreatic function test) can be directly measured to quantify the impaired function, this assessment is invasive and time-consuming, and thus is rarely performed in clinical practice [4]. The Cambridge classification has been used since 1984 as a reference standard to diagnose and grade CP by endoscopic retrograde cholangiopancreatography (ERCP) [5]. However, the use of ERCP as a purely diagnostic tool has declined and is rarely used. The trend is likely due to the introduction of less invasive and more sensitive imaging tools that evaluate both parenchymal and ductal changes [6–8].
Although different etiologies lead to chronic pancreatitis, pancreatic fibrosis is the common histologic finding. This fibrosis might be detected by MR techniques, such as T1 mapping and MR elastography [9, 10]. T1 mapping has been successfully used to assess the extent of myocardial fibrosis, and rapid acquisition in one breath-hold of T1 mapping is currently available for abdominal evaluation [11, 12]. A prior study has reported that the T1 relaxation time of the pancreas is greater in mild CP patients than in patients with a healthy pancreas [9]. MRE has been successfully used to stage hepatic fibrosis in patients with chronic hepatitis; even mild chronic hepatitis can be detected by MRE with high sensitivity [10]. Hence, the purpose of this study was to investigate the diagnostic performance of pancreatic stiffness, determined by MRE, and T1 relaxation time of the pancreas, determined by T1 mapping, for diagnosis of different stages of CP.
Materials and Methods
Population
This retrospective, Health Insurance Portability and Accountability Act–compliant study was approved by the Institutional Review Board of the local hospital. From May 2015 to July 2017, MRE was included in the routine pancreatic MR protocol for each participant with suspected pancreaticobiliary pathology. Informed consent was required to include MRE into any patient’s pancreatic MR protocol. First, we identified 219 adult patients who underwent both MRE and T1 mapping for pancreatic evaluation. We excluded those patients with an acute episode of pancreatitis (n=37), cystic or solid pancreatic tumors (n=68), without evidence of clinical follow-up available (n=14), suboptimal image quality on T1 maps (n=4), elastograms (n=5) or severe atrophy of the pancreatic parenchyma (n=10). Thus, 81 patients were further evaluated. Then, two gastroenterologists, with 10 years and 12 years of experience, respectively, reviewed these patients’ history, ERCP or endoscopic ultrasonography (EUS) results and other imaging findings. According to the Cambridge classification, 16 patients were determined to have moderate/severe CP (mod/sev CP) based on ERCP/EUS or typical CT/MRI findings, which showed main pancreatic duct (MPD) dilation or irregularity with/without parenchymal or ductal calculi, together with clinical symptoms. Patients with normal ductal findings on ERCP (n=4) or EUS (n=31), normal serum amylase and lipase levels, and without history of acute or chronic pancreatitis together with at least a 6-month clinical follow-up, were considered as the control group (n=35). The clinical findings in the control group included choledocholithiasis (n=27), sphincter of Oddi dysfunction (n=7) and primary sclerosing cholangitis (n=1); none had with any form of pancreatitis. The diagnosis of those in the mild CP group (n=30) was based on history of consistent symptomatology (abdominal pain suspected to be of pancreatic origin), concordant ductal findings on ERCP according to the Cambridge classification [5] (n=21) or EUS findings based on the Rosemont classification [13] (n=9). Rosemont classification does not directly classify patients as having “mild CP”; therefore EUS findings were regarded as mild CP based on inhomogeneous echo texture or echogenic strands in the gland. We combined patients with equivocal or mild chronic pancreatitis into one group (mild CP group).
Imaging Technique
Patients fasted overnight for 6–8 hours prior to the MR examination. The patients first underwent clinical pancreatic MR sequences with T1 mapping with a 3.0T Philips scanner (Philips Ingenia, Philips Healthcare, Best, the Netherlands) and then underwent MRE immediately with a 3.0T GE scanner next (Signa HDX system; GE Healthcare, Milwaukee, WI). A modified Look-Locker inversion recovery (MOLLI) 2D imaging sequence was applied for T1 acquisition and quantification using a 5s(3s)3s sampling scheme for non-contrast enhanced application. MOLLI sequence images were obtained using a 32-channel torso coil with the following settings: 2.24 ms/1.01 ms TR/TE; 30cm FOV; 152×150 acquisition matrix ; 10 mm slice thickness; 12s breath-hold duration; 20° flip angle, and 152 × 150 acquisition matrix. The MOLLI sequence was repeated 3~6 times to cover different subregions of the pancreas. MRE data were collected using a Signa Excite HD 3T GE scanner with an 8-element torso-phased array coil. Each patient was imaged using flow-compensated, multi-slice, spin-echo echo-planar-imaging (SE-EPI) pulse sequences. Axial slices were performed using 40-Hz mechanical vibrations. The mechanical vibrations were supplied by an active pneumatic driver system located outside the scanning room and were delivered to the upper abdomen centered on the xiphisternum via a plastic tube that terminated in a passive driver that transmitted the waves into the deep pancreas. The passive drivers were fastened to the upper abdomen using an elastic belt. In patients with BMI >27 kg/m2, a rigid round liver driver, diameter 19 cm, was utilized with 40–50% power amplitude [14] ; in other patients, a rectangular soft driver (19×14 cm) was utilized with 70–100% power amplitude, as previously described [15, 16]. All the drivers were developed by Mayo Clinic (Rochester, MN). The acquisition included four 22-s and one 11-s breath-holds. The imaging parameters settings were 40 Hz frequency; 1375/38.8 ms TR/TE; 32~44 cm FOV; 3 phase offsets; parallel imaging acceleration factor of 3; 96×96 acquisition matrix; 1 signal average; 32 slices; and 3.5 mm slice thickness. The pancreatic thickness was measured from axial breath-hold 2-point DIXON (mdixon) T1-weighted images with TE at 1.32 and 2.3 ms, (3.6 ms TR and 3.5 mm slice thickness). The diameter of the MPD was measured from T2-weighted images, which were acquired using 622.84 ms TR, 70.00 ms TE, and 5 mm slice thickness.
Image Analysis
Wave images were processed on the scanner automatically using the automated direct inversion algorithm (Mayo Clinic, Rochester, MN) as previous reportedly in MRE studies of the pancreas, kidney, and liver [15–18]. T1 maps were calculated based on MOLLI images at a set of inversion recoveries (IR) with increasing TI’s in each IR performed throughout one breath-hold. The T1 maps for pancreas were read. Then, for better illustration, the T1 maps encompassing the pancreatic parenchyma were segmented out and overlaid on the T1 weighted images. One region of interest (ROI) was drawn separately for each subregion (head/uncinate process, body, and tail). The ROIs were drawn to encompass the largest area of pancreatic parenchyma as possible while avoiding the pancreatic border, large vessels, duct, the surrounding tissues, and retroperitoneal fat. The elastograms matching the T1 maps were selected, as close as possible, to draw similar ROIs including pancreatic parenchyma on matching sections. Two experienced abdominal radiologists, with 3 and 5 years of experience with MRE measurement, blinded to the ERCP/EUS data, measured pancreatic stiffness, T1 relaxation time from the head, body, and tail of the pancreas, pancreatic thickness from DIXON sequence, and MPD diameter from T2-weighted images. The final measurements were calculated as the mean value of the two observers after establishing the inter-observer agreement.
Statistical Analysis
The intraclass correlation coefficient (ICC) was calculated to assess inter-observer agreement for stiffness and T1 relaxation time measurements. Continuous variables were summarized as means ± standard deviation (SD), or median with 25th and 75th percentiles of the interquartile range, as appropriate. Categorical variables were expressed as counts and percentages. The clinical characteristics of the subjects in different groups were compared using the Kruskal-Wallis test. The stiffness and T1 relaxation time among different subregions in each group were compared using the Friedman test. Mann-Whitney U test was used for comparisons between two groups. Categorical variables (e.g. gender) were compared using Chi-squared test. Receiver operating characteristic (ROC) curve analysis was performed to determine the diagnostic accuracy of both stiffness and T1 relaxation time for diagnosing mild CP from healthy pancreas, the presence of any CP from healthy pancreas, and mod/sev CP from normal/mild CP. The sensitivity, specificity, and accuracy with 95% confidence intervals (CI) were calculated using a cutoff value determined by maximizing sensitivity and specificity. Between-group comparisons of sensitivity, specificity, and accuracy were based on the McNemar test. AUC comparisons were performed according to the method proposed by DeLong et al [19]. Univariate and multivariate logistic regression analyses were used to analyze the association of imaging factors, including T1 relaxation time, stiffness, pancreatic thickness, MPD size, and demographic characteristics to the presence of mild CP and the presence of any CP (both mild and mod/sev CP vs normal pancreas). The sensitivity by MRE reached up to 100% for diagnosing mod/sev CP, making the results of the logistic regression analyses invalid, therefore, we avoid using logistic regression analyses for diagnosing mod/sev CP. ROC analyses were performed using MedCalc software (MedCalc Software, Mariakerke, Belgium). All other statistical analyses were performed using commercially available SPSS Statistics V 20.0 (IBM Corporate, New York, USA) software. P < 0.05 was indicative of a statistically significant difference. MPD diameter < 3 mm was considered normal, that is, nondilated. Fecal elastase-1 (FE-1) < 200 µg/g was considered normal [20].
Results
The patients’ demographic and clinical characteristics are shown in Table 1. These three groups had similar gender distribution (P = 0.720), BMI (P = 0.162), age (P = 0.453), serum amylase (P = 0.115), and lipase levels (P = 0.171). The mean MPD diameter in the control group was narrower (2.47±0.56 mm) than in the mild CP group (2.94±0.51 mm) (P = 0.002) and mod/sev CP group (5.17±2.30 mm) (P < 0.001). Pancreatic thickness was slightly greater in the control group (19.2±4.2 mm) than in the mild CP group (18.2±3.5 mm) (P = 0.305) and mod/sev CP group (16.2±4.3 mm) (P = 0.015). FE-1 in the mod/sev CP group was significantly lower (121±100 µg/g) than in the control group (312±156 µg/g) (P < 0.001) and mild CP group (285±164 µg/g) (P = 0.001). FE-1 did not differ between the mild CP and control groups (P = 0.418).
Table 1.
Demographic and Clinical Characteristics of Controls and Two Chronic Pancreatitis (CP) Groups
| Control (n=35) | Mild CP (n=30) | Mod/Sev CP(n=16) | |
|---|---|---|---|
| Age (years) | 52 (Range:21–82) | 56 (Range:33–81) | 57 (Range:37–78) |
| Gender (F:M) | 21:14 | 15:15 | 9:7 |
| BMI (Kg/M2) | 23.0 (SD:2.5) | 23.7 (SD:2.7) | 22.3 (SD:2.9) |
| Lipase level (U/L) | 68(50–82)* | 81(56–104)* | 86(62–110)* |
| Amylase level (U/L) | 37(SD:11) | 42(SD:13) | 50(SD:22) |
| FE-1 (µg/g) | 312(SD:156) | 285(SD:164) | 121(SD:100) |
| MPD (mm) | 2.47(SD:0.56) | 2.94(SD:0.51) | 5.17(SD:2.30) |
| Thickness (mm) | 19.2(SD:4.2) | 18.2(SD:3.5) | 16.2(SD:4.3) |
BMI = body-mass index, SD = standard deviation, MPD = main pancreatic duct, FE-1 = Fecal elastase-1
median with 25th and 75th percentiles, Mod/Sev = Mdoerate/Severe.
Correlation of T1 Relaxation Time and Pancreatic Stiffness with CP Category
Three representative figures for normal pancreas, mild CP and moderate CP cases are shown Fig. 1–3, respectively. The corresponding wave images are shown in Fig. 4. The distribution of pancreatic stiffness and T1 relaxation times are shown in Table 2, Fig. 5 and 6. The mean pancreatic stiffness values and T1 relaxation times were significantly different (both P < 0.001) among the study groups. Both the pancreatic stiffness and the T1 relaxation times were consistent among the different pancreatic subregions in all the CP and control groups (all P > 0.05). Thus, the mean values of the three subregions for each patient were used for all calculations and statistical comparisons. Excellent inter-observer agreement was observed for stiffness measurements (ICC = 0.93; 95% CI: 0.90–0.96), and for T1 relaxation time measurements (ICC = 0.94; 95% CI: 0.91–0.96).
Figure 1.
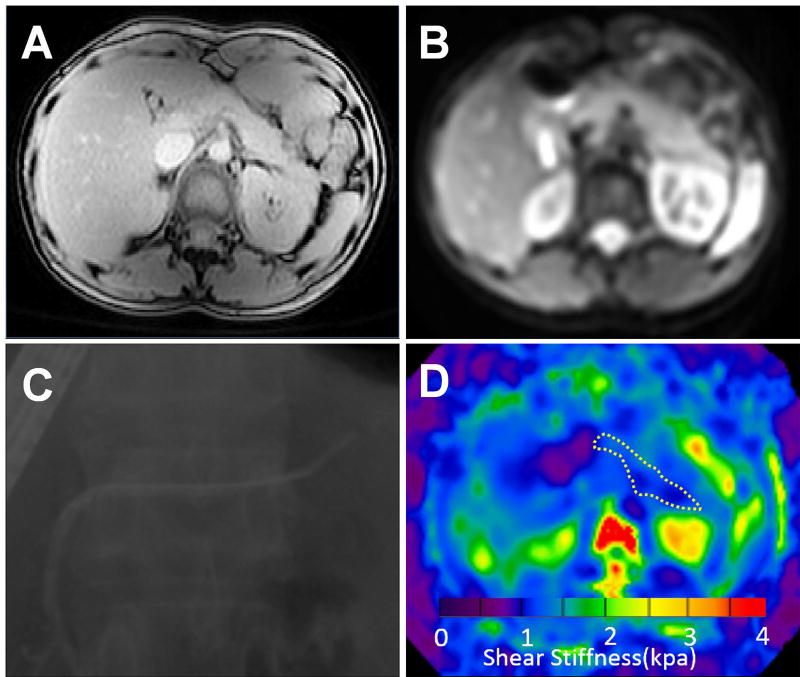
Pancreatic images from a 24-year healthy female volunteer. T1 mapping image (A), MRE magnitude image (B), ERCP image (C), and elastogram (D) at 40 Hz. The pancreatic T1 relaxation time and stiffness value on the elastogram (outlined in white dotted line) were 758±43 ms and 1.18±0.23 kPa, respectively.
Figure 3.
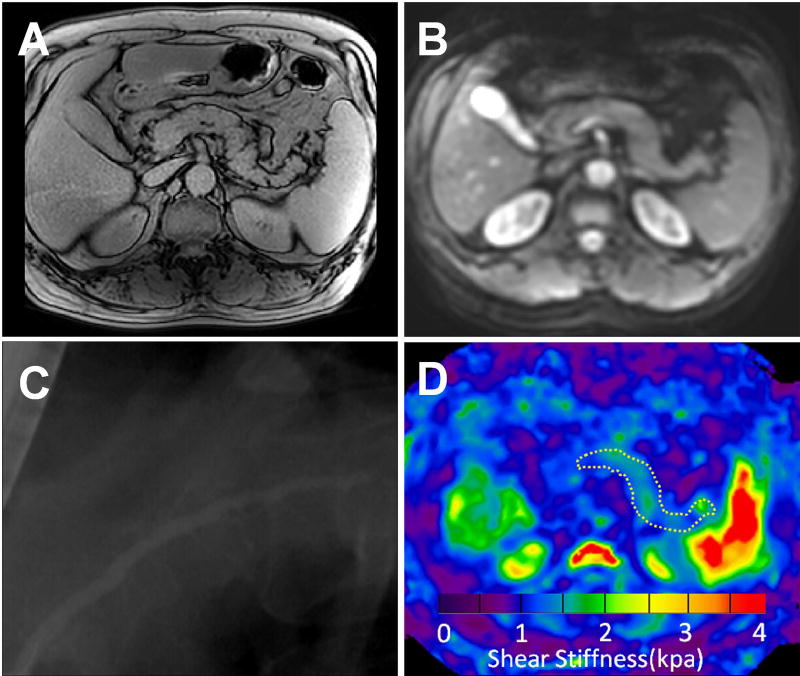
Images from a 52-year male with moderate CP. T1 mapping image (A), magnitude image (B), ERCP image (C), and elastogram (D) at 40 Hz for the pancreas The pancreatic T1 relaxation time (1544±87 ms) and stiffness (1.81±0.27 kPa) value on the elastogram (outlined in white dotted line) were both greater than normal and mild group.
Figure 4.
Representative pancreatic magnitude images (left) and wave images of the x, y, and z (from left to right) components of the vector displacement field generated by MRE. Images were obtained from a 24-year healthy female volunteer (upper row), a 46-year female with mild CP (middle row) and 52-year male with moderate CP (lower row) corresponding to Fig 1, 2 and 3, respectively.
Table 2.
T1 Relaxation Times and Stiffness of the Pancreas of Controls and Two Chronic Pancreatitis (CP) Groups
| T1 relaxation time (ms) | Stiffness (kPa) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Mild CP | Mod/Sev C P(n=16) |
P# | Control | Mild CP | Mod/Sev C P(n=16) |
P# | |
| Mean | 865 (220) | 1075 (221) | 1350 (139) | <0.001 | 1.21 (0.13) | 1.50 (0.15) | 1.90 (0.16) | <0.001 |
| Head | 844 (216) | 1051 (190) | 1317 (149) | <0.001 | 1.18 (0.18) | 1.48 (0.18) | 1.85 (0.16) | <0.001 |
| Body | 884 (242) | 1119 (260) | 1373 (124) | <0.001 | 1.23 (0.16) | 1.53 (0.18) | 1.94 (0.16) | <0.001 |
| Tail | 866 (266) | 1055 (284) | 1358 (154) | <0.001 | 1.22 (0.15) | 1.49 (0.18) | 1.91 (0.17) | <0.001 |
| P* | 0.814 | 0.452 | 0.489 | 0.698 | 0.736 | 0.287 | ||
Data are expressed as mean (standard deviation)
P was calculated by using the Friedman test, denoting differences of the characteristics among different subregions in each group
P was calculated by using the Kruskal-Wallis test, denoting differences of characteristics among different groups.
Figure 5.
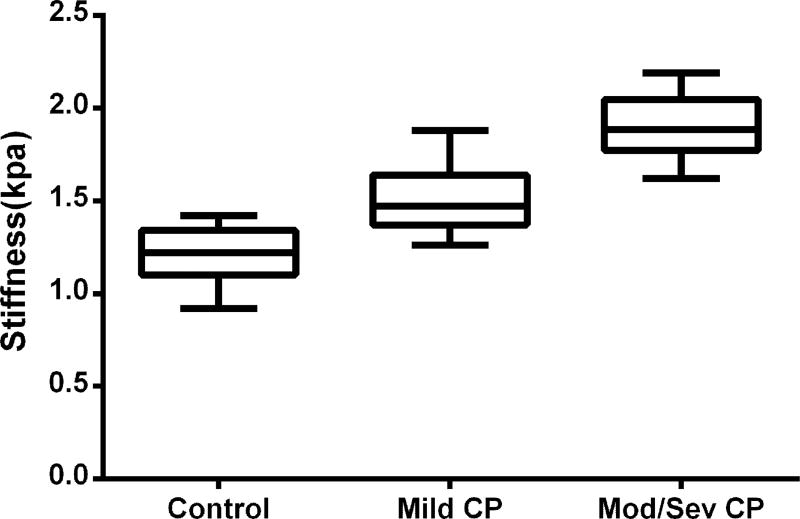
Box plot of pancreatic stiffness in the control (mean = 1.21 kPa, 95% CI: 1.17–1.26), mild CP group (mean = 1.50 kPa, 95% CI: 1.44–1.56) and mod/sev CP (mean = 1.90 kPa, 95% CI: 1.81–1.98). The stiffness of the pancreas in the mod/sev CP and mild CP group were significantly greater than normal group (both P < 0.001).
Figure 6.
Box plot of pancreatic T1 relaxation times in the control (mean = 865 ms, 95% CI: 789–940) and mild CP group (mean = 1075 ms, 95% CI: 993–1158) and mod/sev CP (mean = 1350 ms, 95% CI: 1276–1423). The mean T1 relaxation time of the pancreas in the mild CP and mod/sev CP group were significantly greater than normal group (P = 0.001 and <0.001, respectively).
AUC Analysis for Diagnosis of Chronic Pancreatitis
MRE (pancreatic stiffness >1.34 kPa) achieved significantly better diagnostic performance for distinguishing mild CP from normal pancreas than did T1 mapping (T1 relaxation time >908.4 ms) (AUC: 0.928 vs 0.751, P = 0.011). Although MRE had slightly greater sensitivity (83.3% vs 80.0%, P = 1.000), specificity (85.7% vs 68.6%, P = 0.109), and accuracy (84.6% vs 73.8%, P = 0.167), none of these comparisons reached statistical significance (Table 3 and Fig. 7A). For diagnosing the presence of any CP, pancreatic stiffness (>1.34 kPa) also achieved better diagnostic performance than did T1 mapping (>908.4 ms) (AUC: 0.953 vs 0.822, P = 0.009). For distinguishing mod/sev pancreatitis from normal pancreas or mild CP, pancreatic stiffness (>1.61 kPa) also achieved better diagnostic performance than did T1 mapping (>1,131.6 ms) (AUC: 0.981 vs 0.910, P = 0.033) (Table 3, Fig. 7 B and C).
Table 3.
Diagnostic Performance of T1 Relaxation Time and Stiffness for Diagnosing Different Grades of Chronic Pancreatitis (CP)
| Parameters | Grade of CP | Cut-off | AUC [% (95%CI)] |
Sensitivity [% (95%CI)] |
Specificity [% (95%CI)] |
PPV [% (95%CI)] |
NPV [% (95%CI)] |
|---|---|---|---|---|---|---|---|
| T1 relaxation time (ms) | Normal vs Mild CP | >908.4 | 0.751 (0.629–0.874) | 80.0 (61.4–92.3) | 68.6 (50.7–83.1) | 68.6 (50.7 – 83.1) | 80.0 (61.4 – 92.3) |
| Stiffness (kPa) | >1.34 | 0.928 (0.871–0.985) | 83.3 (65.3–94.4) | 85.7 (69.7–95.2) | 83.3 (65.3 – 94.4) | 85.7 (69.7 – 95.2) | |
| T1 relaxation time (ms) | Normal vs Any CP | >908.4 | 0.822 (0.729– 0.916) | 87.0 (73.7 – 95.1) | 68.6 (50.7 – 83.1) | 78.4 (64.7 – 88.7) | 80.0 (61.4 – 92.3) |
| Stiffness (kPa) | >1.34 | 0.953 (0.914 – 0.991) | 89.1 (76.4 – 96.4) | 85.7 (69.7 – 95.2) | 89.1 (76.4 – 96.4) | 85.7 (69.7 – 95.2) | |
| T1 relaxation time (ms) | Normal/Mild CP vs Mod/Sev CP | >1131.6 | 0.910 (0.862 – 0.984) | 93.8 (69.8 – 99.8) | 75.4 (63.1 – 85.2) | 48.4 (30.2 – 66.9) | 98.0 (89.4 – 99.9) |
| Stiffness (kPa) | >1.61 | 0.981 (0.957 – 1.000) | 100.0 (79.4 – 100.0) | 87.7 (77.2 – 94.5) | 66.7 (44.7 – 84.4) | 100.0 (93.7– 100.0) |
AUC = Area under curve, PPV = Positive predictive value, NPV = Negative predictive value, Mod/Sev = moderate and severe.
Figure 7.
A: Results of ROC curve analysis for T1 relaxation time and stiffness as criteria for diagnosis of mild CP. T1 relaxation time: Threshold value of 908.4 ms was 80.0% sensitive (95% CI: 61.4%–92.3%) and 68.6% specific (95% CI: 50.7%–83.1%) for the diagnosis of mild CP (AUC = 0.751). Stiffness: threshold value of 1.34 kPa was 83.3% sensitive (95% CI: 65.3%–94.4%) and 85.7% specific (95% CI: 69.7%–95.2%) for the diagnosis of mild CP (AUC = 0.928). B: Results of ROC curve analysis for T1 relaxation time and stiffness as criteria for diagnosis of mod/sev CP. T1 relaxation time: Threshold value of 1,131.6 ms was 93.8% sensitive (95% CI: 69.8% – 99.8%) and 75.4% specific (95% CI: 63.1 – 85.2%) for the diagnosis of mod/sev CP (AUC = 0.910). Stiffness: threshold value of 1.61 kPa was 100.0% sensitive (95% CI: 79.4%–100.0%) and 87.7% specific (95% CI: 77.2%–94.5%) for the diagnosis of mod/sev CP (AUC = 0.981). C: Results of ROC curve analysis for T1 relaxation time and stiffness as criteria for diagnosis of CP. T1 relaxation time: Threshold value of 908.4 ms was 87.0% sensitive (95% CI: 73.7%–95.1%) and 68.6% specific (95% CI: 50.7%–83.1%) for the diagnosis of mod/sev CP (AUC = 0.822). Stiffness: threshold value of 1.34 kPa was 89.1% sensitive (95% CI: 76.4%–96.4%) and 85.7% specific (95% CI: 69.7%–95.2%) for the diagnosis of mod/sev CP (AUC = 0.953).
Multiple Regression Analysis for Diagnosis of Chronic Pancreatitis
In the univariate analysis of the diagnoses of mild CP versus normal pancreas, the MPD diameter was significantly associated with mild CP (P = 0.025), but did not reach statistically significance in the multivariate analysis (P = 0.136). In multivariate regression analysis, T1 relaxation time and stiffness were independently associated with the diagnosis of mild CP (P = 0.025 and <0.001, respectively), as shown by Table 4.
Table 4.
Univariate and Multivariate Analysis for the Occurrence of Mild CP
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
| ||||
| Variable | Odds ratio (95%CI) | P | Odds ratio(95%CI) | P |
| MPD diameter(>3mm) | 3.50(1.17–10.47) | 0.025 | 3.45(0.68–17.54) | 0.136 |
| T1 relaxation time(>908.4ms) | 8.73(2.78–27.41) | <0.001 | 5.41 (1.24–23.58) | 0.025 |
| Stiffness(>1.34kPA) | 30.00(7.79–115.54) | <0.001 | 21.00 (4.88–90.27) | <0.001 |
| Age(>61years) | 2.80(0.89–8.84) | 0.080 | --- | --- |
| Gender(Female) | 0.67(0.25–1.79) | 0.667 | --- | --- |
| BMI(>24.95Kg/M2) | 3.32 (0.90–12.21) | 0.071 | --- | --- |
| Lipase level (>101.3U/L) | 3.88(0.93–16.27) | 0.064 | --- | --- |
| Amylase level (>34.0U/L) | 2.77(0.94–8.12) | 0.064 | --- | --- |
| FE-1 (<200µg/g) | 1.44(0.49–4.22) | 0.502 | --- | --- |
| Thickness(≤16.41mm) | 2.58(0.89–7.52) | 0.082 | --- | --- |
The values in bold denote statistical significance, BMI = body-mass index, MPD = main pancreatic duct, FE-1 = Fecal elastase-1.
In the univariate analysis of the diagnosis of any CP (mild or mod/sev) versus normal pancreas, MPD, thickness and FE-1 were significantly associated with any CP (P < 0.001, 0.011 and 0.045, respectively), but did not reach statistically significance in the multivariate analysis (P = 0.067, 0.823 and 0.284, respectively). In multivariate regression analysis, T1 relaxation time and stiffness were the variables independently associated with the presence of any CP (P = 0.024 and <0.001, respectively), as shown by Table 5.
Table 5.
Univariate and Multivariate Analysis for the Occurrence of CP
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
| ||||
| Variable | Odds ratio (95%CI) | P | Odds ratio(95%CI) | P |
| MPD diameter (>3mm) | 6.22(2.25–17.22) | <0.001 | 4.49(0.90–22.35) | 0.067 |
| T1 relaxation time(>908.4ms) | 14.55(4.77–44.40) | <0.001 | 5.69(1.26–25.64) | 0.024 |
| Stiffness(>1.34kPA) | 49.20(13.06–185.29) | <0.001 | 26.15(5.66–120.92) | <0.001 |
| Age(>61years) | 2.58(0.89–7.50) | 0.082 | --- | --- |
| Gender(Female) | 0.67(0.27–1.62) | 0.372 | --- | --- |
| BMI(>24.95Kg/M2) | 2.74(0.80–9.38) | 0.109 | --- | --- |
| Lipase level(>62.5U/L) | 2.45(0.99–6.07) | 0.052 | --- | --- |
| Amylase level(>38.0U/L) | 2.41(0.98–5.93) | 0.057 | --- | --- |
| FE-1 (<200µg/g) | 2.65(1.02–6.87) | 0.045 | 2.52(0.47–13.71) | 0.284 |
| Thickness(≤16.92mm) | 3.44(1.32–8.93) | 0.011 | 1.20(0.24–5.99) | 0.823 |
The values in bold denote statistical significance, BMI = body-mass index, MPD = main pancreatic duct, FE-1 = Fecal elastase-1.
Discussion
We investigated the value of both MR elastography (MRE) and T1 mapping for diagnosing CP. In this study, both MRE and T1 mapping achieved good diagnostic performance for diagnosing different stages of CP; MRE slightly outperformed T1 mapping. In multivariate analysis, both pancreatic stiffness and T1 relaxation time were independent predictors for the diagnosis of mild and of any CP.
CP leads to irreversible parenchymal and ductal changes in the pancreas. In a recent systemic review [21], EUS and ERCP outperformed all other imaging modalities for diagnosing CP. However, EUS and ERCP are invasive techniques; furthermore, diagnostic ERCP has been largely replaced by EUS [22, 23] and non-invasive imaging modalities. Both clinicians and radiologists have accepted noninvasive imaging to evaluate pancreatic parenchyma and pancreatic ducts [6, 7, 24, 25]. In patients with moderate or severe CP, irregularities and dilatations of the MPD and side branches on CT/MR imaging and typical clinical symptoms are expected; hence moderate or severe CP is usually easily diagnosed. In our study, both MRE and T1 mapping also achieved very high diagnostic performance (both AUC > 0.9 and sensitivity > 90%) for distinguishing moderate/severe CP from normal/mild CP. However, in patients with mild CP, findings using conventional imaging may be subtle or even absent [4]. Consequently, those imaging techniques are insensitive for the diagnosis of mild CP.
Previous studies showed that ultrasound elastography can provide relevant information about tissue stiffness and thus may aid the diagnosis of CP [26–29]. Quantitative EUS elastography (EUS-EG) evaluation, including strain ratio and histogram analysis, has been reported to achieve relatively high AUC values for the presence of CP (0.86 and 0.91) [30–33], which are lower than that achieved by MRE in our study (0.953), but higher than those achieved by T1 mapping (0.822). EUS-EG only measures theoretically relative elasticity in ROIs, rather than absolute values of stiffness. Moreover, EUS-EG is a subjective and invasive method, EUS-EG images are markedly affected by the size of ROI and the level applied to the pancreas, and the ROI setting of the strain ratio cannot include the entire pancreatic parenchyma. On the other hand, MRE is objective, non-invasive, and produces the entire pancreatic stiffness map. Individual values of each separate subregion can be analyzed. Hence, MRE has the potential to detect heterogenous changes of CP in different regions. The newly emerged shear wave elastography (SW-EG), measuring the Young’s modulus [28], was also recently used to diagnose CP. However, SW-EG uses US, the probe is more distant from the pancreas compared to EUS, which reduces the resolution of the pancreas image, particularly when large area of gastrointestinal gas or liquid component (e.g. large vessels, stomach) are present, because the shear waves are easily refracted and reflected. In this study, MRE propagated good waves across the entire upper abdomen, the technical failure of wave data was only reported in 5 of 219 cases. We found that the MRE-based stiffness was very sensitive to detect mild CP, showing higher sensitivity (83.3% vs 77.1%) and specificity (85.7 vs 64.9%) than that reported by SW-EG (normal and indeterminate CP vs consistent with CP and suggestive of CP) [28]. Mild CP directly alters the mechanical properties of pancreatic tissue. The histological changes of CP, histological fibrosis, inflammation and changes of the cellular density, lead to increased stiffness detected by MRE, prior to the signal changes detectable by conventional MR imaging. The mean numerical stiffness values (shear modulus) of normal pancreas and mild CP were 1.21 kPa and 1.50 kPa, respectively, whereas the Young’s moduli in EUS studies were 3.5 kPa and approximately 5~7 kPa, respectively. Based on elasticity theories, the Young’s modulus of soft biological tissues is nearly three times as great as shear modulus [17], which might partially explain these differences.
T1 mapping quantitatively measures the T1 relaxation time of the target organ. Similar to stiffness, T1 relaxation time is also a tissue-specific property and independent of imaging parameters. Both stiffness and T1 relaxation times should be interchangeable across MRI machines, vendors and magnet strength. T1 mapping has been successfully used in a number of cardiac diseases including myocardial fibrosis and myocardial deposition diseases. Tirkes et al first compared T1 relaxation times between a control (mean=797 ms) and mild CP group (mean=1099 ms), which were similar to our results (mean=865 ms and 1075 ms, respectively). A T1 relaxation time (>900 ms) was 80% sensitive and 69% specific for the diagnosis of mild CP in Tirkes’ study, which was also consistent with our results. Our study further proved that the performance of MRE was slightly better than T1 mapping for diagnosis of mild CP. MRE was more specific than T1 mapping (85.7% vs 68.6%), with similar sensitivity (83.3% vs 80.0%). In Tirkes’ study [34], combining extracellular volume fraction with T1 mapping yielded a high AUC, 0.94, for diagnosing the presence of CP, which is very similar to our results using MRE (0.953). Compared with secretin-enhanced MRCP, which adds several minutes of imaging time and significant cost, both MRE and T1 mapping acquisition are simple, fast and free of intravenous injection of secretin and should be added to clinical imaging protocols. In those centers when MRE hardware is not available, T1 mapping can provide an alternative choice with reasonable diagnostic accuracy.
Our study has several limitations. First, this is a retrospective study with relatively small subject numbers. Secondly, the diagnosis of CP was not confirmed histologically. The criteria for CP used in this study, particularly EUS criteria, are known to be subjective and not very sensitive for the detection of early CP. Third, MRE has lower resolution than standard MRI pulse sequences. MRE and T1 mapping might not be suitable for detection of severe CP that is more likely to have severe pancreatic atrophy. To control this potential bias, such patients were excluded from this study. We believe that the practical importance of this limitation is mitigated because the diagnosis of severe CP is usually easy and is not likely to require stiffness/T1 measurements. Fourth, we did not evaluate other MR sequences, such as DWI imaging, which might also be useful for CP diagnosis.
In conclusion, both MRE and T1 mapping are fast, cost-effective, and promising MR methods for diagnosing CP; MRE slightly outperformed T1 mapping. Although these methods cannot substitute for EUS or ERCP to detect ductal changes, they offer quantitative measurements of early parenchymal changes. Therefore, these techniques can provide very valuable supplementary information and screening for suspected CP. In practice, patients referred with typical abdominal pain who are being evaluated for CP may undergo MRCP/EUS combined with T1 mapping/MRE to assess for both morphological and mechanical changes, thereby optimizing the detection of minimal changes in CP.
Figure 2.
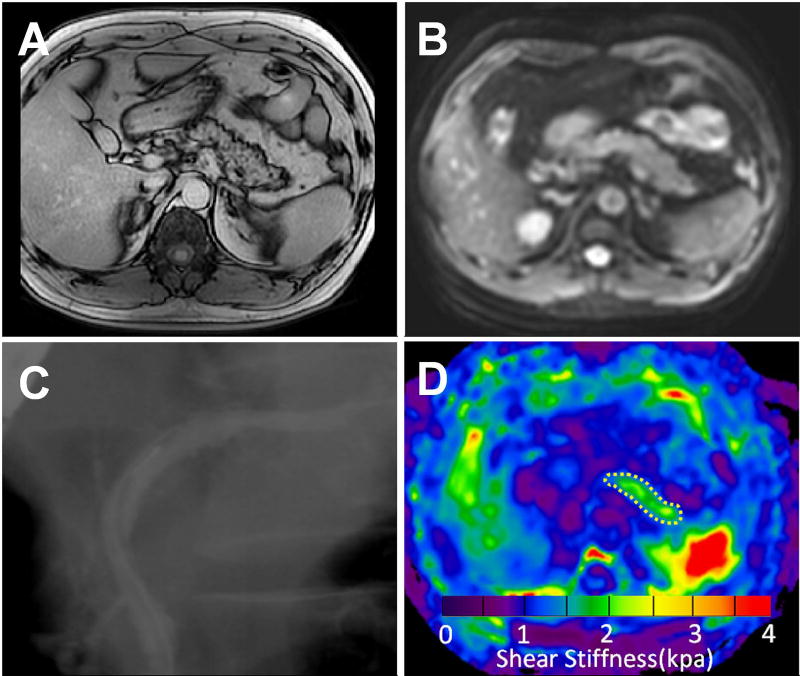
Images from a 46-year female with mild CP. T1 mapping image (A), MRE magnitude image (B), ERCP image (C), and elastogram (D) at 40 Hz for the pancreas The pancreatic T1 relaxation time (1344±103 ms) and stiffness (1.53±0.34 kPa) value on the elastogram (outlined in white dotted line) were both greater than normal.
Acknowledgments
We thank Richard L Ehman, Jun Chen and Kevin J Glaser from the Mayo Clinic for providing the MRE system and the tailored pancreatic MRE drivers.
Grant support:
National Natural Science Foundation of China (81771802, 81771893, 81401376 and 81471718), Outstanding Youth Foundation of China Medical University (YQ20160005) and National Institutes of Health (grant EB001981).
Footnotes
Conflicts of interest:
Xiaoqi Wang is the employee of Philips Healthcare, but he had no control over inclusion of any data or information that might have presented a conflict of interest. There are no actual or potential conflicts of interest to declare in relation to this article. None of the other authors have conflicts of interest or any specific financial interests relevant to the subject of this manuscript.
References
- 1.Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184–1197. doi: 10.1016/S0140-6736(10)61852-1. [DOI] [PubMed] [Google Scholar]
- 2.Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–1573. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Chari ST. Chronic pancreatitis: classification, relationship to acute pancreatitis, and early diagnosis. J Gastroenterol. 2007;42(Suppl 17):58–59. doi: 10.1007/s00535-006-1918-7. [DOI] [PubMed] [Google Scholar]
- 4.Conwell DL, Lee LS, Yadav D, Longnecker DS, Miller FH, Mortele KJ, Levy MJ, Kwon R, Lieb JG, Stevens T, Toskes PP, Gardner TB, Gelrud A, Wu BU, Forsmark CE, Vege SS. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: evidence-based report on diagnostic guidelines. Pancreas. 2014;43:1143–1162. doi: 10.1097/MPA.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25:756–759. doi: 10.1136/gut.25.7.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wathle GK, Tjora E, Ersland L, Dimcevski G, Salvesen OO, Molven A, Njolstad PR, Haldorsen IS. Assessment of exocrine pancreatic function by secretin-stimulated magnetic resonance cholangiopancreaticography and diffusion-weighted imaging in healthy controls. J Magn Reson Imaging. 2014;39:448–454. doi: 10.1002/jmri.24167. [DOI] [PubMed] [Google Scholar]
- 7.Madzak A, Engjom T, Wathle GK, Olesen SS, Tjora E, Njolstad PR, Laerum BN, Drewes AM, Dimcevski G, Frokjaer JB, Haldorsen IS. Secretin-stimulated MRI assessment of exocrine pancreatic function in patients with cystic fibrosis and healthy controls. Abdom Radiol (NY) 2016 doi: 10.1007/s00261-016-0972-8. [DOI] [PubMed] [Google Scholar]
- 8.Erturk SM. Chronic pancreatitis and diffusion-weighted MR imaging. Radiology. 2009;252:316. doi: 10.1148/radiol.2521090396. [DOI] [PubMed] [Google Scholar]
- 9.Tirkes T, Lin C, Fogel EL, Sherman SS, Wang Q, Sandrasegaran K. T1 mapping for diagnosis of mild chronic pancreatitis. J Magn Reson Imaging. 2017;45:1171–1176. doi: 10.1002/jmri.25428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin M, Glaser KJ, Talwalkar JA, Chen J, Manduca A, Ehman RL. Hepatic MR Elastography: Clinical Performance in a Series of 1377 Consecutive Examinations. Radiology. 2016;278:114–124. doi: 10.1148/radiol.2015142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H, Park JB, Yoon YE, Park EA, Kim HK, Lee W, Kim YJ, Cho GY, Sohn DW, Greiser A, Lee SP. Noncontrast Myocardial T1 Mapping by Cardiac Magnetic Resonance Predicts Outcome in Patients With Aortic Stenosis. JACC Cardiovasc Imaging. 2017 doi: 10.1016/j.jcmg.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Curcic J, Sauter M, Schwizer W, Fried M, Boesiger P, Steingoetter A. Validation of a golden angle radial sequence (GOLD) for abdominal T1 mapping during free breathing: demonstrating clinical feasibility for quantifying gastric secretion and emptying. J Magn Reson Imaging. 2015;41:157–164. doi: 10.1002/jmri.24530. [DOI] [PubMed] [Google Scholar]
- 13.Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, Freeman M, Yamao K, Canto M, Hernandez LV. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009;69:1251–1261. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Guo Q, Xia F, Dzyubak B, Glaser KJ, Li Q, Li J, Ehman RL. MR elastography for the assessment of hepatic fibrosis in patients with chronic hepatitis B infection: does histologic necroinflammation influence the measurement of hepatic stiffness? Radiology. 2014;273:88–98. doi: 10.1148/radiol.14132592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Gao F, Li Y, Tao S, Yu B, Liu Z, Liu Y, Glaser KJ, Ehman RL, Guo Q. Differentiation of benign and malignant solid pancreatic masses using magnetic resonance elastography with spin-echo echo planar imaging and three-dimensional inversion reconstruction: a prospective study. Eur Radiol. 2017 doi: 10.1007/s00330-017-5062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Glaser KJ, Venkatesh SK, Ben-Abraham EI, Ehman RL. Feasibility of using 3D MR elastography to determine pancreatic stiffness in healthy volunteers. J Magn Reson Imaging. 2015;41:369–375. doi: 10.1002/jmri.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y, Xia F, Li QJ, Li JH, Yu B, Li Y, An H, Glaser KJ, Tao S, Ehman RL, Guo QY. Magnetic Resonance Elastography for the Evaluation of Liver Fibrosis in Chronic Hepatitis B and C by Using Both Gradient-Recalled Echo and Spin-Echo Echo Planar Imaging: A Prospective Study. Am J Gastroenterol. 2016 doi: 10.1038/ajg.2016.56. [DOI] [PubMed] [Google Scholar]
- 18.Lee CU, Glockner JF, Glaser KJ, Yin M, Chen J, Kawashima A, Kim B, Kremers WK, Ehman RL, Gloor JM. MR elastography in renal transplant patients and correlation with renal allograft biopsy: a feasibility study. Acad Radiol. 2012;19:834–841. doi: 10.1016/j.acra.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 20.Kamath MG, Pai CG, Kamath A, Kurien A. Comparing acid steatocrit and faecal elastase estimations for use in M-ANNHEIM staging for pancreatitis. World journal of gastroenterology. 2017;23:2217–2222. doi: 10.3748/wjg.v23.i12.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issa Y, Kempeneers MA, van Santvoort HC, Bollen TL, Bipat S, Boermeester MA. Diagnostic performance of imaging modalities in chronic pancreatitis: a systematic review and meta-analysis. Eur Radiol. 2017;27:3820–3844. doi: 10.1007/s00330-016-4720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rana SS, Vilmann P. Endoscopic ultrasound features of chronic pancreatitis: A pictorial review. Endosc Ultrasound. 2015;4:10–14. doi: 10.4103/2303-9027.151314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato A, Irisawa A, Bhutani MS, Shibukawa G, Yamabe A, Fujisawa M, Igarashi R, Arakawa N, Yoshida Y, Abe Y, Maki T, Hoshi K, Ohira H. Significance of normal appearance on endoscopic ultrasonography in the diagnosis of early chronic pancreatitis. Endosc Ultrasound. 2017 doi: 10.4103/2303-9027.209870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akisik MF, Sandrasegaran K, Jennings SG, Aisen AM, Lin C, Sherman S, Rydberg MP. Diagnosis of chronic pancreatitis by using apparent diffusion coefficient measurements at 3.0-T MR following secretin stimulation. Radiology. 2009;252:418–425. doi: 10.1148/radiol.2522081656. [DOI] [PubMed] [Google Scholar]
- 25.Dominguez Munoz JE. Diagnosis of chronic pancreatitis: Functional testing. Best Pract Res Clin Gastroenterol. 2010;24:233–241. doi: 10.1016/j.bpg.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Iglesias-Garcia J, Larino-Noia J, Lindkvist B, Dominguez-Munoz JE. Endoscopic ultrasound in the diagnosis of chronic pancreatitis. Rev Esp Enferm Dig. 2015;107:221–228. [PubMed] [Google Scholar]
- 27.Kawada N, Tanaka S. Elastography for the pancreas: Current status and future perspective. World J Gastroenterol. 2016;22:3712–3724. doi: 10.3748/wjg.v22.i14.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuwahara T, Hirooka Y, Kawashima H, Ohno E, Ishikawa T, Yamamura T, Furukawa K, Funasaka K, Nakamura M, Miyahara R, Watanabe O, Ishigami M, Hashimoto S, Goto H. Usefulness of shear wave elastography as a quantitative diagnosis of chronic pancreatitis. J Gastroenterol Hepatol. 2017 doi: 10.1111/jgh.13926. [DOI] [PubMed] [Google Scholar]
- 29.Pozzi R, Parzanese I, Baccarin A, Giunta M, Conti CB, Cantu P, Casazza G, Tenca A, Rosa R, Gridavilla D, Casella G, Conte D, Fraquelli M. Point shear-wave elastography in chronic pancreatitis: A promising tool for staging disease severity. Pancreatology. 2017;17:905–910. doi: 10.1016/j.pan.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Iglesias-Garcia J, Dominguez-Munoz JE, Castineira-Alvarino M, Luaces-Regueira M, Larino-Noia J. Quantitative elastography associated with endoscopic ultrasound for the diagnosis of chronic pancreatitis. Endoscopy. 2013;45:781–788. doi: 10.1055/s-0033-1344614. [DOI] [PubMed] [Google Scholar]
- 31.Yashima Y, Sasahira N, Isayama H, Kogure H, Ikeda H, Hirano K, Mizuno S, Yagioka H, Kawakubo K, Sasaki T, Nakai Y, Tada M, Yoshida H, Omata M, Koike K. Acoustic radiation force impulse elastography for noninvasive assessment of chronic pancreatitis. J Gastroenterol. 2012;47:427–432. doi: 10.1007/s00535-011-0491-x. [DOI] [PubMed] [Google Scholar]
- 32.Llamoza-Torres CJ, Fuentes-Pardo M, Alvarez-Higueras FJ, Alberca-de-Las-Parras F, Carballo-Alvarez F. Usefulness of percutaneous elastography by acoustic radiation force impulse for the non-invasive diagnosis of chronic pancreatitis. Rev Esp Enferm Dig. 2016;108:450–456. doi: 10.17235/reed.2016.4103/2015. [DOI] [PubMed] [Google Scholar]
- 33.Kuwahara T, Hirooka Y, Kawashima H, Ohno E, Ishikawa T, Kawai M, Suhara H, Takeyama T, Hashizume K, Koya T, Tanaka H, Sakai D, Yamamura T, Furukawa K, Funasaka K, Nakamura M, Miyahara R, Watanabe O, Ishigami M, Hashimoto S, Goto H. Quantitative diagnosis of chronic pancreatitis using EUS elastography. J Gastroenterol. 2017;52:868–874. doi: 10.1007/s00535-016-1296-8. [DOI] [PubMed] [Google Scholar]
- 34.Tirkes T, Lin C, Cui E, Deng Y, Territo PR, Sandrasegaran K, Akisik F. Quantitative MR Evaluation of Chronic Pancreatitis: Extracellular Volume Fraction and MR Relaxometry. AJR Am J Roentgenol. 2018:1–10. doi: 10.2214/AJR.17.18606. [DOI] [PMC free article] [PubMed] [Google Scholar]