Abstract
Inflammation plays a major role in progression of rheumatoid arthritis (RA), a disease treated with antagonists of tumor necrosis factor-alpha (TNF-α) and interleukin 1β (IL-1β). New in vitro testing systems are needed to evaluate efficacies of new anti-inflammatory biological drugs, ideally in a patient-specific manner. To address this need, we studied microspheroids containing 10,000 human osteoarthritic primary chondrocytes (OACs) or chondrogenically differentiated mesenchymal stem cells (MSCs), obtained from three donors. Hypothesizing that this system can recapitulate clinically observed effects of anti-inflammatory drugs, spheroids were exposed to TNF-α, IL-1β or to supernatant containing secretome from activated macrophages (MCM). The anti-inflammatory efficacies of anti-TNF-α biologicals adalimumab (ADA), infliximab (IFX) and etanercept (ETA), and the anti-IL-1β agent anakinra (ANA) were assessed in short- term microspheroid and long-term macrospheroid cultures (100,000 OACs). While gene and protein expressions were evaluated in microspheroids, diameters, amounts of DNA, glycosaminoglycans and hydroxiproline were measured in macrospheroids. The tested drugs significantly decreased the inflammation induced by TNF-α or IL-1β. The differences in potency of anti-TNF-α biologicals at 24 hrs and 3 weeks after their addition to inflamed spheroids were comparable, showing high predictability of short-term cultures. Moreover, the data obtained with microspheroids grown from OACs and chondrogenically differentiated MSCs were comparable, suggesting that MSCs could be used for this type of in vitro testing. We propose that in vitro gene expression measured after the first 24 hrs in cultures of chondrogenically differentiated MSCs can be used to determine the functionality of anti-TNF-α drugs in personalized and preclinical studies.
Keywords: human articular chondrocytes, mesenchymal stem cells, 3D tissue spheroids, inflammation, anti-TNF-α biologicals
1. INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disease, causing disability in about 1% of general population.1 RA is characterized by the accumulation of inflammatory cells in synovium, erosion of articular cartilage and subchondral bone, and in severe cases by joint destruction.2 Therapies with anti-TNF-α biologicals are being used with improved clinical outcomes in comparison to traditional anti-rheumatic drugs.3 Anti-TNF-α drugs competitively antagonize TNF-α, a pivotal factor in immune-mediated inflammatory diseases such as RA, psoriatic arthritis (PA) and osteoarthritis (OA).4 Etanercept (ETA; recombinant human TNF-α soluble receptor fusion protein), infliximab (IFX; chimeric mouse/human monoclonal antibody) and adalimumab (ADA; recombinant human monoclonal antibody) are being clinically used for the longest time. However, due to the high cost, these drugs represent a heavy burden to healthcare budgets. Fortunately, their less expensive and therefore more available biosimilar counterparts (biosimilars) are becoming available to patients. But before gaining official approvals for entering clinical trials, these drugs must first demonstrate their in vitro “similarity” to the existing originators. Therefore, the development and application of patho-physiologically relevant and reliable cell culture-based in vitro models for drug testing are of great importance.
Cell-based assays are critical for assessing efficacies of new drugs in preclinical studies, while contributing to reduction of animal testing, in line with the 3R (Replacement, Reduction and Refinement) ethical principle.5 Cell lines are often used in drug research, as they are cost-effective, easy to use, available in unlimited quantities and are can be free of ethical concerns. However, since they are genetically different, either due to natural mutations or planned manipulations, their phenotypes, functionalities and responses to drugs are often different from those obtained with their primary counterparts. Also, after several consecutive in vitro passages, cell lines can experience genetic instabilities.6
Human osteoarthritic primary chondrocytes (OACs), isolated from biomedical waste materials following joint-replacement surgery, represent an accessible and attractive cell source for in vitro drug testing. Importantly, genetic stability during long-term in vitro expansion of OACs has been demonstrated.7,8 Interestingly, gene expression profiles of normal chondrocytes (NCs) and OACs show little difference when cultured in monolayers, suggesting that the biological profile of cells is influenced more by the microenvironment than the disease state of donor’s cartilage.9 We have shown, by analyzing changes in expression of the most important genes involved in inflammation (MCP1, IL6, IL8, PTGS2, VCAM1) and catabolism (ADAMTS4, MMP1, MMP13) in OA, that the inflammatory state of OACs and anti-inflammatory effects of IFX and ETA can be monitored in monolayer cultures using a quantitative reverse transcription-polymerase chain reaction (qRT-PCR).10 We therefore hypothesized that OACs could be used to create three-dimensional (3D) microtissues used for in vitro testing of anti-inflammatory biologicals.
Chondrocytes grown in a 3D environment morphologically and physiologically differ from their counterparts growing in two-dimensional (2D) monolayer cultures. Spatial and physical aspects of a 3D environment are thought to affect a number of cellular processes, including proliferation, differentiation, morphology, gene and protein expression and responsiveness to external stimuli.11,12 As 3D cell culture systems can mimic physiological tissue microenvironments, they could be used as predictive models for drug testing.5 For decades, scaffold-free 3D tissues (spheroids), uniform in cell numbers and sizes, are being successfully generated by self-assembly of cells seeded in hanging drops.5,13 Also, this cell culture procedure can be accomplished by using automated liquid handling systems, thereby enabling high-throughput testing in preclinical drug discovery.
In this study we describe a new in vitro hanging-drop 3D chondrogenic tissue model combined with the qRT-PCR method for assessing potencies of anti-TNF-α (ADA, IFX, and ETA) and anti-IL-1β (ANA) biological drugs. Moreover, our goal was to determine whether chondrogenically differentiated mesenchymal stem cells (MSCs), obtained from the same donors as the OACs, could be equivalently used in the newly developed testing model. Spheroid microtissues were prepared from either 10,000 OACs or a matching number of chondrogenically differentiated MSCs (ndonors = 3) and exposed for 24 h to human recombinant TNF-α, IL-1β or a cytokine rich medium conditioned with activated macrophages (MCM). The specific cytokine-neutralizing potencies of ADA, ETA, IFX and ANA were determined by qRT-PCR. Drug potencies were assessed from the expression levels of the eight most differently expressed genes involved in arthritis.14,15 The changes in gene expression levels measured in differently treated microspheroids were correlated with the concentrations of specific proteins found in microspheroid culture supernatants. The quantities of DNA, glycosaminoglycans (GAG) and hydroxiproline (OHP) were assessed in macrospheroids containing 100,000 OACs following their 3 week-long exposure to TNF-α, IL-1β or MCM, in the presence or absence of a corresponding individual anti-inflammatory biological drug.
2. MATERIALS AND METHODS
2.1. Isolation of mesenchymal stem cells and osteoarthritic chondrocytes
After obtaining the Institutional Review Board approval (IRB-AAC4836), femoral heads were acquired from three patients (2 females and 1 male) aged 57 to 70 years (mean age 63) with advanced osteoarthritis (OA), who underwent hip replacement surgeries at the New York Presbyterian Hospital, Columbia Medical Center, New York, USA. After dissection of cartilage and its storage in phosphate buffered saline (PBS; Mediatech, Manassas, VA, USA) at 4 °C, the trabecular bone was removed using a sterile surgical bone chisel (Dental Wakefield Bone Chisel #1w, ProSurg, USA) and transferred into 50 mL falcon tubes (BD Falcon, Franklin Lakes, NJ, USA), each containing 20 ml of α-Minimum Essential Medium (α-MEM; Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10% (v/v) of heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 1% (v/v) of Antibiotic-Antimycotic solution (10,000 U/mL of penicillin, 10,000 µg/mL of streptomycin and 25 µg/mL of Fungizone®; Gibco, Grand Island, NY, USA) and 1% (v/v) of 200 mM L-Glutamine (Sigma-Aldrich).
The harvested material was agitated for 30 min at 37 °C and 100 rpm, using the Excella E24 incubator shaker (New Brunswick Scientific, Edison, NJ, USA) and then vortexed for 2 min to release bone marrow cells. In order to remove the fatty layer from the cell suspension, the falcon tubes were placed upright for approximately 20 min at room temperature and the upper adipose-rich layer was aspirated using a sterile pipette. The cell suspension was then filtered through a 70 µm cell strainer (Falcon, BD). A volume of 5 mL of filtered cell suspension was then added to 20 mL of a complete α-MEM medium supplemented with 1 ng/mL of basic Fibroblast Growth Factor (bFGF; Peprotech, Rocky Hills, NJ, USA), and used for plating the cells at a density of 50,000/cm2 into 150 cm2 plastic tissue culture T flasks (Costar, Corning, NY, USA). Of all isolated cells, only MSCs adhered to the bottom, and were further cultured at standard conditions (37 °C and 5% CO2 in air) in a humidified MCO-19M Sanyo O2/CO2 incubator (Sanyo, Osaka, Japan).
The cells were expanded until they reached ~90% confluence, during which time the complete medium was changed 3 times per week. Passage one (P1) MSCs were trypsinized using 0.025% trypsin/0.01% Ethylenediaminetetraacetic acid (EDTA) solution (Gibco), washed in PBS and resuspended in 5 mL of expansion medium, i.e. high glucose Dulbecco’s Modified Eagle Medium (DMEM; Gibco), supplemented with 10 % v/v FBS, 50 µg/mL gentamicin (Gibco) and 0.1 ng/mL bFGF (Peprotech). Subsequently the number of viable cells was microscopically determined using 0.4% Trypan blue staining solution (Sigma-Aldrich) and the Neubauer haemocytometer (Fisher Scientific, Hampton, NH, USA).
Next, MSCs were aliquoted and cryopreserved in 10% (v/v) DMSO (Sigma-Aldrich) in FBS, at concentrations of 106 cells/mL and stored in liquid nitrogen. The identity and multipotent nature of isolated MSCs were confirmed by: (a) detection of their characteristic surface molecules CD73, CD90 and CD105 with flow cytometry, and (b) their in vitro differentiation into adipocytes, chondroblasts and osteoblasts (Supplement 1).
Osteoarthritic chondrocytes (OACs) were enzymatically isolated and cryopreserved from cartilage samples as described previously.14 Briefly, samples were rinsed in sterile PBS, minced into small pieces and collected in DMEM Nutrient Mixture F-12 (Gibco), supplemented with 50 µg/mL gentamicin, 2 µg/mL Fungizone® and 1 mg/mL collagenase type II (Gibco). Enzymatic tissue digestion was carried out for 24 h at 37 °C. After washing and resuspending the cells, their numbers were determined as in the case of MSCs. Subsequently, OACs were either grown in chondrogenic medium, composed of high glucose DMEM (Gibco), supplemented with 1% (v/v) Corning® ITS Premix Universal Culture Supplement (Corning, Bedford, MA, USA), 50 µg/mL gentamicin (Gibco), 1% (v/v) 1M HEPES buffer (Mediatech), 0.1 mg/mL sodium pyruvate (Invitrogen, Waltham, MA, USA), 50 µg/mL L-Proline (Sigma-Aldrich), 50 µg/mL L-ascorbic acid (Sigma-Aldrich) and 10 ng/mL transforming growth factor beta 3 (TGF-β3; R&D, Minneapolis, MN, USA), or cryopreserved in liquid nitrogen until use.
2.2. Cell expansion and the formation of micro and macro spheroids
OACs or MSCs were grown in monolayers to 80 – 90% confluence in MSC expansion medium. For short-term experiments cell culture passages P1 – P3 were trypsinized, cells seeded on Perfecta 3D® 96-Well Hanging Drop Plates (3D Biomatrix Inc, Ann Arbor, MI, USA), using a 20 – 200 µL multichannel pipette (Thermo Scientific, Waltham, MA, USA) for delivering 10,000 cells/drop (Vdrop= 40 µL), and then left to form tissue microspheres. At day 3, half volume of the expansion medium, devoid of bFGF, was replaced with the chondrogenic medium. While primary chondrocytes were grown in drops for 5 days, chondrogenic differentiation of MSCs was only achieved within a 3 week culture period. During this time half of the medium in drops was exchanged 3-times a week. Finally, diameters of 30 microspheres/cell type/donor were measured using the Olympus FSX 100 inverted microscope (Olympus, Tokyo, Japan). At the end of the culture period, microspheroids formed in hanging drops were transferred to U-bottomed 96-well microplates (Costar), incubated for an additional 24 h, and then used for further experiments. After fixing and dissecting the microspheroids, chondrogenic differentiation of MSCs and re-differentiation of OACs were determined in histological slices by Alcian blue and collagen II staining (Supplement 1a). For long-term experiments macrospheroids containing 100,000 OACs (P2) were formed by employing the same centrifugation method, as in case of forming spheroids for in vitro characterization of chondrogenic differentiation of isolated MSCs (Supplement 1a).
2.3. THP-1 cell culture and preparation of macrophage conditioned medium (MCM)
Following the established protocol for induction and polarization of the THP-1 human monocytic cell line (American Type Culture Collection - ATCC, Manassas, VA, USA) into M1 inflammatory macrophages, an abundant natural source of TNF-α, IL-1β and numerous other cytokines and growth factors in a form of macrophage conditioned medium (MCM) was obtained.16 THP-1 cell cultures were established at a density of 2 – 4 × 105 cells/mL in 150 cm2 plastic tissue culture T flasks (Costar), using THP-1 culture medium, i.e. RPMI-1640 (Gibco), supplemented with 50 µg/mL of gentamicin (Gibco) and 10% (v/v) of either heat-inactivated human serum (ATCC) or FBS (Gibco). The cells were subcultured 4 – 6 times, at a concentration of 8×105 cells/mL.
Differentiation of monocytes into macrophages was induced with 10 ng/mL (~16 nM) of phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) for 16 h in 25 cm2 ultra-low attachment flasks (Costar), at a concentration of 106 cells/mL. Subsequently, the THP-1 culture medium was replaced with the polarization medium, consisting of 100 ng/mL lipopolysaccharide (LPS; Sigma-Aldrich) and 100 ng/mL of human interferon gamma (IFN-γ; Peprotech) in THP-1 culture medium. The M1 macrophage polarization was achieved after 48 h. Next, the polarization medium was completely removed and substituted with the THP-1 medium. The cells were incubated for 24 h, after which the medium was again replenished. Finally, after additional 24 h incubation the cell culture supernatant (MCM) was collected and stored at −80 °C. Two batches of MCM were prepared, depending on the type of serum used throughout THP-1 cell cultivation and polarization, i.e. MCMFBS and MCMh.s., respectively.
2.4. Inflammatory cytokines and anti-inflammatory biological drugs
The OA inflammatory microenvironment was established by adding 1 ng/mL of recombinant human TNF-α (Peprotech), 1 ng/mL of recombinant human IL-1β (Peprotech) or a working solution of MCM (50% of MCM in 50% chondrogenic medium) to cell cultures. Then, anti-inflammatory biologicals were added to reduce the inflammation (Figure 1). Anti-TNF-α biologicals etanercept (Enbrel®, Immunex Corp., Thousand Oaks, CA, USA) and adalimumab (Humira®, Abbott Laboratories, North Chicago, IL, USA), as well as the anti-IL1β biological drug anakinra (Kineret®, Swedish Orphan Biovitrum AB, Stockholm, Sweden), were kindly donated by Dr. Lisa F. Imundo, MD, head of the Division of Adolescence Rheumatology at Morgan Stanley Children’s Hospital, New York, NY, USA. Another anti-TNF-α drug, infliximab (Remicade®, Janssen Biotech Inc., Horsham, PA, USA), was obtained from the local pharmacy. To avoid excessive freeze-thaw cycles, stock solutions of cytokines, MCM and tested biological drugs were prepared, aliquoted and stored at −80 °C.
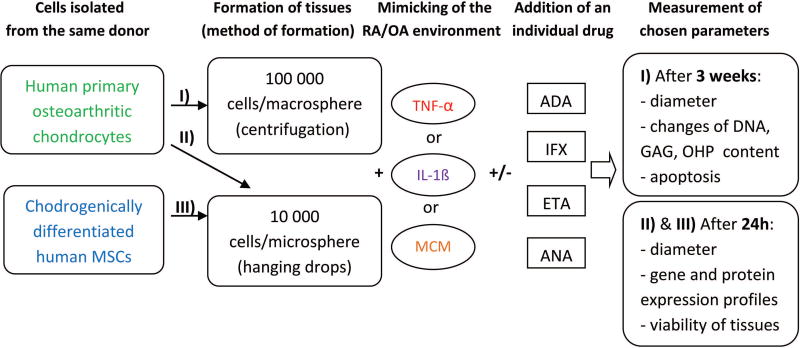
Figure 1. Experimental design.
TNF-α: tumor necrosis factor α; IL-1β: interleukin 1β; MCM: macrophage conditioned medium; ADA: adalimumab; IFX: infliximab; ETA: etanercept; ANA: anakinra; GAG: glycosaminoglycans; OHP: hydroxyproline.
Solutions of human recombinant cytokines in chondrogenic medium, either alone or supplemented with 1 µg/mL of adalimumab (ADA), infliximab (IFX), etanercept (ETA) or anakinra (ANA), were preincubated for 30 min at room temperature, after which they were added to MSC- or OAC-derived chondral microspheroid cultures for 24 h or to OAC macrospheroid cultures for 3 weeks with medium refreshment twice a week. Subsequently, the microspheroids and their corresponding supernatants were collected and stored at −80 °C for further analysis. Sample groups were set up as follows: ø (control 1; chondrogenic medium alone), MCMø (control 2; 50% THP-1 culture medium + 50% chondrogenic medium), TNF-α, IL-1β or MCMh.s. working solution either alone or in conjunction with ADA (TNF-α+ADA; MCM+ADA), IFX (TNF-α+IFX; MCM+IFX), ETA (TNF-α+ETA; MCM+ETA) or ANA (IL-1β+ANA; MCM+ANA).
2.5. Gene and protein expression analyses
Expressions of IL6, IL8, MCP1, MMP1, MMP13, ACAN, SOX9 and VCAM1 genes in microspheroids were determined by qRT-PCR. Total RNA isolation and reverse transcription of mRNA to cDNA were carried out with Fast SYBR® Green Cells-to CT™ Kit (Ambion, Foster City, CA, USA), according to manufacturer’s instructions. DNase I was added to lysis solution and then half of each microspheroid lysate was used for mRNA to cDNA reverse transcription. Next, isolated cDNA was stored at −20 °C. Thawed cDNA samples were diluted with equal volumes of nuclease-free water, provided with the kit. qRT-PCR reactions were carried out using 96-well optical plates (Applied Biosystems, Foster City, CA, USA), containing 15 µL of reaction mixture/well. This was composed of 7.5 µL Fast SYBR® Green Master Mix (Applied Biosystems), 1.9 µL nuclease-free water, 5 µL of 2-times diluted cDNA sample, and 0.3 µL of each, forward and reverse custom-made primers (cprimer = 10 µM; Invitrogen) (Table 1).
Table 1.
Primers used in qRT-PCR experiments
| Gene symbol |
Gene name | Accession number |
Forward sequence (5’→3’) |
|---|---|---|---|
| Reverse sequence (5’→3’) | |||
|
| |||
| GAPDH | Glycarldehyde-3-phosphate dehydrogenase | NM_002046.5 | AAGGTGAAGGTCGGAGTCAAC |
| GGGGTCATTGATGGCAACAATA | |||
|
| |||
| MMP1 | Matrix metallopeptidase 1 (interstitial collagenase) | NM_002421.3 | GGTGTGGTGTCTCACAGCTT |
| GTCCCGATGATCTCCCCTGA | |||
|
| |||
| MMP13 | Matrix metallopeptidase 13 (collagenase 3) | NM_002427.3 | CCCCAGGCATCACCATTCAA |
| CAGGTAGCGCTCTGCAAACT | |||
|
| |||
| MCP1 | Chemokine (C-C motif) ligand 2 | NM_002982.3 | GCTCATAGCAGCCACCTTCATTC |
| GGACACTTGCTGCTGGTGATTC | |||
|
| |||
| IL6 | Interleukin 6 (interferon, beta 2) | NM_000600.3 | ACTCACCTCTTCAGAACGAATTG |
| CCATCTTTGGAAGGTTCAGGTTG | |||
|
| |||
| IL8 | Interleukin 8 | NM_000584.3 | ACTGAGAGTGATTGAGAGTGGAC |
| AACCCTCTGCACCCAGTTTTC | |||
|
| |||
| VCAM1 | Vascular cell adhesion molecule 1 | NM_001078.3 | CATGGAATTCGAACCCAAACA |
| GACCAAGACGGTTGTATCTCTGG | |||
|
| |||
| ACAN | Aggrecan | NM_001135.3 | CCCCTGCTATTTCATCGACCC |
| GACACACGGCTCCACTTGAT | |||
|
| |||
| SOX9 | SRY (sex determining region Y)-box 9 | NM_000346.3 | AGCGAACGCACATCAAGAC |
| CTGTAGGCGATCTGTTGGGG | |||
A total of 40 cycles of qRT-PCR were performed as follows: activation (20 s at 95 °C), denaturation (3 s at 95 °C), annealing and extension (30 s at 60 °C). The results were recorded on StepOne Plus Real-Time PCR System (Applied Biosystems, Singapore) and the amplification products were analyzed with the StepOne Soft program (Applied Biosystems, Singapore). In each sample, the level of particular gene expression was calculated as a ratio of the analyzed gene Cq values versus the stably expressed glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reference gene Cq values and normalized to untreated control group, i.e. ø or MCMø.14 For each group, three samples of cDNA were obtained from three individual microspheroids and for each of them qRT-PCR analysis was performed in duplicate. The resulting Cq values were recorded at 0.3 threshold and changes in gene expressions were calculated as 2−∆∆Cq.17 For low expressed genes a cut-off value or Cq ≥ 35 was used.
To determine the concentrations of TNF-α, IL-1β, IL-6, IL-8 and MCP-1 cytokines, both in MCM, and in supernatants of differently treated microspheroids, enzyme-linked immunosorbent assays (ELISA) were performed according to manufacturers’ instructions. Except for IL-1β ELISA (R&D), all other kits were purchased from Peprotech. Using a TECAN Infinite M200 microplate reader (TECAN, Grödig, Austria) absorbances were measured, either at 405 nm with the wavelength correction set at 650 nm, or at 450 nm with the wavelength correction at 570 nm, for Peprotech kits and the R&D kit, respectively. Concentrations of TNF-α, IL-1β, IL-6, IL-8 and MCP-1 were determined from slopes of standard curves and calculated in ng/mL.
2.6. Assessment of apoptosis
Apoptotic changes were evaluated after a 3 week treatment of chondral macrospheroids composed of 100,000 OACs, with TNF-α, IL-1β or MCM. At that point, macrospheroids were fixed and processed as described in Supplement 1a. Histological sections (5 µm thick) were incubated with antibodies recognizing the cleaved caspase-3 (Cell Signaling, Danvers, MA, USA), according to manufacturer’s instructions. To visualize apoptotic cells, HRP-labeled secondary antibodies and other components of the Vectastain® Universal Elite® ABC Kit (Vector laboratories, Burlingame, CA, USA) were applied, following the manufacturer’s protocol. Images were acquired with the Motic BA210 LED light microscope, equipped with a Moticam 3.0 MP camera, and processed by using the Motic Images Plus 2.0 software (all from Motic Asia, Hong Kong).
2.7. Cell viability assay
To determine the extent of cell viability in microspheroids, which were individually kept in 50 µL of chondrogenic medium within their original wells of a 96-well microtiter plate, 50 µL of CellTiter-Glo® reagent (Promega, Madison, WI, USA) per well was added. The plate was incubated for 30 min at room temperature and then mildly agitated for 2 min on orbital shaker to accelerate cell lysis. The contents of wells were transferred to a clear bottom black 96-well plate and incubated at room temperature for additional 10 min. Luminescence of ATP released from living cells was recorded on the TECAN infinite M200 plate reader (TECAN), with the integration time of 1s.
2.8. DNA, GAG and total collagen quantification
For DNA quantification, each tissue macrospheroid containing 100,000 OACs was digested overnight at 60 °C in 1 mL of papain digestion buffer, containing: 20 µL of papain solution (Papain from papaya latex, Sigma-Aldrich), 8 mg sodium acetate (Sigma-Aldrich), 1.6 mg cysteine hydrochloride (Sigma-Aldrich), 18.6 mg EDTA (Sigma-Aldrich) and up to 1 mL of deionized water. Next, supernatants (100 µL) were transferred in duplicate to clear bottom black 96-well microplates (Costar) and 100 µL of Quanti-iT™ PicoGreen® ds DNA Assay reagent (Molecular Probes, Eugene, OR, USA) was added into each well. Finally, fluorescence was measured using the TECAN Infinite M200 plate reader (TECAN) at 485 nm excitation and 520 nm emission wavelengths, to determine DNA contents.
The quantities of GAG were determined by using the Sulfated Gycosaminoglycan Assay (Blyscan™ Kit, Biocolor, UK). Briefly, 40 µL of supernatants taken from papain digested samples or same volumes of chondroitin-6 sulphate standards, were transferred to clear polystyrene 96-well plates (Costar). Following the addition of 200 µL of 1.9-dimethylmethylene blue solution (Sigma-Aldrich) per well, absorbances were measured at 540 and 595 nm on the TECAN Infinite M200 plate reader (TECAN), within 5 min. The OHP content was determined following a modified protocol of Stegemann and Stalder (Supplement 2).18
2.9. Data analysis
All results are presented as mean ± SEM. Unless otherwise stated, one-way ANOVA with Tukey’s post hoc analysis was used to determine statistically significant differences between the sample groups. Statistical analyses were carried out using GraphPad Prism® 5.0 software (San Diego, CA, USA), with a minimum of 3 biological replicates. P<0.05, P<0.01 and P<0.001 values, shown in figures as *, ** or ***, respectively, were considered statistically significant.
For obtaining gene expression data following neutralization of ADA, ETA, IFX and ANA, the measured Cq values were normalized to those of non-treated (ø) samples. For microspheroids treated with a single recombinant pro-inflammatory cytokine, gene expressions were also normalized to the untreated control group, while in the case of microspheroids treated with MCM, gene expression data were normalized to the MCM ø control group.
3. RESULTS
3.1. Culture of chondrogenic microspheroids
In order to systematically compare the effects of pro-inflammatory agents and anti-inflammatory biologicals on OACs and chondrogenically differentiated MSCs, we isolated both cell types from surgically removed femoral heads (biological waste material) of each of the three available donors (ndonors = 3; mean age = 63 years), whenever possible. However, due to the advanced age of donors and lower viability of MSCs, we were only able to acquire paired sets of MSCs (M1 and M2) and primary OACs (p ch1 and p ch2) samples from two of them. A sample of primary OACs (p ch3) obtained from the third donor, was paired with unrelated MSCs (M3; donor age = 19 years), purchased from Lonza (Walkersville, MD, USA).
To confirm that the isolated and purchased MSCs meet the generally accepted criteria, a tri-lineage in vitro differentiation of each sample (M1, M2, M3) was carried out. Successful adipogenic, chondrogenic and osteogenic differentiation was confirmed by Oil Red O, Alcian Blue and von Kossa staining, respectively (Supplement Figure 1a). In accordance with the established guidelines, MSCs were also phenotypically verified by flow cytometry, following the positive staining of their representative CD73, CD90 and CD105 surface stem cell markers with specific fluorescent antibodies (Supplement Figure 1b).19
After characterization of MSCs and subsequent expansion of MSCs and OACs in monolayer cultures, the cells were used for microspheroid formation in hanging drops. The average diameters of microspheroids formed of OACs (n = 30) and chondrogenically differentiated MSCs (n = 30) were 344 ± 3.2 µm and 247 ± 3.5 µm, respectively. Successful chondrogenic differentiation of MSCs and re-differentiation of OACs in spheroids was confirmed by GAG and collagen II staining using Alcian blue dye and a specific antibody against collagen II, respectively (Supplement Figure 1c).
3.2. Effects of inflammatory cytokines on chondral microspheroids
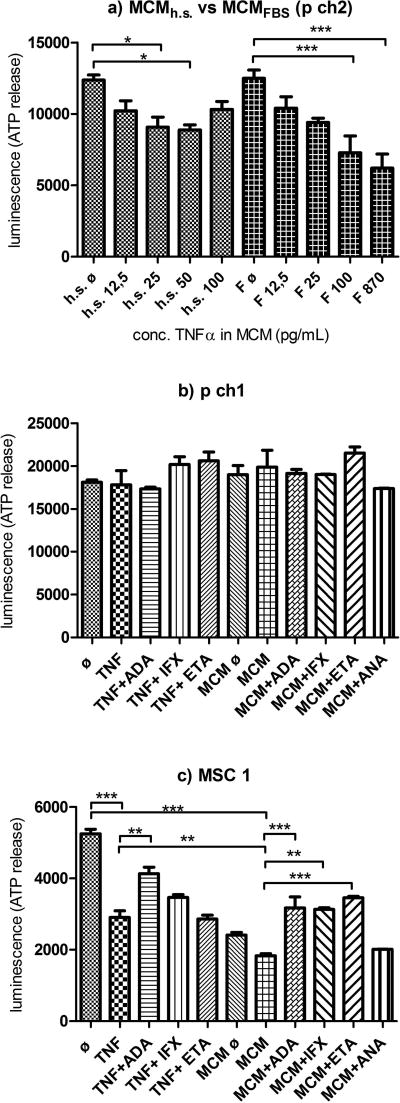
Activated macrophages conditioned medium (MCM) is a very potent pro-inflammatory agent. Prior to being used in experiments, properties of MCMh.s. and MCMFBS, the contents of selected inflammatory cytokines, stability following repeated freeze-thaw cycles, and cell viability within MCM-treated microspheroids were comparatively characterized. Concentrations of TNF-α, IL-1β, IL-6, IL-8 and MCP-1 in MCMh.s. and MCMFBS were determined by ELISA assays (Supplement Figure 2). TNF-α and IL-1β concentrations found in MCMFBS were almost 10- and 2-fold higher than those present in MCMh.s., respectively. After two freeze-thaw cycles, the reproducibility of TNF-α and IL-1β measurements was better in the case of MCMh.s. (results not shown). Therefore, MCMh.s. was selected for further work, aliquoted and then used in most experiments. The concentrations of TNF-α in synovial fluids of patients with OA range between 0.03 and 0.08 ng/mL.20 To obtain similar concentrations, MCMh.s. was diluted 1:1 with chondrogenic medium, to obtain a working solution containing 0.05 ng/mL TNF-α and 0.45 ng/mL IL-1β.
As expected, undiluted MCMFBS and MCMh.s. affected cell viability in OAC microspheroids more strongly than their diluted counterparts (Figure 2a). After 24 h of incubation following their addition to microspheroids, a significant drop in cell viability was observed already at concentrations ≥ 0,025 ng TNF-α/mL in MCM. In addition, the effects on cell viability of TNF-α or MCMh.s. alone or in combination with the three anti-TNF-α biological (ADA, ETA and IFX), as well as the anti-IL-1β agent ANA, were tested on microspheroids composed of OACs (p ch1 and p ch2) or donor matched chondrogenically differentiated MSCs (M1 and M2) (Figures 2b and 2c). For OAC microspheroids that were treated with either TNF-α or MCMh.s. the differences in cell viability were not statistically significant compared to untreated samples. On the other hand, cell viability in TNF-α or MCMh.s. treated microspheroids containing chondrogenically differentiated MSCs, dropped significantly. This cytotoxic effect could clearly be prevented with the addition of any of the three anti-TNF-α agents (ADA, ETA or IFX) or the anti-IL-1β drug ANA.
Figure 2. Cell viability.
a) Impacts of different concentrations of MCMh.s. and MCMFBS on cell viability after 24 h incubation of microspheroids prepared from p ch 2 (h.s. = human serum; F = fetal bovine serum (FBS); h.s. ø or Fø = ½ chondrogenic medium + ½ THP-1 medium supplemented with h.s. or FBS). b) Viability of microspheroids made of p ch1 and c) MSC1 after 24 h incubation with cytokine(s) and tested drugs. (ø - chondrogenic medium; TNF - 1 ng/mL TNF-α; MCMø - ½ chondrogenic medium + ½ THP-1 medium; MCM - working conc. of MCMh.s.; ADA - adalimumab; IFX - infliximab; ETA - etanercept; ANA - anakinra). Data shown as averages of 3 measurements/treatment ± SEM; One-way ANOVA; *, ** and *** indicate statistical significance at p<0.05, p< 0.01 and p<0.001, respectively.
3.3. Effects of inflammatory factors and anti-inflammatory biological drugs on expression of genes and proteins
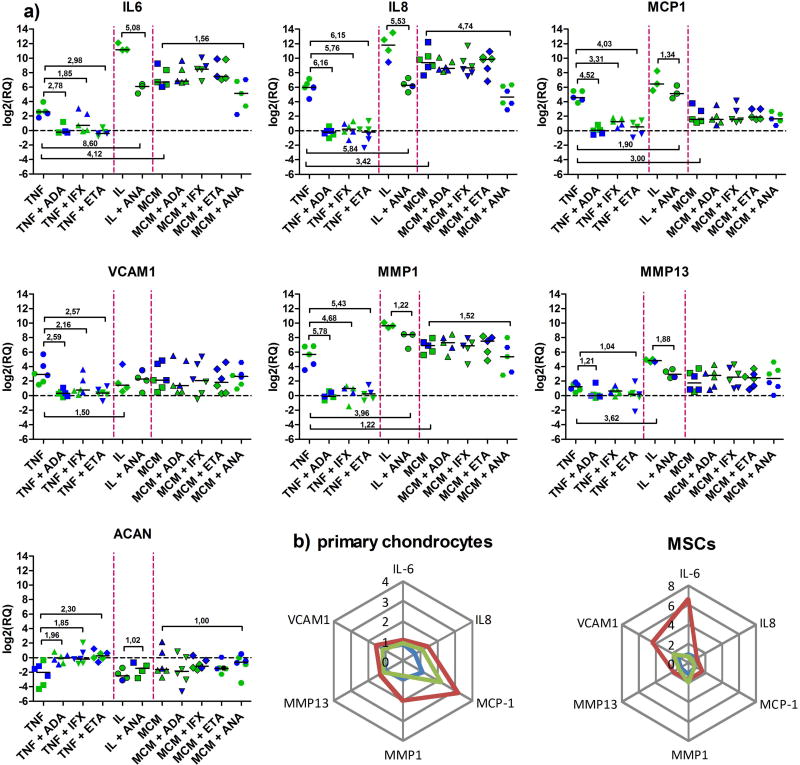
Based on our previous results, the levels of 8 genes that were found to be most differentially expressed in OA (IL6, IL8, MCP1, MMP1, MMP13, VCAM1, ACAN, and SOX9)14 were assessed in microspheroids made of OACs or chondrogenically differentiated MSCs originating from 3 different donors (Figure 3a), following inflammation induction with TNF-α, IL-1β, or MCMh.s. In the case of TNF-α induced inflammation, median gene expressions were increased by >50-fold for IL8 and MMP1, >20-fold for MCP1, >5-fold for IL6 and VCAM1, and >2-fold for MMP13. When IL-1β was used as the inflammation inducer, gene expression levels increased >2,000-fold for IL6 and IL8, >780-fold for MMP1, >80-fold for MCP1, >30-fold for MMP13, and >2-fold for VCAM1. The pro-inflammatory MCMh.s. working solution increased gene expressions by >450-fold for IL8, >100-fold for MMP1, >3-fold for MMP13 and VCAM1, and >2-fold for MCP1. All three inflammation inducing factors efficiently increased the expression of the IL8 gene. At the same time, all three factors were the weakest inducers of ACAN and SOX9 genes, i.e. >0.2-fold and >0.45-fold, respectively. Differences in gene expressions between differently treated chondral microspheroid groups were considered biologically significant when their calculated fold changes were ≥ 2 which is equivalent to the log2 fold change of ≥ 1.
Figure 3. a) Gene expression profiles following the addition of inflammatory mediators TNF-α, IL-1β or MCMh.s. working solution and anti-inflammatory biological drugs adalimumab (ADA), infliximab (IFX), etanercept (ETA) and anakinra (ANA).
Blue and green dots represent values obtained in microspheroid chondral tissues made of MSCs and OACs (3 donors), respectively. Statistically significant changes, i.e. log2(RQ) ≥ 1 and ≤ −1 have been outlined; median values of all groups are also shown. b) Radar graphs representing anti-TNF-α neutralization efficacies of ADA (blue), IFX (red) and ETA (green). Mean RQ values of three biological samples are shown for OAC- and MSC-derived microspheroids. Value 0 represents total inhibition of gene expression.
The inflammatory processes triggered by either TNF-α, IL-1β or the MCMh.s. working solution could always be reversed with the addition of ADA, IFX and ETA, as well as ANA. As shown in Figure 3a, when microspheroid inflammation was triggered by TNF-α, the addition of any of the three tested anti-TNFα biologicals had a remarkable impact on gene expression. In general, the levels of constitutively expressed genes in tested cells were re-established (log2RQ = 0) with the only exception being SOX9, where the observed changes were not statistically significant (data not shown). Similarly, the addition of ANA notably reversed the microspheroid inflammation process induced by IL-1β, but its neutralizing effect was weaker in terms of reaching the constitutive level of monitored gene expressions. As shown previously, the working solution of MCMh.s. caused significant changes in the expression of IL6, IL8, MMP1, MMP13, VCAM1 and ACAN genes. In this case, the presence of ADA, IFX or ETA had no significant impact on the expression of most analyzed genes. On the other hand, the addition of ANA diminished the expression of IL6, IL8 and MMP1 genes and at the same time increased the expression of ACAN.
To compare the anti-inflammatory capacities of ADA, IFX and ETA in a TNF-α-induced inflammatory microspheroid tissue environment, median values of relative quantities, i.e. fold changes of six selected genes, IL8, IL6, MCP1, MMP1, MMP13 and VCAM1, were plotted on radar graphs (Figure 3b). The center of each radar graph (value 0) represents a complete inhibition of gene expression.
Following the addition of TNF-α and individual anti-TNF-α biologicals, ADA, IFX or ETA, to microspheroid tissues composed of OACs or chondrogenically differentiated MSCs, different gene expression profiles were observed. In both types of samples, the anti-inflammatory efficacy of ADA was superior compared to that of ETA or IFX. When tested on microspheroids formed from OACs, IFX was found to be a weaker down-regulator of MCP1, MMP1 and IL8 gene expressions, while in those containing chondrogenically differentiated MSCs it was less efficacious in reducing the expressions of VCAM1, IL6 and MCP1 genes. In comparison to ADA, the TNF-α neutralization effect of ETA was found to be less pronounced in the case of MCP1 and IL8 gene expressions in microsperoid tissues prepared from OACs, while in those made of chondrogenically differentiated MSCs, its weaker effects on the expression of MMP1 and VCAM1 genes were observed (Figure 3b).
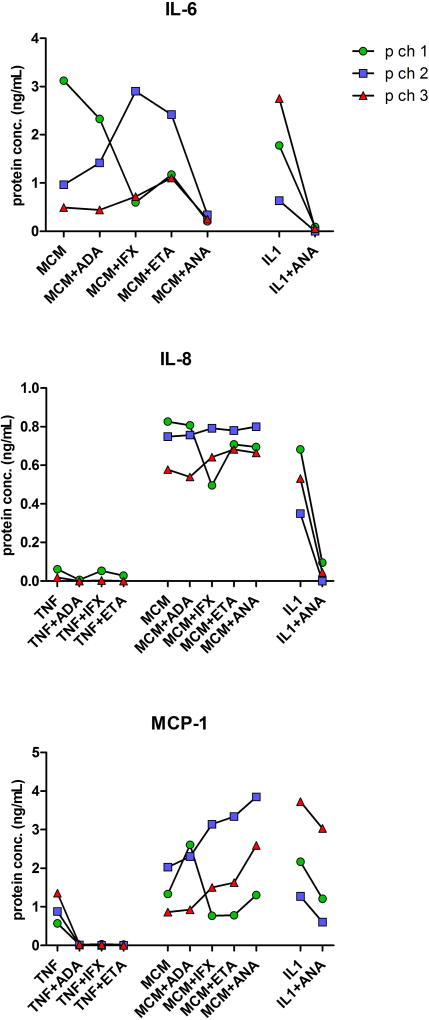
Additionally, we verified whether the observed changes in gene expressions correlated with the corresponding protein levels. For this purpose, IL-6, IL-8 and MCP-1 specific ELISAs were used to test pooled supernatants taken from OAC-derived microspheroid cultures that were previously treated with different inflammatory factors, either alone or in combinations with tested anti-inflammatory biologicals (Figure 4). Compared to the negative control group (ø), the addition of TNF-α, IL-1β or MCMh.s. working solution to microspheroids elicited considerable increases in IL-6, IL-8 and MCP-1 concentrations, with a partial exception of IL-6, where no such changes could be detected when TNF-α alone was used as inflammation inducer. The addition of individual anti-TNF-α (ADA, IFX or ETA) or anti-IL-1β (ANA) biological drugs to microspheroid cultures treated with TNF-α or IL-1β, significantly decreased the amounts of all three cytokines in their supernatants, clearly confirming correlations between particular gene and protein expressions. In cases where MCMh.s. working solution was added to microspheroids, the anti-inflammatory effects of the tested biologicals were less strong, in line with the observed gene expression levels. Except for the samples treated with MCMh.s. working solution, we were unable to detect IL-6, IL-8 and MCP-1 in supernatants of microspheroids formed from chondrogenically differentiated MSCs, treated with TNF-α or IL-1β (data not shown).
Figure 4. Cytokine secretion by chondral microspheroids after their exposure to inflammatory factors and anti-inflammatory biological drugs.
Quantities of IL-6, IL-8 and MCP-1 (ng/mL) detected in supernatants of microspheroid chondral tissues formed from human OACs (p ch1, p ch2 and p ch3) following their 24 h exposure to TNF-α, MCMh.s. working solution or IL-1β alone or combined with individual anti-inflammatory biological drugs, adalimumab (ADA), infliximab (IFX), etanercept (ETA) or anakinra (ANA). Single measurements were carried out in pooled supernants of each differently treated microspheroid group. Please, note differences in scales.
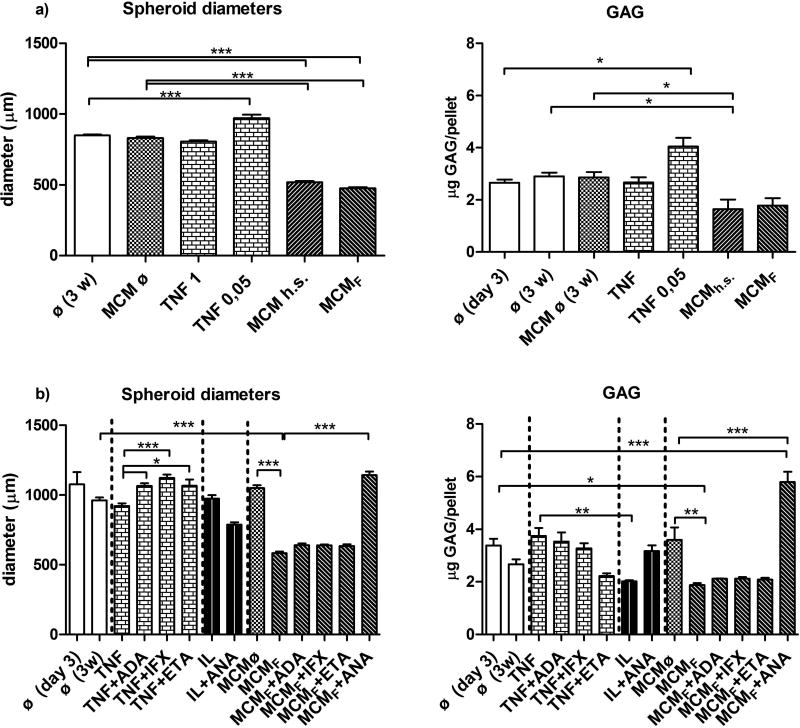
3.4. Effects of inflammatory factors and anti-inflammatory biological drugs on the growth of macrospheroids
The effects of MCMh.s. or MCMFBS working solutions alone or various combinations of MCMFBS with either ADA, IFX, ETA, or ANA on the in vitro growth of macrospheroids made of 100,000 OACs, were monitored over a period of 3 weeks. In comparison to controls, i.e. MCMFBS ø or MCMh.s. ø, the diameters of macrospheroids that were treated with working solutions of MCMFBS or MCMh.s., decreased by almost 40% (Figure 5). In contrast, when macrospheroids were concomitantly treated with combinations of MCMFBS and individual anti-TNFα biological drugs, i.e. ADA, IFX or ETA, their diameters did not change significantly. The only exception was observed when samples were treated with a combination of MCMFBS working solution and anti-IL-1β biological drug ANA, where their diameters increased by ~50%, as compared to the MCMFBS working solution treated controls.
Figure 5. Biological drug testing data.
Differences in diameters and GAG contents of macrospheroids made of 100,000 OACs, which were cultured for 3 weeks in the presence of either TNF-α, IL-1β or working solutions of MCMh.s. (a) or MCMF (b) alone, and combined with tested biological drugs. Diameter measurement results in (a) n=8 and (b) n=6, as well as GAG concentrations (n=3) were compared by one-way ANOVA; p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***). Legend: ø- chondrogenic medium (control 1); MCM ø - ½ chondrogenic medium + ½ THP-1 medium (control 2); MCMh.s. or MCMF - MCM supplemented with human serum or FBS; TNF -TNF-α (1 ng/mL, if not specified otherwise); IL - IL1β (1 ng/mL) ; ADA = adalimumab (1 µg/mL); IFX = infliximab (1 µg/mL); ETA = etanercept (1 µg/mL); ANA = anakinra (1 µg/mL).
The effects of TNF-α and IL-1β either alone or in conjunction with their corresponsive antagonistic biologicals, were also assessed by measuring the diameters of OACs-derived macrospheroids after the 3 weeks observation period. We found that their diameters, after they were treated with 1 ng/mL of TNF-α or 1 ng/mL of IL-1β, were comparable to those measured in the ø negative control group (Figure 5). While the concomitant presence of TNF-α with ADA, IFX or ETA increased the macrospheroid diameters by ~22%, no such effect was observed when the combination of IL-1β and its specific antagonist ANA was applied. To verify whether the changes in macrospheroid sizes were due to apoptosis, their sections were stained with antibodies recognizing specific cleaved caspase III. Apoptotic cells and morphological changes characteristic for apoptosis were only detected in macrospheroids treated exclusively with either MCMh.s. or MCMFBS working solution, and not in those cultured in the presence of 1 ng/mL TNF-α or IL-1β (Supplement Figure 3).
The same 3D tissue model (macrospheroids containing 100,000 OACs) and observation period (3 weeks) were used also for assessing the effects of MCMFBS and MCMh.s. working solutions on DNA, glycosaminoglycan (GAG) and hydoxyproline (OHP) contents (Figure 5, Supplement Figure 4). While in the ø and MCMø negative control groups DNA, GAG and OHP quantities remained comparable over the 3 weeks of culture, a significant drop in DNA quantity was observed in macrospheroids treated with the MCMFBS working solution. This effect was reversed when the combination of MCMFBS working solution and ANA was used. The same negative trend was observed with GAGs. Namely, the GAG content in macrospheroids being treated with IL-1β was significantly lower, as compared to those that were exposed to TNF-α. In the presence of MCMh.s. or MCMFBS working solution, OHP values in macrospheroids decreased significantly after 3 weeks, in comparison to the ø negative control group. However, they were extensively restored when the combination of MCMFBS working solution and ANA was used.
4. DISCUSSION
4.1. Design of the microspheroid cartilage tissue model
The main goal of our study was to develop a new in vitro 3D model for preclinical or patient-specific testing of anti-TNF-α biological drugs adalimumab (ADA), etanercept (ETA) and infliximab (IFX) which are the most prescribed biologicals for treating rheumatoid arthritis (RA). The anti-inflammatory effects of the anti-IL-1β drug anakinra (ANA) were also investigated. To test specific cytokine neutralizing capacities of these drugs, a 3D microspheroid chondral tissue model mimicking human osteoarthritic (OA) pathology was established by using human OA chondrocytes (OACs) or chondrogenically differentiated mesenchymal stem cells (MSCs). The anti-inflammatory efficacies of ADA, ETA, IFX and ANA were assessed by determining the extent of down-regulation of pre-selected genes which have previously been found to be over-expressed in both cell types exposed to potent inflammation inducers (TNF-α, IL-1β or MCMh.s. working solution). The potencies of all three anti-TNF-α biological drugs were compared based on the measured statistically relevant changes in gene expressions (cut-off: ≥ 2-fold change, i.e., log2 fold change ≥ 1). We show that these changes were accompanied by substantial differences in levels of encoded proteins, indicating important functional consequences.21,22
4.2. In vitro effects of TNF-α and IL-1β on OACs and MSCs
Until now, neutralization of soluble TNF-α was evaluated by measuring TNF-α induced cytotoxicity using either animal or human cell lines, which can respond differently than primary human cells23–28 and show poor correlation with the results obtained in clinical studies.6,29–32 Also, measurements of gene expression for evaluation of biological activities of biopharmaceuticals demonstrated higher sensitivity and specificity than a simple in vitro assessment of cell proliferation.33,34 As new anti-TNF-α biosimilars are entering the market and the current in vitro assays used for evaluating their efficacies are still mainly performed using genetically manipulated human or animal cell lines, there is an emerging need for physiologically relevant testing models.
TNF-α and IL-1β are the most important cytokines involved in RA and OA pathologies. It is believed that TNF-α drives acute inflammation whereas IL-1β has a pivotal role in sustaining the inflammation and consequent cartilage erosion.39 In addition to their known catabolic effects, both cytokines inhibit proteoglycan and type II collagen synthesis.40,41 The previously determined in vitro effects of TNF-α and IL-1β on human MSCs42–48 and human and animal chondrocytes 2,35,40,41,45,49–57 served as a basis for designing our 3D OACs/MSCs-derived microspheroid tissue model for efficacy testing of anti-TNF-α biological drugs.14,15,36,37,58–63
4.3. OACs and MSCs in 2D monolayer and 3D spheroid cultures
Although expansion of primary human chondrocytes in monolayer cultures leads to the loss of their chondrogenic phenotype and altered biological behavior, the original features can be restored when the cells are cultured in 3D conditions.12,64 In order to obtain sufficient numbers of OACs for our experiments, the isolated cells were first expanded in monolayer cultures and then transferred into hanging-drops to form 3D chondral microspheroids. The reason for selecting a scaffold-free 3D culture over an alginate-based system was based on superior chondrogenic re-differentiation of primary chondrocytes achieved in hanging drops.65 In addition, tissue formation in hanging-drops mimics the condensation process of MSCs, which is one of the earliest phases of the in vivo cartilage development.13
To avoid permanent de-differentiation of OACs which occurs at passage 5 (P5) in monolayer culture,64 microsphreoids were prepared from passage 2 (P2) cells. The average diameter of microspheroids formed from chondrogenically differentiated MSCs (~250 µm) was 28% smaller than that of microspheroids prepared from OACs (~344 µm). This difference could probably be attributed to chondrogenic differentiation of MSCs which dominated over their proliferative growth in hanging-drop cultures.
OACs isolated from surgically removed biological material showed good proliferation potential in monolayers and were able to re-differentiate in microspheroid cultures68 as also shown in this study Therefore, OACs represent a valuable cell source for in vitro testing of drugs used to treat RA, as already confirmed in monolayer cultures.10 Importantly, OACs can be easily accessed from biological waste material, obtained at knee or hip surgical replacements and are free of ethical concerns. MSCs obtained from RA and OA patients displayed similar chondrogenic potential as MSCs isolated from healthy individuals47 Also, MSCs isolated from bone marrow of OA patients had capacity to produce hyaline cartilage suitable for tissue repair.71 Therefore we see no limitations in using these kinds of cells for in vitro testing of anti-inflammatory drugs.
In previous studies, normal human chondrocytes (NCs) isolated from healthy hyaline cartilage and OACs grown in monolayer cultures displayed only minor differences in their anabolic (ACAN, SOX9, COL1A1, COL2A1), catabolic (MMP1, MMP3. MMP9, MMP13, ADAMTS4) and inflammatory (IL-1β, PTGS2, NOS2) gene, as well as in protein (IL-6, IL-8, MCP-1, IL-1β) expression.9,60,72 Our two earlier studies showed that the exposure of NC and OAC 2D monolayer cultures to recombinant human TNF-α resulted in comparable gene expression patterns.10,14 The superior TNF-α neutralizing efficacy of ETA over IFX was observed in both studies. These results suggest that both NCs and OACs grown in monolayers can b used interchangeably for in vitro testing of anti-TNF-α biologicals.10
4.4. MSCs as a potential cell source for in vitro testing of drugs
High patient-to-patient variability in the chondrogenic potential of both OACs and MSCs demonstrates the importance of using biological material either from the same subject or a large donor population.70 In our study we were able to use paired samples of MSCs and OACs, obtained from two donors. Additionally, we tested a set of mismatched biological samples of OACs (patient) and MSCs (commercially available cells). After comparing gene expression patterns in microspheroids prepared from the paired cell samples we observed only a small variability. Therefore, we propose that MSCs could be used as an alternative to primary chondrocytes, as a more easily accessible cell source that can be rapidly and efficiently expanded in vitro. Accordingly, large numbers of different drugs could be tested with a single batch of MSCs thereby avoiding inter-individual donor variability.
4.5. In vitro effects of inflammatory factors and anti-inflammatory drugs on 3D chondral microspheroids
Microspheroid chondral tissues made of 10,000 human OACs or MSCs were incubated for 24 h with 1 ng/mL of TNF-α ± 1 µg/mL of either ADA, ETA or IFX. This quantity of TNF-α represents the highest concentration measured in synovial fluids of OA and RA patients experiencing severe disease.20 Similarly, the concentration of anti-TNF-α biologicals (1 µg/mL) corresponds to the concentrations recorded in the sera of patients treated with these drugs.23,28 By assessing the expression of key regulatory genes involved in cartilage inflammation (IL6, IL8 and MCP1), matrix degradation (MMP1, MMP13), tissue-specific function (ACAN and SOX9) and adhesion (VCAM1) in microspheroids made using OACs or chondrogenically differentiated MSCs pre-treated with TNF-α, we found that the levels of IL6, IL8, MCP1, MMP1, MMP13 and VCAM1 were significantly increased. Interestingly, these increases were even more pronounced when IL-1β (1 ng/mL) was used as inflammation inducer, while the MCMh.s. working solution elicited only an intermediate inflammatory response compared to the two individually administered cytokines.
Depending on the type of inflammation induction, anti-TNF-α (ADA, ETA and IFX) or anti-IL1β (ANA) biologicals were applied to assess their anti-inflammatory potency. Surprisingly, except for ANA that reversed the increased expression of IL6, IL8, MMP1 and ACAN genes in microspheroids treated with the MCMh.s. working solution, none of the three anti-TNF-α agents demonstrated anti-inflammatory activity. After a 3-week exposure of chondral macrospheroids made of 100,000 OACs to MCMFBS working solution, ANA proved to be a stronger anti-inflammatory agent compared to ADA, ETA and IFX as it significantly increased the GAG and OHP production and resulted in larger spheroids. These findings could be attributed to both the complex compositions of MCMh.s. and MCMFBS working solutions and the higher concentrations of IL-1β they contain (450 pg/mL and 750 pg/mL, respectively) relatively to TNF-α (50 pg/mL and 435 pg/mL, respectively). The concentration of IL-1β in both MCM batches was much higher than in the ynovial fluids of OA and RA patients (28 pg/mL and > 100 pg/mL, respectively).20 For that reason the pro-inflammatory action of MCM working solutions was persistant despite the presence of ADA, ETA or IFX.
A strong long-lasting inflammatory effect of MCMh.s. and MCMFBS working solutions was also observed in histology sections of chondral macrospheroids. Namely, both MCM solutions caused apoptosis that was not observed when either 1 ng/mL of TNF-α or IL-1β was used. Although MCMh.s. and MCMFBS working solutions were excellent in vitro inducers of inflammation, their use in testing anti-inflammatory potencies of biological drugs acting upon defined single cytokine targets could be problematic. This drawback is a consequence of multiple synergistic pro-inflammatory effects of different biogenic factors present in MCM, which do not allow detailed conclusions regarding the potencies of tested anti-inflammatory biologicals.
When inflammation of OACs or chondrogenically differentiated MSC-derived microspheroids was triggered by TNF-α alone, the neutralization efficacies of ADA, ETA and IFX were reflected in different gene expression profiles. Nevertheless, when overall results for the six analyzed genes (IL6, IL8, MCP1, MMP1, MMP13 and VCAM1) were considered, the same conclusions regarding the anti-inflammatory potencies of ANA, ETA and IFX were drawn for both types of microspheroid tissues, although ADA showed slightly higher efficacy over ETA and IFX, and ETA was more efficient than IFX. Except for significantly decreased expression levels of IL6 and IL8 genes, the IL-1β neutralizing effects of ANA on the rest of the six monitored genes were similar to those observed in anti-TNF-α neutralization efficiencies of ADA, ETA or IFX. These effects were also confirmed at the protein level.
Small differences in the effects of ADA, ETA and IFX can be attributed to the differences in their structures and binding affinities.4 Superior efficacy of ADA over ETA and IFX was found in a previous study of Hu et al., where correlation between their molecular structures and efficacies was also considered.73 The primary effect of ADA or IFX as monoclonal antibodies binding to the soluble form of TNF-α is the prevention of its interaction with TNF-α receptor, while ETA is an engineered fusion protein that functions as a decoy receptor for soluble TNF-α.4 At the molecular level, ADA has been reported to bind TNF-α with a relatively higher affinity (Kd = 7.05×10−11) than ETA (Kd = 2.35×10−11) and IFX (Kd = 1.17×10−10).74,75
According to our criterion, a given biological drug would be classified as statistically more efficient in comparison to another if it would cause a ≥ 2-fold decrease in a particular gene expression. However, this was not the case when testing ADA, ETA or IFX. Therefore we assume that the efficacies of all three anti-TNF-α biologicals were comparable in both microspehroid tissue models using either OACs or chondrogenically differentiated MSCs and conclude, that both cell types can be used for their in vitro efficacy testing.
Although ETA was significantly more efficient than IFX in our previous study, where 2D monolayer culture of OACs was used as a testing model,10 in the 3D microspheroid chondral tissue model the differences between these two biologicals were not statistically significant. We believe that this is due to different drug diffusion conditions within the two models. Diffusion is slower in case of 3D chondral microspheroids, but this model better resembles the in vivo conditions. Therefore, monolayer cultures may be useful for gaining preliminary information about anti-inflammatory effects of a particular drug, while the 3D cell/tissue culture model enables insights in drug-tissue interactions and the possible in vivo scenarios. This was actually shown in our 3D microspheroid chondral tissue model, where the efficacies of all three tested anti-TNF-α biological drugs were comparable to the results in clinical studies,76 which was not the case when the 2D monolayer culture model was used.10.
5. CONCLUSION
Although the mechanisms of action of anti-TNF-α biologicals are well documented,4,23,24,28,73,74,78–81 the existing in vitro models for assessing their anti-inflammatory efficacies do not have sufficient physiological relevance. In this regard, we report a new approach to in vitro functional testing of anti-TNF-α biological drugs (ADA, ETA and IFX). We created 3D chondral microspheroids made of only 10,000 osteoarthritic chondrocytes (OACs) or chondrogenically differentiated mesenchymal stem cells (MSCs), and macrospheroids made using 100,000 cells of the same types. Early quantitative changes in inflammation-related gene expressions were successfully assessed and evaluated following their exposure to different pro-inflammatory factors either alone or combined with the tested biological. The main advantages of our approach are the use of small amounts of cells and cytokines of human origin, the possibility of personalized testing approach, and automated operation. Moreover, our model can be used for in vitro functional evaluation of anti-inflammatory biologicals with different mechanisms of action, which was successfully proved by testing the effects of TNF-α (ADA, ETA and IFX) and IL-1β (ANA) antagonists.
Supplementary Material
Acknowledgments
We thank Dr. William B. Macaulay, MD (Center for Hip and Knee Replacement, New York Presbyterian Hospital at Columbia Medical Center, New York, USA) for providing human osteoarthritic tissues, from which the cells were isolated. We also thank Dr. Lisa F. Imundo, MD (Division of Rheumatology, New York Morgan-Stanley Children’s Hospital, New York, USA) for kindly donating adalimumab, etanercept and anakinra for our experiments. We are also thankful to Dr. Martina Sladkova (New York Stem Cell Foundation, New York, USA) for her help with the isolation of MSCs from trabecular bone. We are grateful to Dr. Johnathan Ng (Department of Biomedical Engineering, Columbia University, New York, USA) for performing the FACS analysis. We gratefully acknowledge funding support of this work by the Ministry of Economic Development and Technology of Slovenia, the European Regional Development Fund (OP13.2.1.1.1.05.0048), and NIH (EB002520).
Notation
- 2D
two-dimensional
- 3D
three-dimensional
- ACAN
aggrecan
- ADA
adalimumab
- ADAMTS4
ADAM metallopeptidase with thrombospondin type 1, motif 4
- ANA
anakinra
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- bFGF
basic fibroblast growth factor
- CD
cluster of differentiation
- cDNA
complementary DNA
- Cq
cycle of quantification
- DNA
deoxyribonucleic acid
- ECM
extracellular matrix
- ELISA
enyzme-linked immunosorbent assay
- ETA
etanercept
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- GAG
glycosaminoglycans
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- ICRS
International Cartilage Repair Society
- IFX
infliximab
- IFN-γ
interferon γ
- IL-1β
interleukin 1β
- IL-1Ra
interleukin 1 receptor antagonist
- IL6
interleukin 6
- IL8
interleukin 8
- LPS
lipopolysaccharide
- M
molarity [mol/L]
- M1-3
MSC of donors 1-3
- MCMØ
MCM control medium
- MCMFBS
MCM containing FBS
- MCMh.s.
MCM containing human serum
- MCP-1
monocyte chemotactic protein
- MMP1 or 13
matrix metallopeptidase 1 or 13
- mRNA
messenger RNA
- MSCs
mesenchymal stem cells
- NCs
normal human articular chondrocytes
- NOS2
nitric oxide synthase 2
- OA
osteoarthritis/osteoarthritic
- OACs
osteoarthritic human articular chondrocytes
- OHP
hydroxyproline
- P2
cell passage 2
- p ch 1-3
OACs of donors 1-3
- PMA
phorbol 12-myristate 13-acetate
- PTGS2
prostaglandin-endoperoxide synthase 2
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- RA
rheumatoid arthritis
- RQ
relative quantity of gene expression
- SEM
standard error of the mean
- SOX9
SRY (sex determining region Y) – box 9
- TGFβ3
transforming growth factor β3
- THP-1
human monocytic cell line
- TNF-α
tumor necrosis factor α
- VCAM-1
vascular cell adhesion molecule 1
Footnotes
Authors’ Contributions
SŽB, AB, MJ and GVN conceived and designed the experiments; SŽB performed the experiments; GVN, AB and MJ supervised the study; SŽB analyzed the data; SŽB, GVN, MJ, AB, and MK wrote/reviewed the paper. The authors had access to all data, and have read and approved the final manuscript.
References
- 1.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet Lond Engl. 2001;358(9285):903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 2.Schuerwegh A, Dombrecht E, Stevens W, Van Offel J, Bridts C, De Clerck L. Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis Cartilage. 2003;11(9):681–687. doi: 10.1016/S1063-4584(03)00156-0. [DOI] [PubMed] [Google Scholar]
- 3.Kahlenberg JM, Fox DA. Advances in the Medical Treatment of Rheumatoid Arthritis. Hand Clin. 2011;27(1):11–20. doi: 10.1016/j.hcl.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117(2):244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Rimann M, Graf-Hausner U. Synthetic 3D multicellular systems for drug development. Curr Opin Biotechnol. 2012;23(5):803–809. doi: 10.1016/j.copbio.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Kaur G, Dufour JM. Cell lines. [Accessed March 7, 2016];Spermatogenesis. 2012 2(1) doi: 10.4161/spmg.19885. http://search.ebscohost.com/login.aspx?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=21565554&AN=75235788&h=D64ruTD4cSqyMbcCflBPKVAbbuQxQ5yncZ1aybcxPY%2BH5kv6gm8KEekMfMRgiJ%2FfcyBjvXfjjxv915shTtvXKQ%3D%3D&crl=c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laganà M, Arrigoni C, Lopa S, Sansone V, Zagra L, Moretti M, Raimondi MT. Characterization of articular chondrocytes isolated from 211 osteoarthritic patients. Cell Tissue Bank. 2014;15(1):59–66. doi: 10.1007/s10561-013-9371-3. [DOI] [PubMed] [Google Scholar]
- 8.Neri S, Mariani E, Cattini L, Facchini A. Long-term in vitro expansion of osteoarthritic human articular chondrocytes do not alter genetic stability: a microsatellite instability analysis. J Cell Physiol. 2011;226(10):2579–2585. doi: 10.1002/jcp.22603. [DOI] [PubMed] [Google Scholar]
- 9.Lin Z, Fitzgerald JB, Xu J, Willers C, Wood D, Grodzinsky AJ, Zheng MH. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J Orthop Res Off Publ Orthop Res Soc. 2008;26(9):1230–1237. doi: 10.1002/jor.20523. [DOI] [PubMed] [Google Scholar]
- 10.Žigon-Branc S, Jeras M, Blejec A, Barlič A. Applicability of human osteoarthritic chondrocytes for in vitro efficacy testing of anti-TNFα drugs. Biol J Int Assoc Biol Stand. 2017;45:96–101. doi: 10.1016/j.biologicals.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Glowacki J, Trepman E, Folkman J. Cell shape and phenotypic expression in chondrocytes. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1983;172(1):93–98. doi: 10.3181/00379727-172-41533. [DOI] [PubMed] [Google Scholar]
- 12.Caron MMJ, Emans PJ, Coolsen MME, Voss L, Surtel DAM, Cremers A, van Rhijn LW, Welting TJM. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20(10):1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann M, Martin F, Mannigel K, Kaltschmidt K, Sack U, Anderer U. Three-dimensional scaffold-free fusion culture: the way to enhance chondrogenesis of in vitro propagated human articular chondrocytes. Eur J Histochem. 2013;57(4):31. doi: 10.4081/ejh.2013.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlič A, Žigon S, Blejec A, Kregar Velikonja N. Gene expression of cultured human chondrocytes as a model for assessing neutralization efficacy of soluble TNFα by TNFα antagonists. Biologicals. 2015;43(3):171–180. doi: 10.1016/j.biologicals.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Boyle DL, Rosengren S, Bugbee W, Kavanaugh A, Firestein GS. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003;5(6):R352–R360. doi: 10.1186/ar1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freytes DO, Kang JW, Marcos-Campos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114(1):220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- 17.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta Int J Clin Chem. 1967;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 19.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 20.Westacott CI, Whicher JT, Barnes IC, Thompson D, Swan AJ, Dieppe PA. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann Rheum Dis. 1990;49(9):676–681. doi: 10.1136/ard.49.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva GM, Vogel C. Quantifying gene expression: the importance of being subtle. Mol Syst Biol. 2016;12(10):885. doi: 10.15252/msb.20167325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarca AL, Romero R, Draghici S. Analysis of microarray experiments of gene expression profiling. Am J Obstet Gynecol. 2006;195(2):373–388. doi: 10.1016/j.ajog.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nesbitt A, Fossati G, Bergin M, Stephens P, Stephens S, Foulkes R, Brown D, Robinson M, Bourne T. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis. 2007;13(11):1323–1332. doi: 10.1002/ibd.20225. [DOI] [PubMed] [Google Scholar]
- 24.Gay RD, Clarke AW, Elgundi Z, Domagala T, Simpson RJ, Le NB, Doyle AG, Jennings PA. Anti-TNFα domain antibody construct CEP-37247: Full antibody functionality at half the size. mAbs. 2010;2(6):625–638. doi: 10.4161/mabs.2.6.13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu W-C, Lai Y-P, Chou M-Y. Humanization and characterization of an anti-human TNF-α murine monoclonal antibody. PloS One. 2011;6(1):e16373. doi: 10.1371/journal.pone.0016373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan Q, Guo Q, Fang C, Wang C, Li B, Wang H, Li J, Guo Y. Characterization and comparison of commercially available TNF receptor 2-Fc fusion protein products. mAbs. 2012;4(6):761–774. doi: 10.4161/mabs.22276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camacho-Villegas T, Mata-Gonzalez T, Paniagua-Solis J, Sanchez E, Licea A. Human TNF cytokine neutralization with a vNAR from Heterodontus francisci shark: a potential therapeutic use. mAbs. 2013;5(1):80–85. doi: 10.4161/mabs.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shealy DJ, Cai A, Staquet K, Baker A, Lacy ER, Johns L, Vafa O, Gunn G, Tam S, Sague S, Wang D, Brigham-Burke M, Dalmonte P, Emmell E, Pikounis B, Bugelski PJ, Zhou H, Scallon BJ, Giles-Komar J. Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor α. mAbs. 2010;2(4):428–439. doi: 10.4161/mabs.2.4.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman RA. A human approach to drug development: Opportunities and limitations. Altern Lab Anim. 2010;38(Supplement 1):21–25. doi: 10.1177/026119291003801S06. [DOI] [PubMed] [Google Scholar]
- 30.Burdall SE, Hanby AM, Lansdown MR, Speirs V. Breast cancer cell lines: friend or foe? Breast Cancer Res. 2003;5(2):89–89. doi: 10.1186/bcr577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giannoni P, Crovace A, Malpeli M, Maggi E, Arbicò R, Cancedda R, Dozin B. Species variability in the differentiation potential of in vitro-expanded articular chondrocytes restricts predictive studies on cartilage repair using animal models. Tissue Eng. 2005;11(1–2):237–248. doi: 10.1089/ten.2005.11.237. [DOI] [PubMed] [Google Scholar]
- 32.Schulze-Tanzil G, Müller RD, Kohl B, Schneider N, Ertel W, Ipaktchi K, Hünigen H, Gemeinhardt O, Stark R, John T. Differing in vitro biology of equine, ovine, porcine and human articular chondrocytes derived from the knee joint: an immunomorphological study. Histochem Cell Biol. 2009;131(2):219–229. doi: 10.1007/s00418-008-0516-6. [DOI] [PubMed] [Google Scholar]
- 33.Burns CJ, Silva MMCG, Gray E, Robinson CJ. Quantitative RT-PCR as an alternative to late-stage bioassays for vascular endothelial growth factor. J Pharm Biomed Anal. 2008;47(3):460–468. doi: 10.1016/j.jpba.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Silva MMCG, Lamarre B, Cerasoli E, Rakowska P, Hills A, Bailey MJA, Wheeler JX, Burns CJ, Gaines-Das RE, Jones C, Robinson CJ. Physicochemical and biological assays for quality control of biopharmaceuticals: interferon alpha-2 case study. Biol J Int Assoc Biol Stand. 2008;36(6):383–392. doi: 10.1016/j.biologicals.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Ge Z, Hu Y, Heng BC, Yang Z, Ouyang H, Lee EH, Cao T. Osteoarthritis and therapy. Arthritis Rheum. 2006;55(3):493–500. doi: 10.1002/art.21994. [DOI] [PubMed] [Google Scholar]
- 36.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier J-P, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 37.Fernandes JC, Martel-Pelletier J, Pelletier J-P. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(1–2):237–246. [PubMed] [Google Scholar]
- 38.van de Loo AA, van den Berg WB. Effects of murine recombinant interleukin 1 on synovial joints in mice: measurement of patellar cartilage metabolism and joint inflammation. Ann Rheum Dis. 1990;49(4):238–245. doi: 10.1136/ard.49.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Berg WB. Lessons from animal models of osteoarthritis. Curr Opin Rheumatol. 2001;13(5):452–456. doi: 10.1097/00002281-200109000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Goldring MB, Birkhead J, Sandell LJ, Kimura T, Krane SM. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988;82(6):2026. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Müller PE, Evans CH, Porter RM. Interleukin-1β and tumor necrosis factor α inhibit chondrogenesis by human mesenchymal stem cells through NF-κB-dependent pathways. Arthritis Rheum. 2009;60(3):801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ousema PH, Moutos FT, Estes BT, Caplan AI, Lennon DP, Guilak F, Weinberg JB. The inhibition by interleukin 1 of MSC chondrogenesis and the development of biomechanical properties in biomimetic 3D woven PCL scaffolds. Biomaterials. 2012;33(35):8967–8974. doi: 10.1016/j.biomaterials.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heldens GTH, Blaney Davidson EN, Vitters EL, Schreurs BW, Piek E, van den Berg WB, van der Kraan PM. Catabolic factors and osteoarthritis-conditioned medium inhibit chondrogenesis of human mesenchymal stem cells. Tissue Eng Part A. 2012;18(1–2):45–54. doi: 10.1089/ten.TEA.2011.0083. [DOI] [PubMed] [Google Scholar]
- 45.J Malemud C. Monosodium Urate and Tumor Necrosis Factor-α Increase Apoptosis in Human Chondrocyte Cultures. Rheumatol Curr Res. 2012;2(3) doi: 10.4172/2161-1149.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felka T, Schäfer R, Schewe B, Benz K, Aicher WK. Hypoxia reduces the inhibitory effect of IL-1beta on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthritis Cartilage. 2009;17(10):1368–1376. doi: 10.1016/j.joca.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 47.Dudics V, Kunstár A, Kovács J, Lakatos T, Géher P, Gömör B, Monostori E, Uher F. Chondrogenic Potential of Mesenchymal Stem Cells from Patients with Rheumatoid Arthritis and Osteoarthritis: Measurements in a Microculture System. Cells Tissues Organs. 2009;189(5):307–316. doi: 10.1159/000140679. [DOI] [PubMed] [Google Scholar]
- 48.Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189(3):275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 49.Cillero-Pastor B, Ruiz-Romero C, Caramés B, López-Armada MJ, Blanco FJ. Proteomic analysis by two-dimensional electrophoresis to identify the normal human chondrocyte proteome stimulated by tumor necrosis factor α and interleukin-1β. Arthritis Rheum. 2010;62(3):802–814. doi: 10.1002/art.27265. [DOI] [PubMed] [Google Scholar]
- 50.Caramés B, López-Armada MJ, Cillero-Pastor B, Lires-Dean M, Vaamonde C, Galdo F, Blanco FJ. Differential effects of tumor necrosis factor-alpha and interleukin-1beta on cell death in human articular chondrocytes. Osteoarthritis Cartilage. 2008;16(6):715–722. doi: 10.1016/j.joca.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 51.López-Armada MJ, Caramés B, Lires-Deán M, Cillero-Pastor B, Ruiz-Romero C, Galdo F, Blanco FJ. Cytokines, tumor necrosis factor-α and interleukin-1β, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 2006;14(7):660–669. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto K, Oreffo ROC, Gibson MB, Goldring MB, Roach HI. DNA demethylation at specific CpG sites in the IL1B promoter in response to inflammatory cytokines in human articular chondrocytes. Arthritis Rheum. 2009;60(11):3303–3313. doi: 10.1002/art.24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gebauer M, Saas J, Sohler F, Haag J, Söder S, Pieper M, Bartnik E, Beninga J, Zimmer R, Aigner T. Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1β. Osteoarthritis Cartilage. 2005;13(8):697–708. doi: 10.1016/j.joca.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Relič B, Bentires-Alj M, Ribbens C, Franchimont N, Guerne P-A, Benoît V, Merville M-P, Bours V, Malaise MG. TNF-alpha protects human primary articular chondrocytes from nitric oxide-induced apoptosis via nuclear factor-kappaB. Lab Investig J Tech Methods Pathol. 2002;82(12):1661–1672. doi: 10.1097/01.lab.0000041714.05322.c0. [DOI] [PubMed] [Google Scholar]
- 55.Geng Y, Valbracht J, Lotz M. Selective activation of the mitogen-activated protein kinase subgroups c-Jun NH2 terminal kinase and p38 by IL-1 and TNF in human articular chondrocytes. J Clin Invest. 1996;98(10):2425–2430. doi: 10.1172/JCI119056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henrotin YE, De Groote DD, Labasse AH, Gaspar SE, Zheng SX, Geenen VG, Reginster JY. Effects of exogenous IL-1 beta, TNF alpha, IL-6, IL-8 and LIF on cytokine production by human articular chondrocytes. Osteoarthr Cartil OARS Osteoarthr Res Soc. 1996;4(3):163–173. doi: 10.1016/s1063-4584(96)80012-4. [DOI] [PubMed] [Google Scholar]
- 57.Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995;146(1):75–85. [PMC free article] [PubMed] [Google Scholar]
- 58.Tsuchida AI, Beekhuizen M, t Hart MC, Radstake T, Dhert W, Saris D, van Osch G, Creemers LB. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis Res Ther. 2014;16(5):441. doi: 10.1186/s13075-014-0441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loeser RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006;54(5):1357–1360. doi: 10.1002/art.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Ceuninck F, Dassencourt L, Anract P. The inflammatory side of human chondrocytes unveiled by antibody microarrays. Biochem Biophys Res Commun. 2004;323(3):960–969. doi: 10.1016/j.bbrc.2004.08.184. [DOI] [PubMed] [Google Scholar]
- 61.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11(3):224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2(6):459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 63.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 64.Schulze-Tanzil G, de Souza P, Villegas Castrejon H, John T, Merker H-J, Scheid A, Shakibaei M. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002;308(3):371–379. doi: 10.1007/s00441-002-0562-7. [DOI] [PubMed] [Google Scholar]
- 65.Bernstein P, Sticht C, Jacobi A, Liebers C, Manthey S, Stiehler M. Expression pattern differences between osteoarthritic chondrocytes and mesenchymal stem cells during chondrogenic differentiation. Osteoarthritis Cartilage. 2010;18(12):1596–1607. doi: 10.1016/j.joca.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 66.Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl 1):S58–65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 67.Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4(3):309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- 68.Tallheden T, Bengtsson C, Brantsing C, Sjogren-Jansson E, Carlsson L, Peterson L, Brittberg M, Lindahl A. Proliferation and differentiation potential of chondrocytes from osteoarthritic patients. Arthritis Res Ther. 2005;7(3):R560–8. doi: 10.1186/ar1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jagielski M, Wolf J, Marzahn U, Völker A, Lemke M, Meier C, Ertel W, Godkin O, Arens S, Schulze-Tanzil G. The Influence of IL-10 and TNFα on Chondrogenesis of Human Mesenchymal Stromal Cells in Three-Dimensional Cultures. Int J Mol Sci. 2014;15(9):15821–15844. doi: 10.3390/ijms150915821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agar G, Blumenstein S, Bar-Ziv Y, Kardosh R, Schrift-Tzadok M, Gal-Levy R, Fischler T, Goldschmid R, Yayon A. The Chondrogenic Potential of Mesenchymal Cells and Chondrocytes from Osteoarthritic Subjects: A Comparative Analysis. Cartilage. 2011;2(1):40–49. doi: 10.1177/1947603510380899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kafienah W, Mistry S, Dickinson SC, Sims TJ, Learmonth I, Hollander AP. Three-dimensional cartilage tissue engineering using adult stem cells from osteoarthritis patients. Arthritis Rheum. 2007;56(1):177–187. doi: 10.1002/art.22285. [DOI] [PubMed] [Google Scholar]
- 72.Martin I, Jakob M, Schäfer D, Dick W, Spagnoli G, Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage. 2001;9(2):112–118. doi: 10.1053/joca.2000.0366. [DOI] [PubMed] [Google Scholar]
- 73.Hu S, Liang S, Guo H, Zhang D, Li H, Wang X, Yang W, Qian W, Hou S, Wang H, Guo Y, Lou Z. Comparison of the Inhibition Mechanisms of Adalimumab and Infliximab in Treating Tumor Necrosis Factor α-Associated Diseases from a Molecular View. J Biol Chem. 2013;288(38):27059–27067. doi: 10.1074/jbc.M113.491530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scallon B, Cai A, Solowski N, Rosenberg A, Song X-Y, Shealy D, Wagner C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301(2):418–426. doi: 10.1124/jpet.301.2.418. [DOI] [PubMed] [Google Scholar]
- 75.Granneman RG, Zhang YM, Noertersheuser PA, Velagapudi RB, Awni WM, Locke CS, Fischkoff SA. ARTHRITIS AND RHEUMATISM. Vol. 48. WILEY-LISS DIV JOHN WILEY & SONS INC; 605 THIRD AVE, NEW YORK, NY 10158-0012 USA: 2003. Pharmacokinetic/pharmacodynamic (PK/PD) relationships of adalimumab (HUMIRA (TM), Abbott) in rheumatoid arthritis (RA) patients during phase II/III clinical trials; pp. S140–S141. [Google Scholar]
- 76.Hyrich KL, Lunt M, Watson KD, Symmons DPM, Silman AJ. British Society for Rheumatology Biologics Register. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 2007;56(1):13–20. doi: 10.1002/art.22331. [DOI] [PubMed] [Google Scholar]
- 77.Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, Kollerup G, Linde L, Lindegaard HM, Poulsen UE, Schlemmer A, Jensen DV, Jensen S, Hostenkamp G, Ã~Stergaard M. All Departments of Rheumatology in Denmark. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: Results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62(1):22–32. doi: 10.1002/art.27227. [DOI] [PubMed] [Google Scholar]
- 78.Horiuchi T, Mitoma H, Harashima S-i, Tsukamoto H, Shimoda T. Transmembrane TNF-: structure, function and interaction with anti-TNF agents. Rheumatology. 2010;49(7):1215–1228. doi: 10.1093/rheumatology/keq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benucci M, Saviola G, Manfredi M, Sarzi-Puttini P, Atzeni F. Tumor necrosis factors blocking agents: analogies and differences. Acta Bio-Medica Atenei Parm. 2012;83(1):72–80. [PubMed] [Google Scholar]
- 80.Anderson PJ. Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profiles. Semin Arthritis Rheum. 2005;34(5 Suppl 1):19–22. doi: 10.1016/j.semarthrit.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 81.Kohno T, Tam L-TT, Stevens SR, Louie JS. Binding Characteristics of Tumor Necrosis Factor Receptor-Fc Fusion Proteins vs Anti-Tumor Necrosis Factor mAbs. J Investig Dermatol Symp Proc. 2007;12(1):5–8. doi: 10.1038/sj.jidsymp.5650034. [DOI] [PubMed] [Google Scholar]
- 82.Nesbitt A, Fossati G, Bergin M, Stephens P, Stephens S, Foulkes R, Brown D, Robinson M, Bourne T. Mechanism of action of certolizumab pegol (CDP870): In vitro comparison with other anti-tumor necrosis factor α agents. Inflamm Bowel Dis. 2007;13(11):1323–1332. doi: 10.1002/ibd.20225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.