Abstract
Background
Mass distributions of azithromycin for trachoma have been associated with secondary benefits, including reductions in child mortality.
Methods
In the PRET cluster-randomized trial for trachoma in Niger, 24 communities were randomized to annual treatment of everyone and 24 communities were randomized to biannual treatment of children under 12 for 3 years (clinicaltrials.gov, NCT00792922). Treatment was a single dose of directly observed oral azithromycin (20mg/kg up to 1gm in adults). Vital status was assessed during annual census and monitoring visits. In this pre-specified secondary analysis, we compared the mortality rate among children 6 months to less than 5 years of age by treatment arm using negative binomial regression.
Results
Among children 6 months to less than 5 years of age, 404 deaths occurred during the study period. The mortality rate was 35.6 deaths per 1000 person-years (231 deaths, 95% CI 30.9-40.9) in the annual arm and 29.0 deaths per 1000 person-years (173 deaths, 95% CI 24.8 to 33.8) in the biannual arm. The mortality rate ratio comparing children in the biannual arm to the annual arm was 0.81 (95% CI 0.66 to 1.00, P = 0.07; primary outcome). The mortality rate ratio comparing children who died from infectious causes in the biannual arm to the annual arm was 0.73 (95% CI 0.57 to 0.94, P=0.02). No adverse events were reported.
Conclusions
This secondary analysis of a cluster-randomized trial found a non-significant 19% decrease in mortality among children 6 months to less than 5 years of age who received biannual azithromycin compared with children who received annual azithromycin. This study was conducted in a high mortality, trachoma-endemic area, thus results may be specific to this environment only. In addition, the trial was neither designed nor powered to detect a mortality effect, and we cannot rule out the possibility that mortality differences resulted from bias.
Keywords: azithromycin, mortality, mass drug administration, cluster-randomized trial
INTRODUCTION
Mass distribution of oral azithromycin for trachoma has been associated with both intended and unintended effects. Mass azithromycin has been shown to reduce the prevalence of the Chlamydia trachomatis infection that causes the disease.(1) Secondary benefits include reductions in respiratory and gastrointestinal infections as well as malaria, all of which are among the leading contributors to childhood mortality in areas affected by trachoma.(2-5) On the other hand, the mass distribution of antibiotics is not without potential risks. Mass distribution could theoretically increase resistance of chlamydia to azithromycin, although previous studies have found no evidence of this even after multiple rounds of antibiotics.(6-8) Mass azithromycin selects for macrolide-resistant strains of other organisms, such as Streptococcus pneumoniae, at least until distributions are discontinued.(9-11)
Mass azithromycin distributions for trachoma may reduce childhood mortality.(12, 13) Any potential mortality benefit of mass azithromycin would likely occur by reducing the burden and severity of common childhood infections. Studies finding reductions in infectious disease burden within the first 3 months after azithromycin have also found little to no effect 3 to 6 months after administration.(2, 3) Thus, the annual schedule of mass azithromycin normally distributed for trachoma may not be sufficient for widespread mortality benefits. Multiple treatments per year may increase the time a child is exposed to an antimicrobial while also increasing the chances of coverage during seasonal peaks of different infectious diseases.(14) Here, our objective was to compare the community-level effects of annual versus biannual mass azithromycin on all-cause mortality in children 6 months to less than 5 years of age in a pre-specified secondary analysis of the PRET cluster-randomized trial for trachoma conducted in Niger. A cluster-randomized trial allows us to assess mortality effects at the level of the intervention, the community.
MATERIALS AND METHODS
Study setting and participants
This study was part of the Partnership for the Rapid Elimination of Trachoma (PRET) cluster-randomized trials in The Gambia, Niger, and Tanzania.(15) The Niger trial enrolled participants from 48 grappes (communities) in 6 Centres de Santé Intégrées (CSI) within the Matameye District in the Zinder Region. Detailed eligibility criteria for participation in the Niger trial have been previously reported.(16, 17) Briefly, communities with populations between 250 and 600 per the most recent government census were eligible for participation in the trial. Communities with less than 10% prevalence of active trachoma among children younger than 72 months were excluded.
Institutional Review Board (IRB) approval was obtained from the University of California, San Francisco Committee for Human Research and the Comité d’Ethique du Niger (the Ethical Committee of Niger). Both IRBs approved verbal consent, which was obtained from each local community chief before randomization and from each individual or guardian prior to data collection. The study was implemented in accordance with the Declaration of Helsinki. The Data and Safety Monitoring Committee (DSMC) met annually to review study progress and reported adverse events. PRET was registered at clinicaltrials.gov (NCT00792922).
Randomization and masking
This study employed a 2×2 factorial design to examine both treatment frequency and treatment coverage. Communities were randomized into 4 arms of 12 communities each: 1) annual azithromycin treatment at standard (80%) coverage, 2) annual treatment at enhanced (90%) coverage, 3) biannual treatment at standard (80%) coverage, and 4) biannual treatment at enhanced (90%) coverage. Standard coverage (80%) was chosen as a target for the original trial in line with the World Health Organization’s recommendation for coverage in trachoma control programs.(18) In addition, the randomization was stratified and blocked based on trachoma prevalence in children, as has been described previously.(16, 17) This report compares the 2 annual treatment arms to the 2 biannual treatment arms. The randomization allocation was generated at the community level by Travis C. Porco using R (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org) and the sequence was implemented by study staff at UCSF and in Niger. Study personnel conducting the annual census were not informed of treatment allocation and were different from those conducting treatment visits.
Interventions and procedures
The 24 communities randomized to annual mass distribution of oral azithromycin received 3 rounds of treatment over 3 years in all persons 6 months of age or older. The 24 communities randomized to biannual distribution of oral azithromycin received 6 rounds of treatment in children 6 months to 12 years of age during the 3-year study period. The original trial targeted ages 6 months to 12 years in the biannual arm to ensure those treated twice per year formed a core group.(19) In both arms, treatment was a single dose of directly observed oral azithromycin (20mg/kg up to a maximum dose of 1gm in adults). Children under 6 months of age and those allergic to macrolides were given topical tetracycline ointment (1%) to be applied to both eyes twice a day for 6 weeks. In the annual treatment arm, pregnant women were also offered topical tetracycline.
An annual census was conducted over the 3-year period to enumerate the study population. Treatment was administered based on census data after the completion of data collection. Trachoma was monitored during separate biannual study visits in both arms. Community residents were advised to alert village health workers within 2 weeks following mass treatment if they and/or their children experienced a serious adverse event, defined as diarrhea, nausea and vomiting for more than 2 days, hospitalization for any cause, or death. Residents were referred to the nearest health care center as needed.
Outcomes
The pre-specified outcomes for the present study were: 1) community-level all-cause mortality rate among children 6 months to less than 5 years of age by treatment arm over the 3-year study period; and 2) community-level infectious disease mortality rate among children 6 months to less than 5 years of age by treatment arm over the 3-year study period. This age group was chosen as it is at the highest risk for mortality. Vital status was recorded at each annual census, treatment visit, and biannual trachoma monitoring visit by data collectors masked to treatment arm. Absences of individuals recorded during a previous census were noted along with the reason for the absence, if possible to determine. Treatment coverage was determined based on the study population enumerated during the census directly preceding the treatment period.
For deaths reported during data collection, verbal autopsy was obtained using the 2007 World Health Organization verbal autopsy instrument. Causes of deaths were ascribed using a validated hierarchical expert algorithm.(20) This method utilizes a symptoms-based algorithm developed by experts in global child mortality to ascribe cause of death and, in the case of multiple causes, allocates a single cause of death using a hierarchy of causes. For this study, attributed causes of death were defined as infectious or non-infectious. Attributed infectious causes of death included all primary causes of death as determined by the algorithm except injury, malnutrition, and unspecified.
Children included on the census who were either younger than 6 months or were 5 years to less than 12 years of age were included in exploratory analyses, as were adults 15 years of age and older. Children 12 years to less than 15 years of age were not included in analyses, as this group received treatment in the biannual arm only during part of the study period. An additional analysis combined children 6 months to 12 years of age.
Statistical analysis
The sample size was calculated with respect to the overall primary outcome of the main trial (prevalence of C. trachomatis infection in children), accounting for clustering by community and the factorial design. Assuming a standard deviation in the community-level prevalence of 5%, we estimated that 48 communities (24 communities per arm) would provide greater than 80% power to detect an absolute difference of 6% in the prevalence of infection among children. Note that this would provide 80% power to detect a 34% difference in the mortality rate among children 6 months to less than 5 years of age, assuming a mortality rate of 34.3 deaths per 1000 person-years in the annual arm and a standard deviation of 0.014.
The pre-specified analysis for the present study was negative binomial regression to compare mortality rates among children 6 months to less than 5 years of age at the community level between the biannual and annual treatment arms. The models included count of deaths per community over the 3-year study period as the outcome, log person-time per community as an offset, and treatment arm as a covariate. Person-time at risk was estimated as the inter-census interval in years multiplied by the number of individuals at the prior census, minus half of the number of the individuals who died, moved, or were lost to follow-up. Exploratory analyses were conducted using similar models to compare mortality rate by arm across all age groups as well as the combined group with children 6 months to 12 years of age. Loss to follow-up, including those who moved, was compared between arms for each age group.
To assess whether attributed cause of death differed between the 2 arms, we computed the chi-squared statistic. We used negative binomial regression to compare infectious disease mortality rate among children 6 months to less than 5 years by arm, as pre-specified in the analysis plan and described above. Specifically, we compared mortality rates by arm among children whose ascribed causes of deaths were infectious.
P-values for all analyses were determined by Monte Carlo permutation (n=16,384) at the level of the randomization unit (community), accounting for the stratified design. The dispersion parameter used in negative binomial regression and the permutation P-values account for clustering at the level of the randomization unit, the community.
RESULTS
In May 2010, 48 communities in Niger were randomized to receive either annual or biannual mass azithromycin and were monitored until September 2013 (Figure 1, online only). At baseline, the 24 annually treated communities had a mean of 114 children per community (95% CI 88 to 156) and the 24 biannually treated communities had a mean of 107 children per community (95%CI 89 to 127, Table 1). Baseline characteristics of eligible children 6 months to less than 5 years of age were comparable between treatment arms (Table 1). All study communities were treated per protocol and no communities were lost to follow-up. Among children 6 months to less than 5 years of age, 497 (18.2%) in the annual treatment arm and 567 (22.1%) in the biannual treatment arm moved out of the study area or were lost to follow-up during the study period. No significant differences were identified when comparing loss to follow-up by arm in this age group.
FIGURE 1.
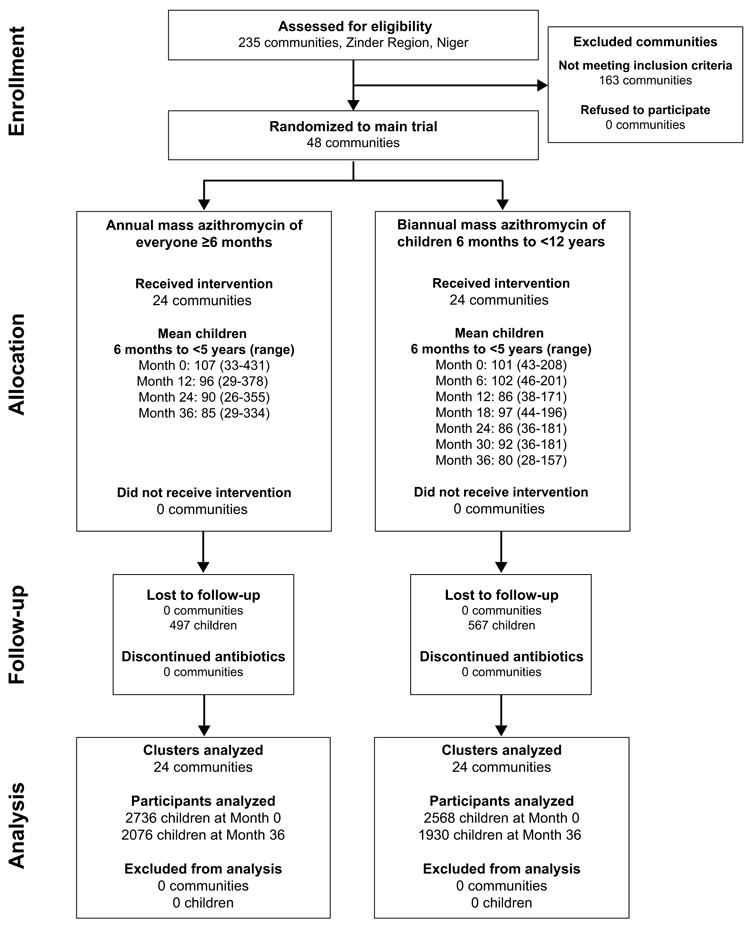
1CONSORT, Consolidated Standards of Reporting Trials.
Table 1.
Baseline characteristics of children 6 months to less than 5 years of age in 48 communities randomized to annual or biannual mass azithromycin in a cluster-randomized trial in Niger
| Characteristic | Mean (95% CI)
|
|
|---|---|---|
| Annual 24 communities | Biannual 24 communities | |
| Participants per community | 114 (88-156) | 107 (89-127) |
| Age (years) | 2.4 (2.3-2.4) | 2.4 (2.4-2.4) |
| Proportion female, % | 51.8% (50.2%-53.3%) | 48.9% (46.7%-51.1%) |
| Prevalence trachoma TF* | 29.5% (23.5%-36.4%) | 26.7% (21.9%-32.8%) |
| Prevalence trachoma TI* | 9.1% (6.3%-12.6%) | 8.4% (6.0%-11.2%) |
TF, trachomatous inflammation – follicular; TI, trachomatous inflammation – intense per the WHO Simplified Grading System.
Antibiotic treatment coverage of children 6 months to less than 5 years of age is shown in Table 2. All communities received 3 mass azithromycin distributions (June/July 2010, June/July 2011, and June/July 2012). The 24 biannual communities were treated an additional 3 times (December 2010/January 2011, December 2011/January 2012, and December 2012/January 2013). The average treatment coverage of children 6 months to less than 5 years of age was greater than 80% across all treatment periods in both arms. No serious adverse events were reported.
Table 2.
Average antibiotic treatment coverage of children 6 months to less than 5 years of age in 48 communities in Niger over 3 years
| Time point (months after study initiation) | Mean (95% CI)
|
|
|---|---|---|
| Annual 24 communities | Biannual 24 communities | |
| 0 | 92.3% (90.3%-94.1%) | 88.4% (84.8%-91.8%) |
| 6 | 85.3% (81.8%-88.6%) | |
| 12 | 89.2% (86.9%-91.3%) | 87.7% (84.7%-90.6%) |
| 18 | 87.3% (83.3%-90.5%) | |
| 24 | 90.1% (88.1%-91.9%) | 87.2% (84.4%-89.4%) |
| 30 | 84.1% (80.6%-87.4%) | |
| 36 | 84.7% (81.2%-88.1%) | 84.6% (81.0%-88.2%) |
Among children 6 months to less than 5 years of age, 231 deaths occurred in the annual arm and 173 deaths occurred in the biannual arm during the 3-year study period. The mortality rate was 35.3 deaths per 1000 person-years (95% CI 30.9 to 40.2) among children in the annual treatment arm and 28.9 deaths per 1000 person-years (95% CI 24.8 to 33.6) among children in the biannual treatment arm (Table 3). The mortality rate ratio comparing children 6 months to less than 5 years old in the biannual arm to the annual arm was 0.81 (95% CI 0.66 to 1.00, P = 0.07, Table 3).
Table 3.
Comparison of community-level mortality rates by treatment arm and age group
| Age group1 | Mortality rates per 1000 person-years (95% CI) [number of deaths]
|
Rate ratio (95% CI)4 | P-value4 | |
|---|---|---|---|---|
| Annual2 24 communities | Biannual3 24 communities | |||
| <6 months old | 83.0 (62.8-109.6) [71] | 76.3 (57.8-100.9) [66] | 0.92 (0.62-1.37) | 0.61 |
| 6 months to <5 years old | 35.6 (30.9-40.9) [231] | 29.0 (24.8-33.8) [173] | 0.81 (0.66-1.00) | 0.07 |
| 5 years to <12 years old | 6.8 (5.2-8.8) [71] | 5.6 (4.1-7.4) [53] | 0.82 (0.56-1.22) | 0.20 |
| ≥15 years old | 9.8 (8.4-11.5) [155] | 8.4 (7.1-10.0) [132] | 0.86 (0.68-1.08) | 0.20 |
Mortality in participants 6 months to less than 5 years of age was a pre-specified outcome of the trial.
In the annually-treated communities, study participants 6 months and older received oral azithromycin. Children younger than 6 months old were offered tetracycline ointment instead of oral azithromycin.
In the biannually-treated communities, study participants 6 months to 12 years of age received oral azithromycin. Children younger than 6 months old were offered tetracycline ointment instead of oral azithromycin.
Rate ratios and confidence intervals estimated by negative binomial regression; P-values estimated by Monte Carlo permutation (n=16,384) at the level of the randomization unit (community), accounting for the stratified design.
Exploratory analyses revealed similar results for the other age groups (Table 3). When children were combined into 1 age group, the mortality rate ratio comparing children 6 months to less than 12 years of age who received biannual azithromycin to those who received annual azithromycin was 0.82 (95% CI 0.67 to 0.99, P = 0.057)
Verbal autopsy results are shown in Table 4. Of the 404 children 6 months to less than 5 years of age who died during the study period, verbal autopsy was available for 362 (89.6%). The most common causes of death in both arms were malaria (45.3%) and diarrhea (24.9%). The distribution of cause of death did not differ between the 2 arms (P = 0.36). The infectious disease mortality rate ratio comparing children who died from infectious causes in the biannual arm to the annual arm was 0.73 (95% CI 0.57 to 0.94, P=0.02).
Table 4.
Causes of death among children 6 months to less than 5 years of age in the annual and biannual treatment arms as ascertained by verbal autopsy.1
| Primary cause of death2 | Annual 24 communities | Biannual 24 communities | Total 48 communities | |||
|---|---|---|---|---|---|---|
| Total | % | Total | % | Total | % | |
| Injury | 1 | 0.5% | 3 | 2.0% | 4 | 1.1% |
| AIDS | 5 | 2.4% | 5 | 3.4% | 10 | 2.8% |
| Measles | 3 | 1.4% | 1 | 0.67% | 4 | 1.1% |
| Meningitis | 4 | 1.9% | 0 | 0.0% | 4 | 1.1% |
| Dysentery | 12 | 5.6% | 6 | 4.0% | 18 | 5.0% |
| Diarrhea | 61 | 28.6% | 29 | 19.5% | 90 | 24.9% |
| Pertussis | 1 | 0.5% | 2 | 1.3% | 3 | 0.8% |
| Pneumonia | 8 | 3.8% | 6 | 4.0% | 14 | 3.9% |
| Malaria | 89 | 41.8% | 75 | 50.3% | 164 | 45.3% |
| Hemorrhagic fever | 0 | 0.0% | 1 | 0.7% | 1 | 0.3% |
| Other infection | 12 | 5.6% | 7 | 4.7% | 19 | 5.2% |
| Malnutrition | 7 | 3.3% | 7 | 4.7% | 14 | 3.9% |
| Unspecified | 10 | 4.7% | 7 | 4.7% | 17 | 4.7% |
| Total | 213 | 100.0% | 149 | 100.0% | 362 | 100.0% |
P-value = 0.36 (chi-squared test for comparison of primary cause of death by arm with permuted P-value).
Causes of death ordered according to expert hierarchical algorithm.
DISCUSSION
In this cluster-randomized trial, we found a non-significant 19% decrease in mortality rate in children 6 months to less than 5 years of age in biannually treated communities compared to annually treated communities (pre-specified analysis). Similar results were noted across the other age groups analyzed. No differences were identified in the distribution of attributed cause of death by arm, but there is a suggestion of a lower rate of mortality from infectious causes of death in the biannual arm.
Previous trachoma studies have found significant reductions in childhood mortality among those receiving mass oral azithromycin compared to no treatment.(12, 13) A cohort study from Ethiopia suggested a direct protective effect of azithromycin after finding a decreased risk of all-cause mortality in children under 5 who received azithromycin compared to household members who did not.(12) A cluster-randomized trial for trachoma in Ethiopia demonstrated a 50% reduction in mortality after mass azithromycin distributions compared to delayed treatment.(13) These prior studies each compared treated subjects to untreated subjects whereas the present study compared communities receiving different treatment frequencies. The trial in Ethiopia did compare varying frequencies of mass azithromycin distribution for trachoma, but these were combined into 1 treated group for the mortality analysis based on power calculations.(13) No prior study has compared mortality among groups receiving different azithromycin regimens.
The mortality effects found in this study were similar in all age groups of the biannually treated communities, even among the untreated age groups. Children younger than 6 months received topical tetracycline in both arms, and adults 15 years and older were not treated in the biannual arm. It is possible that these results are due to indirect effects of biannual treatment on untreated individuals in the community. Oral azithromycin has been associated with indirect effects on trachoma in previous studies, thus it is plausible this phenomenon extends to other childhood illnesses as well.(21) Alternatively, the randomization procedure could have resulted in the biannual treatment arm including communities that, on average, experienced less mortality than communities in the annual treatment arm did. Data were not collected on baseline infectious disease burden or mortality rates, though a comparison of available baseline characteristics did not indicate an imbalance across arms. Systematic bias may also explain reduced mortality seen in the untreated age groups of the biannually-treated communities. These communities received twice as many treatment visits, which may have led to increased interaction with the health care system. However, all study communities received biannual trachoma monitoring visits, which should have limited any Hawthorne-like effect. Finally, it is also possible that these results are simply due to chance as no significant effects of biannual azithromycin on mortality were identified in the primary analysis.
This study is limited by the lack of an untreated or placebo-controlled arm for comparison of baseline mortality rates. There are also limitations inherent in conducting verbal autopsy as well as delayed and incomplete verbal autopsy data, which make it difficult to fully assess cause of death in this population. In addition, Niger has among the highest childhood mortality rates in the world, thus results from this setting may not be generalizable to others.(22) Finally, this study was not powered to assess mortality as a primary outcome. If there were in fact a 20% effect of biannual azithromycin on mortality compared to annual azithromycin, a larger study would be required to have a reasonable chance of detecting it. A large, multi-site, cluster-randomized trial is currently underway to directly assess both the effects of biannual azithromycin on mortality among children under 5 as well as the effect of such an intervention on macrolide resistance (MORDOR, Bill & Melinda Gates Foundation grant # OPP1032340-B, clinicaltrials.gov NCT02047981).
Previous studies have found large, significant reductions in mortality among children receiving oral azithromycin compared to control groups. Biannual azithromycin may confer larger benefits than annual azithromycin by offering greater coverage throughout the year. Our study did not find an effect of biannual treatment in the primary analysis. Secondary analyses provide some evidence that biannual treatment may result in a decrease in mortality from infectious causes of death among children. Given the risks of emerging antibiotic resistance, however, further evidence is required before such a strategy be implemented on a wider scale.
Acknowledgments
The authors would like to thank the Data and Safety Monitoring Committee, including Douglas Jabs, MD, MBA (chair); Antoinette Darville, MD; Maureen Maguire, PhD; and Grace Saguti, MD, who were generous with their time and advice and met before and during the study. The authors thank Kurt Dreger, who designed and helped maintain the database, and all of our colleagues in Niger at Programme National de Santé Oculaire who helped perform the study.
FUNDING
This work was supported by the Bill and Melinda Gates Foundation [48027]; National Institutes of Health [NIH/NEI K23 EYO19881-01, NIH/NCRR/OD UCSF-CTSI KL2 RR024130]; Research to Prevent Blindness; That Man May See; and the Harper-Inglis Trust.
Footnotes
DECLARATION OF INTERESTS
No conflicts of interest exist and none of the authors have any disclosures.
Trial registration: NCT00792922 (clinicaltrials.gov)
References
- 1.Evans JR, Solomon AW. Antibiotics for trachoma. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD001860.pub3. Cd001860. [DOI] [PubMed] [Google Scholar]
- 2.Coles CL, Levens J, Seidman JC, Mkocha H, Munoz B, West S. Mass distribution of azithromycin for trachoma control is associated with short-term reduction in risk of acute lower respiratory infection in young children. Pediatr Infect Dis J. 2012;31:341–346. doi: 10.1097/INF.0b013e31824155c9. [DOI] [PubMed] [Google Scholar]
- 3.Coles CL, Seidman JC, Levens J, Mkocha H, Munoz B, West S. Association of mass treatment with azithromycin in trachoma-endemic communities with short-term reduced risk of diarrhea in young children. Am J Trop Med Hyg. 2011;85:691–696. doi: 10.4269/ajtmh.2011.11-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fry AM, Jha HC, Lietman TM, et al. Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin Infect Dis. 2002;35:395–402. doi: 10.1086/341414. [DOI] [PubMed] [Google Scholar]
- 5.Schachterle SE, Mtove G, Levens JP, et al. Short-term malaria reduction by single-dose azithromycin during mass drug administration for trachoma, Tanzania. Emerg Infect Dis. 2014;20:941–949. doi: 10.3201/eid2006.131302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon AW, Mohammed Z, Massae PA, et al. Impact of mass distribution of azithromycin on the antibiotic susceptibilities of ocular Chlamydia trachomatis. Antimicrob Agents Chemother. 2005;49:4804–4806. doi: 10.1128/AAC.49.11.4804-4806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West SK, Moncada J, Munoz B, et al. Is there evidence for resistance of ocular Chlamydia trachomatis to azithromycin after mass treatment for trachoma control? J Infect Dis. 2014;210:65–71. doi: 10.1093/infdis/jiu046. [DOI] [PubMed] [Google Scholar]
- 8.Hong KC, Schachter J, Moncada J, Zhou Z, House J, Lietman TM. Lack of macrolide resistance in Chlamydia trachomatis after mass azithromycin distributions for trachoma. Emerg Infect Dis. 2009;15:1088–1090. doi: 10.3201/eid1507.081563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho DK, Sawicki C, Grassly N. Antibiotic resistance in Streptococcus pneumoniae after azithromycin distribution for trachoma. J Trop Med. 2015;2015:917370. doi: 10.1155/2015/917370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidman JC, Coles CL, Silbergeld EK, et al. Increased carriage of macrolide-resistant fecal E. coli following mass distribution of azithromycin for trachoma control. Int J Epidemiol. 2014;43:1105–1113. doi: 10.1093/ije/dyu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidman JC, Johnson LB, Levens J, et al. Longitudinal comparison of antibiotic resistance in diarrheagenic and non-pathogenic Escherichia coli from young Tanzanian children. Front Microbiol. 2016;7:1420. doi: 10.3389/fmicb.2016.01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keenan JD, Ayele B, Gebre T, et al. Childhood mortality in a cohort treated with mass azithromycin for trachoma. Clin Infect Dis. 2011;52:883–888. doi: 10.1093/cid/cir069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porco TC, Gebre T, Ayele B, et al. Effect of mass distribution of azithromycin for trachoma control on overall mortality in Ethiopian children: a randomized trial. JAMA. 2009;302:962–968. doi: 10.1001/jama.2009.1266. [DOI] [PubMed] [Google Scholar]
- 14.Findley SE, Medina DC, Sogoba N, Guindo B, Doumbia S. Seasonality of childhood infectious diseases in Niono, Mali. Glob Public Health. 2010;5:381–394. doi: 10.1080/17441690903352572. [DOI] [PubMed] [Google Scholar]
- 15.Stare D, Harding-Esch E, Munoz B, et al. Design and baseline data of a randomized trial to evaluate coverage and frequency of mass treatment with azithromycin: the Partnership for Rapid Elimination of Trachoma (PRET) in Tanzania and The Gambia. Ophthalmic Epidemiol. 2011;18:20–29. doi: 10.3109/09286586.2010.545500. [DOI] [PubMed] [Google Scholar]
- 16.Amza A, Kadri B, Nassirou B, et al. Community risk factors for ocular Chlamydia infection in Niger: pre-treatment results from a cluster-randomized trachoma trial. PLoS Negl Trop Dis. 2012;6:e1586. doi: 10.1371/journal.pntd.0001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amza A, Kadri B, Nassirou B, et al. A cluster-randomized trial to assess the efficacy of targeting trachoma treatment to children. Clin Infect Dis. 2017;64:743–750. doi: 10.1093/cid/ciw810. [DOI] [PubMed] [Google Scholar]
- 18.Solomon A, Zondervan M, Kuper H, Buchan J, Mabey D, Foster A. Trachoma control: a guide for programme managers. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 19.Lietman T, Porco T, Dawson C, Blower S. Global elimination of trachoma: how frequently should we administer mass chemotherapy? Nat Med. 1999;5:572–576. doi: 10.1038/8451. [DOI] [PubMed] [Google Scholar]
- 20.Kalter HD, Perin J, Black RE. Validating hierarchical verbal autopsy expert algorithms in a large data set with known causes of death. Journal of Global Health. 2016;6 doi: 10.7189/jogh.06.010601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.House JI, Ayele B, Porco TC, et al. Assessment of herd protection against trachoma due to repeated mass antibiotic distributions: a cluster-randomised trial. Lancet. 2009;373:1111–1118. doi: 10.1016/S0140-6736(09)60323-8. [DOI] [PubMed] [Google Scholar]
- 22.GBD 2015 Child Mortality Collaborators. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1725–1774. doi: 10.1016/S0140-6736(16)31575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


