Abstract
Spleen tyrosine kinase (SYK) plays a critical role in immune cell signaling pathways and has been reported as a novel biomarker for human hepatocellular carcinoma (HCC). We sought to investigate the mechanism by which SYK promotes liver fibrosis and to evaluate SYK as a therapeutic target for liver fibrosis. We evaluated the cellular localization of SYK and the association between SYK expression and liver fibrogenesis in normal, HBV-infected, HCV-infected and non-alcoholic steatohepatitis (NASH) liver tissue (n=36, 127, 22 and 30, respectively). A PCR array was used to detect the changes in transcription factor expression in hepatic stellate cells (HSCs) with SYK knockdown. The effects of SYK antagonism on liver fibrogenesis were studied in LX-2 cells, TWNT-4 cells, primary human HSCs, and three progressive fibrosis/cirrhosis animal models, including a carbon tetrachloride mouse model, and diethylnitrosamine and bile duct ligation rat models. We found that SYK protein in HSCs and hepatocytes correlated positively with liver fibrosis stage in human liver tissue. HBV or HCV infection significantly increased SYK and cytokine expression in hepatocytes. Increasing cytokine production further induced SYK expression and fibrosis-related gene transcription in HSCs. Up-regulated SYK in HSCs promoted HSC activation by increasing the expression of specific transcription factors related to activation of HSCs. SYK antagonism effectively suppressed liver fibrosis via inhibition of HSC activation, and decreased obstructive jaundice and reduced HCC development in animal models.
Conclusions: SYK promotes liver fibrosis via activation of HSCs and is an attractive potential therapeutic target for liver fibrosis and prevention of HCC development.
Keywords: SYK, hepatic stellate cell, fibrosis, targeting therapy
Introduction
Liver cirrhosis is a major global health problem and accounts for more than 1 million deaths each year worldwide.(1) Liver fibrosis, excessive deposition of extracellular matrix (ECM) in the liver, is the result of the wound-healing response to chronic liver damage triggered by a variety of causes, including hepatitis virus infection, alcohol abuse, or nonalcoholic steatohepatitis,(2, 3) and is a precursor to cirrhosis. Hepatic stellate cells (HSCs) are responsible for producing most of the ECM and play a central role in liver fibrogenesis.(2, 4) HSCs are quiescent and located in the space between hepatocytes and sinusoidal endothelium (space of Disse) as retinoid storage cells.(5) Liver injury or stimuli from the microenvironment activate quiescent HSCs leading to production and accumulation ECM, resulting in liver fibrosis.(3) Activated HSCs lose their retinoid stores, proliferate and produce large amounts of chemokines and cytokines that further increase ECM production.(6, 7) Currently, there are no approved drugs that reverse liver fibrosis, further highlighting an urgent clinical need for anti-fibrotic therapies.(8, 9) An agent that directly inactivates HSCs has potential to be an effective antifibrotic strategy.(10, 11) In this study, we report that spleen tyrosine kinase (SYK) is critical for HSC activation and may represent a therapeutic target for inactivation of HSCs.
SYK is a cytoplasmic nonreceptor tyrosine kinase expressed in either hematopoietic or epithelial cells. It plays a crucial role in diverse biological functions including cellular adhesion, immune recognition, platelet activation and vascular development.(12) Previous studies have identified a pathogenetic role for SYK in renal interstitial fibrosis,(13) rheumatoid arthritis and some types of leukemia.(14–17) SYK has been demonstrated as a potential therapeutic target for some fibrosis-related diseases including scleroderma, renal fibrosis and rheumatoid arthritis.(18–20) However, the biological function of SYK in liver fibrosis has not been well characterized. In this study, we found that SYK is a key molecule in HSC activation and we demonstrated that SYK inhibitors blocked HSC activation, both in cell culture and in animal fibrosis models, including the carbon tetrachloride (CCl4)-induced mouse, diethylnitrosamine (DEN)-induced rat, and bile duct ligation (BDL)-induced rat models. We found that SYK inhibitors effectively blocked HSC activation. In addition, we found that SYK inhibitors potently attenuated liver fibrosis and HCC development in animal models.
Materials and methods
Detailed Materials and Methods are described in Supplementary Information.
Results
Up-regulation of SYK expression in HSCs and hepatocytes is associated with liver fibrosis
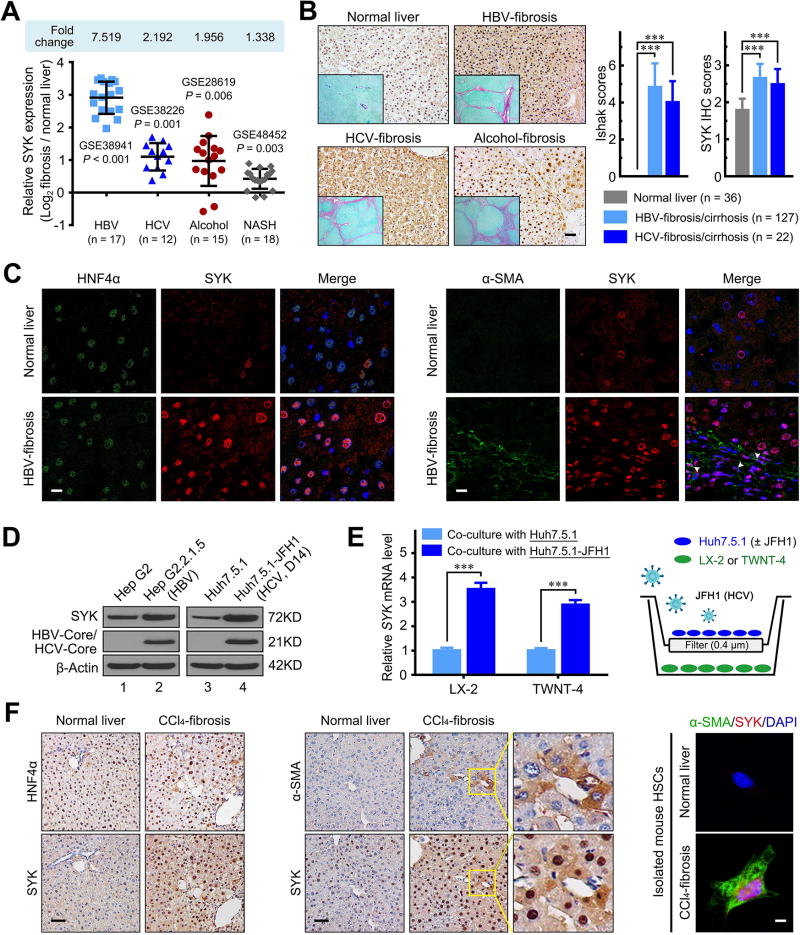
To evaluate whether SYK expression is associated with liver fibrosis/cirrhosis, we analyzed the publicly available National Cancer for Biotechnology Information Gene Expression Omnibus (GEO) database. We found that human SYK mRNA expression was significantly up-regulated by 7.52-fold (HBV infection), 2.19-fold (HCV infection), 1.96-fold (alcohol abuse) and 1.34-fold (non-alcoholic steatohepatitis, NASH) compared to normal liver (Figure 1A and Supplementary Figure 1). Moreover, SYK expression was up-regulated in liver tissues from infants with biliary atresia or other causes of intrahepatic cholestasis (Supplementary Figure 1). We further examined SYK expression by immunostaining in human liver tissue with confirmed fibrosis/cirrhosis. We found that SYK protein expression was markedly higher in HBV-infected (n=127), HCV-infected (n=22), cholestasis (n=7), and NASH (n=30) liver tissue compared to normal liver (n=36) tissue (P values all < 0.01; Figure 1B and Supplementary Figure 1). Similar to the human data, murine fibrotic livers also demonstrated a significant increase in SYK and α-smooth muscle actin (ACTA2) mRNA and/or protein levels compared with controls in CCl4-induced mouse fibrosis, DEN-induced and BDL induced rat fibrosis models (Supplementary Figure 2). Furthermore, we analyzed co-localization in vivo of SYK with HNF4α, α-SMA, CK19, CD31, and CD68, which are cell markers of hepatocytes, activated HSCs, biliary tract cells, endothelial cells, and Kupffer cells, respectively. Compared to normal human liver, the enhanced SYK expression was mainly located in hepatocytes and HSCs of in human fibrotic livers (Figure 1C), but not in other liver cell types (Supplementary Figure 3).
Figure 1. SYK expression up-regulation in HSCs and hepatocytes is associated with fibrosis.
(A) SYK expression was up-regulated in liver biopsies from patients with HBV-associated liver failure (GSE38941), chronic HCV (GSE38226), alcoholic hepatitis (GSE28619) and NASH (GSE48452). Data were shown as log2-ratio of the individual value of fibrosis samples to the mean value of normal control samples. (B) SYK immunohistochemistry (IHC) and Sirius red staining were performed in normal, HBV-infected, HCV-infected and alcoholic liver fibrotic/cirrhotic tissues. Representative images of SYK (magnification 200×) and Sirius red staining (insertion panels, magnification 40×) are shown in the left panel. The right panel shows Ishak scores, and SYK scores quantified by IHC in normal, HBV-infected and HCV-infected liver tissue. Scale bars, 50 µm. (C) Left panel shows representative double immunofluorescence images of HNF4α (green) and SYK (red) in normal and HBV-infected fibrotic liver tissue. Right panel shows images of α-SMA (green) and SYK (red). Scale bars, 10 µm. (D) Immunoblotting confirmation of SYK, HBV/HCV-core and β-actin protein in the indicated cells. (E) HCV in JFH1-infected Huh7.5.1 cells increases SYK mRNA expression levels in LX-2 or TWNT-4 cells in the co-culture model. The right schematic represents the Transwell co-culture model of HSCs and HCV-infected Huh7.5.1 cells. (F) Representative images of HNF4α and SYK (left panel), or α-SMA and SYK (middle panel) staining in the serial liver sections from the CCl4-induced fibrosis mouse model. Scale bars, 50 µm. Representative double immunofluorescence images of α-SMA (green) and SYK (red) in primary HSCs isolated from normal or CCl4-induced fibrosis mouse livers are shown in right panel. Scale bars, 10 µm. Data presented are means ± SD. ***, P < 0.001.
We found higher levels of SYK mRNA and protein expression in HBV or HCV-infected hepatocytes compared to uninfected Huh7.5.1 cells (Figure 1D, Supplementary Figure 4). Higher SYK expression was also observed in HSCs co-cultured with JFH1 HCV-infected Huh7.5.1 compared to uninfected Huh7.5.1 cells (P < 0.001; Figure 1E). Consistent with the results from our human fibrotic liver, we found that up-regulated SYK expression was mainly found in hepatocytes and HSCs by immunostaining of liver sections from CCl4-induced fibrotic mouse liver (Figure 1F). Furthermore, SYK expression was detected in activated HSCs isolated from CCl4-induced fibrotic liver, but not in primary HSCs isolated from normal liver (Figure 1F).
SYK promotes HSC activation
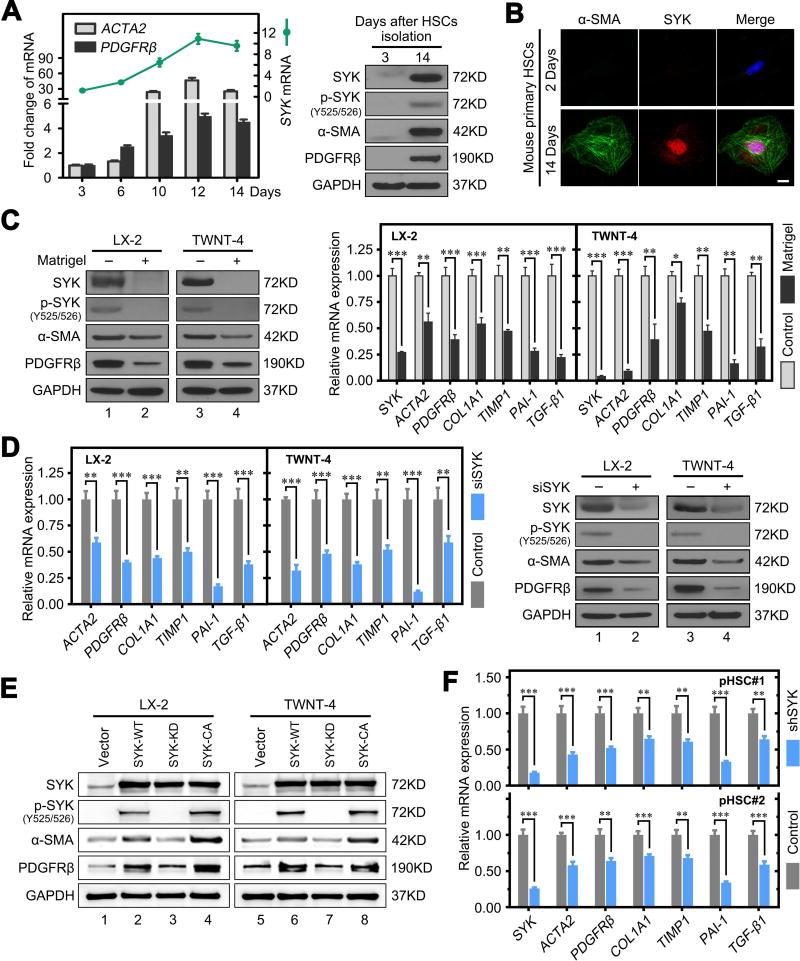
To assess whether SYK expression is related to HSC activation and liver fibrosis, we compared SYK mRNA and protein expression in isolated primary mouse HSCs (mHSCs) from day 2 to 14. It has been reported that isolated primary mHSCs plated on plastic gradually become activated within 2 weeks.(21) We found significantly higher HSC activation maker (ACTA2 and PDGFRβ) mRNA and protein expression at day 14 compared to day 3 post isolation (Figure 2A,B). SYK expression was almost undetectable in the quiescent state early after mHSC isolation. However, we found high expression levels of both SYK and activated SYK [p-SYK (Y525/526)] in primary mHSCs with subsequent plate activation, CCl4-induced fibrotic mouse liver and their primary hepatocytes and HSCs (Figure 2A,B, Supplementary Figure 3E,F).(22) Therefore, we cultured HSCs on Matrigel coated plates to inactivate the cells. We found that Matrigel dramatically decreased SYK and activated SYK expression, along with the HSC activation markers α-SMA and PDGFRβ, to nearly undetectable levels (Figure 2C). Moreover, SYK overexpression significantly promoted HSC activation and enhancement of ACTA2, PDGFRβ, COL1A1, TIMP1, PAI-1 and TGF-β1 expression (Supplementary Figure 5). Matrigel abrogated SYK overexpression-induced HSC activation and the enhancement of fibrosis related gene mRNA and protein expression (Supplementary Figure 5). These findings suggest that HSC quiescence induced by Matrigel suppresses the effect of SYK on HSC activation.
Figure 2. SYK promotes HSC activation.
(A) The left panel shows fold change of SYK, ACTA2 and PDGFRβ mRNA expression in primary HSCs isolated from normal mouse liver cultured for the indicated time intervals. The right panel shows higher protein levels of SYK, activated SYK [p-SYK(Y525/526)], α-SMA and PDGFRβ at day 14 compared to day 3 post isolation of primary mouse HSCs. (B) Double immunofluorescence staining of SYK and α-SMA in primary mouse HSCs cultured for 2 or 14 days. Scale bars, 10 µm. (C) Left panel shows reduced protein levels of SYK, p-SYK, α-SMA and PDGFRβ in LX-2 and TWNT-4 cells cultured on Matrigel. Right panel shows reduced mRNA expression of SYK, and markers of activated HSCs and fibrosis in LX-2 and TWNT-4 cells cultured on Matrigel. (D) Left panel shows SYK siRNA inhibited protein level of SYK, α-SMA and PDGFRβ in LX-2 and TWNT-4 cells. Right panel shows decreased mRNA expression of indicated HSC activation and fibrotic markers in LX-2 and TWNT-4 cells following SYK siRNA (siSYK) compared to Neg control siRNA. (E) Overexpression of wild type (WT) and catalytically activated (CA) mutant SYK, but not kinase dead (KD) mutant SYK increased expression of SYK, p-SYK, and activated HSC markers in LX-2 and TWNT-4 cells. (F) Lentivirus SYK shRNA reduced SYK mRNA expression and several HSC activation and fibrosis markers in primary human HSCs compared to negative scrambled hairpin (Control). Data presented are means ± SD. NS = not significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In addition, we found that knockdown of SYK expression with siRNA (siSYK) in LX-2 or TWNT-4 HSC lines significantly decreased mRNA and protein expression of activated HSC and fibrosis related genes, including α-SMA (ACTA2), PDGFRβ, COL1A1, TIMP1, PAI-1 and TGF-β1 (Figure 2D). In contrast, overexpression of wild type (WT) or catalytically activated (CA) mutant SYK, but not kinase dead (KD) mutant SYK, significantly enhanced expression of these genes in HSCs (Figure 2E, Supplementary Figure 6). We further confirmed that SYK shRNA knockdown attenuated induction of fibrosis related genes in the human primary HSCs in vitro (Figure 2F). These results suggest that activated SYK [p-SYK (Y525/526)] promotes HSC activation.
We previously demonstrated that the SYK two main isoforms, SYK(L) and SYK(S), play different roles in HCC development. We found that both SYK(L) and SYK(S) mRNA were enhanced during fibrosis progression in the CCl4 and DEN induced murine fibrotic liver, however, SYK(L) expressed higher level than SYK(S) (Supplementary Figure 7). Overexpression of SYK(L) or SYK(S) in LX-2 or TWNT-4 cells significantly increased HSC activation markers (ACTA2 and PDGFRβ) and fibrosis associated genes (COL1A1, TIMP1, PAI-1 and TGF-β1). SYK(L) exerted stronger activation effects on liver fibrosis than SYK(S) (Supplementary Figure 8). These data indicate that both SYK(L) and SYK(S) promoted liver fibrosis, and that SYK(L) is a more potent inducer of liver fibrosis progression than SYK(S).
SYK promotes HSC activation via up-regulation of specific transcription factors
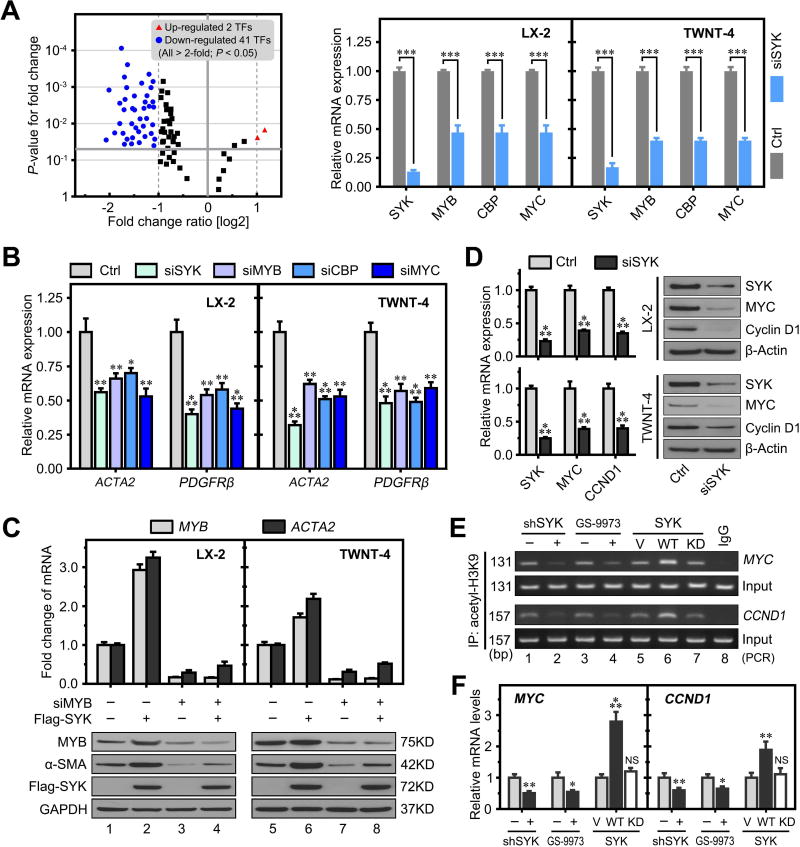
It has been reported that the alternative expression of specific transcription factors is critical for HSC activation.(23–25) To further investigate whether SYK promoted HSC activation by regulating these transcription factors (TFs), we performed a transcription factor PCR array to detect changes in expression of 84 TFs in LX-2 cells following SYK siRNA knockdown. We identified that 41 TFs were significantly down-regulated and 2 that were up-regulated more than 2-fold (P < 0.05) between SYK siRNA and control siRNA (Figure 3A, Supplementary Table 1). We selected three well-reported HSC activation associated genes including the MYB proto-oncogene transcription factor (MYB), CREB binding protein (CBP) and MYC proto-oncogene (MYC), for further validation. MYB directly binds to the promoter region of the ACTA2 gene, increasing ACTA2 gene expression and further activating HSCs.(26) Both CBP and MYC are downstream molecules of the Wnt/β-catenin signaling pathway and play a key role in promoting HSC activation and proliferation.(27–29) We hypothesized that SYK promoted HSC activation via regulation of the expression of specific transcription factors. Our qPCR validation of these 3 TFs confirmed our PCR array results of downregulation of these TFs by SYK siRNA (Figure 3A). We found that siRNAs to MYB, CBP or MYC did not affect SYK expression compared to Neg siRNA in both LX2 and TWNT-4 cells. These results suggest that SYK lies upstream of MYB, CBP and MYC (Supplementary Figure 9). Furthermore, we found that siRNAs to SYK, MYB, CBP or MYC reduced expression of the HSC activation markers ACTA2 and PDGFRβ in LX-2 and TWNT-4 cells, (Figure 3B, Supplementary Figure 10). MYB siRNA blocked SYK overexpression-induced enhancement of MYB and ACTA2 expression in LX-2 and TWNT-4 cells (Figure 3C). These results indicate that SYK promotes HSC activation via up-regulation of these transcription factors.
Figure 3. SYK promotes HSC activation via up-regulation of specific transcription factors.
(A) Left panel shows the volcano plot of SYK-regulated transcription factor genes in a PCR array comparing LX-2 cells transfected with SYK siRNA and negative control siRNA. Right panel shows reduced mRNA expression of SYK, MYB, CBP and MYC in LX-2 and TWNT-4 cells transfected with SYK siRNA compared to negative siRNA control. (B) siRNAs to SYK (siSYK), MYB (siMYB), CBP (siCBP) and MYC (siMYC) reduced mRNA expression of ACTA2 and PDGFRβ (markers of activated HSCs) in LX-2 and TWNT-4 compared to negative control siRNA (Ctrl). (C) MYB siRNA blocked SYK overexpression induced mRNA expression of MYB and ACTA2 in LX-2 and TWNT-4 cells (upper panel), with protein levels shown in the lower panel by immunoblotting. (D) SYK siRNA inhibited protein and mRNA expression of SYK, MYC and CCND1 in LX-2 and TWNT-4 cells compared to negative control siRNA. (E&F) ChIP analyses were performed with an anti-acetyl-H3K9 antibody in LX-2 cells infected with lentivirus expressing SYK shRNA, treated with GS-9973 or overexpressed with plasmids expressing wild type (WT) or kinase dead (KD) SYK (E). Relative mRNA expression of MYC and CCND1 were detected by qRT-PCR (F). Data presented are means ± SD. NS = not significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
MYC and CCND1 are important target effectors of the Wnt/β-catenin signaling pathway to promote the activation and proliferation of HSCs.(28, 29) Histone H3 lysine 9 acetylation (acetyl-H3K9) in the promotor region is important for both MYC and CCND1 expression.(30) We found that SYK siRNA significantly decreased MYC and CCND1 mRNA and protein expression in HSCs (Figure 3D). We performed chromatin immunoprecipitation (ChIP) analyses with an acetylation-H3K9 antibody to determine whether SYK regulates H3K9 acetylation of MYC and CCND1 promoter regions. We found that SYK shRNA and SYK inhibitor (GS-9973) treatment significantly decreased H3K9 acetylation in the CCND1 and MYC promoter regions in LX-2 cells. Moreover, overexpression of SYK-WT, but not SYK-KD in LX-2 cells significantly increased CCND1 and MYC promoter regions H3K9 acetylation and mRNA expression, respectively (Figure 3E,F).
Up-regulation of SYK in hepatocytes promotes HSC activation via crosstalk
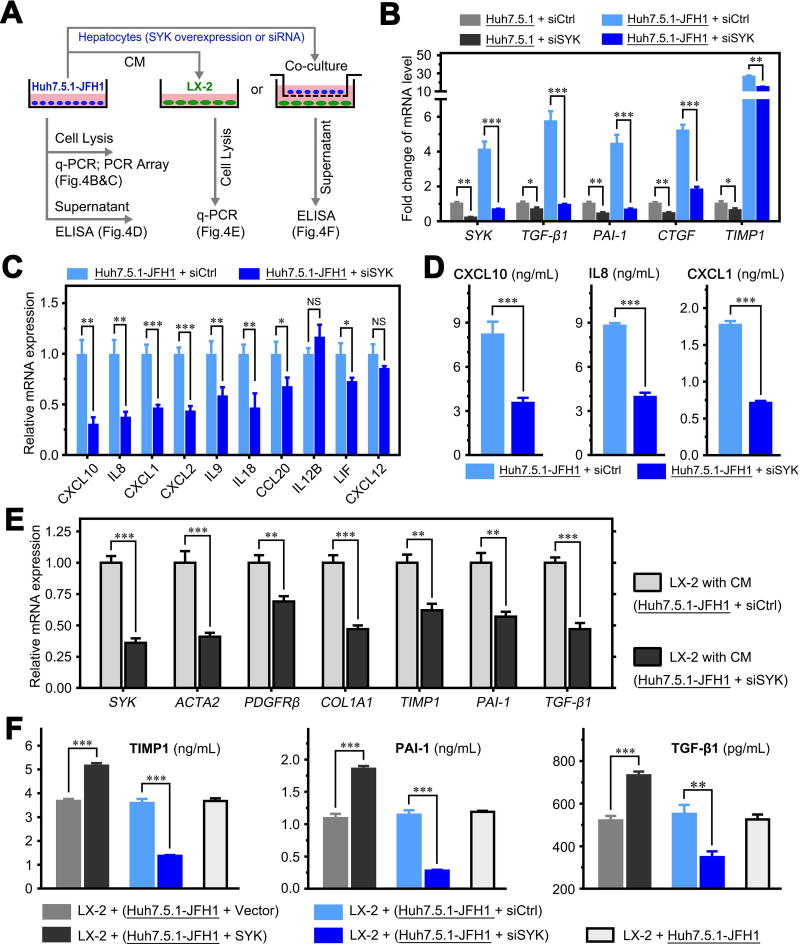
We investigated whether SYK up-regulation in hepatocytes promotes liver fibrosis via crosstalk between hepatocytes and HSCs. We utilized conditioned medium (CM) and a Transwell co-culture system to investigate crosstalk between hepatocytes and HSCs (Figure 4A). We found that JFH1 HCV infection significantly increased SYK, TGF-β1, PAI-1, CTGF and TIMP1 expression, while SYK siRNA blocked this HCV-induced up-regulation in JFH1-infected Huh7.5.1 cells (Figure 4B). To further investigate changes of cytokine by siSYK in JFH1-infected hepatocytes, we performed a PCR array, including 86 cytokine genes. The PCR array identified 10 cytokines with more than 2-fold expression change (P < 0.05) in JFH1-infected Huh7.5.1 cells by siSYK compared to its control. Of the ten cytokines, CXCL10, IL8, CXCL1, CXCL2, IL9, IL18, CCL20, IL12B and LIF were significantly down-regulated and CXCL12 was up-regulated (Supplementary Table 2). Real-time PCR assays indicated that the change in expression of CXCL10, IL8, CXCL1, CXCL2, IL9, IL18, CCL20, and LIF, corresponding with the results of PCR array, but not IL12B and CXCL12 (Figure 4C). We selected CXCL10, IL8 and CXCL1 for further verification by ELISA, and the secreted levels of these cytokines in cell supernatants were decreased significantly (P < 0.001; Figure 4D). Moreover, when SYK-WT and SYK-KD were overexpressed in Huh7.5.1-JFH1 cells, only WT SYK increased the expression of these cytokines (Supplementary Figure 11). These results suggest that SYK promotes cytokine production in Huh7.5.1 cells. Furthermore, we found that SYK siRNA in Huh7.5.1-JFH1 cells significantly reduced, while overexpression of SYK significantly increased TIMP1, PAI-1 and TGF-β1 mRNA and protein levels compared to Neg siRNA in the supernatant when co-cultured with LX2 cells (Figure 4E,F). In fact, activated HSCs expressed and secreted higher levels of TGF-β1, PAI-1 and TIMP1 than hepatocytes (Supplementary Figure 12). Our results suggest that HCV infection-induced SYK and cytokine production enhancement in hepatocytes promotes HSC activation through cytokine crosstalk between hepatocytes and HSCs.
Figure 4. Up-regulation of SYK in hepatocytes promotes HSC activation via crosstalk.
(A) Schematic flow chart of Transwell insert mono- and co-culture models. Supernatant was collected for ELISA and cells were harvested for PCR. CM, conditioned medium. (B) SYK siRNA inhibited HCV-induced up-regulation of mRNA expression of SYK, TGF-β1, PAI-1, CTGF and TIMP1 in JFH1-infected Huh7.5.1 cells. (C) SYK siRNA inhibited HCV-induced up-regulation of mRNA expression of related cytokines and chemokines which identified by PCR array. (D) Protein levels of CXCL10, IL8 and CXCL1 in the supernatant of conditions presented in (C) were validated by ELISA. (E) Relative mRNA expression of SYK and markers of activated HSCs in LX-2 cells cultured with CM from JFH1-infected Huh7.5.1 cells. (F) Protein levels of TIMP1, PAI-1 and TGF-β1 in supernatants from the co-cultured system of LX-2 and JFH1-infected Huh7.5.1 cells transfected with SYK plasmid, vector, SYK siRNA or control siRNA. Data presented are means ± SD. NS = not significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
SYK antagonism has anti-fibrotic activity in vitro
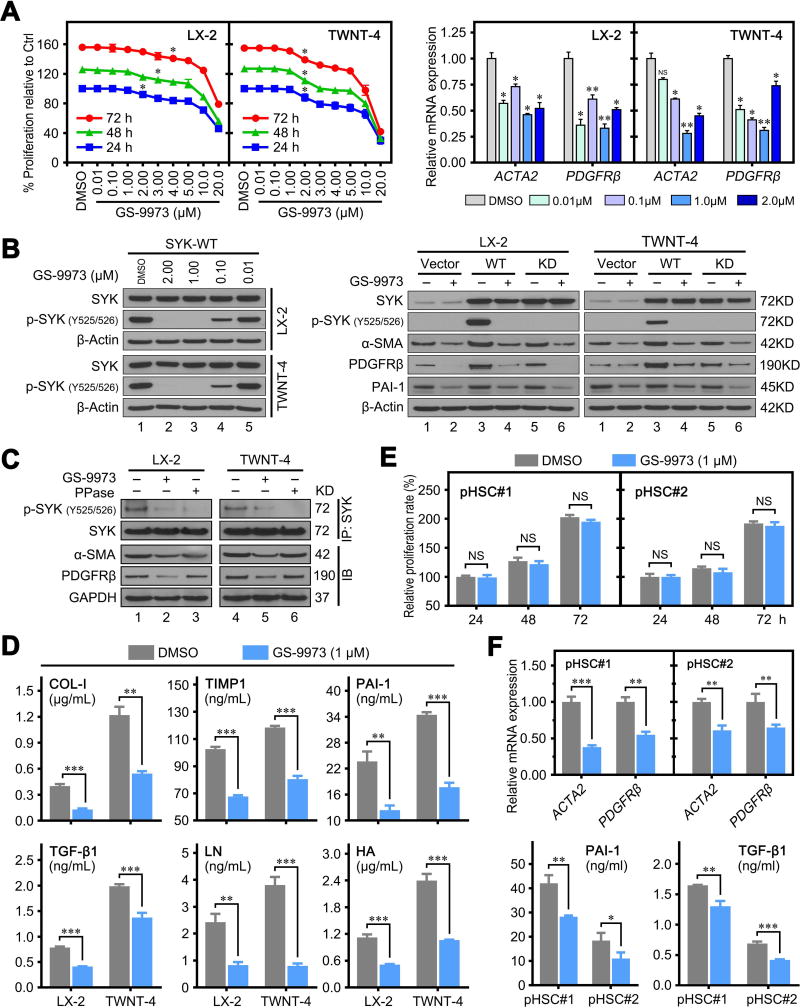
GS-9973 is a selective and potent oral inhibitor of SYK (Supplementary Figure 13A),(31) and has been in clinical evaluation for chronic lymphocytic leukemia.(32) We utilized GS-9973 to investigate whether SYK antagonism has anti-fibrotic activity. We found that GS-9973 treatment inhibited cell proliferation and HSC activation marker (ACTA2 and PDGFRβ) expression in a dose and time dependent manner in LX-2 and TWNT-4 cells (Figure 5A). Our data indicated that 1.0 µM GS-9973 did not affect proliferation in both LX-2 and TWNT-4 cells and was thus selected for further experiments (Figure 5A). We confirmed that GS-9973 (1.0 µM) effectively inhibited SYK phosphorylation at Y525/526 (Figure 5B), the key activation site at the kinase domain of SYK.(33, 34) We also found that GS-9973 down-regulated protein expression of α-SMA, PDGFRβ and PAI-1 (Figure 5B). These results demonstrate that SYK antagonism inhibits HSC activation and exerts an anti-fibrotic activity by inhibiting Y525/526 phosphorylation of SYK. Immunoprecipitation of SYK followed by immunoblotting indicated that both GS-9973 (1.0 µM) and protein phosphatase (PPase, as a positive control) completely inhibited Y525/526 phosphorylation of SYK, and decreased α-SMA and PDGFβ expression in LX-2 and TWNT-4 cells (Figure 5C). GS-9973 also effectively decreased the secreted protein level of COL-1, TIMP1, PAI-1, TGF-β1, LN and HA in cultured supernatants of LX-2 and TWNT-4 cells (Figure 5D). Moreover, we observed similar antifibrotic activity of GS-9973 in primary human HSCs. Treatment of GS-9973 (1.0 µM) did not influence the proliferation of primary human HSCs (Figure 5E), but significantly decreased the mRNA level of HSC activation markers and protein levels of PAI-1 and TGF-β1 in supernatants (Figure 5F).
Figure 5. SYK antagonism has anti-fibrotic activity in vitro.
(A) Left panel shows GS-9973 inhibited cell proliferative effect in LX-2 and TWNT-4 cells in a dose and time dependent manner. Right panel shows the relative mRNA expression of ACTA2 and PDGFRβ in LX-2 and TWNT-4 cells treated with different concentrations of GS-9973. (B) Left panel shows immunoblots of indicated proteins in LX-2 and TWNT-4 cells treated with different concentrations of GS-9973. Right panel shows that GS-9973 (1.0 µM) blocked p-SYK (Y525/526) phosphorylation-induced by overexpression of SYK wild-type (WD) in LX-2 and TWNT-4 cells. (C) GS-9973 (1.0 µM) or PPase blocked SYK phosphorylation in LX-2 and TWNT-4 cells. The lower panel shows the immunoblot of α-SMA, PDGFRβ and GAPDH in HSCs. (D) LX-2 and TWNT-4 cells were treated with DMSO or 1.0 µM GS-9973 for 48 hours. Protein levels of COL-1, TIMP-1, PAI-1, TGF-β1, LN and HA in the supernatant was detected by ELISA. (E) GS-9973 (1.0 µM) treatment did not affect cell proliferation rate of primary human HSCs compared to DMSO treatment. (F) GS-9973 (1.0 µM) treatment for 48 hrs reduced mRNA expression of ACTA2 and PDGFRβ in primary human HSCs (upper panel) and decreased protein levels of PAI-1 and TGF-β1 in the supernatant of primary human HSCs. Data are the mean ± SD. NS = not significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In addition, we found another SYK antagonist, PRT062607 (2.0 µM), also significantly inhibited HSC activation, but with lower efficiency compared to GS-9973 (Supplementary Figure 13). Our results indicate that the SYK antagonists GS-9973 and PRT062607 exhibit anti-fibrotic activity in vitro.
SYK antagonist treatment reduces fibrosis development in three murine models
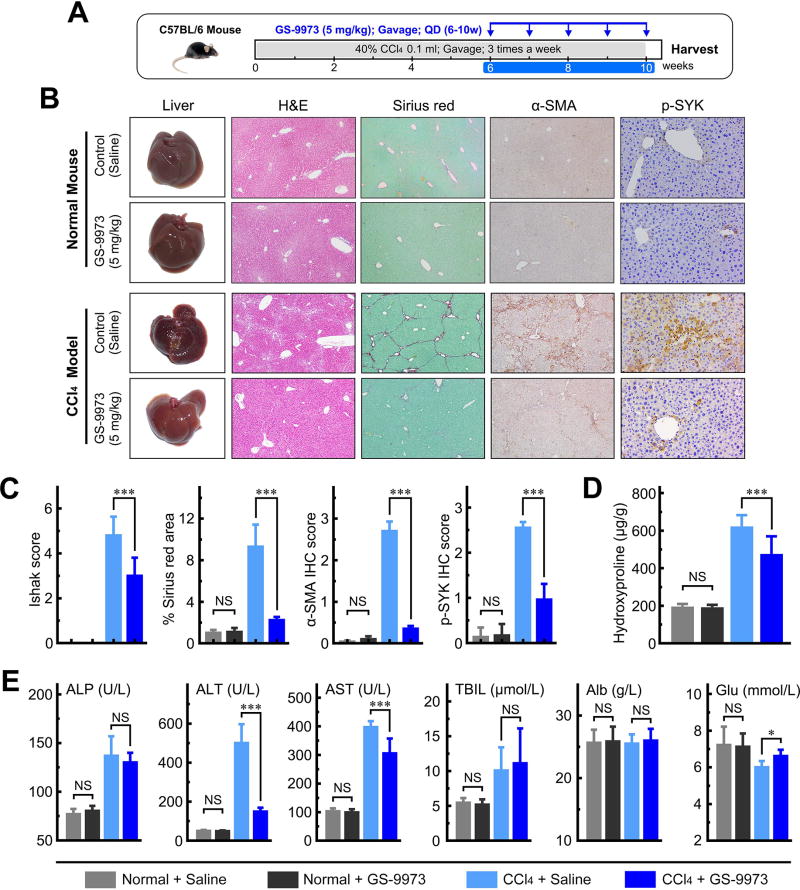
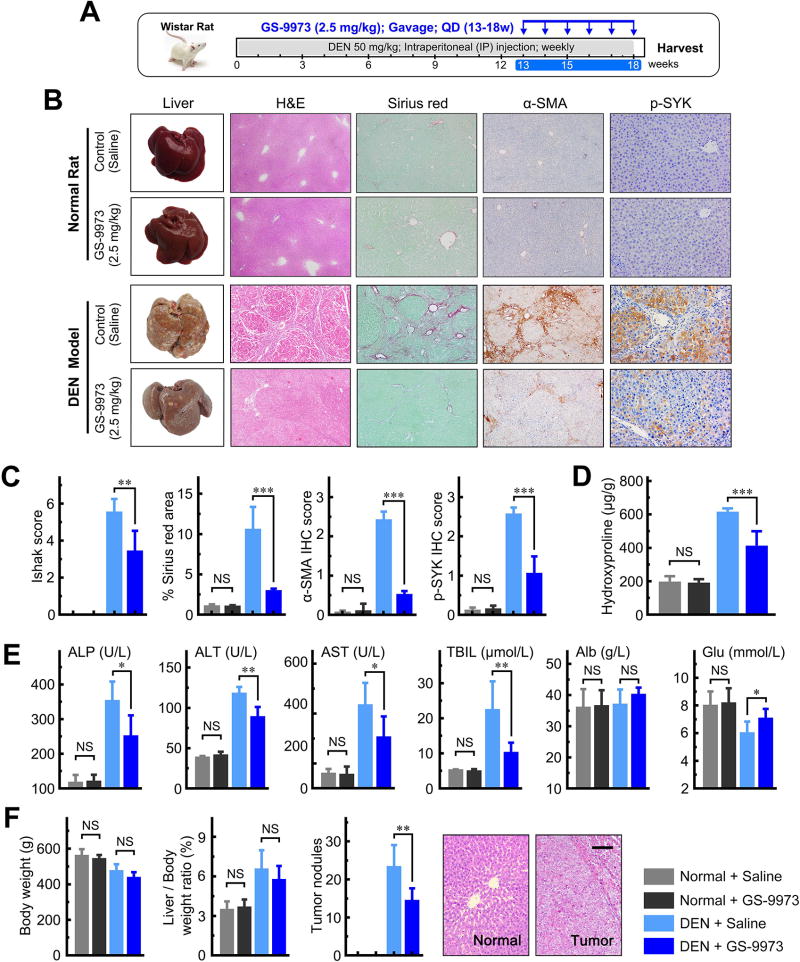
We further tested the effects of the SYK inhibitor GS-9973 on liver fibrogenesis and hepatocellular carcinoma transformation in three distinct animal fibrosis/cirrhosis models, the CCl4 mouse, DEN rat and BDL rat models (Figure 6A, 7A and 8A). In each model, two doses of GS-9973 were used (Supplementary Figure 14–16). We successfully induced liver fibrosis and cirrhosis in all three animal models. The median Ishak fibrosis scores were 5.0 (interquartile range [IQR] 4.0–6.0) for the CCl4 mouse model, and 6.0 (IQR 4.0–6.0) for the DEN and BDL rat models. GS-9973 significantly reduced Ishak fibrosis scores, and Sirius red, α-SMA and p-SYK (Y525/526) staining compared to vehicle controls in all three animal models (all P < 0.01; Figure 6C, 7C and 8B; representative images shown in Figure 6B, 7B and 8B). In addition, GS-9973 also reduced hepatic hydroxyproline levels all three fibrosis models (Figure 6D, 7D and 8D). GS-9973 did not affect fibrosis development in vehicle control animals (Figure 6–8).
Figure 6. GS-9973 reduces fibrosis in a CCl4 mouse model.
(A) Schematic of the experimental design of GS-9973 treatment in a CCl4-induced fibrosis model in mice. (B) Representative images of mouse livers stained with H&E, Sirius red, α-SMA or p-SYK. (C) GS-9973 reduced Ishak fibrosis score, and Sirius red, α-SMA and p-SYK IHC staining. (D) GS-9973 reduced hepatic hydroxyproline levels. (E) Serum levels of ALP, ALT, AST, TBIL, Alb and Glu. Data presented are means ± SD. NS = not significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 7. GS-9973 reduces fibrosis and HCC development in a DEN-induced fibrosis rat model.
(A) Schematic of the experimental design of GS-9973 treatment in a DEN-induced fibrosis model in rats. (B) Representative images of rat livers stained with H&E, Sirius red, α-SMA or p-SYK. (C) GS-9973 reduced Ishak fibrosis scores, and Sirius red, α-SMA and p-SYK IHC staining. (D) GS-9973 reduced hepatic hydroxyproline levels. (E) Serum levels of ALP, ALT, AST, TBIL, Alb and Glu. (F) GS-9973 reduced the number of tumor nodules, but did not significantly affect body weight or liver weight. Scale bars, 100 µm. Data presented are means ± SD. NS = not significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 8. GS-9973 reduces fibrosis in a BDL fibrosis rat model.
(A) Schematic of the experimental design of GS-9973 treatment in a BDL-induced fibrosis model in rats. (B) Left panel shows representative images of rat livers stained with H&E, Sirius red, α-SMA or p-SYK. Right panel shows GS-9973 reduced Ishak fibrosis scores, and Sirius red, α-SMA and p-SYK IHC staining. (C) Serum levels of ALP, ALT, AST, TBIL, Alb and Glu. (D) GS-9973 reduced hepatic hydroxyproline levels and liver/body weight ratio. (E) Representative photomicrographs of CK19 immunostaining in liver tissue from BDL rats treated with vehicle or GS-9973. Scale bar, 100 µm. (F) Proposed model of SYK regulation of liver fibrosis through promoting HSC activation and proliferation. Data presented are means ± SD. NS = not significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
There were no significant differences in liver weight or liver/body weight ratio between GS-9973 treated and vehicle control animals in DEN models (Figure 7F, Supplementary Figure 15C,D). Importantly, we found that GS-9973 significantly decreased the number of HCCs, particularly the number of smaller tumors (<8 mm diameter) after 18 weeks of DEN injury compared to vehicle control (P < 0.05; Figure 7F, Supplementary Figure 15E,F). These findings indicate that GS-9973 inhibits the development of new liver neoplasms associated with liver fibrosis/cirrhosis.
Furthermore, we observed that GS-9973 rescued hepatic function (ALP, ALT, AST, Alb and GLU) in all three fibrosis models (Figure 6E, 7E and 8C). In the BDL model, we found that high dose GS-9973 (10 mg/kg) dramatically decreased jaundice, inhibited proliferation of intrahepatic bile ducts and reduced liver/body weight ratio (all P < 0.01; Figure 8D,E) compared to the sham group. Low dose GS-9973 (2.5 mg/kg) treatment partially rescued hepatic function (ALP, ALT and Glu; all P < 0.05), but did not significantly recover AST, Alb and TBIL levels compared to vehicle control in the BDL model (Supplementary Figure 16F). Moreover, we demonstrated that GS-9973 inhibited Ki-67 expression compared to vehicle control in the CCl4 and BDL models (Supplementary Figure 17).
Hemodynamic adverse effects have been reported with several SYK inhibitors in some clinical trials.(35) Therefore, we measured systemic arterial blood pressure, portal pressure, liver weight, spleen weight and body weight of normal rats treated with vehicle or the SYK antagonist GS-9973. We did not observe any significant hemodynamic changes following GS-9973 treatment (2.5 and 10 mg/kg), for two or five weeks in rats, and no effects on liver weight, spleen weight and body weight in mice treated with GS-9973 (5 and 20 mg/kg) for four weeks (Supplementary Table 3). In addition, there were no effects on hepatic and renal function, or histopathologic changes in murine heart, liver and kidney with GS-9973 treatment (data not shown).
Discussion
Liver fibrosis/cirrhosis is a major health problem worldwide, for which there are currently no approved therapies.(10, 11). Activated HSCs play a central role in liver fibrosis, however, the molecular mechanisms by which HSCs are activated and become fibrogenic are incompletely understood. In this study, we found that enhanced SYK expression associated with liver fibrosis/cirrhosis, especially in liver tissues from patients with viral hepatitis and with NASH. Dual immunofluorescence and IHC staining further demonstrated that SYK upregulation is mainly localized to hepatocytes and HSCs. Moreover, our results showed that HBV/HCV infection significantly increased SYK expression in hepatocytes. We demonstrated that the up-regulation of SYK in hepatocytes induced SYK expression in HSCs and promoted HSCs activation via crosstalk between cytokines secreted by hepatocytes and HSCs. We also found that the induced expression of SYK in HSCs further promoted HSC activation by increasing the expression of specific transcription factors associated with HSC activation and proliferation (Figure 8F). SYK inhibition by the small-molecule inhibitor GS-9973 blocked HSC activation in vitro and significantly reduced fibrogenesis in three independent murine models. SYK may be a potential therapeutic target for liver fibrosis, including in NASH-related liver fibrosis, where there are no approved therapies for NASH in addition to the lack of approved therapies for liver fibrosis.
SYK is a nonreceptor protein tyrosine kinase, which is in clinical development as a therapeutic target for rheumatoid arthritis and lymphocytic leukemia.(15, 20) SYK gene codes two transcripts, the full-length SYK(L) and an alternatively spliced SYK transcript, named SYK(S). The SYK(S) isoform lacks a 69-bp sequence, generating a truncated isoform lacking 23 residues in interdomain B (IDB).(36) We previously found that two isoforms of SYK exerted opposing functions in HCC.(36, 37) SYK(L) was mainly expressed in non-tumor liver tissue, and was significantly down-regulated in HCC. In contrast, SYK(S) expression was very low in non-tumor tissue. Moreover, SYK(L) suppressed the proliferation and invasiveness of HCC cells, while SYK(S) possessed oncogenic activities and promoted invasion and metastases of HCC cells.(36, 37) We found both SYK(L) and SYK(S) overexpression promoted the activation of HSCs and chemically-induced murine liver fibrosis stimulated SYK(L)/(S) expression up-regulation. We found that SYK(L), but not SYK(S) played a major role in liver fibrosis in vitro. SYK(L) exerted greater effects on liver fibrosis development compared to SYK(S) in all three of our animal fibrosis models. We speculate that expression levels of these two SYK isoforms may be useful markers to monitor HCC development.
In this study, we found that four SYK inhibitors GS-9973, PRT062607, R406 and BAY-61-3606 (data of R406 and BAY-61-3606 not shown) had significant in vitro antifibrotic activity. GS-9973 was the most potent SYK inhibitor for blocking SYK phosphorylation at Y525/526. Our data show that GS-9973 significantly reduced liver fibrosis and rescued hepatic function. Furthermore, GS-9973 (10 mg/kg) dramatically decreased jaundice and serum TBIL, inhibited proliferation of intrahepatic bile ducts and reduced liver/body weight ratio in BDL rats. We speculate that this phenomenon is mainly due to the anti-fibrotic and hepatoprotective effects of GS-9973. However, the specific mechanism underlying this phenomenon is not fully elucidated. Further studies to explore the mechanism by which SYK inhibition by GS-9973 leads to lower bilirubin levels in the surgical BDL model are warranted. In addition, we found that GS-9973 decreased the number of HCC tumors in DEN-induced rat fibrosis model. These results suggest that targeting SYK could help to prevent HCC. Further studies to investigate whether SYK inhibition prevents intrahepatic recurrence in patients with a history of HCC are warranted.
We conclude that SYK is a key molecule for HSC activation and liver fibrogenesis. SYK-targeted treatment with a SYK inhibitor resulted in a potent anti-fibrotic effect without concomitant hemodynamic changes in vivo. Therefore, SYK is an attractive novel therapeutic target for liver fibrosis. We therefore propose a model of SYK regulating liver fibrosis (Figure 8F). Chronic liver injury (i.e. HBV, HCV, alcohol) increases expression of SYK in hepatocytes, which promotes HSC activation and up-regulates SYK expression in HSCs via crosstalk. Up-regulated SYK in HSCs further promotes HSC activation by increasing the expression of transcription factors associated with their activation (i.e. CBP, MYB, MYC) and by enhancing the expression of genes associated with HSC proliferation (MYC and CCND1). The up-regulated SYK further promotes the progression of liver fibrosis/cirrhosis and hepatocarcinogenesis.
Supplementary Material
Acknowledgments
Financial Support: National Natural Science Foundation of China (NSFC) (81472265 and 81672320 to J. Hong; 81602067 to L. He), Science and Technology Program of Guangzhou, China (201704020128 to J. Hong), and NIH grants AI069939 and AI082630 (RTC).
List of Abbreviations
- ACTA2
α-smooth muscle actin
- aHSC
activated hepatic stellate cell
- Alb
albumin
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate transaminase
- BDL
bile duct ligation
- CCl4
carbon tetrachloride
- ChIP
chromatin immunoprecipitation
- Col1A1
collagen type 1 alpha 1
- DAPI
40,6-diamidino- 2-phenylindole
- DEN
diethylnitrosamine
- ECM
extracellular matrix
- Glu
glucose
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HSC
hepatic stellate cell
- PCR
polymerase chain reaction
- JFH1
Japanese fulminant hepatitis 1
- pHSC
primary hepatic stellate cell
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- TGF-β1
transforming growth factor beta-1
- TBIL
total bilirubin
- TIMP1
tissue inhibitor of metalloproteinase 1
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
References
- 1.Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, Murray CJ, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, McGinn CM, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577–1590. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology. 2017;65:1039–1043. doi: 10.1002/hep.28948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang DM, Sun W, Ning BF, Zhou TF, Li XF, Zhong W, Cheng Z, et al. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut. 2017 doi: 10.1136/gutjnl-2016-313392. [DOI] [PubMed] [Google Scholar]
- 9.Bottcher K, Pinzani M. Pathophysiology of liver fibrosis and the methodological barriers to the development of anti-fibrogenic agents. Adv Drug Deliv Rev. 2017 doi: 10.1016/j.addr.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017 doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, Pradere JP, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–1083. e1022. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen KH, Hsu HH, Yang HY, Tian YC, Ko YC, Yang CW, Hung CC. Inhibition of spleen tyrosine kinase (syk) suppresses renal fibrosis through anti-inflammatory effects and down regulation of the MAPK-p38 pathway. Int J Biochem Cell Biol. 2016;74:135–144. doi: 10.1016/j.biocel.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Cha HS, Boyle DL, Inoue T, Schoot R, Tak PP, Pine P, Firestein GS. A novel spleen tyrosine kinase inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. J Pharmacol Exp Ther. 2006;317:571–578. doi: 10.1124/jpet.105.097436. [DOI] [PubMed] [Google Scholar]
- 15.Perova T, Grandal I, Nutter LM, Papp E, Matei IR, Beyene J, Kowalski PE, et al. Therapeutic potential of spleen tyrosine kinase inhibition for treating high-risk precursor B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:236ra262. doi: 10.1126/scitranslmed.3008661. [DOI] [PubMed] [Google Scholar]
- 16.Hahn CK, Berchuck JE, Ross KN, Kakoza RM, Clauser K, Schinzel AC, Ross L, et al. Proteomic and genetic approaches identify Syk as an AML target. Cancer Cell. 2009;16:281–294. doi: 10.1016/j.ccr.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohr S, Doebele C, Comoglio F, Berg T, Beck J, Bohnenberger H, Alexe G, et al. Hoxa9 and Meis1 Cooperatively Induce Addiction to Syk Signaling by Suppressing miR-146a in Acute Myeloid Leukemia. Cancer Cell. 2017;31:549–562. e511. doi: 10.1016/j.ccell.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pamuk ON, Can G, Ayvaz S, Karaca T, Pamuk GE, Demirtas S, Tsokos GC. Spleen tyrosine kinase (Syk) inhibitor fostamatinib limits tissue damage and fibrosis in a bleomycin-induced scleroderma mouse model. Clin Exp Rheumatol. 2015;33:S15–22. [PubMed] [Google Scholar]
- 19.Ma FY, Blease K, Nikolic-Paterson DJ. A role for spleen tyrosine kinase in renal fibrosis in the mouse obstructed kidney. Life Sci. 2016;146:192–200. doi: 10.1016/j.lfs.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Weinblatt ME, Kavanaugh A, Genovese MC, Musser TK, Grossbard EB, Magilavy DB. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N Engl J Med. 2010;363:1303–1312. doi: 10.1056/NEJMoa1000500. [DOI] [PubMed] [Google Scholar]
- 21.Mederacke I, Dapito DH, Affo S, Uchinami H, Schwabe RF. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat Protoc. 2015;10:305–315. doi: 10.1038/nprot.2015.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bukong TN, Iracheta-Vellve A, Saha B, Ambade A, Satishchandran A, Gyongyosi B, Lowe P, et al. Inhibition of spleen tyrosine kinase activation ameliorates inflammation, cell death, and steatosis in alcoholic liver disease. Hepatology. 2016;64:1057–1071. doi: 10.1002/hep.28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Tang X, Gong X, Albanis E, Friedman SL, Mao Z. Regulation of hepatic stellate cell activation and growth by transcription factor myocyte enhancer factor 2. Gastroenterology. 2004;127:1174–1188. doi: 10.1053/j.gastro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Mann DA, Smart DE. Transcriptional regulation of hepatic stellate cell activation. Gut. 2002;50:891–896. doi: 10.1136/gut.50.6.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann J, Mann DA. Transcriptional regulation of hepatic stellate cells. Adv Drug Deliv Rev. 2009;61:497–512. doi: 10.1016/j.addr.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Li J, Zhang J, Dai C, Liu X, Wang J, Gao Z, et al. S100A4 promotes liver fibrosis via activation of hepatic stellate cells. J Hepatol. 2015;62:156–164. doi: 10.1016/j.jhep.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Tokunaga Y, Osawa Y, Ohtsuki T, Hayashi Y, Yamaji K, Yamane D, Hara M, et al. Selective inhibitor of Wnt/beta-catenin/CBP signaling ameliorates hepatitis C virus-induced liver fibrosis in mouse model. Sci Rep. 2017;7:325. doi: 10.1038/s41598-017-00282-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su M, Chao G, Liang M, Song J, Wu K. Anticytoproliferative effect of Vitamin C on rat hepatic stellate cell. Am J Transl Res. 2016;8:2820–2825. [PMC free article] [PubMed] [Google Scholar]
- 29.Son G, Hines IN, Lindquist J, Schrum LW, Rippe RA. Inhibition of phosphatidylinositol 3-kinase signaling in hepatic stellate cells blocks the progression of hepatic fibrosis. Hepatology. 2009;50:1512–1523. doi: 10.1002/hep.23186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, Aldape K, et al. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012;150:685–696. doi: 10.1016/j.cell.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Currie KS, Kropf JE, Lee T, Blomgren P, Xu J, Zhao Z, Gallion S, et al. Discovery of GS-9973, a selective and orally efficacious inhibitor of spleen tyrosine kinase. J Med Chem. 2014;57:3856–3873. doi: 10.1021/jm500228a. [DOI] [PubMed] [Google Scholar]
- 32.Barr PM, Saylors GB, Spurgeon SE, Cheson BD, Greenwald DR, O'Brien SM, Liem AK, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016;127:2411–2415. doi: 10.1182/blood-2015-12-683516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speich HE, Grgurevich S, Kueter TJ, Earhart AD, Slack SM, Jennings LK. Platelets undergo phosphorylation of Syk at Y525/526 and Y352 in response to pathophysiological shear stress. Am J Physiol Cell Physiol. 2008;295:C1045–1054. doi: 10.1152/ajpcell.90644.2007. [DOI] [PubMed] [Google Scholar]
- 34.Aouar B, Kovarova D, Letard S, Font-Haro A, Florentin J, Weber J, Durantel D, et al. Dual Role of the Tyrosine Kinase Syk in Regulation of Toll-Like Receptor Signaling in Plasmacytoid Dendritic Cells. PLoS One. 2016;11:e0156063. doi: 10.1371/journal.pone.0156063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lengel D, Lamm Bergstrom E, Barthlow H, Oldman K, Musgrove H, Harmer A, Valentin JP, et al. Prevention of fostamatinib-induced blood pressure elevation by antihypertensive agents. Pharmacol Res Perspect. 2015;3:e00176. doi: 10.1002/prp2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong J, Hu K, Yuan Y, Sang Y, Bu Q, Chen G, Yang L, et al. CHK1 targets spleen tyrosine kinase (L) for proteolysis in hepatocellular carcinoma. J Clin Invest. 2012;122:2165–2175. doi: 10.1172/JCI61380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong J, Yuan Y, Wang J, Liao Y, Zou R, Zhu C, Li B, et al. Expression of variant isoforms of the tyrosine kinase SYK determines the prognosis of hepatocellular carcinoma. Cancer Res. 2014;74:1845–1856. doi: 10.1158/0008-5472.CAN-13-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.