Abstract
This special edition of the Biomedical Journal honors the awarding of the 2016 Nobel Prize in Physiology and Medicine to Yoshinori Ohsumi for his pioneering work on elucidating the mechanisms of autophagy. We also highlight a study reporting a new and simple animal model for a widespread surgical technique called interbody spinal fusion. Finally, this issue also includes two articles reporting protocols that could produce specific cell types for cell based therapies.
Keywords: Autophagy, Nobel Prize in Physiology or Medicine, Interbody spinal fusion
Spotlight on special edition
Food for thought: autophagy researcher wins 2016 Nobel Prize in Physiology or Medicine
This issue of the Biomedical Journal includes three articles to mark the occasion of the 2016 Nobel Prize in Physiology or Medicine, awarded to Japanese cell biologist, Yoshinori Ohsumi, for his work on autophagy. Although at first glance autophagy may appear to be a simple cellular clean up and recycling system in which unnecessary or damaged cytoplasmic components are degraded in lysosomes, it is now known to play important function in many biological processes. These include maintenance of organelle integrity, differentiation, stress responses and as we learn in this issue, even innate and adaptive immunity.
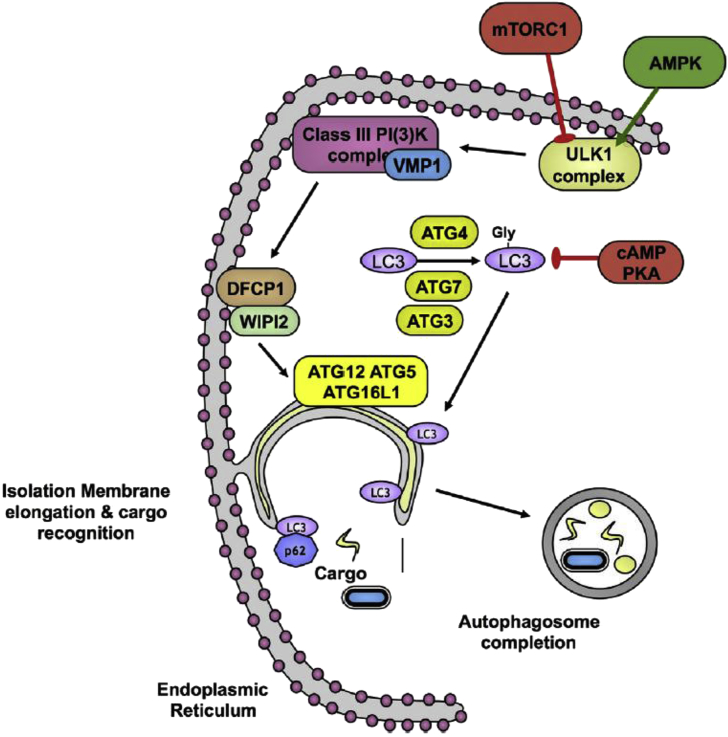
In the first article, Po-yuan Ke [1] outlines the history of research into autophagy in brief. The groundwork for Ohsumi's discoveries was laid in the late 1950s, early 1960s when electron microscopy studies revealed membrane-bound “dense bodies” containing semi-digested cellular organelles and lysosomal enzymes [2], [3]. Christophe de Duve, one of the discoverers of the lysosome, coined the term “autophagy” literally meaning “eat” “oneself” to describe this self-inflicted destruction occurring in normal cells. But the molecular mechanisms underpinning autophagy [Fig. 1] were not worked out until the 1990s, when Ohsumi developed a screen for the genes involved based on temperature-sensitive yeast mutants defective in autophagy [4]. This work set the precedent for the identification of dozens of autophagy genes (ATGs), including LC3, the mammalian homolog of ATG8, the lipidation of which provides an easy means to track autophagy [5].
Fig. 1.
The molecular mechanisms underpinning autophagy. Kindly provided by Harnett et al. [6]. During autophagy an “isolation membrane” develops (probably from the endoplasmic reticulum) to envelop cargo destined for destruction. Autophagy involves several ATG genes and two ubiquitin-like conjugation cascades, the ATG5-ATG12-ATG16 and ATG8 (LC3)-phosphatidylethanolamine conjugation systems, which are critical for autophagosome maturation (see Harnett et al. [6] for more details).
Although autophagy is traditionally studied in the context of starvation-induced stress, Harnett et al. [6] highlight the some of the diverse roles of this process, notably in immunity. Parallels can be drawn between autophagy and host cell defense mechanisms involving the destruction of bacteria, viruses or parasites in phagolysosomes or autophagolysosomes. Autophagy also provides a mechanism by which self and non-self cytosolic antigens can be taken up and expressed by MHC class II molecules, thus effectively linking innate to adaptive immunity. By providing antigen-presenting molecules with a continual pool of self antigens, autophagy also helps to maintain immune tolerance [7]. As a testament to this link, disruption of ATG genes in animal models leads to autoimmunity [8] and ATG polymorphisms have been linked to autoimmune diseases such as Crohn's disease [9] and asthma [10].
As an example of the role of autophagy during immunity, in the final article of this special issue, Latré de Laté et al. [11] describe how autophagy defends against intracellular Apicomplexan parasites like Plasmodium, which causes malaria. Some of these parasites, including the bovine parasite Theileria seem to have developed some tricks up their sleeve to manipulate and even evade host autophagy. Unlike other Apicomplexan parasites, Theileria does not reside in a parasitophorous vacuole and are thus completely exposed within the host cell cytoplasm. Surprisingly however, the host cell's autophagy machinery does not appear to react to the parasite. How the parasite is able to hide in plain sight is still unclear, although this may involve manipulation of host LC3, whereby the parasite promotes the phosphorylation and hence inactivation of LC3 [12] and perhaps even takes up and sequesters the protein [11].
Last year's award has put the spotlight of the research community firmly on autophagy and its every increasing list of functions in both normal and pathological contexts. Perhaps the medical field will draw inspiration from the Apicomplexan parasites and develop pharmacological manipulators of autophagy to boost immune responses.
Spotlight on original articles
New model of interbody spinal fusion surgery involving the rat tail
In this issue of the Biomedical Journal, Yeh et al. [13] develop a new animal model for interbody spinal fusion surgery, which could provide a simple method for future research to test improvements to this widespread surgical technique.
During conditions such as degenerative disc disease or spondylolisthesis, the spinal vertebra may become mobile or slide other one another. This causes pinching or squeezing of the spinal cord or nerve roots, followed by severe back pain. Stability is restored by fusing mobile vertebra together, most commonly through a technique called interbody spinal fusion. During this procedure, interbody spacers packed with bone graft are fixed between the vertebrae to be fused. Eventually the bone graft grows around the implants to form a bone bridge that connects the vertebra. Several animal models have been proposed to study interbody fusion, including a porcine model [14] and a rabbit model [15]. Although most models involve lumbar discs, rat tail discs are becoming an attractive alternative in disc research owing to their strong resemblance to human discs [16], [17]. Yeh et al. set out to develop a new simple and efficient animal model of interbody fusion based on the rat tail.
Surgery was performed on 12-week-old female anesthetized rats. Following an incision to the tail, the underlying tendons were partially removed to access the caudal vertebrae. The caudal disc between the third and fourth coccygeal vertebrae was completely removed and a commercial bone graft was placed in the disc space as a fusion material. To stabilize the surgical site, a sterile surgical tube was attached to the rat tail. X-rays taken at 12-weeks post-surgery revealed the formation of a bone bridge at the fusion site in all rats. Further histological examination showed that the anatomical structure of the rat tail was maintained after surgery. Twelve weeks after surgery, the rats were killed and their tails were subjected to a three-point bending test. Significantly more force was required to bend fusions tails than control tails, indicating that fusion surgery increased tail stiffness.
Small animal models offer several advantages over their larger counterparts, including lower costs. Moreover, the use of caudal discs instead over lumbar discs offers further benefits such as ease of manipulation. Differences in mechanics and geometry between rat caudal discs and human lumbar discs could limit findings from this model, and the small size of the tail prohibits the use of pedicle screws or cages that are often implanted to stabilize the vertebrae during surgery in humans. Nonetheless, because of the rapid recovery time and high metabolic rate, the rat tail model reported here is ideal for testing the effect of drugs or adjuvant treatments like growth factor supplementation.
Also in this issue:
Original articles
A vitamin D-based recipe for keratinocytes
Coaxing stem cells to differentiate along particular developmental trajectories holds great promise for generating autologous transplants for regenerative medicine. Here, Joulai Veijouye et al. [18] take advantage of easily extractable stem cells from the hair follicle bulge, and show that these cells can be coaxed to differentiate into keratinocytes in vitro when supplied with the active metabolite of vitamin D, 1,25(OH)2D3. These findings have important implications for skin grafting and tissue engineering.
Status of mismatch repair proteins predicts recurrence in colorectal cancer
Although stage I and II colorectal cancer is considered to be cured by surgical resection, around 10–20% of patients relapse or develop metastasis [19]. Biomarkers are thus needed to select patients who could benefit from post-operative chemotherapy. In this retrospective analysis of biopsies from 120 patients, Huang et al. [20] find that the overexpression of the DNA mismatch repair protein MLH1 is correlated with short survival, whereas overexpression of the related protein MSH2 is associated with longer survival. If confirmed in a larger setting, MLH1/MSH2 status could help to identify CRC patients with a high risk of recurrence.
Generating B regulatory cells for cell-based therapy
Regulatory B cells are a small but powerful population of B cells that act to dampen immune responses predominantly through secreting the anti-inflammatory cytokine interleukin 10. The introduction of these cells prevents or limits the development of autoimmune diseases in animal models [21], [22] and they hold great promise for cell-based therapy. Reasoning that these cells could bring about tolerance in transplant recipients, Gupte et al. [23] set out to generate them in vitro from donor adipose tissue derived mesenchymal stem cells (AD-MSC) and renal allograft recipient (RAR) peripheral blood mononuclear cells (PBMC). The protocol that they report here could serve as a basis to generate B-regs for transplant recipients.
Risk factors for relapse of non-small-cell lung cancer
Between 30 and 55% of patients with N2 non-small-cell lung cancer (NSCLC) develop recurrence, even after curative surgical resection [24]. It is not clear however which patients will relapse or why. In this retrospective analysis of 90 patients who underwent surgical resection for NSCLC, Wen et al. [25] used medical records, image studies, and pathology reports to identify risk factors for relapse. According to their analysis, patients at the highest risk of relapse were those with visceral pleural invasion, skip mediastinal lymph node involvement and those who received neoadjuvant therapy.
Optimizsing treatment planning for removable partial dentures
Removable partial dentures are a common treatment for individuals lacking some of their teeth. Such dentures may be placed using the rotational path of insertion whereby one segment of the partial denture is seated first, after which the remainder is rotated into position. The projections of the soft tissues, bone or tilted teeth, can interfere with seating of a rotating framework and must be considered in treatment planning. Huang et al. [26] examine how location of the rotational center and the morphology of teeth interfere with the path of insertion. Their results reveal under which conditions an interference test should be performed during treatment planning.
Conflicts of interest
The author declares that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Ke P.Y. Horning cell self-digestion: autophagy wins the 2016 Nobel Prize in Physiology or Medicine. Biomed J. 2017;40:5–8. doi: 10.1016/j.bj.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark S.L., Jr. Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J Biophys Biochem Cytol. 1957;3:349–362. doi: 10.1083/jcb.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novikoff A.B. The proximal tubule cell in experimental hydronephrosis. J Biophys Biochem Cytol. 1959;6:136–138. doi: 10.1083/jcb.6.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeshige K., Baba M., Tsuboi S., Noda T., Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 12 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harnett M.M., Pineda M.A., Latré de Laté P., Eason R.J., Besterio S., Langsley From Christian de Duve to Yoshinori Ohsumi: more to autophagy than just dining at home. Biomed J. 2017;40:9–22. doi: 10.1016/j.bj.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lunemann J.D., Munz C. Autophagy in CD4+ T-cell immunity and tolerance. Cell Death Differ. 2009;16:79–86. doi: 10.1038/cdd.2008.113. [DOI] [PubMed] [Google Scholar]
- 8.Nedjic J., Aichinger M., Emmerich J., Mizushima N., Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 9.Barrett J.C., Hansoul S., Nicolae D.L., Cho J.H., Duerr R.H., Rioux J.D. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin L.J., Gupta J., Jyothula S.S., Butsch Kovacic M., Biagini Myers J.M., Patterson T.L. Functional variant in the autophagy-related 5 gene promotor is associated with childhood asthma. PLoS One. 2012;7:e33454. doi: 10.1371/journal.pone.0033454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latré de Laté P., Pineda M.A., Harnett M.M., Harnett W., Besteiro S., Langsley G. Apicomplexan autophagy and modulation of autophagy in parasite-infected host cells. Biomed J. 2017;40:23–30. doi: 10.1016/j.bj.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guergnon J., Dessauge F., Traincard F., Cayla X., Rebollo A., Bost P.E. A PKA survival pathway inhibited by DPT-PKI, a new specific cell permeable PKA inhibitor, is induced by T. annulata in parasitized B-lymphocytes. Apoptosis. 2006;11:1263–1273. doi: 10.1007/s10495-006-7702-6. [DOI] [PubMed] [Google Scholar]
- 13.Yeh Y.C., Yang C.C., Tai C.L., Tsai T.T., Lai P.L., Chen W.J. Characterization of a novel caudal vertebral interbody fusion in a rat tail model: an implication for future material and mechanical testing. Biomed J. 2017;40:62–68. doi: 10.1016/j.bj.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H., Zou X., Xue Q., Egund N., Lind M., Bunger C. Anterior lumbar interbody fusion with carbon fiber cage loaded with bioceramics and platelet-rich plasma. An experimental study on pigs. Eur Spine J. 2004;13:354–358. doi: 10.1007/s00586-003-0647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yalcin N., Ozturk A., Ozkan Y., Celimli N., Ozocak E., Erdogan A. The effects of zoledronic acid and hyperbaric oxygen on posterior lumbar fusion in a rabbit model. J Bone Jt Surg Br. 2011;93:793–800. doi: 10.1302/0301-620X.93B6.24257. [DOI] [PubMed] [Google Scholar]
- 16.Beckstein J.C., Sen S., Schaer T.P., Vresilovic E.J., Elliott D.M. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine. 2008;33:E166–E173. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- 17.Showalter B.L., Beckstein J.C., Martin J.T., Beattie E.E., Espinoza Orias A.A., Schaer T.P. Comparison of animal discs used in disc research to human lumbar disc: torsion mechanics and collagen content. Spine. 2012;37:E900–E907. doi: 10.1097/BRS.0b013e31824d911c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joulai Veijouye S., Mashayekhi F., Yari A., Heidari F., Sajedi N., Nobakht M. In vitro induction effect of 1,25(OH)2D3 on differentiation of hair follicle stem cell into Q6 keratinocyte. Biomed J. 2017;40:31–38. doi: 10.1016/j.bj.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gertler R., Rosenberg R., Schuster T., Friess H. Defining a highrisk subgroup with colon cancer stages I and II for possible adjuvant therapy. Eur J Cancer. 2009;45:2992–2999. doi: 10.1016/j.ejca.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Huang S.C., Huang S.F., Chen Y.T., Chang Y., Chiu Y.T., Chen J.S. Overexpression of MutL homolog 1 and MutS homolog 2 proteins have reversed prognostic Q5 implications for stage IeII colon cancer patients. Biomed J. 2017;40:39–48. doi: 10.1016/j.bj.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauri C., Gray D., Mushtaq N., Londei M. Prevention of arthritis by interleukin 10 producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushita T., Yanaba K., Bouaziz J.D., Fujimoto M., Tedder T.F. Regulatory B-cells inhibit EAE initiation in mice while other B-cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupte K.S., Vanikar A.V., Trivedi H.L., Patel C.N., Patel J.V. In-vitro generation of interleukin-10 secreting B-regulatory cells from donor adipose tissue derived mesenchymal stem cells and recipient peripheral blood mononuclear cells for potential cell therapy. Biomed J. 2017;40:49–54. doi: 10.1016/j.bj.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uramoto H., Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3:242–249. doi: 10.3978/j.issn.2218-6751.2013.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen C.T., Fu J.Y., Wu C.F., Liu Y.H., Wu C.T., Tsai Y.H. Risk factors for relapse of resectable pathologic N2 non small lung cancer and prediction model for time-to-progression. Biomed J. 2017;40:55–61. doi: 10.1016/j.bj.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C.T., Liu F.C., Luk K.C. Interference factors regarding the path of insertion of rotational-path removable partial dentures. Biomed J. 2017;40:69–75. doi: 10.1016/j.bj.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]