Abstract
In the normal non-diseased lung, various macrophage populations maintain homeostasis and sterility by ingesting and clearing inhaled particulates, pathogens and apoptotic cells from the local environment. This process of phagocytosis leads to the degradation of the internalized material, coordinated induction of gene expression, antigen presentation and cytokine production, implicating phagocytosis as a central regulator of innate immunity. Phagocytosis is extremely efficient and any perturbation of this function is deleterious. In inflammatory lung diseases such as chronic obstructive pulmonary disease (COPD), despite their increased numbers, macrophages demonstrate significantly reduced phagocytic capacity of bacteria and apoptotic cells. This defect could play a role in dysbiosis of the lung microbiome contributing to disease pathophysiology. In this review, we will discuss lung macrophages, describe phagocytosis and its related downstream processes and the reported phagocytosis defects in COPD. Finally, we will briefly examine current strategies that focus on restoring the phagocytic capabilities of lung macrophages that may have utility in COPD.
Keywords: Bacterial clearance, COPD, Inflammation, Macrophages, Phagocytosis, Superinfections
Phagocytes in the lung
The lung is an organ at the interface between the environment and host, crucial in mounting and regulating immunological responses against pathogens and particulate matter. The cellular system of the lung is well characterised but the complexity of the interactions regulating homeostasis is not clearly defined [Fig. 1]. At steady state, interplay between macrophages, dendritic cells, regulatory T cells and epithelial cells maintain ‘status quo’ [1], [2]. On exposure to noxious agents and damage to the epithelia, the tissue resident innate immune cells mount a strong immune response. Macrophages within the human lung are key sentinels in mediating this response and consist of atleast three different populations: bronchial (bronchial wash-derived), interstitial (lung–tissue derived) and alveolar cells (bronchial-alveolar lavage (BAL)-derived) [1], [2], [3], [4]. Localisation of these cells within distinct compartments of the lung alludes to divergent cellular functions of these macrophage populations. However, due to the lack of access to ‘normal’ human lung samples, the function of these populations, particularly the interstitial macrophages, are less characterised. A recent study utilising a multi parameter analysis in non-diseased human lung identified four distinct mononuclear phagocytic cells resident in the lung that differed in their phenotype, expression and cellular location in addition to alveolar macrophages [5]. This seminal work describes lung resident monocytes, monocyte-derived cells and dendritic cells that differ from other previously described mononuclear phagocytes in human blood, highlighting diversity of the myeloid population in health. However, in chronic inflammatory states, there could be changes to the lung resident populations of mononuclear phagocytes and contribution from differentiated circulating monocytic cells to these. Regardless of the origin, phagocytosis, the clearance of pathogens, cellular debris and particulate matter are an integral function of these mononuclear phagocytes, particularly macrophages, which will be discussed in more detail in the following sections.
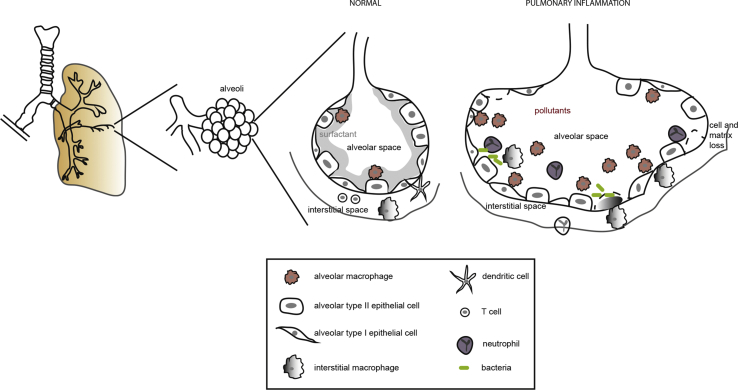
Fig. 1.
The lung architecture with division of bronchi that terminate in alveolar ducts made up of alveolar sacs. In the non-inflamed state, the resident alveolar cells are alveolar epithelial cells type I and II and alveolar macrophages. Constant exposure of the alveoli to toxic compounds and pollutants cause chronic inflammation. This is correlated with increased numbers of macrophages and the presence of neutrophils. Cells present in the interstitial tissue are interstitial macrophages, dendritic cells and lymphocytes.
Receptors mediating phagocytosis
Phagocytosis is defined as the mechanism of internalisation of large particulate material that are approximately 1 μm or greater. It is also crucial during development to remove cell debris and eliminate cells undergoing programmed cell death [6], [7], [8]. The whole process of phagocytosis leads to the degradation of the internalized material, coordinated induction of expression of hundreds of new genes, antigen presentation and cytokine production [Fig. 2], which implicates phagocytosis as a central regulator in innate immunity.
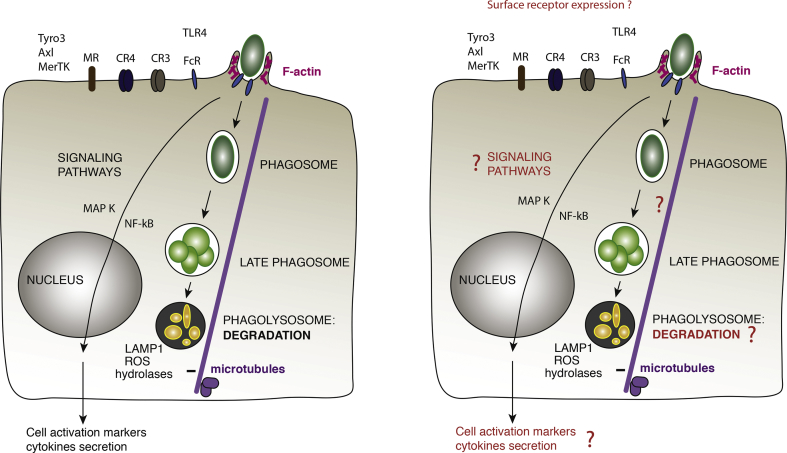
Fig. 2.
Schematic representation of the different processes of phagocytosis in the steady state (left) and their potential impairment under chronic lung inflammation (right). Phagocytic receptors are represented on the surface of the cell, leading to a dynamic remodelling of the actin cytoskeleton required for phagosome formation. The closed compartment evolves by fusion and fission steps while moving along microtubules to acquire the degradative properties of the phagolysosome. Parallel signaling pathways allow the phagocyte to secrete cytokines and participate in the activation of the immune response.
Phagocytosis is initiated by receptors on the plasma membrane of phagocytes that can be divided in non-opsonic or opsonic receptors. Non-opsonic receptors can directly recognize molecular groups on the surface of the targets including sugars, lipids, protein sequences and concentrations of charge that are unique to pathogens (so-called pathogen-associated molecular pattern). Among these receptors there are lectin-like recognition molecules e.g. CD169, CD33, related C-type lectins e.g. Dectin-2, Mincle or DNGR-1, scavenger receptors, Dectin-1 and the mannose receptor CD206. Other receptors, such as SR-A or CD36, can recognize both apoptotic and microbial polyanionic ligands, but their associated signalling is not well described. Scavenger receptors were initially described for their capacity to internalise oxidized low density lipoproteins [9], [10]. A new nomenclature and classification has been proposed collegially for scavenger receptors that recognize altered molecules of the organism but also pathogen determinants [11]. In addition, toll-like receptors (TLRs) are detectors for foreign particles, but they do not function as phagocytic receptors per se. However, TLRs often collaborate with other receptors to induce uptake and modulate the fate of the internal phagosomes and the concomitant signalling.
Opsonic receptors are the most studied receptors and therefore best characterized. The receptors for the Fc portion of the immunoglobulins (Ig) belong to a complex family of receptors that is heterogeneous as they perform different functions in different species. The complement receptors are integrins like CR3 (also known as αMβ2, CD11bCD18 or Mac-1) or CR4 (αXβ2, CD11cCD18) that can recognize complement-coated particles.
Initial signalling to cytoskeleton reorganisation
To dissect the signalling pathways and morphological changes leading to phagocytosis, the phagocytic receptors have been expressed ectopically in model cells, a strategy that allows to trigger signalling from a single phagocytic receptor [12]. Another approach to target a specific receptor is to coat or opsonize inert particles such as beads or red blood cells with a single ligand of interest, i.e., Ig or complement. Zymosan, a heat-inactivated yeast has also been used as opsonized particle to target FcR or CR3, although direct binding of the yeast cell surface ligands to other phagocytic receptors like Dectin or mannose receptors cannot be excluded [6], [7].
Phagocytic FcR (FcγRII and FcγRIII) belong to the immunoreceptor family using ITAM motifs to transduce signalling, which are either built in the receptor intracellular tail or in the associated common γ-chain [13]. Hence, early signalling events involve the Src kinases Lyn, Hck and Fgr. However, phagocytosis was significantly reduced but not abolished in cells from triple knockout mice, suggesting the existence of further redundancy or alternative triggering mechanisms [14]. On the other hand, Syk knockout resulted in a complete block of phagocytosis, indicating the indispensable role of this kinase [15]. More recently, Syk was implicated in actin remodelling allowing the lateral mobility of the phagocytic receptors [16].
The β-glucan receptor Dectin-1, a member of the C-type lectin receptor family that displays an ITAM-like sequence, also induces sequential activation of Src and Syk kinases after clustering and exclusion of the CD45 and CD148 phosphatases [17]. Interestingly, the protein tyrosine phosphatase SHP-2 was also implicated in bridging Syk to Dectin-1 and other C-type lectin receptors [18].
The molecular mechanisms underlying integrin-dependent phagocytosis, like that elicited by complement-coated particles binding to CR3, have been largely unexplored. Integrin binding to their ligand requires prior activation via a conformational change induced by “inside-out signalling”. This priming phase is induced by inflammatory cytokines or pathogen-specific signals leading to the activation of the small GTPase Rap1 [19]. Active Rap1 induces the recruitment of RapL, RIAM and talin to integrin cytoplasmic tails, triggering the switch of integrins to their extended conformation that can bind ligands with high affinity [20]. Then, ligand-bound integrins transmit “outside-in” signals that drive actin polymerization and downstream activation. These steps involve several effectors including the protein kinases FAK (or Pyk2) and ILK, non-muscle myosin II and Rho GTPases [21]. A crosstalk between integrin activation and ITAM bearing receptors allows a diffusion barrier to be established with active integrins and intact actin cytoskeleton to exclude phosphatases from the contact zone [17], [22], [23].
In all cases, actin polymerization is strictly required for phagosome formation as a force driving plasma membrane deformation. Signalling downstream of the phagocytic receptors is translated into molecular switches including the small GTPases of the Rho/Rac/Cdc42 family and their effectors that can promote the activity of actin nucleators. The activity of Arp2/3 and the formin family of proteins has been implicated in the phagocytic cup formation [6], [7], [8], [24]. Formins are especially important for the CR3-mediated phagocytosis [25]. The actin ring diameter progressively shrinks until the two membrane extensions eventually fuse. This step probably involves a constriction activity generated by Myosin II, and possibly other myosins [8]. Our recent work showed that the GTPase dynamin2 is required during the early steps of phagosome formation and actin remodelling, as well as to mediate the constriction and scission of the closed phagosome from the plasma membrane [26].
Phagosome maturation, clearance and activation of phagocytes
Once sealed, the phagosomal compartment evolves like an endocytic compartment in a process called maturation. This is accompanied by an acidification of the compartment and its migration on microtubules towards the cell center to reach a perinuclear localisation. The early phagosome is similar to early endosomes, bearing the Rab5 marker and its effector the early endosome antigen 1 (EEA1). It has a pH of 6.1–6.5 and is poorly degradative. The Ser and Thr kinase p150 allows the recruitment of hVPS34, a class III phosphoinositide 3 kinase (PI3K). The phosphoinositide 3 phosphate (PI3P) produced is recognized by sorting nexins, Hrs or p40phox involved in membrane sorting and reactive oxygen species (ROS) production. The products of PI3K are involved in the dissociation of Rab5 but are not essential for the recruitment of Rab7 on the phagosome [27]. A Rab5 effector in Caenorhabditis elegans, Mon1, revealed a link between Rab5 and Rab7. In a complex with Ccz1, it plays an important role in attracting and activating Rab7 on phagosomes [28]. The GEF for Rab7 is still not fully characterized although the homotypic fusion and protein sorting (HOPS) complex is a good candidate [29]. The complex is composed of Vps11, Vps16, Vps18, Vps33, Vps39 and Vps41. In yeast, Vps39 activates Rab7. The Vps41 protein is required for the stabilization of the HOPS complex on the endosomal membrane prior to fusion with the vacuole. The conversion from a Rab5 to a Rab7 positive compartment is a crucial step in phagosome maturation [30], as it leads to acquisition of proton-pumping v-ATPases and acidification to a pH of 5.5–6.0. It is enriched in proteases and lysosomal-associated proteins (LAMPs). Various Rab7 effectors are then recruited to the late phagosome, including RILP and ORP1L, which act as adaptors for dynein [31], [32]. The coordination between the microtubule based movements and the fusion machineries is probably crucial for an efficient maturation. Our recent work indeed showed that the plus-end microtubule-binding protein EB1 is critical to load the minus-end directed motors that will drive the centripetal phagosome movement during phagosome maturation [33].
The host microbicidal mechanisms include NOX2/gp91phox NADPH oxidase, inducible NO synthase (iNOS), iron scavengers and exporters, such as lactoferrin and natural resistance-associated macrophage protein 1 (NRAMP1; also known as SLC11A1) as well as antimicrobial peptides and proteins that permeabilize and degrade the bacteria [34]. The importance of NADPH oxidase and ROS production in bacterial clearance are evidenced by individuals with mutations that cause partial or total inactivation of the oxidase. Hydrolases delivered to the phagosomal lumen by late endosomes and lysosomes contribute to degradation of the ingested material. Peptides derived from this processing are then loaded onto major histocompatibility complex (MHC) class II molecules. MHC class II (HLA-DR in humans) molecules are then expressed on the surface of the phagocytic cell to activate CD4+ T lymphocytes. Phagocytosed antigens can also be presented by MHC class I molecules and activate cytotoxic CD8+ T lymphocytes in a process called “cross-presentation”. Activated macrophages express the co-stimulatory molecules CD80, CD86 and CD40 on their surface. The early response genes, which include a number of classical inflammatory cytokines such as TNF, are subject to regulation by the MAP kinases, NFkB pathways and transcription factors. Late response genes are regulated by autocrine factors including TNF and type 1 interferon and regulated transcription factors [35], [36], [37], [38]. Macrophages therefore are considered as regulators of acute inflammatory responses and critical for the resolution of inflammation. Of note, the alveolar macrophages were reported not to upregulate activation markers such as CD86 or CCR7, and despite expression of HLA-DR, to be poor inducers of T cell activation [3]. They indeed express high levels of TGFβ receptors, signal regulatory protein alpha (SIRPα) and interleukine 10 receptors that contribute to negatively regulate them [1], [3], [39]. Uptake of surfactant by CD36 has also been implicated in promoting the growth of intracellular Mycobacterium tuberculosis [40], further showing that the clearance functions of macrophages are modified by the alveolar environment.
Impaired phagocytosis in chronic lung inflammatory disease
In chronic inflammatory diseases of the lung including COPD, asthma and cystic fibrosis homeostasis is disrupted, contributing to a vicious cycle of inflammation [41], [42], [43]. It is increasingly apparent in these inflammatory lung conditions that, despite the increased numbers of macrophages [44], [45], their function is dysregulated. This contributes to dysbiosis of the normal lung microbiome, which is being recognised as drivers of diseases such as COPD [46]. This review will now focus herein on reported phagocytic defects in COPD, a multifactorial progressive disease characterized by an abnormal inflammatory response within the lung environment and destruction of the lung parenchyma resulting in “airflow limitation that is only partially reversible” [47]. Long term exposure of lung irritants including cigarette smoke, particulate matter and pollutants are risk factors for COPD [48]. In contrast to stable disease, exacerbations of COPD are characterised by increase in airflow limitations and worsening of symptoms triggered by abnormal inflammatory events usually associated with respiratory pathogens. Exacerbations cause significant mortality and morbidity in disease [49], [50]. Whether defective phagocytosis is fundamental to driving exacerbations of disease remains unclear and will be discussed in the following sections.
Phagocytosis defects in COPD-patient derived macrophages
The first report of defective function of alveolar macrophages in COPD was in 2003 [51]. In this article, the authors utilised UV-exposed apoptotic airway epithelial cells (AEC) as ‘bait’ to BAL-derived alveolar macrophages and quantified phagocytosis utilising a flow cytometry-based method. In COPD alveolar macrophages, a marked defect in phagocytosis of AEC was reported in patients (all current smokers) as opposed to never smokers of a similar age. Interestingly, they reported no defect in the COPD-derived cells when utilising polystyrene beads, highlighting differences of responses based on the trigger for phagocytosis. This defective function of clearance was attributed to either failure to recognise apoptotic markers on the epithelial cells or to the satiety hypothesis suggesting previous clearance by the cells results in downregulation of subsequent phagocytosis mediated by the same receptors.
The link to bacterial persistence or colonisation in lung samples of patients with COPD were subsequently reported [52]. In this seminal study, the authors studied alveolar macrophages from non-smokers and ex-smokers with and without COPD to clear non-typeable Haemophilus influenzae (NTHI). NTHI is common respiratory pathogen that is frequently isolated with episodes of COPD exacerbations. Utilising clinical strains of the bacteria, the authors demonstrated a defect in phagocytosis of NTHI by former smokers with COPD versus patients without COPD, implicating a global defect of NTHI uptake in disease. The study also examined blood-derived macrophages from the same patients and did not observe any difference in phagocytosis of NTHI. The authors implicate a compartmentalised defect in phagocytic capabilities of alveolar macrophages and propose this defect as instrumental for colonisation of pathogenic bacteria in disease.
This concept was challenged by the work of Taylor et al. [53] in GM-CSF differentiated monocyte-derived macrophages. This work demonstrated a phagocytosis defect in blood-derived macrophages from COPD patients versus blood-derived macrophages from non-smokers and smokers when respiratory pathogens were used as bait. Their work challenged the concept of compartmentalised defect proposed earlier and suggests a new hypothesis of an inherent defect even in the circulating precursor cells of the macrophages, thus identifying a need to clearly understand if in disease the precursor cells are affected.
Further work was subsequently initiated to understand the effects of smoking and disease [54]. In this study, the authors compared phagocytic defects in current smokers and ex-smokers with COPD, ex-smokers without COPD and non-smoker alveolar macrophages. In COPD alveolar macrophages a defect in clearance of AEC was seen irrespective of smoking status in comparison to never smoker-derived macrophages. Interestingly, they suggest an improvement in phagocytosis in ex-smokers with disease in comparison to current smokers with disease implicating a potential role for active smoking in suppressing phagocytosis. Expression of CD14 that is more highly expressed in peripheral monocytes (in comparison to alveolar macrophages) was increased in COPD current smokers and smokers without COPD, suggesting a role for replenishment of tissue resident macrophages by peripheral precursor cells. This was further supported by the increase in the cellular proliferation marker Ki67 in samples obtained from current smokers with and without COPD. In induced sputum samples obtained from COPD patients after exacerbation, an increased presence of ‘small’ alveolar macrophages versus control subjects were also reported [55]. Similar to the observations of Hodge et al., these small macrophages had increased expression of CD14 and HLA-DR markers versus large macrophages. These findings could imply an important role for peripheral precursor cells potentially in contributing to disease with their lower phagocytic capacity.
Expression of markers such as CD31, CD71, CD44 and CD91 were suppressed in current smoker macrophages with or without disease [54]. They were further inhibited by exposure of the cells to cigarette smoke extract confirming a direct impact of smoke on cell surface recognition molecules [54]. Interestingly, stratification of the COPD current smokers by disease severity did not show any differentiation between markers suggesting a stronger impact for smoke exposure rather than disease on the down regulation of these markers. Another important observation was that in ex-smokers with COPD, there was an apparent increase in phagocytic ability versus current smokers further strengthening the authors’ hypothesis of an acute effect of smoking. The effect of smoking on phagocytosis function is however not concordant with other reports [52], [53], [56]. In monocyte-derived macrophages obtained from COPD patients, a smoke-related effect on phagocytosis was not observed [53].
Furthermore, in a large cohort of COPD patients consisting of current and ex-smokers with the disease a differential effect of smoking was not apparent when a trigger of NTHI or Moraxella catarrhalis was used [56]. However, consistent with previous literature a clear phagocytic defect was apparent in mild to moderate COPD patients as opposed to healthy non-smokers. Regression analysis of the defect to COPD severity suggested a strong correlation between defect and disease [56], further strengthening a causal role for the defective function of these cells and disease. These numerous reports have established in BAL-derived alveolar macrophages defective phagocytosis of apoptotic epithelia and bacterial pathogens. This suggests different recognition mechanisms playing a role in regulating this defect. It is also unclear whether the defect established in alveolar macrophages is mirrored in other lung resident macrophages. Further studies dissecting the mechanism and potential drivers of this defect in alveolar macrophages/other lung resident macrophages are clearly required.
Implications of viral infections in exacerbations
Exacerbations in COPD are multifactorial. In addition to exacerbations attributed solely to pathogenic bacteria or viruses, bacterial and viral co-infections have also been linked with more severe exacerbations [57], [58], [59], [60]. Although the role of alveolar macrophages in mediating airway antiviral response has been overshadowed by airway epithelium, they are critical players in pathogen antiviral responses. In vitro exposure of alveolar macrophages to human rhinovirus (HRV) (implicated as key drivers of COPD exacerbations) have demonstrated a significant inhibition of the inflammatory response of these cells to either lipopolysaccharide (LPS) or lipoteichoic acid (LTA) challenge [61]. These cells also demonstrated an impaired clearance of bacteria but not inert beads. Furthermore, infectious rhinovirus and rhinoviral RNA were detected for up to a period of ten days post-infection, implicating a low level replication of the virus within these cells, potentially acting as viral sinks. The global effect of viral infection on inflammatory responses coupled with phagocytic defect and prolonged low grade viral infection could be considered as key drivers of exacerbations in COPD. In experimental infection of COPD patients with HRV, an increase in secondary bacterial infections were observed concurrent with a decrease in antimicrobial peptide production, implicating a possible direct effect of the virus on the innate immune system. This effect was not observed in smokers or non-smoker without COPD [62]. Further studies utilising experimental infection of COPD patients confirmed an increase in bacterial burden and an outgrowth of bacterial species already present in the lung [63], confirming an interplay between multiple pathogens in driving disease pathology. How respiratory viruses drive increased bacterial colonisation and “super-infections” still remain the source of much debate, but defective phagocytosis and clearance could be a key contributor.
Pharmacological restoration of phagocytosis
Macrophage phagocytosis dysfunction in COPD could be a key driver of disease pathophysiology and agents that could ‘restore’ their normal homeostatic functions could be beneficial in disease. We will be briefly discussing pharmacological agents reported to restore phagocytosis defect and their potential clinical utility.
Modulating cell surface receptor expression could be one way that these agents can affect phagocytosis. For example, azithromycin, a macrolide antibiotic, was found to restore COPD alveolar macrophage efferocytosis towards a range of targets [64], [65], [66] and to lead to increased surface expression of the mannose receptors both invitro and in a small cohort of COPD patients [67]. This effect on scavenger receptors expression highlights the pleiotrophic roles of macrolides like azithromycin that are currently in clinical use for COPD. Other modulators of phagocytosis include calcium that increases expression of CD16 and MARCO at the cell surface on COPD monocyte-derived macrophages [68] and Sulforaphane that is similarly described to increase the expression of MARCO [69].
There has been a lot of interest in looking if inhibiting downstream pathways of phagocytosis can improve macrophage function in COPD as these pathways can have different effects [70], [71]. Previous research as part of COPD MAP cohort demonstrated that inhibition of the individual subunits of PI3K or ROCK pathways did not alter phagocytosis or intracellular killing of bacteria by alveolar macrophages [72]. However ROCK inhibition reversed defective efferocytosis highlighting potential differences in pathways regulating bacterial phagocytosis versus efferocytosis [72]. The team of Sandra Hodge recently also described an effect for thymoquinone on improved macrophage efferocytosis and phagocytosis in COPD [73]. The mode of action of thymoquinone is unknown but data suggests it modulates the sphingosine 1 phosphate signalling system, which is critical for various macrophage functions [74]. This study was a follow up to previous work where they demonstrated procysteine improved macrophage efferocytosis presumably through increased availability of glutathione [75]. The clinical utility of these downstream pathway modulators remain to be established.
Conclusions
Defective phagocytic functions in COPD alveolar macrophages in the uptake and processing of respiratory pathogens and cellular debris have been conclusively proven. Although some conflicting data exists on the role of receptors regulating phagocytosis, there is no conclusive data on mechanism (s) regulating this defect, which highlights a gap in the current knowledge. Given the emerging importance of dysbiosis of the lung microbiome in disease, it is also important to consider the effect of multi-pathogen exposure on macrophage phagocytic function. Finally, it is yet unclear whether the defect described in the alveolar macrophage population is a global defect of all lung resident macrophages in disease and we should also consider immune cells in the context of their unique microenvironment in interaction with other cell types.
Conflicts of interest
NK is employed by the commercial company “AstraZeneca”, and AstraZeneca provided support in the form of salaries for JJ as part of a collaborative grant with FN. There are no patents, products in development or marketed products to declare.
Acknowledgements
Work in the laboratory of FN is supported by the French National Center for Scientific Research (CNRS), the National Institute for Health and Medical Research (INSERM), Université Paris Descartes and a collaborative grant with AstraZeneca/Inserm Transfert.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Garbi N., Lambrecht B.N. Location, function, and ontogeny of pulmonary macrophages during the steady state. Pflugers Arch. 2017;469:561–572. doi: 10.1007/s00424-017-1965-3. [DOI] [PubMed] [Google Scholar]
- 2.Kopf M., Schneider C., Nobs S.P. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16:36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 3.Baharom F., Rankin G., Blomberg A., Smed-Sorensen A. Human lung mononuclear phagocytes in health and disease. Front Immunol. 2017;8:499. doi: 10.3389/fimmu.2017.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balhara J., Gounni A.S. The alveolar macrophages in asthma: a double-edged sword. Mucosal Immunol. 2012;5:605–609. doi: 10.1038/mi.2012.74. [DOI] [PubMed] [Google Scholar]
- 5.Desch A.N., Gibbings S.L., Goyal R., Kolde R., Bednarek J., Bruno T. Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung-draining lymph nodes. Am J Respir Crit care Med. 2016;193:614–626. doi: 10.1164/rccm.201507-1376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flannagan R.S., Jaumouille V., Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. 2012;7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 7.Niedergang F. Phagocytosis. In: Bradshaw Ralph A., Stahl Philip D., editors. vol. 2. Academic Press; Waltham, MA: 2016. pp. 751–757. (Encyclopedia of cell biology). [Google Scholar]
- 8.Swanson J.A. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown M.S., Goldstein J.L., Krieger M., Ho Y.K., Anderson R.G. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol. 1979;82:597–613. doi: 10.1083/jcb.82.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canton J., Neculai D., Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 11.Prabhudas M., Bowdish D., Drickamer K., Febbraio M., Herz J., Kobzik L. Standardizing scavenger receptor nomenclature. J Immunol. 2014;192:1997–2006. doi: 10.4049/jimmunol.1490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groves E., Dart A.E., Covarelli V., Caron E. Molecular mechanisms of phagocytic uptake in mammalian cells. Cell Mol Life Sci. 2008;65:1957–1976. doi: 10.1007/s00018-008-7578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daeron M. Fc receptors as adaptive immunoreceptors. Curr Top Microbiol Immunol. 2014;382:131–164. doi: 10.1007/978-3-319-07911-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzer-Attas C.J., Lowry M., Crowley M.T., Finn A.J., Meng F., DeFranco A.L. Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191:669–682. doi: 10.1084/jem.191.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowley M.T., Costello P.S., Fitzer-Attas C.J., Turner M., Meng F., Lowell C. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaumouille V., Farkash Y., Jaqaman K., Das R., Lowell C.A., Grinstein S. Actin cytoskeleton reorganization by syk regulates fcgamma receptor responsiveness by increasing its lateral mobility and clustering. Dev Cell. 2014;29:534–546. doi: 10.1016/j.devcel.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodridge H.S., Reyes C.N., Becker C.A., Katsumoto T.R., Ma J., Wolf A.J. Activation of the innate immune receptor Dectin-1 upon formation of a 'phagocytic synapse'. Nature. 2011;472:471–475. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng Z., Ma S., Zhou H., Zang A., Fang Y., Li T. Tyrosine phosphatase SHP-2 mediates C-type lectin receptor-induced activation of the kinase Syk and anti-fungal TH17 responses. Nat Immunol. 2015;16:642–652. doi: 10.1038/ni.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caron E., Self A.J., Hall A. The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr Biol. 2000;10:974–978. doi: 10.1016/s0960-9822(00)00641-2. [DOI] [PubMed] [Google Scholar]
- 20.Dupuy A.G., Caron E. Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci. 2008;121:1773–1783. doi: 10.1242/jcs.018036. [DOI] [PubMed] [Google Scholar]
- 21.Liu S., Calderwood D.A., Ginsberg M.H. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113:3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- 22.Freeman S.A., Goyette J., Furuya W., Woods E.C., Bertozzi C.R., Bergmeier W. Integrins form an expanding diffusional barrier that coordinates phagocytosis. Cell. 2016;164:128–140. doi: 10.1016/j.cell.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jongstra-Bilen J., Harrison R., Grinstein S. Fcgamma-receptors induce Mac-1 (CD11b/CD18) mobilization and accumulation in the phagocytic cup for optimal phagocytosis. J Biol Chem. 2003;278:45720–45729. doi: 10.1074/jbc.M303704200. [DOI] [PubMed] [Google Scholar]
- 24.Freeman S.A., Grinstein S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev. 2014;262:193–215. doi: 10.1111/imr.12212. [DOI] [PubMed] [Google Scholar]
- 25.Colucci-Guyon E., Niedergang F., Wallar B.J., Peng J., Alberts A.S., Chavrier P. A role for mammalian diaphanous-related formins in complement receptor (CR3)-mediated phagocytosis in macrophages. Curr Biol. 2005;15:2007–2012. doi: 10.1016/j.cub.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 26.Marie-Anais F., Mazzolini J., Herit F., Niedergang F. Dynamin-actin cross talk contributes to phagosome formation and closure. Traffic. 2016;17:487–499. doi: 10.1111/tra.12386. [DOI] [PubMed] [Google Scholar]
- 27.Vieira O.V., Bucci C., Harrison R.E., Trimble W.S., Lanzetti L., Gruenberg J. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol. 2003;23:2501–2514. doi: 10.1128/MCB.23.7.2501-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinchen J.M., Ravichandran K.S. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature. 2010;464:778–782. doi: 10.1038/nature08853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T., Ming Z., Xiaochun W., Hong W. Rab7: role of its protein interaction cascades in endo-lysosomal traffic. Cell Signal. 2011;23:516–521. doi: 10.1016/j.cellsig.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Rink J., Ghigo E., Kalaidzidis Y., Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 31.Harrison R.E., Bucci C., Vieira O.V., Schroer T.A., Grinstein S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol Cell Biol. 2003;23:6494–6506. doi: 10.1128/MCB.23.18.6494-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson M., Rocha N., Zwart W., Jordens I., Janssen L., Kuijl C. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumas A., Le-Bury G., Marie-Anais F., Herit F., Mazzolini J., Guilbert T. The HIV-1 protein Vpr impairs phagosome maturation by controlling microtubule-dependent trafficking. J Cell Biol. 2015;211:359–372. doi: 10.1083/jcb.201503124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flannagan R.S., Cosio G., Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 35.Aschenbrenner A.C., Schultze J.L. New ‘programmers’ in tissue macrophage activation. Pflugers Arch. 2017;469:375–383. doi: 10.1007/s00424-017-1943-9. [DOI] [PubMed] [Google Scholar]
- 36.Hume D.A., Wells C.A., Ravasi T. Transcriptional regulatory networks in macrophages. Novartis Found Symp. 2007;281:2–18. doi: 10.1002/9780470062128.ch2. discussion -24, 50–3, 208–9. [DOI] [PubMed] [Google Scholar]
- 37.Medzhitov R., Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 38.Wells C.A., Ravasi T., Hume D.A. Inflammation suppressor genes: please switch out all the lights. J Leukoc Biol. 2005;78:9–13. doi: 10.1189/jlb.1204710. [DOI] [PubMed] [Google Scholar]
- 39.Hussell T., Bell T.J. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 40.Dodd C.E., Pyle C.J., Glowinski R., Rajaram M.V., Schlesinger L.S. CD36-mediated uptake of surfactant lipids by human macrophages promotes intracellular growth of Mycobacterium tuberculosis. J Immunol. 2016;197:4727–4735. doi: 10.4049/jimmunol.1600856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brennan S., Sly P.D., Gangell C.L., Sturges N., Winfield K., Wikstrom M. Alveolar macrophages and CC chemokines are increased in children with cystic fibrosis. Eur Respir J. 2009;34:655–661. doi: 10.1183/09031936.00178508. [DOI] [PubMed] [Google Scholar]
- 42.Liang Z., Ni R., Zhou J., Mao S. Recent advances in controlled pulmonary drug delivery. Drug Discov Today. 2015;20:380–389. doi: 10.1016/j.drudis.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro S.D. The macrophage in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S29–S32. doi: 10.1164/ajrccm.160.supplement_1.9. [DOI] [PubMed] [Google Scholar]
- 44.Finkelstein R., Fraser R.S., Ghezzo H., Cosio M.G. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med. 1995;152:1666–1672. doi: 10.1164/ajrccm.152.5.7582312. [DOI] [PubMed] [Google Scholar]
- 45.Hogg J.C., Chu F., Utokaparch S., Woods R., Elliott W.M., Buzatu L. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z., Bafadhel M., Haldar K., Spivak A., Mayhew D., Miller B.E. Lung microbiome dynamics in COPD exacerbations. Eur Respir J. 2016;47:1082–1092. doi: 10.1183/13993003.01406-2015. [DOI] [PubMed] [Google Scholar]
- 47.Cosio M.G., Saetta M., Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 48.Mannino D.M., Buist A.S. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 49.Rennard S.I., Farmer S.G. Exacerbations and progression of disease in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:88–92. doi: 10.1513/pats.2306026. [DOI] [PubMed] [Google Scholar]
- 50.Wedzicha J.A., Seemungal T.A. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodge S., Hodge G., Scicchitano R., Reynolds P.N., Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol. 2003;81:289–296. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- 52.Berenson C.S., Garlipp M.A., Grove L.J., Maloney J., Sethi S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis. 2006;194:1375–1384. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 53.Taylor A.E., Finney-Hayward T.K., Quint J.K., Thomas C.M., Tudhope S.J., Wedzicha J.A. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35:1039–1047. doi: 10.1183/09031936.00036709. [DOI] [PubMed] [Google Scholar]
- 54.Hodge S., Hodge G., Ahern J., Jersmann H., Holmes M., Reynolds P.N. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am J Respir cell Mol Biol. 2007;37:748–755. doi: 10.1165/rcmb.2007-0025OC. [DOI] [PubMed] [Google Scholar]
- 55.Frankenberger M., Menzel M., Betz R., Kassner G., Weber N., Kohlhaufl M. Characterization of a population of small macrophages in induced sputum of patients with chronic obstructive pulmonary disease and healthy volunteers. Clin Exp Immunol. 2004;138:507–516. doi: 10.1111/j.1365-2249.2004.02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berenson C.S., Kruzel R.L., Eberhardt E., Sethi S. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis. 2013;208:2036–2045. doi: 10.1093/infdis/jit400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papi A., Bellettato C.M., Braccioni F., Romagnoli M., Casolari P., Caramori G. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 58.Bafadhel M., McKenna S., Terry S., Mistry V., Reid C., Haldar P. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 59.MacDonald M., Korman T., King P., Hamza K., Bardin P. Exacerbation phenotyping in chronic obstructive pulmonary disease. Respirology. 2013;18:1280–1281. doi: 10.1111/resp.12197. [DOI] [PubMed] [Google Scholar]
- 60.Wilkinson T.M., Hurst J.R., Perera W.R., Wilks M., Donaldson G.C., Wedzicha J.A. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;129:317–324. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oliver B.G., Lim S., Wark P., Laza-Stanca V., King N., Black J.L. Rhinovirus exposure impairs immune responses to bacterial products in human alveolar macrophages. Thorax. 2008;63:519–525. doi: 10.1136/thx.2007.081752. [DOI] [PubMed] [Google Scholar]
- 62.Mallia P., Footitt J., Sotero R., Jepson A., Contoli M., Trujillo-Torralbo M.B. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:1117–1124. doi: 10.1164/rccm.201205-0806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molyneaux P.L., Mallia P., Cox M.J., Footitt J., Willis-Owen S.A., Homola D. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:1224–1231. doi: 10.1164/rccm.201302-0341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hodge S., Hodge G., Brozyna S., Jersmann H., Holmes M., Reynolds P.N. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur Respir J. 2006;28:486–495. doi: 10.1183/09031936.06.00001506. [DOI] [PubMed] [Google Scholar]
- 65.Xu G., Fujita J., Negayama K., Yuube K., Hojo S., Yamaji Y. Effect of macrolide antibiotics on macrophage functions. Microbiol Immunol. 1996;40:473–479. doi: 10.1111/j.1348-0421.1996.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 66.Yamaryo T., Oishi K., Yoshimine H., Tsuchihashi Y., Matsushima K., Nagatake T. Fourteen-member macrolides promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrob Agents Chemother. 2003;47:48–53. doi: 10.1128/AAC.47.1.48-53.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodge S., Hodge G., Jersmann H., Matthews G., Ahern J., Holmes M. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:139–148. doi: 10.1164/rccm.200711-1666OC. [DOI] [PubMed] [Google Scholar]
- 68.Provost K.A., Smith M., Arold S.P., Hava D.L., Sethi S. Calcium restores the macrophage response to nontypeable haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2015;52:728–737. doi: 10.1165/rcmb.2014-0172OC. [DOI] [PubMed] [Google Scholar]
- 69.Harvey C.J., Thimmulappa R.K., Sethi S., Kong X., Yarmus L., Brown R.H. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med. 2011;3:78ra32. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henry P.J., Mann T.S., Goldie R.G. A rho kinase inhibitor, Y-27632 inhibits pulmonary eosinophilia, bronchoconstriction and airways hyperresponsiveness in allergic mice. Pulm Pharmacol Ther. 2005;18:67–74. doi: 10.1016/j.pupt.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 71.To Y., Ito K., Kizawa Y., Failla M., Ito M., Kusama T. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:897–904. doi: 10.1164/rccm.200906-0937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bewley M.A., Belchamber K.B., Chana K.K., Budd R.C., Donaldson G., Wedzicha J.A. Differential effects of p38, MAPK, PI3K or rho kinase inhibitors on bacterial phagocytosis and efferocytosis by macrophages in COPD. PLoS One. 2016;11:e0163139. doi: 10.1371/journal.pone.0163139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barnawi J., Tran H.B., Roscioli E., Hodge G., Jersmann H., Haberberger R. Pro-phagocytic effects of thymoquinone on cigarette smoke-exposed macrophages occur by modulation of the sphingosine-1-phosphate signalling system. COPD. 2016;13:653–661. doi: 10.3109/15412555.2016.1153614. [DOI] [PubMed] [Google Scholar]
- 74.Rivera J., Proia R.L., Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hodge S., Matthews G., Mukaro V., Ahern J., Shivam A., Hodge G. Cigarette smoke-induced changes to alveolar macrophage phenotype and function are improved by treatment with procysteine. Am J Respir Cell Mol Biol. 2011;44:673–681. doi: 10.1165/rcmb.2009-0459OC. [DOI] [PubMed] [Google Scholar]