Abstract
Pyroptosis is a lytic type of programmed cell death that was traditionally associated with the involvement of inflammatory caspases, such as caspase-1. These inflammatory caspases are activated within multi-protein complexes called inflammasomes that are assembled in response to invading pathogens and/or danger signals. Pyroptotic cell death was suggested to evolve via the formation of pores in the plasma membrane, but the exact mechanism underlying the formation of these pores remained unclear. Recently, gasdermin D, a member of the gasdermin protein family was identified as a caspase substrate and essential effector of pyroptosis, being identified as the protagonist of membrane pore formation. Gasdermins have emerged as a family of new class of cell death inducers, but many questions remain unanswered. Here, we present an overview of recent work being done in the area of programmed cell death and the latest evidence regarding the role and participation of gasdermin D as an effector of pyroptosis.
Keywords: Pyroptosis, Apoptosis, Inflammasome, Gasdermin
Many ways of cell death
Pyroptosis is an inflammatory, caspase-dependent (caspase-1 or caspase-4/-5/-11) way of cell death, initiated by the formation of the so-called inflammasome in response to infection or danger signals. This form of death occurs mainly in innate immune cells, such as monocytes and macrophages and is characterized by swelling and ultimately, lysis of the cell. Inflammatory caspases, including caspase-1, -4, -5 and -11, are crucial mediators of inflammation and cell death. Caspase-1 is found in humans and mice. Caspase-4 and -5 are found in humans and the orthologue caspase-11 is found in mice. Inflammatory caspases form part of this dynamic multi-protein complex known as the inflammasome, which orchestrates proteolytic processing of the classic pro-inflammatory cytokines IL-1β and IL-18. The exact molecular mechanisms by which cell swelling, and hence pyroptosis, occur were unknown until recently [1]. Chen et al., 2016 recently revealed morphological differences between pyroptosis and necroptosis, and provided mechanistic explanations for these differences. This study showed that necroptotic cell death is a process involving cell explosion, whereas pyroptotic cells undergo cytoplasm flattening caused by plasma membrane leakage [2].
Although both necroptosis and pyroptosis display plasma membrane disruption which is clearly different from apoptosis (known as a tightly regulated non-lytic and non-inflammatory form of programmed cell death), the morphologies of cells undergoing necroptosis and pyroptosis were shown to be different from each other. Both necroptosis and pyroptosis require oligomerization and translocation of their executor proteins to the plasma membrane. The translocation of their executor protein, Mixed Lineage Kinase Domain Like Pseudokinase (MLKL) and gasdermin D (GSDMD), respectively, to the plasma membrane was required for cell death. However, MLKL forms ion selective channels, whereas GSDMD forms pores that lack ion selectivity. These GSDMD pores are 10–20 nm in size, and formation of these pores in cells drives pyroptosis [3], [4], [5]. These mechanistic differences determine the morphological differences between necroptosis and pyroptosis; and the different ways of plasma membrane rupture suggest that the in vivo functions of necroptosis and pyroptosis can also be different [2].
Activation of inflammasomes often requires a priming signal induced by activation of Toll-like receptors (TLRs). Different subsets of inflammasomes contain different cytosolic pattern-recognition receptors and their assembly is initiated by the sequence of first and second triggers.
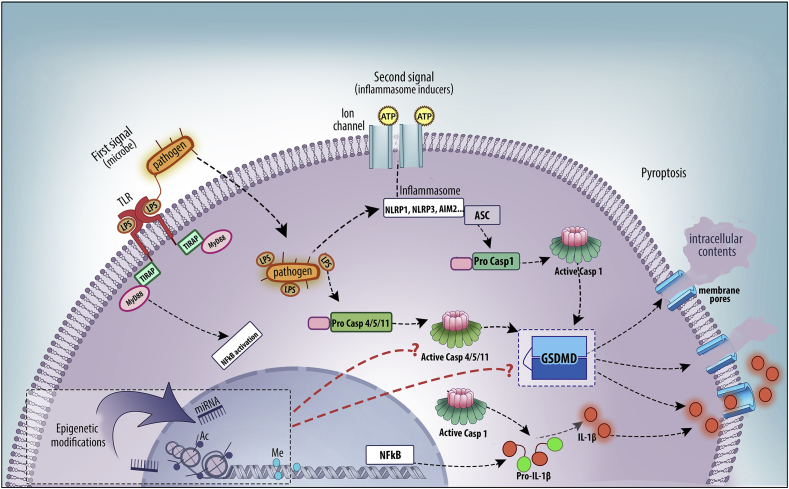
Usually, the first signal may represent a pathogen-associated molecular pattern (PAMP) such as LPS which binds to a TLR, leading to activation of NF-kB and expression of pro-IL-1β and pro-IL-18. The second signal may consist of a DAMP such as ATP which is released from damaged or stressed cells [Fig. 1]. Also, inflammasome complex formation, activation of caspase-1, and cleavage and secretion of active IL-1β and IL-18 happen not only in response to tissue injury or metabolic perturbations but also following contact with microbes and environmental toxins. For this reason, inflammasome activation has been linked with a wide range of diseases associated with chronic inflammation (e.g., atherosclerosis, cancer, infection, obesity, and type 2 diabetes), which have been reviewed elsewhere [6], [7]. Plasma membrane permeabilization is a hallmark of cell death. In this context, pyroptosis is an inflammatory form of programmed cell death that occurs in response to microbial products in the cytoplasm or to cellular perturbations caused by diverse stimuli, including crystalline substances, toxins, and extracellular ATP [8], [9]. Pyroptosis also plays a critical role in the clearance of intracellular bacteria [10], but may also contribute to autoinflammatory and autoimmune disease pathology. According to Shi et al. [11], for a long time, pyroptosis was suggested to be an auxiliary event to IL-1β secretion, a critical inflammatory response in monocytes.
Fig. 1.
Summary of Gasdermin D participation in Caspases-induced pyroptosis. The first signal for inflammasome activation may represent a PAMP such as LPS which binds to a TLR, leading to activation of NF-kB and expression of pro-IL-1β. The second signal may consist of a DAMP such as ATP which is released from damaged or stressed cells. The canonical inflammasome sensors (e.g., NLRP1, NLRP3, AIM2…) can detect several microbial signals and activate caspase-1 through the ASC adaptor. Caspase-4, 5, and 11 are activated by direct binding to LPS. Active caspase-1 and caspase-4/5/11 cleave GSDMD which is able to generate membrane pores allowing extravasation of intracellular contents and secretion of mature IL-1β. There is a lack of information about how epigenetic modifications such as DNA methylation, acetylation or the action of micro-RNAs could regulate the function of GSDMD or caspases 1/4/5/11. Abbreviations: TLR: Toll-like receptor; TIRAP: TIR domain containing adaptor protein; MyD88: myeloid differentiation primary response 88; LPS: lipopolysaccharide; ATP: Adenosine triphosphate; NFκB: nuclear factor kappa B; NLRP1: NLR family pyrin domain containing 1; NLRP3: NLR family pyrin domain containing 3; AIM2: Absent in melanoma 2; ASC: adaptor apoptosis associated speck-like proteins containing a CARD; Casp: Caspase; GSDMD: gasdermin D; IL: interleukin; Ac: Acetylation; Me: Methylation; miRNA: micro-RNA.
Characterization of various inflammasomes has established the paramount importance of caspase-1 in innate immune defenses. The discovery of caspase-11 and caspase-4/5 function has expanded the notion of pyroptosis mediators from caspase-1 to the inflammatory caspase group, which also reveals that pyroptosis is not limited to monocytic cells. In fact, Zhu et al. [12] mechanistically characterized the NLR Nlrp9b inflammasome that is specifically expressed in intestinal epithelial cells. This work demonstrated that conditional depletion of Nlrp9b or other inflammasome components in the intestine in vivo resulted in enhanced susceptibility of mice to rotavirus replication [12] and highlighted an important immune signalling pathway that functions in intestinal epithelial cells. Alternative functions of the inflammasomes have been described in a recent mini-review, including the role of non-canonical inflammasome activation and GSDMD in pyroptosis [13]. In this recent review, authors emphasized the importance of the inflammasome complex formation in many physiological processes which extend beyond modulation of inflammation, such as autophagy, metabolism, eicosanoids production and phagosome maturation [13].
The role of gasdermin D as a potent substrate and effector of pyroptosis
In 2015, Shi et al. [14] and Kayagaki et al. [15] independently discovered that the orphan protein gasdermin D (GSDMD) was the central mediator of pyroptotic cell death downstream of both caspase-1 and caspase-11. It was shown that GSDMD is a substrate of caspase-1 and that its cleavage at the predicted site during inflammasome activation was required for pyroptosis and IL-1β secretion [16]. The two groups also found that GSDMD is cleaved by these caspases into a 31 kDa N-terminal fragment (GSDMD-Nterm) and a 22 kDa C-terminal fragment (GSDMD-Cterm), and that the N-terminus by itself had the ability to induce pyroptosis when expressed ectopically. In other words, they revealed that proteolytic cleavage of Gsdmd by mouse caspase-11 or human caspase-4 is essential for pyroptosis of innate immune cells and endothelial cells harbouring LPS-tainted cytoplasm. Cleaved Gsdmd also triggers NLRP3-dependent activation of caspase-1 through a cell-intrinsic pathway. At this point, the exact function of the p31 fragment was not clear yet. In fact, Kayagaki et al., 2015 showed in vitro and in vivo that caspase-11 relies exclusively on Gsdmd to promote pyroptosis, caspase-1 activation and LPS-induced lethal sepsis. Their robust data on Gram-negative bacteria confirmed GsdmD as a critical target of caspase-11 and a key mediator of the host response against Gram-negative bacteria [15].
Aglietti RA et al., 2016 then showed that human GsdmD p30 forms functional pores within membranes and they discussed that IL-1β and IL-18 could exit the pyroptotic cell through GsdmD p30 pores even before catastrophic membrane rupture by osmotic lysis. Precisely how GsdmD p30 triggers pyroptosis has not been established [3]. This work demonstrated that the human GsdmD p30 fragment liberated by active caspase-11 forms ring-like structures within membranes that function as pores. This work elegantly proposed that p30 subunit kills cells by directly compromising the integrity of cellular membranes. The resulting imbalance would elicit cell lysis and release of intracellular components that can alert other immune cells to the threat of infection. Also, another work showed that the N-terminal fragment of GSDMD can actually form pores with average diameters of 21 nm in artificial liposomes, as well as large pores in the plasma membrane of cells [5]. They also confirmed that the formation of the GSDMD pore does not require other proteins as co-factors or membrane receptors, demonstrating that GSDMD-Nterm is the sole and final executor of pyroptotic cell death [5]. Interestingly, Wright et al., 2016 suggested that the N-terminus of GSDMD is an effective killer of eukaryotic and, potentially, prokaryotic cells, although it is not clear whether it is able to kill intracellular bacteria directly, or whether this is a consequence of host cell death. Nonetheless, they suggest that gasdermin-derived peptides could be potentially developed as antimicrobial agents [1].
It is important to mention that caspase-1 appears to only partly require GSDMD for pyroptosis induction, because the canonical inflammasome activators can still trigger caspase-1-dependent plasma membrane damage in Gsdmd knockout macrophages [17]. Pyroptosis is simply delayed, and not totally abolished. These observations suggest that caspase-11 activation engages a noncanonical inflammasome that requires GSDMD, while caspase-1 canonical inflammasome activation targets redundant death executioners that merely include GSDMD. This distinction between the canonical and non-canonical inflammasomes is consistent with the observation that GSDMD and caspase-11 only occur in mammals, while caspase-1 is conserved among all vertebrates, as recently reviewed by Aglieti et al. [17]. The role of GSDMD was also recently investigated by Lei X et al. [18] in a model of Enterovirus infection (EV71). In this interesting work, authors reported EV71 infection decreases GSDMD expression and that protease 3C of enterovirus 71 cleaves GSDMD and generates a 20-kDa NH2 fragment which is unable to trigger pyroptosis [18]. These results revealed the ability of this enterovirus to evade the antiviral response through cleavage of GSDMD.
Recently, Taabazuing et al. [19] proposed a bidirectional model of crosstalk between pyroptosis and apoptosis in monocytes and macrophages. They found that in the absence of GSDMD, caspase-1 activates caspase-3 and -7 and induces apoptosis, confirming that GSDMD is the only caspase-1 substrate that induces pyroptosis. They also demonstrated that during apoptosis, caspase-3/-7 specifically block pyroptosis by cleaving GSDMD at a distinct site from the inflammatory caspases that inactivates the protein. There is no doubt that the discovery of GSDMD as the pyroptotic substrate of caspase-1/4/5/11 redefined pyroptosis as gasdermin-mediated programmed lytic cell death or programmed necrosis. However, it seems that pyroptosis mediated by other members of the gasdermin family could also play an important role, as recently shown in a model of chemotherapy drug-induced toxicity [20]. In this referred work, GSDME was shown to be cleaved and activated specifically by caspase-3 and cause pyroptosis. This was suggested to change the paradigm of understanding programmed cell death as caspase-3 is long regarded as the hallmark of apoptosis. Indeed, it was proposed that the expression level of GSDME would determine the form of cell death in caspase-3- activated cells. While GSDME-high cells should undergo pyroptosis upon “apoptotic stimulation” like chemotherapy drug, cells lacking sufficient GSDME develop secondary necrosis after apoptosis [20]. The physiological function of other gasdermins is largely unknown and was recently reviewed elsewhere [11].
Concluding remarks
Gasdermins have emerged as a family of new class of cell death inducers, but many questions remain unanswered. For example, it is still unclear how other family members beside GSDMD are activated and how this is regulated at the post-translation level. Furthermore, if and how these proteins are implicated in physiological and pathological cell death pathways, will be extremely important to address [21]. The recent advances regarding pyroptosis have now firmly established the sequence of events leading to this lytic form of cell death. The proinflammatory caspase-11 (caspases-4 and -5 in humans) is activated upon sensing of cytosolic LPS to cleave GSDMD. Liberation of the N-terminal domain of GSDMD causes GSDMD-N to relocate to the plasma membrane, therefore producing large oligomeric pores that lead to cell death. The challenge for the future is to understand the molecular details of each GSDM protein, and how those details fit into the larger context of programmed cell death. Also, there is a lack of information about how epigenetic modifications such as DNA methylation, acetylation or the action of micro-RNAs could regulate the function of GSDM proteins or caspases 1/4/5/11. Fig. 1 summarizes the main points discussed in this review regarding the participation of GSDMD in caspases-induced pyroptosis. The physiological relevance of GSDMD and pyroptosis should be examined further in a range of diseases, including cancer and inflammatory conditions.
Conflicts of interest
The authors declare that there are no conflicts of interest related to this study.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Wright J.A., Bryant C.E. The killer protein gasdermin D. Cell Death Differ. 2016;23:1897–1898. doi: 10.1038/cdd.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X., He W.T., Hu L., Li J., Fang Y., Wang X. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007–1020. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aglietti R.A., Estevez A., Gupta A., Ramirez M.G., Liu P.S., Kayagaki N. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sborgi L., Ruhl S., Mulvihill E., Pipercevic J., Heilig R., Stahlberg H. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamkanfi M., Dixit V.M. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 7.Strowig T., Henao-Mejia J., Elinav E., Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 8.Cookson B.T., Brennan M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 9.Lamkanfi M., Dixit V.M. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Miao E.A., Leaf I.A., Treuting P.M., Mao D.P., Dors M., Sarkar A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi J., Gao W., Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Zhu S., Ding S., Wang P., Wei Z., Pan W., Palm N.W. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature. 2017;546:667–670. doi: 10.1038/nature22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martel J., Lai H.C., Ko Y.F., Young J.D., Ojcius D.M. Alternative functions for the multifarious inflammasome. Biomed J. 2016;39:183–187. doi: 10.1016/j.bj.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 15.Kayagaki N., Stowe I.B., Lee B.L., O'Rourke K., Anderson K., Warming S. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 16.He W.T., Wan H., Hu L., Chen P., Wang X., Huang Z. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aglietti R.A., Dueber E.C. Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions. Trends Immunol. 2017;38:261–271. doi: 10.1016/j.it.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Lei X., Zhang Z., Xiao X., Qi J., He B., Wang J. Enterovirus 71 inhibits pyroptosis through cleavage of gasdermin D. J Virol. 2017;91 doi: 10.1128/JVI.01069-17. e01069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taabazuing C.Y., Okondo M.C., Bachovchin D.A. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol. 2017;24:507–514. doi: 10.1016/j.chembiol.2017.03.009. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Gao W., Shi X., Ding J., Liu W., He H. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 21.Ruhl S., Broz P. The gasdermin-D pore: executor of pyroptotic cell death. Oncotarget. 2016;7:57481–57482. doi: 10.18632/oncotarget.11421. [DOI] [PMC free article] [PubMed] [Google Scholar]