Abstract
Alzheimer's Disease (AD) is a chronic neurodegenerative disorder and the most common type of dementia (60–80% of cases). In 2016, nearly 44 million people were affected by AD or related dementia. AD is characterized by progressive neuronal damages leading to subtle and latter obvious decline in cognitive functions including symptoms such as memory loss or confusion, which ultimately require full-time medical care. Its neuropathology is defined by the extracellular accumulation of amyloid-β (Aβ) peptide into amyloid plaques, and intraneuronal neurofibrillary tangles (NFT) consisting of aggregated hyper- and abnormal phosphorylation of tau protein. The latter, identified also as Tau pathology, is observed in a broad spectrum of neurological diseases commonly referred to as “Tauopathies”. Besides these lesions, sustained neuroinflammatory processes occur, involving notably micro- and astro-glial activation, which contribute to disease progression. Recent findings from genome wide association studies further support an instrumental role of neuroinflammation. While the interconnections existing between this innate immune response and the amyloid pathogenesis are widely characterized and described as complex, elaborated and evolving, only few studies focused on Tau pathology. An adaptive immune response takes place conjointly during the disease course, as indicated by the presence of vascular and parenchymal T-cell in AD patients' brain. The underlying mechanisms of this infiltration and its consequences with regards to Tau pathology remain understudied so far. In the present review, we highlight the interplays existing between Tau pathology and the innate/adaptive immune responses.
Keywords: Alzheimer's disease, Tau, Inflammation, Glia, Astrocytes, Microglia
Tau: from the gene to the protein, an overview
Tau belongs to the family of microtubule-associated proteins (MAP) and is mainly expressed by neurons with a preferential axonal localization [1]. The gene mapt encoding Tau protein is located at locus 17q21, contains 16 exons and can undergo an alternative splicing of the exons 2, 3 and 10 in human brain, generating 6 major isoforms. Depending on the inclusion of the exon 10, the C-terminal microtubule-binding region (MBR) of Tau contains 3 or 4 repeat motifs (3R and 4R tau), ensuring the assembly and stabilization of axonal microtubules through their interaction with heterodimers of α- and β-tubulin. Tau was observed in vitro to promote tubulin polymerization and decrease the rate of transition between growing and shrinking phases, also called catastrophe, generating a stable but still dynamic state in microtubules [2], [3]. Although a large proportion of Tau is located in the axons, a small amount is physiologically distributed in dendrites. The postsynaptic function of Tau remains ill-defined but it may be implicated in synaptic plasticity [4], [5], [6], [7], [8]. Besides axons and dendrites, a nuclear function of Tau has been discovered [9]. Nuclear Tau may regulate transcriptional activity and maintain DNA/RNA integrity under physiological and stress conditions [10], [11], [12]. Recent data also emphasize the role of Tau as a signaling molecule, owing to a large number of protein partners [13]. For instance, the ability of Tau to regulate brain insulin pathway was observed through a direct interaction and tonic inhibition of the phosphatase PTEN [14].
Tau hyperphosphorylation
Cellular functions of Tau and interactions with its protein partners are impacted by multiple post-translational modifications (PTMs) including acetylation, glycation, glycosylation, methylation, nitration, truncation, ubiquitination and phosphorylation, the most commonly described [15], [16]. Tau contains 85 putative phosphorylation sites mainly located in the MBR and the proline-rich domain of the protein [17], [18]. Tau phosphorylation state is under the control of many serine/threonine or tyrosine kinases as well as phosphatases; this homeostasis is disrupted in tauopathies favoring Tau hyper-phosphorylation [19], [20]. Using mass spectroscopy or phospho-specific tau antibodies, an extensive listing of tau phosphorylation sites was obtained, some of being restricted to pathological conditions [16], [17]. Interestingly, tau phosphorylation in Alzheimer's Disease (AD) can be viewed as a hierarchical process: some sites are phosphorylated earlier in the disease course generating structural changes promoting the action of secondary kinases and the formation of conformational epitopes. For instance, the epitopes detected by the antibody AT100 and recognizing paired-helical filaments (PHF) was shown to result from a sequential phosphorylation by GSK3-β and PKA at Thr212 and Ser214, in addition to Ser199, Ser202 and Thr205 phosphorylation (AT8 epitope, redefined recently and including also Ser208) [[21], [22], [23]]. Truncation at Asp may facilitate the transition from a natural highly soluble to differential aggregated forms of Tau (oligomers, pre-tangle, tangles), generating the late conformational epitopes AT-100 or Alz50 [24], [25], [26] Tau phosphorylation can generate epitopes recognized by immune cells as it will be discussed further. Expression of Tau by microglial cells themselves was also shown to promote their activation [27]. Together, the exact cascade leading to Tau phosphorylation remains ill-defined but subsequent structural changes induce its detachment from microtubules and produce higher levels of soluble free tau. Appearing prior to the formation of NFT [28], Tau hyper-phosphorylation favors a dynamic and progressive self-assembly of Tau into oligomeric forms and insoluble materials as PHF along the disease with different degree of neurotoxicity.
Tau species-driven neurotoxicity
The identification of Tau species responsible for neurotoxicity is still a matter of debate. Post-mortem studies showed that density of NFTs was correlated with cognitive impairments characterizing AD patients [29], [30]. Recently, imaging studies using selective Tau Positron Emission Tomography (PET) tracers replicate the spreading of pathologic Tau along the disease as defined by Braak stages and observed as well a positive correlation between aggregated Tau and cognitive decline, suggesting a toxic function of insoluble Tau [31], [32]. NFT are not inert end products but may be directly detrimental per se by disrupting cell metabolism, like proteasome activity as observed in vitro using HEK293 cell line transfected with human Tau [33]. In addition, PHF-Tau isolated from AD brains interacts with the 20S-subunit of the proteasome and inhibits its activity [34]. The decline of proteasome activity by NFT may lead to an abnormal accumulation of proteins and initiates a cascade of events ending by neuronal death [35]. Post-synaptic redistribution of pathologic Tau as observed in AD can be involved in neurotoxicity as well. In that view, dendritic Tau was observed in vivo to interact with Fyn and mediates amyloid-β toxicity through a Fyn/NMDA receptors (NR)/PSD95 coupling responsible of excitotoxicity [5]. Pathological aggregation of Tau reduces the level of native soluble Tau and consequently its physiological functions, inducing indirectly detrimental effects. Therefore, interactions of Tau with partners are compromised, disrupting microtubule network and axonal transport, RNA/DNA integrity or cell signaling. Also, brain insulin signaling impairments as observed in AD could be explained by a loss of function of Tau [14]. Other studies however revealed that NFT are not a central element of the neurotoxic cascade in comparison with soluble oligomeric Tau. Indeed, using the mouse model of Tauopathy rTg4510, which reversibly expresses the human Tau with P301L mutation that cause inherited frontotemporal dementia, it was found a regional dissociation between neuronal loss and NFT accumulation; suppressing the transgene restored memory formation and stabilized neuron numbers without affecting the accumulation of NFTs [36], [37].
Tau secretion
Regardless Tau species driving neurotoxicity, the increase of extracellular cerebrospinal fluid (CSF)-Tau in AD patients was accepted for a long time to be the consequence of a passive release of pathologic Tau from dead neurons generating ghost tangles, even if Tau is also found at low levels in CSF of healthy individuals [38]. However, compelling observations indicate more an active process of Tau secretion [39], [40]. Consistent with this view, a longitudinal decrease of CSF Tau phosphorylated at Thr181 was observed in the late stages of AD process, in a context of widespread neuronal death [41]. Also, Tau was found in the CSF of wild type mice in absence of any sign of neurodegeneration and in vitro evidences show a physiological Tau secretion upon neuronal activity, in particular after AMPA receptors stimulation [42], [43]. Moreover, truncation at Asp421 site and hyper-phosphorylation of Tau were observed to favor its secretion in vitro [44]. Interestingly, exosomes-associated Tau were detected in the CSF of AD patients [45], [46]. Containing oligomeric pThr181-Tau, exosomal Tau was found in a larger extent in early AD (Braak stage 3) compared to advanced disease progression stage defined by important neuronal loss (Braak stage 5) [45]. Extracellular Tau could then be detected by the immune system and initiates an antigen-driven immune response. For instance, active immunization of the mouse model of Tauopathy rTg4510 with WT or mutated P301L Tau protein induces robust humoral immune responses accompanied by anti-tau antibodies, shown to target 5 immunogenic epitopes found in multiple sites of Tau sequence [47]. Also, circulating tau-specific antibodies were identified in healthy individuals prone to recognize pathological Tau and block in vitro Tau aggregation seeding, through notably the cytosolic Fc receptor TRIM21 [48], [49]. Therefore, with the aim to achieve successful Tau-immunotherapy and slow down the disease progression, identifying the most immunogenic tau epitopes and the interplay existing between Tau and the immune system remain necessary [50].
Tau inter-cellular transfer
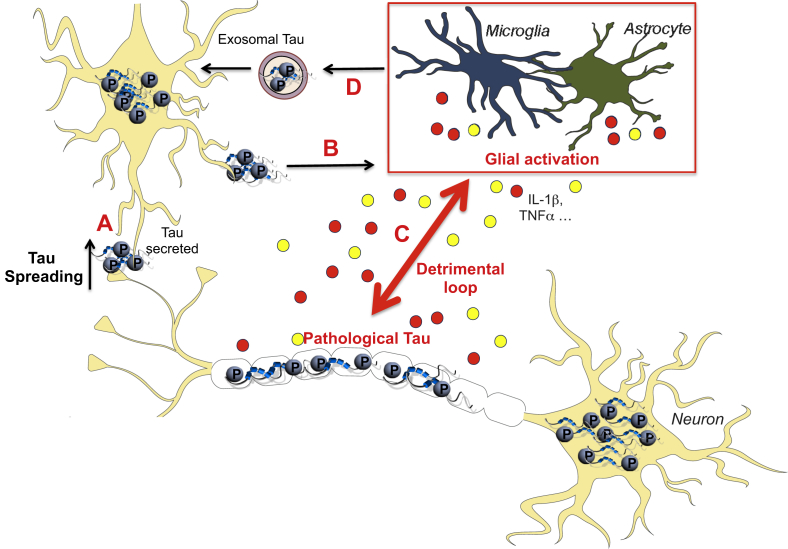
The post-mortem observation of AD brains reveals a characteristic distribution pattern of NFT lesions in the disease course, starting in the transentorhinal cortex and progressively affecting the hippocampus, temporal cortex and polymodal association areas [28], [31], [32], [51]. This sequential and hierarchical pathway defined the six Braak stages and a positive correlation between the affected areas and the clinical symptoms was observed, suggesting an instrumental role in synaptic dysfunction [30], [52]. The modalities of the propagation of Tau pathology were investigated. Experimental transfer of brain homogenates containing pathologic Tau from transgenic P301S mice promotes the accumulation of NFT in wild-type (WT) recipient animals in a stereotypical and time-dependent fashion. Tau spreading occurs at distance of the injection site and concerns area connected anatomically instead of affecting proximal structures. However, transcellular propagation of Tau in a prion-like fashion was observed as well in the P301S mouse model of Tauopathy or in AD brain [53], [54]. Tau seeding activity was shown to be an early manifestation, present in multiple brain regions and associated with disease progression and cognitive decline [53]. Moreover, insoluble Tau is more efficient to be propagated; all without any sign of neurodegeneration, suggesting that molecular forms of Tau responsible for propagation and neurotoxicity are different [55], [56]. Of interest, using a lentiviral approach, a trans-synaptic transfer of WT Tau in a dephosphorylated state can also be observed [57]. The cellular mechanisms of this prion-like trans-synaptic transmission were evaluated and different pathways were identified, including free forms, Tunneling nanotubes (TNTs) structures, ectosomes or exosomes [46], [58]. Finally, a study revealed a role played by microglial cells, the resident phagocytes of the central nervous system (CNS), in Tau transmission using two different models of Tauopathy: P301S mice and adeno-associated virus (AAV) expressing mutated P301L Tau [59]. Authors observed that microglial cells phagocyte aggregated Tau and that its exosomal secretion is readily transmissible to neurons (Fig. 1). Pharmacological depletion of microglial cells and exosomes synthesis inhibits Tau propagation; highlighting the critical role of microglia in Tau spreading and posit it as a valuable target to slow down disease progression.
Fig. 1.
Innate Immune response and Tau pathology: a vicious circle. Hyperphosphorylated pathological Tau species can be secreted extracellularly, explaining the progressive spread of tauopathy (A). Therefore, it promotes microglial activation/reactive astrocytes which release cytokines or neurotoxic inflammatory molecules including IL1β or TNFα (B). By a modulation of Tau kinases (p38, cdk5…), glial activation enhances Tau pathology, self-perpetuating the detrimental circle (C). Also, microglia was observed to be involved in Tau propagation by releasing exosomal Tau once pathological Tau phagocytosed (D).
AD, Tauopathies and inflammation: genetic evidences
Early onset forms of familial AD concern less than 1% of total cases and are associated with mutations in presenilin 1/2 (PS-1/2) and amyloid precursor protein (APP) genes. By contrast, the susceptibility of developing sporadic forms of the disease, also called Late-Onset Alzheimer's Disease (LOAD), are conditioned by a combination of environmental and genetic factors. APOE4 polymorphism is the major genetic risk factor for LOAD, known to intensify brain amyloid-β pathology and described to modulate Aβ-inducing neuroinflammation [60], [61]. It was also shown recently to exacerbate Tau-mediated disease and neuroinflammation independently of Aβ pathology using P301S mice [62]. Large genome-wide and rare variant association studies were performed with the purpose of identifying other candidate genes. Authors found a greater amount of single-nucleotide polymorphisms in genes related to immune system, translating an etiology of this pathway in AD development [63], [64], [65]. For instance CR1, CD33, MS4A4/MS4A6E, ABCA7, CD2AP, CD33 or EPHA1 genes are involved in complement activation or innate immune response (for review see Ref. [66]). Similarly, polymorphisms in ABI family member 3 (ABI3) and Phospholipase C gamma 2 (PLCG2) genes were found both highly expressed in microglia [67]. PLCγ2 hydrolyzes the membrane phospholipid PIP2 to form the secondary messenger inositol triphosphate (IP3), which promotes the release of calcium into the cytoplasm. Functional alterations of PLCγ2 impact the IP3/Ca2+ signaling pathway, found determinant for microglial properties [68]. Of interest, Tau misfolding could also impact IP3 production, as it was shown that physiological Tau exerts a tonic inhibition on the phosphatase PTEN reducing the formation of PIP2 [14]. In addition, variants of the triggering receptor expressed on myeloid cells 2 (TREM2) gene were also found associated with an increased predisposition to AD [67], [69], [70], [71]. TREM2 is an immunoglobulin receptor exclusively expressed by microglial cells in the brain, reinforcing the interest on the function of microglia in AD. TREM2 was observed necessary for aging-dependent microglial expansion and for microglial activation and phagocytosis in the context of demyelination [72], [73]. Although TREM2 was described to bind apolipoprotein/phospholipids, promoting microglial survival/activation and increasing amyloid clearance [74], [75], its role toward Tau is controversial. Using model of amyloid pathology 5xFAD or APP/PS1 mice, TREM2 was observed to increase or decrease Tau hyper-phosphorylation [76], [77]. In addition, while TREM2 mRNA level increases during disease progression in P301S mice, silencing TREM2 by a lentiviral approach was observed to exacerbate Tau pathology, enhance Tau kinase activity, worsening neuroinflammatory response and memory deficits [78]. Also, post mortem analysis of human cortical samples from AD indicates an increase of TREM2 protein associated with Tau pathology and synaptic loss. Finally, TREM2 adapter protein TYROBP or DNAX-activating protein 12 (DAP12) signaling was observed to promote Tau pathology and neuron injuries in APP/PS1 mice [79], [80]. Altogether, these genetic studies highlight the critical role played by immune system, in particular microglial cells, in AD pathogenesis even if some evidences indicate an involvement of the adaptive immune system as well. For instance, CR1 and EPHA1 can be expressed on lymphocytes subsets, ABCA7 was shown to promote CD1d surface expression inducing NKT function and ABI3 can indirectly induce T cell activation [67], [81], [82], [83]. Also, polymorphisms in HLA-DR region were found associated in both AD and fronto-temporal dementia (FTD) susceptibility [65], [84]. GWAS studies have also been conducted similarly in Corticobasal Degeneration (CBD) or Progressive Supranuclear Palsy (PSP), 2 rare tauopathies [85]. An overlap in genetic risk factors was observed with variants found in genes encoding Tau protein or the myelin-associated oligodendrocyte basic protein (MOBP). PSP susceptibility genes STX6 or EIF2AK3 encoding respectively syntaxin 6 and perk were observed as well, highlighting the importance of a dysfunction of vesicular trafficking dysfunction in this disorder [86]. However, no genetic risk factor related to immune function was observed so far in CBD or PSP. Thus, even if microglial activation plays a role in the pathogenesis of these Tauopathies, it might not be a causal factor [87], [88], [89], [90].
Glial cells in AD
Besides amyloid and Tau pathologies, another histological feature of AD is the accumulation of reactive astrocytes and microglia in the vicinity of amyloid deposits, commonly referred to as neuroinflammatory response. In the healthy brain, astrocytes provide neuronal energy supply (lactate shuttle hypothesis), participate in synaptic function (recapture/release transmitter – inter-astrocytic communication through calcium wave), induce synaptic pruning and the release of neurotrophic factors [91], [92]. However, during neuroinflammatory conditions, activated microglia-driven IL-1α, Tumor Necrosis Factor (TNFα) and C1q release was shown to favor the formation of a neurotoxic subset of reactive astrocytes called A1. A1 astrocytes lose their normal functions and their ability to promote synapse formation, but instead, kill CNS neurons via the secretion of harmful factors [93], [94]. A higher proportion of A1 astrocytes producing complement protein C3 was observed in AD brain, suggesting a gain of toxic functions and a loss of physiological properties that could contribute to detrimental effects of reactive astrocytes in AD [94]. Concerning tauopathies, neuronal Tau misfolding as observed in AD and some FTDs is sufficient to induce morphological changes in astrocytes impacting their physiological role. They shift towards an inflammatory profile, as indicated by GFAP upregulation and the secretion of pro-inflammatory factors, which contribute to the pathogenesis [95], [96]. Astrocytic Tau inclusions are pathological hallmarks of PSP and CBD, respectively called tufted astrocytes and astrocytic plaques. To reproduce these pathological features, transgenic mice overexpressing human Tau gene under the GFAP promoter were generated [97]. These mice develop an age-dependent Tau pathology in astrocytes associated with blood-brain-barrier disruption and focal neuron loss, pointing the important role of reactive astrocytes in tauopathies.
Microglia plays a critical role in AD and Tauopathies as well. Microglial cells exhibit highly motile and ramified processes allowing a dynamic and continual survey of the healthy brain as observed using in vivo two-photon imaging [98]. They sample, detect and eliminate debris or apoptotic neurons by phagocytosis but this ability is considerably decreased in a pro-inflammatory context [99]. Microglia is involved in multiple processes such as neurogenesis, synapse elimination – in a complement-dependent manner- or synapse plasticity [100]. Moreover, neuronal secretion of CX3CL1 (fractalkine), CD200, the colony-stimulating factor 1 (CSF1) or transforming growth factor-β (TGF-β), among others, favor an inhibitory signaling once fixed to their microglial cognate receptors, keeping these cells in a quiescent state [101], [102]. The involvement of microglia on AD pathogenesis was essentially studied in the light of the amyloid side and largely reviewed elsewhere [103], [104]. Briefly, these cells appear to have a complex, dynamic and time-dependent impact on amyloid pathology, either promoting the clearance of deposits or associated with neurotoxicity and disease progression due notably to the release of pro-inflammatory cytokines. This is strengthened by the measure of longitudinal changes in microglial activation using imaging and positron emission tomography (PET) scans during the disease course. An initial peak was observed in patients exhibiting mild-cognitive impairments (MCI), and at a latter stage of the disease [105], [106]. Although this model will require a larger cohort of patients, the two peaks of activation could reflect a biphasic role of microglia. Therefore, therapeutic avenue targeting microglia require in-depth understanding and a better characterization. In that view, heterogeneity of microglia was deciphered using single-cell RNA sequencing. An individual mapping of immune cell subset identified, in mouse models, a protective and dynamic disease-associated microglia (DAM) involving key genes along the course of AD progression [107]. AD progression can also be impacted by the locus coeruleus (LC), a brain structure early affected (asymptomatic stage) and producing norepinephrine (NE), an anti-inflammatory neurotransmitter (for review see Ref. [108]). Its degeneration induces a disinhibiting effect favoring the establishment of microglial activation and, due to its widespread projections all the major brain structures, facilitates the inflammatory reaction [109], [110]. Finally, brain infiltration of peripheral innate immune subsets has been suggested to contribute to AD pathogenesis. For instance, neutrophil infiltration was shown in cerebral parenchyma of AD patients and associated with cognitive damage and an increased Tau/amyloid pathology in 3xTg-AD mice, even if others observed opposite effects [111], [112]. Also, recruitment of circulating monocytes via the chemoattractant protein CCL2 and its cognate receptor CCR2 could positively impact the phenotype of APP models. Indeed, deletion of CCR2 in Tg2576 APP mice increases accumulation of microglia around blood vessels possibly through the recruitment of mononuclear phagocytes from the blood and bone marrow and promotes perivascular Aβ deposition [113]. Another study also demonstrated detrimental effect on memory in APP/PS1 mice deficient for CCR2 [113], [114]. However, involvement of circulation monocytes in AD remains subject to controversy as most experimental models involved irradiation procedure which open blood-brain barrier. Of interest, the reduction of monocyte infiltration following ccr2 deficiency was involved in Tau hyper-phosphorylation in a different disorder called Traumatic Brain Injury (TBI) [115]. Together, innate immune system was shown to participate in disease progression and bidirectional detrimental connections are notably observed with regards to Tau pathology.
Innate immune response and Tau pathology: a vicious circle
Compelling studies revealed that the exclusive presence of Tau pathology is prone to induce microglial/astrocytic activation. For instance, FTD patients carrying the P301S mutation display activated CD68 positive microglial cells around neurons containing hyper-phosphorylated Tau [116]. A strong neuroinflammatory response was also measured as indicated by the upregulation of interleukin-1β (IL1β) and cyclooxygenase-2 (Cox2). Microglial activation and reactive GFAP astrocytes have been also reported in Pick's disease [117], and a microglia-driven IL-1β production was observed in the substantia nigra of PSP patients [87]. Tau pathology is thus prone to directly favor the development of neuroinflammation. Indeed, using different transgenic models of Tauopathy, age-dependent astrogliosis/microglial activation and pathological neuroinflammatory changes were observed in CNS structures bearing Tau pathology at a stage where neuronal loss is absent [95], [118], [119], [120]. The nature of Tau species involved in this process was raised. Since the innate immune response was initiated before the formation of hippocampal NFT, soluble Tau species are more susceptible to be implicated [120]. In that view, a co-localization of activated microglial cells and reactive astrocytes with tau oligomers was observed in both mouse models of Tauopathy and AD/frontotemporal lobar dementia (FTLD) patients' brains [121]. Moreover, it was observed in microglial cultures that misfolded truncated Tau is sufficient to induce pro-inflammatory cytokines (IL-6, IL-1β, TNFα) upregulation through NF-κB and MAPK signaling pathways [122]. Of interest, recent data emphasize that pathological Tau could promote IL-1β secretion by activating the inflammasome, a microglial component of the neuroinflammatory response previously shown to be important regarding amyloid accumulation [123] [124]. Finally, strategies modulating Tau pathology were observed to impact immune response. For instance, active immunization with WT or P301L mutated Tau protein reduces Tau pathology of rTg4510 mice and the number of activated microglia/astrocytic cells [47]. In a similar manner, blockade of adenosine A2A receptors reduce both hippocampal Tau phosphorylation and neuroinflammatory response [125].
Reciprocally, the neuroinflammatory response was seen to impact Tau pathogenesis. Indeed, the intra-cerebral administration of LPS, a powerful pro-inflammatory component acting on the myeloid receptor TLR4, promotes a microglial activation and hyper-phosphorylation of Tau as well as the formation of tangles in the conditional rTg4510 model [126]. In addition, microglial activation was observed to be an early event occurring prior the formation of NFTs in P301S mice. Then, it could partially initiate the disease progression since a parallel exists between early synaptic defects and microglial activation in the hippocampus of P301S mice [120]. The underlying mechanisms, which direct the exacerbation of Tau pathology ensuing brain inflammation, were studied and appear mediated by a modulation of Tau kinases. The 3xTg-AD mice, a transgenic model harboring mutations in APP, PS1 and tau gene, received LPS intraperitoneally, which aggravate Tau pathology via CDK5 activation [127]. However, amyloid pathology is present in this model and may create a bias by favoring directly Tau pathology [128]. Transgenic hTau mice, overexpressing human tau gene and depleted for endogenous mouse tau leading to the development of Tau pathology were also used. Genetic deficiency of CX3CR1 induces microglial activation in this model, as the CX3CL1-CX3CR1 regulatory signaling of neuronal-microglial communication is disrupted. Consequently, higher degree of hippocampal Tau phosphorylation/aggregation, loss of synaptic integrity and behavior impairment were observed [119], [129]. This is in accordance with the reduced Tau-mediated disease observed in rTg4510 mice overexpressing fractalkine, emphasizing CX3CL1-CX3CR1 axis in the pathogenesis [130]. Notably, it was recently observed that microglial phagocytosis at extracellular Tau is possible through CX3CR1. However, in later stage of AD, both hyper-phosphorylation of Tau and the progressive overexpression of CX3CL1, which competes with Tau in CX3CR1 binding, contribute to Tau clearance impairment by microglia [131]. Interestingly, hTau CX3CR1−/− mice display p38 MAPK upregulation, especially at an early stage [119], [129]. Previous observations revealed that LPS-inducing hyper-phosphorylated Tau and synaptic defects in microglial–neuronal co-culture are mediated by the IL-1β/p38 axis [132]. Indeed LPS administration in mice deficient for IL-1 receptor fails to induce Tau hyper-phosphorylation [129]. In addition, experimental transfer of purified microglia from hTau CX3CR1−/− mice is unable to trigger Tau hyper-phosphorylation after the blockade of IL-1β/p38 signaling pathway [119]. Together, these results suggest that activated microglia is prone to secrete IL1β, or others pro-inflammatory cytokines including TNFβ known to favor Tau pathology [133]. Reducing the amount of pro-inflammatory mediator secretion using minocycline treatment was observed to impact directly cortical Tau phosphorylation in hTau mice [134], [135]. Both Tau misfolding and neuroinflammatory response favor the progression of pathological changes as loss of synaptic and neuronal integrity, and behavior impairments. In a reciprocal way, Tau is instrumental in LPS-inducing neurotoxicity and neuro-immune response in CX3CR1−/− mice as suggested by the significant reduction of microglial IL-1β secretion and annexin-5/caspase-3 positive neurons in CX3CR1−/− mapt−/− mice [136]. Finally, recent observations indicate that microglia is directly implicated in Tau spreading via the formation of exosomes containing Tau, directly transmissible to neurons [59]. Microglia is then implicated in all the different steps occurring in Tau-driven disease formation (Tau phosphorylation, aggregation, propagation and synaptic alteration), posit it has a valuable target to modulate AD and related Tauopathies progression (Fig. 1). Also, by generating a potent inflammatory response, glial cells can impact adult neurogenesis by reducing progenitor proliferation or neuronal differentiation as extensively detailed elsewhere [137]. A recent study has notably shown that stress inducing Tau hyper-phosphorylation reduces dentate gyrus neurogenesis in vivo, a phenomenon counteracted by Tau deficiency [138]. Together, it may suggest a possible detrimental loop between neurogenesis, inflammation and Tau pathology.
Adaptive immune response and Tau pathology
Adaptive immune response, in particular brain-infiltrated T-cells, was observed in AD patients and was mainly studied in the light of amyloid pathology [139], [140], [141]. So far, little is known on the relationships existing between T cells and Tau pathology. Previous observations are in accordance with such Tau pathology driven T cell process. In AD patients, a positive correlation between the number of parenchymal CD3+ T cell and phospho-Tau load was found [141]. Also, a greater amount of activated HLA-DR+ CD8 T cells was measured in the CSF of AD patients, associated with structural MRI changes and cognitive deterioration, suggesting a detrimental impact of T cell infiltration [142]. Other findings indicated functional changes in circulating lymphocytes from AD patients. For instance, a greater percentage of late-differentiated CD4 T cells (CD28−CD27−CD45RA+CD45RO+) was observed compared to age-matched controls [143]. In a similar manner, the proportion of activated HLA-DR positive CD4 and CD8 T cells was increased in AD patients' blood [142]. These modifications might result of the chronic stimulation by circulating Aβ while a role of peripheral Tau cannot be excluded. We recently investigated the immune profile of CD4 and CD8 T cells in the blood of Tau transgenic mice (THY-Tau22). Using CD44 and CD62L markers, the proportion of naïve and memory subsets, as well as the amount of interferon-γ (IFNγ) or TNFα was unchanged compared to WT [95]. Regarding Tau, the notion of antigen-specificity remains to be further evaluated. Current results suggest that specific T cell recruitment rather occurs once the CNS is reached, possibly due to the presence of Tau pathology and neuroinflammatory processes, rather than to a preliminar peripheral activation.
Leukocytes trafficking, including tissue-infiltrating T cells, is favored by inflammatory chemoattractant molecules named chemokines. The potential role of chemokines on Aβ pathogenesis and their direct/indirect effects on neurons has been studied and reviewed elsewhere [144]. These mediators are involved in the Tau-driven neuro-inflammatory response characterizing Tauopathies as previously described. Indeed, transcriptome analysis in acute model of rats overexpressing Tau indicates an overexpression of chemokines (C-X-C motif) ligand 10 (CXCL10) and CXCL16 [145]. Moreover, upregulation of chemokines such as CCL3, CCL4, CCL12 and CXCL2 release was observed in the cortex of hTau mice [134]. In addition, early hippocampal upregulation of microglial CCL3, but also CCL4 and CCL5 were observed in our transgenic mouse model of Tauopathy (THY-Tau22) associated with Tau pathology, and memory impairments [95], [146]. Also, a positive correlation was observed between the level of CCL2 and phospho-Tau in the CSF of AD patients, and CCL2, CCL3 and CCL5 were found upregulated in the AD brain supporting the idea of a determinant role of chemokines in chronic inflammation [144], [147], [148]. Finally, besides their implications in numerous immune functions, chemokines are prone to modulate synaptic plasticity [149]. For instance, ex-vivo CCL3 treatment was found to decrease hippocampal LTP in a CCR5-dependent manner. In addition, intra-cerebroventricular injection of CCL3 alters hippocampal synaptic transmission and plasticity, in association with memory impairments, the latter being reversed by CCR5 antagonist administration [150]. Thus, early release of CCL3, acting on its cognate receptor CCR5, may contribute per se to progressive synaptic/memory alterations exhibited by THY-Tau22 mice. Of interest, APOE4 genotype, the strongest genetic risk factor for sporadic AD, was observed to favor astrocytic CCL3 production [151].
CCL3-inducing CNS T cell infiltration may also explain the detrimental effects of the chemokine. Using an in vitro model of Blood-Brain Barrier (BBB), CCL3 overexpression in AD T cells favors their transendothelial migration by acting on its endothelial cognate receptor CCR5. In addition, cortical T cell infiltration, following acute Aβ administration in rats, is considerably reduced once CCL3 function is neutralized [152]. Therefore, CCL3 could promote brain-infiltrated CD8+ T-cell through the BBB as observed in our THY-Tau22 model, the chemokine being secreted by microglial cells at a stage where T cell infiltration is not present yet [95] (Fig. 2).
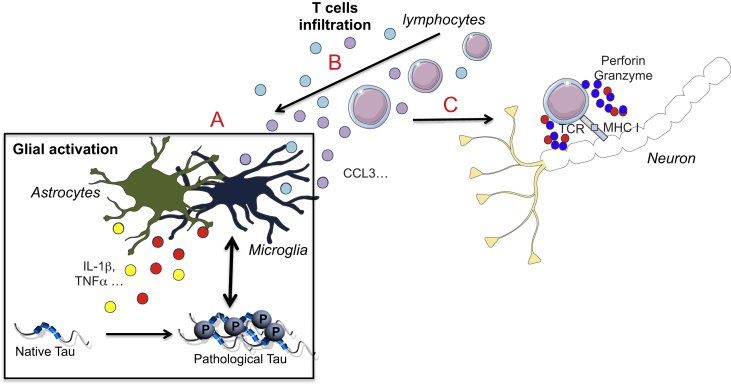
Fig. 2.
Consequences of T cell infiltration in Tauopathies. Along the disease formation, innate immune subsets release chemokines (eg. CCL3…) favoring brain T Cell infiltration (A). Consequently, either beneficial or detrimental effects may occur depending on the infiltrated subset. It can modulate the neuroimmune response which may impact tau pathology and synaptic functions (B). Activated T cells can also dampen neurons integrity by the release of neurotoxic factors (granzyme, perforin…) using TCR/MHC I interaction (C). TCR. T-cell receptor, MHC I. Major histocompatibility complex.
Unlike the large majority of tissues, BBB, which is a highly specified structure, restricts drastically the diffusion of cellular components, and so immune cells, from the circulation to brain parenchyma, which confine leukocytes in the vascular compartment in non-pathological conditions [153]. Leukocyte extravasation across the BBB is a multi-step process whose molecular mechanisms have been largely studied in the context of multiple sclerosis [154], [155], [156]. BBB disruption has been observed in a certain number of tauopathies including progressive supranuclear palsy (PSP) [157]. In vitro experiments found a toxic gain of function of Tau misfolding on BBB integrity [158]. In a similar manner, aged rTg4510 mice exhibit hippocampal Tau accumulation, notably in vascular structure, accompanied by BBB disruption as observed by immunoglobulin G (IgG) extravasation, and CD4+ T cell infiltration, suggesting a passive mechanism of infiltration due to BBB leakage. Tau suppression using doxycycline administration in this conditional model, reverse BBB dysfunction in association with a significant lower number of infiltrated T cells [159]. However, CD8+ T cell infiltration observed in THY-Tau22 mice occur in absence of any BBB disruption, no alteration in tight junction organization or IgG extravasation was observed, suggesting an active model of diapedesis in brain-restricted neuroinflammatory processes [95]. Therefore, even if BBB alteration can occur in association with Tau pathogenesis, it does not appear essential for T cells infiltration.
The functional impact of T cell infiltration on Tau-mediated disease progression has never been evaluated so far. To this end, we chronically suppressed circulating CD3+ T cells using anti-CD3 depleting antibody treatment prior to the development of pathological alterations characterizing THY-Tau22 mice. Paralleling the significant drop of hippocampal CD8 T cells infiltration, treated THY-Tau22 animals displayed a reduction of spatial memory impairments and a recovery of synaptic function, as indicated by the hiuppocampal normalization of Arc and 14.3.3. expressions [95]. Interestingly, the neuroinflammatory response and notably astrocytic/microglial activation was found reduced while no change was observed in term of Tau pathology. Together, this suggests an instrumental role of CD8 T cell infiltration towards Tau-induced cognitive deficits, independent of Tau misfolding, but rather related to a modulate of the innate immune response. This is strongly evocative of previous observations made in the context of amyloid pathology. As a matter of facts, antigen-specific reactive Th1 cells induced by Aβ immunization in APP/IFNγ transgenic mice migrate at site of amyloid plaques to the brain and induce an upregulation of microglial phagocytic markers such as TREM2 and signal regulatory protein-β1 (SIRPβ1) [160]. In a similar manner, pharmacological depletion of FoxP3+ regulatory T cells (Treg) in APP/PS1 mice reduces the amount of plaque-associated microglia and shifts the functionality of microglial cells as observed by transcriptome analysis, all without affecting amyloid deposition [161]. Also, Treg activation induced by IL-2 treatment was associated with an astrocytic recruitment around amyloid plaques in APP/PS1 mice [162]. Although T cell infiltration may impact on microglial activation, a direct effect cannot be excluded to explain the detrimental effects of lymphocytes (Fig. 2). Notably, antigen-specific CD8 T cells may interact with neurons expressing MHCI and exert cytotoxic functions by the release of lytic granules containing granzyme A, B, perforin. The engagement of Fas ligand/Fas signaling pathway and secretion of IFN-γ/TNFα could promote neuronal death as well [163]. Also, CD8 T cells were observed to have an inhibitory effect on neurites outgrowth in vitro independently of apoptosis mechanisms [164]. Finally, the impact of regulatory CD4 Treg was evaluated in the light of Aβ pathology. In the amyloid-driven disease model 5xFAD, authors induced a breaking of immune tolerance by a systemic transient depletion of FoxP3+ Treg or programmed death-1 (PD1) immune checkpoint blockade. In both conditions, it favors the trafficking of immunoregulatory myeloid cells and/or Treg cells across the choroid plexus in an IFNγ-dependent pathway, which mitigate amyloid plaque burden and memory impairments [165], [166]. This is in apparent discrepancy with the acceleration of cognitive deficits observed in APP/PS1 early depleted in systemic FoxP3+ Treg [161]. Similarly, IL-2 treatment was observed to expand FoxP3+ Treg in the blood and the brain of APP/PS1 mice and rescue hippocampal spatial memory impairments, synaptic defect and amyloid pathology [162], which could actually suggest a dual and time-dependent function of this population [161]. The function of this population is unknown in Tau mediated pathology and more generally deciphering the role played by activated/regulatory subsets along the disease course is necessary to custom therapeutical strategies.
Conclusion
Pathological Tau misfolding as defined in tauopathies and neuroinflammatory processes generated by innate/adaptive components form a detrimental vicious circle and act together in the progression of pathogenesis. Underlying mechanisms are multiples but can be summarized as a toxic gain of function including Tau aggregation, release of inflammatory molecules and a loss of physiological functions such as adult neurogenesis, glial phagocytosis and trophic factor release, Tau-driven axonal transport and RNA/DNA integrity. Also, environmental disease modifiers (for instance obesity, stress, caffeine consumption) can act through an alteration of the neuroimmune response and impact upon the aforementioned vicious circle. Therefore, better understanding of the spatiotemporal pattern of Tau-dependent neuroinflammation in Tauopathies is necessary to better delineate to which extent these innate and adaptive pathways constitute future therapeutical targets for the treatments of AD and Tauopathies.
Conflicts of interest
Authors declare no conflict of interest regarding the present manuscript.
Acknowledgments
Our laboratory is supported by grants from France Alzheimer/Fondation de France (InsTauBrain project), FHU VasCog research network (Lille, France) and programs d'investissements d'avenir LabEx (excellence laboratory) DISTALZ (Development of Innovative Strategies for a Transdisciplinary approach to ALZheimer's disease). Our laboratory is also supported by ANR (ADORATAU to DB, SPREADTAU to LB), Fondation pour la Recherche Médicale, LECMA/Alzheimer Forschung Initiative, Fondation Plan Alzheimer as well as Inserm, CNRS, Université Lille 2, Lille Métropole Communauté Urbaine, Région Nord/Pas-de-Calais, FEDER, DN2M and FUI MEDIALZ.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Binder L.I., Frankfurter A., Rebhun L.I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drechsel D.N., Hyman A.A., Cobb M.H., Kirschner M.W. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weingarten M.D., Lockwood A.H., Hwo S.Y., Kirschner M.W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frandemiche M.L., De Seranno S., Rush T., Borel E., Elie A., Arnal I. Activity-dependent tau protein translocation to excitatory synapse is disrupted by exposure to amyloid-beta oligomers. J Neurosci. 2014;34:6084–6097. doi: 10.1523/JNEUROSCI.4261-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ittner L.M., Ke Y.D., Delerue F., Bi M., Gladbach A., van Eersel J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Kimura T., Whitcomb D.J., Jo J., Regan P., Piers T., Heo S. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2013;369:20130144. doi: 10.1098/rstb.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee G., Newman S.T., Gard D.L., Band H., Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci. 1998;111:3167–3177. doi: 10.1242/jcs.111.21.3167. [DOI] [PubMed] [Google Scholar]
- 8.Tai H.C., Wang B.Y., Serrano-Pozo A., Frosch M.P., Spires-Jones T.L., Hyman B.T. Frequent and symmetric deposition of misfolded tau oligomers within presynaptic and postsynaptic terminals in Alzheimer's disease. Acta Neuropathol Commun. 2014;2:146. doi: 10.1186/s40478-014-0146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sultan A., Nesslany F., Violet M., Begard S., Loyens A., Talahari S. Nuclear tau, a key player in neuronal DNA protection. J Biol Chem. 2011;286:4566–4575. doi: 10.1074/jbc.M110.199976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansuroglu Z., Benhelli-Mokrani H., Marcato V., Sultan A., Violet M., Chauderlier A. Loss of Tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin. Sci Rep. 2016;6:33047. doi: 10.1038/srep33047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Violet M., Chauderlier A., Delattre L., Tardivel M., Chouala M.S., Sultan A. Prefibrillar Tau oligomers alter the nucleic acid protective function of Tau in hippocampal neurons in vivo. Neurobiol Dis. 2015;82:540–551. doi: 10.1016/j.nbd.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Violet M., Delattre L., Tardivel M., Sultan A., Chauderlier A., Caillierez R. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front Cell Neurosci. 2014;8:84. doi: 10.3389/fncel.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arendt T., Stieler J.T., Holzer M. Tau and tauopathies. Brain Res Bull. 2016;126:238–292. doi: 10.1016/j.brainresbull.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Marciniak E., Leboucher A., Caron E., Ahmed T., Tailleux A., Dumont J. Tau deletion promotes brain insulin resistance. J Exp Med. 2017;214:2257–2269. doi: 10.1084/jem.20161731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo T., Noble W., Hanger D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017;133:665–704. doi: 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris M., Knudsen G.M., Maeda S., Trinidad J.C., Ioanoviciu A., Burlingame A.L. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat Neurosci. 2015;18:1183–1189. doi: 10.1038/nn.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanger D.P., Seereeram A., Noble W. Mediators of tau phosphorylation in the pathogenesis of Alzheimer's disease. Expert Rev Neurother. 2009;9:1647–1666. doi: 10.1586/ern.09.104. [DOI] [PubMed] [Google Scholar]
- 18.Sergeant N., Bretteville A., Hamdane M., Caillet-Boudin M.L., Grognet P., Bombois S. Biochemistry of Tau in Alzheimer's disease and related neurological disorders. Expert Rev Proteomics. 2008;5:207–224. doi: 10.1586/14789450.5.2.207. [DOI] [PubMed] [Google Scholar]
- 19.Martin L., Latypova X., Wilson C.M., Magnaudeix A., Perrin M.L., Yardin C. Tau protein kinases: involvement in Alzheimer's disease. Ageing Res Rev. 2013;12:289–309. doi: 10.1016/j.arr.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Papon M.A., El Khoury N.B., Marcouiller F., Julien C., Morin F., Bretteville A. Deregulation of protein phosphatase 2A and hyperphosphorylation of tau protein following onset of diabetes in NOD mice. Diabetes. 2013;62:609–617. doi: 10.2337/db12-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malia T.J., Teplyakov A., Ernst R., Wu S.J., Lacy E.R., Liu X. Epitope mapping and structural basis for the recognition of phosphorylated tau by the anti-tau antibody AT8. Proteins. 2016;84:427–434. doi: 10.1002/prot.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng-Fischhofer Q., Biernat J., Mandelkow E.M., Illenberger S., Godemann R., Mandelkow E. Sequential phosphorylation of Tau by glycogen synthase kinase-3beta and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired-helical-filament-like conformation. Eur J Biochem. 1998;252:542–552. doi: 10.1046/j.1432-1327.1998.2520542.x. [DOI] [PubMed] [Google Scholar]
- 23.Bussiere T., Hof P.R., Mailliot C., Brown C.D., Caillet-Boudin M.L., Perl D.P. Phosphorylated serine422 on tau proteins is a pathological epitope found in several diseases with neurofibrillary degeneration. Acta Neuropathol. 1999;97:221–230. doi: 10.1007/s004010050978. [DOI] [PubMed] [Google Scholar]
- 24.Luna-Munoz J., Chavez-Macias L., Garcia-Sierra F., Mena R. Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer's disease. J Alzheimers Dis. 2007;12:365–375. doi: 10.3233/jad-2007-12410. [DOI] [PubMed] [Google Scholar]
- 25.Mondragon-Rodriguez S., Basurto-Islas G., Santa-Maria I., Mena R., Binder L.I., Avila J. Cleavage and conformational changes of tau protein follow phosphorylation during Alzheimer's disease. Int J Exp Pathol. 2008;89:81–90. doi: 10.1111/j.1365-2613.2007.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondragon-Rodriguez S., Perry G., Luna-Munoz J., Acevedo-Aquino M.C., Williams S. Phosphorylation of tau protein at sites Ser(396-404) is one of the earliest events in Alzheimer's disease and Down syndrome. Neuropathol Appl Neurobiol. 2014;40:121–135. doi: 10.1111/nan.12084. [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Jiang Q., Chu J., Lin L., Li X.G., Chai G.S. Expression of Tau40 induces activation of cultured rat microglial cells. PLoS One. 2013;8:e76057. doi: 10.1371/journal.pone.0076057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 29.Arriagada P.V., Growdon J.H., Hedley-Whyte E.T., Hyman B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 30.Duyckaerts C., Bennecib M., Grignon Y., Uchihara T., He Y., Piette F. Modeling the relation between neurofibrillary tangles and intellectual status. Neurobiol Aging. 1997;18:267–273. doi: 10.1016/s0197-4580(97)80306-5. [DOI] [PubMed] [Google Scholar]
- 31.Cho H., Choi J.Y., Hwang M.S., Lee J.H., Kim Y.J., Lee H.M. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology. 2016;87:375–383. doi: 10.1212/WNL.0000000000002892. [DOI] [PubMed] [Google Scholar]
- 32.Pontecorvo M.J., Devous M.D., Sr., Navitsky M., Lu M., Salloway S., Schaerf F.W. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 2017;140:748–763. doi: 10.1093/brain/aww334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren Q.G., Liao X.M., Chen X.Q., Liu G.P., Wang J.Z. Effects of tau phosphorylation on proteasome activity. FEBS Lett. 2007;581:1521–1528. doi: 10.1016/j.febslet.2007.02.065. [DOI] [PubMed] [Google Scholar]
- 34.Keck S., Nitsch R., Grune T., Ullrich O. Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer's disease. J Neurochem. 2003;85:115–122. doi: 10.1046/j.1471-4159.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- 35.Ying Z., Wang H., Wang G. The ubiquitin proteasome system as a potential target for the treatment of neurodegenerative diseases. Curr Pharm Des. 2013;19:3305–3314. doi: 10.2174/1381612811319180013. [DOI] [PubMed] [Google Scholar]
- 36.Santacruz K., Lewis J., Spires T., Paulson J., Kotilinek L., Ingelsson M. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spires T.L., Orne J.D., SantaCruz K., Pitstick R., Carlson G.A., Ashe K.H. Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am J Pathol. 2006;168:1598–1607. doi: 10.2353/ajpath.2006.050840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsson B., Lautner R., Andreasson U., Ohrfelt A., Portelius E., Bjerke M. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 39.Hall G.F., Saman S. Death or secretion? The demise of a plausible assumption about CSF-tau in Alzheimer Disease? Commun Integr Biol. 2012;5:623–626. doi: 10.4161/cib.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medina M., Avila J. The role of extracellular Tau in the spreading of neurofibrillary pathology. Front Cell Neurosci. 2014;8:113. doi: 10.3389/fncel.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seppala T.T., Koivisto A.M., Hartikainen P., Helisalmi S., Soininen H., Herukka S.K. Longitudinal changes of CSF biomarkers in Alzheimer's disease. J Alzheimers Dis. 2011;25:583–594. doi: 10.3233/JAD-2011-101911. [DOI] [PubMed] [Google Scholar]
- 42.Pooler A.M., Phillips E.C., Lau D.H., Noble W., Hanger D.P. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013;14:389–394. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada K., Cirrito J.R., Stewart F.R., Jiang H., Finn M.B., Holmes B.B. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci. 2011;31:13110–13117. doi: 10.1523/JNEUROSCI.2569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plouffe V., Mohamed N.V., Rivest-McGraw J., Bertrand J., Lauzon M., Leclerc N. Hyperphosphorylation and cleavage at D421 enhance tau secretion. PLoS One. 2012;7:e36873. doi: 10.1371/journal.pone.0036873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saman S., Kim W., Raya M., Visnick Y., Miro S., Saman S. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Balaji V., Kaniyappan S., Kruger L., Irsen S., Tepper K. The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener. 2017;12:5. doi: 10.1186/s13024-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selenica M.L., Davtyan H., Housley S.B., Blair L.J., Gillies A., Nordhues B.A. Epitope analysis following active immunization with tau proteins reveals immunogens implicated in tau pathogenesis. J Neuroinflammation. 2014;11:152. doi: 10.1186/s12974-014-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McEwan W.A., Falcon B., Vaysburd M., Clift D., Oblak A.L., Ghetti B. Cytosolic Fc receptor TRIM21 inhibits seeded tau aggregation. Proc Natl Acad Sci U S A. 2017;114:574–579. doi: 10.1073/pnas.1607215114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pascual G., Wadia J.S., Zhu X., Keogh E., Kukrer B., van Ameijde J. Immunological memory to hyperphosphorylated tau in asymptomatic individuals. Acta Neuropathol. 2017;133:767–783. doi: 10.1007/s00401-017-1705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agadjanyan M.G., Petrovsky N., Ghochikyan A. A fresh perspective from immunologists and vaccine researchers: active vaccination strategies to prevent and reverse Alzheimer's disease. Alzheimers Dement. 2015;11:1246–1259. doi: 10.1016/j.jalz.2015.06.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delacourte A., David J.P., Sergeant N., Buee L., Wattez A., Vermersch P. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology. 1999;52:1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- 52.Grober E., Dickson D., Sliwinski M.J., Buschke H., Katz M., Crystal H. Memory and mental status correlates of modified Braak staging. Neurobiol Aging. 1999;20:573–579. doi: 10.1016/s0197-4580(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 53.Furman J.L., Vaquer-Alicea J., White C.L., 3rd, Cairns N.J., Nelson P.T., Diamond M.I. Widespread tau seeding activity at early Braak stages. Acta Neuropathol. 2017;133:91–100. doi: 10.1007/s00401-016-1644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holmes B.B., Furman J.L., Mahan T.E., Yamasaki T.R., Mirbaha H., Eades W.C. Proteopathic tau seeding predicts tauopathy in vivo. Proc Natl Acad Sci U S A. 2014;111:E4376–E4385. doi: 10.1073/pnas.1411649111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmed Z., Cooper J., Murray T.K., Garn K., McNaughton E., Clarke H. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 2014;127:667–683. doi: 10.1007/s00401-014-1254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clavaguera F., Bolmont T., Crowther R.A., Abramowski D., Frank S., Probst A. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dujardin S., Lecolle K., Caillierez R., Begard S., Zommer N., Lachaud C. Neuron-to-neuron wild-type Tau protein transfer through a trans-synaptic mechanism: relevance to sporadic tauopathies. Acta Neuropathol Commun. 2014;2:14. doi: 10.1186/2051-5960-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tardivel M., Begard S., Bousset L., Dujardin S., Coens A., Melki R. Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol Commun. 2016;4:117. doi: 10.1186/s40478-016-0386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asai H., Ikezu S., Tsunoda S., Medalla M., Luebke J., Haydar T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 61.Tai L.M., Ghura S., Koster K.P., Liakaite V., Maienschein-Cline M., Kanabar P. APOE-modulated Abeta-induced neuroinflammation in Alzheimer's disease: current landscape, novel data, and future perspective. J Neurochem. 2015;133:465–488. doi: 10.1111/jnc.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi Y., Yamada K., Liddelow S.A., Smith S.T., Zhao L., Luo W. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523–527. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hollingworth P., Harold D., Sims R., Gerrish A., Lambert J.C., Carrasquillo M.M. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones L., Holmans P.A., Hamshere M.L., Harold D., Moskvina V., Ivanov D. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer's disease. PLoS One. 2010;5:e13950. doi: 10.1371/journal.pone.0013950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lambert J.C., Grenier-Boley B., Chouraki V., Heath S., Zelenika D., Fievet N. Implication of the immune system in Alzheimer's disease: evidence from genome-wide pathway analysis. J Alzheimers Dis. 2010;20:1107–1118. doi: 10.3233/JAD-2010-100018. [DOI] [PubMed] [Google Scholar]
- 66.Tosto G., Reitz C. Genome-wide association studies in Alzheimer's disease: a review. Curr Neurol Neurosci Rep. 2013;13:381. doi: 10.1007/s11910-013-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sims R., van der Lee S.J., Naj A.C., Bellenguez C., Badarinarayan N., Jakobsdottir J. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer's disease. Nat Genet. 2017;49:1373–1384. doi: 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma P., Ping L. Calcium ion influx in microglial cells: physiological and therapeutic significance. J Neurosci Res. 2014;92:409–423. doi: 10.1002/jnr.23344. [DOI] [PubMed] [Google Scholar]
- 69.Guerreiro R., Hardy J. TREM2 and neurodegenerative disease. N Engl J Med. 2013;369:1569–1570. doi: 10.1056/NEJMc1306509. [DOI] [PubMed] [Google Scholar]
- 70.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seshadri S., Fitzpatrick A.L., Ikram M.A., DeStefano A.L., Gudnason V., Boada M. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cantoni C., Bollman B., Licastro D., Xie M., Mikesell R., Schmidt R. TREM2 regulates microglial cell activation in response to demyelination in vivo. Acta Neuropathol. 2015;129:429–447. doi: 10.1007/s00401-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poliani P.L., Wang Y., Fontana E., Robinette M.L., Yamanishi Y., Gilfillan S. TREM2 sustains microglial expansion during aging and response to demyelination. J Clin Invest. 2015;125:2161–2170. doi: 10.1172/JCI77983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Cella M., Mallinson K., Ulrich J.D., Young K.L., Robinette M.L. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yeh F.L., Hansen D.V., Sheng M. TREM2, microglia, and neurodegenerative diseases. Trends Mol Med. 2017;23:512–533. doi: 10.1016/j.molmed.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Jay T.R., Miller C.M., Cheng P.J., Graham L.C., Bemiller S., Broihier M.L. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer's disease mouse models. J Exp Med. 2015;212:287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y., Ulland T.K., Ulrich J.D., Song W., Tzaferis J.A., Hole J.T. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med. 2016;213:667–675. doi: 10.1084/jem.20151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang T., Zhang Y.D., Chen Q., Gao Q., Zhu X.C., Zhou J.S. TREM2 modifies microglial phenotype and provides neuroprotection in P301S tau transgenic mice. Neuropharmacology. 2016;105:196–206. doi: 10.1016/j.neuropharm.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 79.Haure-Mirande J.V., Audrain M., Fanutza T., Kim S.H., Klein W.L., Glabe C. Deficiency of TYROBP, an adapter protein for TREM2 and CR3 receptors, is neuroprotective in a mouse model of early Alzheimer's pathology. Acta Neuropathol. 2017;134:769–788. doi: 10.1007/s00401-017-1737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi H., Klein Z.A., Bhagat S.M., Kaufman A.C., Kostylev M.A., Ikezu T. Opposing effects of progranulin deficiency on amyloid and tau pathologies via microglial TYROBP network. Acta Neuropathol. 2017;133:785–807. doi: 10.1007/s00401-017-1668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Erdei A., Isaak A., Torok K., Sandor N., Kremlitzka M., Prechl J. Expression and role of CR1 and CR2 on B and T lymphocytes under physiological and autoimmune conditions. Mol Immunol. 2009;46:2767–2773. doi: 10.1016/j.molimm.2009.05.181. [DOI] [PubMed] [Google Scholar]
- 82.Holen H.L., Nustad K., Aasheim H.C. Activation of EphA receptors on CD4+CD45RO+ memory cells stimulates migration. J Leukoc Biol. 2010;87:1059–1068. doi: 10.1189/jlb.0709497. [DOI] [PubMed] [Google Scholar]
- 83.Nowyhed H.N., Chandra S., Kiosses W., Marcovecchio P., Andary F., Zhao M. ATP binding cassette transporter ABCA7 regulates NKT cell development and function by controlling CD1d expression and lipid raft content. Sci Rep. 2017;7:40273. doi: 10.1038/srep40273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferrari R., Hernandez D.G., Nalls M.A., Rohrer J.D., Ramasamy A., Kwok J.B. Frontotemporal dementia and its subtypes: a genome-wide association study. Lancet Neurol. 2014;13:686–699. doi: 10.1016/S1474-4422(14)70065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kouri N., Ross O.A., Dombroski B., Younkin C.S., Serie D.J., Soto-Ortolaza A. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun. 2015;6:7247. doi: 10.1038/ncomms8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoglinger G.U., Melhem N.M., Dickson D.W., Sleiman P.M., Wang L.S., Klei L. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernandez-Botran R., Ahmed Z., Crespo F.A., Gatenbee C., Gonzalez J., Dickson D.W. Cytokine expression and microglial activation in progressive supranuclear palsy. Parkinsonism Relat Disord. 2011;17:683–688. doi: 10.1016/j.parkreldis.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerhard A., Trender-Gerhard I., Turkheimer F., Quinn N.P., Bhatia K.P., Brooks D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in progressive supranuclear palsy. Mov Disord. 2006;21:89–93. doi: 10.1002/mds.20668. [DOI] [PubMed] [Google Scholar]
- 89.Gerhard A., Watts J., Trender-Gerhard I., Turkheimer F., Banati R.B., Bhatia K. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in corticobasal degeneration. Mov Disord. 2004;19:1221–1226. doi: 10.1002/mds.20162. [DOI] [PubMed] [Google Scholar]
- 90.Ishizawa K., Dickson D.W. Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J Neuropathol Exp Neurol. 2001;60:647–657. doi: 10.1093/jnen/60.6.647. [DOI] [PubMed] [Google Scholar]
- 91.Liddelow S., Barres B. SnapShot: astrocytes in health and disease. Cell. 2015;162:1170–1170.e1. doi: 10.1016/j.cell.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 92.Stobart J.L., Anderson C.M. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front Cell Neurosci. 2013;7:38. doi: 10.3389/fncel.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liddelow S.A., Barres B.A. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 94.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laurent C., Dorothee G., Hunot S., Martin E., Monnet Y., Duchamp M. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain. 2017;140:184–200. doi: 10.1093/brain/aww270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rodriguez-Arellano J.J., Parpura V., Zorec R., Verkhratsky A. Astrocytes in physiological aging and Alzheimer's disease. Neuroscience. 2016;323:170–182. doi: 10.1016/j.neuroscience.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 97.Forman M.S., Lal D., Zhang B., Dabir D.V., Swanson E., Lee V.M. Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J Neurosci. 2005;25:3539–3550. doi: 10.1523/JNEUROSCI.0081-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 99.Koenigsknecht-Talboo J., Landreth G.E. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci. 2005;25:8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paolicelli R.C., Bisht K., Tremblay M.E. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walker D.G., Lue L.F. Understanding the neurobiology of CD200 and the CD200 receptor: a therapeutic target for controlling inflammation in human brains? Future Neurol. 2013;8:321–332. doi: 10.2217/fnl.13.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wohleb E.S. Neuron-microglia interactions in mental health disorders: “For Better, and For Worse”. Front Immunol. 2016;7:544. doi: 10.3389/fimmu.2016.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guillot-Sestier M.V., Doty K.R., Town T. Innate immunity fights Alzheimer's disease. Trends Neurosci. 2015;38:674–681. doi: 10.1016/j.tins.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heneka M.T., Golenbock D.T., Latz E. Innate immunity in Alzheimer's disease. Nat Immunol. 2015;16:229–236. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- 105.Fan Z., Brooks D.J., Okello A., Edison P. An early and late peak in microglial activation in Alzheimer's disease trajectory. Brain. 2017;140:792–803. doi: 10.1093/brain/aww349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fan Z., Okello A.A., Brooks D.J., Edison P. Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer's disease. Brain. 2015;138:3685–3698. doi: 10.1093/brain/awv288. [DOI] [PubMed] [Google Scholar]
- 107.Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K. A unique microglia type associated with restricting development of Alzheimer's disease. Cell. 2017;169:1276–1290e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 108.Simic G., Babic Leko M., Wray S., Harrington C.R., Delalle I., Jovanov-Milosevic N. Monoaminergic neuropathology in Alzheimer's disease. Prog Neurobiol. 2017;151:101–138. doi: 10.1016/j.pneurobio.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heneka M.T., Nadrigny F., Regen T., Martinez-Hernandez A., Dumitrescu-Ozimek L., Terwel D. Locus ceruleus controls Alzheimer's disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci U S A. 2010;107:6058–6063. doi: 10.1073/pnas.0909586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kelly S.C., He B., Perez S.E., Ginsberg S.D., Mufson E.J., Counts S.E. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer's disease. Acta Neuropathol Commun. 2017;5:8. doi: 10.1186/s40478-017-0411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gabbita S.P., Johnson M.F., Kobritz N., Eslami P., Poteshkina A., Varadarajan S. Oral TNFalpha modulation alters neutrophil infiltration, improves cognition and diminishes tau and amyloid pathology in the 3xTgAD mouse model. PLoS One. 2015;10:e0137305. doi: 10.1371/journal.pone.0137305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zenaro E., Pietronigro E., Della Bianca V., Piacentino G., Marongiu L., Budui S. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 113.El Khoury J., Toft M., Hickman S.E., Means T.K., Terada K., Geula C. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 114.Naert G., Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31:6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gyoneva S., Kim D., Katsumoto A., Kokiko-Cochran O.N., Lamb B.T., Ransohoff R.M. Ccr2 deletion dissociates cavity size and tau pathology after mild traumatic brain injury. J Neuroinflammation. 2015;12:228. doi: 10.1186/s12974-015-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bellucci A., Bugiani O., Ghetti B., Spillantini M.G. Presence of reactive microglia and neuroinflammatory mediators in a case of frontotemporal dementia with P301S mutation. Neurodegener Dis. 2011;8:221–229. doi: 10.1159/000322228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schofield E., Kersaitis C., Shepherd C.E., Kril J.J., Halliday G.M. Severity of gliosis in Pick's disease and frontotemporal lobar degeneration: tau-positive glia differentiate these disorders. Brain. 2003;126:827–840. doi: 10.1093/brain/awg085. [DOI] [PubMed] [Google Scholar]
- 118.Bellucci A., Westwood A.J., Ingram E., Casamenti F., Goedert M., Spillantini M.G. Induction of inflammatory mediators and microglial activation in mice transgenic for mutant human P301S tau protein. Am J Pathol. 2004;165:1643–1652. doi: 10.1016/S0002-9440(10)63421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maphis N., Xu G., Kokiko-Cochran O.N., Jiang S., Cardona A., Ransohoff R.M. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain. 2015;138:1738–1755. doi: 10.1093/brain/awv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoshiyama Y., Higuchi M., Zhang B., Huang S.M., Iwata N., Saido T.C. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 121.Nilson A.N., English K.C., Gerson J.E., Barton Whittle T., Nicolas Crain C., Xue J. Tau oligomers associate with inflammation in the brain and retina of tauopathy mice and in neurodegenerative diseases. J Alzheimers Dis. 2017;55:1083–1099. doi: 10.3233/JAD-160912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kovac A., Zilka N., Kazmerova Z., Cente M., Zilkova M., Novak M. Misfolded truncated protein tau induces innate immune response via MAPK pathway. J Immunol. 2011;187:2732–2739. doi: 10.4049/jimmunol.1100216. [DOI] [PubMed] [Google Scholar]
- 123.Heneka M.T., Kummer M.P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang S, Maphis N, Binder J, Weston L, Bigio E, Geula C, Bhaskar K. Pathological tau activates inflammasomes (and IL-1b) and contributes to neuroinflammation relevant to tauopathies. Poster #61, 5th Venusberg Meeting on Neuroinflammation, Bonn. 2017/05/11–13.
- 125.Laurent C., Burnouf S., Ferry B., Batalha V.L., Coelho J.E., Baqi Y. A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol Psychiatry. 2016;21:97–107. doi: 10.1038/mp.2014.151. [DOI] [PubMed] [Google Scholar]
- 126.Lee D.C., Rizer J., Selenica M.L., Reid P., Kraft C., Johnson A. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J Neuroinflammation. 2010;7:56. doi: 10.1186/1742-2094-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kitazawa M., Oddo S., Yamasaki T.R., Green K.N., LaFerla F.M. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bennett R.E., DeVos S.L., Dujardin S., Corjuc B., Gor R., Gonzalez J. Enhanced tau aggregation in the presence of amyloid beta. Am J Pathol. 2017;187:1601–1612. doi: 10.1016/j.ajpath.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bhaskar K., Konerth M., Kokiko-Cochran O.N., Cardona A., Ransohoff R.M., Lamb B.T. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68:19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nash K.R., Lee D.C., Hunt J.B., Jr., Morganti J.M., Selenica M.L., Moran P. Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy. Neurobiol Aging. 2013;34:1540–1548. doi: 10.1016/j.neurobiolaging.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bolos M., Llorens-Martin M., Perea J.R., Jurado-Arjona J., Rabano A., Hernandez F. Absence of CX3CR1 impairs the internalization of Tau by microglia. Mol Neurodegener. 2017;12:59. doi: 10.1186/s13024-017-0200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y., Liu L., Barger S.W., Griffin W.S. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gorlovoy P., Larionov S., Pham T.T., Neumann H. Accumulation of tau induced in neurites by microglial proinflammatory mediators. FASEB J. 2009;23:2502–2513. doi: 10.1096/fj.08-123877. [DOI] [PubMed] [Google Scholar]
- 134.Garwood C.J., Cooper J.D., Hanger D.P., Noble W. Anti-inflammatory impact of minocycline in a mouse model of tauopathy. Front Psychiatry. 2010;1:136. doi: 10.3389/fpsyt.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Noble W., Garwood C., Stephenson J., Kinsey A.M., Hanger D.P., Anderton B.H. Minocycline reduces the development of abnormal tau species in models of Alzheimer's disease. FASEB J. 2009;23:739–750. doi: 10.1096/fj.08-113795. [DOI] [PubMed] [Google Scholar]
- 136.Maphis N., Xu G., Kokiko-Cochran O.N., Cardona A.E., Ransohoff R.M., Lamb B.T. Loss of tau rescues inflammation-mediated neurodegeneration. Front Neurosci. 2015;9:196. doi: 10.3389/fnins.2015.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Borsini A., Zunszain P.A., Thuret S., Pariante C.M. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015;38:145–157. doi: 10.1016/j.tins.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 138.Dioli C., Patricio P., Trindade R., Pinto L.G., Silva J.M., Morais M. Tau-dependent suppression of adult neurogenesis in the stressed hippocampus. Mol Psychiatry. 2017;22:1110–1118. doi: 10.1038/mp.2017.103. [DOI] [PubMed] [Google Scholar]
- 139.Rogers J., Luber-Narod J., Styren S.D., Civin W.H. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 140.Togo T., Akiyama H., Iseki E., Kondo H., Ikeda K., Kato M. Occurrence of T cells in the brain of Alzheimer's disease and other neurological diseases. J Neuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 141.Zotova E., Bharambe V., Cheaveau M., Morgan W., Holmes C., Harris S. Inflammatory components in human Alzheimer's disease and after active amyloid-beta42 immunization. Brain. 2013;136:2677–2696. doi: 10.1093/brain/awt210. [DOI] [PubMed] [Google Scholar]
- 142.Lueg G., Gross C.C., Lohmann H., Johnen A., Kemmling A., Deppe M. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer's disease. Neurobiol Aging. 2015;36:81–89. doi: 10.1016/j.neurobiolaging.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 143.Pellicano M., Larbi A., Goldeck D., Colonna-Romano G., Buffa S., Bulati M. Immune profiling of Alzheimer patients. J Neuroimmunol. 2012;242:52–59. doi: 10.1016/j.jneuroim.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 144.Liu C., Cui G., Zhu M., Kang X., Guo H. Neuroinflammation in Alzheimer's disease: chemokines produced by astrocytes and chemokine receptors. Int J Clin Exp Pathol. 2014;7:8342–8355. [PMC free article] [PubMed] [Google Scholar]
- 145.Wang D.B., Dayton R.D., Zweig R.M., Klein R.L. Transcriptome analysis of a tau overexpression model in rats implicates an early pro-inflammatory response. Exp Neurol. 2010;224:197–206. doi: 10.1016/j.expneurol.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burnouf S., Martire A., Derisbourg M., Laurent C., Belarbi K., Leboucher A. NMDA receptor dysfunction contributes to impaired brain-derived neurotrophic factor-induced facilitation of hippocampal synaptic transmission in a Tau transgenic model. Aging Cell. 2013;12:11–23. doi: 10.1111/acel.12018. [DOI] [PubMed] [Google Scholar]
- 147.Sokolova A., Hill M.D., Rahimi F., Warden L.A., Halliday G.M., Shepherd C.E. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer's disease. Brain Pathol. 2009;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tripathy D., Thirumangalakudi L., Grammas P. RANTES upregulation in the Alzheimer's disease brain: a possible neuroprotective role. Neurobiol Aging. 2010;31:8–16. doi: 10.1016/j.neurobiolaging.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rostene W., Dansereau M.A., Godefroy D., Van Steenwinckel J., Reaux-Le Goazigo A., Melik-Parsadaniantz S. Neurochemokines: a menage a trois providing new insights on the functions of chemokines in the central nervous system. J Neurochem. 2011;118:680–694. doi: 10.1111/j.1471-4159.2011.07371.x. [DOI] [PubMed] [Google Scholar]
- 150.Marciniak E., Faivre E., Dutar P., Alves Pires C., Demeyer D., Caillierez R. The Chemokine MIP-1alpha/CCL3 impairs mouse hippocampal synaptic transmission, plasticity and memory. Sci Rep. 2015;5:15862. doi: 10.1038/srep15862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cudaback E., Yang Y., Montine T.J., Keene C.D. APOE genotype-dependent modulation of astrocyte chemokine CCL3 production. Glia. 2015;63:51–65. doi: 10.1002/glia.22732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Man S.M., Ma Y.R., Shang D.S., Zhao W.D., Li B., Guo D.W. Peripheral T cells overexpress MIP-1alpha to enhance its transendothelial migration in Alzheimer's disease. Neurobiol Aging. 2007;28:485–496. doi: 10.1016/j.neurobiolaging.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 153.Zenaro E., Piacentino G., Constantin G. The blood-brain barrier in Alzheimer's disease. Neurobiol Dis. 2017;107:41–56. doi: 10.1016/j.nbd.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Engelhardt B., Ransohoff R.M. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 155.Engelhardt B., Vajkoczy P., Weller R.O. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 156.Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. doi: 10.1126/science.aag2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bartels A.L., Willemsen A.T., Kortekaas R., de Jong B.M., de Vries R., de Klerk O. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson's disease, PSP and MSA. J Neural Transm (Vienna) 2008;115:1001–1009. doi: 10.1007/s00702-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kovac A., Zilkova M., Deli M.A., Zilka N., Novak M. Human truncated tau is using a different mechanism from amyloid-beta to damage the blood-brain barrier. J Alzheimers Dis. 2009;18:897–906. doi: 10.3233/JAD-2009-1197. [DOI] [PubMed] [Google Scholar]
- 159.Blair L.J., Frauen H.D., Zhang B., Nordhues B.A., Bijan S., Lin Y.C. Tau depletion prevents progressive blood-brain barrier damage in a mouse model of tauopathy. Acta Neuropathol Commun. 2015;3:8. doi: 10.1186/s40478-015-0186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fisher Y., Nemirovsky A., Baron R., Monsonego A. T cells specifically targeted to amyloid plaques enhance plaque clearance in a mouse model of Alzheimer's disease. PLoS One. 2010;5:e10830. doi: 10.1371/journal.pone.0010830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dansokho C., Ait Ahmed D., Aid S., Toly-Ndour C., Chaigneau T., Calle V. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain. 2016;139:1237–1251. doi: 10.1093/brain/awv408. [DOI] [PubMed] [Google Scholar]
- 162.Alves S., Churlaud G., Audrain M., Michaelsen-Preusse K., Fol R., Souchet B. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer's disease mice. Brain. 2017;140:826–842. doi: 10.1093/brain/aww330. [DOI] [PubMed] [Google Scholar]
- 163.Liblau R.S., Gonzalez-Dunia D., Wiendl H., Zipp F. Neurons as targets for T cells in the nervous system. Trends Neurosci. 2013;36:315–324. doi: 10.1016/j.tins.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 164.Pool M., Rambaldi I., Darlington P.J., Wright M.C., Fournier A.E., Bar-Or A. Neurite outgrowth is differentially impacted by distinct immune cell subsets. Mol Cell Neurosci. 2012;49:68–76. doi: 10.1016/j.mcn.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 165.Baruch K., Deczkowska A., Rosenzweig N., Tsitsou-Kampeli A., Sharif A.M., Matcovitch-Natan O. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer's disease. Nat Med. 2016;22:135–137. doi: 10.1038/nm.4022. [DOI] [PubMed] [Google Scholar]
- 166.Baruch K., Rosenzweig N., Kertser A., Deczkowska A., Sharif A.M., Spinrad A. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology. Nat Commun. 2015;6:7967. doi: 10.1038/ncomms8967. [DOI] [PMC free article] [PubMed] [Google Scholar]