Abstract
This systematic literature review investigates factors impacting on clinicians’ decisions to offer genetic tests in their practice, and maps them to a theoretical behaviour change framework. Better understanding of these factors will inform the design of effective interventions to integrate genomics tests into clinical care. We conducted a narrative synthesis of empirical research of medical specialists’ perspectives on and experiences of offering genetic tests to their patients. This review was based upon the PRISMA statement and guidelines for reviewing qualitative research. Four electronic data sources were searched—MEDLINE, EMBASE, CINAHL, PubMed. Studies were independently assessed by two authors. Content analysis was applied to map the findings of included studies to a framework validated for behaviour and implementation research, the Theoretical Domains Framework (TDF). The TDF describes 14 factors known to influence behaviour and has been applied in diverse clinical settings to understand and/or modify health professional behaviour. Thirty-four studies published in 39 articles met inclusion and quality criteria. Most studies were published after 2011 (54%), Northern American (82%), quantitative in design (68%) and addressed familial cancer genetic tests (53%). Of the 14 TDF factors, 13 were identified. The three most common factors were: Environmental Context and Resources (n = 33), Beliefs about Consequences (n = 26), and Knowledge (n = 23). To support the adoption of genomic tests beyond specialist services, nuanced interventions targeting considerations beyond clinician education are needed. For instance, interventions addressing organisational constraints which may restrict clinicians’ ability to offer genomic tests are required alongside those targeting factors intrinsic to the clinician.
Introduction
Genomic technologies have significantly increased the breadth of genomic variation that can be accessed in a clinically relevant timeframe, and have potential to radically transform healthcare service provision across multiple, if not all clinical specialties [1]. While specialist genetic and cancer services are currently at the forefront of introducing genomic tests, it is likely that use of this testing will be applied in the ‘mainstream’ of other specialist healthcare over time. Early prospective evidence supports the cost-effectiveness of genomic tests in specific contexts [2]. However, as with many clinical research findings, realising patient outcomes is dependent on clinicians adopting the test into usual practice, and integrating the resultant information appropriately into clinical management decisions.
Experts have identified numerous barriers to adoption of genomic tests, one of which is lack of clinician preparedness [3]. It is anticipated that specialists in clinical genetics would be early adopters of new genomic technologies where possible [4], but this may not be the case for other medical disciplines. Previous research assessing the introduction of single-gene tests described ‘clinical inertia’ [5] between test development and its integration into mainstream healthcare findings [6–8]. Concerns around the introduction of genomic tests echo these [5]. Health professionals remain underprepared to appropriately incorporate even single-gene genetic testing into their practice, let alone more extensive multigene panels and genomic sequencing [9–14]. This is important as over-use and under-use of genomic tests by medical practitioners could reduce benefits to patients and cost-effectiveness of testing [15–17]. A common, but limited, reflex response to ‘clinical inertia’ has been to focus on educating clinicians to address a knowledge gap [6, 9]. Hamilton et al. investigated the successful implementation of genetic tests in the Veterans Health Administration system as a surrogate for genomic tests, finding that this relied on knowledgeable clinicians and organisational-level facilitators [18]. Medical practitioners’ decision-making regarding genetic or genomic testing in mainstream practice however, has not been systematically examined. Klitzman et al.s' observation remains pertinent; ‘Many questions remain concerning whether, when and how physicians order genetic tests and what factors are involved in their decisions’ [19].
As has been identified within the Cochrane Effective Practice and Organization of Care (EPOC) group [20–22], overcoming ‘clinical inertia’ to maximise appropriate use of testing requires deliberate, targeted interventions to optimise behaviour in the relevant clinical context. So, to effectively integrate genomic testing into mainstream medicine, factors which impact on medical practitioners’ decisions to offer a genomic test must be systematically identified, then optimised [23]. Such an approach has been recommended by the UK’s Chief Medical Officer in the 2016 annual report, Generation Genome [24], but a systematic review identified a lack of relevant research [23].
The Theoretical Domains Framework (TDF) provides a validated agnostic tool by which factors influencing clinicians’ decisions to offer a genomic test can be systematically identified. Based on behaviour change theory, this framework categorises drivers and barriers of behaviour change into 14 key domains such as social influences, knowledge, skill and environmental context (Table 1) [25]. Three key aspects make it particularly suitable for research into clinician behaviour and decision-making around genomic testing. First, the TDF has been extensively used to investigate clinicians’ practices and is thus well validated in this field. Second, it is agnostic to clinical specialty—an important consideration given the potential breadth of application of genomics in clinical practice. Finally, the TDF sits at the core of the Behaviour Change Wheel: developed by Michie et al. [26]; the Behaviour Change Wheel maps the 14 TDF factors onto a clinician’s capability, opportunity and motivation to offer a test, and then identifies evidence-based intervention mechanisms targeted to these areas. Through the Behaviour Change Wheel, the TDF not only provides a direct framework with which to identify barriers and facilitators of genomic test integration, but also indirectly identifies plausible, evidence-based interventions to optimise its integration.
Table 1.
Description of TDF domains grouped into the COM-B (capability, opportunity and motivation influence behaviour) model (Cane et al.) and descriptions as operationalized in the context of this study
| COM-B component | TDF domain | TDF domain definition [26] | TDF definition in context | |
|---|---|---|---|---|
| Capability | Knowledge | An awareness of the existence of something | Clinicians’ awareness and understanding (through education/training) of the principles and process of offering genetic testing | |
| Skills | An ability or proficiency acquired though practice | Clinicians’ physical and psychological ability or proficiency acquired through practice to make decisions whether or not to offer genetic testing | ||
| Memory, attention and decision processes | The ability to retain information focus selectively on aspects of the environment and choose between two or more alternatives | Clinicians’ ability to remember to consider genetic testing alongside other interventions for health risk identification, diagnosis, management and therapy | ||
| Behavioural regulation | Anything aimed at managing or changing objectively observed or measured actions | Clinicians’ self-created or self-imposed regulation to help make decisions about offering genetic tests | ||
| Opportunity | Social influences | Those interpersonal processes that can cause individuals to change their thoughts, feelings or behaviours | Interpersonal interactions between professionals that can influence clinicians’ thoughts, feelings or behaviour regarding offering genetic testing | |
| Environmental context and resources | Any circumstance of a person’s situation or environment that discourages or encourages the development of skills and abilities independence, social competence and adaptive behaviour | Any external circumstance of a clinicians’ situation or environment that clinicians consider discourages or encourages them to offer genetic testing | ||
| New sub-domain (code) | Description in context | |||
| Genetic test factors (E1) | Factors relating to the genetic test itself | |||
| Internal clinician factors (E2) | All factors relating to clinician other than internal factors within Capability and Motivation | |||
| Patient factors (E3) | Factors relating to a specific patient or objective patient characteristics | |||
| Organisational factors (E5) | Factors relating to the hospital organisation context (culture) and resources | |||
| Professional regulatory factors (E6) | Professional factors other than interpersonal interactions within Social Influences | |||
| Ethical, legal and social context factors (E7) | Factors relating to perceived ethical, legal and social contexts generally surrounding the test | |||
| Motivation | Social/professional role and identity | A coherent set of behaviours and displayed personal qualities of an individual in a social or work setting | Clinicians’ perceived professional role and identity in relation to offering genetic tests | |
| Beliefs about Capabilities | Acceptance of the truth, reality or validity about at ability, talent or facility that a person can put to constructive use | Clinicians’ perception about their own capability to consider genetic testing | ||
| Optimism | The confidence that things will happen for the best or that desired goals will be attained | Clinicians’ optimism or pessimism that genetic testing will be appropriately integrated into clinical practice and will improve healthcare generally | ||
| Beliefs about consequences | Acceptance of the truth, reality or validity about outcomes of a behaviour in a given situation | Clinicians’ perceptions about the value of offering genetic testing in clinical practice—whether it is worthwhile in that it will improve patient outcomes in their own practice | ||
| Intentions | A conscious decision to perform a behaviour or a resolve to act in a certain way | Clinicians’ intentions to consider genetic testing | ||
| Goals | Mental representations of outcomes or end states that an individual wants to achieve | Whether clinicians offering genetic testing is a priority within their practice | ||
| Reinforcement | Increasing the probability of a response by arranging a dependent relationship, or contingency, between the response and a given stimulus | Incentives, rewards, sanctions, reinforcement at any level that encourage or increase clinicians’ decisions to offer genetic testing | ||
| Emotion | A complex reaction pattern, involving experiential, behavioural and physiological elements, by which the individual attempts to deal with a personally significant matter | Clinicians’ feelings when they consider genetic testing | ||
Rationale and objective
Interventions to support adoption of current genomic tests will be most effective if two conditions are met. First, they are informed by a comprehensive understanding of factors influencing medical practitioners’ use of single-gene testing. Second, they are based upon a well-established, practical framework designed to optimise practitioner behaviour [23]. We sought to identify key, modifiable mediators of behaviour change which can guide the adoption of new technologies within the healthcare system, applying the Theoretical Domains Framework. This review systematically examines existing published literature to identify factors that impact upon medical specialists’ decisions to offer single-gene tests in clinical practice. To address the adoption of genomics beyond specialist genetic services, we focus on the use of tests by other medical specialists caring for people who either have a clinical condition or are at high risk of a hereditary disease.
Methods
Design
Guided by: the PRISMA statement for reporting systematic reviews [27] and Economic and Social Research Council UK guidelines for reviews incorporating qualitative research [28].
Eligibility criteria
Studies were eligible for inclusion if they satisfied the following criteria (developed by HL, AT, CG):
original primary peer-reviewed research;
obtained evidence about factors which impacted on medical specialists’ decisions to offer genetic tests;
and genetic test was germline and currently available for the patients’ clinical care.
Studies were excluded if they:
were not published in English;
involved a clinical scenario for a test that did not exist;
concerned return of results or family communication;
involved only medical (clinical) geneticists;
or concerned a prenatal, population, somatic or direct-to-consumer (DTC) genetic test.
Prenatal or preimplantation tests were excluded as this review focuses on medical specialists’ decision-making behaviour regarding genetic tests used to determine management of that person. Population screening programmes were also excluded as no clinician-based decision-making is required.
Search
Four electronic databases were selected using a detailed search strategy developed for MEDLINE and adapted for other databases (Table 2).
Table 2.
Electronic search results
| Search terma | ‘Genetic testing/’, ‘Physician’s Practice Patterns/’ and ‘Health Knowledge, Attitudes, Practice/’. | |||
|---|---|---|---|---|
| Electronic databases searched | Date searched | Search type | Years | Result |
| MEDLINE | 29/09/2015 | MeSH Topics | All | 339 |
| Embase | 29/09/2015 | MeSH Topics | All | 418 |
| CINAHL | 29/09/2015 | MeSH Topics | All | 263 |
| PubMed | 29/09/2015 | Keyword | All | 146 |
| TOTAL | 1166 | |||
| Duplicates removed: | TOTAL | 1026 | ||
aFull search term available upon request
Study selection
Studies were assessed for inclusion by two reviewers (a third used if discordance was encountered) as follows: exclusion based on title, abstract and screening the remaining full-texts for inclusion. Reference lists were hand-searched during study screening and selection.
Data extraction
Identifying information was extracted: first author, publication year, country of origin and study design (quantitative, qualitative or mixed methods). The following was also collected: principal objective, clinician population (including clinician type and sample size), response rate, clinical domain (e.g. oncology, paediatrics or multiple domains), indication for test (e.g. predictive or diagnostic) and genetic testing platform (traditional single-gene testing or whole-genome testing). Using deductive content analysis [29], study findings were mapped in a non-mutually exclusive manner to the 14 TDF domains (Table 1) and further sub-grouped by clinician’s capability, opportunity and motivation according to the Behaviour Change Wheel framework. Findings which were acting on the medical specialists’ decision to offer genetic testing were investigated and mapped to the TDF. Coding was conducted by two authors (HL and JP) and discussed and amended among all authors. Findings could be coded to multiple domains. The QSR International software NVivo10 facilitated data management and storage [30].
Risk of bias in individual studies
Each article was independently evaluated for methodological quality (HL, JP). Due to heterogeneity in study design and analytical approaches, a quality appraisal tool, based upon QualSyst [31] as described by Paul and colleagues [32], was adapted and applied to best assess the elements relevant to the aims of the review and study design. Briefly, all studies were assessed using eight standard questions plus additional questions specific to the underlying methodology: qualitative or quantitative. The total study score was divided by the total possible score. The questions applied and scoring scheme are provided in Supplementary Table 1. Only those with an average score above 0.70 were included.
Synthesis of results
A meta-analysis was not feasible due to study heterogeneity in primary aims, design, method and populations. A narrative synthesis [28] was therefore conducted to allow for comprehensive description and interpretation. Findings are presented in descriptive frequencies of TDF domains and narrative form. As a finding may map to more than one domain, its discussion in regard to one domain below does not exclude its inclusion in other domains. The unit of analysis, or ‘study’, was the series of published articles reported from a single data set.
Results
Search outcome
From the total articles retrieved (Table 2), most were removed at title or abstract stage, while only five were included through reference-searching. Eight were excluded based upon quality score, leaving a total of 39 articles from 34 studies [15, 19, 33–69] (Fig. 1).
Fig. 1.
PRISMA flow demonstrating study selection
Characteristics of included studies
Table 3 summarises characteristics of the 34 studies included. The majority were published within the last five years (54%), originated from Northern America (82%), and were quantitative in design (68%). Collectively, they spanned many medical disciplines and included a mixture of medical specialists (53%), although were highly enriched for studies based within oncology (53%). A wide spectrum of genetic tests was explored, the majority being predictive genetic tests (65%).
Table 3.
Study characteristics
| Publication date | N (% of 39 articles) |
|---|---|
| ≤2000 | 4 (10%) |
| 2001–2005 | 8 (21%) |
| 2006–2010 | 6 (15%) |
| 2011–2015 | 21 (54%) |
| Country of origin | N (% of 34 studies) |
| North America | 28 (82%) |
| Europe | 4 (12%) |
| Australasia | 1 (3%) |
| Asia | 1 (3%) |
| Study design | N (34 studies) |
| Quantitative | 23 (68%) |
| Qualitative | 10 (29%) |
| Mixed methods | 1 (3%) |
| Clinician population a | N (34 studies) |
| Mixed clinical population | 18 (53%) |
| Obstetricians/gynaecologists | 11 (32%) |
| Internists | 10 (29%) |
| Oncologists | 9 (27%) |
| Family physicians/GPs | 9 (27%) |
| Surgeons | 8 (24%) |
| Paediatricians | 5 (15%) |
| Neurologists | 3 (9%) |
| Psychiatrists | 3 (9%) |
| Not specified in study | 2 (6%) |
| Other (including urologists, endocrinologists, geriatricians, cardiologists) | 6 (18%) |
| Clinical domain a | N (34 studies) |
| Oncology | 18 (53%) |
| Multiple clinical domains | 11 (32%) |
| Paediatrics | 6 (18%) |
| Neurology | 5 (15%) |
| Pre-pregnancy care | 5 (15%) |
| Other (including psychiatry, haematology, endocrinology, immunology) | 10 (29%) |
| Indication for test a | N (34 studies) |
| Predictive | 22 (65%) |
| Diagnostic | 9 (27%) |
| Various indications | 8 (24%) |
| Pharmacogenetic | 7 (21%) |
| Carrier status | 4 (12%) |
aCategories not mutually exclusive
Overview of study designs and quality
Most studies investigated clinicians’ knowledge, attitudes or beliefs about genetics and testing in clinical practice (Table 4, Study Details), using exploratory cross-sectional surveys and descriptive statistics. Twenty-five explored perspectives and knowledge of traditional diagnostic genetic testing techniques, while seven examined pharmacogenetics to guide drug dosage in various health conditions [37, 46, 52, 56, 60, 61, 64]. Only two studies explored use of newer genetic tests such as microarray testing [35, 58]. No studies examined the use of genomic tests (multigene panel, exome or genome sequencing). Many studies had low response rates, three being below 10% [39, 47, 59, 61]. Few provided clear justifications for their methodological approach and most lacked an explicit theoretical model in their design; only one qualitative study described using ‘grounded theory’ [35]. Studies generally included limited detail regarding approaches to data collection, clinicians’ demographics and study setting.
Table 4.
Study details
| Study number | First author, year of publication, Country of origin |
Clinical domain indication for test Testing platform (conventional unless noted) |
Principal objective of study Study design (data collection and analysis) |
Relevant medical specalist population* and sample size Quality appraisal score Response Rate (RR) (quantitative studies only) *excluding genetic specialists or non-hospital-based clinicians |
TDF domain codes mapped to study findings |
|---|---|---|---|---|---|
| Quantitative studies | |||||
| 1 | Acharya, 2013 USA |
Paediatrics: Fragile X associated disorders Various: Diagnostic, Carrier status |
Investigate clinicians’ familiarity with and attitudes toward Fragile X genetic testing (GT). Cross-sectional survey: closed and open questions, descriptive and analytical. |
294 Paediatricians 0.85 RR 61% |
C–K O–E (3, 5, 6, 7), SI M–PI, BCap, BCons |
| 2 | Beitsch, 2014 USA |
Oncology: familial breast and ovarian cancer Predictive |
Assess U.S. breast surgeons’ current practice regarding GT Cross-sectional survey: online, closed questions, descriptive and analytical |
907 Surgeons (breast) 0.75 RR 35% |
C–K, MAD O–E (3, 5, 6, 7) M–PI, BCap, BCons, G, I |
| 3 | Chase, 2002 USA |
Neurology: Alzheimer’s disease Predictive |
Examine knowledge, preferences and use of GT. Survey: 6 domains, closed questions, descriptive and analytical. |
171 Clinicians: Internists (32%), Family physicians (30%), Neurologists (18%), Psychiatrists (11%), Geriatricians (5%), Other specialists (4%) 0.85 RR 40% |
C–K O–E (1, 2, 3, 5) M–BCons |
| 4 | Cox, 2012 USA |
Oncology: familial breast and ovarian cancer, familial colorectal cancer Predictive |
Evaluate use and knowledge of BRCA1/2 GT. Cross-sectional survey: closed questions, descriptive and analytical. |
630 Clinicians: 333 Obstetricians/Gynaecologists (28%), 297 Other specialists (25%) 0.95 RR 57% |
C–K O–E (1, 2, 3, 6, 7) M–BCap, BCons |
| 5 | Darcy, 2011 USA |
Pre-pregnancy care: Cystic Fibrosis (CF) Carrier status |
Determine clinicians’ awareness of practice guidelines, variety in practice and level of knowledge regarding CF genetics and result interpretation. Explore potential barriers to offering screening and whether academic affiliation or type of practice influence outcome. Survey: 21 items, closed questions, descriptive and analytical. |
156 Obstetricians/Gynaecologists 0.70 RR 8% |
C–K O–E (3, 5, 6, 7), SI M–BCap, BCons |
| 6 | Doksum, 2001 USA |
Pre-pregnancy care: CF Carrier status |
Assess impact of NIH statement and factors associated with clinicians’ GT practice patterns. 2 surveys: brief pre-conference survey, longer post-conference survey, 4 domains, closed questions, descriptive and analytical |
107 Obstetricians/Gynaecologists 0.93 RR 51% |
C–K O–E (3, 5, 6, 7) M–BCap, O, BCons |
| 7 | Doksum, 2003 USA |
Oncology: familial breast and ovarian cancer Predictive |
Assess whether knowledge about genetics of breast cancer is greater in non-genetic clinicians who had discussed and/or ordered GT rather than those who had not discussed or ordered GT. Cross-sectional survey: 4 domains, closed questions, descriptive and analytical |
803 Clinicians: 257 Oncologists, 312 Obstetricians/Gynaecologists, 234 Internists 0.98 RR 40% |
C–K, MAD, S O–E (1, 2, 3, 6, 7), SI M–BCap, BCons, R |
| 8 | a. Freedman, 2003 b. Vadaparampil, 2005 c. Wideroff, 2003 USA |
Oncology: familial breast and ovarian cancer; familial colorectal cancer Predictive |
a. Assess clinicians’ opinions of a range of issues related to GT for breast cancer. b. Determine proportion of clinicians who received advertisements for breast cancer GT when clinically introduced (1999–2000); assess associated characteristics; and measure perceived importance of commercial advertisements in clinicians’ decisions to recommend GT. c. Determine prevalence and clinician associated characteristics of GT use among clinicians in selected specialties. Survey: 15 min, closed questions, descriptive and analytical. |
431 Clinicians: 221 Oncologists, 123 Surgeons, 45 Urologists, 42 Internists (Gastroenterologists) 0.88 RR 72% |
O–E (2, 3, 5, 6, 7) M–BCap, I |
| 9 | a. Geller, 1998 b. James, 1998 USA |
Oncology: familial breast and ovarian cancer Predictive |
a. Determine attitudes and beliefs regarding causes of breast cancer, benefits and harms of GT, opinions regarding topics to be discussed pre-tests, and preferred role in testing decisions. b. Explore readiness to meet guidelines for informed consent for GT. Survey: case-based scenario, closed questions, descriptive and analytical. |
230 Clinicians: 69 Internists, 47 Oncologists, 59 Obstetricians/Gynaecologists, 55 Surgeons 0.95 RR 51% |
C–K O–E (1, 2, 3, 5, 7) M–PI, BCons |
| 10 | Harris, 2014 USA |
Paediatrics: Brugada syndrome (BrS) Diagnostic |
Describe current practice for diagnosis and treatment of BrS Web-based survey, closed and open questions, descriptive analysis. |
83 Paediatricians (electrophysiologist specialists) 0.70 RR 41% |
C–MAD M–BCons |
| 11 | Kadafour, 2009 USA |
Haematology: drug therapy for thromboembolic conditions Pharmacogenetic |
Assess perception and knowledge of warfarin pharmacogenetic testing, and identify barriers to its use in clinical practice. Survey: 25 item, closed questions, descriptive and analytical. |
38 Internists (anticoagulation provider) 0.80 RR 22% |
C–S O–E (1, 3, 5, 6, 7) M–BCap, O, BCons |
| 12 | Klitzman, 2013 USA |
Multiple: 20 different diseases over many specialties Various indications |
Explore clinicians’ behaviour regarding GT and factors which may be influence their decisions. Survey: 44 item, closed questions, descriptive and analytical. |
220 Internists 0.78 RR 20% |
C–K O–E (3, 5, 6, 7), SI M–Em, PI, BCap |
| 13 | a. Klitzman, 2014 b. Salm, 2014 USA |
a. Psychiatry b. Neurology Various: Diagnostic, Predictive |
a. Examine psychiatrists’ views of genetic influences and use of GTs in psychiatry. b. Explores neurologists’ and psychiatrists’ knowledge, attitudes and practices concerning GTs. Cross-sectional survey: web-based. Binary and multiple logistic regression analysis. Survey developed through prior study, literature review and clinical experience |
a. 372 Psychiatrists b. 535 Clinicians: 31% Neurologists (163), 69% Psychiatrists (372) 0.80 RR Neurologists 8%, Psychiatrists 7% |
C–K, MAD O–E (2, 3, 5, 7), SI M–BCons, I, G |
| 14 | Myers, 2006 USA |
Oncology: familial breast and ovarian cancer Predictive Conventional and Genomic testing |
Assess impact of DTC marketing by a biotechnology company on clinicians’ knowledge and practice patterns. Survey: 35 item, closed questions, descriptive and analytical. |
1052 Clinicians: 113 Oncologists, 241 Obstetricians/Gynaecologists, 321 Internists, 377 Family physicians 0.90 RR 66% |
C–K O–E (2, 3, 4, 5, 6, 7) M–G |
| 15 | Ngoi, 2013 Singapore |
Oncology: familial breast and ovarian cancer Various: Predictive, Pharmacogenetic |
Evaluate awareness, attitudes and concerns of breast cancer patients and cancer clinicians regarding new GTs CYP2D6 and Oncotype DX, compared to traditional BRCA1/2 GT. Survey: closed questions, descriptive and analytical. |
Cancer clinicians: 61% Oncologists, 15% Surgeons (Breast), 15% Oncologists (radiation) and 9% Other specialists 0.73 RR N/A (convenience sampling) |
C–K, S O–E (1, 3, 7) M–BCons |
| 16 | Nyrhinen, 2007 Finland |
Multiple: Paediatrics, Pre-Pregnancy care, Oncology Various: Diagnostic, Predictive |
Identify ways in which privacy and equality are viewed according to adult patients, the parents of child patients and clinicians in pre-analytic and post-analytic phases of diagnostic GT. Survey: 9 item, closed questions, descriptive and analytical. |
20 Clinicians: Neurologists, Obstetricians/Gynaecologists 0.85 RR 32% |
O–E (3, 5, 7) M–BCap |
| 17 | Pal, 2013 USA |
Oncology: familial breast and ovarian cancer Predictive |
Describe clinician knowledge and practices related to BRCA testing and management. Explore how training may contribute to practice patterns. Survey: 4 domains, closed questions, descriptive. |
53 Oncologists, Surgeons, Obstetricians/Gynaecologists, Other specialists 0.83 RR 21% |
C–K, S O–E6 M–PI, BCons |
| 18 | Peppercorn, 2013 USA |
Oncology: familial breast and ovarian cancer Various: Predictive, Pharmacogenetic |
Assess clinical use and attitudes of CYP2D6 GT. Survey: closed questions, descriptive. |
201 Oncologists 1.00 RR 44% |
C–K O–E (1, 5, 6, 7), SI M–BCons |
| 19 | Plon, 2011 USA |
Oncology: familial breast and ovarian cancer Predictive |
Comparison of GT and cancer risk management recommendations for healthy at-risk women when GT identifies a deleterious mutation or VUS in their relative with cancer. Survey: case-based scenarios and closed questions, descriptive and analytical. |
225 Clinicians: Family physicians, Internists, Surgeons, Obstetricians/Gynaecologists, Oncologists 0.80 RR 30% |
C–K, S O–E (1, 3, 6), SI M–BCons |
| 20 | Selkirk, 2013 USA |
Multiple clinical domains Various: Diagnostic, Predictive, Pharmacogenetic |
Assess clinician preparedness to incorporate genomic and pharmacogenetic testing into practice (knowledge, experience, comfort level, barriers and expectations for practice). Survey: 30 items, closed questions, descriptive and analytical. |
Surgeons, Internists, Paediatricians 0.80 RR 13% |
C–S O–E (3, 5, 6), SI M–Em, PI, R, G |
| 21 | Stanek, 2012 USA |
Multiple: Neurology, Oncology, Psychiatry Pharmacogenetic |
Obtain baseline measure of adoption of testing, including assessment of knowledge, training, beliefs, readiness, concerns and preferred information sources. Evaluate characteristics that predispose clinicians to be early or late adopters of GT. Survey: 26 items, closed questions, descriptive and analytical. |
10,303 Clinicians: 3917 Family physicians, 5065 Non-surgical specialists, 353 Surgeons, 130 Oncologist, 532 Other specialists 0.75 RR 3% |
C–K, S O–E (1, 2, 6, 7), SI M–PI, BCap, BCons |
| 22 | Walden, 2015 Canada |
Psychiatry Pharmacogenetic |
Explore clinicians’ opinions of pharmacogenetic testing. Cross-sectional survey: closed questions, ANCOVA to test responses to clinician demographics. |
168 Clinicians: Psychiatrists (34%), Family physicians (41%), Other specialists (2%) and Unknown specialties (16%) 0.70 RR 38% |
C–K O–E (1, 2) M–O |
| 23 | a. Wilkins-Haug, 2000a b. Wilkins-Haug, 2000b USA |
Multiple: Oncology (familial breast and ovarian cancer), Neurology (Alzheimer’s Disease, Huntington Disease), Pre-Pregnancy care Predictive |
a. Explore clinician knowledge, training and practice experience with GT. b. Describe current practice patterns and opinions on genetic screening and perceived importance of genetic screening within individual practices Survey: closed questions, descriptive and analytical |
428 Obstetricians/Gynaecologists 0.80 RR 45% |
C–K, S O–E (1, 3, 5, 7), SI M–O, BCons, G |
| Qualitative studies | |||||
| 24 | Carroll, 2003 Canada |
Oncology: familial breast and ovarian cancer Predictive |
Explore Family Physicians’ experiences in dealing with genetic susceptibility to cancer. Focus groups: semi-structured, exploratory, constant comparative analysis |
40 Family Physicians 0.90 |
O–E (1, 2, 3, 7) M–Em, PI, BCap, O, BCons, R |
| 25 | Corkindale, 2007 Australia |
Multiple: Oncology (colorectal cancer, childhood leukaemia), Cardiology (Angina), Immunology (Inflammatory bowel disease, HIV/AIDS), Haematology (anticoagulant therapy, thromboembolic conditions) Pharmacogenetic |
Investigate reasons for clinical (non-) adoption of pharmacogenetic testing in Australia. Compare with reasons reported in Europe. Identify any specific new influences on the adoption of innovations that are peculiar to the research context. Semi-structured interviews: exploratory, interpretive descriptive analysis |
4 Oncologists 0.85 |
O–E (1, 3, 6, 7), SI M–PI, BCons |
| 26 | Lubin, 2009 USA |
Multiple: Pre-pregnancy care (Cystic Fibrosis Haematology (Hemaglobinopathies), Paediatrics (Fragile X, Turner, Angelman Syndromes), Oncology (familial breast and ovarian cancer; colorectal cancer) Various: Diagnostic, Carrier status, Predictive |
To seek input and develop a ‘model test results’ report to facilitate effective communication of clinically relevant information from laboratory to clinical setting. Focus groups: simulated testing scenarios, analytical methods not clear |
25 Clinicians: 8 Paediatricians, 11 Obstetricians/Gynaecologists, 6 Family physicians 0.73 |
C–K O–E (2, 5, 6, 7), SI M–PI, BCap, R, G |
| 27 | McGowan, 2014 USA |
Multiple: ‘genomic risk profiling’ Predictive Genomic testing (whole-genome sequencing) |
Assess how and why early clinical users of genomic risk assessment incorporate these tools in their clinical practices and how they interpret genomic information for their patients. In-depth interviews, thematic analysis. |
18 Clinician ‘partners’ 0.70 |
C–K, S O–E (1, 5) M–Em, PI, BCap, O, R, I, G |
| 28 | Najafzadeh, 2013 Canada |
Multiple: no specific clinical domain Predictive Genomic and traditional GT |
Investigate clinicians’ perceptions regarding future role of personalised medicine; identify factors that influence their decision to use GT. Semi-structured focus groups: exploratory, constant comparative analysis |
28 Physicians 0.88 |
O–E (1, 2, 3, 5, 6, 7), SI M–PI, BCap, BCons, G |
| 29 | Nyrhinen, 2004 Finland |
Multiple: Neurology and Paediatrics Diagnostic |
Determine views on a range of ethical issues involved in GT. Semi-structured interviews: content analysis (computer analysis management model, index trees) |
4 Clinicians (specialties not reported) 0.90 |
O–E (2, 3, 7) M–Em, BCons, G |
| 30 | Reiff, 2014 USA |
Paediatrics Diagnostic Genomic testing (chromosomal microarray, CMA) |
Investigate impact of CMA on clinical practice, including implications for providers, patients and families. Semi-structured interviews: convenience sampling, qualitative content analysis. |
3 Paediatricians 0.70 |
C–K, MAD, S O–E (1, 5, 7) M–Em, PI, BCap, O, BCons |
| 31 | Van De Zwaag, 2014 Netherlands |
Cardiology/Endocrinology: Maturity onset diabetes of the young (MODY) Diagnostic |
Determine clinicians’ views on factors which may influence the current practice of GT for MODY and explore next steps toward best practice. Semi-structured interviews: purposeful recruitment, content analysis |
12 Clinicians: Endocrinologists, Internists, Cardiologists, Paediatricians, General Practitioner 0.70 |
C–K, MAD O–E (1, 3, 6, 7) M–Em, PI, O, BCons, I |
| 32 | Walsh, 2012 USA |
Oncology: Lynch syndrome Predictive |
Establish key characteristics that patients, community members and clinicians regard as central to decisions about GT and personalised medicine. Focus groups: exploratory, content analysis |
12 Clinicians 0.75 |
O–E (1, 3, 5, 6, 7) M–BCons |
| 33 | Will, 2010 UK |
Cardiology/Endocrinology: familial hypercholesterolaemia Predictive |
Explore ways in which clinicians manage and communicate genetic information Semi-structured interviews: appears exploratory, content or thematic analysis |
6 Endocrinologists (lipid specialists) 0.85 |
O–E (1, 2, 3) M–BCons, R, I, G |
| Mixed methods | |||||
| 34 | Birmingham, 2013 USA |
Oncology: prostate cancer Predictive Genomic testing (SNP array) |
Examine attitudes, knowledge and intentions regarding genomic testing. Focus groups: thematic analysis, grounded theory. Survey: closed questions, descriptive |
14 Urologists 0.85 |
C–K, S O–E (1, 3, 5) M–Em, BCap, BCons |
Overview of TDF factors identified
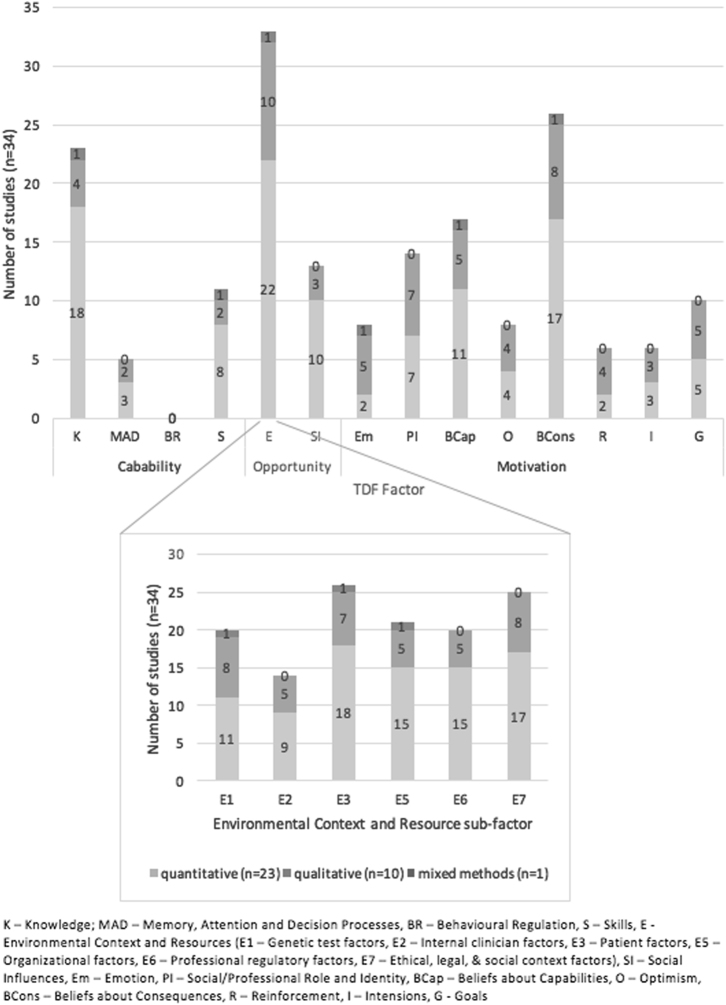
Thirteen of the 14 (TDF) factors (Table 1, overview of TDF factors) were identified from studies’ findings as impacting clinicians’ ability to offer genetic testing; only Behavioural Regulation was not represented (Fig. 2). The most commonly identified factors were: Environmental Context and Resources (n = 33), Beliefs about Consequences (n = 26) and Knowledge (n = 23), elaborated below.
Fig. 2.
Representation of TDF factors identified in studies (n = 34)
Clinicians consider numerous ‘environmental context and resource’ parameters (n = 33)
This domain comprises six further components, based upon healthcare practice-change research [70, 71] (see Table 1 for full descriptions). All six sub-domains were identified (Fig. 2), patient factors being most common (n = 26), followed by ethical, legal and social-based factors (n = 25). Clinicians’ characteristics were identified least (n = 14). In order of frequency, environmental and resources constructs identified were:
Patient factors
Patient factors relates to both tangible patient characteristics and clinicians’ considerations of an individual patient. Clinicians considered patient characteristics, such as age [33], gender [41], family history [44, 50, 57], or particular health condition and indication for testing [33, 37, 67, 68], when deciding if testing should be offered. Across several studies, patient interest and patients’ ‘escalating expectations’ [36] acted as facilitators for clinicians considering offering genetic testing [36, 41, 42, 56, 63, 66–68], even when contrary to guidelines [15]. In contrast, clinician-based perceptions that a specific patient may lack the ability to pay or experience a negative reaction to the test outcome, acted as barriers to offering genetic testing [38, 40].
Ethical, legal and social context factors
Clinicians were concerned about potential non-medical implications of testing, including privacy [60], health insurance [34, 38, 60] (U.S. studies) and equity of access in terms of test costs [38, 49, 51, 52]. In addition to consideration of impacts relating to an individual patient (included as ‘patient factors’ above), it was evident that clinicians’ general perceptions of the potential impact of testing may influence their decisions, for example the potential ‘psychological stress’ of an incidental finding [58] or the potential for a reduction in stigma [63]. This may result from clinicians’ previously perceived negative experiences of genetic testing for their patients, specifically with predictive testing for Huntington [36] or Alzheimer disease when there is no available treatment [67]. Clinicians were less willing to offer testing if they believed testing might produce false-positive results [15].
Organisational resources
Clinicians reported a lack of organisational resources at a health-service level as a barrier to testing [39], particularly, limited time for considering testing within their current practice [35, 51, 60], especially if the testing process was not clear [48, 67, 68] or not the standard of care at their practice [39], and extra time required for professional development [51, 58]. Long turnaround time for results also acted as a barrier to offering a genetic test [43, 46]. Other perceived barriers included: seeing few cases requiring testing [17]; or if the hospital was based in a regional location [40]. Alternatively, genetic counsellor availability or a close working relationship with genetic specialists and testing laboratories [48, 58, 61] could act as a facilitator [34]. Having an organisational policy for access to consent forms, educational literature for families [58] and requesting tests [50] could also influence clinicians’ decisions to offer testing.
Genetic test factors
Perceptions of clinical utility were a positive influence on clinicians’ decisions [15, 37, 43, 56, 67, 68], while novelty and complexity of the test could act as a barrier [36, 37, 51]. Similarly, clinicians were hesitant to consider tests where medical implications of results were uncertain [37, 58], or if predictive or prognostic value of the test was perceived as uncertain [36, 51, 52, 63, 69].
Professional regulatory factors
Many studies identified that clinicians reported being highly influenced by professional guidelines [34, 37, 39, 40, 50, 56, 60, 65]. If genetic testing was new or unfamiliar, clinicians believed that guidelines were not always complete [63] or available [38, 51], and they also perceived there to be a lack of (access to) relevant training [51, 53], and literature [46]. Where available, resources such as professional development and training were considered helpful when considering genetic testing [38, 41].
Clinician factors
The reported clinician-based parameters associated with requesting genetic tests were: (1) being an oncologist [15, 66]; (2) clinicians in U.S. hospitals who had completed their training in the United States [38]; (3) greater length of time since graduation [38]; and (4) working in a larger urban setting [38]. Parameters such as: clinician age; importance of religious values on decision-making, and personal experience with a genetic condition showed no associations.
Beliefs about potential consequences when deciding to offer testing (n = 26)
The second most frequently identified domain related to clinicians’ perceived value of offering genetic testing within their clinical practice. This intersects with environmental factors pertaining to ethical, legal and social considerations, with medical or clinical consequences placed in this code. Overall, clinicians had positive attitudes towards offering genetic tests, believing it could improve their patients’ health. This acted as a facilitator to offering genetic testing. This perception varied based upon the type of test, the health condition and the availability of other tools for risk assessment, management or treatment.
Clinicians reported requesting genetic tests with the belief that results would guide future treatment, screening or management options [35, 38, 45, 52, 56, 61], including reproductive information [33]. They requested tests when they perceived it would reduce ‘uncertainty’ [43, 65], even when contrary to guidelines, for example clinicians considering breast cancer susceptibility testing in young [43] and/or unaffected relatives [55], regardless of uncertain results identified in the proband [57]. Conversely, clinicians did not consider genetic tests when they perceived such tests would not be relevant to their patients [38, 39], or did not believe results would change treatment [44, 52, 69]. Some clinicians were concerned about potential consequences of tests perceived as ‘too new’ [54, 63], believing a clear benefit had not yet been proven [37].
Knowledge about genetics, testing processes and guidelines is varied (n = 23)
Clinician knowledge was assessed in studies across two main areas: (1) the genetic condition and test; and (2) clinical guidelines and test-requesting. Most studies which addressed these areas were quantitative in design and involved surveys. Overall, clinicians had good knowledge of basic genetics associated with conditions and an awareness of an appropriate genetic test [39, 40, 47, 55, 56, 61, 63]. However, there were significant differences in knowledge about different tests [52] and result types [58]. Knowledge was also found to be related to clinical indication [33], and clinician gender [64]. Several studies reported clinicians rated their knowledge of risk estimates and genetics as low [15, 50, 63], clinicians believing they lacked sufficient knowledge to perform pre-test or post-test genetic counselling [34]. Clinicians’ awareness and knowledge of current available guidelines was variable [40, 41, 57, 68]. They often did not know the process for requesting genetic testing [48, 61]. However, other findings also revealed that clinicians had a good understanding of appropriate clinical use [35, 68]. Only two studies investigated relationships between knowledge and test-requesting behaviour [19, 41]. Klitzman et al.’s study found knowledge of the requesting process was a facilitator of testing [19] but did not investigate if testing was provided appropriately. Doksum and colleagues found that perfect knowledge scores were associated with discussing or requesting BRCA1/2 genetic testing only for oncologists and not other disciplines within their study [41].
Discussion
This review identifies a complex network of factors which impact on clinicians’ self-reported decisions to offer patients a genetic test. We found clinicians’ decisions to offer testing is a nuanced process influenced by 13 parameters, nearly all of those known to influence behaviour change. Although behavioural regulation (that is, anything which clinicians do to adjust their own behaviour) was the only TDF factor not identified, this likely reflects the nature of the studies examined, rather than the factor’s relevance. Our findings suggest that for effective adoption of genomic information into healthcare, a multi-level approach targeting institutional change as well as individuals will be required.
Much of the literature identified in this systematic review proposed educating clinicians as a means by which to change their practice in genetics [15, 19, 33, 34, 36–42, 46–48, 50, 51, 53, 56, 57, 60, 61, 63, 67, 68]—indeed it is usually the only strategy proposed. Strikingly, none of these papers provided evidence for education as a means to change clinician behaviour. We did not identify any studies within the parameters of this systematic review that directly examined the effects of education on genetic testing requests. Furthermore, the studies did not draw on clinician behaviour change frameworks. This is most likely because the description of clinicians’ test request patterns was usually only a minor element of larger studies. In fact, most studies only investigated clinician knowledge and opinions with no consideration given to alternative factors known to affect clinician behaviour. Interestingly, a systematic review to identify effective knowledge translation interventions in genetic testing found the majority focus on patient decision making, while evidence in other clinical areas indicates targeting both the health professional and the patient may be most effective [72].
Our findings support the principle that a clinician’s decision to offer a genomic test will be a complex process resulting from an interplay of factors [23]. However, the studies identified in this review did not seek to explain clinician practice patterns or behaviours, looking instead at attitudes and potential associations between clinician characteristics and use of tests. This is an important gap in the literature. The offer of a new test may be a novel behaviour for the clinician, but it occurs in the context of decisions that the clinician already makes about the patient’s management. Decisions about genomic testing could be seen in this light as a modification of an existing decision-making process, rather than being an entirely new decision. However, studies examined the decision to offer a genetic test in isolation from clinicians’ usual patient care decision-making. There is a need for theoretically informed research studies specifically designed to make explicit the nature of these decisions.
Not all TDF factors identified are within clinician control. We found that organisational constraints had a significant impact on the offer of a test, suggesting institutional-based restructuring is necessary to support adoption. In view of this, it is significant that there was a total absence of studies identified which applied implementation science methodology. This scientific framework pragmatically and rigorously examines processes and services to determine how they may be optimised to improve a specific outcome. A roundtable discussion in the US recently advocated for an implementation science approach to genomic healthcare delivery [5].
It is tempting to offer suggestions of specific interventions based on the three most commonly identified factors—clinician knowledge, belief about consequences, and environmental context and resources. However, there is a paucity of high quality studies identified in this review to currently support the effectiveness of any intervention. Furthermore, the frequency of factors identified in this review is likely to relate to the design and purpose of the studies identified in this review, rather than their relative importance.
There is an inherent assumption in our systematic review that the provision of genomic tests for medical care will mirror that of genetic tests for hereditary disease, with primary care practitioners continuing to refer to other practitioners for both testing and specialised medical management of people with a condition. It is possible that genomic testing will be more widely used in primary care, particularly to predict the risk of disease in healthy people at population risk. However, the findings of our review are not applicable to that scenario, which is already being explored in pilot trials [73]. Nonetheless, we encourage research designed to specifically investigate primary care providers clinical decision-making and use of frameworks such as the TDF to enable insights into similarities and differences in decision-making across disciplines and clinical scenarios.
Many limitations arise from studies identified in this review. There were no longitudinal studies and most were largely reactive to the introduction of a genetic test. They therefore do not provide insights into changes that may occur over time, for example as clinicians become more familiar with tests or as costs decrease. Response rates were generally low, which was attributed in some cases to lack of interest by clinicians and suggests a possible bias in results. Findings from these studies may have over-estimated clinician knowledge and positive attitudes, as less interest in testing has been associated with lower knowledge [54] and less interested individuals may not have been motivated to participate. The study settings also introduce limitations as most the studies were from the U.S. healthcare system and examined inherited predisposition to cancer. No findings were unique to cancer; however, our findings may not be generalisable to other healthcare systems. The data provide a basis for hypotheses generation which can be prospectively tested in future studies.
Limitations arise from the systematic review methodology. As this is the first review to investigate clinician behaviour regarding genetic testing using the TDF, it was important to include studies with varied approaches to document the extent of published findings related to the topic. Thus, the heterogeneity of included studies precluded a meta-synthesis and we instead conducted a narrative analysis of findings from qualitative and quantitative studies which met quality standards. Finally, the role of medical specialists in relation to genetic testing was not always clearly articulated in the reviewed studies. It is possible that medical specialists in some studies would have to refer patients for testing, rather than being in a position to order tests themselves.
Conclusions
We sought to identify strategies which proved successful in the adoption of genetic testing to guide the introduction of genomics into clinical practice. Instead, we identified flaws in strategies widely espoused to support adoption of genomics by clinicians in mainstream healthcare. We find little evidence to support strong assertions of the value of education in changing clinician behaviour with respect to genetic or genomic tests. Education to build knowledge is an aspect of a clinician’s capability, but this should not be relied on alone. We find the TDF provides a useful and comprehensive framework. The results of our study can now inform more nuanced interventions, such as organisational constraints, which may restrict clinicians’ ability to offer genomic testing in the future, alongside those targeting factors intrinsic to the clinician.
Electronic supplementary material
Acknowledgements
This study was supported by the Murdoch Children’s Research Institute and the Victorian Government’s Operational Infrastructure Support Program. The authors are grateful to Brenda Wilson for formative discussions and to Natalie Taylor for comments on the manuscript. The authors would like to acknowledge Margaret Sahhar and Jan Hodgson for supporting the original development of this research. All authors have no conflicts of interest to disclose.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
The online version of this article (10.1038/s41431-018-0190-7) contains supplementary material, which is available to authorized users.
References
- 1.Weitzel KW, Alexander M, Bernhardt BA, et al. The IGNITE network: a model for genomic medicine implementation and research. BMC Med Genom. 2016;9:1. doi: 10.1186/s12920-015-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stark Z, Tan TY, Chong B, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18:1090–6. doi: 10.1038/gim.2016.1. [DOI] [PubMed] [Google Scholar]
- 3.Manolio TA, Abramowicz M, Al-Mulla F, et al. Global implementation of genomic medicine: we are not alone. Sci Transl Med. 2015;7:290ps213. doi: 10.1126/scitranslmed.aab0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Directors ABo. Scope of practice: a statement of the American College of Medical Genetics and Genomics (ACMG) Genet Med. 2015;17:e3. doi: 10.1038/gim.2014.76. [DOI] [PubMed] [Google Scholar]
- 5.Addie S, Olson S, Beachy SH. Workshop Summary—Roundtable on translating genomic-based research for health; board on health sciences policy; health and medicine division; national academies of sciences, engineering, and medicine. Washington, DC: National Academies Press; 2016. Applying an implementation science approach to genomic medicine. [PubMed] [Google Scholar]
- 6.Feero WG, Green ED. Genomics education for health care professionals in the 21st century. J Am Med Assoc. 2011;306:989–90. doi: 10.1001/jama.2011.1245. [DOI] [PubMed] [Google Scholar]
- 7.Khoury MJ, Berg A, Coates R, Evans J, Teutsch SM, Bradley LA. The evidence dilemma in genomic medicine. Health Aff. 2008;27:1600–11. doi: 10.1377/hlthaff.27.6.1600. [DOI] [PubMed] [Google Scholar]
- 8.Rogowski WH, Grosse SD, Schmidtke J, Marckmann G. Criteria for fairly allocating scarce health-care resources to genetic tests: Which matter most. Eur J Hum Genet. 2014;22:25–31. doi: 10.1038/ejhg.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunham LR, Hayden MR. Medicine. Whole-genome sequencing: the new standard of care? Science. 2012;336:1112–3. doi: 10.1126/science.1220967. [DOI] [PubMed] [Google Scholar]
- 10.Gonzaga-Jauregui C, Lupski JR, Gibbs RA. Human genome sequencing in health and disease. Annu Rev Med. 2012;63:35–61. doi: 10.1146/annurev-med-051010-162644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hastings R, de Wert G, Fowler B, et al. The changing landscape of genetic testing and its impact on clinical and laboratory services and research in Europe. Eur J Hum Genet. 2012;20:911–6. doi: 10.1038/ejhg.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerner-Ellis JP. The clinical implementation of whole genome sequencing: a conversation with seven scientific experts. J Inherit Metab Dis. 2012;35:689–93. doi: 10.1007/s10545-012-9463-4. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy JJ, McLeod HL, Ginsburg GS. Genomic medicine: a decade of successes, challenges, and opportunities. Sci Transl Med. 2013;5:189sr184. doi: 10.1126/scitranslmed.3005785. [DOI] [PubMed] [Google Scholar]
- 14.Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. J Am Med Assoc. 2008;299:1320–34. doi: 10.1001/jama.299.11.1320. [DOI] [PubMed] [Google Scholar]
- 15.Chase GA, Geller G, Havstad SL, Holtzman NA, Bassett SS. Physicians’ propensity to offer genetic testing for Alzheimer’s disease: Results from a survey. Genet Med. 2002;4:297–303. doi: 10.1097/00125817-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Van Riel E, Warlam-Rodenhuis C, Verhoef S, Rutgers E, Ausems M. BRCA testing of breast cancer patients: medical specialists’ referral patterns, knowledge and attitudes to genetic testing. Eur J Cancer Care. 2010;19:369–76. doi: 10.1111/j.1365-2354.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 17.White DB, Bonham VL, Jenkins J, Stevens N, McBride CM. Too many referrals of low-risk women for BRCA1/2 genetic services by family physicians. Cancer Epidemiol Biomark Prev. 2008;17:2980–6. doi: 10.1158/1055-9965.EPI-07-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2014;16:238–45. doi: 10.1038/gim.2013.101. [DOI] [PubMed] [Google Scholar]
- 19.Klitzman R, Chung W, Marder K, et al. Attitudes and practices among internists concerning genetic testing. J Genet Couns. 2013;22:90–100. doi: 10.1007/s10897-012-9504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Registered Nurses Association of Ontario. Toolkit: implementation of clinical practice guidelines, 2002. http://rnao.ca/sites/rnao-ca/files/BPG_Toolkit_0.pdf.
- 21.Brand CA, Barker AL, Morello RT, et al. A review of hospital characteristics associated with improved performance. Int J Qual Health Care. 2012;24:483–94. doi: 10.1093/intqhc/mzs044. [DOI] [PubMed] [Google Scholar]
- 22.Scott I. What are the most effective strategies for improving quality and safety of health care? Intern Med J. 2009;39:389–400. doi: 10.1111/j.1445-5994.2008.01798.x. [DOI] [PubMed] [Google Scholar]
- 23.Roberts MC, Kennedy AE, Chambers DA, Khoury MJ. The current state of implementation science in genomic medicine: opportunities for improvement. Genet Med. 2017;19:858–63. doi: 10.1038/gim.2016.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Department of Health. Annual report of the Chief Medical Officer 2016: generation genome, 2017. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/631043/CMO_annual_report_generation_genome.pdf.
- 25.Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012;7:37–53. doi: 10.1186/1748-5908-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42–53. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. The PG: preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popay J, Roberts H, Sowden A et al. Guidance on the conduct of narrative synthesis in systematic reviews: a product from the ESRC methods programme. Lancaster:Lancaster University; 2006. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.178.3100&rep=rep1&type=pdf
- 29.Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62:107–15. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- 30.QSR International. NVivo qualitative data analysis Software, Version 10; Melbourne, VIC:QSR International Pty Ltd.; 2012.
- 31.Kmet LM, Lee RC, Cook LS. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. Edmonton: Alberta Heritage Foundation for Medical Research; 2004. [Google Scholar]
- 32.Paul J, Metcalfe S, Stirling L, Wilson B, Hodgson J. Analyzing communication in genetic consultations—a systematic review. Patient Educ Couns. 2015;98:15–33. doi: 10.1016/j.pec.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Acharya K, Schindler A. Developmental and behavioral pediatricians’ attitudes toward screening for fragile X. Am J Intellect Dev Disabil. 2013;118:284–93. doi: 10.1352/1944-7558-188.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beitsch PD, Whitworth PW. Can breast surgeons provide breast cancer genetic testing? An American Society of Breast Surgeons survey. Ann Surg Oncol. 2014;21:4104–8. doi: 10.1245/s10434-014-3711-9. [DOI] [PubMed] [Google Scholar]
- 35.Birmingham WC, Agarwal N, Kohlmann W, et al. Patient and provider attitudes toward genomic testing for prostate cancer susceptibility: a mixed method study. BMC Health Serv Res. 2013;13:279. doi: 10.1186/1472-6963-13-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll JC, Brown JB, Blaine S, Glendon G, Pugh P, Medved W. Genetic susceptibility to cancer. Family physicians’ experience. Cancer Fam Physician. 2003;49:45–52. [PMC free article] [PubMed] [Google Scholar]
- 37.Corkindale D, Ward H, McKinnon R. Low adoption of pharmacogenetic testing: an exploration and explanation of the reasons in Australia. Pers Med. 2007;4:191–9. doi: 10.2217/17410541.4.2.191. [DOI] [PubMed] [Google Scholar]
- 38.Cox SL, Zlot AI, Silvey K, et al. Patterns of cancer genetic testing: a randomized survey of Oregon clinicians. J Cancer Epidemiol. 2012;2012:1–11. doi: 10.1155/2012/294730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darcy D, Tian L, Taylor J, Schrijver I. Cystic fibrosis carrier screening in obstetric clinical practice: knowledge, practices, and barriers, a decade after publication of screening guidelines. Genet Test Mol Biomark. 2011;15:517–23. doi: 10.1089/gtmb.2010.0228. [DOI] [PubMed] [Google Scholar]
- 40.Doksum T, Bernhardt BA, Holtzman NA. Carrier screening for cystic fibrosis among Maryland obstetricians before and after the 1997 NIH Consensus Conference. Genet Test. 2001;5:111–6. doi: 10.1089/109065701753145565. [DOI] [PubMed] [Google Scholar]
- 41.Doksum T, Bernhardt BA, Holtzman NA. Does knowledge about the genetics of breast cancer differ between nongeneticist physicians who do or do not discuss or order BRCA testing? Genet Med. 2003;5:99–105. doi: 10.1097/01.GIM.0000055198.63593.32. [DOI] [PubMed] [Google Scholar]
- 42.Freedman AN, Wideroff L, Olson L, et al. US physicians’ attitudes toward genetic testing for cancer susceptibility. Am J Med Genet A. 2003;120A:63–71. doi: 10.1002/ajmg.a.10192. [DOI] [PubMed] [Google Scholar]
- 43.Geller G, Bernhardt BA, Doksum T, Helzlsouer KJ, Wilcox P, Holzman NA. Decision-making about breast cancer susceptibility testing: how similar are the attitudes of physicians, nurse practitioners, and at-risk women? J Clin Oncol. 1998;16:2868–76. doi: 10.1200/JCO.1998.16.8.2868. [DOI] [PubMed] [Google Scholar]
- 44.Harris BU, Miyake CY, Motonaga KS, Dubin AM. Diagnosis and management of pediatric Brugada syndrome: a survey of pediatric electrophysiologists. Pacing Clin Electrophysiol. 2014;37:638–42. doi: 10.1111/pace.12346. [DOI] [PubMed] [Google Scholar]
- 45.James C, Geller G, Bernhardt BA, Docksum T, Holtzman NA. Are practicing and future physicians prepared to obtain informed consent? The case of genetic testing for susceptibility to breast cancer. Community Genet. 1998;1:203–12. doi: 10.1159/000016165. [DOI] [PubMed] [Google Scholar]
- 46.Kadafour M, Haugh R, Posin M, Kayser SR, Shin J. Survey on warfarin pharmacogenetic testing among anticoagulation providers. Pharmacogenomics. 2009;10:1853–60. doi: 10.2217/pgs.09.117. [DOI] [PubMed] [Google Scholar]
- 47.Klitzman R, Abbate KJ, Chung WK, et al. Psychiatrists’ views of the genetic bases of mental disorders and behavioral traits and their use of genetic tests. J Nerv Ment Dis. 2014;202:530–8. doi: 10.1097/NMD.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lubin IM, McGovern MM, Gibson Z, et al. Clinician perspectives about molecular genetic testing for heritable conditions and development of a clinician-friendly laboratory report. J Mol Diagn. 2009;11:162–71. doi: 10.2353/jmoldx.2009.080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGowan ML, Fishman JR, Settersten RA, Lambrix MA, Juengst ET. Gatekeepers or intermediaries? The role of clinicians in commercial genomic testing. PLoS ONE. 2014;9:e108484. doi: 10.1371/journal.pone.0108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myers MF, Chang MH, Jorgensen C, et al. Genetic testing for susceptibility to breast and ovarian cancer: evaluating the impact of a direct-to-consumer marketing campaign on physicians’ knowledge and practices. Genet Med. 2006;8:361–70. doi: 10.1097/01.gim.0000223544.68475.6c. [DOI] [PubMed] [Google Scholar]
- 51.Najafzadeh M, Davis JC, Joshi P, Marra C. Barriers for integrating personalized medicine into clinical practice: a qualitative analysis. Am J Med Genet A. 2013;161A:758–63. doi: 10.1002/ajmg.a.35811. [DOI] [PubMed] [Google Scholar]
- 52.Ngoi N, Lee SC, Hartman M, Khin LW, Wong A. Interest and attitudes of patients, cancer physicians, medical students and cancer researchers towards a spectrum of genetic tests relevant to breast cancer patients. Breast. 2013;22:47–52. doi: 10.1016/j.breast.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Nyrhinen T, Hietala M, Puukka P, Leino-Kilpi H. Privacy and equality in diagnostic genetic testing. Nurs Ethics. 2007;14:295–308. doi: 10.1177/0969733007075864. [DOI] [PubMed] [Google Scholar]
- 54.Nyrhinen T, Leino-Kilpi H, Hietala M. Ethical issues in the diagnostic genetic testing process. New Genet. 2004;23:73–87. doi: 10.1080/1463677042000189570. [DOI] [PubMed] [Google Scholar]
- 55.Pal T, Cragun D, Lewis C, et al. A statewide survey of practitioners to assess knowledge and clinical practices regarding hereditary breast and ovarian cancer. Genet Test Mol Biomark. 2013;17:367–75. doi: 10.1089/gtmb.2012.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peppercorn J, Hamilton E, Marcom PK, Beskow L, Lyman GH. Pharmacogenetic testing in the face of unclear clinical efficacy: lessons from cytochrome P450 2D6 for tamoxifen. Cancer. 2013;119:3703–9. doi: 10.1002/cncr.28263. [DOI] [PubMed] [Google Scholar]
- 57.Plon SE, Cooper HP, Parks B, et al. Genetic testing and cancer risk management recommendations by physicians for at-risk relatives. Genet Med. 2011;13:148–54. doi: 10.1097/GIM.0b013e318207f564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reiff M, Mueller R, Mulchandani S, Spinner N, Pyeritz R, Bernhardt B. A Qualitative Study of Healthcare Providers’ Perspectives on the implications of genome-wide testing in pediatric clinical practice. J Genet Couns. 2014;23:474–88. doi: 10.1007/s10897-013-9653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salm M, Abbate K, Appelbaum P, et al. Use of genetic tests among neurologists and psychiatrists: knowledge, attitudes, behaviors, and needs for training. J Genet Couns. 2014;23:156–63. doi: 10.1007/s10897-013-9624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Selkirk CG, Weissman SM, Anderson A, Hulick PJ. Physicians’ preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genet Test Mol Biomark. 2013;17:219–25. doi: 10.1089/gtmb.2012.0165. [DOI] [PubMed] [Google Scholar]
- 61.Stanek EJ, Sanders CL, Taber KAJ, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin Pharmacol Ther. 2012;91:450–8. doi: 10.1038/clpt.2011.306. [DOI] [PubMed] [Google Scholar]
- 62.Vadaparampil ST, Wideroff L, Olson L, Viswanath K, Freedman AN. Physician exposure to and attitudes toward advertisements for genetic tests for inherited cancer susceptibility. Am J Med Genet A. 2005;135:41–46. doi: 10.1002/ajmg.a.30681. [DOI] [PubMed] [Google Scholar]
- 63.Van Der Zwaag AM, Weinreich SS, Bosma AR, et al. Current and best practices of genetic testing for maturity onset diabetes of the young: views of professional experts. Public Health Genomics. 2014;18:52–59. doi: 10.1159/000367963. [DOI] [PubMed] [Google Scholar]
- 64.Walden LM, Brandl EJ, Changasi A, et al. Physicians’ opinions following pharmacogenetic testing for psychotropic medication. Psychiatry Res. 2015;229:913–8. doi: 10.1016/j.psychres.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 65.Walsh J, Arora M, Hosenfeld C, Ladabaum U, Kuppermann M, Knight SJ. Preferences for genetic testing to identify hereditary colorectal cancer: perspectives of high-risk patients, community members, and clinicians. J Cancer Educ. 2012;27:112–9. doi: 10.1007/s13187-011-0286-z. [DOI] [PubMed] [Google Scholar]
- 66.Wideroff L, Freedman AN, Olson L, et al. Physician use of genetic testing for cancer susceptibility: results of a national survey. Cancer Epidemiol Biomark Prev. 2003;12:295–303. [PubMed] [Google Scholar]
- 67.Wilkins-Haug L, Erickson K, Hill L, Power M, Holzman GB, Schulkin J. Obstetrician-gynecologists’ opinions and attitudes on the role of genetics in women’s health. J Women Health Gend Based Med. 2000;9:873–9. doi: 10.1089/152460900750020900. [DOI] [PubMed] [Google Scholar]
- 68.Wilkins-Haug L, Hill LD, Power ML, Holzman GB, Schulkin J. Gynecologists’ training, knowledge, and experiences in genetics: a survey. Obstet Gynecol. 2000;95:421–4. doi: 10.1016/s0029-7844(99)00581-5. [DOI] [PubMed] [Google Scholar]
- 69.Will CM, Armstrong D, Marteau TM. Genetic unexceptionalism: clinician accounts of genetic testing for familial hypercholesterolaemia. Soc Sci Med. 2010;71:910–7. doi: 10.1016/j.socscimed.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 70.Flottorp SA, Oxman AD, Krause J, et al. A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci. 2013;8:35. doi: 10.1186/1748-5908-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rainbird K, Sanson-Fisher R, Buchan H. Identifying barriers to evidence uptake. Melbourne: National Insitute of ClinicalStudies; 2006. [Google Scholar]
- 72.Légaré F, Robitaille H, Gane C, Hébert J, Labrecque M, Rousseau F. Improving decision making about genetic testing in the clinic: an overview of effective knowledge translation interventions. PLoS ONE. 2016;11:e0150123. doi: 10.1371/journal.pone.0150123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vassy JL, Christensen KD, Schonman EF, et al. The impact of whole-genome sequencing on the primary care and outcomes of healthy adult patients: a pilot randomized trial. Ann Intern Med. 2017;167:159–69. doi: 10.7326/M17-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.