Abstract
DMSO, glycerol, and ascorbic acid (AA) are used in pharmaceuticals and known to display radioprotective effects. The present study investigates radioprotective properties of novel glyceryl glucoside, ascorbic acid 2-glucoside, glyceryl ascorbate, and palmitoyl ascorbic acid 2-glucoside (PA). Gamma-rays or high-LET carbon-ions were irradiated in the presence of tested chemicals. Lambda DNA damage, cell survival, and micronuclei formation of CHO cells were analyzed to evaluate radioprotective properties. Radiation-induced Lambda DNA damage was reduced with chemical pre-treatment in a concentration-dependent manner. This confirmed tested chemicals were radical scavengers. For gamma-irradiation, enhanced cell survival and reduction of micronuclei formation were observed for all chemicals. For carbon-ion irradiation, DMSO, glycerol, and PA displayed radioprotection for cell survival. Based on cell survival curves, protection levels by PA were confirmed and comparable between gamma-rays and high-LET carbon-ions. Micronuclei formation was only decreased with AA and a high concentration of glycerol treatment, and not decreased with PA treatment. This suggests that mechanisms of protection against high-LET carbon-ions by PA can differ from normal radical scavenging effects that protect DNA from damage.
Introduction
Ionizing radiation (IR) of living cells is a known cause of cancer, mutations, and aging1–4. IR can produce highly reactive free radicals. Most free radicals that damage cellular systems are reactive oxygen species (ROS)1–4. Free radicals disrupt cell’s DNA and produce single and double strand DNA breaks, which result in chromosome aberrations and cell death1,3,4. IR effects can be reduced by the pre-treatment of antioxidants. Antioxidants can neutralize oxidative stress by scavenging free radicals prior to damaging DNA2. Dimethyl sulfide (DMSO), glycerol, and ascorbic acid (AA) are well known radical scavengers5–10. However, they display cellular toxicity with continuous exposure11–13. Less toxic radioprotectors may be useful to reduce the damaging effects of IR on healthy tissues surrounding tumors during radiotherapy. Moreover, practical radioprotectors may also be useful to protect radiation workers who are exposed to low levels of IR periodically.
Recently, glycosyl and glyceryl modified chemicals are available for glycerol and AA. They are stable in water and showed less cytotoxicity. In our previous study, glycosylation has a positive effect for flavonoids radioprotection in vitro14. Glyceryl glucoside (GG) is a glycosyl derivative of glycerol and glucose15,16. GG exhibits antioxidant properties by decreasing ROS and is associated with cell regeneration and renewal17. GG is naturally present in traditional Japanese foods brewed using koji (i.e. sake and miso), wine, and cosmetics (i.e. anti-aging and skin moisturizing) as an osmoprotectant16–19. Our previous study displayed GG has lower cytotoxicity compared to DMSO and glycerol16. A recent toxicology study reported that in vitro and in vivo, GG exhibited non-mutagenic and non-cytotoxic properties17. Glycosylated and glycerylated AA, which are used in food and supplements as vitamins and antioxidants20 are: ascorbic acid 2-glucoside (2G); glycerol ascorbic acid (GA); and palmitoyl ascorbic acid-2-glucoside (PA). Previous studies showed AA modified chemicals maintained their antioxidant properties and are expected to be potential practical radioprotectors9,21,22.
In this study, we hypothesized that chemically modified classical radioprotectors are also radical scavengers and have the potential to display radioprotective effects. The current study explored the radioprotective properties of DMSO, glycerol, GG, AA, 2 G, GA, and PA (Fig. 1) by investigating CHO cells and Lambda DNA in terms of cell survival, micronuclei formation, and DNA damage after irradiation of gamma-rays and high-LET carbon-ions.
Figure 1.
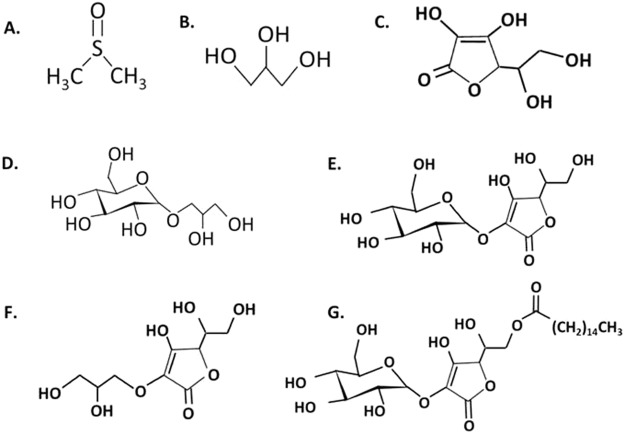
Chemical structures of (A) dimethyl sulfoxide, (B) glycerol, (C) ascorbic acid, (D) glyceryl glucoside, (E) ascorbic acid 2-glucoside, (F) glyceryl ascorbate, and (G) palmitoyl ascorbic acid 2-glucoside.
Results
In vitro radioprotective ability of tested chemicals
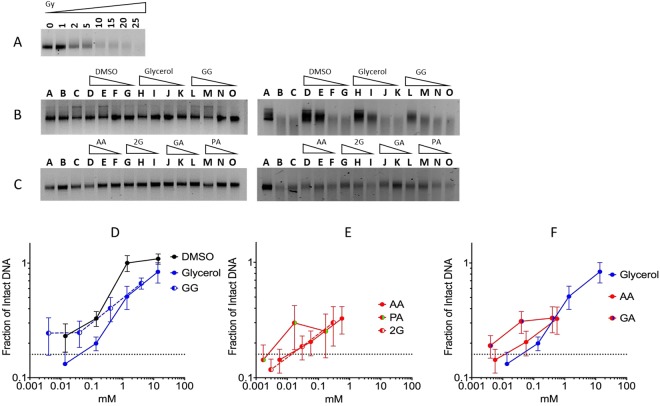
In the gel electrophoresis assay, irradiated and fragmented Lambda DNA migrated further and was detected as a smear in a dose dependent manner (Fig. 2A). In Fig. 2A, Lambda DNA was damaged at 20 Gy. We confirmed the tested chemicals, alone, did not produce DNA damage (Fig. 2B left, and C left). Chemical dose-dependent protection was observed as a reduction of DNA damage (Fig. 2B right, and C right). All agents displayed positive radioprotective abilities at 0.1 mM concentrations compared to the control (Fig. 2D–F). DMSO displayed the highest radioprotective properties at 1.4 and 14 mM. Glycerol and GG had nearly identical protection at 1 mM and above (Fig. 2D). AA and 2 G exhibited similar protective properties (Fig. 2E), as well as GA and PA (Fig. 2E,F). Gel electrophoresis results confirm the tested chemicals are radical scavengers.
Figure 2.
Gel electrophoresis of Lambda DNA. (A) Radiation dose-dependent DNA damage. (B left, and C left) Effects of tested chemicals on DNA without irradiation. (B right) Radioprotective effects of pre-treated DMSO, glycerol, and GG. Triangles indicate concentration gradient. Lane A: control water without irradiation; lane B and C water with irradiation; lane D–G DMSO at 14 mM, 1.4 mM, 0.14 mM, and 0.014 mM; lane H-K: glycerol at 13.7 mM, 1.37 mM, 0.137 mM, and 0.0137 mM; lane L–O: GG at 4 mM, 0.4 mM, 0.04 mM, and 0.004 mM. (C right) Radioprotective effects of AA, 2G, GA, and PA. Triangles indicate concentration gradient. Lane A: control water without irradiation; lane B and C: water with irradiation; lane D–F AA at 0.56 mM, 0.056 mM, and 0.0056 mM; lane G–I: 2G at 0.3 mM, 0.03 mM, and 0.003 mM; lane J–L: GA at 0.4 mM, 0.04 mM, and 0.004 mM; lane M–O: PA at 0.17 mM, 0.017 mM, and 0.0017 mM. (D–G) Fraction of intact irradiated Lambda DNA with chemicals. Error bars indicate standard error of the means. At least three independent experiments were carried out. The cropped gel images were used in the figure, and full-length gel images are presented in Supplementary Fig. S1.
Radioprotection of cell survival
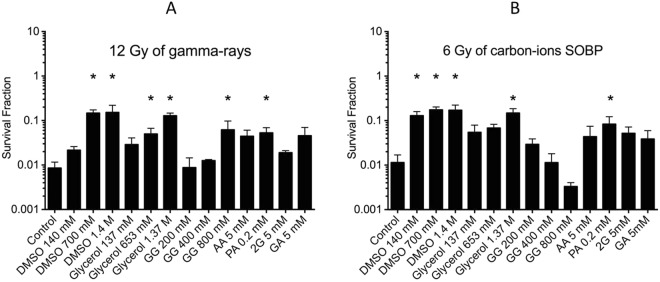
To evaluate the role of DMSO, glycerol, GG, AA and its derivatives as potential radioprotectors, cell survival was investigated using colony formation assay. CHO cells were treated with various non-toxic chemical concentrations for 0.5 h and then exposed to 12 Gy gamma-rays or 6 Gy high-LET carbon-ions. For gamma-rays (12 Gy), without chemicals cell survival fraction was approximately 0.0086 (Fig. 3A). In Fig. 3A, cells were pre-treated with various chemicals and then irradiated with 12 Gy gamma-rays; all displayed an overall increased cell survival compared to the control. CHO cells pre-treated with DMSO and glycerol presented radioprotection, significantly increasing cell survival above 100 mM (P < 0.05). However, GG required a concentration greater than 400 mM to show similar protection (P = 0.0195). 5 mM of AA and its derivatives (2G, GA) and 0.2 mM PA demonstrated radioprotection. Especially PA showed significant protection. For high-LET carbon-ion radiation (6 Gy), without chemicals cell survival fraction was approximately 0.018 (Fig. 3B). Cells pre-treated with greater than 140 mM DMSO and 1.37 M glycerol, then irradiated with 6 Gy high-LET carbon-ions, revealed significant radioprotection (P = 0.00327 for 140 mM DMSO, P = 0.0033 for 1.37 M glycerol). GG, AA, 2G, PA and GA did not show significant radioprotection in the tested concentrations. 0.2 mM PA showed increased cell survival, but it was not statistically significant (P = 0.3324).
Figure 3.
DMSO, glycerol, GG, AA and its derivatives on radioprotective effects for cell survival of CHO cells. (A) Under 12 Gy gamma-ray irradiation, cells irradiated without chemicals induced a 0.0086 cell survival fraction. (B) Under 6 Gy high-LET carbon-ion irradiation, cells irradiated without chemicals stimulated a 0.018 cell survival rate. * indicate statistical significance (P < 0.05). Error bars indicate standard error of the means. At least three independent experiments were carried out.
Micronuclei formation
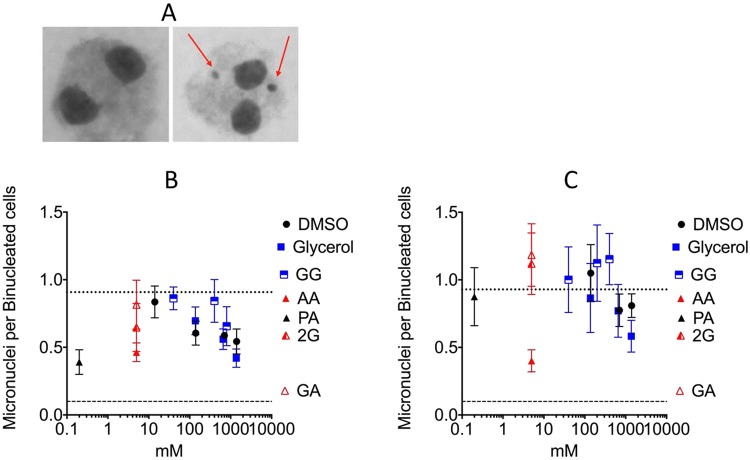
To investigation the role of DMSO, glycerol, GG, AA and its derivatives, in the prevention of radiation-induced genotoxicity, micronuclei formation was analyzed. For gamma-ray irradiation (6 Gy), without chemical treatment CHO cells show a level of 0.91 micronuclei per binucleated cell (Fig. 4B). All pre-treated chemicals demonstrated radioprotection against micronuclei formation compared to the control. 1.4 mM DMSO and 1.37 mM glycerol displayed a strong reduction of radiation-induced micronuclei formation (P = 0.0263, P = 0.037 respectively). 5 mM of AA and 0.2 mM of PA reduced micronuclei formation over 50% compared to the control. The most significant reduction in micronuclei formation was displayed by 0.2 mM of PA (P = 0.0142), with 0.26 micronuclei per binucleated cell. For high-LET carbon-ion radiation (3 Gy), without chemical treatment CHO cells produced 0.93 micronuclei per binucleated cell (Fig. 4C). At varying concentrations, all chemicals did not reduce micronuclei formation compared to the control, with the exception of AA (5 mM) and glycerol (1.37 mM). AA (5 mM) reduced micronuclei formation by 50% with 0.42 micronuclei per binucleated cell, but it was not significant (P = 0.2294).
Figure 4.
Radioprotective effects of DMSO, glycerol, GG, AA, 2G, GA, and PA by micronuclei assay. (A) On the left is a normal binucleated cell. On the right is binucleated cell with micronuclei formation indicated by red arrows. (B) Micronuclei formation per binucleated CHO cell with pre-treated chemicals after gamma-ray irradiation. Horizontal dashed line indicates non-irradiated control (less than 0.1 micronuclei per binucleated cells for each condition). Horizontal dotted line indicates the control (0.91 micronuclei per binucleated cells). (C) Micronuclei formation after high-LET carbon-ion irradiation. Horizontal dashed line indicates non-irradiated control (less than 0.1 micronuclei per binucleated cells for each condition). Horizontal dotted line indicates the control (0.93 micronuclei per binucleated cells). Error bars indicate standard error of the means. At least three independent experiments were carried out.
Survival curve for palmitoyl ascorbic acid-2-glucoside
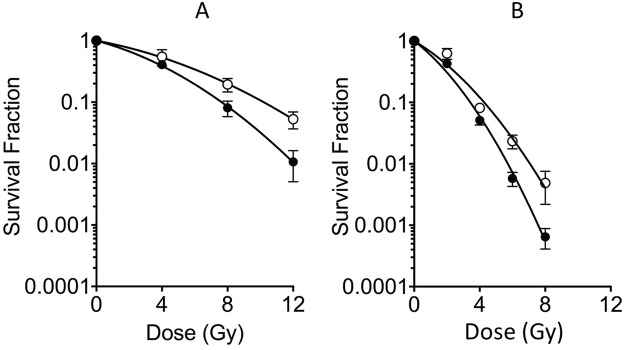
In order to confirm the radioprotective properties of PA, survival curves with and without 0.2 mM PA treatment were created. PA showed radioprotective properties for both gamma-rays and high-LET carbon-ions SOBP (Spread out Bragg peak) irradiation (Fig. 5A,B). D10 values were increased from 7.64 Gy to 9.64 Gy for gamma-ray irradiation and 3.43 Gy to 4.27 Gy for high-LET carbon-ion irradiation. The PER was 1.26 for gamma-rays and 1.24 for high-LET carbon-ion irradiation. Therefore, the level of protection by PA was similar between gamma-rays and high-LET carbon-ions.
Figure 5.
Cell survival curves with and without 0.2 mM palmitoyl ascorbic acid 2-glucoside (PA). (A) gamma-ray irradiation. (B) Carbon-ions irradiation. Closed circles indicate control; open circles indicate PA. Curves were fitted to liner-quadratic model. Error bars indicate standard error of the means. At least three independent experiments were carried out.
Discussion
As expected, DMSO, glycerol and AA exhibited radioprotection under gamma-ray (low-LET) and carbon-ion (high-LET) irradiation. GG demonstrated radical scavenging effects in vitro at 4 mM solution, but not for cell survival. This may be due to glycosylation preventing entry into the cell membrane in vivo, since glycosylated chemicals are more water soluble, making them less bioavailable23,24. GG is glycosylated; thus, we suggest deglycosylation must occur prior or during cell-chemical uptake in order for α-glucosidase to initiate digestion, increasing cellular uptake in vivo24–26. In this study, cells were pre-treated 0.5 hour prior to irradiation, which was sufficient for DMSO, glycerol, and AA to display radioprotective properties. It is possible that GG requires a longer pre-treatment to efficiently be bioavailable. Perhaps, a greater concentration of GG is needed to be radioprotective for the current treatment time, although concentrations of GG greater than 800 mM display cytotoxic effects14. Thus, GG does not display comparable radioprotection like DMSO or glycerol in non-toxic concentrations. It is important to note that the concentration of each chemical largely relied on in vitro DNA damage versus cell culture experiments. In vitro DNA experiments required more dilute concentrations than cell culture experiments, due to the ease that chemicals have when entering DNA compared to the cell membrane (Figs 2 and 4).
Furthermore, 2G did not display strong radioprotection (Fig. 4B,C), as well as AA because glucoside inhibits cell entry. We expected enhanced protection by GA because both AA and glycerol presented radioprotection. However, GA exhibited protection only for gamma-rays (Fig. 4A) and displayed better protection for DNA damage (Fig. 2D), as it is a glycerylated AA. Cell survival for GA was not increased as expected since GA may not have been up-taken by the cell. This may be due to glycerol modification of AA not enhancing bioavailability14. On the other hand, PA is a great radioprotector (Figs 2E, 3A,B and 4B,C) even with glycosyl substituent as 2G. PA is a liposoluble ester of ascorbic acid20, and we confirmed PA displays protection at all doses (Fig. 5). It is possible that liposoluble properties enhanced PA’s cellular uptake, or PA remains in the cell membrane and can protect from damage; thus, explaining PA’s significant protection from micronuclei formation and cell survival after gamma-rays.
Due to high density of ionization, high-LET carbon-ion radiation produces complex types of DNA damage and results in higher relative biological effectiveness. Carbon-ion radiation does not have a high fraction of indirect effects, but radical scavengers, DMSO and glycerol, could protect cells from cell death with low-LET gamma-irradiation (Fig. 5). These results may suggest that high-LET radiation can be protected by high concentration of radical scavengers by reducing the indirect effect. It is also possible that long-lived free radicals may contribute to DNA damage27,28. Since PA is lipophilic, the protection against carbon-ion radiation observed by PA can be explained by scavenging these long-live free radicals.
In conclusion, radical scavenging capacity is well related to DNA damage. GG and AA derivatives can reduce radiation-induced damage and cell death for gamma-rays. Among modified chemicals, only PA significantly reduced cell death induced by high-LET carbon-ion radiation. Further studies are required to verify the mechanisms of radioprotection by palmitoyl ascorbic acid 2-glucoside for high-LET carbon-ion radiation.
Material and Methods
Chemicals
DMSO was purchased from Fisher Scientific Co. (Fair Lawn, NJ). Glycerol was purchased from Mallinckrodt Specialty Chemicals Co. (St. Louis, MO). Glyceryl glucoside, ascorbic acid and its derivatives were synthesized and obtained by Tokyo Sugar Co., Ltd. (Tokyo, Japan) and Carlit Holding Co., Ltd. (Tokyo, Japan). Chemical structures were illustrated in Fig. 1. Concentrations on chemicals are weight per volume percentages. In vitro DNA experiment requires more dilute concentrations on chemicals than cell culture experiments. This is due to the ease chemicals have when entering the cell membrane. Higher concentrations of chemicals will lead to cytotoxic effects. Stock solution was prepared in 1% solution such as AA (56.7 mM), AA2G (30 mM), GA (40 mM), and PA (17 mM). Lambda DNA was purchased from Nippon Gene Co., Ltd. (Tokyo, Japan).
Irradiation
Gamma-ray irradiation was performed at Colorado State University (Fort Collins, CO) with a J.L. Shepherd Model Mark I-68 nominal 6000 Ci 137Cs irradiator (J.L. Shepherd and Associates, San Fernando, CA) and used at room temperature (20 °C)14,29. The dosage rate was 2.5 Gy/min for cell survival and micronuclei experiments and 12.5 Gy/min for gel electrophoresis experiments. At the National Institute of Radiological Sciences (NIRS) in Chiba, Japan, particle-based irradiation experiments were carried out. For high-LET carbon-ion exposure, the Heavy-Ion Medical Accelerator in Chiba (HIMAC; Chiba, Japan) irradiated accelerated carbon ions at room temperature (20 °C)29. Specifics in regards to the beam characteristics of the particle radiation, biological irradiation procedures, and dosimetry have been depicted previously30–32. Carbon ions were accelerated at 290 MeV/nucleon of initial energy and spread out with a ridge filter for 6 cm width of SOBP29. The monolayer cell culture was irradiated at the center (50 keV/μm of average LET) within the SOBP at a distance of 119 mm from the entrance33. Dose rates for high-LET carbon-ions was set at 1 and 5 Gy/min respectively.
Cell culture
Chinese hamster ovary (CHO), CHO10B2 (wild type) were provided by Dr. Joel Bedford at Colorado State University (Fort Collins, CO). CHO cells were maintained in culture in α-minimum essential medium (Gibco, Grand Island, NY), and supplemented with 10% heat inactivated fetal bovine serum (Sigma, St. Louis, MO) and supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin and 25 ng/ml Amphotericin B at 37 °C and 5% CO2. The CHO cell doubling time is approximately 12 h.
Electrophoresis and DNA analysis
One percent agarose gel with ethidium bromide and 1X TAE buffer was used. Freshly prepared for each experiment, 10 μl of the DNA solution consisted of 23 ng Lambda DNA (stock concentration 460 ng/μl), and 10 mM Tris-HCl buffer with DMSO, glycerol, GG, AA, 2G, GA, or PA. Each sample was exposed to gamma-rays, and run through electrophoresis after adding 2 μl of 6X DNA loading dye (15% Ficoll (w/v), 10% glycerol (v/v), 0.25% bromophenol blue (w/v), and 0.25% xylene cyanol FF (w/v) in distilled water). Electrophoresis was carried out at 100 V for 60 minutes in 1X TAE buffer. After electrophoresis, the gel was immersed with distilled water and placed in a 4 °C cold room for a maximum of 24 hours. After the cold room, the gel images were obtained with the Molecular Imager Gel Doc XR system with Image Lab software (Bio-Rad Laboratories, Inc., Hercules, CA). Images were analyses by pixel intensities. The amount of remaining intact DNA was calculated by dividing the treated samples by the control.
Cell survival assay
Clonogenic assay was used to measure cell survival. To investigate the radioprotective cell survival after irradiation, CHO cells were pre-treated with DMSO, glycerol, GG, AA, 2G, GA, or PA for 0.5 h and irradiated with gamma-rays or high-LET carbon-ions. Cells were replated at a density devised to yield about 100 viable colony–forming cells/P–60 cell culture dish34. Seven to eight days after treatment, colonies were scored. To fix colonies, dishes were treated with 100% ethanol and stained with 0.1% crystal violet solution. Colonies encompassing greater than 50 cells were recorded as non-toxic reproductively viable surviving cells34. Cell survival fraction was obtained by dividing the irradiated samples by the control. Cell survival curves were produced using linear quadratic regression equations with Prism 6 (GraphPad Software, Inc., La Jolla, CA). The dose to achieve 10% survival fractions (D10 values) were obtained by interpolation of cell survival. Protection Enhancement Ratio (PER) values were calculated by D10,control divided by D10,drug treatment.
Micronuclei assay
To investigate the induction of micronuclei by irradiation, CHO cells were pre-treated with DMSO, glycerol, GG, AA, 2G, GA, or PA for 0.5 h and irradiated with gamma-rays or high-LET carbon-ions. Cells were treated with 4 μg/ml of Cytochalasin B (Sigma, St. Louis, MO) for 22 h16. Cells were then harvested in 5 ml of 75 mM KCl solution, centrifuged at 1,000 rpm for 5 min, and then fixed in 3:1 methanol: acetic acid solution and formaldehyde (Fisher Scientific, Fair Lawn, NJ)16. Next, cells were dropped onto slides and air dried at room temperature. Slides were stained in filtered 5% Giemsa solution in GURR solution (Gibco, St. Louis, MO) for 5 min16. Three hundred binucleated cells were scored per treatment dosage using a Zeiss Axioskop microscope. Images were taken by SPOT CCD camera RT 2.3.1 with SPOT basic software (Diagnostic Instruments. Sterling Heights, MI).
Statistical analysis
All experiments were carried out more than three times, independently. Statistical comparison of mean values was performed using a one-way analysis of variance (ANOVA) with GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA). P < 0.05 was considered to indicate a statistically significant difference. Error bars indicate the standard error of the mean.
Electronic supplementary material
Acknowledgements
The present study was partially supported by Carlit Holdings Co. Ltd. and Toyo Sugar Refining Co. Ltd., Dr. Akiko Ueno Radiobiology Research Fund (T.A.K.) and by MEXT Grant-in-Aid for Scientific Research on Innovative Areas, Grant Number 15H05935 and JP15K21745 (F.A.). We would like to thank HIMAC for helping support our research and making this study possible.
Author Contributions
T.A.K. conceived and designed the experiment. A.H.H., D.J.B., H.H. and T.A.K. performed experiment, data analysis, and wrote the manuscript. A.H.H., A.F., Y.A., T.A.K. performed data analysis and interpretation and critical revision of the article. All authors approved final version of manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31747-1.
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- 3.Husain N, Kumar A. Reactive oxygen species and natural antioxidants: a review. Adv. Biores. 2012;3:164–175. [Google Scholar]
- 4.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiebe JP, Dinsdale CJ. Inhibition of cell proliferation by glycerol. Life Sci. 1991;48:1511–1517. doi: 10.1016/0024-3205(91)90275-G. [DOI] [PubMed] [Google Scholar]
- 6.Millar BC, Sapora O, Fielden EM, Loverock PS. The application of rapid-lysis techniques in radiobiology. IV. The effect of glycerol and DMSO on Chinese hamster cell survival and DNA single-strand break production. Radiat Res. 1981;86:506–514. doi: 10.2307/3575466. [DOI] [PubMed] [Google Scholar]
- 7.Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol. 2003;65:1035–1041. doi: 10.1016/S0006-2952(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 8.Brustad T, Singsaas B. On the time scale for radioprotection by and permeation of glycerol in anoxic cells of E. coli B. Radiat Res. 1971;45:94–109. doi: 10.2307/3573084. [DOI] [PubMed] [Google Scholar]
- 9.Fujii Y, et al. Ascorbic acid gives different protective effects in human cells exposed to X-rays and heavy ions. Mutat Res Genet Toxicol Environ Mutagen. 2010;699:58–61. doi: 10.1016/j.mrgentox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Peak MJ, Peak JG. Protection by glycerol against the biological actions of near-ultraviolet light. Radiat Res. 1980;83:553–558. doi: 10.2307/3575336. [DOI] [PubMed] [Google Scholar]
- 11.Galvao J, et al. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2013;28:1317–1330. doi: 10.1096/fj.13-235440. [DOI] [PubMed] [Google Scholar]
- 12.Aye M, et al. Assessment of the genotoxicity of three cryoprotectants used for human oocyte vitrification: Dimethyl sulfoxide, ethylene glycol and propylene glycol. Food and Chemical Toxicology. 2010;48:1905–1912. doi: 10.1016/j.fct.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012;1826:443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, et al. In vitro screening of radioprotective properties in the novel glucosylated flavonoids. Int J Mol Med. 2016;38:1525–1530. doi: 10.3892/ijmm.2016.2764. [DOI] [PubMed] [Google Scholar]
- 15.Weber TM, Kausch M, Rippke F, Schoelermann AM, Filbry AW. Treatment of xerosis with a topical formulation containing glyceryl glucoside, natural moisturizing factors, and ceramide. J Clin Aesthet Dermatol. 2012;5:29–39. [PMC free article] [PubMed] [Google Scholar]
- 16.Su C, Allum AJ, Aizawa Y, Kato TA. Novel glyceryl glucoside is a low toxic alternative for cryopreservation agent. Biochem Biophys Res Commun. 2016;476:359–364. doi: 10.1016/j.bbrc.2016.05.127. [DOI] [PubMed] [Google Scholar]
- 17.Schagen, S. K., Overhagen, S. & Bilstein, A. New data confirm skin revitalizing and stress protection by Glycoin® natural. Euro Cosmetics, 14–17 (2017).
- 18.Schrader A, et al. Effects of glyceryl glucoside on AQP3 expression, barrier function and hydration of human skin. Skin Pharmacol Physiol. 2012;25:192–199. doi: 10.1159/000338190. [DOI] [PubMed] [Google Scholar]
- 19.Takenaka F, Uchiyama H. Synthesis of alpha-D-glucosylglycerol by alpha-glucosidase and some of its characteristics. Biosci Biotechnol Biochem. 2000;64:1821–1826. doi: 10.1271/bbb.64.1821. [DOI] [PubMed] [Google Scholar]
- 20.Authority EFS. Scientific Opinion on the safety and efficacy of vitamin C (ascorbic acid, sodium ascorbate, calcium ascorbate, ascorbyl palmitate, sodium calcium ascorbyl phosphate and sodium ascorbyl phosphate) as a feed additive for all animal species based on a dossi. EFSA. 2013;11:3104. doi: 10.2903/j.efsa.2013.3104. [DOI] [Google Scholar]
- 21.Mathew D, et al. Ascorbic acid monoglucoside as antioxidant and radioprotector. J. Radiat. Res. 2007;48:369–376. doi: 10.1269/jrr.07007. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekharan DK, Kagiya TV, Nair CK. Radiation protection by 6-palmitoyl ascorbic acid-2-glucoside: studies on DNA damage in vitro, ex vivo, in vivo and oxidative stress in vivo. J Radiat Res. 2009;50:203–212. doi: 10.1269/jrr.08090. [DOI] [PubMed] [Google Scholar]
- 23.Engen A, et al. Induction of cytotoxic and genotoxic responses by natural and novel quercetin glycosides. Mutat Res Genet Toxicol Environ Mutagen. 2015;784–785:15–22. doi: 10.1016/j.mrgentox.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Hostetler G, et al. Flavone deglycosylation increases their anti-inflammatory activity and absorption. Mol Nutr Food Res. 2012;56:558–569. doi: 10.1002/mnfr.201100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollman PCH. Absorption, bioavailability, and metabolism of flavonoids. Pharm Biol. 2004;42:74–83. doi: 10.3109/13880200490893492. [DOI] [Google Scholar]
- 26.Yamamoto, I. & Muto, N. Bioavailability and Biological Activity of L-Asorbic Acid 2-O-α-Glucoside. J Nutr Sci Vitaminol (Tokyo), 161–164 (1992). [DOI] [PubMed]
- 27.Kumagai J, et al. Long-lived mutagenic radicals induced in mammalian cells by ionizing radiation are mainly localized to proteins. Radiation Research. 2003;160:95–102. doi: 10.1667/RR3015. [DOI] [PubMed] [Google Scholar]
- 28.Waldren CA, Vannais DB, Ueno AM. A role for long-lived radicals (LLR) in radiation-induced mutation and persistent chromosomal instability: counteraction by ascorbate and RibCys but not DMSO. Mutat Res. 2004;551:255–265. doi: 10.1016/j.mrfmmm.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Maeda J, et al. Relative biological effectiveness in canine osteosarcoma cells irradiated with accelerated charged particles. Oncology letters. 2016;12:1597–1601. doi: 10.3892/ol.2016.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki M, Kase Y, Yamaguchi H, Kanai T, Ando K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. International Journal of Radiation Oncology*Biology*Physics. 1999;48:241–250. doi: 10.1016/S0360-3016(00)00568-X. [DOI] [PubMed] [Google Scholar]
- 31.Kamada T, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20:4466–4471. doi: 10.1200/JCO.2002.10.050. [DOI] [PubMed] [Google Scholar]
- 32.Cartwright IM, et al. Effects of targeted phosphorylation site mutations in the DNA-PKcs phosphorylation domain on low and high LET radiation sensitivity. Oncology letters. 2015;9:1621–1627. doi: 10.3892/ol.2015.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMillan DD, et al. Validation of 64Cu-ATSM damaging DNA via high-LET Auger electron emission. J Radiat Res. 2015;56:784–791. doi: 10.1093/jrr/rrv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunada S, et al. Monoglucosyl-rutin as a potential radioprotector in mammalian cells. Mol Med Rep. 2014;10:10–14. doi: 10.3892/mmr.2014.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.