Abstract
Natural killer T-cells, with an invariant T-cell antigen receptor α-chain (iNKT cells), are unique and conserved subset of lymphocytes capable of altering the immune system through their rapid and potent cytokine responses. They are reactive to lipid antigens presented by the CD1d molecule, an antigen-presenting molecule that is not highly polymorphic. iNKT cell responses frequently involve mixtures of cytokines that work against each other, and therefore attempts are underway to develop synthetic antigens that elicit only strong interferon-gamma (IFNγ) or only strong interleukin-4 responses but not both. Strong IFNγ responses may correlate with tighter binding to CD1d and prolonged stimulation of iNKT cells, and this may be useful for vaccine adjuvants and for stimulating anti-tumor responses. iNKT cells are self-reactive although the structure of the endogenous antigen is controversial. By contrast, bacterial and fungal lipids that engage the T-cell receptor and activate IFNγ from iNKT cells have been identified from both pathogenic and commensal organisms and the responses are in some cases highly protective from pathogens in mice. It is possible that the expanding knowledge of iNKT cell antigens and iNKT cell activation will provide the basis for therapies for patients suffering from infectious and immune diseases and cancer.
Keywords: Glycolipid, Immune system, Natural killer T-cells

Introduction
General background
Initially described in mice [1], natural killer T-cells (NKT cells) have characteristics of both innate and adaptive immune cells. In mice, they were originally identified as cells that express NK 1.1, a marker for NK cells, which are a type of innate lymphocyte, expressed together with a αβ T-cell receptor (TCR), characteristic of adaptive immune cells. It is now known that all NKT cells do not express NK 1.1, and a subset of these lymphocytes expresses an invariant TCR α-chain that imparts a particular glycolipid specificity that is described further below. Cells with this phenotype were later confirmed to be present also in humans [2]. As in the mouse, the human NKT cell population is marked by a NK cell receptor, in this case, CD161, and it also expresses the αβ TCR with an invariant α-chain that shares significant homology with its mouse counterpart [3]. Comprising approximately 1% of peripheral lymphocytes in mice, NKT cells numbers in the peripheral blood mononuclear cells of humans tend to be less frequent, with a wide variation ranging from under 0.01% to 1% in healthy donors [4]; however, using CD161 and TCR expression as a definition of NKT cells, the frequency is much higher.
Because they express the αβ TCR, it was originally assumed that NKT cells would recognize peptides, similar to conventional T-lymphocytes that recognize peptides presented by major histocompatibility complex (MHC)-encoded antigen presenting proteins. Although early data and some recent data [5], [6] indicated that NKT cells may be able to recognize peptides, it has been widely confirmed that NKT cells recognize and respond to lipids, mostly glycolipids. These antigens are presented not by MHC-encoded molecules but by CD1d, a related protein encoded outside the MHC gene complex [7]. This review will provide a brief overview of NKT cells, some of their known antigens and properties of the CD1d molecule.
Type I and Type II natural killer T-cells
Although there are T-lymphocytes having different specificities that express NK receptors, NKT cells are now generally defined as T-cells that recognize CD1d. The most commonly studied NKT cells are invariant NKT cells (iNKT cells) or Type I NKT cells, which express a nearly fixed or invariant TCR α-chain encoded by a Vα14-Jα18 rearrangement. This α-chain is co-expressed most frequently with a Vβ8.2, Vβ7, or Vβ2 TCR β-chain in mice. In humans, the Type I NKT cell TCR is formed by a nearly fixed Vα24-Jα18 (TRAV1-2-TRAJ18) rearrangement paired with a Vβ11 β-chain. The mouse and human invariant α-chains are true homologs, as are human Vβ11 and mouse Vβ8. As a consequence; the specificity of mouse and human iNKT cells is highly conserved. This Type I NKT cell TCR has a conserved parallel binding motif radically different than the binding orientation of the TCR of mainstream CD4 or CD8 T-cells [8]. Type II NKT cells recognize CD1d as well; however, the α-chain is not highly restricted in diversity. Therefore, they do not have a single specificity, and they have been much less studied [9]. Here, we will focus on Type I NKT cells or iNKT cells.
Biological response of invariant natural killer T-cells
iNKT cells are very rapid responders when stimulated through their TCR, providing a cytokine burst within 90 min of in vivo stimulation [10]. Though TCR recognition of a lipid antigen presented by CD1d [Fig. 1], iNKT cells can induce a wide range of cytokines including T-helper Type 1 (Th1), T-helper Type 2 (Th2), and other responses. Activated iNKT cells not only secrete these cytokines but also induce other cells to secrete cytokines. The results from a number of studies demonstrate that the totality of the iNKT cell-induced immune response is dependent on the structure of the lipid antigen that is presented and recognized. Certain lipid antigens cause the production of predominately Th1 cytokines such as interferon-gamma (IFN-γ) and tumor necrosis factor, and other lipids lead to a more Th2 skewed pattern of cytokines that includes interleukin (IL-4), IL-5, and IL-13 [11].
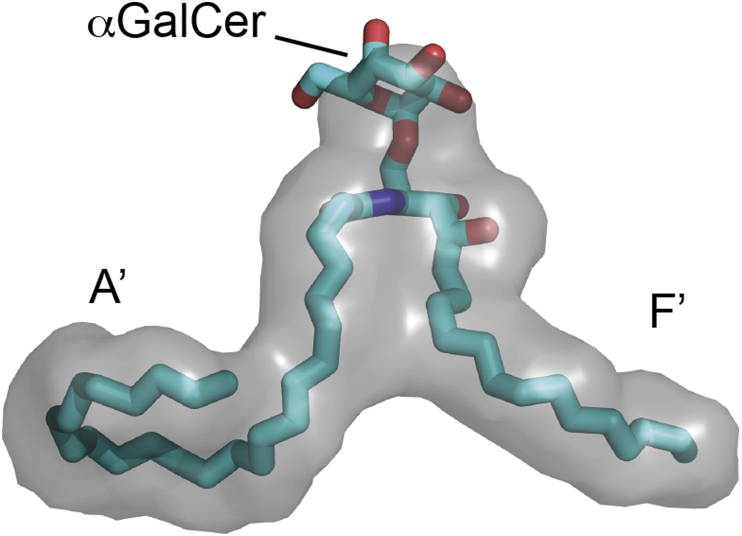
Fig. 1.
CD1d lipid binding pocket showing A′ and F′ grooves with α-galactosylceramide bound to CD1d for reference.
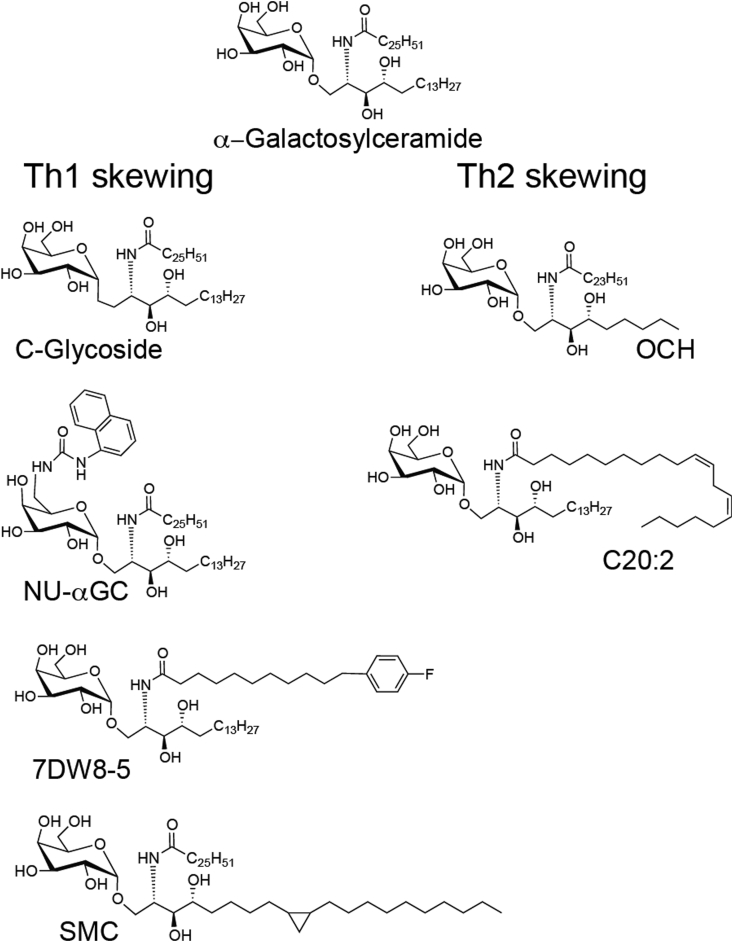
α-Galactosylceramide
The most studied glycolipid that activates iNKT cells, also the first discovered, is α-galactosylceramide (αGalCer) [Fig. 1, Fig. 2]. This is sometimes considered Th0 skewing lipid as iNKT cells that respond to this lipid robustly produce both IFN-γ and IL-4. αGalCer was originally identified by the Kirin Pharmaceutical Company in a screen of natural extracts for substances that prevent metastases of the mouse B16 melanoma, and it was shown to reduce liver metastases [12]. The structure was then synthesized and optimized by medicinal chemistry using the tumor metastases assay [13], [14]. αGalCer has α-linked galactose, a phytosphingoid base chain with 18 carbons, and an acyl chain containing 26 carbons. αGalCer has not yet proven highly successful in human cancer studies, which may be due to the fact that it leads to both Th1 and Th2 cytokine responses [15], [16]. These opposing responses may not promote an optimal anti-tumor response, which is more Th1-dependent. There are other explanations for reduced efficacy including the lower affinity of αGalCer/CD1d complexes for the human TCR compared to the mouse TCR [17]. For these reasons, there have been extensive efforts to develop other iNKT cell-activating lipids that can skew the cytokine response, especially in a Th1 direction. In addition, the type of antigen-presenting cell (APC) targeted may be critical, and in clinical trails, transfer of dendritic cells (DC) incubated with αGalCer generated a more robust iNKT cell response than αGalCer alone [16], [18], [19]. Continuing efforts to develop more effective glycolipids, delivery systems, and cell-based therapies using αGalCer remain underway.
Fig. 2.
Some representative Th1 and Th2 cytokine skewing lipids compared to α-galactosylceramide.
CD1d
CD1d antigen-presenting molecule is a member of the family of CD1 proteins. This family is divided into two groups: Group 1 CD1 proteins (CD1a, CD1b, and CD1c) and Group 2 CD1 (CD1d) [20]. There is also a third, intermediate group member (CD1e). Whereas CD1a, CD1b, CD1c, and CD1d are found on the cell surface; CD1e is an intracellular protein that facilitates glycolipid processing and presentation [21]. These proteins are found in humans and most other mammals; however, the mouse genome contains only two copies of the CD1d gene and no Group 1 CD1 proteins.
CD1d has a heterodimeric structure similar to MHC Class I antigen presenting molecules, with a heavy chain having three extracellular domains and a conserved β2-microglobulin subunit [22]. However, whereas MHC Class I molecules have shallow binding grooves capable of binding peptides that are typically nine amino acids in length; CD1d has a much deeper, narrower, and more hydrophobic groove containing two pockets, delineated as A′ and F′ [Fig. 1]. This groove is perfectly suited to bind glycosphingolipids (GSLs) that have two hydrophobic chains that can anchor deeply within it. The phytosphingoid base chain of GSLs is localized to the smaller F′ pocket, whereas the amide-linked fatty acid chain binds in the A′ pocket. Within the A′ pocket, the lipid chain must curl around a central point created by Cys12 and Phe70 [21]. The binding of the lipid chains within CD1d exposes the saccharide head group that is recognized and forced into a fixed orientation by the iNKT cell TCR [23].
CD1d is synthesized in the endoplasmic reticulum (ER) and binds to self-phospholipids that allow it to traffic to endosomal compartments and the cell surface [21]. It has a tyrosine-containing cytoplasmic tail motif that mediates internalization to endosomes and eventually to lysosomes before recycling back to the cell surface. Exchange of self-antigens obtained in the ER with exogenous glycolipids and with self-lipids involved in the positive selection of iNKT cells in the thymus occurs within endosomal compartments [24], [25], and for some exogenous antigens, also on the cell surface [26]. CD1d is expressed on a wide variety of hematopoietic series cells including B-cells, DC, macrophages, Langerhans cells, monocytes, T-cells, and iNKT cells [27]. Some nonhematopoietic cells including hepatocytes and intestinal epithelial cells also express it [28], [29]. Data indicate that DCs are the key activators of iNKT cells for exogenous glycolipids, except that various types of macrophages are important for presenting glycolipids in particulate form such as on beads or in liposomes [30]. Recently, the CD8α+ DEC-205+ DC subset was identified as the APC type responsible for inducing both Th1 and Th2 responses [31]. According to these findings, increased expression of cell surface markers Rae-1 and CD86 by APCs leads to a Th1 cytokine profile while increased APC expression of PD-L2 serves to promote a Th2 cytokine profile [31].
Synthetic antigens
Head group modifications
The hydrophobic chains of iNKT cell lipid antigens are usually bound deep within the pockets of CD1d, and a polar head group is exposed for recognition by the iNKT cell TCR. The CD1d molecule stabilizes this head group through interactions with α1 and α2 helices [22]. In humans, position 153 of the α2 helix is a tryptophan amino acid, instead of a glycine in the homologous position (155) of mouse CD1d. Tryptophan, being a much bulkier amino acid, shifts the sugar head group into a slightly different position [23], [32]. This is the main distinction between the structures of the GSL-CD1d complexes expressed by mice and humans.
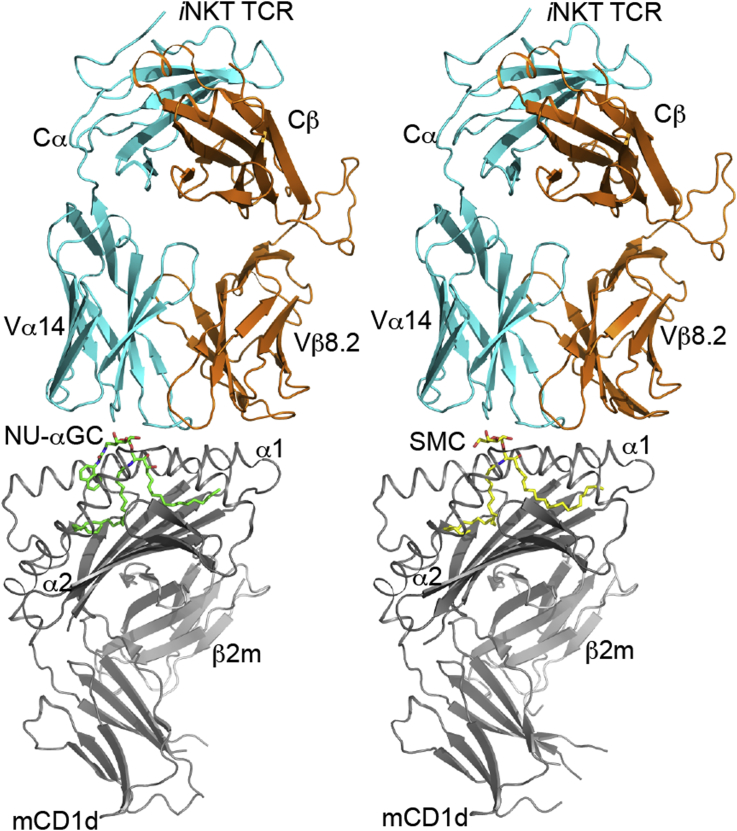
Contacts between Asp153 and Thr156 of mouse CD1d and the 2′ and 3′-hydroxyls of the αGalCer saccharide head group as well as the αGalCer O-glycosidic oxygen are important for ordering the sugar head group [33], [34]. Because iNKT cell TCR recognizes the galactose head groups of GSLs, it is not surprising that altering this head group had deleterious effects on TCR binding. Modifications of the 2′, 3′, or 4′ positions, particularly 2′ diminished iNKT cell responses and TCR binding [35], [36]. For example, while αGalCer and α-glucosyl ceramide (αGluCer) could both activate iNKT cells, which indicated that the axial versus equatorial orientation of the 4′-hydroxyl group is not critical; αGalCer was the most potent antigen. In contrast, α-mannosyl ceramide (αManCer) did not activate iNKT cells, indicating that the equatorial 2′-hydroxyl is critical [37]. Additional studies demonstrated the importance of the 2′-hydroxyl group in TCR recognition, as synthetic analogs of αGalCer with modifications of this position lacked antigenic activity [38]. The 3′ position is more permissive as modifications at this position decreased but did not completely diminish activity [38], [39], [40]. Modifications of the 6′ position are the most tolerated. This makes sense as crystal structures of the CD1d-lipid-TCR complex show that the 6′ position does not make contacts with the TCR [17], [41], [42], [43]. Tolerance for modifications at the 6′ position were revealed in a study that compared responses to Gal (α1-2) GalCer with Gal (α1-6) GalCer, which are both GSLs with a disaccharide as opposed to monosaccharide head groups. While Gal (α1-2) GalCer required carbohydrate processing to remove the terminal galactose in order for antigenic activity, Gal (α1-6) GalCer did not [44]. Indeed, modification of the 6′-hydroxyl proved to be beneficial when PBS57, a 6′-deoxy-6′-acetamide αGalCer analog, was synthesized [45]. PBS57 was not only more easily solubilized than αGalCer, and it also induced a more potent in vivo cytokine response [46]. The 6′ position is permissive for the addition of other bulky chemical groups as exemplified by the napthylurea αGalCer molecule (NU-αGC) [Fig. 2, Fig. 3] [47]. NU-αGC causes a robust Th1 cytokine response and reduces tumor metastases in mice even more effectively than αGalCer. It is very likely that NU-αGC binds in a more stable fashion to CD1d than αGalCer as the NU group serves as a “third anchor” of the lipid for binding to the CD1d molecule. It binds on top instead of deep in the CD1d groove, but without impeding the TCR [48]. Other 6′ modifications such as NC-αGalCer, 4ClPhC-αGalCer, and PyrC-αGalCer are also antigens that induce strong Th1 cytokine responses, correlated with a marked reduction of B16 melanoma metastasis to the liver [49]. PyrC-αGalCer, the most potent of these lipids, also shows novel and increased contacts with the both CD1d and the iNKT cell TCR. The 6′ modifications have also served as tools for labeling GSLs with biotin or fluorophore, which has permitted analyses of their progress through endosomal compartments in cells [50].
Fig. 3.
Two T-helper Type 1 skewing lipids crystallized in complex with mouse CD1d and the invariant Natural Killer T-cell receptor. PDB files: napthylurea, α-galactosylceramide (PDB code: 3QUZ), SMC (PDB code: 3TVM).
Other synthetic alterations have been used to create α-carba-GalCer analogs [51]. In 2009, carbasugar and cyclitol analogs of αGalCer were generated, and another three analogs, RCAI-56 (a carba-α-d-galactose analog), RCAI-59 (a 1-deoxy-neo-inositol analog), and RCAI-92 (a 1-O-methylated analog), also were found to be Th1 skewing lipids [52]. The α-carba-GalCer analog (RCAI-56), with the oxygen atom of d-galactose replaced with a methyl group, was shown to be an effective Th1 skewing molecule [53]. The authors proposed two mechanisms for this response. First, the methylene group replacing the oxygen atom interaction of the sugar head group makes the lipid less susceptible to degradation by hydroxylation, therefore allowing it to be presented for longer. Second, the oxygen of αGalCer may lead to a repulsive interaction with Pro28 of the TCR α-chain whereas the carbon of α-carba-GalCer would lead to an enhanced hydrophobic interaction [53]. This lipid also was tested in collagen-induced arthritis, an autoimmune model of rheumatoid arthritis in mice, and it was shown to provide protection against Th17-mediated autoimmune arthritis through the induction of Th1 cytokines [54].
HS44 is another GSL with head group modifications of αGalCer that induces a strong Th1 cytokine response. HS44 is an aminocyclitol molecule in which the sugar head group is a carba cyclitol ring that mimics glucose instead of galactose, and which has the O-glycosidic linkage replaced with an amide group [55]. Structural analysis and binding assays by surface plasmon resonance showed that when bound to CD1d this compound had a 14-fold weaker interaction with the iNKT cell TCR compared to αGalCer. Despite this, it caused a strong Th1 cytokine response and was effective at preventing tumor metastases [56].
Linkage modifications
As previously mentioned [55], the O-glycosidic linkage of αGalCer can be altered, and in doing so, can lead to some unique properties. In 2003, the oxygen bond was altered to a CH2, theorizing that this GSL would be more resistant to α-galactosidase-mediated catabolism [57], [58]. This compound, referred as C-glycoside [Fig. 2], was the first glycolipid shown to induce a strong Th1 cytokine response in mice [59], but it did not stimulate human iNKT cells very strongly. The ternary structure of C-glycoside/CD1d complexes bound to the iNKT cell TCR demonstrated the importance of hydrogen bonding of the oxygen in the O-glycosidic linkage to CD1d [48], [60]. Without this linkage, the galactose headgroup was in a suboptimal orientation when bound to CD1d, resulting in a lower TCR affinity compared to αGalCer/CD1d complexes.
Several C-glycoside analogs with a double bond were synthesized to try to generate a glycolipid providing a more fixed orientation of the galactose headgroup, in the expectation that this would activate iNKT cells to produce a strong Th1 response [61]. One of these, GCK127 had an E-olefin linker instead of a CH2 glycosidic linkage. This molecule activated both mouse and human iNKT cells, albeit it was less potent than αGalCer [61]. The O-glycosidic linkage has also been modified by replacing the oxygen with a sulfur atom. The α-S-GC molecule induced a more Th2 skewed cytokine profile in mice and did not lead to increased survival in a tumor model [62]. A sulfur linkage was proposed to have a similar conformation to an oxygen bond, because although a sulfur atom is larger than oxygen, the bond angle is less than an O-glycosidic linkage and a thioglycosidic linkage would be less susceptible to enzymatic cleavage [63]. However, in vivo studies in mice indicated that α-S-GC did not lead to iNKT cell proliferation or cytokines. The authors proposed that the α-S-GC compound would lack some key hydrogen bonds when bound to CD1d, but this remains a conjecture. Another group synthesized the same compound and showed that although it did not stimulate mouse iNKT cells, it could activate human iNKT cells, causing cytokine secretion and iNKT cell-induced maturation signals in human DCs [64]. This is an intriguing counter example of species specificity of iNKT cell reactivity, compared to C-glycoside, which only works to activate mouse iNKT cells but not those from humans.
Lipid chain modifications
The sphingoid base is a defining property of the GSL antigens that activate iNKT cells. The most commonly found sphingosine chain length in animal tissues is the aliphatic C: 18 chains; however, the number of carbon lengths can range from 14 to more than 27, and the sphingosine can have a wide variety of saturation levels and branched modifications [65]. The phytosphingosine of αGalCer [Fig. 2], with a hydroxyl group at C-4, differs from the more common natural sphingosine, which typically contains a trans double bond between C-4 and C-5. OCH is a compound with a phytosphingosine shortened by several carbon atoms [Fig. 2] [66]. This lipid antigen induced a more IL-4 dominated or Th2 profile. When injected into mice, OCH was shown to reduce the symptoms of experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis [66]. The further shortening of the sphingoid chain also led to a Th2 cytokine profile [67]. Different alterations of the sphingoid base can have an opposite polarizing effect on the cytokine response. For example, SMC124 is a synthetic Th1 skewing GSL similar to αGalCer but with a sphingoid base chain length increased to 22 linear carbons with the addition of a cyclopropyl group at C11-12 [Fig. 2, Fig. 3]. It was designed to mimic partially a naturally occurring GSL called plakoside A [68]. The results from structural studies of OCH and SMC124 bound to mouse CD1d suggest that the length of the lipid chain packed within the F′ pocket of CD1d may alter the presentation of the GSL [68]. The longer, bulkier chain of SMC124 may anchor the lipid more deeply within the antigen-presenting molecule, thereby prolonging antigen presentation, which may, for reasons that have not been completely elucidated, contribute to Th1 cytokine skewing. Conversely, OCH does not have a long chain to anchor CD1d and may have a reduced antigen presentation time.
The acyl chain of a number of synthetic GSL antigens is typically longer than the sphingoid base. For αGalCer, the acyl chain is an unbranched and fully saturated 26 carbons long. C20:2 is a GSL antigen containing a di-unsaturated and shorter, 20 carbon, and acyl chain [Fig. 2] [69]. This compound was identified in a screen of multiple αGalCer analogs with shorter acyl chains, all of which induced an enhanced Th2 cytokine profile [67]. The decrease in the carbon chains of the lipid, either in the acyl chain or in the sphingoid base, is proposed to destabilize the interactions between the lipid and CD1d. Indeed, when the lipid chains become too short, the interaction of the lipid and the CD1d is too unstable for any stimulation of iNKT cells [67]. Conversely, the elongation of the carbon chains, or the addition of bulky groups to the acyl chain, caused a Th1 profile that may be due to stabilization of the CD1d/lipid interaction. For example, the addition of an aromatic group to the terminus of the fatty acid created a potent IFN-γ inducing lipid in human cells [70]. 7DW8-5, which contains a C10 length fatty acyl chain with a fluorinated benzene ring at the end, is planned for use in clinical trials as an adjuvant for a malaria vaccine [Fig. 2] [71]. 7DW8-5 was shown to exhibit a higher binding affinity to both mouse and human CD1d molecules than αGalCer, resulting in a more potent stimulatory activity for iNKT cells [72]. Ultimately, 7DW8-5 could display a stronger adjuvant effect than αGalCer for malaria vaccine testing in mice [72] and nonhuman primates. EF77 is a companion lipid to SMC124 because it is also partially modeled after αGalCer and plakoside A. EF77 is identical to αGalCer but with an elongated acyl chain containing a cyclopropyl group. Like SMC124, EF77 also induced a strong Th1 cytokine response in mice [68]. Both of these GSL antigens were shown to form more stable complexes in vivo with CD1d on the surface APCs, at least when compared to αGalCer, consistent with the hypothesis that stronger or prolonged antigen interactions with CD1d favor IFN-γ production in vivo. [68].
Endogenous ligands
When the initial antigens for iNKT cells were discovered, it was predicted that the linkage of the sugar to the lipid could only be α-anomeric, and a β-linkage would not lead to iNKT cell activation [37], [73]. This was presumed as logical as the α-anomeric form as well as D-glycosylceramides were not thought to be detected in mammals and are therefore “foreign” epitopes. However, the analysis of iNKT cell differentiation in the thymus indicated that there had to be a self-ligand that could positively select these cells. A β-Linkage of sugar to the ceramide lipid does occur naturally in mammals, β-anomeric GSLs are part of cellular membranes [74], [75]. β-linkages therefore were considered to be candidates for the major self-ligands [76]. Mice lacking βGalCer synthase, and thus lacking βGalCer, have normal iNKT cell development [77], and while mice treated with this compound have decreased iNKT cells in vivo, there was no detectable cytokine signal [78]. βGluCer, having glucose instead of galactose, is both an anabolic and catabolic GSL pathway metabolic intermediate [79], and it decreases in vitro iNKT cell proliferation [80] and is accumulated in patients with Gaucher's disease, which have an increase in iNKT cells [81]. Surprisingly, while αManCer fails to activate iNKT cells, βManCer is an iNKT cell ligand capable of inducing a protective anti-tumor response [82]. This anti-tumor ligand operates through the induction of TNFα and nitric oxide, without IFN-γ, and it does not induce anergy, a side effect of some other strong iNKT cell ligands [83].
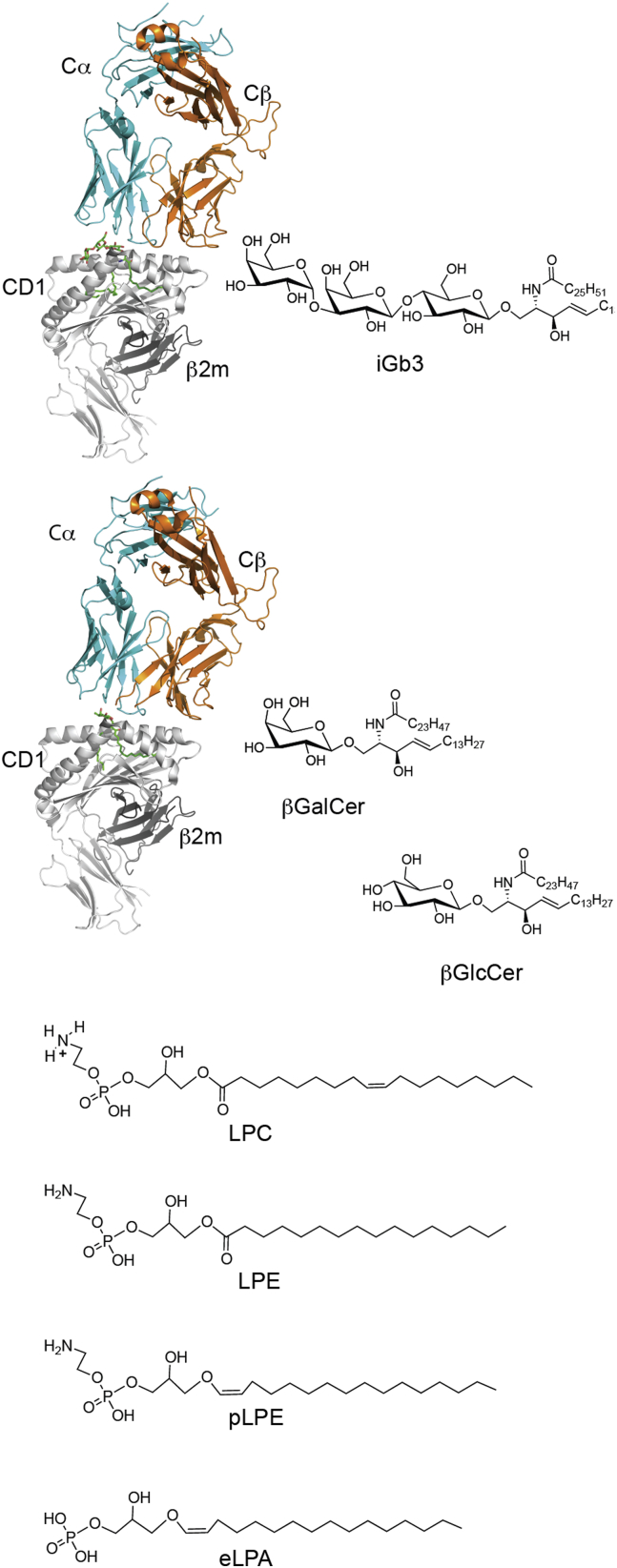
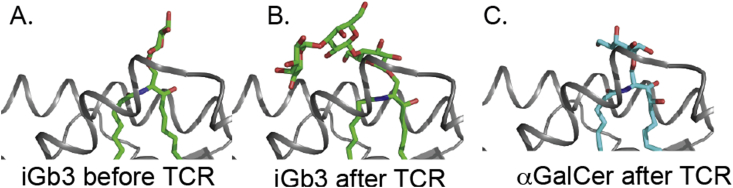
Because iNKT cells originate in the thymus, it was assumed that there must be some endogenous ligand(s) that participate in their positive selection. The hunt for endogenous ligands turned up several candidates including, isoglobotrihexosylceramide (iGb3), plasmalogen lysophosphatidylethanolamine [84], lysophosphatidylcholine, lysophosphatidylethanolamine, phosphatidylinositol, phosphatidylcholine, phosphatidylserine, phosphatidic acid βGluCer, βGalCer [85], and even a peptide from mouse collagen [6], [75], [81], [86], [87], [88], [89]. The structures of some of these are shown in [Fig. 4] iGb3, the first identified self-ligand [86], was crystallized, and it was shown that the mouse CD1d molecule can bind iGb3 [Fig. 5] [90]. When CD1d is loaded with iGb3, it forms a complex with the iNKT cell TCR in which the lipid-proximal sugar is moved into a conformation similar to α-linked ligands. The terminal sugar of iGb3 forming novel contacts with CD1d [Fig. 5] [91], [92]. However, the validity of such a compound as an endogenous ligand has been clouded by reports that suggest mice lacking iGb3 synthase, essential for iGb3 formation, still have a normal iNKT cell population [93]. In addition, it has been claimed that human CD1d cannot present iGb3 because of the glycine-tryptophan difference mentioned above [94], and iGb3 is not present in mouse or human thymus [95]. Therefore, iGb3 cannot be a required self-ligand for iNKT cells. β-Glucosyl ceramide was identified as another endogenous ligand for iNKT cells. With a structure very similar to αGalCer, and data indicating that this lipid accumulated in the presence of a microbial infection [96], as well as tumor prevention data [97], it seemed very logical that this ligand could activate iNKT cells. However, recent data has indicated that the activation of iNKT cells in this study could be due to contaminating trace α-anomeric lipids, possibly generated during synthesis of β-glycolipids [98], [99]. Regardless of the antigen, it is entirely possible that these self-ligands serve as not only a selection mechanism but also a means to get over an activation threshold when iNKT cells are activated by cytokines such as IL-12, IL-18, or IFN-1 [100], [101], [102], [103].
Fig. 4.
Proposed endogenous ligands for invariant natural killer T-cells. Top: Crystal structures of tri-molecular complexes and the corresponding glycosphingolipid antigen structures are indicated. Bottom: Phospholipid-containing putative self-antigens.
Fig. 5.
Induced fit by the invariant natural killer T-cell receptor with iGb3 showing the carbohydrate head group before and after T-cell receptor engagement. In A, only the sugar linked to the ceramide lipid could be resolved, presumably because the position of the two distal sugars was not fixed in the crystal. iGB3 before engagement (PDB code: 2Q7Y) and after engagement (PDB code: 3RZC) shown in comparison to α-galactosylceramide (PDB code: 3HE6).
Bacterial antigens for invariant natural killer T-cells
Mycobacterium tuberculosis
The first lipid antigens identified for any T-lymphocytes were lipids from Mycobacteria presented by Group 1 CD1 molecules [104], [105], [106]. These bacteria have an unusual outer waxy coating, comprised of different lipids, which is used to evade immune clearance. A screen of CD1d binding mammalian and Mycobacterium lipids identified phosphatidylinositol tetramannoside (PIM4) [107] isolated from a related bacterium, Mycobacterium bovis bacillus, a cause of bovine tuberculosis. The structure of a similar lipid, PIM2, bound to CD1d allowed for modeling of the PIM4 molecule in the CD1d groove [108]. However, a synthetic PIM4 molecule was shown to not stimulate iNKT cells [109], highlighting the need to compare the outcomes with synthetic and purified lipids. Subsequent work showed that Type II NKT cells with diverse TCRs could recognize different phospholipids from Mycobacterium tuberculosis. [110].
Sphingomonas spp.
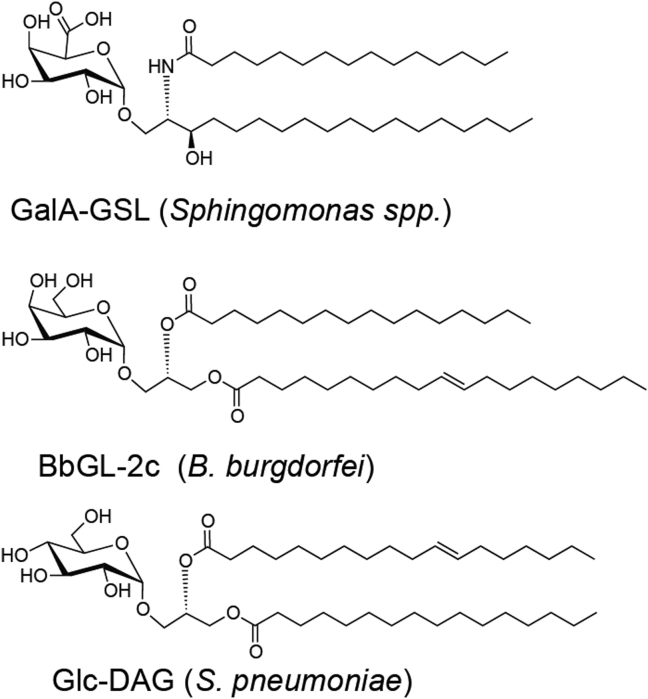
Sphingomonas spp. was the first bacteria unambiguously shown to have antigens for iNKT cells. Sphingomonas are commensal bacteria of the human intestine, and there is evidence that commensal bacteria affect the number and function of iNKT cells [73], [111], [112], [113]. Although not highly pathogenic, Sphingomonas includes more than 30 species that live in a wide variety of environments including soil and seawater. Sphingomonas paucimobilis is the most pathogenic, and it may be an opportunistic pathogen associated with suppression of the immune system [114]. As early as 2000, purification of GSLs from Sphingomonas bacteria was performed and it was noted that bacterial synthesis of different lipids can change depending on environmental factors [115], [116], [117]. There is an unusually high presence of GSLs in this α-Proteobacteria, instead of the LPS that is otherwise commonly found in Gram-negative bacteria [118]. In 2005, using both purified and synthetic material, several strains of Sphingomonas bacteria were shown to have antigens for iNKT cells including S. paucimobilis, Sphingomonas capsulate, and Sphingomonas yanoikuyae [73], [119]. From these organisms, GSLs were isolated that had very strong structural similarities to αGalCer. For example, from S. paucimobilis, GSL-1 was isolated α-glucuronosylceramide compound, and from S. yanoikuyae α-galacturonosylceramide originally called GSL-1′. These two lipids are highly similar but differ only in their carbohydrate head groups; having a glucuronic acid versus galacturonic acid (GalA). Since their discovery, GSL-1′ has been renamed as GalA-GSL to indicate the presence of GalA [Fig. 6]. This family of Sphingomonas-derived compounds also contains some with additional saccharide groups including tri-and tetra-saccharides known as GSL-3 and GSL-4, respectively. While the monosaccharide-containing GSL antigens activate iNKT cells in vivo and in vitro, [73], [119], [120] they are all weaker than αGalCer. For example, the iNKT cell TCR affinity for GalA-GSL/CD1d is 50-fold weaker than for the αGalCer/CD1d complex [23]. The GSLs with oligosaccharide headgroups are not antigenic or do not activate as well as their monosaccharide counterparts, possibly due to a failure of the APCs to reduce the structures to a monosaccharide form in lysosomal compartments [46], [121].
Fig. 6.
Invariant natural killer T-cell microbial lipids that have been crystallized in complex with mouse CD1d and the mouse invariant natural killer T-cell receptor.
Borrelia burgdorferi
Whereas Sphingomonas spp. is not considered pathogenic, another source of iNKT cell antigens is from the pathogenic spirochete Borrelia burgdorferi. These bacteria, transmitted to humans by Ioxedes scapularis (deer tick) bites, are the cause of Lyme disease, which is currently the most common vector-borne disease in the United States [122]. The immune response of mice to B. burgdorferi infection is partially CD1d dependent as a strain of mice resistant to Lyme arthritis had symptoms when the gene encodingCD1d was deleted [123]. Furthermore, mice lacking CD1d had decreased pathogen-specific antibodies [124]. B. burgdorferi-induced pathogenesis was increased in Jα18 deficient mice, which cannot form the invariant iNKT cell α-chain. In C57BL/6 mice, the absence of iNKT cells led to increased replication of spirochetes in the joint, delayed clearance, and arthritis [125], [126] while Jα18 deficient mice on the BALB/c background had increased carditis [127]. Analysis of lipids from B. burgdorferi detected a series of abundant galactosyl diacylglycerols (DAGs) including BbGL-2 [109]. BbGL-2 was shown to activate iNKT cells in purified and synthetic forms. BbGL-2 was the first reported iNKT cell antigen that is not a GSL; it contains a d-galactose saccharide group with an α-anomeric glycosidic bond and is a DAG lipid, with lipid chains that vary in length and degree of saturation. The most highly potent of these in mice was BbGL-2c [Fig. 6], with C18:1 oleic acid in the sn-1 position and C16:0 palmitic acid in the sn-2 position [109] [Fig. 6]. Structural characterization of multiple different DAG antigens showed how alteration of the lipid chains determines the binding mode. The sn-1 linked fatty acid is capable of binding to either A′ or F′ pockets of mouse CD1d, with C18:1 oleic acid preferring the A′ pocket [23]. The different binding modes determine how the exposed saccharide group is oriented for recognition by the TCR. Structural analysis demonstrated that the orientation of the BbGL-2c allowed for an optimal configuration compared to another DAG lipid with two unsaturated fatty acid chains [23]. Interestingly, in humans, the optimal sn-1 and sn-2 fatty acids are different because of the role that the position 153 tryptophan in the human CD1d α2 helix plays in orienting the exposed sugar.
Streptococcus pneumonia
Streptococcus pneumonia is a Gram-positive bacterium that not only leads to pneumonia, but also causes potentially lethal bacteremia, otitis media, and meningitis. iNKT cells were shown to be protective in mice following pulmonary S. pneumonia infection [128], and this protective response was linked to IFN-γ production by the iNKT cells [129]. The lipids of this bacterium were subjected to electrospray mass spectrometry analysis and two highly abundant DAGs were isolated: a monosaccharide linked DAG, α-glucosyldiacylglycerol (GlcDAG) [Fig. 6], and a disaccharide linked DAG, α-galactosyl GlcDAG (GalGlcDAG) [130]. Like the B. burgdorferi antigen, the DAG from S. pneumoniae has α-linked hexose sugar in the sn-3 position but is a glucose rather than galactose. This antigen has palmitic acid (C16:0) in the sn-1 position, and a vaccenic acid, a C18:1 fatty acid with a C11-C12 unsaturated bond, in the sn-2 position. The ternary crystal structure of GlcDAG bound to mouse CD1d with the iNKT cell TCR engaged shows that the vaccenic acid allows for a novel conformational change causing a re-orienting of the axial 4′-hydroxyl group of glucose to allow for the TCR to engage in a conserved binding motif [131].
Helicobacter pylori
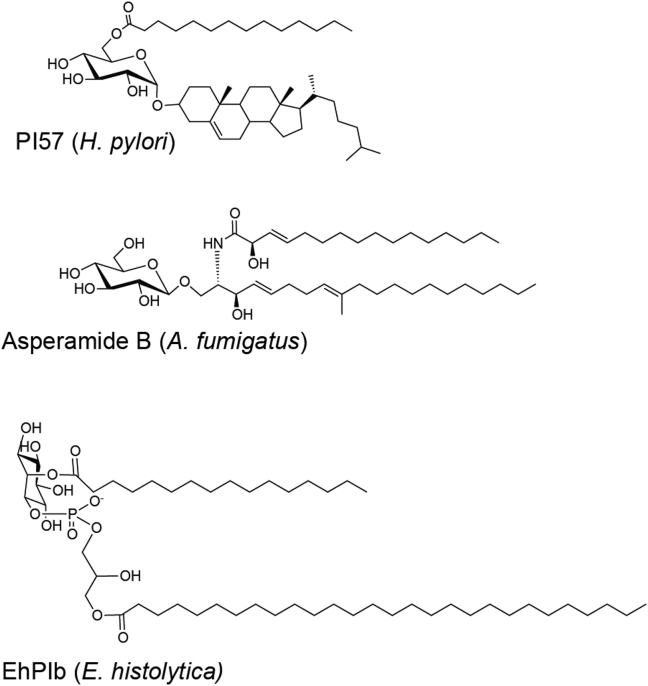
Helicobacter pylori is a common, Gram-negative spiral bacterium that causes stomach problems in some individuals including gastritis, peptic ulcer, duodenal ulcer, lymphoma, and gastric cancer [132]. Certain H. pylori lipid extracts have been shown to expand iNKT cells and to play a beneficial role in clearing the bacterium [133], [134]. This bacterium synthesizes cholesteryl α-glucosides using host-derived cholesterol. Although one study presented data indicating that cholesterol glucosylation allows H. pylori to evade phagocytosis by macrophages [135], these compounds also were shown to be recognized by iNKT cells, and the absence of iNKT cells in mice was correlated with increased H. pylori infection [134]. In a different study, mice were protected from asthma symptoms by administering an H. pylori, cholesterol-containing, synthetic glycolipid called PI57 [Fig. 7] [133], although this compound activated iNKT cells in both mice and humans, as determined by immune assays, biochemical analysis of TCR interaction with the cholesteryl α-glucoside complex with CD1d, and structural analysis showing how a cholesterol-containing antigen binds to CD1d, have not been reported.
Fig. 7.
Invariant natural killer T-cell microbial lipids that currently have not been crystallized in complex with CD1d and the mouse invariant natural killer T-cell receptor.
Fungal antigens for invariant natural killer T-cells
Recently, it was reported that Aspergillus fumigatus contains a glycolipid antigen that can activate iNKT cells. Aspergillus is a ubiquitous fungus, and although exposure through inhalation is almost a daily occurrence, the spores or conidia can lead to infections, most commonly in the lungs and sinuses, and furthermore, Aspergillus exposure can contribute to asthma [136]. Previously, it had been shown that iNKT cells were activated by β-1,3-glucans from Aspergillus, which triggered IL-12 secretion from APCs that mediated the activation of iNKT cells, but which also required recognition CD1d-presented self-antigens [137]. In the more recent study implicating a microbial lipid in iNKT cell activation, the lipids of the two most common strains of Aspergillus, A. fumigatus, and Aspergillus niger were fractionated and tested against primary iNKT cell lines, and the GSL asperamide B [Fig. 7] was identified as an antigen for mouse and human iNKT cells. Asperamide B is a β-linked glucosylceramide, which is different from any of the previously described α-linked exogenous ligands. It possesses a 9-methyl-4,8-sphingadienine chain, commonly found in fungi [65] and a β-γ unsaturated acyl chain with an α hydroxyl group [138]. Both purified and synthetic asperamide B were able to activate iNKT cells, and mice exposed to asperamide B experienced airway hyperreactivity, a feature of asthma, within 24 h [139].
Protozoan antigens for invariant natural killer T-cells
The glucosylphosphoshatidylinositol (GPI) anchors of surface proteins from Plasmodium falciparum and Typanosoma brucei could expand iNKT cells, and this expansion was CD1d dependent [140]. A subsequent study, however, contradicted the earlier finding that reported that the production of Plasmodium anti-circumsporozoite IgG was MHC Class II-independent, and by implication, iNKT cell dependent. These investigators also reported that GPI anchors could not induce iNKT cell activation [141]. More recently, a phosphoinositol antigen known as EhPI [Fig. 7] was isolated from a pool of lipopeptidophosphoglycans from Entamoeba histolytica trophozoites and was shown to activate iNKT cells [142]. E. histolytica can be fatal, especially in the developing world, as it leads to amoebiasis, a diarrheal disease [143]. There are two isoforms of EhPI, a and b, with the only difference between them being the acylation of inositol. Only the diacylated EhPIb [Fig. 7] stimulated iNKT cells. This lipid caused IFN-γ activation of iNKT cells that was dependent not only on CD1d but also on IL-12 production through Toll-like receptor signaling of APCs [142].
Summary
In conclusion, over the past two decades, knowledge about the specificity of iNKT cells has grown dramatically. Synthetic agonists have been synthesized that bind with different degrees of stability to CD1d and that are able to preferentially skew the global immune response. Several natural antigens for iNKT cells have been identified, and they are both self-antigens that originate from antigen-presenting cells or foreign antigens from commensal or pathogenic microbes. It has been shown in several cases that the protective responses to pathogenic microbes depend on iNKT cell activation. The iNKT cell TCR can recognize these different structures, which are mostly glycolipids. A conserved docking motif of the iNKT cell TCR has been characterized through dozens of structural studies and is nearly always present, in some cases through a large accommodation of the antigen. Much work has been done to understand the mechanism of antigen recognition and the therapeutic potential of iNKT cells [36], [144], [145], [146], and the hope is that this detailed biochemical knowledge can be harnessed to realize the therapeutic potential of these cells.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Yankelevich B., Knobloch C., Nowicki M., Dennert G. A novel cell type responsible for marrow graft rejection in mice. T cells with NK phenotype cause acute rejection of marrow grafts. J Immunol. 1989;142:3423–3430. [PubMed] [Google Scholar]
- 2.Spada F.M., Koezuka Y., Porcelli S.A. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Exley M.A., Garcia J., Balk S.P., Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4-CD8- T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee P.T., Putnam A., Benlagha K., Teyton L., Gottlieb P.A., Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest. 2002;110:793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tangri S., Brossay L., Burdin N., Lee D.J., Corr M., Kronenberg M. Presentation of peptide antigens by mouse CD1 requires endosomal localization and protein antigen processing. Proc Natl Acad Sci U. S. A. 1998;95:14314–14319. doi: 10.1073/pnas.95.24.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y., Teige A., Mondoc E., Ibrahim S., Holmdahl R., Issazadeh-Navikas S. Endogenous collagen peptide activation of CD1d-restricted NKT cells ameliorates tissue-specific inflammation in mice. J Clin Invest. 2011;121:249–264. doi: 10.1172/JCI43964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendelac A., Lantz O., Quimby M.E., Yewdell J.W., Bennink J.R., Brutkiewicz R.R. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Girardi E., Wang J., Yu E.D., Painter G.F., Kronenberg M. The Vα14 invariant natural killer T cell TCR forces microbial glycolipids and CD1d into a conserved binding mode. J Exp Med. 2010;207:2383–2393. doi: 10.1084/jem.20101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godfrey D.I., MacDonald H.R., Kronenberg M., Smyth M.J., Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda J.L., Naidenko O.V., Gapin L., Nakayama T., Taniguchi M., Wang C.R. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.East J.E., Kennedy A.J., Webb T.J. Raising the roof: the preferential pharmacological stimulation of Th1 and Th2 responses mediated by NKT cells. Med Res Rev. 2014;34:45–76. doi: 10.1002/med.21276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natori T., Morita M., Akimoto K., Koezuka Y. Agelasphins, novel antitumor and immunostimulatory cerebrosides from the marine sponge agelas-mauritianus. Tetrahedron. 1994;50:2771–2784. [Google Scholar]
- 13.Akimoto K., Natori T., Morita M. Synthesis and stereochemistry of agelasphin-9b. Tetrahedron Lett. 1993;34:5593–5596. [Google Scholar]
- 14.Morita M., Motoki K., Akimoto K., Natori T., Sakai T., Sawa E. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38:2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 15.Uchida T., Horiguchi S., Tanaka Y., Yamamoto H., Kunii N., Motohashi S. Phase I study of α-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57:337–345. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa A., Motohashi S., Ishikawa E., Fuchida H., Higashino K., Otsuji M. A phase I study of α-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 17.Gadola S.D., Koch M., Marles-Wright J., Lissin N.M., Shepherd D., Matulis G. Structure and binding kinetics of three different human CD1d-α-galactosylceramide-specific T cell receptors. J Exp Med. 2006;203:699–710. doi: 10.1084/jem.20052369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieda M., Okai M., Tazbirkova A., Lin H., Yamaura A., Ide K. Therapeutic activation of Vα24 Vβ11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 19.Chang D.H., Osman K., Connolly J., Kukreja A., Krasovsky J., Pack M. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen N.R., Garg S., Brenner M.B. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 21.Barral D.C., Brenner M.B. CD1 antigen presentation: how it works. Nat Rev Immunol. 2007;7:929–941. doi: 10.1038/nri2191. [DOI] [PubMed] [Google Scholar]
- 22.Zajonc D.M., Kronenberg M. CD1 mediated T cell recognition of glycolipids. Curr Opin Struct Biol. 2007;17:521–529. doi: 10.1016/j.sbi.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J., Li Y., Kinjo Y., Mac T.T., Gibson D., Painter G.F. Lipid binding orientation within CD1d affects recognition of Borrelia burgorferi antigens by NKT cells. Proc Natl Acad Sci U. S. A. 2010;107:1535–1540. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cernadas M., Sugita M., van der Wel N., Cao X., Gumperz J.E., Maltsev S. Lysosomal localization of murine CD1d mediated by AP-3 is necessary for NK T cell development. J Immunol. 2003;171:4149–4155. doi: 10.4049/jimmunol.171.8.4149. [DOI] [PubMed] [Google Scholar]
- 25.Zhou D., Cantu C., 3rd, Sagiv Y., Schrantz N., Kulkarni A.B., Qi X. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Im J.S., Arora P., Bricard G., Molano A., Venkataswamy M.M., Baine I. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–898. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dougan S.K., Kaser A., Blumberg R.S. Springer Berlin Heidelberg; Berlin, Heidelberg: 2007. T cell activation by CD1 and lipid antigens. [Google Scholar]
- 28.Canchis P.W., Bhan A.K., Landau S.B., Yang L., Balk S.P., Blumberg R.S. Tissue distribution of the non-polymorphic major histocompatibility complex class I-like molecule, CD1d. Immunology. 1993;80:561–565. [PMC free article] [PubMed] [Google Scholar]
- 29.Blumberg R.S., Terhorst C., Bleicher P., McDermott F.V., Allan C.H., Landau S.B. Expression of a nonpolymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J Immunol. 1991;147:2518–2524. [PubMed] [Google Scholar]
- 30.Barral P., Polzella P., Bruckbauer A., van Rooijen N., Besra G.S., Cerundolo V. CD169+ macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat Immunol. 2010;11:303–312. doi: 10.1038/ni.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arora P., Baena A., Yu K.O., Saini N.K., Kharkwal S.S., Goldberg M.F. A single subset of dendritic cells controls the cytokine bias of natural killer T cell responses to diverse glycolipid antigens. Immunity. 2014;40:105–116. doi: 10.1016/j.immuni.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch M., Stronge V.S., Shepherd D., Gadola S.D., Mathew B., Ritter G. The crystal structure of human CD1d with and without α-galactosylceramide. Nat Immunol. 2005;6:819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 33.Kamada N., Iijima H., Kimura K., Harada M., Shimizu E., Si Motohashi. Crucial amino acid residues of mouse CD1d for glycolipid ligand presentation to Vα14 NKT cells. Int Immunol. 2001;13:853–861. doi: 10.1093/intimm/13.7.853. [DOI] [PubMed] [Google Scholar]
- 34.Brossay L., Naidenko O., Burdin N., Matsuda J., Sakai T., Kronenberg M. Structural requirements for galactosylceramide recognition by CD1-restricted NK T cells. J Immunol. 1998;161:5124–5128. [PubMed] [Google Scholar]
- 35.Trappeniers M., Goormans S., Van Beneden K., Decruy T., Linclau B., Al-Shamkhani A. Synthesis and in vitro evaluation of α-GalCer epimers. ChemMedChem. 2008;3:1061–1070. doi: 10.1002/cmdc.200800021. [DOI] [PubMed] [Google Scholar]
- 36.Girardi E., Zajonc D.M. Molecular basis of lipid antigen presentation by CD1d and recognition by natural killer T cells. Immunol Rev. 2012;250:167–179. doi: 10.1111/j.1600-065X.2012.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 38.Wu D., Xing G.W., Poles M.A., Horowitz A., Kinjo Y., Sullivan B. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci U. S. A. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franchini L., Matto P., Ronchetti F., Panza L., Barbieri L., Costantino V. Synthesis and evaluation of human T cell stimulating activity of an α-sulfatide analogue. Bioorg Med Chem. 2007;15:5529–5536. doi: 10.1016/j.bmc.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 40.Xing G.W., Wu D., Poles M.A., Horowitz A., Tsuji M., Ho D.D. Synthesis and human NKT cell stimulating properties of 3-O-sulfo-α/β-galactosylceramides. Bioorg Med Chem. 2005;13:2907–2916. doi: 10.1016/j.bmc.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Kjer-Nielsen L., Borg N.A., Pellicci D.G., Beddoe T., Kostenko L., Clements C.S. A structural basis for selection and cross-species reactivity of the semi-invariant NKT cell receptor in CD1d/glycolipid recognition. J Exp Med. 2006;203:661–673. doi: 10.1084/jem.20051777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borg N.A., Wun K.S., Kjer-Nielsen L., Wilce M.C., Pellicci D.G., Koh R. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 43.Pellicci D.G., Patel O., Kjer-Nielsen L., Pang S.S., Sullivan L.C., Kyparissoudis K. Differential recognition of CD1d-α-galactosyl ceramide by the Vβ8.2 and Vβ7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prigozy T.I., Naidenko O., Qasba P., Elewaut D., Brossay L., Khurana A. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y., Goff R.D., Zhou D., Mattner J., Sullivan B.A., Khurana A. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Long X., Deng S., Mattner J., Zang Z., Zhou D., McNary N. Synthesis and evaluation of stimulatory properties of Sphingomonadaceae glycolipids. Nat Chem Biol. 2007;3:559–564. doi: 10.1038/nchembio.2007.19. [DOI] [PubMed] [Google Scholar]
- 47.Pauwels N., Aspeslagh S., Vanhoenacker G., Sandra K., Yu E.D., Zajonc D.M. Divergent synthetic approach to 6′-modified α-GalCer analogues. Org Biomol Chem. 2011;9:8413–8421. doi: 10.1039/c1ob06235b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aspeslagh S., Li Y., Yu E.D., Pauwels N., Trappeniers M., Girardi E. Galactose-modified iNKT cell agonists stabilized by an induced fit of CD1d prevent tumour metastasis. EMBO J. 2011;30:2294–2305. doi: 10.1038/emboj.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aspeslagh S., Nemcovic M., Pauwels N., Venken K., Wang J., Van Calenbergh S. Enhanced TCR footprint by a novel glycolipid increases NKT-dependent tumor protection. J Immunol. 2013;191:2916–2925. doi: 10.4049/jimmunol.1203134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou X.T., Forestier C., Goff R.D., Li C., Teyton L., Bendelac A. Synthesis and NKT cell stimulating properties of fluorophore- and biotin-appended 6′′-amino-6′′-deoxy-galactosylceramides. Org Lett. 2002;4:1267–1270. doi: 10.1021/ol025565+. [DOI] [PubMed] [Google Scholar]
- 51.Yu S.H., Park J.J., Chung S.K. Practical syntheses of optically active carbagalactose and their potential application to the carbocyclic analogues of KRN7000. Tetrahedron Asymmetry. 2006;17:3030–3036. [Google Scholar]
- 52.Tashiro T., Nakagawa R., Hirokawa T., Inoue S., Watarai H., Taniguchi M. RCAI-37, 56, 59, 60, 92, 101, and 102, cyclitol and carbasugar analogs of KRN7000: their synthesis and bioactivity for mouse lymphocytes to produce Th1-biased cytokines. Bioorg Med Chem. 2009;17:6360–6373. doi: 10.1016/j.bmc.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 53.Tashiro T., Sekine-Kondo E., Shigeura T., Nakagawa R., Inoue S., Omori-Miyake M. Induction of Th1-biased cytokine production by α-carba-GalCer, a neoglycolipid ligand for NKT cells. Int Immunol. 2010;22:319–328. doi: 10.1093/intimm/dxq012. [DOI] [PubMed] [Google Scholar]
- 54.Yoshiga Y., Goto D., Segawa S., Horikoshi M., Hayashi T., Matsumoto I. Activation of natural killer T cells by α-carba-GalCer (RCAI-56), a novel synthetic glycolipid ligand, suppresses murine collagen-induced arthritis. Clin Exp Immunol. 2011;164:236–247. doi: 10.1111/j.1365-2249.2011.04369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrak Y., Barra C.M., Bedia C., Delgado A., Castaño A.R., Llebaria A. Aminocyclitol-substituted phytoceramides and their effects on iNKT cell stimulation. ChemMedChem. 2009;4:1608–1613. doi: 10.1002/cmdc.200900193. [DOI] [PubMed] [Google Scholar]
- 56.Kerzerho J., Yu E.D., Barra C.M., Alari-Pahissa E., Girardi E., Harrak Y. Structural and functional characterization of a novel nonglycosidic type I NKT agonist with immunomodulatory properties. J Immunol. 2012;188:2254–2265. doi: 10.4049/jimmunol.1103049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmieg J., Yang G., Franck R.W., Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. J Exp Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franck R.W., Tsuji M. α- C-galactosylceramides: synthesis and immunology. Acc Chem Res. 2006;39:692–701. doi: 10.1021/ar050006z. [DOI] [PubMed] [Google Scholar]
- 59.Fujii S., Shimizu K., Hemmi H., Fukui M., Bonito A.J., Chen G. Glycolipid α-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci U. S. A. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel O., Cameron G., Pellicci D.G., Liu Z., Byun H.S., Beddoe T. NKT TCR recognition of CD1d-α-C-galactosylceramide. J Immunol. 2011;187:4705–4713. doi: 10.4049/jimmunol.1100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X., Chen G., Garcia-Navarro R., Franck R.W., Tsuji M. Identification of C-glycoside analogues that display a potent biological activity against murine and human invariant natural killer T cells. Immunology. 2009;127:216–225. doi: 10.1111/j.1365-2567.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang Y.J., Huang J.R., Tsai Y.C., Hung J.T., Wu D., Fujio M. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci U. S. A. 2007;104:10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blauvelt M.L., Khalili M., Jaung W., Paulsen J., Anderson A.C., Brian Wilson S. α-S-GalCer: synthesis and evaluation for iNKT cell stimulation. Bioorg Med Chem Lett. 2008;18:6374–6376. doi: 10.1016/j.bmcl.2008.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hogan A.E., O'Reilly V., Dunne M.R., Dere R.T., Zeng S.G., O'Brien C. Activation of human invariant natural killer T cells with a thioglycoside analogue of α-galactosylceramide. Clin Immunol. 2011;140:196–207. doi: 10.1016/j.clim.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 65.Merrill A.H. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem Rev. 2011;111:6387–6422. doi: 10.1021/cr2002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyamoto K., Miyake S., Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 67.Goff R.D., Gao Y., Mattner J., Zhou D., Yin N., Cantu C., 3rd Effects of lipid chain lengths in α-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 68.Tyznik A.J., Farber E., Girardi E., Birkholz A., Li Y., Chitale S. Glycolipids that elicit IFN-γ-biased responses from natural killer T cells. Chem Biol. 2011;18:1620–1630. doi: 10.1016/j.chembiol.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu K.O., Im J.S., Molano A., Dutronc Y., Illarionov P.A., Forestier C. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc Natl Acad Sci U. S. A. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujio M., Wu D., Garcia-Navarro R., Ho D.D., Tsuji M., Wong C.H. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: tuning the adjuvant versus immunosuppression activity. J Am Chem Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 71.Padte N.N., Boente-Carrera M., Andrews C.D., McManus J., Grasperge B.F., Gettie A. A glycolipid adjuvant, 7DW8-5, enhances CD8+ T cell responses induced by an adenovirus-vectored malaria vaccine in non-human primates. PLoS One. 2013;8:e78407. doi: 10.1371/journal.pone.0078407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X., Fujio M., Imamura M., Wu D., Vasan S., Wong C.H. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci U. S. A. 2010;107:13010–13015. doi: 10.1073/pnas.1006662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sriram V., Du W., Gervay-Hague J., Brutkiewicz R.R. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 74.Goñi F.M., Alonso A. Biophysics of sphingolipids I. Membrane properties of sphingosine, ceramides and other simple sphingolipids. Biochim Biophys Acta. 2006;1758:1902–1921. doi: 10.1016/j.bbamem.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 75.Sonnino S., Mauri L., Chigorno V., Prinetti A. Gangliosides as components of lipid membrane domains. Glycobiology. 2007;17:1R–13R. doi: 10.1093/glycob/cwl052. [DOI] [PubMed] [Google Scholar]
- 76.Parekh V.V., Singh A.K., Wilson M.T., Olivares-Villagómez D., Bezbradica J.S., Inazawa H. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct α- and β-anomeric glycolipids. J Immunol. 2004;173:3693–3706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 77.Stanic A.K., De Silva A.D., Park J.J., Sriram V., Ichikawa S., Hirabyashi Y. Defective presentation of the CD1d1-restricted natural Va14Ja18 NKT lymphocyte antigen caused by β-D-glucosylceramide synthase deficiency. Proc Natl Acad Sci. 2003;100:1849–1854. doi: 10.1073/pnas.0430327100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ortaldo J.R., Young H.A., Winkler-Pickett R.T., Bere E.W., Jr., Murphy W.J., Wiltrout R.H. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J Immunol. 2004;172:943–953. doi: 10.4049/jimmunol.172.2.943. [DOI] [PubMed] [Google Scholar]
- 79.Lalazar G., Preston S., Zigmond E., Ben Yáacov A., Ilan Y. Glycolipids as immune modulatory tools. Mini Rev Med Chem. 2006;6:1249–1253. doi: 10.2174/138955706778742722. [DOI] [PubMed] [Google Scholar]
- 80.Margalit M., Abu Gazala S., Alper R., Elinav E., Klein A., Doviner V. Glucocerebroside treatment ameliorates ConA hepatitis by inhibition of NKT lymphocytes. Am J Physiol Gastrointest Liver Physiol. 2005;289:G917–G925. doi: 10.1152/ajpgi.00105.2005. [DOI] [PubMed] [Google Scholar]
- 81.Balreira A., Lacerda L., Miranda C.S., Arosa F.A. Evidence for a link between sphingolipid metabolism and expression of CD1d and MHC-class II: monocytes from Gaucher disease patients as a model. Br J Haematol. 2005;129:667–676. doi: 10.1111/j.1365-2141.2005.05503.x. [DOI] [PubMed] [Google Scholar]
- 82.O'Konek J.J., Illarionov P., Khursigara D.S., Ambrosino E., Izhak L., Castillo B.F., 2nd Mouse and human iNKT cell agonist ß-mannosylceramide reveals a distinct mechanism of tumor immunity. J Clin Invest. 2011;121:683–694. doi: 10.1172/JCI42314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O'Konek J.J., Kato S., Takao S., Izhak L., Xia Z., Illarionov P. ß-mannosylceramide activates type I natural killer t cells to induce tumor immunity without inducing long-term functional anergy. Clin Cancer Res. 2013;19:4404–4411. doi: 10.1158/1078-0432.CCR-12-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ni G., Li Z., Liang K., Wu T., De Libero G., Xia C. Synthesis and evaluation of immunostimulant plasmalogen lysophosphatidylethanolamine and analogues for natural killer T cells. Bioorg Med Chem. 2014;22:2966–2973. doi: 10.1016/j.bmc.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Mallevaey T., Clarke A.J., Scott-Browne J.P., Young M.H., Roisman L.C., Pellicci D.G. A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity. 2011;34:315–326. doi: 10.1016/j.immuni.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou D., Mattner J., Cantu C., 3rd, Schrantz N., Yin N., Gao Y. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 87.Fox L.M., Cox D.G., Lockridge J.L., Wang X., Chen X., Scharf L. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rauch J., Gumperz J., Robinson C., Sköld M., Roy C., Young D.C. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J Biol Chem. 2003;278:47508–47515. doi: 10.1074/jbc.M308089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gumperz J.E., Roy C., Makowska A., Lum D., Sugita M., Podrebarac T. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 90.Zajonc D.M., Savage P.B., Bendelac A., Wilson I.A., Teyton L. Crystal structures of mouse CD1d-iGb3 complex and its cognate Vα14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J Mol Biol. 2008;377:1104–1116. doi: 10.1016/j.jmb.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pellicci D.G., Clarke A.J., Patel O., Mallevaey T., Beddoe T., Le Nours J. Recognition of ß-linked self glycolipids mediated by natural killer T cell antigen receptors. Nat Immunol. 2011;12:827–833. doi: 10.1038/ni.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu E.D., Girardi E., Wang J., Zajonc D.M. Cutting edge: structural basis for the recognition of ß-linked glycolipid antigens by invariant NKT cells. J Immunol. 2011;187:2079–2083. doi: 10.4049/jimmunol.1101636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Porubsky S., Speak A.O., Luckow B., Cerundolo V., Platt F.M., Gröne H.J. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci U. S. A. 2007;104:5977–5982. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanderson J.P., Brennan P.J., Mansour S., Matulis G., Patel O., Lissin N. CD1d protein structure determines species-selective antigenicity of isoglobotrihexosylceramide (iGb3) to invariant NKT cells. Eur J Immunol. 2013;43:815–825. doi: 10.1002/eji.201242952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Speak A.O., Salio M., Neville D.C., Fontaine J., Priestman D.A., Platt N. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci U. S. A. 2007;104:5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brennan P.J., Tatituri R.V., Brigl M., Kim E.Y., Tuli A., Sanderson J.P. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12:1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Inafuku M., Li C., Kanda Y., Kawamura T., Takeda K., Oku H. Beta-glucosylceramide administration (i.p.) activates natural killer T cells in vivo and prevents tumor metastasis in mice. Lipids. 2012;47:581–591. doi: 10.1007/s11745-012-3666-1. [DOI] [PubMed] [Google Scholar]
- 98.Brennan P.J., Tatituri R.V., Heiss C., Watts G.F., Hsu F.F., Veerapen N. Activation of iNKT cells by a distinct constituent of the endogenous glucosylceramide fraction. Proc Natl Acad Sci U. S. A. 2014;111:13433–13438. doi: 10.1073/pnas.1415357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kain L., Webb B., Anderson B.L., Deng S., Holt M., Costanzo A. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian α-linked glycosylceramides. Immunity. 2014;41:543–554. doi: 10.1016/j.immuni.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tyznik A.J., Tupin E., Nagarajan N.A., Her M.J., Benedict C.A., Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paget C., Mallevaey T., Speak A.O., Torres D., Fontaine J., Sheehan K.C. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 102.Nagarajan N.A., Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 103.Wesley J.D., Tessmer M.S., Chaukos D., Brossay L. NK cell-like behavior of Vα14i NK T cells during MCMV infection. PLoS Pathog. 2008;4:e1000106. doi: 10.1371/journal.ppat.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beckman E.M., Porcelli S.A., Morita C.T., Behar S.M., Furlong S.T., Brenner M.B. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 105.Moody D.B., Ulrichs T., Mühlecker W., Young D.C., Gurcha S.S., Grant E. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 106.Porcelli S., Morita C.T., Brenner M.B. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 107.Fischer K., Scotet E., Niemeyer M., Koebernick H., Zerrahn J., Maillet S. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci U. S. A. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zajonc D.M., Ainge G.D., Painter G.F., Severn W.B., Wilson I.A. Structural characterization of mycobacterial phosphatidylinositol mannoside binding to mouse CD1d. J Immunol. 2006;177:4577–4583. doi: 10.4049/jimmunol.177.7.4577. [DOI] [PubMed] [Google Scholar]
- 109.Kinjo Y., Tupin E., Wu D., Fujio M., Garcia-Navarro R., Benhnia M.R. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 110.Tatituri R.V., Watts G.F., Bhowruth V., Barton N., Rothchild A., Hsu F.F. Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc Natl Acad Sci U. S. A. 2013;110:1827–1832. doi: 10.1073/pnas.1220601110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wei B., Wingender G., Fujiwara D., Chen D.Y., McPherson M., Brewer S. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J Immunol. 2010;184:1218–1226. doi: 10.4049/jimmunol.0902620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wingender G., Stepniak D., Krebs P., Lin L., McBride S., Wei B. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology. 2012;143:418–428. doi: 10.1053/j.gastro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nieuwenhuis E.E., Matsumoto T., Lindenbergh D., Willemsen R., Kaser A., Simons-Oosterhuis Y. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest. 2009;119:1241–1250. doi: 10.1172/JCI36509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ryan M.P., Adley C.C. Sphingomonas paucimobilis: a persistent gram-negative nosocomial infectious organism. J Hosp Infect. 2010;75:153–157. doi: 10.1016/j.jhin.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 115.Kawahara K., Moll H., Knirel Y.A., Seydel U., Zähringer U. Structural analysis of two glycosphingolipids from the lipopolysaccharide-lacking bacterium Sphingomonas capsulata. Eur J Biochem. 2000;267:1837–1846. doi: 10.1046/j.1432-1327.2000.01189.x. [DOI] [PubMed] [Google Scholar]
- 116.Kawahara K., Lindner B., Isshiki Y., Jakob K., Knirel Y.A., Zähringer U. Structural analysis of a new glycosphingolipid from the lipopolysaccharide-lacking bacterium Sphingomonas adhaesiva. Carbohydr Res. 2001;333:87–93. doi: 10.1016/s0008-6215(01)00111-2. [DOI] [PubMed] [Google Scholar]
- 117.Kawahara K., Kubota M., Sato N., Tsuge K., Seto Y. Occurrence of an alpha-galacturonosyl-ceramide in the dioxin-degrading bacterium Sphingomonas wittichii. FEMS Microbiol Lett. 2002;214:289–294. doi: 10.1111/j.1574-6968.2002.tb11361.x. [DOI] [PubMed] [Google Scholar]
- 118.Alexander C., Rietschel E.T. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167–202. [PubMed] [Google Scholar]
- 119.Kinjo Y., Wu D., Kim G., Xing G.W., Poles M.A., Ho D.D. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 120.Mattner J., DeBord K.L., Ismall N., Goff R.D., Cantu C., 3rd, Zhou D. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 121.Kinjo Y., Pei B., Bufali S., Raju R., Richardson S.K., Imamura M. Natural Sphingomonas glycolipids vary greatly in their ability to activate natural killer T cells. Chem Biol. 2008;15:654–664. doi: 10.1016/j.chembiol.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tilly K., Rosa P.A., Stewart P.E. Biology of infection with Borrelia burgdorferi. Infect Dis Clin North Am. 2008;22:217–234. doi: 10.1016/j.idc.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kumar H., Belperron A., Barthold S.W., Bockenstedt L.K. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J Immunol. 2000;165:4797–4801. doi: 10.4049/jimmunol.165.9.4797. [DOI] [PubMed] [Google Scholar]
- 124.Belperron A.A., Dailey C.M., Bockenstedt L.K. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J Immunol. 2005;174:5681–5686. doi: 10.4049/jimmunol.174.9.5681. [DOI] [PubMed] [Google Scholar]
- 125.Lee W.Y., Moriarty T.J., Wong C.H., Zhou H., Strieter R.M., van Rooijen N. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol. 2010;11:295–302. doi: 10.1038/ni.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tupin E., Benhnia M.R., Kinjo Y., Patsey R., Lena C.J., Haller M.C. NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc Natl Acad Sci U. S. A. 2008;105:19863–19868. doi: 10.1073/pnas.0810519105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Olson C.M., Jr., Bates T.C., Izadi H., Radolf J.D., Huber S.A., Boyson J.E. Local production of IFN-γ by invariant NKT cells modulates acute Lyme carditis. J Immunol. 2009;182:3728–3734. doi: 10.4049/jimmunol.0804111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kawakami K., Yamamoto N., Kinjo Y., Miyagi K., Nakasone C., Uezu K. Critical role of Vα14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003;33:3322–3330. doi: 10.1002/eji.200324254. [DOI] [PubMed] [Google Scholar]
- 129.Nakamatsu M., Yamamoto N., Hatta M., Nakasone C., Kinjo T., Miyagi K. Role of interferon-γ in Vα14+ natural killer T cell-mediated host defense against Streptococcus pneumoniae infection in murine lungs. Microbes Infect. 2007;9:364–374. doi: 10.1016/j.micinf.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 130.Kinjo Y., Illarionov P., Vela J.L., Pei B., Girardi E., Li X. Invariant natural killer T cells recognize glycolipids from pathogenic gram-positive bacteria. Nat Immunol. 2011;12:966–974. doi: 10.1038/ni.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Girardi E., Yu E.D., Li Y., Tarumoto N., Pei B., Wang J. Unique interplay between sugar and lipid in determining the antigenic potency of bacterial antigens for NKT cells. PLoS Biol. 2011;9:e1001189. doi: 10.1371/journal.pbio.1001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Momtaz H., Dabiri H., Souod N., Gholami M. Study of Helicobacter pylori genotype status in cows, sheep, goats and human beings. BMC Gastroenterol. 2014;14:61. doi: 10.1186/1471-230X-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chang Y.J., Kim H.Y., Albacker L.A., Lee H.H., Baumgarth N., Akira S. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ito Y., Vela J.L., Matsumura F., Hoshino H., Tyznik A., Lee H. Helicobacter pylori cholesteryl α-glucosides contribute to its pathogenicity and immune response by natural killer T cells. PloS One. 2013;8:e78191. doi: 10.1371/journal.pone.0078191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wunder C., Churin Y., Winau F., Warnecke D., Vieth M., Lindner B. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12:1030–1038. doi: 10.1038/nm1480. [DOI] [PubMed] [Google Scholar]
- 136.Agarwal R., Aggarwal A.N., Gupta D., Jindal S.K. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2009;13:936–944. [PubMed] [Google Scholar]
- 137.Cohen N.R., Tatituri R.V., Rivera A., Watts G.F., Kim E.Y., Chiba A. Innate recognition of cell wall ß-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe. 2011;10:437–450. doi: 10.1016/j.chom.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chaudhary V., Albacker L.A., Deng S., Chuang Y.T., Li Y., Umetsu D.T. Synthesis of fungal glycolipid asperamide B and investigation of its ability to stimulate natural killer T cells. Org Lett. 2013;15:5242–5245. doi: 10.1021/ol4024375. [DOI] [PubMed] [Google Scholar]
- 139.Albacker L.A., Chaudhary V., Chang Y.J., Kim H.Y., Chuang Y.T., Pichavant M. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat Med. 2013;19:1297–1304. doi: 10.1038/nm.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schofield L., McConville M.J., Hansen D., Campbell A.S., Fraser-Reid B., Grusby M.J. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 141.Molano A., Park S.H., Chiu Y.H., Nosseir S., Bendelac A., Tsuji M. Cutting edge: the IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: exploring the role of GPIs in NK T cell activation and antimalarial responses. J Immunol. 2000;164:5005–5009. doi: 10.4049/jimmunol.164.10.5005. [DOI] [PubMed] [Google Scholar]
- 142.Lotter H., González-Roldán N., Lindner B., Winau F., Isibasi A., Moreno-Lafont M. Natural killer T cells activated by a lipopeptidophosphoglycan from Entamoeba histolytica are critically important to control amebic liver abscess. PLoS Pathog. 2009;5:e1000434. doi: 10.1371/journal.ppat.1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ralston K.S., Solga M.D., Mackey-Lawrence N.M., Somlata, Bhattacharya A., Petri W.A., Jr. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature. 2014;508:526–530. doi: 10.1038/nature13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Laurent X., Bertin B., Renault N., Farce A., Speca S., Milhomme O. Switching invariant natural killer T (iNKT) cell response from anticancerous to anti-inflammatory effect: molecular bases. J Med Chem. 2014;57:5489–5508. doi: 10.1021/jm4010863. [DOI] [PubMed] [Google Scholar]
- 145.Anderson B.L., Teyton L., Bendelac A., Savage P.B. Stimulation of natural killer T cells by glycolipids. Molecules. 2013;18:15662–15688. doi: 10.3390/molecules181215662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Banchet-Cadeddu A., Hénon E., Dauchez M., Renault J.H., Monneaux F., Haudrechy A. The stimulating adventure of KRN 7000. Org Biomol Chem. 2011;9:3080–3104. doi: 10.1039/c0ob00975j. [DOI] [PubMed] [Google Scholar]