Abstract
As incidence of Alzheimer's disease (AD) and other neurodegenerative diseases rise, there is increasing interest in environmental factors which may contribute to disease onset and progression. Air pollution has been known as a major health hazard for decades. While its effects on cardiopulmonary morbidity and mortality have been extensively studied, growing evidence has emerged that exposure to polluted air is associated with impaired cognitive functions at all ages and increased risk of AD and other dementias in later life; this association is particularly notable with traffic related pollutants such as nitrogen dioxide, nitrous oxide, black carbon, and small diameter airborne solids and liquids known as particulate matter. The exact mechanisms by which air pollutants mediate neurotoxicity in the central nervous system (CNS) and lead to cognitive decline and AD remain largely unknown. Studies using animal and cell culture models indicate that amyloid-beta processing, anti-oxidant defense, and inflammation are altered following the exposure to constituents of polluted air. In this review, we summarize recent evidence supporting exposure to air pollution as a risk for cognitive decline at all ages and AD at later lifetime. Additionally, we review the current body of work investigating the molecular mechanisms by which air pollutants mediate damage in the CNS. Understanding of the neurotoxic effects of air pollution and its constituents is still limited, and further studies will be essential to better understand the cellular and molecular mechanisms linking air pollution and cognitive decline.
Keywords: Particulate matter, Cognition, Dementia
Alzheimer's disease (AD) is the most common form of dementia, with the estimated number of patients in the U.S. over 5 million, and over 35 million patients worldwide [1], [2]. AD and other dementias accounted for about 1.5 million deaths in 2015 and are currently ranked at the seventh leading cause of death worldwide [3]. These numbers will double or even triple as the aged population rapidly increases in next few decades. Such a rapid increase in AD cases will create major socioeconomic burdens for society unless effective therapeutic interventions to slow, halt, or cure this devastating disease are developed. In lieu of conquering the disease, the most expedient and potentially actionable course to curb the predicted rise in AD cases is to identify and limit major risk factors for the disease. Aging is the single greatest risk factor for AD, and mounting evidence also indicates a strong contribution from genetic predispositions, particularly the presence of apolipoprotein E ε4 (APOE ε4) allele as, by far, the major genetic risk factor for the late-onset AD [4], [5]. While both aging and genetic risk factors are virtually perpetual within each individual, they do not fully explain the cause of every AD case, and homozygotic and heterozygotic twin studies reveal key involvement of additional environmental and modifiable risk factors in AD etiology [6], [7]. These include, but are not limited to, lifestyle, disease history, educational background, dietary habits, and exposure to environmental and occupational hazards [8], [9]. Environmental risk factors, such as metals and other toxic contaminants in drinking water, agricultural chemicals on food, and air pollution, could potentially impact large sections of the population and become public health concerns. Chronic exposure to environmental factors has been shown to increase the risk for developing AD in both epidemiological studies and in animal models [10], [11], [12].
In this review, we summarize recent evidence indicating that chronic exposure to polluted air is a major environmental risk factor for AD. There is a significant body of epidemiological works unveiling a strong correlation between exposure to particulate matter (PM) and associated air pollutants with accelerated cognitive decline across multiple stages of life, most prominently when exposed at young or old ages. More recently, growing evidence indicates increased risk of AD and other dementias following chronic PM exposure [11], [12]. Reports examining the effects of PM exposure in cell culture, animal models, and human patients show changes in inflammatory and oxidative stress markers [13], [14], [15]. Changes in AD specific pathology, such as abnormal buildup of amyloid-beta (Aβ) plaques, have also been reported [10], [16], [17]. The purpose of this review is to discuss the recent evidence for the role of air pollutants, with a focus on PM, in AD risk at an epidemiological level, in AD pathogenesis in in vitro and in vivo models and humans, and to suggest future areas of research in this field.
Sources and chemistry of PM
Exposure to unhealthy levels of polluted air is a worldwide problem, particularly in heavily urbanized areas in developing or developed countries where the ambient levels of air pollution can be over 10 times as concentrated as health guidelines recommend [18]. Major constituents of air pollution are various sizes of PM, nitrogen oxide species (NOx), sulfur oxide species (SOx), carbon monoxide, ozone, hydrocarbons, volatile organic compounds (VOCs), metals, and other inorganic chemicals. While pollutants from natural sources, such as volcanic activities, wildfires, dust, and coastal aerosols, are difficult to reduce, those released by human activities may be more reasonably curbed if found to adversely impact health to a sufficient degree. The major sources of human contribution are traffic and industrial related combustion of fossil fuels, as well as mining, agricultural activities, and burning fuels for cooking and heating [19], [20], [21], [22]. PM found in the atmosphere is either generated directly from these sources as primary PM or a result of complex photochemical reactions of NOx, SOx, and ammonia released from motor vehicles, industrial combustion, and agricultural activities, respectively, as secondary PM [23]. This gas-to-particle chemical conversion occurs within water droplets and aerosols in the atmosphere and produces ammonium nitrate as a nucleation step for PM formation. The ammonium nitrate core eventually grows and ages together with other constituents to form mature PM [24]. In certain regions, ammonium nitrate could take up over 50% of all chemical mass present in PM [25].
PM includes a wide variety of microscopic liquid or solid matter in the atmosphere. Particulate contaminants include biological elements such as pollen, bacteria, viruses, and spores, as well as suspended non-biological solids such as dust and smoke. The exact composition of PM varies considerably based on size, location, weather, the season, time of day, and a multitude of other factors. The major components of airborne particulates worldwide are sulfates, nitrates, ammonium, chlorides, elemental and organic carbons, biological materials, and minerals and dust [23], [26]. The US EPA primarily divides PM into an “ultrafine” designation for PM < 100 nm (PM0.1), a “fine” fraction of PM of diameter 2.5 microns or less (PM2.5), and a “coarse” fraction of PM between 2.5 and 10 microns (PM10) [27]. While most components of PM can be found in all size fractions, the smaller PM fractions generally contain higher amounts of black carbon, gases, and other products of combustion, while the coarse fraction contains more metals, inorganic material, and other debris and dust from mechanical processes [28], [29] [Table 1].
Table 1.
Particulate matter size fractions.
| Size fraction | Designation | Diameter range (μm) | Major constituents | Minor constituents |
|---|---|---|---|---|
| Coarse PM | PM10 | 2.5–10 | Metals, inorganic ions | Organic matter |
| Fine PM | PM2.5 | 0.1–2.5 | Inorganic ions | Metals, organic matter |
| Ultra-Fine PM | PM0.1 | ≤0.1 | Organic matter | Metals, inorganic ions |
Table 1 shows the three common size fractions of PM as designated by the US EPA. Major (>25%) and minor constituents (<25%) are estimated by particle constituent mass from USC studies [28], [29]; note that composition of PM may vary considerably with time and location, and that this is only a general estimate of the components of each size fraction.
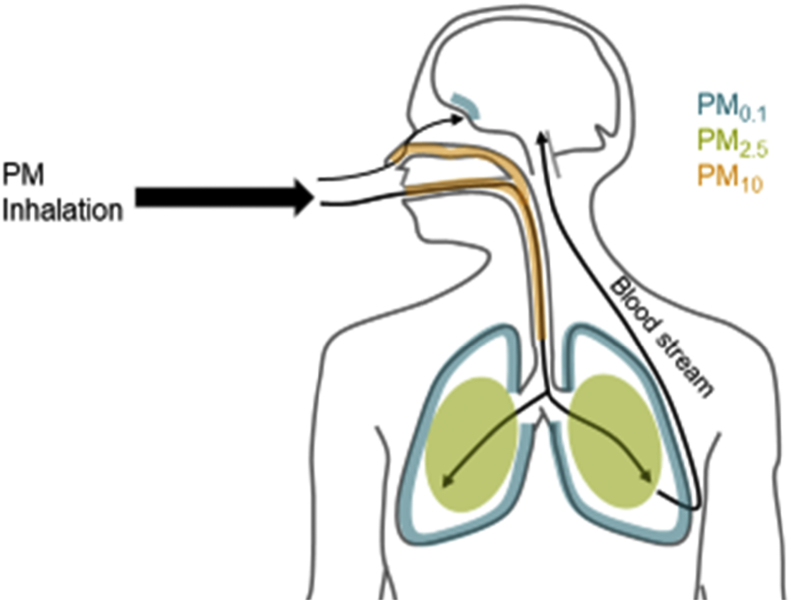
The outlined size classifications are commonly used at least in part as the size and weight of the particles plays a major role in determining inhalability and particle deposition location in the respiratory tract. While larger particles tend to deposit in and affect the upper respiratory tract, it is largely PM2.5 and, particularly ultrafine PM that penetrate and deposit in the deep lung tissues [30], [31] [Fig. 1]. Once in the lung, these particulates are taken up by cells [32], [33], [34] and enter the blood stream [35], [36]. PM uptake by these lung cells, macrophages, and blood also facilitates the absorption of potentially toxic chemicals on the surface of the PM into the cell and tissue to exert toxicity [37], [38]. Importantly, ultrafine PM represents the majority of both the total particle number and available surface area of typical PM mixtures [39], [40], [41]. In part due to these traits, which increase relative ability to carry other agents and available surface for reactions, ultrafine PM is generally considered the most toxic form of PM [23], [40]. Research shows that the ultrafine PM fraction causes increased oxidative stress and mitochondrial damage compared to larger PM sizes in macrophages and epithelial cells [42]. Recent evidence suggests that the ultrafine PM can directly infiltrate to the brain through olfactory nerves, and potentially penetrate to the central nervous system (CNS) via systemic uptake [43], [44]. Once in the CNS, PM may lead to inflammation response and oxidative damage similar to what is seen in macrophages and the lungs [45], [46]. Vehicle exhaust is one of the most significant human contributions to PM2.5 and PM0.1 levels [47], [48]. As PM tends to aggregate and increase in size over time [49], sources of PM0.1 to which people are immediately exposed, such as vehicle exhaust in traffic, have a higher impact on human exposure than more distant sources.
Fig. 1.
A depiction of particulate matter (PM) primary deposition areas in the body and potential routes to affect the CNS. Larger particles (PM10, orange) tend to be trapped in the upper respiratory tract, while the fine (PM2.5, green) and ultra-fine (PM0.1, blue) fractions can reach deep in the lungs [30]. Ultra-fine PM deposit in the alveoli and can cross into the interstitium and blood, where they may cause systemic effects [35], [36]. In addition, ultra-fine PM can directly cross the olfactory epithelium and enter the CNS [43], [44].
In the U.S., it is estimated that over 43 million people live in areas where the air concentration of PM2.5 exceeds the EPA's limit of 35 μg/m3 for a short periods of time, and 20 million people in the US live with exposure levels higher than the EPA long-term exposure standard of 12 μg/m3 year round [50]. These areas include major metropolitan regions such as the Los Angeles basin, which had a 2015 average PM2.5 level of 12.4 μg/m3, with 24 h averages up to 70 μg/m3 [51]. Worldwide, the WHO estimates that 92% of the population lives in areas where WHO air quality guidelines (10 μg/m3 for maximum yearly average and 25 μg/m3 for maximum 24 h average) are not met [18]. The organization attributed an estimated 3 million premature deaths to ambient air pollution in 2012. PM levels in developing countries are still rising [52], leaving many people at risk of being exposed to unhealthy levels. Highly polluted areas such as Delhi, India, and Xingtai, China, experienced annual average PM2.5 level of over 120 μg/m3 [52]. Together, these data indicate the importance of exploring and understanding of the health ramifications of PM exposure.
Epidemiology
Since major incidents during the 20th century, such as the 1952 London Great Smog event, it has been well-documented that exposure to polluted air and particulates is associated with cardiopulmonary mortality and morbidity [53], [54]. It is only in the 21st century that epidemiological studies have begun to uncover a correlation between air pollution and accelerated cognitive decline and dementia outcomes. This correlation has been well studied at various ages of exposure and testing, and in-depth reviews of the topic have been published elsewhere [55], [56], [57]. An overview of the literature and major findings on this subject will be provided here to facilitate discussion. Table 2 provides a summary of all papers included.
Table 2.
Epidemiological studies of air pollutants and cognitive impairment.
| Year | Measured exposure | Study population | Participants | Age at testing | Outcomes | Citation |
|---|---|---|---|---|---|---|
| In utero exposure | ||||||
| 2006 | PAH levels in third trimester and cord blood. | Mother-child pairs Black or Dominican-American mothers in New York City. | 183 | 1, 2, and 3 years. | No effect at 1 and 2 years of age. Reduced BSID-II scores at 3 years with high PAH. | Perera et al., 2006 [58] |
| 2009 | PAH levels in third trimester and cord blood. | Mother-child pairs Black or Dominican-American mothers in New York City. | 249 | 5 years. | ∼4 point reduction in full-scale and verbal IQ with high PAH exposure. | Perera et al., 2009 [59] |
| 2010 | PAH levels over 48 h during. second or third trimester of pregnancy. | Mother-child pairs in Krakow, Poland. | 214 | 5 years. | ∼4 point reduction in non-verbal IQ associated with PAH exposure. | Edwards et al., 2010 [60] |
| 2014 | NO2, NOx, PM2.5, and PM10 levels. | Metaanalysis of studies with European children from Germany, France, Italy, Greece, and Spain | 9482 | 1–6 years. | Higher NO2 exposure associated with reduced psychomotor development. No pollutant associated with reduced cognitive development. | Guxens et al., 2014 [62] |
| 2014 | NO2, SO2, O3, non-methane hydrocarbons, THC, and CO exposure during pregnancy. | Taiwanese mother–child pairs multiple villages in Taiwan. | 533 | 6 and 18 months. | Non-methane hydrocarbons exposure during 2nd and 3rd trimester associated with reduction in gross motor skills. No other exposure showed significant effect. | Lin et al., 2014 [65] |
| 2015 | PM2.5, NO2, benzene, and distance of residence to major roadway. | Spanish mother–child pairs in the Guipuzcoa region. | 438 | 2 years. | Reduced BSID motor score with increasing PM2.5, decreased mental score with NO2, no changes with benzene or distance to roadway. | Lertxundi et al., 2015 [63] |
| 2015 | PM2.5, black carbon, and distance of residence to major roadway during third trimester, from birth to age 6, and the year before assessment. | Mother-child pairs in eastern Massachusettes area. | 1109 | Mean age 8 years. | Prenatal lower distance to major roadway associated with decreased non-verbal IQ and visual motor ability. Other exposures showed no significant changes. | Harris et al., 2015 [66] |
| 2016 | NO2, PM2.5, PMcoarse, and PM10 levels at birth, as well as distance of residence to major roadway. | Italian mother–child pairs. | 719 | 7 years. | Increasing NO2 levels and decreasing distance to roadway associated with decreased verbal IQ and verbal comprehension IQ by WISC-III. | Porta et al., 2016 [64] |
| Early life exposure | ||||||
| 2008 | General air pollution by residence in high pollution versus low pollution areas. | Children in Mexico City, Mexico, and Polotitlán, Mexico. | 73 | Mean age 9.5 years. | Residence in Mexico City associated with reduced performance age in verbal IQ, full scale IQ, and multiple sub-tests by WISC-R assessment. | Calderón-Garcideuñas et al., 2008a [45] |
| 2008 | Black carbon levels at residence from birth to testing. | Mother-child pairs in the Boston, Massachusetts area. | 202 | 8–11 years. | Higher BC exposure associated with decreased matrices and composite performance in the Kaufman Brief Intelligence Test. | Suglia et al., 2008 [70] |
| 2009 | General air pollution by residence for at least 3 years in high pollution versus low pollution areas. | Second and third grade students either in central or northern districts of Quanzhou, Fujian Province, China. | 861 | 8–10 years. | Residence in the higher pollution central area associated with impaired performance risk for Visual Simple Reaction Time, Continuous Performance, Digit Symbol, Pursuit Aiming, and Sign Register by Neurobehavioral Evaluation System and Jinyi Psychomotor Test Battery tests. | Wang et al., 2009 [72] |
| 2010 | NO2 exposure over 1 year. | Spanish male children age 4 | 210 | 5 years. | Decreases in general performance, motor function, and perceptual performance in MSCA tests comparing lowest NO2 exposure group to highest. | Freire et al., 2010 [67] |
| 2011 | General air pollution by residence in high pollution versus low pollution areas. | Children in Mexico City, Mexico, and Polotitlán, Mexico. | 30 | Mean age 7 years. | Decreased vocabulary and memory performance by WISC-R assessment. | Calderón-Garcideuñas et al., 2011 [71] |
| 2012 | NO2 and PM10 exposure at school and homel. | Children in the area around Schipol-Amsterdam airport, Schipol, Netherlands. | 485 | 9–11 years. | NO2 levels at school, but not at home, linked to small memory span reduction in digit memory span test. | van Kempen et al., 2012 [68] |
| 2013 | Black carbon levels at residence from birth to testing. | Mother-child pairs in the Boston, Massachusetts area. | 174 | 7–14 years. | Male, but not female, children in higher 3 quartiles of BC exposure showed increased hit reaction time and commission errors in Connor's Continuous Performance Test. | Chiu et al., 2013 [69] |
| 2015 | PAH levels in cord blood and 48 h PAH monitoring at home at 3 years of age. | Children in Krakow, Poland. | 170 | 7 years of age. | Depressed verbal IQ index (WISC-R – vIQ score). RR = 3.0 with ln-unit increase in cord blood PAH, RR = 1.6 with postnatal exposure. | Jedrychowski et al., 2015 [61] |
| 2015 | Benzene biomarker trans,trans-muconic acid in urine, distance weighted traffic density, and time spent in traffic before testing. | 9th grade highschool students in Flanders, Belgium. | 606 | Mean age 15 years. | One SD increase in approximated traffic exposure associated with 0.26 SD decrease in sustained attention by Neurobehavioral Evaluation System. No other metrics significant. | Kicinski et al., 2015 [73] |
| 2016 | Lifelong residence in Mexico City Metro Area. | Children in Mexico City, Mexico with either APOE ɛ3/ɛ3 or APOE ɛ3/ɛ4 genotypes. | 105 | Mean age 12 years. | APOE ɛ3/ɛ4 females showed decreased performance IQ and full scale IQ by WISC-R assessment compared to APOE ɛ3/ɛ3. Male children showed no statistician difference. | Calderón-Garcideuñas et al., 2016 [74] |
| Adult to elderly exposure | ||||||
| 2006 | General air pollution by residence in high or low pollution areas for at least 10 years. | Elderly from either Mexico City, Mexico, or Actopan, Mexico. | 189 | 60 years or greater. | Increased cognitive impairment in high pollution area patients age 80 + by MMSE, as well as increased lipoperoxides. | Sánchez-Rodríguez et al., 2006 [75] |
| 2008 | Air pollution index at residence compared to region gross domestic product. | Elderly from 22 different provinces in China. | 7358 | 65 years or greater. | Higher air pollution index associated with lower performance in activities of daily living and cognition as measure by MMSE. Effect was exacerbated by residence in higher GDP areas. | Sun and Gu 2008 [76] |
| 2009 | Lifetime PM10 and ozone exposure estimated from annual exposure metrics. | Adults living in the United States | 1764 | Mean age 37 years. | No statistically significant effect seen in simplem reaction time, symbol-digit substitution, or serial-digit learning tests with PM or ozone exposure. | Chen and Schwartz 2009 [87] |
| 2009 | PM10 levels by 5 year average, and distance of residence to major roadway. | Elderly women living in the Ruhr district and surrounding areas in Germany. | 399 | Mean age 74 years. | Participants age 74 + showed impaired performance in CERAD-plus, Stroop, and sniffing tests associated with increasing traffic exposure. | Ranft et al., 2009 [88] |
| 2010 | Air pollution index at residence. | Elderly from 22 different provinces in China. | 15,973 | 65 years or greater. | Residence in higher air pollution index areas associated with increased cognitive deficit by MMSE, decreased performance in activities of daily living, and increased cumulative deficits index. | Zeng et al., 2010 [78] |
| 2011 | Black carbon levels at residence. | United States male veterans in the Boston, Massachusettes area. | 680 | Mean age 71 years. | Each doubling of BC increases risk for MMSE score ≤ 25 OR 1.3. | Power et al., 2011 [79] |
| 2012 | Distance of residence to nearest major roadway and black carbon levels. | Seniors from Boston, Massachusettes area. | 765 | Mean age 78 years. | Distance of residence to major roadway inversely correlated with immediate and delayed recall on Hopkins Verbal Learning Test, trailmaking test performance, and letter and category fluency. Black carbon exposure associated with impaired immediate recall, but no other metrics. | Wellenius et al., 2012 [77] |
| 2012 | PM2.5 and PMcoarse at residence for the preceeding month, year, 2 years, 5 years, and since 1988. | Women residing in the United States | 19,307 | 70-81 (mean 74) years. | Cognitive decline determined by telephone interview for cognitive status per 2 year assessment period increased with highest quintile of exposure for both PM fractions. PM10 exposures long than the preceeding month associated with impaired cognition, PM2.5 only with exposure since 1988. | Weuve et al., 2012 [83] |
| 2014 | PM2.5 at residence averaged for 2004. | United States adults. | 13,996 | 50 years or greater. | Residence in areas with higher PM levels linked with reduced cognition by the telephone interview for cognitive status. | Ailshire and Crimmins 2014 [82] |
| 2014 | NO2, PM2.5, and Ozone levels at residence. | Elderly from the Los Angeles Basin area. | 1496 | Mean age 60.5 years. | High PM2.5 exposure associated with reduced verbal learning by California Verbal Learning Test. No other exposure showed significance. | Gatto et al., 2014 [80] |
| 2014 | PM2.5 and PM10 from traffic and all sources at residence. | London, England civil servants. | 2867 | Mean age 66 years. | 5 year decline in memory score with 20 word free recall test when examining only participants who remained in London between study points with all source exposures. | Tonne et al., 2014 [85] |
| 2015 | PM2.5 levels at residence. | United States adults. | 780 | 55 years or greater. | Reduced cognitive function as measured by errors on abbreviated Short Portable Mental Status Questionnaire associated with increasing PM exposure. | Aishire and Clarke 2015 [81] |
| 2015 | NOx, NO2, PM2.5, PM10, and traffic load within 100 m of residence. | Elderly women living in the Ruhr district and surrounding areas in Germany. | 789 | Mean age 73 years. | All exposures linked to decreased figure drawing scores in CERAD testing, however traffic exposure was only significant in those with at least one APOE ɛ4 allele. No other CERAD subtest showed significant change with any exposure. | Schikowski et al., 2015 [89] |
| 2016 | PM2.5 levels at residence. Neighborhood stressors were measured as a second variable. | United States adults. | 779 | 55 years or greater. | PM2.5 levels or high neighborhood stress alone were not correlated with increased errors on abbreviated SPMSQ, but exposure to both results in increased risk of errors. | Ailshire et al., 2017 [86] |
| 2017 | PM2.5 by satellite data and geocoding. | Adults in China, Ghana, India, Mexico, South Africa, and Russia. | 45,625 | Mean age 58 years. | Increased overall disability score by World Health Organization Disability Assessment Schedule | Lin et al., 2017 [84] |
| AD and dementia outcomes | ||||||
| 2015 | O3 and PM2.5 levels. | Elderly in Taiwan. | 95,690 | 65 years and older. | Strong association of both O3 (HR 3.11 per 10.91 ppb) and PM2.5 (HR 2.38 per 4.43 μg/m3) increases with AD incidence over follow up. | Jung et al., 2015 [11] |
| 2015 | 12 year PM10 and 14 year O3 exposure at residence. | Taiwanese retirees. | AD 249, VaD 125, Control 497. | 60 years or greater. | AD and VaD risk increased with higher exposure to either pollutant (OR highest vs. lowest exposure tertile AD 4.17 for PM, 2 for O3; VaD 3.61 PM and 2.09 O3). | Wu et al., 2015 [94] |
| 2016 | City of residence average PM2.5 from 1999 to 2010. | Medicare enrollees in the United States east coast. | 9,817,806 | 65 years or greater | Increased risk of PD (HR 1.08), AD (HR1.15), and dementia (HR 1.08) per 1 μg/m3 increase in airborne | Kioumourtzoglou et al., 2016 [93] |
| 2016 | Annual mean NOx concentrations at residence. | Residents in the area of Umeå, Sweden. | 1806 | 55–85 years | Increased risk of AD and VaD in highest exposure quartile vs. lowest (HR 1.38 AD, 1.47 VaD) | Oudin et al., 2016 [12] |
| 2016 | NOx, NO2, PM2.5, PMcoarse, and PM2.5 absorbance levels at residence. | Adults from the Ruhr area of Germany. | 2050 | 50–80 years. | PM2.5 levels associated with overall and amnestic MCI incidence (HR 1.16 and 1.22 per IQR), while PM 10 (1.07) and NO2 (1.13) were only associated with amnestic MCI risk. | Tzivian et al., 2016 [92] |
| 2017 | PM2.5 at residence. | Elderly women of European ancestry in the United States. | 3647 | 65–79 years | Exposure above 12 μg/m3 associated with increased cognitive delcine (HR 1.81) and dementia (HR 1.92) APOE allele status modulated risk. | Cacciottolo et al., 2017 [10] |
| 2017 | Distance of residence to nearest major roadway. | Residents of Ontario, Canada of at least 5 years. | 2,165,268 | 55 years or greater. | Living 200 m or closer to the nearest major roadway increased risk of dementia incidence, up to HR 1.07 when living within 50 m. Incidence of PD not affected. | Chen et al., 2017a [90] |
| 2017 | PM2.5, NO2, and O3 levels at residence. | Residents of Ontario, Canada of at least 5 years. | 2,066,639 | 55 years or greater. | Increase dementia incidence risk with PM2.5 (HR 1.04 per IQR increase) and NO2 (HR 1.1 per IQR increase), but not O3 exposure. | Chen et al., 2017b [91] |
Prenatal PM exposure and its impact on cognition
The adverse effect of polluted air on cognition in young children can be caused by exposure as early as in utero. Exposure to polycyclic aromatic hydrocarbons (PAHs), a component of the PM organic carbon fraction, during pregnancy among African-American and Dominican women in New York City was found to correlate with reduced Bayley scale of infant development (BSID-II) scores in the children 3 years old and reduced verbal and full IQ at 5 years [58], [59]. Similar studies performed in Poland also reported a decrease in non-verbal IQ scores at 5 years of age and verbal IQ at 7 years of age in a group of children with high PAH exposure in utero compared to a low exposure group [60], [61]. These studies suggest that PAH exposure during development exhibits delayed impairment of performance during childhood, as in both cases negative cognitive effects were not observed at earlier time points.
In addition to PAH, motor vehicle traffic associated gases are also commonly associated with decreased cognitive ability of children if exposed in utero [62], [63], [64]. NO2 exposure, PM2.5 exposure, and traffic intensity are correlated with reduced IQ performance in young children, while PM10, benzene, or reduced distance to roadways during pregnancy are not [63], [64]. A meta-analysis of six other European studies examining the effects of PM2.5, PM10, and NO2 exposure during pregnancy on children 1–6 years old found the only significant association to be between NO2 levels and psychomotor development deficit [62]. On the other hand, exposure to high levels of SO2 and non-methane hydrocarbons, but not NO2 and other pollutants, during 2nd or 3rd trimester pregnancy is found to be associated with reduced motor skills in infants at 6 and 18 months of age in Taiwan [65]. Verbal and social-personal scores do not appear to be correlated with any measured pollutant in this study. A recent U.S. based study found limited association between prenatal traffic related pollution exposure in late pregnancy [66]. Using distance to major roadway and EPA estimated PM and black carbon levels, and measuring cognitive ability of children at an average 8 years of age, only distance to roadway had significant effect on cognition. Lack of personal monitoring of the specific pollutants and more generalized exposure metrics or the higher age of the tested children in this study may account for the discrepancy with other studies. Overall, these studies show that exposures to certain constituents of polluted air during pregnancy exhibit adverse effects on cognitive performance in infants. However, the inconsistency between studies of whether any given active constituent causes impaired cognitive functions requires further investigation. Differences in study populations, the concentrations of pollutants seen in the studies, and times of exposure and endpoint testing may account for the majority of these discrepancies. Another interesting possibility raised is that the overall mixture of air pollutants, depending on the constituents and concentrations, may elicit a complex and novel toxicity in the body.
Early-life childhood PM exposure and cognition
Adverse effect of air pollution on cognition is not limited to in utero exposure. Increasing levels of NO2 are associated with decreased gross motor skills in a 1 year longitudinal study of 5 year old male Spanish children [67]. In addition, increased levels of NO2 at school, but not at home, are associated with a reduction in memory span among 9–11 year old school children [68]. Although the exact reason why NO2 at school specifically correlates with the children's cognitive performance remains unknown, the authors speculate that either additional unmeasured factors in the air at school or increased activity at school may be responsible. The same group also found that road and airway noise levels negatively affect switching attention test scores. This indicates that other environmental hazards outside of PM pollution often associated with heavy traffic may be important considerations for confounding factors in determining the effects of PM.
Two U.S. based studies in children focus on black carbon, which is often associated with diesel engines or organic matter fuels such as wood burning. Environmental levels of black carbons are inversely associated with various intellectual performances including vocabulary, composite intelligence, and visual skills of learning and memory in children [69], [70]. The finding by Suglia et al. of reduced memory ability shows that exposure to the black carbon fraction of PM can impact brain functions also affected by AD, and potentially has neurotoxic effect in the regions associated with those functions [70]. Long term studies following from childhood to senescence are highly challenging, but it is interesting to consider that early life PM exposure extends its adverse effect in later life and triggers neurodegenerative diseases like AD.
The remaining studies of air pollution exposure effects on child cognition focus primarily on using proxy measurements of air pollution exposure, such as rural versus city residence or distance of residence to roadway, rather than measurements of the individual constituents of air pollution. While these measures offer relatively simple ways to approximate all source air pollutant exposure, they leave considerable room for interference of confounding factors and exposure variance to inhibit interpreting results. Measuring performance of children living in Mexico City, where very high levels of air pollution, including PM2.5, are found, versus Polotitlán, a rural area of Mexico with relatively little pollution, unveils that children in Mexico City are behind age normalized levels of multiple intelligence subscales, including full scale IQ and vocabulary [45], [71]. Another study comparing children 8–10 years of age living at least 3 years in either northern or heavily polluted central Quanzhou reports that children living in the heavily polluted central area have increased risk of poor psychomotor stability, motor coordination, and response time tests [72]. Researchers also found an association between sustained attention in 13–17 year old adolescents with distance weighted traffic density-a mixture of distance to roadway and roadway traffic density used to approximate traffic related pollutant exposure [73]. Cognitive performance was, however, non-significantly impacted. Data using the Project Viva cohort in the U.S. indicated decreased non-verbal IQ in 8 year olds whose mothers lived in residences nearer to major roadways at the time of birth to child age 6 as compared to children from mothers who lived further from major roadways [66]. In this study, prenatal or childhood exposure 1 year before the test administration were not associated with cognitive deficits. The lack of effect seen at the later exposure point may indicate that either long term or very heavy exposure is required to reduce cognition. There was no association when black carbon or PM2.5 levels were assessed individually.
Genotype may impart particular sensitivity to air pollutants, as female, but not male, children living in Mexico City with one copy of the ApoE ε4 allele are found to perform worse than those homozygous for the APOE ε3 allele [74]. While this study did not include less polluted controls outside of Mexico City, limiting the ability to determine whether the genotype and environment are specifically interacting, this finding is critical as it indicates that certain subpopulations may be more vulnerable to the effects of air pollutants on cognition. This in turn provides possible genetic confounding factors to account for variant and sometimes conflicting results seen between many of the studies reviewed. Additional studies in this area are required to determine the strength and consistency of these effects.
Adult PM exposure and risk for dementia
Adults who are exposed to polluted air also experience accelerated cognitive impairment. In China, Mexico and the U.S., elderly residents over 65 years old who live in areas with high air pollution generally performed significantly worse on a mini-mental state examination (MMSE), one of common cognitive tests to assess dementia, than those living in cleaner areas [75], [76], [77], [78]. Black carbon [79], and PM2.5 [80] are particularly associated with poor performance on MMSE among elderly. It is estimated that every 10 μg/m3 increase in black carbon exposure is equivalent to an extra two years of cognitive decline by aging [79]. Measuring NO2, PM2.5, and O3 exposure by geocoding in the U.S. in a 1500 person sample of mean age 60, Gatto et al. found a reduction in verbal learning associated only with PM2.5 levels [80]. PM2.5 is also found to have a strong association with increased rate of errors in tests of working memory and orientation in adults age 55 or older [81], and with reduced episodic memory as compared to those with lower PM2.5 exposure [82]. These associations did not carry over to overall mental status as determined by composite scores on multiple tests.
Elderly women exposed to varying PM2.5-10 levels for up to 14 years, as estimated by modeling of US EPA environmental data, have greater decline in global cognitive function than those in the lowest exposure level [83]. PM2.5 exposure was also linked to increase in overall disability, as determined by the World Health Organization Disability Assessment Schedule, and cognitive disability specifically in a study of populations from lower income countries [84]. Other population-based studies find that PM2.5 and PM10 levels associate with reduced memory scores in multiple tests [85], [86]. Interestingly, PM2.5 and cognitive decline are more strongly correlated when neighborhood stressor factors, such as empty lots and abandoned buildings, are included in analysis [87]. This raises the potentially important point that other environmental factors play a role in modulating the effects of PM, and must be considered when determining the impact of PM on cognition in the elderly. It is also of note that a study focusing on younger adults, mean age approximately 37, found no significant association between PM10 exposure levels and reduced cognition [88]. This could indicate that PM exposure is a more significant risk factor in vulnerable populations, such as the elderly or children, than it is in healthy adults, though additional research in this age group is required to be sure they are not vulnerable.
Recent works demonstrate a correlation between PM exposure and risk for neurological disease outcomes, with particular focus on AD. A study in Sweden observed that general dementia incidence over a 15 year period correlated to nitrogen oxide exposure levels [12]. Associations between levels of NOx and AD or vascular dementia (VaD) specifically were non-significant, however there was significant association between the highest quartile of exposure (>26 μg/m3) and general dementia diagnosis. Considering that the previously mentioned study evaluating cognitive changes in older groups exposed to NO2 found no association [80], there is a possibility that additional factors impact the relation between NOx and cognitive decline. Whether this discrepancy is due to differences in study populations and conditions, such as genetic factors or non-air pollution environmental factors, or if it is due to the specific compositions and concentrations of the air pollutants involved, is a potential area of interest for further research. Another study of elderly women (<75 years) in Germany revealed lower executive and olfactory function, and reduced Consortium to Establish a Registry for Alzheimer's Disease (CERAD) test scores, for those living within 50 m of a busy roadway as compared to those living further from roadways [88]. Levels of PM10 were not associated with reductions in any of these measures-potentially due to reduced systemic effects resulting from the inability of coarse fraction PM to infiltrate to the bloodstream or directly to the brain. A later study using the same study cohort found no association between overall traffic related pollution exposure and CERAD score in the general population but did see significant decreases in the figure drawing subtest associated with PM2.5 and PM10 exposure levels specifically [89]. Schikowski et al. used a larger base group, 789 participants compared to 399 in Ranft et al., presumably as more participants had responded by the later date, and so it is possible that the variations in the study cohort account for the differences. However, as very little changed in terms of average age, education level, or other descriptive statistics within the cohort between the two studies, analytical differences or exposure variations are more likely reasons for the divergent outcomes. These divergent results highlight the precarious nature of using proxy measurements for air pollution exposures when the actual composition changes daily. Increasing the body of epidemiological studies on this topic and the complimentary use of controlled animal studies that can control for such variables will be helpful in mitigating these factors.
Additional studies indirectly support a link between PM exposure and AD, one of which focuses on unspecified dementia cases and the other examining an association with mild cognitive impairment (MCI). A Canadian population based cohort of 2.2 million residents age 55–85 residing in Ontario for five or more years was examined for incidence rates of dementia and Parkinson disease (PD) related to distance of residence to the nearest major roadway, as well as dementia incidence related to PM2.5, O3, and NO2 exposure [90], [91]. The adjusted hazard ratio (HR) for incident dementia was significant at 1.07 for those living within 50 m of the nearest major roadway, decreasing with distance to 1.04 at 51–100 m, 1.02 at 101–200 m, and 1 at 201–300 m compared to those living more than 300 m from the nearest major roadway. PM2.5 and NO2 levels were significantly associated with increased dementia incidence as well, with HR values of 1.04 and 1.1, respectively. There was no association seen between PD and distance to roadway, implying a potential vulnerability of AD-related brain regions against air pollution. Another study examined the link between various sizes of PM and nitrogen oxides with MCI in a German population-based cohort ages 47–75 from the Ruhr area [92]. PM2.5 was significantly associated with increased overall MCI incidence and incidence of amnestic MCI at a five year follow up exam. NO2 was only significantly associated with amnestic MCI. This study is also notable for comparing the effects of noise level concurrently with air pollution and finding that noise was also associated with MCI incidence. This supports results seen in Ailshire et al. [86] that other environmental concerns can be a strong confounding factor with many tests of cognitive ability used in the epidemiological studies reviewed-particularly as many of these studies rely on air pollution metrics that would be associated with increased noise pollution, such as distance to major roadways.
Examination of >64 year old Medicare enrollees in multiple U.S. cities over an 11-year period, found that for every 1 μg/m3 increase in annual city wide PM2.5 concentration, there was a 1.08 HR for all cause dementia, 1.15 HR for AD, and 1.08 HR for PD first time hospital admission [93]. A Taiwanese 9-year cohort study of individuals age 65 or higher to determine the relationship between O3 and PM2.5 exposure and AD risk found a 211% increased risk of AD incidence per 10.91 ppb increase in O3 over the follow up period, and a 138% increased risk of AD incidence per 4.34 μg/m3 of PM2.5 [11]. A case control study in Taiwan enrolling AD and VaD patients used PM10 and O3 data from the Taiwan EPA for the past 12 and 14 years, respectively, to determine exposure [94]. As with the previous study, both O3 and PM were associated with and increased odds ratio of AD and VaD; the highest tertile exposure group for PM10 (>49.23 μg/m3) had an adjusted OR of 4.17 for AD and 3.61 for VaD. The highest tertile exposure group for O3 (>21.56 ppb) had AD an adjusted odds ratios of 2 and 2.09 for AD and VaD, respectively. Compared to Oudin et al. [12], who found no association between NOx and either AD or VaD alone, it is interesting that both diseases are associated with PM and O3 here, both of which are also tied to traffic related pollution. This again suggests that additional factors or specific composition of the air pollutants may affect cognitive outcomes. Together, these studies show consistent evidence that AD risk is increased with exposure to higher levels of PM. As VaD is also shown to be linked to PM exposure, it remains an open question whether AD risk is elevated more than other forms of dementia.
Genetic predisposition may play an important role in air pollution dementia risk. The APOE gene remains the strongest known genetic risk factor [4], [5], and a few studies have examined whether air pollutant exposure risk is modulated by APOE allele status. Within the Women's Health Initiative Memory Study cohort patients living in areas of high PM2.5 concentrations, which are over the EPA recommended long term exposure limit of 12 μg/m3, are found to have increased risk of dementia incidence [10]. The risk is exacerbated by APOE ε4 status, with increasing risk when exposed to high levels PM in carriers of one copy of the allele and the highest risk with two copies. This strengthens the previously mentioned Mexico City study [74], which found increased risk of cognitive deficits in children when APOE ε4 carrier status and higher air pollution were both present as compared to either factor alone. While AD risk was not assessed, the ability of APOE status to modulate risk of dementia and impaired cognition strongly suggests a gene–environment interaction in determining the likelihood of developing dementia. In a study of a cohort of elderly women in Germany, there was no association between PM or NO2 exposure and reduced overall cognition or MMSE scores regardless of APOE status [89]. However, traffic pollutant exposure, which was measured as a product of the number of cars passing and length of road close to the residence, was linked to impaired visuospatial function, but only in carriers of at least one APOE ε4 allele, further supporting that genetic factors may be critical in assessing risk for air pollution exposure.
Human clinical and pathological studies
Correlation between exposure to air pollution and cognitive impairments found by these epidemiological studies is partly supported by studies examining brain imaging and biochemical assays in younger age groups performed by comparing Mexico City residents to those living in less polluted areas of Mexico [45], [71], [95], [96], [97], [98], [99], [100]. MRI scans show increased white-matter hyperintensities in Mexico City children and young adults compared to controls, and minor decreases in bilateral and parietal temporal lobe white matter volume, while necropsy tissue shows white matter lesions and disruption of the blood–brain barrier based on sonula occludens-1 (ZO-1) staining of tight junctions [45], [71], [96], [99]. Blood, urine, and necropsy tissue sample from children and adults showed increases in multiple cytokines, inflammatory response markers, and oxidative stress markers, including cyclooxygenase-2 (COX-2), interleukin-1β (IL-1β), interleukin-12 (IL-12), nuclear factor-κB (NF-κB), CD14, and tumor necrosis factor-α (TNF-α), and down-regulation of prion-related protein PrPC in multiple brain tissues for those living in Mexico City [95], [96], [99], [100]. Children and young adults living in the high pollution area exhibited greater amounts of Aβ42 immunoreactivity, Aβ diffuse plaques, and hyperphosphorylated tau pre-tangles in the olfactory bulb, hippocampus, and cortical neurons than subjects in low pollution areas [95], [96], [98], [100]. Critically, subjects homozygous for APOE ε4 had more Aβ plaque pathology, Aβ42 immunoreactivity, and hyperphosphorylated tau, and performed worse in olfaction tests, than those with the APOE ε3 allele [96], [100]. A separate group performed a MRI study of children ages 8–12 and found a correlation between higher levels of PM2.5 elemental carbon and NO2 with lower functional integration and segregation in the default mode network and stimulus driven operations, both indications of slower maturation [101]. These studies indicate that PM exposure can affect amyloid processing and inflammation response in the human brain. They also demonstrate overt neurotoxicity and vascular damage in the brain, suggesting a potential mechanism for cognitive impairment due to pollution exposure in humans. However, as much of this work has been performed by one group in the same area and populations, additional studies with expanded scope may be required for extrapolation to the population at large. These findings strengthen the epidemiological evidence of PM exposure affecting the CNS function by providing potential pathways for that interaction to occur and further suggest that genetic risk factors may act in concert with environmental exposure to exacerbate disease pathology. These studies also provide a starting platform for animal model and in vitro studies examining the effect of air pollutants on the CNS by indicating the molecular pathways that are likely involved.
Animal and cell culture studies
Growing bodies of epidemiological studies have unveiled pathological link between PM exposure and cognitive decline to AD. Underlying cellular and molecular mechanisms are being investigated using in vivo and in vitro models to provide powerful insight into understanding the etiopathogenesis of AD. See Table 3, Table 4 for summaries of animal model and in vitro works examining effects of PM and other air pollutant exposures.
Table 3.
Animal Model Studies of PM effects on the CNS.
| Animal Models | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Model | Exposure type and source | Sex | Start age (weeks) | Exposure duration (weeks) | Exposure hours/day, days/week | Conc. (μg/m3)a | Age at sacrifice (months) | Findings | Citation |
| 2002 | Mongrel dogs | Mexico City (high pollution) or Tlaxcala (low pollution) residence | M,F | N/A | N/A | N/A | N/A | 0–12 years | Increased Nf-κB, iNOS, NFTs, non-neuritic plaques, and neuronal morphology changes in Mexico city animals. | Calderón-Garcideuñas et al., 2002 [102] |
| 2003 | Mongrel dogs | Mexico City (high pollution) or Tlaxcala (low pollution) residence | M,F | N/A | N/A | N/A | N/A | 0–10 years | Increased Nf-κB, iNOS, COX2, Aβ plaques, APP expression, and AP DNA sites in Mexico City animals. | Calderón-Garcideuñas et al., 2003 [103] |
| 2005 | BALB/c mice | PM2.5 and UF PM collected near I-10 freeway in the Los Angeles, CA area. | M | 7 | 2 | 4 h/day, 5 days/week | 282.5 | 2.5 | Whole brain Nf-κB, IL-1α up in both PM2.5 and UF exposures, TNF-α only significantly increased in PM2.5 exposure. | Campbell et al., 2005 [104] |
| 2008 | Mongrel dogs | Mexico City (high pollution) or Tlaxcala (low pollution) residence | M,F | N/A | N/A | N/A | N/A | 12, 15, or 19 | Increased COX2, IL-1β, GFAP, and white matter lesions in Mexico City animals. | Calderón-Garcideuñas et al., 2008 [45] |
| 2008 | APOE−/− C57BL/6J mice | UF PM collected near I-10 freeway in the Los Angeles, CA area. | M | 6 | 6 | 5 h/day, 3 days/week | 30.4 or 114.2 | 3 | Whole brain AP1 increase with both exposures, NFKB only with high exposure. GFAP level and pJNK to JNK ratio increased in low exposure only. | Kleinman et al., 2008 [46] |
| 2008 | BALB/c mice | Nano-particle enriched Diesel exhaust. LTA injection second variable. | M | 7 | 4 | 5 h/day, 5 days/week | 148.86 | 3 | Increased espace latency in MWM. Increased GluN1, GluN2A, GluN2B, IL-1β, and TNF-α mRNA in the hippocampus. | Win-Shwe et al., 2008 [112] |
| 2010 | Fischer F344/DUCRL rats | Diesel particulate matter, O3 pre-exposure. Nose only. | M | 15 | 4 | 6 h/day, 5 days/week | 173 | 4.5 | TNF-α, IL-1β increase in the striatum. No Nf-κB change. | Gerlofs-Nijland et al., 2010 [109] |
| 2010 | Wistar rats | Ambient air pollution at Porto Alegre, Brazil. | M | 0 | Pre-natal to PND21 and/or PND21 to 22 weeks. | 24 h/day, 7 days/week | 16.2 | 5 | Decreased SOD and MDA in animals treated both pre and postnatally. tGSH decreased in PND21 to adulthood group only. Decreased performance in spontaneous nonmatching-to-sample recognition test for continuous exposure and PND21 to adulthood. | Zanchi et al., 2010 [113] |
| 2011 | C57BL/6J mice | PM2.5 collected from the Columbus, OH area. | M | 4 | 30 | 6 h/day, 5 days/week | 94.38 | 9.5, testing at 8.5 | Reduced learning ability and memory in Barnes maze task, reduced time in open maze center, and increased depressive response in forced swim test. Reduced spine density and dendrite length in the CA1. IL-1β, TNF-α, and HO1 expression increased in the hippocampus. | Fonken et al., 2011 [16] |
| 2011 | Sprague–Dawley rats | Diesel particulate matter | M,F | 12 | 26 | 6 h/day, 7 days/week | 30, 100, 311, or 992 | 9 | TNF-α increase in olfactory bulb, midbrain, frontal lobe, temporal lobe in highest treatment, with 311 μg/m3 treatment increased in midbrain. IL-1β, α-synuclein increased in midbrain with highest exposure, along with a-synuclein, MIP-1α down. Aβ-42 and tau increased in frontal lobe with highest exposure. | Levesque et al., 2011 [110] |
| 2011 | C57BL/6J mice | UF PM collected near highway 101 in the Los Angeles, CA area. | M | 12 | 10 | 5 h/day, 3 days/week | 468 | 7 | GluR1 decrease and increase in CD14, CD68, GFAP, IL-1β, and IL-6 in the hippocampus. | Morgan et al., 2011 [15] |
| 2012 | FVBN mice | Nickle Nanoparticles | M,F | 8 | 1 time exposure | 3 h | 1000 | 2 | Aβ load increased. | Kim et al., 2012 [111] |
| 2013 | C57BL/6J mice | UF PM collected in the Cambridge, MA area | M,F | 1 | PND 4–7, 10–13, 56–60. | 4 h/day | 96 | 2.5 test | Increased fixed ration operant wait time, no change in locomotor behaviors. | Allen et al., 2013 [114] |
| 2013 | Sprague–Dawley rats | PM10, PM2.5, UF PM collected in Mexico City, Mexico | M | 6 | 8 | 5 h/day, 4 days/week | PM10 32, PM2.5 178, UFPM 107 | 5 | HO-1 and SOD2 mRNA increased in multiple brain regions with all exposures. Nrf-2, IL-1β, adn TNF-α increased in striatum and hippocampus only with UF PM. Nf-κB increased in striatum with UF PM. | Guerra et al., 2013 [14] |
| 2014 | C57BL/6J mice | UF PM collected in the Cambridge, MA area | M,F | 1 | PND 4–7, 10–13. | 4 h/day | 96 | PND14, PND55 | Ventriculomegaly in males. Decreased GFAP in males, increased GFAP in PND14 females. Increased IBA1 in males. Variable changes in neurotransmitters and cytokines based on sex and brain region. | Allen et al., 2014a [115] |
| 2014 | C57BL/6J mice | UF PM collected in the Cambridge, MA area | M,F | PND 4–7 and 10–13, and/or PND56-60. | 4 h/day | 15 to 240 | 9 | Decreased NOR test performance with early exposure. Variable neurotransmitter changes based on sex, region, and treatment. | Allen et al., 2014b [116] | |
| 2015 | C57BL/6J mice | PM2.5 collected from the Columbus, OH area. | M | 8 | 13 or 39 | 6 h/day, 5 days/week | 65.7 | 5 or 11 | Temporal Cortex AB load increased in 39 week treatment, APP decreased, BACE increased, no change in tau load. PSD95 increased in both treatments. Cytokine assay showed increase in chemoattractants, but not IL-1β, IL-6, or TNF-α. No change in GFAP, VCAM, IBA1. Cox1 and Cox2 increased. | Bhatt et al., 2015 [17] |
| 2015 | Wistar rats | Diesel particulate matter Nano-particles. Nose only. | M,F | 12 | 12 | 4 h/day, 5 days/week | 0.33, 0.5, or 1 | 3.5 | With highest exposure, IL-6, IL-1β increased with highest exposure in temporal lobe, COX2 increased the in midbrain and temporal lobe, and TNF-α and Aβ-42 increased in multiple brain tissues. DNA damage, ROS, and H2O2 increases observed in frontal, temporal lobes. | Durga et al. 2015[108] |
| 2016 | C57BL/6J mice | NanoPM collected by the I-110 in the Los Angeles, CA region. | M | 12 | 1, 4, or 9 days | 5 h/day, 3 days/week | 343 | 3 | Increased IBA-1 in the olfactory bulb. Increased TNF-α in the cortex and cerebellum, and CD68 in the cerebellum. | Cheng et al., 2016 [13] |
| 2017 | 5xFAD ± APOE ɛ3 or ɛ4, and C57BL/6J mice | UF PM collected near highway 101 in the Los Angeles, CA area. | F | 8 | 15 FAD, 10 wt | 5 h/day, 3 days/week | 468 | 7 | Increase in Aβ protein load in APOE ɛ4 Cerebral Cortex. Lowered CA1 neuron density in wild type and APOE ɛ3. GluR1 reduced in all models. | Cacciottolo et al., 2017 [10] |
Concentrations represent average exposures.
Table 4.
In vitro studies of PM effects on CNS derived cells.
| Cell culture studies | ||||||
|---|---|---|---|---|---|---|
| Year | Cells used | Exposure | Concentration | Exposure duration | Outcomes | Citation |
| 2007 | BV2 | Fine PM collected from Sterling Forest State Park in Tuxedo, NY. PM divided into high and low potency samples based on Nf-κB response in epithelial cells. | 25–100 μg/mL antioxidants and inflammatory marker tests. 6–50 μg/mL for mitochondrial membrane depolarization, 34–500 μg/mL for ATP level tests. 75 μg/mL for gene regulation tests. | 1.5 h antioxidants. 6 h inflammatory markers. 15 min ATP, mitochondria. 4 h gene regulation. | Upregulation of Nf-κB, inflammatory response, and oxidative stress response genes with treatment. Increased TNF-α and IL-6 with higher concentrations of treatment. Decreased GSH, NPSH with high potency samples. Decreased intracellular ATP and increased mitochondrial membrane depolarization with higher concentration treatments. | Sama et al., 2007 [120] |
| 2008 | Brain capillaries from male Sprague–Dawley rats, C57BL/J6 mice, and TNF-R1 deficient mice. | Diesel particulate matter. | 5, 50, and 200 μg/mL. | 30 min or 6 h | Increased P-glycoprotein expression and activity after 6 h exposure. Increased NADPH subunit gp91PHOX after 6 h and ROS by CM-DCF after 30 min exposure. Inhibition of NADPH oxidase or addition of SOD blocked P-glycoprotein changes. | Hartz et al., 2008 [123] |
| 2011 | Hippocampal slices from post natal day 10–12 rats, primary glial cells from neonatal day 3 and primary neurons from embryonic day 18 rat cerebral cortex. | nanoPM suspension collected from near the CA-I110 in Los Angeles, CA | 1–20 μg/mL. | 24–72 h | nPM treatment increased LDH in media and increased propidium iodide uptake in brain slices. Neuron cultures had reduced viability and neurite outgrowth. Glial cultures showed increased TNF-α and IL-1α mRNA. Treatment of neuron culture with media from glial culture showed reduced neurite outgrowth and neuron loss. | Morgan et al., 2011 [15] |
| 2013 | Hippocampal slices from 1 month Male C57BL/6J mice and primary neuron culture from Sprague–Dawley embryonic day 18 rats. | nanoPM suspension collected from near the CA-I110 in Los Angeles, CA | 1, 5, and 10 μg/mL | 2 h | Increased GluA1, GluN2A, GluN2B, PSD95, and spinophilin in CA1 but not DG in brain slices. Increased nitric oxide radicals in both slices and neurons. Increased GluN2A nitrosylation and dephosphorylation of GluN2B and GluA1 in CA1 but not DG. | Davis et al., 2013 [121] |
| 2013 | N27 neurons and primary culture from Sprague–Dawley embryonic striatum. | Fine (0.18–1 μm) and ultrafine (<0.18 μm) PM collected from Sterling Forrest State Park in Tuxedo, NY. | 12.5, 25, and 50 μg/mL for toxicity. 8 μg/mL (UF) and 80 μg/mL (Fine) for morphometry. | 24 h | Neuronal loss for all tested concentrations of UF PM, and for 50 μg/mL Fine PM. Apoptotic morphology observed for both treatments, but NSE staining only reduced in UF treatment. | Gillespie et al., 2013 [126] |
| 2016 | Primary mixed glia from cerebral cortex of Sprague–Dawley rats. | nanoPM suspension collected from near the CA-I110 in Los Angeles, CA | 12 μg/mL | 2 h | Increased iNOS and delayed increase in TNF-α. | Cheng et al., 2016 [13] |
| 2017 | Neuroblastoma N2a cells with Swedish mutant APP. | nanoPM suspension collected from near the CA-I110 in Los Angeles, CA | 10 μg/mL | 24 h | Increased Aβ42 compared and sAPPβ/α ratio in exposed N2a cells. | Cacciottolo et al., 2017 [10] |
Animal model studies
Dogs living in Mexico City, which has high levels of ambient air pollution, develop white matter lesions, damage to the blood–brain barrier, degenerating neurons, oxidative damage, glial activation and neuroinflammation compared to dogs living in less polluted rural areas [45], [102], [103]. Diffuse Aβ plaques and neurofibrillary tangles-two key pathological hallmarks of AD were also observed in Mexico city dogs [102], [103]. These studies support the hypothesis that PM adversely impacts neuroanatomical and neuropathological changes in the brain, leading to the development of AD-like pathology via multifactorial mechanisms, including neurodegeneration, altered glial cell levels, amyloid processing, and immune response. However, as in the similarly structured epidemiological studies, the lack of tight control on the exposure paradigm leaves considerable room to question which components of air pollution are critical in these processes.
In vivo studies provide significant insights into the neurotoxic effect of PM exposure and help decipher underlying mechanisms linking exposure to the development of dementia and AD. Earlier studies demonstrate that high levels of ultra-fine PM for short durations (2–6 weeks) in mice elicit significant increases in pro-inflammatory cytokines IL-1α and TNF-α, Glial responses, and activation of NF-κB and AP-1 transcriptional factors in brain tissue [46], [104]. These studies show that, under a controlled exposure environment, even acute PM exposure can cause changes in the brain inflammatory responses to the levels similarly found in the lungs, the primary target organ for PM exposure. As inflammation has been implicated as an important pathway in AD and dementia [105], the connection between PM exposure and neuro-inflammation establishes strong a potential linking pathway between PM exposure and AD risk.
Increased brain inflammation, measured by IL-1α, IL-1β, TNFα, heme oxygenase-1 (HO-1), glial fibrillary acidic protein (GFAP), CD14, or CD68 mRNA, is also observed in C57BL/6 mice and Wistar rats exposed to nanoscale PM, PM0.1, or PM2.5 for the courses of 6–10 weeks [13], [14], [15]. Cellular antioxidant machinery proteins nuclear factor erythroid 2–related factor 2 (Nrf2) and superoxide dismutase-2 (SOD-2) are increased in the hippocampus of animals exposed to the ultra-fine PM, indicating that it causes region-specific toxicity. These data strengthen the evidence that immune activation may be a critical pathway for air pollutants to affect the CNS and establish a role for glial cells and oxidative damage in the brain in response to PM exposure. Glial cells, particularly microglia and astrocytes, are well established to play important roles in the pathogenesis of AD [106], [107]. Establishing the capability of PM exposure to alter activation of these cells provides another pathway by which exposure can affect AD risk. Longer exposure (30–39 weeks) to concentrated PM2.5 in young mice provokes similar changes in inflammatory responses, as measured by TNF-α, IL-10, IL-13, eotaxins, and HO-1 mRNA or protein in the brain [16], [17]. These exposed mice additionally develop a significant loss of dendritic spine density and dendrite length in the CA1 region of the hippocampus, which correlates with impaired cognitive outcomes [16]. Buildup of Aβ plaques is also observed [17]. PM exposure increases BACE expression and reduces amyloid precursor protein (APP) in the brain, and this upregulation of BACE is suspected to promote the amyloidogenic pathway of APP processing and increase the production of Aβ in the exposed mice [17]. These findings bridge the association between chronic exposure to PM and inflammation and the development of AD-like neuropathology, demonstrating that PM exposure can lead to neuronal loss akin to what is seen in AD and other neurodegenerative diseases in animal models over longer exposures.
Exposure to diesel exhaust particles also leads to increased inflammatory cytokines, such as TNF-α and IL-1β, increased DNA damage, and generation of ROS species in the striatum, midbrain, and frontal lobe of rats [108], [109], [110]. Increased levels of Aβ42 and phosphorylated tau in multiple brain regions are also observed in the highest exposure group (∼1 mg/m3) [110]. Mice exposed to a similar concentration of nickel nanoparticles also exhibit an increased Aβ42 load [111], suggesting that the effect on Aβ buildup in the brain may be, in part, due to the concentration of particulates exposed, rather than the its chemical constituents. Exposure to diesel exhaust has also been shown to negatively affect performance of seven week old mice in the Morris Water Maze task [112]. However, the association was minimal without the addition of bacterial derived lipoteichoic acid. This study additionally found that exposure to diesel exhaust with lipoteichoic acid increased mRNA levels of NMDA subunits GluN1, GluN2A, and GluN2B, as well as IL-1β, TNF-α, and HO-1. These effects trended in the same direction, but were non-significant, for either diesel exhaust exposure or lipoteichoic acid alone. These findings serve as a reminder of the pitfalls of filtered pure PM exposures, as the total mixture and interactions of air pollutants may be critical in toxicity. The potential importance of overall air pollutant mixture may also explain discrepancies between studies, as potentially un-monitored factors in the PM mixture contribute to effect.
Although there is strong epidemiological evidence that developmental and early life PM exposures can have significant effects on cognitive function, there are currently limited animal model studies examining pre-natal and during development exposures. Rats exposed to PM2.5 for 24 h per day either during gestation, postnatally until testing and sacrifice at 5 months of age, or both, showed changes in oxidative damage indicated by MDA, SOD, and tGSH protein levels, and reduced short-term discriminative memory and habituation [113]. The results in this study were generally only significant when then animals were exposed in utero and during adult life, suggesting early life exposure as a possible potentiating factor to effects of exposure later in life. If this is the case, total life exposure may need consideration as a factor in future epidemiological studies examining the effects of late life exposure to PM. Mice exposed to concentrated ambient ultra-fine (<100 nm) particulates either during post-natal days 4–7 and 10–13, post-natal days 56–60, or both showed deficiencies in the novel object recognition (NOR) and Fixed-interval schedule control performance tasks at 10 weeks of age [114], [115], [116]. NOR discrimination was decreased in all mice exposed during development, while the male mice showed reduced rates in the fixed-interval tasks only in those exposed during postnatal day (PND) 4–7 and 10–13, but not PND 56–60, while female mice showed increased rates but only for those exposed at PND 56–60 and not the earlier time points [114], [115]. Levels of multiple neurotransmitters, cytokines, and GFAP were also altered with exposures, though there was considerable variance in which exposure groups saw significant changes and large variance between sexes [115], [116]. As the tissues were not harvested until the mice were 9 months of age, it is difficult to directly relate the protein changes in the brain with the behavior performances measured at 10 weeks. The differences between exposure groups may have arisen from natural changes in the PM concentration and composition over the course of the experiments, highlighting the fluid nature of PM exposure and difficulties in strict control over experimental conditions. These studies also demonstrate the response to PM induced insult may vary considerably between sexes. Determining the extent of these differences is crucial in assessing at-risk populations.
To our knowledge, currently only one study has examined the effects of PM exposure on an AD mouse model. Cacciottola et al. used female 5xFAD mice with either the ε3 or ε4 allele of APOE [10]. Ultra-fine ambient concentrated PM was selected as the exposure for this study due to the generally higher toxicity of smaller PM fractions [23], [40], as well as its ability to infiltrate the CNS via the olfactory epithelium [43], [44], making it the most likely candidate to affect AD pathology. Cerebral cortex sections showed significant increases in Aβ plaques by 4G8 antibody and thioflavin S staining, and decreased AMPA receptor subunit GluR1. There was an increase in Aβ oligomer in protein samples, but only in the 5xFAD ApoE ε4 mice. Reduced neuron density in the CA1 region of the hippocampus in the PM exposed mice vs the unexposed was seen for both wild type control mice and 5xFAD ApoE ε3 mice; the ApoE ε4 mice showed reduced CA1 neuronal density in both exposed and unexposed mice, making determination of the effects of the air pollution difficult. These data demonstrate that PM exposure exacerbates AD pathology progression in 5xFAD mice, as shown by the 4G8 and thioflavin S, providing a mechanistic link between exposure and risk. Further, the increase of Aβ oligomer only in the APOE ε4 allele carriers show that PM exposure effects on AD pathogenesis can be increased with susceptible genotypes. These findings demonstrate pathological changes that may explain the link between APOE ε4 carrier status and increased risk for cognitive impairment seen in epidemiological studies [12], [74], [89]. Since exacerbation of Aβ load has also been shown in wild type mice as described above [17], [110], [111], determining whether PM acts through AD specific pathways in addition to inflammation response and ROS generation requires further study.
In vitro studies
In vitro studies provide insights into in-depth cellular and molecular mechanisms by which PM exposure promotes cellular damage and abnormality linking to AD. Buildup of Aβ is one of the indispensable pathological hallmarks for AD and is suspected to be mediated by increased production, increased aggregation, and/or decreased clearance of Aβ. As described above, several studies from animals have demonstrated an increased production of Aβ in mice chronically exposed to PM2.5. Recent in vitro work using neuroblastoma N2a cells shows that treatment with 10 μg/ml PM2.5 increased Aβ production [10]. This supports in vivo findings, though detailed underlying mechanisms by which amyloid production is increased have yet to be determined.
Brain resident immune cell lineages, such as microglia and astrocytes, and neuroinflammatory states play a critical role in the metabolism and clearance of Aβ, which has been supported by numerous studies [105], [117], [118], [119]. Not surprisingly, high levels of PM applied to murine microglial BV2 cells showed cytotoxicity and increased secretion of pro-inflammatory cytokines, TNF-α and IL-6, implicating dysregulation of inflammatory responses [120]. Nanoscale PM induced spikes in TNF-α mRNA and protein, as well as iNOS and IL-1α mRNA, in rat derived mixed astrocyte/microglia culture [13], [15]. Aside from Aβ buildup, PM evokes a concentration-dependent, NMDA receptor-mediated neurotoxicity, concomitant with increased inflammation in hippocampal slice culture [15]. Increased oxidative damage and decreased neurite outgrowth are also observed following PM exposure [15], [121]. Impairment of neurite outgrowth was also observed when PM-free media taken from a PM exposed mixed glial culture was added to neuron culture [13]. As TNF-α is known to reduce neurite outgrowth in culture [122], it is suggested as a possible causative factor of the observed effect using transferred media. However, as the contents of the transferred media were not examined in this study, it is unknown if TNF-α protein levels were increased in the media, or what other factors may be involved. Still, together these data indicate that PM toxicity can act both directly on neurons and indirectly through glial cells, providing two possible pathways to link to neurodegeneration.
A study of the effects of diesel exhaust particles on isolate rat brain capillaries found significant up-regulation of the receptor protein P-glycoprotein (p-gp) [123]. P-glycoprotein (p-gp) is expressed in endothelial cells and is well-studied membrane-bound receptor that mediates transcytosis of Aβ from brain parenchyma to blood through the blood–brain barrier [124], [125]. As increases in p-gp could reduce Aβ buildup in the CNS [124], [125], this finding is incongruous with reported animal model studies showing increased Aβ after PM and diesel exposure [17], [110]. It could be explained that the alterations to inflammation response [11], [13], [14], [15] and APP processing [15] caused by PM exposure outweigh the effects of increased p-gp on Aβ accumulation. Additional research is required to determine if other PM fractions can cause this change in p-gp, or if it is specific to diesel particles, as mock particles did not elicit an increase in p-gp [123]. Examination of p-gp and other epithelial transport proteins such as LRP-1 in in vivo exposure models is also warranted.
Overall, these in vitro studies suggest PM induced increases in Aβ production, oxidative stress, and glial cell mediated inflammatory responses as potential mechanisms leading to neurotoxicity, which may then link PM exposure to reduced cognition and increased risk of neurodegenerative disease as seen in epidemiological studies. However, the number of available studies examining the effects of PM on CNS related cells is too small to arrive at definitive conclusions. This is exacerbated by the variable nature of PM and the specificity of cell culture work, as the five listed studies examine three different PM fractions in four different in vitro models. By weight, the ultrafine fraction of PM carries greater toxicity than PM2.5 in neurons [126], suggesting that analyses focusing on the smallest PM fractions may produce the strongest results.
Conclusion and discussion
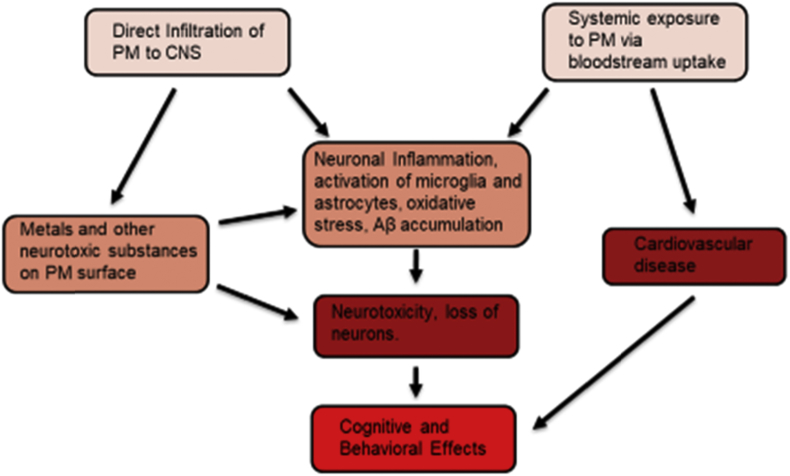
The studies presented above represent a strong foundation for the case of PM and other air pollutants as key risk factors for AD and other neurodegenerative disease etiologies. Epidemiological studies provide evidence for risk association of PM exposure with reduced cognitive abilities across the age spectrum, with exposure at older ages having the strongest and most consistent effects [55], [56], [57]. There is burgeoning evidence of direct risk association between PM2.5 exposure and AD incidence [10], [11]. Studies in vitro and in vivo, both animal model and human, show PM exposure involvement in inflammatory pathways [45], [46], [102], [103], [104] oxidative stress [95], [108], [113], and amyloidogenesis [10], [45], [95], [98], [100], [110], as well as negative effect on cognition and behaviors in animals [16], [114], [115], providing potential mechanisms by which PM exposure can increase AD risk [Fig. 2]. Damage to the brain vasculature [102] and systemic effects such as hypertension may also be important contributors to cognitive risk from PM exposure [127]. Infiltration of PM to the CNS may provide a pathway for metals and other neurotoxic substances to accumulate in the brain, though to what extent this occurs is currently unknown. Additional research is required to determine which of these pathways are the major factors in increasing AD risk, and whether the risk association between PM exposure and AD is specific to AD, or largely caused by general toxic insult to the CNS.
Fig. 2.
Proposed pathways by which particulate matter exposure leads to neurotoxicity and cognitive deficits. Direct infiltration of PM to the brain [43], [44] can provide a pathway for metals and other neurotoxic chemicals to accumulate in neural tissue, and potentially provide a reactive surface as occurs in the lungs [37]. Systemic effects from PM infiltrating the blood via the alveoli include cardiovascular disease [53], [54], which can lead to impaired cognition and promote AD pathology [127]. Both pathways potentially contribute to the inflammatory, glial, and amyloid pathology responses observed in animal models [8], [97], [101], cell culture [10], [15], [120], and human studies [95], [96], [100]. The cascade from these responses to neurotoxicity and cognitive loss is well documented [105], [106], [107], and consistent with results showing neuronal toxicity [10], [15], [126] and behavioral effects [112], [113], [116] observed with PM exposure.
While the epidemiological evidence for air pollutants and particulates to negatively affect cognition is robust, the studies tend to have significant variation in which cognitive functions are affected. Results often indicate dysfunction in entirely different brain regions or functions, particularly for early life exposures. A possible explanation for these results is that PM exposure may not be a targeted risk factor for damage in a specific brain region or for a given disease, but rather a condition that creates general challenge to the brain which then manifests based on an individual's condition. Studies showing a link between PM exposure and general dementia, or other forms of dementia, as opposed to AD specifically [12], [90], [92], indicate that the changes occurring may not be highly specific. Studies showing the effects of APOE genotype on modulating the effects of PM exposure [11], [96], [100], may additionally support this view by demonstrating the importance of genotype of the strength of the associations seen, suggesting that AD risk due to PM is highest in those already with increased risk of AD. However, there is evidence in vivo that PM exposure can alter APP processing [12], which suggests at least some specificity to AD disease progression, though to what extent is unknown. More epidemiological studies focusing on examining the effects of risk factor genes for AD other dementias are required. Additional studies including these risk factor genes as confounding factors would help to determine whether genetic or other predispositions to certain diseases are determinants in the disease outcomes of PM exposure and if exposure can be linked specifically to AD in absence of these factors. In terms of in vitro and in vivo studies, much more work needs to be done in AD model animals to determine the extent to which PM exposure can alter Aβ and tau pathology. Tau pathology particularly requires further investigation, as the few papers that investigate tau load and phosphorylation [17], [99], [110] have found conflicting results. Whether the changes caused by PM exposure occur directly through changes in APP processing and other AD specific pathways, or if inflammation and oxidative stress are the primary causes, is a question that must be answered. Currently available studies tend to only test one age endpoint in the animal models. Studies with multiple age endpoints may be useful to determine whether the alterations to amyloid processing or inflammatory and oxidative stress systems occur first and are therefore the likely ultimate cause.
More robust evidence linking AD to PM levels may be required before policy action can be taken to try to reduce PM exposure in at risk populations. A key area of investigation required for policy making is whether specific air pollutant factors and PM components are critical for affecting risk. As regulation of a given factor is less likely to heavily impact industry compared to requiring reduction of total emission, it may be easier to enact policy if such a factor can be identified. Due to difficulties in teasing out which specific air pollutant may be critical in epidemiological studies, for example NO2 and PM2.5 levels are both related to traffic pollution exposure, animal and cell culture model studies will be of particular use here, as given substituents of the total PM can be separated and administered as Kim et al. [113] demonstrated with Ni particles. This approach has a few noticeable pitfalls, however. Effects of particulate exposure may be dependent on their ability to act as a shuttle for other chemicals [37], particularly given their ability to directly infiltrate to brain tissues [102], [103], [128]. Sub-fractionation of the PM may also eliminate key interactions needed to influence disease progression, such what is observed with LTA levels and diesel particles seen by Win-Shwe et al. [112]. Studies of PM in animal models also generally tend to occur in one of a small handful of areas with sufficiently equipped research facilities, and as these groups use concentrated ambient air for exposure, interactions specific to the PM mixture of regions without these facilities may be overlooked. Related to this, it is also a possibility that a macro scale interaction of the environment plays a role in the overall risk of AD incidence in relation to PM exposure, as suggested by the effects of noise pollution and neighborhood stability [86], [92], and these interactions are difficult to guess and implement in animal model studies without sufficient epidemiology.
Finally, research exploring the effects of early life or long-term exposure to PM on AD risk in late life is an area of potential interest. While there is considerable evidence that exposure in development or early childhood can negatively affect cognition later in life [62], [63], [64], currently, very few epidemiological studies have focused on the effects of long term exposure to PM and AD risk specifically [83]. Studies examining the effects of exposure during mid-life or even early-life on AD incidence in late-life are warranted, particularly given that the exact point of critical development for AD is still unknown. Given the difficulties and time required in such long-term studies, however, a more immediate solution may be to use animal models that can be exposed and grown into late life on a much shorter time scale, as demonstrated by Allen et al. [114], [116], [117] and Zanci et al. [113]. Further studies exposing AD model animals in utero or while young and then examining AD pathology in adulthood could prove to be powerful tools for determining the risks of exposure in young and at-risk populations for later life complications. Of particular interest would be if relatively short exposures during development are sufficient to potentiate AD and other dementias in later life, or if life-long or closer to endpoint exposure is required.
Conflicts of interest
None declared.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bj.2018.06.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Alzheimer’s Association. 2017 Alzheimer's disease facts and figures. http://www.alz.org/facts/, [Accessed 20 October 2017].
- 2.Alzheimer’s Disease International . vol. 87. Alzheimer’s Disease International; London, UK: 2015. (World Alzheimer report 2015). https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf, [Accessed 20 October 2017] [Google Scholar]
- 3.World Health Organziation. “The top 10 causes of death.” WHO fact sheet. http://www.who.int/mediacentre/factsheets/fs310/en/, [Accessed 20 October 2017].
- 4.Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 5.Raber J., Huang Y., Ashford J.W. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25:641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Gatz M., Reynolds C.A., Fratiglioni L., Johansson B., Mortimer J.A., Berg S. Role of genes and environments for explaining alzheimer disease. Arch Gen Psychiatr. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan P.F., Daly M.J., O'Donovan M. Genetic architecture of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campdelacreu J. Parkinson's disease and Alzheimer disease: environmental risk factors. Neurologia. 2014;29:541–549. doi: 10.1016/j.nrl.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Yegambaram M., Manivannan B., Beach T.G., Halden R.U. Role of environmental contaminants in the etiology of Alzheimer's disease: a review. Curr Alzheimer Res. 2015;12:116–146. doi: 10.2174/1567205012666150204121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cacciottolo M., Wang X., Driscoll I., Woodward N., Saffari A., Reyes J. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatr. 2017;7:e1022. doi: 10.1038/tp.2016.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung C.R., Lin Y.T., Hwang B.F. Ozone, particulate matter, and newly diagnosed Alzheimer's disease: a population-based cohort study in Taiwan. J Alzheim Dis. 2015;44:573–584. doi: 10.3233/JAD-140855. [DOI] [PubMed] [Google Scholar]
- 12.Oudin A., Forsberg B., Adolfsson A.N., Lind N., Modig L., Nordin M. Traffic-related air pollution and dementia incidence in Northern Sweden: a longitudinal study. Environ Health Perspect. 2016;124:306–312. doi: 10.1289/ehp.1408322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng H., Saffari A., Sioutas C., Forman H.J., Morgan T.E., Finch C.E. Nanoscale particulate matter from urban traffic rapidly induces oxidative stress and inflammation in olfactory epithelium with concomitant effects on brain. Environ Health Perspect. 2016;124:1537–1546. doi: 10.1289/EHP134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerra R., Vera-Aguilar E., Uribe-Ramirez M., Gookin G., Camacho J., Osornio-Vargas A.R. Exposure to inhaled particulate matter activates early markers of oxidative stress, inflammation and unfolded protein response in rat striatum. Toxicol Lett. 2013;222:146–154. doi: 10.1016/j.toxlet.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan T.E., Davis D.A., Iwata N., Tanner J.A., Snyder D., Ning Z. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ Health Perspect. 2011;119:1003–1009. doi: 10.1289/ehp.1002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonken L.K., Xu X., Weil Z.M., Chen G., Sun Q., Rajagopalan S. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatr. 2011;16:987–995. doi: 10.1038/mp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatt D.P., Puig K.L., Gorr M.W., Wold L.E., Combs C.K. A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain. PLoS One. 2015;10:1–20. doi: 10.1371/journal.pone.0127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . 2016. “Ambient (outdoor) air quality and health.” WHO fact sheet.http://www.who.int/mediacentre/factsheets/fs313/en/ [Google Scholar]
- 19.Craig L., Brook J.R., Chiotti Q., Croes B., Gower S., Hedley A. Air pollution and public health: a guidance document for risk managers. J Toxicol Environ Health. 2008;71:588–698. doi: 10.1080/15287390801997732. [DOI] [PubMed] [Google Scholar]
- 20.Karagulian F., Belis C.A., Dora C., Prüss-Ustün A.M., Bonjour S., Adair-Rohani H. Contributions to cities' ambient particulate matter (PM): a systematic review of local source contributions at global level. Atmos Environ. 2015;120:475–483. [Google Scholar]
- 21.Mazzei F., D'Alessandro A., Lucarelli F., Nava S., Prati P., Valli G. Characterization of particulate matter sources in an urban environment. Sci Total Environ. 2008;401:81–89. doi: 10.1016/j.scitotenv.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Donahuea N.M., Robinsona A.L., Pandis S.N. Atmospheric organic particulate matter: from smoke to secondary organic aerosol. Atmos Environ. 2009;43:94–106. [Google Scholar]
- 23.Valavanidis A., Fiotakis K., Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C. 2008;26:339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R., Wang G., Guo S., Zamora M.L., Ying Q., Lin Y. Formation of urban fine particulate matter. Chem Rev. 2015;115:3803–3855. doi: 10.1021/acs.chemrev.5b00067. [DOI] [PubMed] [Google Scholar]
- 25.Kelly K.E., Kotchenruther R., Kuprov R., Silcox G.D. Receptor model source attributions for Utah's Salt Lake City airshed and the impacts of wintertime secondary ammonium nitrate and ammonium chloride aerosol. J Air Waste Manag Assoc. 2013;63 doi: 10.1080/10962247.2013.774819. [DOI] [PubMed] [Google Scholar]
- 26.Adams K., Greenbaum D.S., Shaikh R., van Erp A.M., Russell A.G. Particulate matter components, sources, and health: systematic approaches to testing effects. J Air Waste Manag Assoc. 2015;65:544–558. doi: 10.1080/10962247.2014.1001884. [DOI] [PubMed] [Google Scholar]
- 27.U.S. EPA . U.S. Environmental Protection Agency; Washington, DC: 2004. Air quality criteria for particulate matter (final report, 2004) EPA 600/P-99/002aF-bF. [Google Scholar]
- 28.Kam W., Liacos J.W., Schauer J.J., Delfino R.J., Sioutas C. Size-segregated composition of particulate matter (PM) in major roadways and surface streets. Atmos Environ. 2012;55:90–97. [Google Scholar]
- 29.Sardar S.B., Fine P.M., Sioutas C. Seasonal and spatial variability of the size-resolved chemical composition of particulate matter (PM10) in the Los Angeles Basin. J Geophys Res Atmosphere. 2005;110 D07S08. [Google Scholar]
- 30.Brauer M., Avila-Casado C., Fortoul T.I., Vedal S., Stevens B., Churg A. Air pollution and retained particles in the lung. Environ Health Perspect. 2001;109:1039–1043. doi: 10.1289/ehp.011091039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Churg A., Brauer M. Ambient atmospheric particles in the airways of human lungs. Ultrastruct Pathol. 2000;24:353–361. doi: 10.1080/019131200750060014. [DOI] [PubMed] [Google Scholar]
- 32.Bermudez E., Mangum J.B., Wong B.A., Asgharian B., Hext P.M., Warheit D.B. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci. 2004;77:347–357. doi: 10.1093/toxsci/kfh019. [DOI] [PubMed] [Google Scholar]
- 33.Müller A.K., Farombi E.O., Møller P., Autrup H.N., Vogel U., Wallin H. DNA damage in lung after oral exposure to diesel exhaust particles in Big Blue® rats. Mutat Res Fund Mol Mech Mutagen. 2004;550:123–132. doi: 10.1016/j.mrfmmm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Stearns R.C., Paulauskis J.D., Godleski J.J. Endocytosis of ultrafine particles by A549 cells. Am J Respir Cell Mol Biol. 2001;24:108–115. doi: 10.1165/ajrcmb.24.2.4081. [DOI] [PubMed] [Google Scholar]
- 35.Kreyling W.G., Semmler M., Erbe F., Mayer P., Takanaka S., Schulz H. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 36.Neymar A., Hoet P., Vanquickenborne B., Dinsdale D., Thomeer M., Hoylaerts M. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 37.Seaton A., MacNee W., Donaldson K., Godden D. Particulate air pollution and acute health effects. Lancet. 1995;345:176–178. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 38.Lundborg M., Johard U., Låstbom L., Gerde P., Camner P. Human alveolar macrophage phagocytic function is impaired by aggregates of ultrafine carbon particles. Environ Res. 2001;86:244–253. doi: 10.1006/enrs.2001.4269. [DOI] [PubMed] [Google Scholar]
- 39.Chang M., Sioutas C., Cassee F.R., Fokkens P.H.B. Field evaluation of a mobile high-capacity particle size classifier (HCPSC) for separate collection of coarse, fine and ultrafine particles. J Aerosol Sci. 2001;32:139–156. [Google Scholar]
- 40.Kim S., Jaques P.A., Chang M., Barone T., Xiong C., Friedlander S.K. Versatile Aerosol Concentration Enrichment System (VACES) for simultaneous in vivo and in vitro evaluation of toxic effects of ultrafine, fine and coarse ambient particles. Part II: field evaluation. J Aerosol Sci. 2001;32:1299–1314. [Google Scholar]
- 41.Zhang K.M., Wexler A.S., Niemeier D.A., Zhu Y.F., Hinds W.C., Sioutas C. Evolution of particle number distribution near roadways. Part III: traffic analysis and on-road size resolved particulate emission factors. Atmos Environ. 2005;39:4155–4166. [Google Scholar]
- 42.Li N., Sioutas C., Cho A., Schmitz D., Misra C., Sempf J. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Block M.L., Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation & CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.González-Maciel A., Reynoso-Robles R., Torres-Jardón R., Mukherjee P.S., Calderón-Garcidueñas L. Combustion-derived nanoparticles in key brain target cells and organelles in young urbanites: culprit hidden in plain sight in Alzheimer's disease development. J Alzheim Dis. 2017;59:189–208. doi: 10.3233/JAD-170012. [DOI] [PubMed] [Google Scholar]
- 45.Calderón-Garcidueñas L., Mora-Tiscareño A., Ontiveros E., Gómez-Garza G., Barragán-Mejía G., Broadway J. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cognit. 2008;68:117–127. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Kleinman M.T., Araujo J., Nel A., Sioutas C., Campbell A., Cong P.Q. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicol Lett. 2008;178:127–130. doi: 10.1016/j.toxlet.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schauer J.J., Rogge W.F., Hildemann L.M., Mazurek M.A., Cass G.R., Simoneit B.R.T. Source apportionment of airborne particulate matter using organic compounds as tracers. Atmos Environ. 1996;30:3837–3855. [Google Scholar]
- 48.Viana M., Kuhlbusch T.A.J., Querol X., Alastuey A., Harrison R.M., Hopke P.K. Source apportionment of particulate matter in Europe: a review of methods and results. J Aerosol Sci. 2008;39:827–849. [Google Scholar]
- 49.Zhu Y., Hinds W.C., Kim S., Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52:1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- 50.American Lung Association . 2016. State of the air 2016.http://www.lung.org/assets/documents/healthy-air/state-of-the-air/sota-201 [Google Scholar]
- 51.South Coast Air Quality Management District . AQMD; 2016. Final 2016 air quality management plan.http://www.aqmd.gov/docs/default-source/clean-air-plans/air-quality-management-plans/2016-air-quality-management-plan/final-2016-aqmp/final2016aqmp.pdf?sfvrsn=15 [Accessed 06 November 2017] [Google Scholar]
- 52.van Donkelaar A., Martin R.V., Brauer M., Boys B.L. Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environ Health Perspect. 2014;110:135–143. doi: 10.1289/ehp.1408646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khafaie M.A., Yajnik C.S., Salvi S.S., Ojha A. Critical review of air pollution health effects with special concern on respiratory health. J Air Pollut Health. 2016;1:123–136. http://japh.tums.ac.ir Retrieved from: [Google Scholar]
- 54.Pope C.A., Dockery D.W. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 55.Clifford A., Lang L., Chen R., Anstey K.J., Seaton A. Exposure to air pollution and cognitive functioning across the life course – a systematic literature review. Environ Res. 2016;147:383–398. doi: 10.1016/j.envres.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 56.Power M.C., Adar S.D., Yanosky J.D., Weuve J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology. 2016;56:235–253. doi: 10.1016/j.neuro.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu X., Ha S.U., Basnet R. A review of epidemiological research on adverse neurological effects of exposure to ambient air pollution. Front Publ Health. 2016;4:157. doi: 10.3389/fpubh.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perera F.P., Rauh V., Whyatt R.M., Tsai W.Y., Tang D., Diaz D. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perera F.P., Li Z., Whyatt R., Hoepner L., Wang S., Camann D. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124:195–202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edwards S.C., Jedrychowski W., Butscher M., Camann D., Kieltyka A., Mroz E. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children's intelligence at 5 years of age in a prospective cohort study in Poland. Environ Health Perspect. 2010;118:1326–1331. doi: 10.1289/ehp.0901070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jedrychowski W.A., Perera F.P., Camann D., Spengler J., Butscher M., Mroz E. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environ Sci Pollut Res. 2015;22:3631–3639. doi: 10.1007/s11356-014-3627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guxens M., Garcia-Esteban R., Giorgis-Allemand L., Forns J., Badaloni C., Ballester F. Air pollution during pregnancy and childhood cognitive and psychomotor development. Epidemiology. 2014;25:636–647. doi: 10.1097/EDE.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 63.Lertxundi A., Baccini M., Lertxundi N., Fano E., Aranbarri A., Martínez M.D. Exposure to fine particle matter, nitrogen dioxide and benzene during pregnancy and cognitive and psychomotor developments in children at 15 months of age. Environ Int. 2015;80:33–40. doi: 10.1016/j.envint.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Porta D., Narduzzi S., Badaloni C., Bucci S., Cesaroni G., Colelli V. Air pollution and cognitive development at age 7 in a prospective Italian birth cohort. Epidemiology. 2016;27:228–236. doi: 10.1097/EDE.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 65.Lin C.C., Yang S.K., Lin K.C., Ho W.C., Hsieh W.S., Shu B.C. Multilevel analysis of air pollution and early childhood neurobehavioral development. Int J Environ Res Publ Health. 2014;11:6827–6841. doi: 10.3390/ijerph110706827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris M.H., Gold D.R., Rifas-Shiman S.L., Melly S.J., Zanobetti A., Coull B.A. Prenatal and childhood traffic-related pollution exposure and childhood cognition in the project viva cohort (Massachusetts, USA) Environ Health Perspect. 2015;123:1072–1078. doi: 10.1289/ehp.1408803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freire C., Ramos R., Puertas R., Lopez-Espinosa M.J., Julvez J., Aguilera I. Association of traffic-related air pollution with cognitive development in children. J Epidemiol Community Health. 2010;64:223–228. doi: 10.1136/jech.2008.084574. [DOI] [PubMed] [Google Scholar]
- 68.van Kempen E., Fischer P., Janssen N., Houthuijs D., van Kamp I., Stansfeld S. Neurobehavioral effects of exposure to traffic-related air pollution and transportation noise in primary schoolchildren. Environ Res. 2012;115:18–25. doi: 10.1016/j.envres.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Chiu Y.H.M., Bellinger D.C., Coull B.A., Anderson S., Barber R., Wright R.O. Associations between traffic-related black carbon exposure and attention in a prospective birth cohort of urban children. Environ Health Perspect. 2013;121:859–864. doi: 10.1289/ehp.1205940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suglia S.F., Gryparis A., Wright R.O., Schwartz J., Wright R.J. Association of black carbon with cognition among children in a prospective birth cohort study. Am J Epidemiol. 2008;167:280–286. doi: 10.1093/aje/kwm308. [DOI] [PubMed] [Google Scholar]
- 71.Calderón-Garcidueñas L., Engle R., Antonieta Mora-Tiscareño A.M., Styner M., Gómez-Garza G., Zhu H. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cognit. 2011;77:345–355. doi: 10.1016/j.bandc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Wang S., Zhang J., Zeng X., Zeng Y., Wang S., Chen S. Association of traffic-related air pollution with children's neurobehavioral functions in Quanzhou, China. Environ Health Perspect. 2009;117:1612–1618. doi: 10.1289/ehp.0800023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kicinski M., Vermeir G., Van Larebeke N., Den Hond E., Schoeters G., Bruckers L. Neurobehavioral performance in adolescents is inversely associated with traffic exposure. Environ Int. 2015;75:136–143. doi: 10.1016/j.envint.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 74.Calderón-Garcidueñas L., Jewells V., Galaz-Montoya C., van Zundert B., Pérez-Calatayud A., Ascencio-Ferrel E. Interactive and additive influences of gender, BMI and Apolipoprotein 4 on cognition in children chronically exposed to high concentrations of PM2.5 and ozone. APOE 4 females are at highest risk in Mexico City. Environ Res. 2016;150:411–422. doi: 10.1016/j.envres.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 75.Sánchez-Rodríguez M.A., Santiago E., Arronte-Rosales A., Vargas-Guadarrama L.A., Mendoza-Núñes V.M. Relationship between oxidative stress and cognitive impairment in the elderly of rural vs. urban communities. Life Sci. 2006;78:1682–1687. doi: 10.1016/j.lfs.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Sun R., Gu D. Air pollution, economic development of communities, and health status among the elderly in urban China. Am J Epidemiol. 2008;168:1311–1318. doi: 10.1093/aje/kwn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wellenius G.A., Boyle L.D., Coull B.A., Milberg W.P., Gryparis A., Schwartz J. Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: results from the mobilize Boston study. J Am Geriatr Soc. 2012;60:2075–2080. doi: 10.1111/j.1532-5415.2012.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng Y., Gu D., Purser J., Hoenig H., Christakis N. Associations of environmental factors with elderly health and mortality in China. Am J Publ Health. 2010;100:298–305. doi: 10.2105/AJPH.2008.154971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Power M.C., Weisskopf M.G., Alexeeff S.E., Coull B.A., Avron S., Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011;119:682–687. doi: 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gatto N.M., Henderson V.W., Hodis H.N., St John J.A., Lurmann F., Chen J.C. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology. 2014;40:1–7. doi: 10.1016/j.neuro.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ailshire J.A., Clarke P. Fine particulate matter air pollution and cognitive function among U. S. older adults. J Gerontol B Psychol Sci Soc Sci. 2015;70:322–328. doi: 10.1093/geronb/gbu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ailshire J.A., Crimmins E.M. Fine particulate matter air pollution and cognitive function among older US adults. Am J Epidemiol. 2014;180:359–366. doi: 10.1093/aje/kwu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weuve J., Puett R.C., Schwartz J., Yanosky J.D., Laden F., Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172:219–227. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin H., Guo Y., Zheng Y., Zhao X., Cao Z., Rigdon S.E. Exposure to ambient PM2.5 associated with overall and domain-specific disability among adults in six low- and middle-income countries. Environ Int. 2017;104:69–75. doi: 10.1016/j.envint.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 85.Tonne C., Elbaz A., Beevers S., Singh-Manoux A. Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology. 2014;25:674–681. doi: 10.1097/EDE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ailshire J., Karraker A., Clarke P. Neighborhood psychosocial stressors, air pollution, and cognitive function among older U.S. adults. Soc Sci Med. 2017;172:56–63. doi: 10.1016/j.socscimed.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen J.C., Schwartz J. Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology. 2009;30:231–239. doi: 10.1016/j.neuro.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 88.Ranft U., Schikowski T., Sugiri D., Krutmann J., Krämer U. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009;109:1004–1011. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 89.Schikowski T., Vossoughi M., Vierkötter A., Schulte T., Teichert T., Sugir D. Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ Res. 2015;142:10–16. doi: 10.1016/j.envres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 90.Chen H., Kwong J.C., Copes R., Tu K., Villeneuve P.J., van Donkelaar A. Living near major roads and the incidence of dementia, Parkinson's disease, and multiple sclerosis: a population-based cohort study. Lancet. 2017;389:718–726. doi: 10.1016/S0140-6736(16)32399-6. [DOI] [PubMed] [Google Scholar]
- 91.Chen H., Kwong J.C., Copes R., Hystad P., van Donkelaar A., Tu K. Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int. 2017;108:271–277. doi: 10.1016/j.envint.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 92.Tzivian L., Dlugaj M., Winkler A., Weinmayr G., Hennig F., Fuks K.B. Long-term air pollution and traffic noise exposures and mild cognitive impairment in older adults: a cross-sectional analysis of the Heinz Nixdorf recall study. Environ Health Perspect. 2016;124:1361–1368. doi: 10.1289/ehp.1509824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kioumourtzoglou M.A., Schwartz J.D., Weisskopf M.G., Melly S.J., Wang Y., Dominici F. Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environ Health Perspect. 2016;124:23–29. doi: 10.1289/ehp.1408973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Y.C., Lin Y.C., Yu H.L., Chen J.H., Chen T.F., Sun Y. Association between air pollutants and dementia risk in the elderly. Alzheimer's Dement Diagn Assess Dis Monit. 2015;1:220–228. doi: 10.1016/j.dadm.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Calderón-Garcidueñas L., Reed W., Maronpot R.R., Henriquez-Roldán C., Delgado-Chavez R., Calderón-Garcidueñas A. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32:650–658. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- 96.Calderón-Garcidueñas L., Solt A.C., Henríquez-Roldán C., Torres-Jardón R., Nuse B., Herritt L. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β-42 and α-synuclein in children and young adults. Toxicol Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 97.Calderón-Garcidueñas L., Macías-Parra M., Hoffmann H.J., Valencia-Salazar G., Henríquez-Roldán C., Osnaya N. Immunotoxicity and environment: immunodysregulation and systemic inflammation in children. Toxicol Pathol. 2009;37:161–169. doi: 10.1177/0192623308329340. [DOI] [PubMed] [Google Scholar]
- 98.Calderón-Garcidueñas L., Franco-Lira M., Henríquez-Roldán C., Osnaya N., González-Maciel A., Reynoso-Robles R. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp Toxicol Pathol. 2010;62:91–102. doi: 10.1016/j.etp.2009.02.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calderón-Garcidueñas L., Kavanaugh M., Block M.L., D'Angiulli A., Delgado-Chávez R., Torres-Jardón R. Neuroinflammation, hyperphosphorilated tau, diffuse amyloid plaques and down-regulation of the cellular prion protein in air pollution exposed children and adults. J Alzheimer Dis. 2012;28:93–107. doi: 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- 100.Calderón-Garcidueñas L., Mora-Tiscareño A., Styner M., Gómez-Garza G., Zhu H., Torres-Jardón R. White matter hyperintensities, systemic inflammation, brain growth, and cognitive functions in children exposed to air pollution. J Alzheimer Dis. 2012;31:183–191. doi: 10.3233/JAD-2012-120610. [DOI] [PubMed] [Google Scholar]
- 101.Pujol J., Martínez-Vilavella G., Macià D., Fenoll R., Alvarez-Pedrerol M., Rivas R. Traffic pollution exposure is associated with altered brain connectivity in school children. NeuroImage. 2016;129:175–184. doi: 10.1016/j.neuroimage.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 102.Calderón-Garcidueñas L., Azzarelli B., Acuna H., Garcia R., Gambling T., Osnaya N. Air pollution and brain damage. Toxicol Pathol. 2002;30:373–389. doi: 10.1080/01926230252929954. [DOI] [PubMed] [Google Scholar]
- 103.Calderon-Garciduenas L., Maronpot R.R., Torres-Jardon R., Henriquez-Roldan C., Schoonhoven R., Acuna-Ayala H. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol. 2003;31:524–538. doi: 10.1080/01926230390226645. [DOI] [PubMed] [Google Scholar]
- 104.Campbell A., Oldham M., Becaria A., Bondy S.C., Meacher D., Sioutas C. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26:133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 105.Heppner F.L., Ransohoff R.M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 106.Dzamba D., Harantova L., Butenko O., Anderova M. Glial cells – the key elements of Alzheimer's disease. Curr Alzheimer Res. 2016;13:894–911. doi: 10.2174/1567205013666160129095924. [DOI] [PubMed] [Google Scholar]
- 107.Lopategui Cabezas I., Herrera Batista A., Pentón Rol G. The role of glial cells in Alzheimer disease: potential therapeutic implications. Neurologia. 2014;29:305–309. doi: 10.1016/j.nrl.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 108.Durga M., Devasena T., Rajasekar A. Determination of LC50 and sub-chronic neurotoxicity of diesel exhaust nanoparticles. Environ Toxicol Pharmacol. 2015;40:615–625. doi: 10.1016/j.etap.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 109.Gerlofs-Nijland M.E., Totlandsdal A.I., Kilinç E., Boere A J.F., Fokkens P.H.B., Leseman DL A.C. Pulmonary and cardiovascular effects of traffic-related particulate matter: 4-week exposure of rats to roadside and diesel engine exhaust particles. Inhal Toxicol. 2010;22:1162–1173. doi: 10.3109/08958378.2010.531062. [DOI] [PubMed] [Google Scholar]
- 110.Levesque S., Surace M.J., McDonald J., Block M.L. Air pollution & the brain: subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation. 2011;8:105. doi: 10.1186/1742-2094-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim S.H., Knight E.M., Saunders E.L., Cuevas A.K., Popovech M., Chen L.C. Rapid doubling of Alzheimer's amyloid-β40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution. F1000 Res. 2012;1:70. doi: 10.12688/f1000research.1-70.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Win-Shwe T.T., Yamamoto S., Fujitani Y., Hirano S., Fujimaki H. Spatial learning and memory function-related gene expression in the hippocampus of mouse exposed to nanoparticle-rich diesel exhaust. Neurotoxicology. 2008;29:940–947. doi: 10.1016/j.neuro.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 113.Zanchi A.C.T., Fagundes L.S., Barbosa F., Bernardi R., Rhoden C.R., Saldiva P.H.N. Pre and post-natal exposure to ambient level of air pollution impairs memory of rats: the role of oxidative stress. Inhal Toxicol. 2010;22:910–918. doi: 10.3109/08958378.2010.494313. [DOI] [PubMed] [Google Scholar]
- 114.Allen J.L., Conrad K., Oberdörster G., Johnston C.J., Sleezer B., Cory-Slechta D.A. Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environ Health Perspect. 2013;121:32–38. doi: 10.1289/ehp.1205505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Allen J.L., Liu X., Pelkowski S., Palmer B., Conrad K., Oberdörster G. Early postnatal exposure to ultrafine particulate matter air pollution: persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environ Health Perspect. 2014;122:939–945. doi: 10.1289/ehp.1307984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Allen J.L., Liu X., Weston D., Prince L., Oberdörster G., Finkelstein J.N. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol Sci. 2014;140:160–178. doi: 10.1093/toxsci/kfu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barger S.W., Hörster D., Furukawa K., Goodman Y., Kreiglstein J., Mattson M.P. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. PNAS. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Griffin W.S.T., Sheng J.G., Royston M.C., Gentleman S.M., McKenzie J.E., Graham D.I. Glial-neuronal interactions in Alzheimer's disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nagele R.G., Wegiel J., Venkataraman V., Imaki H., Wang K.C., Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 120.Sama P., Long T.C., Hester S., Tajuba J., Parker J., Chen L.C. The cellular and genomic response of an immortalized microglia cell line (BV2) to concentrated ambient particulate matter. Inhal Toxicol. 2007;19:1079–1087. doi: 10.1080/08958370701628721. [DOI] [PubMed] [Google Scholar]
- 121.Davis D.A., Akopian G., Walsh J.P., Sioutas C., Morgan T.E., Finch C.E. Urban air pollutants reduce synaptic function of CA1 neurons via an NMDA/NO· pathway in vitro. J Neurochem. 2013;127:509–519. doi: 10.1111/jnc.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neumann H., Schweigreiter R., Yamashita T., Rosenkranz K., Wekerle H., Barde Y.A. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dep. J Neurosci. 2002;22:854–862. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hartz A.M.S., Bauer B., Block M.L., Hong J.S., Miller D.S. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. Faseb J. 2008;22:2723–2733. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bruckmann S., Brenn A., Grube M., Niedrig K., Holtfreter S., von Bohlen und Halbach O. Lack of P-glycoprotein results in impairment of removal of beta-amyloid and increased intraparenchymal cerebral amyloid angiopathy after active immunization in a transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res. 2017;14:656–667. doi: 10.2174/1567205013666161201201227. [DOI] [PubMed] [Google Scholar]
- 125.Mohamed L.A., Keller J.N., Kaddoumi A. Role of P-glycoprotein in mediating rivastigmine effect on amyloid-β brain load and related pathology in Alzheimer's disease mouse model. Biochim Biophys Acta. 2016;1862:778–787. doi: 10.1016/j.bbadis.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gillespie P., Tajuba J., Lippman M., Chen L.C., Veronesi B. Particulate matter toxicity in culture is size dependent. Neurotoxicology. 2013;36:112–117. doi: 10.1016/j.neuro.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Iadecola C., Yaffe K., Biller J., Bratzke L., Faraci F., Gorelick P. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension (Dallas, Tex : 1979) 2016;68:e67–e94. doi: 10.1161/HYP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oberdörster G., Sharp Z., Atudorei V., Elder A., Gelein R., Kreyling W. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.