Abstract
Pigment epithelium-derived factor (PEDF) is a secreted glycoprotein that has anti-angiogenic, anti-proliferative, neurotrophic and immunomodulatory properties. PEDF has recently emerged as a critical metabolic regulatory protein since the discovery of its modulatory activities in the lipolytic pathway by binding to adipose triglyceride lipase (ATGL). Despite being beneficial in maintaining the homeostasis of hepatic lipid accumulation, PEDF has been uncovered an unfavorable role associated with insulin resistance. The molecular events that connect these two apparent distinct observations have been controversial and remained largely unknown. Therefore in this short review, we attempt to summarize the current findings of PEDF regarding its lipid metabolic functions and provide perspectives in identifying PEDF as a potential therapeutic target in lipid disorders.
Keywords: Pigment epithelium-derived factor, Adipose triglyceride lipase, Liver, Adipose tissue, Insulin resistance
Pigment epithelium-derived factor (PEDF), encoded by the serpinf1 gene, is a secreted glycoprotein that belongs to the serine protease inhibitor superfamily although it does not have any anti-proteolytic activity [1]. Research on PEDF began around early 1990s when PEDF was first identified in the conditioned media of human fetal retinal pigment epithelial cells (hence its name) as a neurotrophic factor for retinoblastoma cells [2], [3]. Nearly a decade later, an important discovery showing PEDF as a potent inhibitor of angiogenesis (guarding ocular function) sparked the area of research of its anti-tumor properties [4]. Numerous models have been established to link decreased PEDF expression to increased tumor-associated vasculature. In fact, PEDF is also growth inhibitory as deficiency of PEDF causes epithelial hyperplasia in several organs [4], [5]. Poorly differentiated tumors are often characterized by loss of PEDF [6], [7]. Recently, PEDF has received much attention for its metabolic regulatory activity. This short review will specifically summarize the proposed mechanisms by which PEDF modulates lipid metabolism.
PEDF biochemistry
The PEDF gene, serpinf1, is located on chromosome 17p13 in humans, encoding an approximately 50 kDa protein with 418 amino acids in length (including a 20-amino acid signal peptide) [8]. The protease-sensitive serpin signature sequence is located near the C-terminus but however lacks the conformational change when cleaved as is observed in a typical inhibitory serpin [1], [9]. PEDF is expressed in most tissues examined, with more prominent levels in the liver, testis, uterus, adipose tissue and skeletal muscle. As a secreted soluble protein, PEDF can also be detected in body fluids such as blood, tears, cerebrospinal fluids and aqueous/vitreous humour [10], [11], [12], [13], [14].
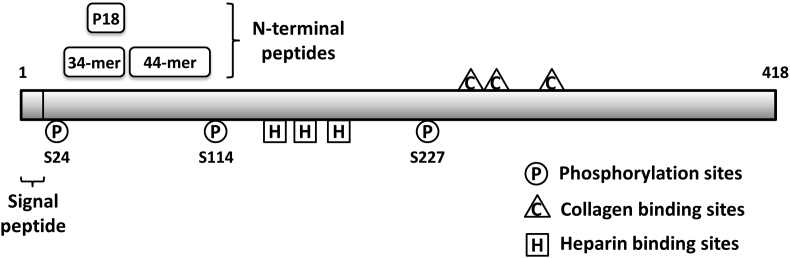
PEDF is known as a multifunctional protein. The molecular mechanisms by which PEDF exerts its diverse biological activities remain largely unidentified and are thought to be based on the interactions with different cell surface receptors that trigger distinct signaling pathways. A number of putative PEDF binding partners have been characterized so far, including membrane-bound phospholipase adipose triglyceride lipase (ATGL), laminin receptor, a cell-surface F1-ATP synthase, Wnt co-receptor LRP6, and more recently characterized PLXDC1/PLXDC2 receptors [15], [16], [17], [18], [19]. PEDF has also been shown to bind extracellular matrix (ECM) components such as heparin/heparan sulfate proteoglycans, collagens and hyaluronan [20], [21], [22]. The amino acids involved in these interactions have been mapped on human PEDF [Fig. 1]. These binding properties may contribute to retainment of PEDF in the ECM to facilitate its anti-tumor/antiangiogenic effects. Furthermore, studies have also revealed two functional epitopes: a 34-mer peptide (residues 44–77), which confers anti-angiogenic and apoptotic properties, and a 44-mer peptide (residues 78–121), which exhibits neurotrophic activity [23], [24]. An even shorter peptide derived from the 34-mer designated as P18 (residues 60–77) has been proved to be more effective in blocking angiogenesis and tumor xenograft growth [Fig. 1] [25]. PEDF can be phosphorylated at specific serines by casein kinase 2 and protein kinase A [Fig. 1] [26]. Differential phosphorylation at these sites acts as a molecular switch to regulate the biological activity. Phosphomimetic mutants of PEDF have been shown to contain enhanced anti-angiogenic potency as an anti-tumor agent [27].
Fig. 1.
Schematic representation displaying functional peptides and key amino acid residues of PEDF. The anti-angiogenic 34-mer, P18, and the neurotrophic 44-mer are marked. Phosphorylation sites, collagen and heparin binding sites are also indicated.
PEDF in hepatic lipid metabolism
As described earlier, liver is one of the highest PEDF producing organs. Despite its abundance, the functional role of PEDF has not been fully resolved. Being a powerful anti-angiogenic agent, PEDF has been shown to be crucial in the development and maintenance of hepatic vascular architecture [28]. In that regard, PEDF can have immense therapeutic implications for treatment of hepatocellular carcinoma (HCC), a typical hypervascular tumor. Indeed, a number of preclinical cancer models have provided evidence that PEDF administration by various means can inhibit tumor vasculature or metastasis from other organs [29], [30], [31]. However, research on a direct anti-tumor effect on HCC gives more divergent results, which depend a lot upon cell models used and receptor compositions [32], [33].
The metabolic role of PEDF was first established in knockout animals in which PEDF deficient mice demonstrated liver steatosis, with an accompanying increase in body mass and visceral fat deposition [34]. PEDF null hepatocytes had pronounced accumulation of triglyceride compared to age-matched wild-type controls; this increase could be rescued by treatment with recombinant PEDF. Reduced PEDF levels and elevated hepatic triglyceride content have also been associated in an animal model and clinical cases of ethanol-induced steatosis. Ethanol exposure creates a hypoxic environment and induces activity of metalloproteinases-2 and -9, which in turn deplete PEDF via proteolytic degradation [35]. Conversely, overexpression of PEDF via adenoviral delivery has been shown to ameliorate hepatic lipid accumulation in a non-alcoholic fatty liver disease model, at least in part, by reduction of oxidative stress [36].
The mechanisms by which PEDF deficiency leads to lipid accumulation is thought to be due to decreased activity of ATGL. ATGL is the enzyme that specifically removes the first fatty acid in the step-wise triglyceride hydrolysis. PEDF has been shown to bind avidly to ATGL [18] and co-localize at the surface of adiposomes in hepatocytes [34]. Interestingly, ATGL-null mice also exhibit enlarged fat deposit and triglyceride accumulation in the liver and multiple other tissues, similar to what is observed in PEDF knockouts [37]. Many of the established functions of PEDF have been shown to be through ATGL, suggesting dependency of ATGL activity on PEDF levels [38], [39]. Downstream action of PEDF in hepatic lipid metabolism has yet to be determined. One study has revealed an interaction between PEDF and PPARα in vitro, thereby regulating PPARα transactivation [40]. As PEDF is a secreted glycoprotein, whether this interaction occurs in a more physiologically relevant condition needs further validation. However, PEDF indeed has a nuclear localization motif and its nuclear presence have been documented [41], [42]. Further investigation may be facilitated to elucidate the relationship between PEDF localization and biological function.
PEDF in the adipose tissue
PEDF is also highly expressed by adipose tissue. In fact, PEDF is one of the most abundant proteins secreted by human primary adipocytes, which may be regarded as a major source for the circulating levels [43]. The expression pattern of PEDF during adipogenic differentiation remains a controversy. While a number of studies have shown a decrease in PEDF mRNA and protein during differentiation in 3T3-L1 pre-adipocytes [44], [45] and adipose-derived stem cells [46], others have shown a differentiation dependent increase [43]. This discrepancy has not been resolved except for the knowledge that these assays were conducted in cells from different species. Despite these differences in findings, studies have revealed that exposure of adipose tissue to PEDF stimulates lipolysis, in accordance with its role in triglyceride catabolism in the liver [47], [48]. In addition, recombinant and adenoviral PEDF attenuate adipogenic differentiation of 3T3-L1 pre-adipocytes, as evidenced by down-regulation of adipocyte markers and decreased lipid accumulation. PEDF also promotes osteoblast differentiation of mesenchymal stem cells [46]. Interestingly, the inhibition by PEDF is only effective at early stages of differentiation, allowing suppression of early ERK1/2 and subsequent C/EBP-β activation [45]. These results may explain why as studies have reported PEDF elevation in metabolic disorders [49], no apparent physiological effect on adipogenesis and body fat content can be observed. A recent report has demonstrated that adipose tissue-specific overexpression of PEDF only enhances adipocyte lipolysis but has no effect on local vascularization and systemic adiposity [50]. Additional functions of PEDF in adipose tissue need to be further elucidated.
PEDF and insulin resistance
Based on what is described above, we know that PEDF is a potentially beneficial metabolic regulatory protein and may be an appropriate candidate for drug development. However, the implications of PEDF in insulin resistance make therapeutic applications less feasible and require further characterization. The role of PEDF in insulin resistance and metabolic disorders is not well understood and remains largely controversial. Circulating levels of PEDF have been found to be elevated in various metabolic disorders such as obesity, type 2 diabetes [51], polycystic ovarian syndrome [52] and metabolic syndrome [49], [53]. In contrast, PEDF levels decrease significantly after weight loss [54]. Some researchers conclude that the elevation of PEDF may act as a counter measure against obesity-related metabolic derangement while some suggest otherwise. Whether PEDF is the cause or effect of impaired metabolism remains debated.
In an HCC cell line Hep3B, PEDF has been shown to improve insulin resistance in cells exposed to advanced glycation end products (AGEs), reactive derivatives associated with several vascular and neurological complications in diabetic patients. PEDF blocks AGE-induced activation of Rac-1 GTPase and subsequent phosphorylation of insulin receptor substrate-1 (IRS-1) at serine-307, JNK, c-Jun and IκB kinase. JNK is an inhibitory serine/threonine kinase that phosphorylates IRS-1, which in turn interferes with the interaction between IRS-1 and insulin receptor, thus preventing tyrosine phosphorylation of IRS-1. PEDF also inhibits AGE-dependent decrease in IRS-1 tyrosine phosphorylation, thereby increasing the association of p85 subunit of phosphatidylinositol 3-kinase (PI3K) with IRS-1, causing glycogen synthesis in the presence of insulin [55]. Moreover, PEDF administration ameliorates AGE-induced platelet activation and shortened tail vein bleeding time in diabetic rats and protects against early phase of experimental diabetic retinopathy. These effects may result from an outcome of anti-oxidative properties through suppression of NADPH oxidase-driven superoxide generation [56], [57]. PEDF null mice recapitulate features of metabolic syndrome including increased adiposity, liver steatosis, impaired glucose tolerance, and increased circulating pro-inflammatory metabolites. PEDF deficient hepatocytes treated with recombinant PEDF protein can suppress IL-1β-mediated stress mediators such as JNK and p38 by normalizing IRS-1 and Akt signaling [58]. These findings identify the role of PEDF in maintaining homeostasis in metabolic syndrome.
In contrast with the effect on hepatocytes and diabetic retinopathy, the link between PEDF and insulin resistance has been established in an extensive series of cellular and animal studies. In human and mouse adipocytes and human skeletal muscle cells, PEDF causes insulin resistance [43]. Prolonged PEDF exposure in lean mice reduces insulin sensitivity. This decrease is accompanied by activation of pro-inflammatory kinases JNK and ERK1/2 in the muscle and adipose tissue, which in turn attenuates insulin signaling. Insulin sensitivity is enhanced after administration of a PEDF neutralizing antibody to obese mice over 5 days while skeletal muscle and liver triglyceride content is decreased [47]. These results suggest a causal role of PEDF in obesity-induced insulin resistance. To pharmacologically alleviate insulin resistance that involves PEDF, Yang and coworkers have found that rosiglitazone, a thiazolidinedione (TZD) family of diabetes medication often used as an insulin sensitizer, inhibits PEDF expression and secretion, with an improvement of insulin resistance [59]. Metformin, another common diabetes medication, also has a lowering effect on serum PEDF levels and ameliorates insulin resistance via increased AMP activated protein kinase (AMPK) activation [60]. However, clinical studies using metformin suggest more divergent outcomes [61].
The underlying mechanisms behind these apparently distinct observations are not clear. One possible explanation is that these observations may have been made under different cellular contexts. As described in the previous section, ATGL, the putative PEDF receptor, is a triglyceride lipase that is critical in lipid homeostasis. ATGL null mice exhibit several phenotypes that are very similar to PEDF knockouts. However, a recent study using ATGL null mice has shown that many of the metabolic actions exerted by PEDF administration, including increased adipose tissue lipolysis, suppression of skeletal muscle fatty acid oxidation and insulin resistance, are diminished [38]. Hence the adverse effects of PEDF on insulin actions are dependent upon the presence of ATGL. Moreover, PEDF itself can attenuate ATGL protein accumulation via proteasomal degradation in adipocytes, adding another level of regulation [48]. Therefore, dysregulation of the PEDF-ATGL interaction may result in insulin resistance, as seen in type 2 diabetes and other conditions characterized by elevated circulating PEDF levels. An anti-inflammatory or anti-oxidative effect of PEDF described in hepatocytes and other cell types may reflect relatively low abundance of ATGL (although present) in these tissues compared with the adipocytes. PEDF may also transmit these signals via different binding partners other than ATGL and this requires further studies to confirm.
Concluding remarks
PEDF is a multifunctional protein that is produced by various tissues and released in soluble form in the circulation. Through interactions with multiple binding receptors, PEDF can initiate a wide range of cellular responses that regulate angiogenesis, proliferation, differentiation, energy metabolism, inflammation and oxidative stress. It has also been implicated in a variety of chronic diseases, including cancer, metabolic syndrome and vascular diseases.
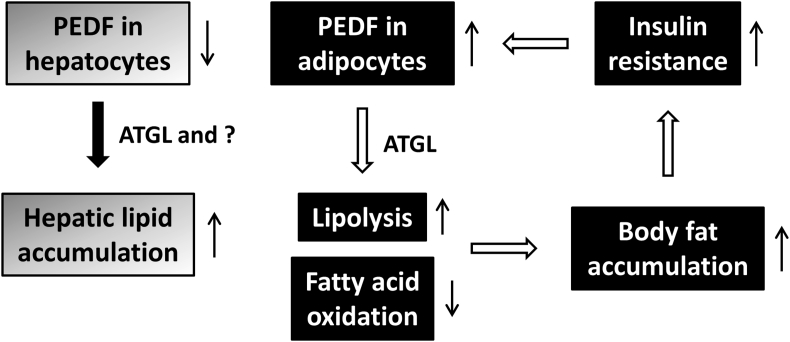
In terms of therapeutic potential, the majority of the preclinical models demonstrate a beneficial outcome such as amelioration of ocular neovascular disease [62] and inhibition of tumor progression. However, the role of PEDF in glucose and lipid metabolism remains largely uncertain as clinical studies reveal positive correlations between serum PEDF levels and several major metabolic disorders. While PEDF shows anti-inflammatory and anti-oxidative properties in various systems, increased PEDF, via ATGL, promotes adipocyte lipolysis and decreases the capacities of skeletal muscle to oxidize fatty acids, leading to ectopic accumulation of excessive fatty acids and their metabolites and eventually insulin resistance. As prolonged insulin exposure promotes PEDF production by adipocytes [43], a feed-forward vicious circle can be initiated and ultimately contribute to development of metabolic disorders such as type 2 diabetes [Fig. 2]. Therefore in order to make PEDF a successful drug without unwanted secondary effects, generation of small active peptides that confer distinct functions may be a way to go. The crystal structure of PEDF provides information regarding important amino acid residues or epitopes responsible for receptor binding and the diverse biological activities that have been identified [9]. Including the 34-mer, 44-mer and P18 described in the previous section, more PEDF peptide mimetics have been produced to test their efficacy for treatment of different clinical conditions. Optimization of these active peptides/fragments to maintain strong biological activity and bioavailability needs immediate efforts. Studies on PEDF binding partners will further extend our knowledge of the underlying mechanisms that can lead to better treatment strategies.
Fig. 2.
Distinct functions of PEDF in lipid metabolic disorders. On one hand, PEDF exerts its anti-inflammatory and anti-oxidative properties in maintaining the homeostasis of hepatic lipid accumulation. On the other hand, increased PEDF in adipose tissue has the potential to initiate a cascade of events that may eventually lead to insulin resistance.
Conflicts of interest
The authors have no conflict of interest to declare regarding this manuscript.
Acknowledgements
This work was supported by grants from Chang Gung Memorial Hospital (CMRPG8C1151, CMRPG8D1032 and CMRPG8D1033 to KTH; CMRPG8A1203 and CMRPG8E1651 to CCL; CMRPG8F0661 and CMRPG8F0662 to MCT; CMRPG8D0561, CMRPG8D0751, CMRPG8D1022 and CMRPG8D1023 to KDC; CMRPG8D1001 and CMRPG8F0621 to KWC).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Becerra S.P., Sagasti A., Spinella P., Notario V. Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J Biol Chem. 1995;270:25992–25999. doi: 10.1074/jbc.270.43.25992. [DOI] [PubMed] [Google Scholar]
- 2.Steele F.R., Chader G.J., Johnson L.V., Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A. 1993;90:1526–1530. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tombran-Tink J., Chader G.G., Johnson L.V. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53:411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 4.Dawson D.W., Volpert O.V., Gillis P., Crawford S.E., Xu H., Benedict W. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 5.Daubriac J., Pandya U.M., Huang K.T., Pavlides S.C., Gama P., Blank S.V. Hormonal and growth regulation of epithelial and stromal cells from the normal and malignant endometrium by pigment epithelium-derived factor. Endocrinology. 2017;158:2754–2773. doi: 10.1210/en.2017-00028. [DOI] [PubMed] [Google Scholar]
- 6.Grippo P.J., Fitchev P.S., Bentrem D.J., Melstrom L.G., Dangi-Garimella S., Krantz S.B. Concurrent PEDF deficiency and Kras mutation induce invasive pancreatic cancer and adipose-rich stroma in mice. Gut. 2012;61:1454–1464. doi: 10.1136/gutjnl-2011-300821. [DOI] [PubMed] [Google Scholar]
- 7.Orgaz J.L., Ladhani O., Hoek K.S., Fernandez-Barral A., Mihic D., Aguilera O. Loss of pigment epithelium-derived factor enables migration, invasion and metastatic spread of human melanoma. Oncogene. 2009;28:4147–4161. doi: 10.1038/onc.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tombran-Tink J., Pawar H., Swaroop A., Rodriguez I., Chader G.J. Localization of the gene for pigment epithelium-derived factor (PEDF) to chromosome 17p13.1 and expression in cultured human retinoblastoma cells. Genomics. 1994;19:266–272. doi: 10.1006/geno.1994.1057. [DOI] [PubMed] [Google Scholar]
- 9.Simonovic M., Gettins P.G., Volz K. Crystal structure of human PEDF, a potent anti-angiogenic and neurite growth-promoting factor. Proc Natl Acad Sci U S A. 2001;98:11131–11135. doi: 10.1073/pnas.211268598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdiu O., Van Setten G. Antiangiogenic activity in tears: presence of pigment-epithelium-derived factor. New insights and preliminary results. Ophthalmic Res. 2008;40:16–18. doi: 10.1159/000111153. [DOI] [PubMed] [Google Scholar]
- 11.Kuncl R.W., Bilak M.M., Bilak S.R., Corse A.M., Royal W., Becerra S.P. Pigment epithelium-derived factor is elevated in CSF of patients with amyotrophic lateral sclerosis. J Neurochem. 2002;81:178–184. doi: 10.1046/j.1471-4159.2002.00813.x. [DOI] [PubMed] [Google Scholar]
- 12.Ortego J., Escribano J., Becerra S.P., Coca-Prados M. Gene expression of the neurotrophic pigment epithelium-derived factor in the human ciliary epithelium. Synthesis and secretion into the aqueous humor. Investig Ophthalmol Vis Sci. 1996;37:2759–2767. [PubMed] [Google Scholar]
- 13.Petersen S.V., Valnickova Z., Enghild J.J. Pigment-epithelium-derived factor (PEDF) occurs at a physiologically relevant concentration in human blood: purification and characterization. Biochem J. 2003;374:199–206. doi: 10.1042/BJ20030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y.Q., Becerra S.P. Proteolytic activity directed toward pigment epithelium-derived factor in vitreous of bovine eyes. Implications of proteolytic processing. Investig Ophthalmol Vis Sci. 1996;37:1984–1993. [PubMed] [Google Scholar]
- 15.Bernard A., Gao-Li J., Franco C.A., Bouceba T., Huet A., Li Z. Laminin receptor involvement in the anti-angiogenic activity of pigment epithelium-derived factor. J Biol Chem. 2009;284:10480–10490. doi: 10.1074/jbc.M809259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng G., Zhong M., Kawaguchi R., Kassai M., Al-Ubaidi M., Deng J. Identification of PLXDC1 and PLXDC2 as the transmembrane receptors for the multifunctional factor PEDF. eLife. 2014;3:e05401. doi: 10.7554/eLife.05401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Notari L., Arakaki N., Mueller D., Meier S., Amaral J., Becerra S.P. Pigment epithelium-derived factor binds to cell-surface F(1)-ATP synthase. FEBS J. 2010;277:2192–2205. doi: 10.1111/j.1742-4658.2010.07641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notari L., Baladron V., Aroca-Aguilar J.D., Balko N., Heredia R., Meyer C. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- 19.Park K., Lee K., Zhang B., Zhou T., He X., Gao G. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol. 2011;31:3038–3051. doi: 10.1128/MCB.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberdi E., Hyde C.C., Becerra S.P. Pigment epithelium-derived factor (PEDF) binds to glycosaminoglycans: analysis of the binding site. Biochemistry. 1998;37:10643–10652. doi: 10.1021/bi9802317. [DOI] [PubMed] [Google Scholar]
- 21.Becerra S.P., Perez-Mediavilla L.A., Weldon J.E., Locatelli-Hoops S., Senanayake P., Notari L. Pigment epithelium-derived factor binds to hyaluronan. Mapping of a hyaluronan binding site. J Biol Chem. 2008;283:33310–33320. doi: 10.1074/jbc.M801287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer C., Notari L., Becerra S.P. Mapping the type I collagen-binding site on pigment epithelium-derived factor. Implications for its antiangiogenic activity. J Biol Chem. 2002;277:45400–45407. doi: 10.1074/jbc.M208339200. [DOI] [PubMed] [Google Scholar]
- 23.Bilak M.M., Becerra S.P., Vincent A.M., Moss B.H., Aymerich M.S., Kuncl R.W. Identification of the neuroprotective molecular region of pigment epithelium-derived factor and its binding sites on motor neurons. J Neurosci : the official journal of the Society for Neuroscience. 2002;22:9378–9386. doi: 10.1523/JNEUROSCI.22-21-09378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filleur S., Volz K., Nelius T., Mirochnik Y., Huang H., Zaichuk T.A. Two functional epitopes of pigment epithelial-derived factor block angiogenesis and induce differentiation in prostate cancer. Canc Res. 2005;65:5144–5152. doi: 10.1158/0008-5472.CAN-04-3744. [DOI] [PubMed] [Google Scholar]
- 25.Mirochnik Y., Aurora A., Schulze-Hoepfner F.T., Deabes A., Shifrin V., Beckmann R. Short pigment epithelial-derived factor-derived peptide inhibits angiogenesis and tumor growth. Clin Canc Res : an official journal of the American Association for Cancer Research. 2009;15:1655–1663. doi: 10.1158/1078-0432.CCR-08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maik-Rachline G., Shaltiel S., Seger R. Extracellular phosphorylation converts pigment epithelium-derived factor from a neurotrophic to an antiangiogenic factor. Blood. 2005;105:670–678. doi: 10.1182/blood-2004-04-1569. [DOI] [PubMed] [Google Scholar]
- 27.Konson A., Pradeep S., Seger R. Phosphomimetic mutants of pigment epithelium-derived factor with enhanced antiangiogenic activity as potent anticancer agents. Canc Res. 2010;70:6247–6257. doi: 10.1158/0008-5472.CAN-10-0434. [DOI] [PubMed] [Google Scholar]
- 28.Sawant S., Aparicio S., Tink A.R., Lara N., Barnstable C.J., Tombran-Tink J. Regulation of factors controlling angiogenesis in liver development: a role for PEDF in the formation and maintenance of normal vasculature. Biochem Biophys Res Commun. 2004;325:408–413. doi: 10.1016/j.bbrc.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 29.Gao Y., Yao A., Zhang W., Lu S., Yu Y., Deng L. Human mesenchymal stem cells overexpressing pigment epithelium-derived factor inhibit hepatocellular carcinoma in nude mice. Oncogene. 2010;29:2784–2794. doi: 10.1038/onc.2010.38. [DOI] [PubMed] [Google Scholar]
- 30.Lattier J.M., Yang H., Crawford S., Grossniklaus H.E. Host pigment epithelium-derived factor (PEDF) prevents progression of liver metastasis in a mouse model of uveal melanoma. Clin Exp Metastasis. 2013;30:969–976. doi: 10.1007/s10585-013-9596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto K., Ishikawa H., Nishimura D., Hamasaki K., Nakao K., Eguchi K. Antiangiogenic property of pigment epithelium-derived factor in hepatocellular carcinoma. Hepatology. 2004;40:252–259. doi: 10.1002/hep.20259. [DOI] [PubMed] [Google Scholar]
- 32.Hou J., Ge C., Cui M., Liu T., Liu X., Tian H. Pigment epithelium-derived factor promotes tumor metastasis through an interaction with laminin receptor in hepatocellular carcinomas. Cell Death Dis. 2017;8:e2969. doi: 10.1038/cddis.2017.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawaguchi T., Yamagishi S., Itou M., Okuda K., Sumie S., Kuromatsu R. Pigment epithelium-derived factor inhibits lysosomal degradation of Bcl-xL and apoptosis in HepG2 cells. Am J Pathol. 2010;176:168–176. doi: 10.2353/ajpath.2010.090242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung C., Doll J.A., Gattu A.K., Shugrue C., Cornwell M., Fitchev P. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL) J Hepatol. 2008;48:471–478. doi: 10.1016/j.jhep.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Chung C., Shugrue C., Nagar A., Doll J.A., Cornwell M., Gattu A. Ethanol exposure depletes hepatic pigment epithelium-derived factor, a novel lipid regulator. Gastroenterology. 2009;136 doi: 10.1053/j.gastro.2008.09.065. 331–40.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida T., Akiba J., Matsui T., Nakamura K., Hisamoto T., Abe M. Pigment epithelium-derived factor (PEDF) prevents hepatic fat storage, inflammation, and fibrosis in dietary steatohepatitis of mice. Dig Dis Sci. 2017;62:1527–1536. doi: 10.1007/s10620-017-4550-x. [DOI] [PubMed] [Google Scholar]
- 37.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 38.Borg M.L., Andrews Z.B., Duh E.J., Zechner R., Meikle P.J., Watt M.J. Pigment epithelium-derived factor regulates lipid metabolism via adipose triglyceride lipase. Diabetes. 2011;60:1458–1466. doi: 10.2337/db10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He T., Hu J., Yan G., Li L., Zhang D., Zhang Q. Pigment epithelium-derived factor regulates microvascular permeability through adipose triglyceride lipase in sepsis. Clin Sci (Lond) 2015;129:49–61. doi: 10.1042/CS20140631. [DOI] [PubMed] [Google Scholar]
- 40.Chung C., Doll J.A., Stellmach V.M., Gonzales J., Surapureddi S., Cornwell M. Pigment epithelium-derived factor is an angiogenesis and lipid regulator that activates peroxisome proliferator-activated receptor alpha. Adv Exp Med Biol. 2008;617:591–597. doi: 10.1007/978-0-387-69080-3_61. [DOI] [PubMed] [Google Scholar]
- 41.Anguissola S., McCormack W.J., Morrin M.A., Higgins W.J., Fox D.M., Worrall D.M. Pigment epithelium-derived factor (PEDF) interacts with transportin SR2, and active nuclear import is facilitated by a novel nuclear localization motif. PLoS One. 2011;6:e26234. doi: 10.1371/journal.pone.0026234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tombran-Tink J., Aparicio S., Xu X., Tink A.R., Lara N., Sawant S. PEDF and the serpins: phylogeny, sequence conservation, and functional domains. J Struct Biol. 2005;151:130–150. doi: 10.1016/j.jsb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Famulla S., Lamers D., Hartwig S., Passlack W., Horrighs A., Cramer A. Pigment epithelium-derived factor (PEDF) is one of the most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cells. Int J Obes (Lond) 2011;35:762–772. doi: 10.1038/ijo.2010.212. [DOI] [PubMed] [Google Scholar]
- 44.Kratchmarova I., Kalume D.E., Blagoev B., Scherer P.E., Podtelejnikov A.V., Molina H. A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics: MCP. 2002;1:213–222. doi: 10.1074/mcp.m200006-mcp200. [DOI] [PubMed] [Google Scholar]
- 45.Wang M., Wang J.J., Li J., Park K., Qian X., Ma J.X. Pigment epithelium-derived factor suppresses adipogenesis via inhibition of the MAPK/ERK pathway in 3T3-L1 preadipocytes. Am J Physiol Endocrinol Metabol. 2009;297:E1378–E1387. doi: 10.1152/ajpendo.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gattu A.K., Swenson E.S., Iwakiri Y., Samuel V.T., Troiano N., Berry R. Determination of mesenchymal stem cell fate by pigment epithelium-derived factor (PEDF) results in increased adiposity and reduced bone mineral content. FASEB J: official publication of the Federation of American Societies for Experimental Biology. 2013;27:4384–4394. doi: 10.1096/fj.13-232900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crowe S., Wu L.E., Economou C., Turpin S.M., Matzaris M., Hoehn K.L. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metabol. 2009;10:40–47. doi: 10.1016/j.cmet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Dai Z., Qi W., Li C., Lu J., Mao Y., Yao Y. Dual regulation of adipose triglyceride lipase by pigment epithelium-derived factor: a novel mechanistic insight into progressive obesity. Mol Cell Endocrinol. 2013;377:123–134. doi: 10.1016/j.mce.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Yamagishi S., Adachi H., Abe A., Yashiro T., Enomoto M., Furuki K. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metabol. 2006;91:2447–2450. doi: 10.1210/jc.2005-2654. [DOI] [PubMed] [Google Scholar]
- 50.Lakeland T.V., Borg M.L., Matzaris M., Abdelkader A., Evans R.G., Watt M.J. Augmented expression and secretion of adipose-derived pigment epithelium-derived factor does not alter local angiogenesis or contribute to the development of systemic metabolic derangements. Am J Physiol Endocrinol Metabol. 2014;306:E1367–E1377. doi: 10.1152/ajpendo.00046.2014. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura K., Yamagishi S., Adachi H., Kurita-Nakamura Y., Matsui T., Inoue H. Serum levels of pigment epithelium-derived factor (PEDF) are positively associated with visceral adiposity in Japanese patients with type 2 diabetes. Diabetes Metab Res Rev. 2009;25:52–56. doi: 10.1002/dmrr.820. [DOI] [PubMed] [Google Scholar]
- 52.Yang S., Li Q., Zhong L., Song Y., Tian B., Cheng Q. Serum pigment epithelium-derived factor is elevated in women with polycystic ovary syndrome and correlates with insulin resistance. J Clin Endocrinol Metab. 2011;96:831–836. doi: 10.1210/jc.2010-2140. [DOI] [PubMed] [Google Scholar]
- 53.Chen C., Tso A.W., Law L.S., Cheung B.M., Ong K.L., Wat N.M. Plasma level of pigment epithelium-derived factor is independently associated with the development of the metabolic syndrome in Chinese men: a 10-year prospective study. J Clin Endocrinol Metab. 2010;95:5074–5081. doi: 10.1210/jc.2010-0727. [DOI] [PubMed] [Google Scholar]
- 54.Sabater M., Moreno-Navarrete J.M., Ortega F.J., Pardo G., Salvador J., Ricart W. Circulating pigment epithelium-derived factor levels are associated with insulin resistance and decrease after weight loss. J Clin Endocrinol Metab. 2010;95:4720–4728. doi: 10.1210/jc.2010-0630. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida T., Yamagishi S., Nakamura K., Matsui T., Imaizumi T., Takeuchi M. Pigment epithelium-derived factor (PEDF) ameliorates advanced glycation end product (AGE)-induced hepatic insulin resistance in vitro by suppressing Rac-1 activation. Horm Metab Res = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2008;40:620–625. doi: 10.1055/s-0028-1083785. [DOI] [PubMed] [Google Scholar]
- 56.Yamagishi S., Matsui T., Takenaka K., Nakamura K., Takeuchi M., Inoue H. Pigment epithelium-derived factor (PEDF) prevents platelet activation and aggregation in diabetic rats by blocking deleterious effects of advanced glycation end products (AGEs) Diabetes Metab Res Rev. 2009;25:266–271. doi: 10.1002/dmrr.906. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida Y., Yamagishi S., Matsui T., Jinnouchi Y., Fukami K., Imaizumi T. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes Metab Res Rev. 2009;25:678–686. doi: 10.1002/dmrr.1007. [DOI] [PubMed] [Google Scholar]
- 58.Gattu A.K., Birkenfeld A.L., Iwakiri Y., Jay S., Saltzman M., Doll J. Pigment epithelium-derived factor (PEDF) suppresses IL-1beta-mediated c-Jun N-terminal kinase (JNK) activation to improve hepatocyte insulin signaling. Endocrinology. 2014;155:1373–1385. doi: 10.1210/en.2013-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang S., Luo T., Zhou H., Lv Q., Liu L., Zhang W. Rosiglitazone inhibits expression and secretion of PEDF in adipose tissue and liver of male SD rats via a PPAR-gamma independent mechanism. Endocrinology. 2014;155:941–950. doi: 10.1210/en.2013-1813. [DOI] [PubMed] [Google Scholar]
- 60.Yang S., Lv Q., Luo T., Liu L., Gao R., Chen S. Metformin inhibits expression and secretion of PEDF in adipocyte and hepatocyte via promoting AMPK phosphorylation. Mediat Inflamm. 2013;2013:429207. doi: 10.1155/2013/429207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akin S., Aksoy D.Y., Cinar N., Aydin K., Karaagaoglu E., Ariyurek M. Pigment epithelium-derived factor increases in type 2 diabetes after treatment with metformin. Clin Endocrinol. 2012;77:852–856. doi: 10.1111/j.1365-2265.2012.04341.x. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y., Leo L.F., McGregor C., Grivitishvili A., Barnstable C.J., Tombran-Tink J. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol Med. 2012;18:1387–1401. doi: 10.2119/molmed.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]