Abstract
Metabolic syndrome (MetS) is a highly prevalent complex trait despite recent advances in pathophysiology and pharmacological treatment. MetS can begin in early life by so-called the developmental origins of health and disease (DOHaD). The DOHaD concept offers a novel approach to prevent MetS through reprogramming. High fructose (HF) intake has been associated with increased risk of MetS. HF diet becomes one of the most commonly used animal model to induce MetS. This review discusses the maternal HF diet induced programming process and reprogramming strategy to prevent MetS of developmental origin, with an emphasis on: (1) an overview of metabolic effects of fructose consumption on MetS; (2) insight from maternal HF animal models on MetS-related phenotypes; (3) impact of HF consumption induces organ-specific transcriptome changes; and (4) application of reprogramming strategy to prevent maternal HF consumption-induced MetS. Research into the preventions and treatments of MetS that begin early in life will have a lifelong impact and profound savings in disease burden and financial costs.
Keywords: Developmental origins of health and disease (DOHaD), Fructose, Hypertension, Metabolic syndrome, Obesity
The worldwide per capita fructose consumption has grown in the last half-century and its growth has been paralleled by an increase in metabolic syndrome (MetS)-related disorders [1], [2]. MetS is a common complex trait comprising of a cluster of medical conditions including hypertension, obesity, dyslipidemia, hyperglycemia, fatty liver, and insulin resistance [3]. Emerging evidence suggests that the origins of susceptibility for MetS in adult can be traced back to the early life, referred to the developmental origins of health and disease (DOHaD) [4]. On the other hand, the DOHaD concept offers a novel approach to prevent MetS through reprogramming, to shift therapeutic interventions from adulthood to early life, even before clinical symptoms are evident [5]. A number of dietary, genetic, surgical, and pharmacological models have been developed to explore the pathophysiology and underlying mechanisms of developmental programming of MetS [6], [7]. As rodents fed with a fructose-enriched diet exhibit many features of the MetS, high-fructose (HF) diet becomes one of the most commonly used animal model to induce MetS.
This review provides an overview of maternal HF consumption-induced programming process contributing to MetS-related phenotypes, with an emphasis on the following areas: metabolic effects of fructose on MetS; effects of maternal HF consumption on developmental programming of MetS-related phenotypes; maternal HF consumption induces transcriptome changes; and application of reprogramming interventions to prevent maternal HF-induced MetS-related disorders.
Metabolic effects of fructose on metabolic syndrome
Fructose is one of the monosaccharides along with glucose and galactose. Fructose is found in all fruits and vegetables. The human body obtains fructose through exogenous supply or endogenously produces fructose from glucose through aldose reductase pathway [8]. Nowadays, most of the increase in fructose consumption is derived from refined sugars and processed foods.
Fructose is absorbed in the intestine through specific glucose transporters such as glucose transporter 5 (Glut 5) and Glut 2. Fructose metabolism differs markedly from glucose metabolism because these two sugars require different enzymes in the initial steps of metabolism. A growing body of evidence indicates that HF diet causes various features of MetS, such as obesity, adiposity, hypertension, hypertriglyceridemia, dyslipidemia, glucose intolerance and decreased insulin sensitivity [2], [6], [7]. Also, previous studies indicated that glucose or starch-feeding is not as effective as fructose-feeding to induce MetS [2].
Unlike glucose, which is metabolized widely in the body, fructose is converted into glucose, glycogen, lactate, and fatty acids mainly in the liver [8]. Since fructose can be transported and produced by the placenta [9], [10], it is considered that the fetal programming process is driven not only by fructose but also by its metabolites [11].
Effects of maternal fructose consumption on developmental programming of metabolic syndrome-related phenotypes
Although a number of epidemiological studies support an association between fructose consumption and adult MetS [12], limited studies have explored the effects of early-life fructose consumption on fetus and disease risk in adult offspring. So far, only a limited number of human studies have shown an association between excessive sweetened food and beverage consumption and poor pregnancy outcome [13]. Notably, human studies have not yet established the direct cause-and-effect relationship between excessive fructose consumption and MetS-related disorders. It stands to reason that the use of animal models is essential to investigate MetS-related programming process and identify reprogramming strategy for further translational research.
Our previous reports showed that adult offspring rats of mothers exposed to 60% HF diet during pregnancy and lactation developed MetS-related comorbidities [14], [15], [16], [17], [18], [19], [20], which is in agreement with the results of earlier studies involving fructose-fed adult rats [21]. Fructose appears to induce MetS in part by increasing uric acid [2]. Unlike fructose-induced uric acid generation that induces oxidative stress and nitric oxide (NO) deficiency in adult rats [2], [8], we observed that these abnormalities are not present in adult offspring exposed to maternal HF intake [14]. It is speculated that mechanisms underlying maternal HF consumption-induced fetal programming of MetS in offspring might be different from those underlying fructose feeding-induced MetS in adult rats [11].
It is noteworthy that adverse effects of fructose feeding depend on the amount and duration of fructose consumption [22]. Because rats express uricase (which degrades uric acid), fructose does not increase uric acid level very effectively [2]. Despite being viewed as far in excess of a relevant load, most animal studies have been performed using diets containing 50%–60% fructose [8]. However, a recent meta-analysis study showed that various features of MteS can be achieved using diets with as little as 10% w/v fructose in drinking water, independent of variations in study design and duration [21].
This review will primarily be limited to MetS-related phenotypes induced by HF consumption in early life in rodent animal models, some of which are listed in Table 1. Despite fructose alone can alter fetal programming to induce numerous features of MetS [17], [18], [19], [23], [25], [26], [28], [29], [30], some animal studies have often used fructose as a part of diet along with salt [20], [24] or fat [27].
Table 1.
Models of developmental programming of metabolic syndrome-related phenotypes exposed to maternal high-fructose consumption.
| Types of fructose intake | Species | Phenotypes related to MetS | Age at evaluation | Ref. |
|---|---|---|---|---|
| 10% w/v fructose in drinking water throughout lactation | Male Sprague–Dawley rats | Obesity, insulin resistance | 8 weeks | [23] |
| 10% w/v fructose plus 4% NaCl in drinking water 28 days before conception and throughout gestation and lactation | Male Sprague–Dawley rats | Hypertension | 9 weeks | [24] |
| 60% HF diet throughout pregnancy and lactation | Male and female Sprague–Dawley rats | Hypertension | 3 months | [14], [15], [17], [19] |
| 60% HF diet throughout pregnancy and lactation | Male Sprague–Dawley rats | Hypertension, insulin resistance, dyslipidemia | 3 months | [16] |
| 60% HF diet throughout pregnancy and lactation plus 1% NaCl in drinking water from weaning to 3 months of age | Male Sprague–Dawley rats | Hypertension | 3 months | [20] |
| 60% HF diet throughout pregnancy and lactation | Male Sprague–Dawley rats | Bladder dysfunction | 3 months | [18] |
| 10% w/v high fructose corn syrup (HFCS-55) in drinking water throughout pregnancy and lactation | Male and female Sprague–Dawley rats | Adiposity, dyslipidemia | 3 months | [25] |
| 10% w/v fructose in drinking water throughout gestation | Male Sprague–Dawley rats | Insulin resistance | 3 months | [26] |
| 56.7% HF/high-fat diet throughout pregnancy and lactation | Male Sprague–Dawley rats | Hypertension, hyperglycemia, kidney disease | 4 months | [27] |
| 60% HF diet throughout pregnancy and lactation | Male Sprague–Dawley rats | Dyslipidemia, hepatic steatosis | 5 months | [28] |
| 10% w/v fructose in drinking water throughout pregnancy | Female Sprague–Dawley rats | Dyslipidemia, hepatic steatosis | 8 months | [29] |
| 10% w/v fructose in drinking water throughout pregnancy and lactation | C57BL/6J mice | Hypertension, insulin resistance, obesity, hepatic steatosis | 12 months | [30] |
Studies have been tabulated according to the age at which the effects were measured.
HF diet induces hypertension in adult rats have been well reviewed elsewhere [31], [32]. However, limited data are available on the effects of maternal HF induced hypertension in adult offspring. Studies listed in Table 1 indicate that consumption of HF alone or as a part of diet by rodent mothers induces programmed hypertension in adult offspring of both sexes [14], [15], [16], [17], [19], [24], [27], [30]. Several mechanisms have been proposed to interpret HF-induced hypertension, including oxidative stress, NO deficiency, increased sodium absorption, endothelial dysfunction, activation of the renin-angiotensin system (RAS) activation, and sympathetic nervous system stimulation [11].
An obesogenic effect of HF intake was also observed in animal studies [23], [30]. Consumption of fructose has been reported to induce obesity by several mechanisms, such as direct effects on adipose tissue, indirect actions on the appetite control and feeding behavior, and by disrupting neuroendocrine signaling between adipose tissue and the hypothalamus [33]. Additionally, often considered the hepatic manifestation of the MetS, non-alcoholic fatty liver disease (NAFLD) is defined as hepatic steatosis in the absence of heavy alcohol use. As shown in Table 1, maternal HF exposure has been reported to induce hepatic steatosis in adult offspring [28], [29], [30]. Fructose consumption can upregulate the hepatic lipogenesis program, which is further amplified by hyperinsulinemia in the context of insulin resistance. Also, consumption of HF by rodent mothers induces insulin resistance in adult offspring [16], [23], [26], [30]. Metabolites from fructose metabolism can directly affecting tissue and organ functions; among these uric acid, free fatty acids and lactate play important roles in mediating insulin resistance in systemic and local tissue/organ [34]. Moreover, in several animal models of MetS, bladder overactivity is the major phenotype of voiding behavior associated with metabolic bladder dysfunction [35], [36]. These findings are supported by our recent work showing that male adult offspring of mothers exposed to 60% HF diet during pregnancy and lactation developed MetS-related bladder dysfunction [18]. However, not all studies in rodents have demonstrated deleterious effects of excess fructose consumption [37].
HF consumption induces transcriptome changes
Previously, we have utilized RNA next-generation sequencing (NGS) technology to analyze the transcriptome expression in several organs from male rat offspring exposed to maternal HF diet [11], [15], [16]. We observed that maternal HF intake can induce long-term transcriptome changes. Importantly, different organs react differently to developmental programming, leading to organ-specific transcriptional modification of gene cascades [16].
There are several shared differentially expressed genes (DEGs) related to fructose metabolism, glycolysis/gluconeogenesis, fatty acid metabolism, and insulin signaling in offspring at 1 day of age [16], including liver-type 6-phosphofructokinase (Pfkl), peroxisome proliferator-activated receptor gamma coactivator1-α (Ppargc1a), glucose transporter1 (Slc2a1), insulin receptor substrate 2 (Irs2), lactate dehydrogenase A (Ldha), sterol regulatory element-binding transcription factor 1 (Srebf1), hexokinase 2 (Hk2), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (Pfkfb3), suppressor of cytokine signaling 3 (Socs3), liver glycogen phosphorylase (Pygl), forkhead box protein O1 (Foxo1), and short/branched chain specific acyl-CoA dehydrogenase (Acadsb). Since fructose and its metabolites are important cellular nutrients, our NGS data suggest that the nutrient-sensing signaling might be crucial for the response of different organs to maternal fructose consumption, leading to differential phenotypes of MetS.
Also, we identified 14 significantly regulated Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways shared by at least two different organs at 1 day of age. Among them, peroxisome proliferator-activated receptor (PPAR) signaling pathway can serve as a nutrient-sensing signaling linking nutritional programming to MetS and hypertension [38]. Additionally, we found PPAR signaling pathway is a significant KEGG pathway shared by one-day, three-week, and three-month-old offspring kidney exposed to maternal HF intake [15], which supports previous studies showing that PPARs might be attractive drug targets for treating MetS [39], [40]. Another significant KEGG pathway shared by three different developmental stages is arachidonic acid metabolism [14]. Soluble epoxide hydrolase (SEH) plays a role in arachidonic acid metabolism. SEH can metabolize vasodilatory epoxyeicosatrienoic acid to vasoconstrictive dihydroxye-icosatrienoic acids [41]. In this aspect, in another study we found that the protein level and activity of SEH are induced by maternal HF exposure in offspring at three months of age. These observations implicate that PPAR signaling pathway and SEH might be therapeutic targets for MetS programmed by maternal fructose consumption.
Reprogramming strategy to prevent maternal HF consumption-induced metabolic syndrome
MetS affects more than one in five adults, and its prevalence is growing worldwide despite progress made in recent years in improving the care of patients with MetS [42]. Since MetS may take origin from early-life insults, a better understanding of the programming mechanisms underlying fructose consumption that lead to MetS may aid in developing early reprogramming intervention to halt the globally growing epidemic of MetS-related disorders. Several intervention strategies, including taurine, arginine, resveratrol, grape-derived polyphenols, sardine protein, vitamin E and α-lipoic acid, have been examined to prevent the adverse metabolic effects of excess fructose consumption in adults [43]. While none of these strategies has thus far been examined as a potential reprogramming strategy in maternal HF consumption-induced MetS of developmental origins.
A growing body of evidence indicates that PPARs ligands have therapeutic potential in treating MetS-related comorbidities [39], [40]. Thus far, however, only a few studies explored the impact of PPAR modulators as reprogramming strategies to prevent MetS of developmental origins [38], especially in models of excess maternal fructose consumption. Therefore, there is a need for studies to address PPAR modulators in maternal HF-induced MetS animal models and a need for prospective cohort data linking biomarkers in PPAR signal pathway to clinical outcomes or surrogate biomarker endpoints in humans.
Our data suggest that several mechanisms are involved in maternal HF-induced programmed hypertension, such as epigenetic regulation, glucocorticoid effects, RAS and sodium transporter alterations, and oxidative stress [11]; these mechanisms, thus, may serve as potential reprogramming strategies. Previously, we prevented hypertension development in adult offspring exposed to maternal HF diet by using three reprogramming approaches, namely, SEH inhibitor [17], melatonin [14], and renin inhibitor aliskiren [19]. First, our NGS data indicate that the SEH is involved in maternal HF consumption-induced renal programming and programmed hypertension [14]. In agreement with this finding, early postnatal SEH inhibitor 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) treatment ameliorated hypertension in maternal HF consumption-induced hypertension [17]. However, it would be interesting to see whether SEH inhibition also prevents other phenotypes of MetS. Second, most reprogramming strategies have focused on restoring the balance of NO and reactive oxygen species (ROS) to prevent cardiovascular disease [44]. Melatonin, an endogenous indoleamine produced by the pineal gland, has pleiotropic bioactivities those are beneficial in many DOHaD-related disorders, as reviewed elsewhere [45]. Our data showed that maternal melatonin treatment can prevent HF consumption-induced programmed hypertension in adult offspring [14]. Thus, reprogramming strategies that restore the NO–ROS balance can be applied in MetS-related disorders, such as hypertension. Last, MetS increases cardiovascular risk and the RAS plays an essential role in cardiovascular homeostasis. Blockade of RAS in young offspring from ages 2–4 weeks have been reported to protect against fetal programming of hypertension in a variety of programming animal models [46], [47]. We recently found that early postnatal aliskiren administration prevented maternal HF consumption-induced programmed hypertension in adult offspring [19]. Therefore, a better understanding of the underlying mechanisms of early-life fructose consumption-induced MetS-related phenotypes in the developing reprogramming strategy to prevent MetS is pretty warranted.
Conclusions
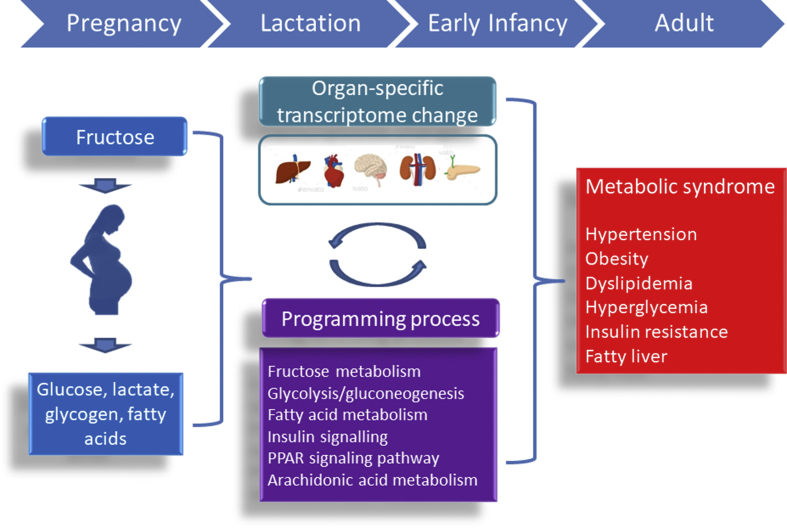
Overconsumption of fructose in early life is a risk factor for the epidemic of MetS with dysfunctions in multiple tissues and organs in later adulthood. A schematic summarizing the links between early-life fructose consumption, mechanisms underlying programming process, and MetS of developmental origin is presented in Fig. 1. Despite results from animal models indicate that maternal HF intake plays a role in the developmental programming of MetS, early-life fructose–gene interactions in humans might be more complex and multifactorial. Although major progress has been made in animal research on fructose consumption induced MetS of developmental origin, these potential pharmacological interventions need to be validated clinically. Future research should aim to bridge the translational gap between animal models and human therapeutics. Underlying the DOHaD concept, research into effective reprogramming strategies for MetS-related programming process that begin early in life will have a profound impact on economic burden of MetS-related disorders over the next century.
Fig. 1.
Schematic illustration of the interplay between early-life fructose consumption and programming process to increase the vulnerability to metabolic syndrome-related phenotypes in later life, of which can be prevented by reprogramming strategies.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by CMRPG8F0022 and CMRPG8G0191 (Y.L.T.), and CGMPG8F0031 and CGMPG8F0032 (J.Y.H.C.) from the Chang Gung Medical Foundation, and MOST 106-2314-B182A-100 (W.C.L.) from the Ministry of Science and Technology of the Republic of China, Taiwan.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Bray G.A., Nielsen S.J., Popkin B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 2.Johnson R.J., Segal M.S., Sautin Y., Nakagawa T., Feig D.I., Kang D.H. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 3.Grundy S.M., Brewer H.B., Jr., Cleeman J.I., Smith S.C., Jr., Lenfant C., American Heart Association, National Heart, Lung, and Blood Institute Definition of metabolic syndrome: report of the National heart, lung, and blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 4.Bruce K.D., Hanson M.A. The developmental origins, mechanisms, and implications of metabolic syndrome. J Nutr. 2010;140:648–652. doi: 10.3945/jn.109.111179. [DOI] [PubMed] [Google Scholar]
- 5.Tain Y.L., Joles J.A. Reprogramming: a preventive strategy in hypertension focusing on the kidney. Int J Mol Sci. 2015;17:E23. doi: 10.3390/ijms17010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMillen I.C., Robinson J.S. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 7.de Gusmão Correia M.L., Volpato A.M., Águila M.B., Mandarim-de-Lacerda C.A. Developmental origins of health and disease: experimental and human evidence of fetal programming for metabolic syndrome. J Hum Hypertens. 2012;26:405–419. doi: 10.1038/jhh.2011.61. [DOI] [PubMed] [Google Scholar]
- 8.Tappy L., Lê K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 9.Holmberg N.G., Kaplan B., Karvonen M.J., Lind J., Malm M. Permeability of human placenta to glucose, fructose, and xylose. Acta Physiol Scand. 1956;36:291–299. doi: 10.1111/j.1748-1716.1956.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 10.Hagerman D.D., Roux J., Villee C.A. Studies of the mechanism of fructose production by human placenta. J Physiol. 1959;146:98–104. doi: 10.1113/jphysiol.1959.sp006180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tain Y.L., Chan J.Y., Hsu C.N. Maternal fructose intake affects transcriptome changes and programmed hypertension in offspring in later life. Nutrients. 2016;8:E757. doi: 10.3390/nu8120757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelishadi R., Mansourian M., Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition. 2014;30:503–510. doi: 10.1016/j.nut.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Regnault T.R., Gentili S., Sarr O., Toop C.R., Sloboda D.M. Fructose, pregnancy and later life impacts. Clin Exp Pharmacol Physiol. 2013;40:824–837. doi: 10.1111/1440-1681.12162. [DOI] [PubMed] [Google Scholar]
- 14.Tain Y.L., Leu S., Wu K.L., Lee W.C., Chan J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: roles of nitric oxide and arachidonic acid metabolites. J Pineal Res. 2014;57:80–89. doi: 10.1111/jpi.12145. [DOI] [PubMed] [Google Scholar]
- 15.Tain Y.L., Wu K.L., Lee W.C., Leu S., Chan J.Y. Maternal fructose-intake-induced renal programming in adult male offspring. J Nutr Biochem. 2015;26:642–650. doi: 10.1016/j.jnutbio.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Chao Y.M., Tain Y.L., Leu S., Wu K.L., Lee W.C., Chan J.Y. Developmental programming of the metabolic syndrome: next-generation sequencing analysis of transcriptome expression in a rat model of maternal high fructose intake. Sheng Li Xue Bao. 2016;68:557–567. [PubMed] [Google Scholar]
- 17.Tain Y.L., Lee W.C., Wu K.L.H., Leu S., Chan J.Y.H. Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J Nutr Biochem. 2016;38:86–92. doi: 10.1016/j.jnutbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Lee W.C., Tain Y.L., Wu K.L., Leu S., Chan J.Y. Maternal fructose exposure programs metabolic syndrome-associated bladder overactivity in young adult offspring. Sci Rep. 2016;6:34669. doi: 10.1038/srep34669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu C.N., Wu K.L., Lee W.C., Leu S., Chan J.Y., Tain Y.L. Aliskiren administration during early postnatal life sex-specifically alleviates hypertension programmed by maternal high fructose consumption. Front Physiol. 2016;7:299. doi: 10.3389/fphys.2016.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tain Y.L., Lee W.C., Leu S., Wu K., Chan J. High salt exacerbates programmed hypertension in maternal fructose-fed male offspring. Nutr Metabol Cardiovasc Dis. 2015;25:1146–1151. doi: 10.1016/j.numecd.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Toop C.R., Gentili S. Fructose beverage consumption induces a metabolic syndrome phenotype in the rat: a systematic review and meta-analysis. Nutrients. 2016;8:E577. doi: 10.3390/nu8090577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai S., McNeill J.H. Fructose-induced hypertension in rats is concentration- and duration-dependent. J Pharmacol Toxicol Methods. 1995;33:101–107. doi: 10.1016/1056-8719(94)00063-a. [DOI] [PubMed] [Google Scholar]
- 23.Alzamendi A., Castrogiovanni D., Gaillard R.C., Spinedi E., Giovambattista A. Increased male offspring's risk of metabolic-neuroendocrine dysfunction and overweight after fructose-rich diet intake by the lactating mother. Endocrinology. 2010;151:4214–4223. doi: 10.1210/en.2009-1353. [DOI] [PubMed] [Google Scholar]
- 24.Gray C., Gardiner S.M., Elmes M., Gardner D.S. Excess maternal salt or fructose intake programmes sex-specific, stress- and fructose-sensitive hypertension in the offspring. Br J Nutr. 2016;115:594–604. doi: 10.1017/S0007114515004936. [DOI] [PubMed] [Google Scholar]
- 25.Toop C.R., Muhlhausler B.S., O'Dea K., Gentili S. Impact of perinatal exposure to sucrose or high fructose corn syrup (HFCS-55) on adiposity and hepatic lipid composition in rat offspring. J Physiol. 2017;595:4379–4398. doi: 10.1113/JP274066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez L., Otero P., Panadero M.I., Rodrigo S., Álvarez-Millán J.J., Bocos C. Maternal fructose intake induces insulin resistance and oxidative stress in male, but not female, offspring. J Nutr Metab. 2015;2015:158091. doi: 10.1155/2015/158091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada-Obara N., Yamagishi S.I., Taguchi K., Kaida Y., Yokoro M., Nakayama Y. Maternal exposure to high-fat and high-fructose diet evokes hypoadiponectinemia and kidney injury in rat offspring. Clin Exp Nephrol. 2016;20:853–861. doi: 10.1007/s10157-016-1265-9. [DOI] [PubMed] [Google Scholar]
- 28.Ching R.H., Yeung L.O., Tse I.M., Sit W.H., Li E.T. Supplementation of bitter melon to rats fed a high-fructose diet during gestation and lactation ameliorates fructose-induced dyslipidemia and hepatic oxidative stress in male offspring. J Nutr. 2011;141:1664–1672. doi: 10.3945/jn.111.142299. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez L., Panadero M.I., Rodrigo S., Roglans N., Otero P., Álvarez-Millán J.J. Liquid fructose in pregnancy exacerbates fructose-induced dyslipidemia in adult female offspring. J Nutr Biochem. 2016;32:115–122. doi: 10.1016/j.jnutbio.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Saad A.F., Dickerson J., Kechichian T.B., Yin H., Gamble P., Salazar A. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am J Obstet Gynecol. 2016;215:378. doi: 10.1016/j.ajog.2016.03.038. e1–6. [DOI] [PubMed] [Google Scholar]
- 31.Tran L.T., Yuen V.G., McNeill J.H. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem. 2009;332:145–159. doi: 10.1007/s11010-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 32.Klein A.V., Kiat H. The mechanisms underlying fructose-induced hypertension: a review. J Hypertens. 2015;33:912–920. doi: 10.1097/HJH.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goran M.I., Dumke K., Bouret S.G., Kayser B., Walker R.W., Blumberg B. The obesogenic effect of high fructose exposure during early development. Nat Rev Endocrinol. 2013;9:494–500. doi: 10.1038/nrendo.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D.M., Jiao R.Q., Kong L.D. High dietary fructose: direct or indirect dangerous factors disturbing tissue and organ functions. Nutrients. 2017;9:E335. doi: 10.3390/nu9040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong Y.C., Cheng J.T. Alterations of M2,3-muscarinic receptor protein and mRNA expression in the bladder of the fructose fed obese rat. J Urol. 2007;178:1537–1542. doi: 10.1016/j.juro.2007.05.114. [DOI] [PubMed] [Google Scholar]
- 36.Lee W.C., Chien C.T., Yu H.J., Lee S.W. Bladder dysfunction in rats with metabolic syndrome induced by long-term fructose feeding. J Urol. 2008;179:2470–2476. doi: 10.1016/j.juro.2008.01.086. [DOI] [PubMed] [Google Scholar]
- 37.Lineker C., Kerr P.M., Nguyen P., Bloor I., Astbury S., Patel N. High fructose consumption in pregnancy alters the perinatal environment without increasing metabolic disease in the offspring. Reprod Fertil Dev. 2016;28:2007–2015. doi: 10.1071/RD15119. [DOI] [PubMed] [Google Scholar]
- 38.Tain Y.L., Hsu C.N., Chan J.Y. PPARs link early life nutritional insults to later programmed hypertension and metabolic syndrome. Int J Mol Sci. 2015;17:E20. doi: 10.3390/ijms17010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azhar S. Peroxisome proliferator-activated receptors, metabolic syndrome and cardiovascular disease. Future Cardiol. 2010;6:657–691. doi: 10.2217/fca.10.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monsalve F.A., Pyarasani R.D., Delgado-Lopez F., Moore-Carrasco R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediat Inflamm. 2013;2013:549627. doi: 10.1155/2013/549627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imig J.D. Epoxyeicosatrienoic acids, hypertension, and kidney injury. Hypertension. 2015;65:476–482. doi: 10.1161/HYPERTENSIONAHA.114.03585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danaei G., Finucane M.M., Lu Y., Singh G.M., Cowan M.J., Paciorek C.J., Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 43.Sloboda D.M., Li M., Patel R., Clayton Z.E., Yap C., Vickers M.H. Early life exposure to fructose and offspring phenotype: implications for long term metabolic homeostasis. J Obes. 2014;2014:203474. doi: 10.1155/2014/203474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tain Y.L., Hsu C.N. Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int J Mol Sci. 2017;18:E841. doi: 10.3390/ijms18040841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tain Y.L., Huang L.T., Chan J.Y. Transcriptional regulation of programmed hypertension by melatonin: an epigenetic perspective. Int J Mol Sci. 2014;15:18484–18495. doi: 10.3390/ijms151018484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherman R.C., Langley-Evans S.C. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 2000;98:269–275. [PubMed] [Google Scholar]
- 47.Hsu C.N., Lee C.T., Huang L.T., Tain Y.L. Aliskiren in early postnatal life prevents hypertension and reduces asymmetric dimethylarginine in offspring exposed to maternal caloric restriction. J. Renin Angiotensin Aldosterone Syst. 2015;16:506–513. doi: 10.1177/1470320313514123. [DOI] [PubMed] [Google Scholar]