Abstract
Chlamydiae are obligate intracellular bacterial pathogens, and as such are sensitive to alterations in the cellular physiology of their hosts. Chlamydial infections often cause pathologic consequences due to prolonged localized inflammation. Considerable advances have been made in the last few years regarding our understanding of how two key inflammation-associated signaling pathways influence the biology of Chlamydia infections: inflammation regulating purinergic signaling pathways significantly impact intracellular chlamydial development, and inflammasome activation modulates both chlamydial growth and infection mediated pro-inflammatory cytokine production. We review here elements of both pathways, presenting the latest developments contributing to our understanding of how chlamydial infections are influenced by inflammasomes and purinergic signaling.
Keywords: Innate immunity, Chlamydia, Immunology, Inflammation, Infection
The bacterial family Chlamydiaceae includes several species which promiscuously or sporadically infect humans. Chlamydia trachomatis is the most common nationally notifiable infection in the USA [1], the most common bacterial cause of sexually transmitted infection worldwide [2], and also a prominent cause of preventable blindness following repeated conjunctival infections in the developing world [3]. C. trachomatis can also cause pneumonia in infants following exposure during birth [4]. Chlamydophila pneumoniae is one of the leading causes of pneumonia in the developed world [5] and may increase the risk of developing atherosclerotic lesions in coronary artery disease [6]. Chlamydophila psittaci, while primarily an avian pathogen, sporadically causes human pneumonia [7], [8]. Chlamydophila abortus and Chlamydophila caviae zoonotic infections have been reported in humans but are rare [9], [10]. Untreated C. trachomatis genital tract infection in women may ascend the endometrial endothelium to reach the fallopian tubes, with the associated chronic inflammation leading to pelvic inflammatory disease (PID), which may also cause miscarriage [11], ectopic pregnancy [12], or tubal scarring and infertility [13]. Repeated and chronic infection of the conjunctiva with C. trachomatis leads to recruitment of lymphocytes and the formation of follicles and inflammation mediated conjunctival thickening, which subsequently causes the deformation of the eyelids and corneal damage via scraping of the cornea by in-turned eyelashes [14]. Pneumonia caused by C. pneumoniae develops slowly and leads to inflammation of the lungs but with limited production of purulent sputum. C. pneumoniae also causes infections of the upper respiratory tract including pharyngitis, sinusitis, and bronchitis. Inflammation due to repeated infections with C. pneumoniae, or unrecognized and untreated infections, may contribute to chronic obstructive pulmonary disorder (COPD) [15], [16]. Human disease following infection with Chlamydiaceae species bears a consistent hallmark: chronic, localized inflammation.
Two major pathways relevant to the induction and regulation of localized inflammation have recently received considerable research emphasis and have been demonstrated to be particularly relevant during Chlamydia infection: purinergic signaling, and the formation of macro-molecular inflammasomes. Here we review the basic concepts of purinergic signaling in the context of immune function and inflammation regulation, and inflammasome mediated inflammatory cytokine production, followed by an examination of the recent literature evaluating the impact of host purinergic signaling and inflammasome activation on chlamydial infection.
Extracellular purines and purinergic receptors
A wide range of extracellular purine concentrations are physiologically relevant [17], and they are met by a similarly broad spectrum of sensory affinity in the purinergic receptor families [18]. ATP is released from cells under normal physiologic conditions reaching nanomolar to low micromolar concentrations in the immediately adjacent extracellular space [19], [20], [21]. Higher concentrations of ATP or ADP result from various forms of cell stress [22], platelet degranulation [23], or are present in tumor microenvironments [24]. The concentration of ATP in cells ranges from 3 to 10 mM, and thus in the context of cell damage or necrosis the neighboring cells are exposed to low millimolar levels of extracellular ATP and purine metabolites. ATP may be released via degranulation in cell types which produce ADP or ATP rich granules, via pannexin channels [25], [26], or following cell damage. At colonized mucosal epithelial surfaces, ATP may also be directly released by bacteria [27]. ATP is also released from cervical epithelial cells in vitro during C. trachomatis infection, particularly during the late stages of inclusion development when there is likely more cellular stress [28].
Receptor mediated purine signaling is an evolutionarily conserved cellular function, and is involved in a wide variety of physiological processes in mammals including neurologic signaling, vascular function, and immune cell regulation. Receptors which recognize purine nucleotides and nucleosides are termed purinergic receptors. Purinergic receptors are grouped into families based on functional similarity: P1 receptors are engaged by the purine nucleoside adenosine, while P2 receptors are activated by nucleotides and are further subdivided into gated ion channels (P2X) or G-protein coupled seven transmembrane receptors (P2Y).
Adenosine receptors couple via G-proteins to adenylyl cyclase to modulate cAMP generation in cells. A1 and A3 receptors associate with Gi proteins to inhibit adenylyl cyclase and prevent cAMP upregulation, whereas A2a and A2b receptors interact with Gs proteins to activate adenylyl cyclase, leading to elevated intracellular cAMP. Adenosine receptors are expressed in a wide variety of cell types, with particularly well described roles for A1 and A3 receptors in cardiac function, and for A2a receptors in immune cell function and A2b on epithelial and endothelial cells. A2b also mediates intracellular signaling via Gq and phospholipase C [29]. Recent excellent reviews have organized the great depth of studies related to adenosine receptor mediated regulation of immune cells [30], [31].
P2X purinergic receptors are ligand gated ion channels which are activated by ATP, and possibly ADP in the case of P2X4, and are increasingly recognized to play a role in inflammation and immune cell function [32]. There are seven described P2X receptors, the best characterized being P2X7. P2X7 plays a critical role in the production of IL-1β and other inflammatory cytokines both in the context of infection and sterile inflammation [33], [34]. Initial stimulation of P2X receptors leads to the opening of small-cation permeable pores (approximately 0.85 nm for P2X7), while prolonged ligation then leads to increased permeability to larger molecules (greater than 1 nm for P2X7) [35], either by P2X7 pore dilation or by P2X7 coupling to pannexin1 and the pannexin pore.
P2Y receptors respond to purine and pyrimidine nucleotides and mediate intracellular signaling via regulation of cAMP or activation of PLC. Eight P2Y receptors have been characterized (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14), and while their activity relevant to inflammation and immune cell function is less well characterized than for P2X receptors, some P2Y receptors are expressed on immune cells [18], [36], [37].
While there is nuance to the roles that purine signaling plays in local and systemic immune regulation, in general, ATP mediates pro-inflammatory responses, and adenosine receptor stimulation is anti-inflammatory [38]. Thus soluble or cell surface associated extracellular enzymes play a critical role in determining the response to extracellular purines. Adenine nucleotides released from cells are dephosphorylated by members of several families of purine enzymes to generate adenosine. Enzymes such as CD39 (ENTPD1) and alkaline phosphatase dephosphorylate ATP and ADP to generate AMP, and CD73 (ecto-5′-nucleotidase) or alkaline phosphatase dephosphorylates AMP to generate adenosine. Adenosine can be captured by adjacent cells via nucleoside transporters for purine salvage, or metabolized by adenosine deaminase to produce inosine. Enzymes regulating extracellular purine metabolism have been more completely described in recent reviews [39], [40].
Purinergic receptor stimulation during chlamydial infection
In the last 10 years, there have been many investigations into the impact of purinergic receptor stimulation on chlamydial development in vitro.
After work by other groups showed that purinergic receptor stimulation altered mycobacterial infection in host cells [41], [42], we demonstrated that P2X7 ligation (5 mM ATPe) reduced growth of C. psittaci in J774 macrophages, while at the same time the chlamydial infection partially inhibited ATPe mediated apoptosis of host cells [43]. We then demonstrated that ATP stimulation of P2X7 on murine macrophages led to killing of intracellular Chlamydia muridarum [44], the murine equivalent of C. trachomatis. Ligation of P2X7 receptor with 0.5–5.0 mM ATPe caused fusion of chlamydial inclusions with host-cell lysosomes, leading to chlamydial death, an effect which was dependent on activation of phospholipase D (PLD). Additionally, P2X7 ligation suppresses C. muridarum infection in the preferred target cell of genitotropic Chlamydia species – cervical epithelial cells [45]. As was the case in murine macrophages, ATPe/P2X7 mediated chlamydial inhibition in murine cervical epithelial cells was at least partially dependent on PLD activation. In animal experiments with P2X7 wild-type and P2X7−/− mice, P2X7 expression reduced the intensity, but not the duration, of vaginal infection, and pathology scores based on histological evaluation of endocervix, oviduct, and mesosalpinx tissue indicated increased inflammation in the P2X7−/− mice following chlamydial infection [45].
While millimolar concentrations of ATPe are required for P2X7 mediated effects on Chlamydia infection, micromolar concentrations of ADPe and ATPe reversibly inhibit chlamydial development in human cervical epithelial cells via P2X4 ligation [28]. One hundred μM ADPe or ATPe applied to C. trachomatis infected epithelial cells at 1 h post-infection (hpi) significantly inhibited chlamydial inclusion development through 24 h of infection, although by 48 hpi inclusion development was proceeding normally. If, however, repeated applications of ADPe or ATPe were made, inclusion development did not occur by 48 hpi unless the media was changed at 24 hpi. Thus, duration of exposure to micromolar concentrations of ADP or ATP determined the extent of impairment of chlamydial development. In the context of inflammation, ATPe (pro-inflammatory) and ADOe (anti-inflammatory) often have paradoxical effects; however, both inhibit development of C. trachomatis in human cervical epithelial cells at micromolar concentrations. ADOe suppression of chlamydial development is reversible, and is mediated by A2b receptor stimulation [46]. ADOe and ATPe also inhibit the development of C. trachomatis serovar E and Chlamydia pecorum in human epithelial cells [47]. It should be noted that two independent investigations have also demonstrated that millimolar concentrations of extracellular cyclic adenosine monophosphate (cAMP) inhibit chlamydial development, leading to the production of aberrant chlamydial bodies [47], [48]. As no receptor for extracellular cAMP has been described, and cAMP is not cell permeable, this effect may possibly be mediated by purinergic receptor stimulation following degradation of extracellular cAMP. The mechanism by which purinergic stimulation of host cells alters chlamydial development has not been elucidated, and it is also not clear whether the alterations described in vitro would be deleterious or advantageous for chlamydial propagation in vivo. As an obligate intracellular bacterium with a substantially reduced genome, chlamydiae are particularly sensitive to changes in the metabolic environment of the host cell. Indeed, in the best described in vitro model of chlamydial persistence, it has been demonstrated that IFN-γ stimulation of human epithelial host cells leads to restriction of intracellular tryptophan for which chlamydiae are auxotrophic [49], [50]. Future studies are needed to evaluate the molecular basis for the impairment in chlamydial development following host cell exposure to elevated extracellular purines.
Inflammasomes and inflammation
The innate immune system mounts a rapid response to unique molecular signatures from the invading pathogen termed pathogen associated molecular patterns (PAMPs), where it utilizes a set of germ-line encoded pattern recognition receptors (PRRs) to initiate a measured and appropriate inflammatory response in order to slow down the spread of infection and thereby allowing and contributing to the initiation of adaptive immunity. Furthermore, PRRs can detect danger signals termed danger associated molecular patterns (DAMPs) released by damaged host cells (for a review, see Ref. [51]). PRRs can be membrane-bound like the Toll-like receptors (TLRs) and C-type lectin receptors (CLRs), which sample the extracellular milieu from the plasma membrane and within endosomes, or cytosolic like the nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs), retinoid acid-inducible gene I (RIG-I)-like receptors (RLRs) and AIM2-like receptors (ALRs), which survey intracellular PAMPs and DAMPs (reviewed in Refs. [52], [53], [54]).
Typically, most PRRs (e.g. TLRs), following recognition of their PAMP, initiate distinct intracellular signaling cascades, which lead to induction of various programs of gene transcription including, but not limited to, co-stimulatory molecules which activate components of the adaptive immune response, production of reactive oxygen and nitrogen species, and expression of pro-inflammatory cytokines and chemokines [53], [54]. On the other hand, NLRs – a family of 22 genes in humans and 34 genes in mice –can detect not only intracellular PAMPs (e.g. NOD1, NOD2) [55], but also can sense endogenous DAMPs [52], [56], [57]. These latter NLRs, which include NLRP and NLRC proteins, are components of a macromolecular protein complex – the inflammasome – required for the activation of caspase-1 and the subsequent secretion of potent pro-inflammatory cytokines, including Interleukin (IL)-1β (IL-1β) and IL-18 (reviewed in Ref. [58]).
Due to their high pro-inflammatory potency and their significant role in innate defense to invading pathogens, secretion of both IL-1β and IL-18 is a highly regulated process in order to avoid diseases associated with their excessive and chronic production, like autoimmune disorders and septic shock [59]. Both cytokines are produced as inactive and leaderless forms, pro-IL-1β and pro-IL-18, and require cleavage by caspase-1 to promote the unconventional secretion of their mature and biologically active forms [60], [61], [62]. Caspase-1, along with caspase-4 and caspase-5 in humans and caspase-11 in mice, constitute a family of inflammatory caspases that exist as inactive zymogens (pro-caspases). Activation of these caspases requires the activation of inflammasomes, which are a multimeric assembly of pro-caspase-1 and an NLR family member, either directly through CARD–CARD interaction or indirectly via the adapter protein ASC [63], [64]. These studies led the way for the identification of additional means to nucleate the inflammasome by members of the RLR and ALR families, and the assembly of non-canonical inflammasomes that lead to the activation of caspase-11 in mice and caspase-4/5 in humans.
The NLRP3 inflammasome (also known as NALP3, cryopyrin) is the best-characterized inflammasome to date and can be activated by wide variety of stimuli including PAMPs, DAMPs, pore-forming toxins, whole pathogens and environmental stressors (reviewed in Refs. [65], [66], [67]). Due to the large number of triggers and in order to prevent inadvertent activation, the NLRP3 inflammasome requires two signals for its optimal induction. PRR agonists (e.g. LPS) provide the first signal and cause NF-κB mediated upregulation of pro-IL-1β and components of the inflammasome, including NLRP3 [68]. The second signal can be provided by a large repertoire of ‘danger signals’ that include host derived DAMPs (e.g. ATP, adenosine, hyaluronan, HMGB1, gout-associated uric acid crystals and amyloid-β fibrils [69], [70], [71], [72], [73], [74]), environmental stressors (e.g. asbestos, silica crystals [75], [76], [77]), and PAMPs that gain access to the cytosol. These PAMPs can be derived from bacteria (e.g. toxins, RNA:DNA hybrids, type III secretion system effectors, cyclic-dinucleotides [58], [67], [70], [78], [79], [80], [81], [82], [83], [84], [85]), viruses (e.g. dsRNA, viral proteins [86], [87], [88], [89], [90]), fungi (e.g. hyphae, zymosan, mannan [91], [92], [93], [94]), or Plasmodium, a malaria causing parasite (e.g. hemozoin [95]). Importantly, some intracellular pathogens can activate the NLRP3 inflammasome by providing both signals (e.g. C. trachomatis [58]).
Despite this wide array of NLRP3 activators, researchers in the field generally agree that NLRP3 does not directly sense any of these stimuli, but instead detects specific host-derived molecular event(s), which then cause the assembly of the NLRP3 inflammasome. These upstream molecular events can be summarized into three main categories. (1) Events that perturb the homeostasis of intracellular cations, and include an increase in intracellular Ca2+ [79], [96], [97] or K+ efflux, which usually occurs through the activated ATP-gated ion channel P2X7 or toxin-induced pores in plasma membrane [70], [98], [99], [100]. (2) Events that cause reactive oxygen species (ROS)-mediated oxidative stress and mitochondrial perturbation. Although the exact mechanism of ROS-mediated NLRP3 inflammasome activation remains controversial, there is a general consensus that mitochondrial ROS (mROS) production is the upstream trigger for NLRP3 activation following stimulation with several inducers (e.g. ATP, uric acid crystals, alum, nigericin, certain pathogens [65], [101]). Elevated and prolonged release of mROS by damaged mitochondria [101] leads to oxidation of mitochondrial DNA, which when leaked to the cytosol binds directly to NLRP3, thereby promoting its activation [102], [103]. (3) Release of Cathepsins from destabilized lysosomes following ‘frustrated phagocytosis’ of relatively large ‘sterile’ molecules (e.g. silica, alum and uric acid crystals, asbestos, amyloid-β-fibrils, malarial hemozoin [69], [77]) also activate the NLRP3 inflammasome. Again, the exact mechanism of how this leads to NLRP3 inflammasome activation remains poorly understood.
Chlamydiae and inflammasomes
In response to Chlamydia infection, epithelial cells produce pro-inflammatory cytokines and chemokines in order to activate and recruit innate immune cells into the site of infection [104]. These immune cells, which include dendritic cells, macrophages, neutrophils and natural killer cells, in turn secrete cytokines including TNF-α and IL-1β in response to the infection [105], [106], [107], [108], [109], [110]. Consistently, IL-1β deficient mice infected with C. muridarum displayed delayed clearance of the infection [111]. However, chronic and excessive production of these pro-inflammatory cytokines has been touted as the dominant culprit causing the pathology associated with chlamydial infections [112], [113], [114], [115]. Consistently, caspase-1 deficient mice displayed reduced inflammatory damage in the urogenital tract, suggesting that it may contribute to the pathology of infection by Chlamydia [116]. Therefore, revealing the mechanism of IL-1β production has important implications for understanding pathology associated with Chlamydia infection.
Previous studies have shown that caspase-1 could be activated during chlamydial infection [107], [109], [111], [116], [117]. Indeed, C. trachomatis infection of cervical epithelial cells caused caspase-1 activation in a manner dependent on NLRP3 inflammasome activation [58]. Subsequent studies showed that human monocytic cell lines infected with C. trachomatis and C. muridarum secreted IL-1β following caspase-1 activation in an NLRP3-dependent fashion [78], while C. pneumoniae was the most potent inducer of IL-1β secretion in bone marrow derived murine macrophages [118]. These studies showed that Chlamydia infection alone was sufficient for inflammasome-dependent caspase-1 activation likely because an active Chlamydia infection can provide both signal one via the TLR2/MyD88 axis [119], [120], [121] in addition to signal two, activating caspase-1. The mechanism whereby chlamydial infections provide the second signal for NLRP3 inflammasome activation was thoroughly investigated. C. trachomatis induced inflammasome activation is dependent on K+ efflux [58] although the role of the P2X7 receptor, which is required for K+ efflux mediated inflammasome activation [122] and plays an important role in Chlamydia infection [45], remains to be investigated. Chlamydia-induced loss of intracellular potassium seems to trigger production of ROS, which appears to be essential for proper activation of the NLRP3 inflammasome [67]. Importantly, the mitochondrial associated protein, NLRX1, plays an important role in mediating ROS production following infection with C. trachomatis [78]. Moreover, C. pneumoniae mediated capase-1 activation required lysosomal acidification and cathepsin B release [118]. The ability of chlamydiae to activate the inflammasome is dependent on the chlamydial type III secretion system (T3SS) [58]. Intriguingly, a chlamydial plasmid encoded protein, pORF5 (a.k.a. pgp3), which is secreted into the host cytosol [123], might play a role in inflammasome activation [124]. Finally, the Rho family GTPase, Rac1, was shown to play a role in C. pneumoniae induced inflammasome activation [125].
The role of the inflammasome in the control of chlamydial infection is still controversial. In a mouse model of C. pneumoniae lung infection, results showed that caspase-1 deficient mice displayed delayed pulmonary bacterial clearance of infection, which was associated with increased mortality compared to wild type mice, thereby indicating that inflammasome activation has an important role in the host response to the infection [83]. On the other hand, lung fibroblasts from ASC−/− and caspase−/− mice displayed resistance to infection by C. trachomatis, a result which was recapitulated by the caspase-1 inhibitor, YVAD [126]. In agreement, a recent report showed that IL-10 mediated increase in tubal damage and infertility in Chlamydia-infected mice is mediated by its ability to enhance NLRP3 inflammasome activation in infected DCs [127]. Furthermore, in vivo challenge with C. muridarum in IL-1R deficient mice showed that, while IL-1β plays an important role for clearance of genital chlamydial infection, infection of NLRP3−/− and ASC−/− mice showed that the inflammasome plays a limited role in this model [128].
Chlamydia is described as the ‘perfect pathogen’ due to its ability to evade host-cell defenses and utilize host resources to promote its own growth [129], [130], [131]. Consistently, Chlamydia subverts host lipids from the Golgi by inducing caspase-mediated fragmentation of the Golgi apparatus [132]. Intriguingly, inflammasome-mediated caspase-1 activation following C. trachomatis infection is required for optimal intracellular growth of this pathogen [58]. Similarly, bone-marrow derived macrophages from NLRP3, ASC or caspase-1 deficient mice showed severely impaired intracellular growth of C. pneumoniae [133]. Intriguingly, both the caspase-1 inhibitor, YVAD, and caspase-4/5 (murine caspase-11 ortholog) inhibitor, WEHD, inhibited chlamydial growth, suggesting possible roles for both caspase-1 and non-canonical caspases (caspase-4/5 or caspase-11) in this process [58]. In fact, a recent study suggested that guanylate binding protein (GBP), which promotes caspase-11-dependent pyroptosis, might play a partial role in regulating the kinetics of inflammasome activation and affecting the ratio of IL-1β vs IL-18 production following C. muridarum infection [134]. Furthermore, treatment with probenecid, a pannexin-1 channel blocker that inhibits P2X7 receptor mediated inflammasome activation following K+ efflux [135], directly inhibits chlamydial development in a dose-dependent and reversible manner, although a direct role for pannexin-1 is not clear [136].
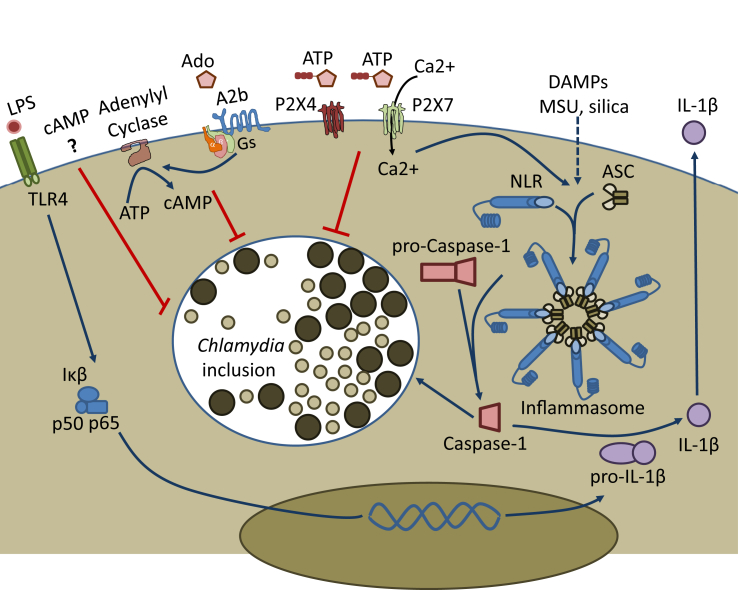
In conclusion, although results from different laboratories show consistently that an NLRP3 inflammasome is activated by infection with different species of Chlamydia and that the inflammasome may be involved in the immune response to infection, much remains to be known of the effects that both canonical and non-canonical inflammasomes may have on metabolism of infected cells [Fig. 1]. In particular, elucidation of the mechanism through which Chlamydia-mediated caspase-1 activation may stimulate lipid metabolism for the purpose of aiding chlamydial growth is an unexplored topic and interesting avenue of future research.
Fig. 1.
Purinergic receptor stimulation and inflammasome activation alter chlamydial development. Prolonged adenosine exposure suppresses chlamydial development via stimulation of host Gs protein- and adenylyl cyclase-coupled A2b receptors, and ADP or ATP mediated activation of P2X4 and P2X7 receptors also leads to reduced chlamydial growth or P2X7 mediated chlamydial killing, depending on the concentration of purine nucleotides and host cell type. Assembly of pro-caspase-1, NLR-family member sensor proteins, and ASC adaptor proteins into multimeric inflammasomes is required to generate active caspase-1, and follows combinatorial signaling events activating multiple upstream pathways, which may include ATP stimulation of P2X7 receptors, or other DAMP-dependent signaling pathways. Inflammatory triggers such as lipopolysaccharide (LPS) activation of TLR4 leads to production of pro-IL-1β, but inflammasome mediated production of active caspase-1 is required for generation of secreted pro-inflammatory IL-1β. Inflammasome activation mediated cleavage of pro-caspase-1, yielding active caspase-1, also contributes directly to growth of intracellular chlamydiae.
Conflicts of interest
None of the authors have a conflict of interest with this manuscript.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Centers for Disease C, Prevention CDC Grand Rounds: Chlamydia prevention: challenges and strategies for reducing disease burden and sequelae. MMWR Morb Mortal Wkly Rep. 2011;60:370–373. [PubMed] [Google Scholar]
- 2.Organization WH . World Health Organization; 2012. Global incidence and prevalence of selected curable sexually transmitted infections-2008. [Google Scholar]
- 3.Mariotti S.P., Pascolini D., Rose-Nussbaumer J. Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol. 2009;93:563–568. doi: 10.1136/bjo.2008.148494. [DOI] [PubMed] [Google Scholar]
- 4.Beem M.O., Saxon E.M. Respiratory-tract colonization and a distinctive pneumonia syndrome in infants infected with Chlamydia trachomatis. N Engl J Med. 1977;296:306–310. doi: 10.1056/NEJM197702102960604. [DOI] [PubMed] [Google Scholar]
- 5.Blasi F., Tarsia P., Aliberti S. Chlamydophila pneumoniae. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2009;15:29–35. doi: 10.1111/j.1469-0691.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- 6.Campbell L.A., Kuo C.C. Chlamydia pneumoniae–an infectious risk factor for atherosclerosis? Nat Rev Microbiol. 2004;2:23–32. doi: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- 7.Williams J., Tallis G., Dalton C., Ng S., Beaton S., Catton M. Community outbreak of psittacosis in a rural Australian town. Lancet. 1998;351:1697–1699. doi: 10.1016/S0140-6736(97)10444-5. [DOI] [PubMed] [Google Scholar]
- 8.Schlossberg D. Churchill Livingstone; NewYork, NY: 2000. Chlamydia psittaci (psittacosis). Principles and practise of infectious diseases; pp. 2004–2006. [Google Scholar]
- 9.Pospischil A., Thoma R., Hilbe M., Grest P., Gebbers J. Abortion in woman caused by caprine Chlamydophila abortus (Chlamydia psittaci serovar 1) Swiss Med Wkly. 2002:64–66. doi: 10.4414/smw.2002.09911. [DOI] [PubMed] [Google Scholar]
- 10.Hartley J., Stevenson S., Robinson A., Littlewood J., Carder C., Cartledge J. Conjunctivitis due to Chlamydophila felis (Chlamydia psittaci feline pneumonitis agent) acquired from a cat: case report with molecular characterization of isolates from the patient and cat. J Infect. 2001;43:7–11. doi: 10.1053/jinf.2001.0845. [DOI] [PubMed] [Google Scholar]
- 11.Baud D., Goy G., Jaton K., Osterheld M.C., Blumer S., Borel N. Role of Chlamydia trachomatis in miscarriage. Emerg Infect Dis. 2011;17:1630–1635. doi: 10.3201/eid1709.100865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaer A., Mert I., Cavkaytar S., Batioglu S. Serological investigation of the role of selected sexually transmitted infections in the aetiology of ectopic pregnancy. Eur J Contracept Reprod Health Care Off J Eur Soc Contracept. 2013;18:68–74. doi: 10.3109/13625187.2012.744818. [DOI] [PubMed] [Google Scholar]
- 13.Kavanagh K., Wallace L.A., Robertson C., Wilson P., Scoular A. Estimation of the risk of tubal factor infertility associated with genital chlamydial infection in women: a statistical modelling study. Int J Epidemiol. 2013;42:493–503. doi: 10.1093/ije/dyt011. [DOI] [PubMed] [Google Scholar]
- 14.Hu V.H., Holland M.J., Burton M.J. Trachoma: protective and pathogenic ocular immune responses to Chlamydia trachomatis. PLoS Negl Trop Dis. 2013;7:e2020. doi: 10.1371/journal.pntd.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choroszy-Krol I., Frej-Madrzak M., Hober M., Sarowska J., Jama-Kmiecik A. Infections caused by Chlamydophila pneumoniae. Adv Clin Exp Med Off Organ Wroc Med Univ. 2014;23:123–126. doi: 10.17219/acem/37035. [DOI] [PubMed] [Google Scholar]
- 16.Kauppinen M., Saikku P. Pneumonia due to Chlamydia pneumoniae: prevalence, clinical features, diagnosis, and treatment. Clin Infect Dis Off Publ Infect Dis Soc Am. 1995;21(Suppl. 3):S244–S252. doi: 10.1093/clind/21.supplement_3.s244. [DOI] [PubMed] [Google Scholar]
- 17.Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009;2:pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 18.Bours M.J., Swennen E.L., Di Virgilio F., Cronstein B.N., Dagnelie P.C. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Beigi R., Kobatake E., Aizawa M., Dubyak G.R. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol. 1999;276:C267–C278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- 20.Lazarowski E.R., Boucher R.C., Harden T.K. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 21.Yegutkin G.G., Mikhailov A., Samburski S.S., Jalkanen S. The detection of micromolar pericellular ATP pool on lymphocyte surface by using lymphoid ecto-adenylate kinase as intrinsic ATP sensor. Mol Biol Cell. 2006;17:3378–3385. doi: 10.1091/mbc.E05-10-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprague R.S., Ellsworth M.L., Stephenson A.H., Lonigro A.J. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol. 1996;271:H2717–H2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- 23.von Papen M., Gambaryan S., Schutz C., Geiger J. Determination of ATP and ADP secretion from human and mouse platelets by an HPLC assay. Transfus Med Hemother Offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2013;40:109–116. doi: 10.1159/000350294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegatti P., Raffaghello L., Bianchi G., Piccardi F., Pistoia V., Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schenk U., Westendorf A.M., Radaelli E., Casati A., Ferro M., Fumagalli M. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 26.Locovei S., Bao L., Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atarashi K., Nishimura J., Shima T., Umesaki Y., Yamamoto M., Onoue M. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 28.Pettengill M.A., Marques-da-Silva C., Avila M.L., d'Arc dos Santos Oliveira S., Lam V.W., Ollawa I. Reversible inhibition of Chlamydia trachomatis infection in epithelial cells due to stimulation of P2X(4) receptors. Infect Immun. 2012;80:4232–4238. doi: 10.1128/IAI.00441-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feoktistov I., Polosa R., Holgate S.T., Biaggioni I. Adenosine A2B receptors: a novel therapeutic target in asthma? Trends Pharmacol Sci. 1998;19:148–153. doi: 10.1016/s0165-6147(98)01179-1. [DOI] [PubMed] [Google Scholar]
- 30.Hasko G., Cronstein B. Regulation of inflammation by adenosine. Front Immunol. 2013;4:85. doi: 10.3389/fimmu.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonioli L., Blandizzi C., Pacher P., Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13:842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 32.Burnstock G. P2X ion channel receptors and inflammation. Purinergic Signal. 2016;12:59–67. doi: 10.1007/s11302-015-9493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Virgilio F. Purinergic signalling in the immune system. A brief update. Purinergic Signal. 2007;3:1–3. doi: 10.1007/s11302-006-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes J.P., Hatcher J.P., Chessell I.P. The role of P2X (7) in pain and inflammation. Purinergic Signal. 2007;3:163–169. doi: 10.1007/s11302-006-9031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rokic M.B., Stojilkovic S.S. Two open states of P2X receptor channels. Front Cell Neurosci. 2013;7:215. doi: 10.3389/fncel.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rayah A., Kanellopoulos J.M., Di Virgilio F. P2 receptors and immunity. Microbes Infect Institut Pasteur. 2012;14:1254–1262. doi: 10.1016/j.micinf.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coutinho-Silva R., Ojcius D.M., Gorecki D.C., Persechini P.M., Bisaggio R.C., Mendes A.N. Multiple P2X and P2Y receptor subtypes in mouse J774, spleen and peritoneal macrophages. Biochem Pharmacol. 2005;69:641–655. doi: 10.1016/j.bcp.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Eltzschig H.K., Sitkovsky M.V., Robson S.C. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughn B.P., Robson S.C., Burnstock G. Pathological roles of purinergic signaling in the liver. J Hepatol. 2012;57:916–920. doi: 10.1016/j.jhep.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longhi M.S., Robson S.C., Bernstein S.H., Serra S., Deaglio S. Biological functions of ecto-enzymes in regulating extracellular adenosine levels in neoplastic and inflammatory disease states. J Mol Med. 2013;91:165–172. doi: 10.1007/s00109-012-0991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lammas D.A., Stober C., Harvey C.J., Kendrick N., Panchalingam S., Kumararatne D.S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 42.Molloy A., Laochumroonvorapong P., Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coutinho-Silva R., Perfettini J.L., Persechini P.M., Dautry-Varsat A., Ojcius D.M. Modulation of P2Z/P2X(7) receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol Cell Physiol. 2001;280:C81–C89. doi: 10.1152/ajpcell.2001.280.1.C81. [DOI] [PubMed] [Google Scholar]
- 44.Coutinho-Silva R., Stahl L., Raymond M.N., Jungas T., Verbeke P., Burnstock G. Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity. 2003;19:403–412. doi: 10.1016/s1074-7613(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 45.Darville T., Welter-Stahl L., Cruz C., Sater A.A., Andrews C.W., Jr., Ojcius D.M. Effect of the purinergic receptor P2X7 on Chlamydia infection in cervical epithelial cells and vaginally infected mice. J Immunol. 2007;179:3707–3714. doi: 10.4049/jimmunol.179.6.3707. [DOI] [PubMed] [Google Scholar]
- 46.Pettengill M.A., Lam V.W., Ojcius D.M. The danger signal adenosine induces persistence of chlamydial infection through stimulation of A2b receptors. PLoS One. 2009;4:e8299. doi: 10.1371/journal.pone.0008299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leonard C.A., Schoborg R.V., Borel N. Damage/Danger Associated Molecular Patterns (DAMPs) modulate Chlamydia pecorum and C. trachomatis Serovar E inclusion development in vitro. PLoS One. 2015;10:e0134943. doi: 10.1371/journal.pone.0134943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaul R., Wenman W.M. Cyclic AMP inhibits developmental regulation of Chlamydia trachomatis. J Bacteriol. 1986;168:722–727. doi: 10.1128/jb.168.2.722-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beatty W.L., Belanger T.A., Desai A.A., Morrison R.P., Byrne G.I. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leonhardt R.M., Lee S.J., Kavathas P.B., Cresswell P. Severe tryptophan starvation blocks onset of conventional persistence and reduces reactivation of Chlamydia trachomatis. Infect Immun. 2007;75:5105–5117. doi: 10.1128/IAI.00668-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Fritz J.H., Ferrero R.L., Philpott D.J., Girardin S.E. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 54.Werts C., Girardin S.E., Philpott D.J. TIR, CARD and PYRIN: three domains for an antimicrobial triad. Cell Death Differ. 2006;13:798–815. doi: 10.1038/sj.cdd.4401890. [DOI] [PubMed] [Google Scholar]
- 55.Girardin S.E., Boneca I.G., Carneiro L.A.M., Antignac A., Jéhanno M., Viala J. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 56.Reed J.C., Doctor K., Rojas A., Zapata J.M., Stehlik C., Fiorentino L. Comparative analysis of apoptosis and inflammation genes of mice and humans. Genome Res. 2003;13:1376–1388. doi: 10.1101/gr.1053803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ting J.P., Lovering R.C., Alnemri E.S., Bertin J., Boss J.M., Davis B.K. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdul-Sater A.A., Koo E., Hacker G., Ojcius D.M. Inflammasome-dependent caspase-1 activation in cervical epithelial cells stimulates growth of the intracellular pathogen Chlamydia trachomatis. J Biol Chem. 2009;284:26789–26796. doi: 10.1074/jbc.M109.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dinarello C.A. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 60.Alnemri E.S., Livingston D.J., Nicholson D.W., Salvesen G., Thornberry N.A., Wong W.W. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 61.Cerretti D.P., Kozlosky C.J., Mosley B., Nelson N., Van Ness K., Greenstreet T.A. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 62.Thornberry N.A., Bull H.G., Calaycay J.R., Chapman K.T., Howard A.D., Kostura M.J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 63.Mariathasan S., Newton K., Monack D.M., Vucic D., French D.M., Lee W.P. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 64.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 65.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 66.de Zoete M.R., Palm N.W., Zhu S., Flavell R.A. Inflammasomes. Cold Spring Harb Perspect Biol. 2014;6:a016287. doi: 10.1101/cshperspect.a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abdul-Sater A.A., Said-Sadier N., Ojcius D.M., Yilmaz O., Kelly K.A. Inflammasomes bridge signaling between pathogen identification and the immune response. Drugs Today (Barc) 2009;45(Suppl. B):105–112. [PMC free article] [PubMed] [Google Scholar]
- 68.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halle A., Hornung V., Petzold G.C., Stewart C.R., Monks B.G., Reinheckel T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mariathasan S., Weiss D.S., Newton K., McBride J., O'Rourke K., Roose-Girma M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 71.Martinon F., Petrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 72.Shi Y., Evans J.E., Rock K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 73.Sitkovsky M.V., Lukashev D., Apasov S., Kojima H., Koshiba M., Caldwell C. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 74.Yamasaki K., Muto J., Taylor K.R., Cogen A.L., Audish D., Bertin J. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem. 2009;284:12762–12771. doi: 10.1074/jbc.M806084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dostert C., Petrilli V., Van Bruggen R., Steele C., Mossman B.T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Franchi L., Eigenbrod T., Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hornung V., Bauernfeind F., Halle A., Samstad E.O., Kono H., Rock K.L. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abdul-Sater A.A., Said-Sadier N., Lam V.M., Singh B., Pettengill M.A., Soares F. Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial nod-like family member NLRX1. J Biol Chem. 2010;285:41637–41645. doi: 10.1074/jbc.M110.137885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdul-Sater A.A., Tattoli I., Jin L., Grajkowski A., Levi A., Koller B.H. Cyclic-di-GMP and cyclic-di-AMP activate the NLRP3 inflammasome. EMBO Rep. 2013;14:900–906. doi: 10.1038/embor.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Broz P., Newton K., Lamkanfi M., Mariathasan S., Dixit V.M., Monack D.M. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duncan J.A., Gao X., Huang M.T., O'Connor B.P., Thomas C.E., Willingham S.B. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kailasan Vanaja S., Rathinam V.A., Atianand M.K., Kalantari P., Skehan B., Fitzgerald K.A. Bacterial RNA: DNA hybrids are activators of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2014;111:7765–7770. doi: 10.1073/pnas.1400075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimada K., Crother T.R., Karlin J., Chen S., Chiba N., Ramanujan V.K. Caspase-1 dependent IL-1beta secretion is critical for host defense in a mouse model of Chlamydia pneumoniae lung infection. PLoS One. 2011;6:e21477. doi: 10.1371/journal.pone.0021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toma C., Higa N., Koizumi Y., Nakasone N., Ogura Y., McCoy A.J. Pathogenic vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-kappa B signaling. J Immunol. 2010;184:5287–5297. doi: 10.4049/jimmunol.0903536. [DOI] [PubMed] [Google Scholar]
- 85.Yilmaz O., Sater A.A., Yao L., Koutouzis T., Pettengill M., Ojcius D.M. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell Microbiol. 2010;12:188–198. doi: 10.1111/j.1462-5822.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Delaloye J., Roger T., Steiner-Tardivel Q.G., Le Roy D., Knaup Reymond M., Akira S. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Ichinohe T., Lee H.K., Ogura Y., Flavell R., Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ichinohe T., Pang I.K., Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010;11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kanneganti T.D., Body-Malapel M., Amer A., Park J.H., Whitfield J., Franchi L. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 90.Thomas P.G., Dash P., Aldridge J.R., Jr., Ellebedy A.H., Reynolds C., Funk A.J. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gross O., Poeck H., Bscheider M., Dostert C., Hannesschlager N., Endres S. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 92.Hise A.G., Tomalka J., Ganesan S., Patel K., Hall B.A., Brown G.D. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joly S., Ma N., Sadler J.J., Soll D.R., Cassel S.L., Sutterwala F.S. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lamkanfi M., Malireddi R.K., Kanneganti T.D. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284:20574–20581. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tiemi Shio M., Eisenbarth S.C., Savaria M., Vinet A.F., Bellemare M.J., Harder K.W. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5:e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee G.S., Subramanian N., Kim A.I., Aksentijevich I., Goldbach-Mansky R., Sacks D.B. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murakami T., Ockinger J., Yu J., Byles V., McColl A., Hofer A.M. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Franchi L., Kanneganti T.D., Dubyak G.R., Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 99.Munoz-Planillo R., Kuffa P., Martinez-Colon G., Smith B.L., Rajendiran T.M., Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 101.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 102.Nakahira K., Haspel J.A., Rathinam V.A., Lee S.J., Dolinay T., Lam H.C. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rasmussen S.J., Eckmann L., Quayle A.J., Shen L., Zhang Y.X., Anderson D.J. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carlin J.M., Weller J.B. Potentiation of interferon-mediated inhibition of Chlamydia infection by interleukin-1 in human macrophage cultures. Infect Immun. 1995;63:1870–1875. doi: 10.1128/iai.63.5.1870-1875.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Entrican G., Wilkie R., McWaters P., Scheerlinck J., Wood P.R., Brown J. Cytokine release by ovine macrophages following infection with Chlamydia psittaci. Clin Exp Immunol. 1999;117:309–315. doi: 10.1046/j.1365-2249.1999.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gervassi A., Alderson M.R., Suchland R., Maisonneuve J.F., Grabstein K.H., Probst P. Differential regulation of inflammation cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection. Infect Immun. 2004;72:7231–7239. doi: 10.1128/IAI.72.12.7231-7239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heinemann M., Susa M., Simnacher U., Marre R., Essig A. Growth of Chlamydia pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect Immun. 1996;64:4872–4875. doi: 10.1128/iai.64.11.4872-4875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ojcius D.M., Souque P., Perfettini J.L., Dautry-Varsat A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J Immunol. 1998;161:4220–4226. [PubMed] [Google Scholar]
- 110.Rothermel C.D., Schachter J., Lavrich P., Lipsitz E.C., Francus T. Chlamydia trachomatis-induced production of interleukin-1 by human monocytes. Infect Immun. 1989;57:2705–2711. doi: 10.1128/iai.57.9.2705-2711.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prantner D., Darville T., Sikes J.D., Andrews C.W., Jr., Brade H., Rank R.G. Critical role for interleukin-1beta (IL-1beta) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1beta in mouse macrophages. Infect Immun. 2009;77:5334–5346. doi: 10.1128/IAI.00883-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Darville T., Andrews C.W., Laffoon K.K., Shymasani W., Kishen L.R., Rank R.G. Mouse strain-dependent variation in the course and outcome of chlamydial genital infection is associated with differences in host response. Infect Immun. 1997;65:3064–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Darville T., Andrews C.W., Sikes J.D., Fraley P.L., Rank R.G. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect Immun. 2001;69:3556–3561. doi: 10.1128/IAI.69.6.3556-3561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ward M.E. Mechanisms of Chlamydia-induced disease. In: Stephens R.S., editor. Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press; Washington, D.C.: 1999. pp. 171–210. [Google Scholar]
- 115.Hvid M., Baczynska A., Deleuran B., Fedder J., Knudsen H.J., Christiansen G. Interleukin-1 is the initiator of Fallopian tube destruction during Chlamydia trachomatis infection. Cell Microbiol. 2007;9:2795–2803. doi: 10.1111/j.1462-5822.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 116.Cheng W., Shivshankar P., Li Z., Chen L., Yeh I.T., Zhong G. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect Immun. 2008;76:515–522. doi: 10.1128/IAI.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu H., Shen C., Brunham R.C. Chlamydia trachomatis infection of epithelial cells induces the activation of caspase-1 and release of mature IL-18. J Immunol. 2000;165:1463–1469. doi: 10.4049/jimmunol.165.3.1463. [DOI] [PubMed] [Google Scholar]
- 118.He X., Mekasha S., Mavrogiorgos N., Fitzgerald K.A., Lien E., Ingalls R.R. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. 2010;184:5743–5754. doi: 10.4049/jimmunol.0903937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Darville T., O'Neill J.M., Andrews C.W., Jr., Nagarajan U.M., Stahl L., Ojcius D.M. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171:6187–6197. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- 120.Netea M.G., Kullberg B.J., Galama J.M., Stalenhoef A.F., Dinarello C.A., Van der Meer J.W. Non-LPS components of Chlamydia pneumoniae stimulate cytokine production through toll-like receptor 2-dependent pathways. Eur J Immunol. 2002;32:1188–1195. doi: 10.1002/1521-4141(200204)32:4<1188::AID-IMMU1188>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 121.Prebeck S., Kirschning C., Durr S., da Costa C., Donath B., Brand K. Predominant role of toll-like receptor 2 versus 4 in Chlamydia pneumoniae-induced activation of dendritic cells. J Immunol. 2001;167:3316–3323. doi: 10.4049/jimmunol.167.6.3316. [DOI] [PubMed] [Google Scholar]
- 122.Kanneganti T.D., Lamkanfi M., Kim Y.G., Chen G., Park J.H., Franchi L. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 123.Li Z., Chen D., Zhong Y., Wang S., Zhong G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008;76:3415–3428. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cao W., Zou Y., Su S., He Z., Liu Y., Huang Q. Chlamydial plasmid-encoded protein pORF5 induces production of IL-1beta and IL-18 via NALP3 inflammasome activation and p38 MAPK pathway. Int J Clin Exp Med. 2015;8:20368–20379. [PMC free article] [PubMed] [Google Scholar]
- 125.Eitel J., Meixenberger K., van Laak C., Orlovski C., Hocke A., Schmeck B. Rac1 regulates the NLRP3 inflammasome which mediates IL-1beta production in Chlamydophila pneumoniae infected human mononuclear cells. PLoS One. 2012;7:e30379. doi: 10.1371/journal.pone.0030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jorgensen I., Bednar M.M., Amin V., Davis B.K., Ting J.P., McCafferty D.G. The Chlamydia protease CPAF regulates host and bacterial proteins to maintain pathogen vacuole integrity and promote virulence. Cell Host Microbe. 2011;10:21–32. doi: 10.1016/j.chom.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Omosun Y., McKeithen D., Ryans K., Kibakaya C., Blas-Machado U., Li D. Interleukin-10 modulates antigen presentation by dendritic cells through regulation of NLRP3 inflammasome assembly during Chlamydia infection. Infect Immun. 2015;83:4662–4672. doi: 10.1128/IAI.00993-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nagarajan U.M., Sikes J.D., Yeruva L., Prantner D. Significant role of IL-1 signaling, but limited role of inflammasome activation, in oviduct pathology during Chlamydia muridarum genital infection. J Immunol. 2012;188:2866–2875. doi: 10.4049/jimmunol.1103461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carabeo R.A., Mead D.J., Hackstadt T. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc Natl Acad Sci U S A. 2003;100:6771–6776. doi: 10.1073/pnas.1131289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hackstadt T., Fischer E.R., Scidmore M.A., Rockey D.D., Heinzen R.A. Origins and functions of the chlamydial inclusion. Trends Microbiol. 1997;5:288–293. doi: 10.1016/S0966-842X(97)01061-5. [DOI] [PubMed] [Google Scholar]
- 131.Hackstadt T., Scidmore M.A., Rockey D.D. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci U S A. 1995;92:4877–4881. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heuer D., Lipinski A.R., Machuy N., Karlas A., Wehrens A., Siedler F. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature. 2009;457:731–735. doi: 10.1038/nature07578. [DOI] [PubMed] [Google Scholar]
- 133.Itoh R., Murakami I., Chou B., Ishii K., Soejima T., Suzuki T. Chlamydia pneumoniae harness host NLRP3 inflammasome-mediated caspase-1 activation for optimal intracellular growth in murine macrophages. Biochem Biophys Res Commun. 2014;452:689–694. doi: 10.1016/j.bbrc.2014.08.128. [DOI] [PubMed] [Google Scholar]
- 134.Finethy R., Jorgensen I., Haldar A.K., de Zoete M.R., Strowig T., Flavell R.A. Guanylate binding proteins enable rapid activation of canonical and noncanonical inflammasomes in Chlamydia-infected macrophages. Infect Immun. 2015;83:4740–4749. doi: 10.1128/IAI.00856-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Silverman W.R., de Rivero Vaccari J.P., Locovei S., Qiu F., Carlsson S.K., Scemes E. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McKuen M.J., Dahl G., Fields K.A. Assessing a potential role of host Pannexin 1 during Chlamydia trachomatis infection. PLoS One. 2013;8:e63732. doi: 10.1371/journal.pone.0063732. [DOI] [PMC free article] [PubMed] [Google Scholar]