Abstract
The inflammasome has been mainly studied in innate immune cells in which it senses microbes and cellular damage, and induces secretion of pro-inflammatory cytokines. This process induces an inflammatory response that is critical for the resolution of infections and repair of tissue damage following injury. Recent studies indicate that inflammasome complex formation also participates in many other cellular and physiological processes beyond modulation of inflammation, such as autophagy, metabolism, eicosanoids production, and phagosome maturation.
Keywords: Autophagy, Inflammation, Innate immunity, NLRP3, Non-canonical inflammasome
The inflammasome has been mainly studied in innate immune cells in which it senses microbes and cellular damage, activates caspase-1, and induces secretion of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 [see Box 1 and Fig. 1A] [1], [2], [3]. This process stimulates an inflammatory response that is critical for the resolution of infections and repair of tissue damage following injury. The inflammasome is thus involved in various diseases associated with chronic inflammation, including Alzheimer's disease, atherosclerosis, cancer, gout, inflammatory bowel disease, microbial infections, and type 2 diabetes [1], [2], [3]. Recent studies indicate that inflammasome complex formation participates in many other cellular and physiological processes beyond modulation of inflammation, such as autophagy, metabolism, eicosanoids production, and phagosome maturation [4].
Box 1. Canonical inflammasome activation.
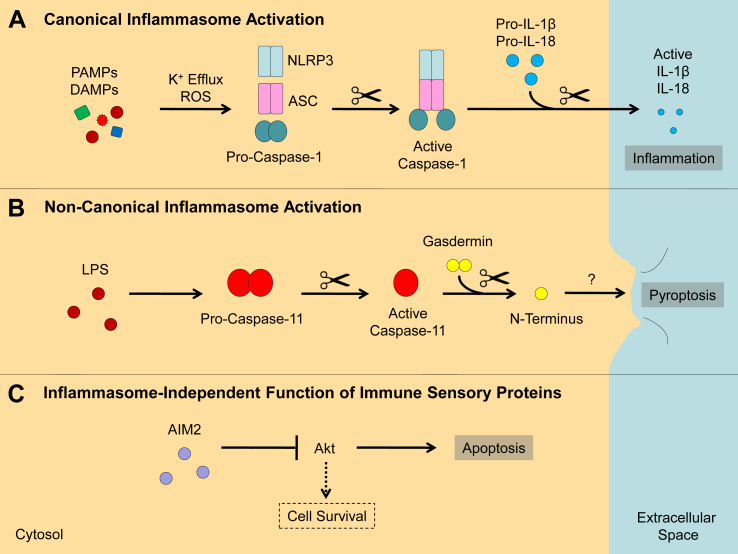
Inflammasomes are intracellular protein complexes that contain an immune sensor protein (e.g., AIM2, NLRC4, NLRP1, or NLRP3), caspase-1, and, in many cases, the adaptor protein ASC. Some inflammasomes are directly activated by pathogen-associated molecular patterns (PAMPs). For example, the AIM2 inflammasome is activated by cytosolic double-strand DNA, whereas NLRC4 is stimulated by bacterial flagellin. In contrast, the NLRP3 inflammasome is activated by a wide range of stimuli, including PAMPs such as lipopolysaccharide (LPS), as well as danger-associated molecular patterns (DAMPs) including extracellular adenosine triphosphate (ATP) and uric acid crystals. The observation that diverse stimuli activate the NLRP3 inflammasome suggests that PAMPs and DAMPs may not interact with NLRP3 but may instead activate the inflammasome indirectly via other cellular pathways such as potassium efflux or production of reactive oxygen species (ROS).
Two signals are usually needed to induce secretion of IL-1β and IL-18 in innate immune cells. The first signal may represent a PAMP such as LPS which binds to a Toll-like receptor (TLR), leading to activation of NF-κB and expression of pro-IL-1β and pro-IL-18. The second signal may consist of a DAMP such as ATP which is released from damaged or stressed cells. In this case, ATP may bind to the purinergic receptor P2X7 and result in inflammasome complex formation, activation of caspase-1, and cleavage and secretion of active IL-1β and IL-18. These cytokines induce inflammation not only in response to tissue injury or metabolic perturbations but also following contact with microbes and environmental toxins. For this reason, inflammasome activation has been linked with a wide range of diseases associated with chronic inflammation (e.g., atherosclerosis, cancer, infection, obesity, and type 2 diabetes).
Fig. 1.
Mode of activation of the inflammasome and immune sensory proteins. (A) Pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) may induce potassium (K+) efflux and production of reactive oxygen species (ROS) which in turn induce formation of the inflammasome complex (containing NLRP3 in the case depicted here). Canonical inflammasome activation leads to activation of caspase-1 which converts pro-IL-1β and pro-IL-18 into their active forms. The pro-inflammatory cytokines are secreted by the cell and may induce an inflammatory response. (B) Non-canonical inflammasome activation may involve bacterial lipopolysaccharide (LPS) which binds and activates caspase-11 in mice (or caspase-4/5 in humans). One substrate of this enzyme is gasdermin, whose cleaved N-terminus induces cell death by pyroptosis via a mechanism that remains to be elucidated. (C) Immune sensory proteins such as AIM2 also have inflammasome-independent functions. In the case illustrated here, AIM2 inhibits Akt, preventing the effect of the latter protein on cell survival and leading to cell death by apoptosis.
Autophagy has been recognized as an important cellular process involving the destruction and recycling of unnecessary or dysfunctional proteins and organelles. Autophagy inhibits inflammasome activation by removing damaged mitochondria which produce inflammasome-activating reactive oxygen species (ROS) [5], [6]. Furthermore, autophagy mediates the removal of inflammasome complexes, thereby inhibiting caspase-1 activation [7]. Conversely, inflammasome activation inhibits autophagy. For instance, when macrophages are infected with Shigella flexneri bacteria, activation of the NLCR4 inflammasome and caspase-1 inhibits autophagy and autophagosome formation [8]. Given that autophagy is normally associated with cell survival in response to stress whereas inflammasome activation is associated with rapid demise of the cell (as when pyroptosis occurs), regulation of these processes may determine cellular fate in response to environmental stimuli.
A recent study showed that autophagy is involved in IL-1β secretion following activation of the AIM2 inflammasome [9]. Activation of AIM2, which forms a caspase-1-activating inflammasome upon binding to cytosolic DNA, was shown to engage end-binding protein 1 (EB1), leading to co-localization with the microtubule-organizing center (MTOC) and incorporation within autophagosomes destined for secretion. Another study showed that the NLRP3 inflammasome binds to the centrosome-associated proteins tubulin and centrin in a caspase-1-dependent manner [10]. Interaction between these proteins leads to breakdown of the microtubule network, a process that may be required for inflammasome-mediated cell death by pyroptosis.
Metabolism is also associated with inflammasome activation. A proteomic-based search for the substrates of caspase-1 identified many glycolysis enzymes such as aldolase, triose-phosphate isomerase, glyceraldehyde-3-phosphate dehydrogenase, α-enolase, and pyruvate kinase [11]. The authors of this study suggested that inhibition of glycolysis in bacteria-infected cells may lead to cell death and limit the growth of intracellular bacteria. Moreover, caspase-1 can activate sterol regulatory element-binding proteins (SREBPs) in cells treated with bacterial pore-forming toxins [12]. In this context, activation of SREBPs, which activate transcription of genes involved in cholesterol and fatty acid biosynthesis, was associated with repair of the plasma membrane following damage by pore-forming toxins.
Eicosanoids are lipid molecules that include leukotrienes, prostaglandins, and thromboxanes. Produced from 20-carbon fatty acids, they are involved in various physiological functions such as inflammation, immunity against pathogens, pain sensation, smooth muscle contraction, and vasodilation. Stimulation of the NLRP1 and NLRC4 inflammasomes activates caspase-1, which in turn leads to membrane pore formation, intracellular calcium influx, and phospholipase A2 activation [13]. This enzyme releases arachidonic acid from phospholipids present in the cell membrane. Arachidonic acid is converted into active eicosanoids by cyclooxygenase and lipoxygenases. This “eicosanoid storm” is produced mainly by peritoneal macrophages and is associated with vasodilation, fluid loss from blood, diarrhea, hypothermia and even death in response to inflammasome triggers such as bacterial lipopolysaccharide (LPS) [13]. Notably, this inflammatory response is independent of IL-1β and IL-18, which are commonly secreted in response to inflammasome activation.
The inflammasome also contributes to phagosome maturation. The NLRP3 inflammasome is activated following phagocytosis of bacteria by macrophages, possibly due to the cytosolic production of ROS by the NADPH oxidase Nox2 [14]. Activated caspase-1 molecules accumulate on phagosomes harboring bacteria, leading to assembly of the NADPH oxidase complex on the phagosomal membrane. The NADPH enzyme then acidifies the lumen of the phagosome, activating proteases that degrade bacterial proteins [14]. The NLRP3 inflammasome therefore participates in the killing of bacteria engulfed by macrophages.
The inflammasome also plays important functions in epithelial cells, which have attracted less attention than cells of hematopoietic origin (reviewed recently [15]). In this context, the inflammasome may induce processing of pro-inflammatory cytokines in response to pathogens and cellular damage, similar to its role in innate immune cells. On the other hand, activation of the inflammasome in epithelial cells is involved in other cellular and physiological processes, including tissue repair and regulation of pathogen growth. For instance, mice that are deficient in the inflammasome component NLRP3 show reduced healing in response to cutaneous wounds [16]. In addition, our group has reported that Chlamydia trachomatis shows reduced growth in the absence of caspase-1 in human HeLa cervical cancer cells [17]. These studies demonstrate that the functions of inflammasomes are by no means limited to pro-inflammatory functions in innate immune cells.
Recent evidence indicates that inflammasome components are required for several functions independently of their capacity to form inflammasome complexes. For instance, NLRP3 binds to the transcription factor IRF4 in CD4+ T cells, leading to activation of the Th2 cell differentiation program [18]. Accordingly, mice lacking NLRP3 show reduced growth of melanoma tumors and lower levels of asthma-like symptoms compared to wild-type mice, and these effects are due to NLRP3 deficiency in CD4+ T cells but not in myeloid cells. Furthermore, AIM2 inhibits the proliferation of colon tumors by preventing activation of Akt [Fig. 1C], an important regulator of cell survival and proliferation [19].
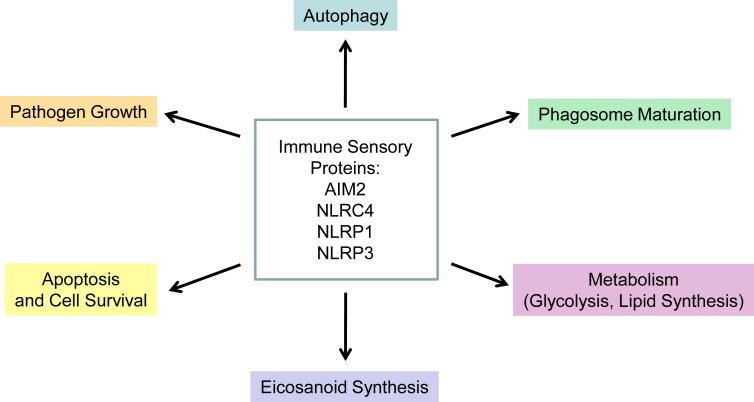
IL-1β and IL-18 were initially thought to be secreted due to a simple two-step mechanism involving detection of the microbial product, followed by danger-induced activation of an inflammasome and caspase-1 [Box 1] [1], [2], [3]. Subsequently, non-canonical and alternative inflammasomes involving caspase-11 or caspase-8 were described [Fig. 1B] [20], [21], [22]. Finally, the inflammasomes are now known to play many roles that are unrelated to inflammation [Fig. 2]. Perhaps it is time to specify which inflammasome activity is being characterized, such as the metabolic inflammasome or inflammatory inflammasome, or to rename this protein complex with a broader term corresponding to its broader functions. Even without a new name, this awesome protein complex is certain to reveal more novel functions in the future.
Fig. 2.
Alternative functions of inflammasomes and immune sensory proteins.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Martinon F., Mayor A., Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 2.Strowig T., Henao-Mejia J., Elinav E., Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 3.Saïd-Sadier N., Ojcius D.M. Alarmins, inflammasomes and immunity. Biomed J. 2012;35:437–449. doi: 10.4103/2319-4170.104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rathinam V.A.K., Fitzgerald K.A. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165:792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine B., Mizushima N., Virgin H.W. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rathinam V.A., Vanaja S.K., Fitzgerald K.A. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–342. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi C.S., Shenderov K., Huang N.N., Kabat J., Abu-Asab M., Fitzgerald K.A. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T., Franchi L., Toma C., Ashida H., Ogawa M., Yoshikawa Y. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L.J., Huang H.Y., Huang M.P., Liou W., Chang Y.T., Wu C.C. The microtubule-associated protein EB1 links AIM2 inflammasomes with autophagy-dependent secretion. J Biol Chem. 2014;289:29322–29333. doi: 10.1074/jbc.M114.559153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis M., Fairgrieve M., Gale M. 2016. A surprising link between the inflammasome and the centrosome (abstract): May 13–17; Annual Meeting of the American Association of Immunologists. Seattle, WA. [Google Scholar]
- 11.Shao W., Yeretssian G., Doiron K., Hussain S.N., Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282:36321–37229. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 12.Gurcel L., Abrami L., Girardin S., Tschopp J., van der Goot F.G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 13.von Moltke J., Trinidad N.J., Moayeri M., Kintzer A.F., Wang S.B., van Rooijen N. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokolovska A., Becker C.E., Ip W.K., Rathinam V.A., Brudner M., Paquette N. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat Immunol. 2013;14:543–553. doi: 10.1038/ni.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santana P.T., Martel J., Lai H.C., Perfettini J.L., Kanellopoulos J.M., Young J.D. Is the inflammasome relevant for epithelial cell function? Microbes Infect. 2016;18:93–101. doi: 10.1016/j.micinf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Weinheimer-Haus E.M., Mirza R.E., Koh T.J. Nod-like receptor protein-3 inflammasome plays an important role during early stages of wound healing. PLoS One. 2015;10:e0119106. doi: 10.1371/journal.pone.0119106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdul-Sater A.A., Koo E., Hacker G., Ojcius D.M. Inflammasome-dependent caspase-1 activation in cervical epithelial cells stimulates growth of the intracellular pathogen Chlamydia trachomatis. J Biol Chem. 2009;284:26789–26796. doi: 10.1074/jbc.M109.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruchard M., Rebe C., Derangere V., Togbe D., Ryffel B., Boidot R. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16:859–870. doi: 10.1038/ni.3202. [DOI] [PubMed] [Google Scholar]
- 19.Wilson J.E., Petrucelli A.S., Chen L., Koblansky A.A., Truax A.D., Oyama Y. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med. 2015;21:906–913. doi: 10.1038/nm.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 21.Kayagaki N., Wong M.T., Stowe I.B., Ramani S.R., Gonzalez L.C., Akashi-Takamura S. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 22.Chung H., Vilaysane A., Lau A., Stahl M., Morampudi V., Bondzi-Simpson A. NLRP3 regulates a non-canonical platform for caspase-8 activation during epithelial cell apoptosis. Cell Death Differ. 2016;23:1331–1346. doi: 10.1038/cdd.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]