Abstract
Human schistosomiasis is a chronic inflammatory disease caused by blood fluke worms belonging to the genus Schistosoma. Health metrics indicate that the disease is related to an elevated number of years lost-to-disability and years lost-to-life. Schistosomiasis is an intravascular disease that is related to a Th1 and Th2 immune response polarization, and the degree of polarization affects the outcome of the disease. The purinergic system is composed of adenosine and nucleotides acting as key messenger molecules. Moreover, nucleotide-transforming enzymes and cell-surface purinergic receptors are obligatory partners of this purinergic signaling. In mammalian cells, purinergic signaling modulates innate immune responses and inflammation among other functions; conversely purinergic signaling may also be modulated by inflammatory mediators. Moreover, schistosomes also express some enzymes of the purinergic system, and it is possible that worms modulate host purinergic signaling. Current data obtained in murine models of schistosomiasis support the notion that the host purinergic system is altered by the disease. The dysfunction of adenosine receptors, metabotropic P2Y and ionotropic P2X7 receptors, and NTPDases likely contributes to disease morbidity.
Keywords: Purinergic receptor, NTPDases, Schistosomiasis, Macrophages, Endothelial cell, Inflammation
Schistosomiasis
Human schistosomiasis (or bilharzia) is a chronic inflammatory disease caused by blood fluke worms belonging to the genus Schistosoma. According to the World Health Organization (WHO), more than 200 million people worldwide suffer from chronic schistosomiasis and approximately 800 million people live in schistosomiasis-endemic areas. Schistosomiasis is therefore considered to be one of the world's most prevalent infectious diseases (http://www.who.int/schistosomiasis/en/, as of June 2016). This neglected tropical disease largely affects people living in poverty. The Disability-Adjusted Life-Year (DALY), a time-based measure used as a health metric, considers the number of years lost-to-disability and the years lost-to-life of a specific disease. According to recent data, schistosomiasis is related to a DALY of 3.3 million and is associated with a substantial socioeconomic burden in low- and middle-income countries [1], [2].
Schistosoma lifecycle
The majority of cases of human schistosomiasis are caused by three main species of the genus Schistosoma: Schistosoma mansoni, Schistosoma japonicum and Schistosoma hematobium. Each species has a geographical and pathological importance: S. mansoni (in Africa, the Middle East and the Americas) and S. japonicum (in South and Middle Asia) cause intestinal and hepatosplenic schistosomiasis; S. hematobium (in Africa and the Middle East) causes urinary schistosomiasis [3].
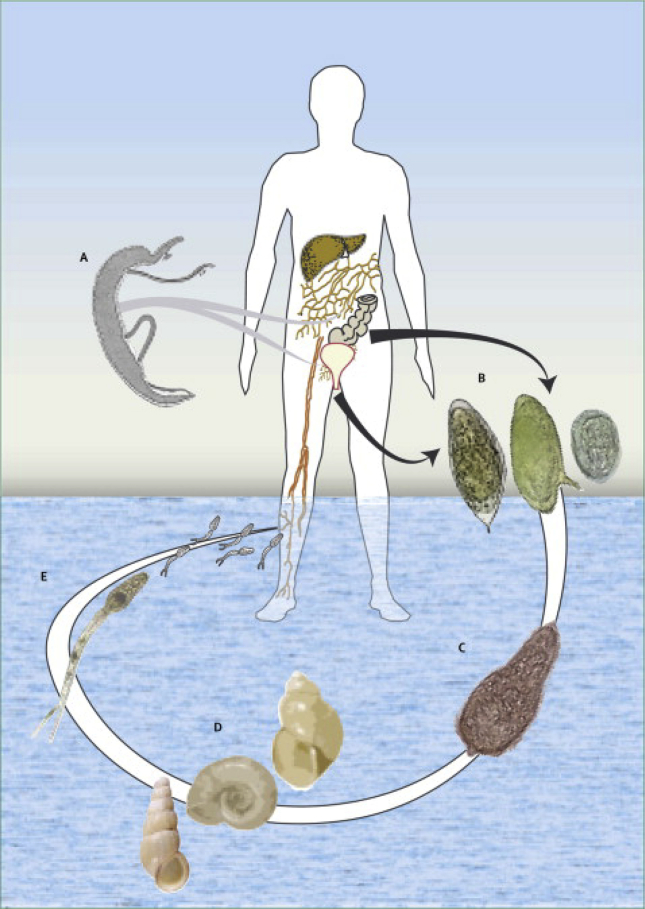
The parasite lifecycle includes an intermediate (snail) and a definitive (mammalian) host [3] and shows remarkable features of adaptive biology. Infected aquatic snails release cercariae that are highly infective for the definitive host. The human infection starts when an individual comes into contact with a body of water infested with cercariae. These larval forms explore host thermostatic gradient or chemical signals, attach to the epidermis and penetrate human skin. Both the head and acetabular cercarial glands secrete enzymes such as elastases that are serine proteases and immunomodulators involved in epidermis and dermis penetration [4]. After entering the dermis, cercariae must reach a venule or a lymphatic vessel while transforming into schistosomula [5], [6]. Perivascular CD4+ T cells are found in human skin accompanied by interleukin (IL)-7 production that seems to favor skin invasion and worm survival [7]. Next, during schistosomula migration via the heart and lungs to their specific vascular site, they undergo a series of structural and physiologic transformations before becoming adults. Adult worms mate in the vessels of the intestine (S. mansoni and S. japonicum) or the vesical plexus around the bladder (S. hematobium) and then become permanent pairs and start oviposition. Each egg contains a miracidium larva and secretes proteolytic enzymes that favor egg migration into the lumen of the intestine or bladder; these eggs are then consequently excreted in feces or urine, respectively. In due course, eggs that are not eliminated are trapped in the organs and result in immune responses. Voided eggs, once in contact with freshwater, release the free-swimming miracidium that infects snails and a new lifecycle is started [Fig. 1]. The asexual reproduction of miracidium in the snails produces hundreds to thousands of cercariae [3]. In light of the schistosoma lifecycle, the host immune system is actually challenged by the three stages of the parasite (cercariae, migrating schistosomula and adult worms) and also by eggs [8], [9].
Fig. 1.
Illustrated schematic of the Schistosoma lifecycle. Infected snails (D) release the larval form cercariae (E) in a body of water. Human schistosomiasis starts after the cercariae penetration the skin in direction to a venule or lymphatic vessel (A). The worms navigate through the cardiovascular system reaching vessels from the gut (S. mansoni and S. japonicum) or bladder (S. hematobium). Adult worms mate, and females lay hundreds of eggs daily (B). The eggs release miracidium in the water (C), which is infective for snails. The asexual reproduction of a miracidium generates several cercariae that are released via the snail. Adult male worms are about 1 cm long. They have smooth muscle layers beneath the external tegument and two suckers by which they attach to the blood vessel wall.
Reproduced from Ref. [3] with permission.
Host immunologic responses during schistosomal infection
Human and schistosomes co-evolved, which favored worm survival in the host [10]. Some abilities of schistosomes have been noted to favor parasite survival (e.g., the capacity to regenerate the outer tegument, molecular mimicry, acquirement of host antigens and immunomodulation) [8]. Murine infection with S. mansoni resembles human infection, and therefore, several lines of data have derived from this experimental model [8].
Worm- and egg-derived antigens recognized by T and B lymphocytes modulate host immune system by down- or up-regulating cellular and humoral immune responses [8], [10], [11], [12], [13], [14], [15], [16], [17]. Briefly, the host immune response polarizes to a Th1-cell response in the first five weeks after infection. At this stage, Th1 cells and peripheral mononuclear blood cells produce large amounts of tumor necrosis factor (TNF)-α, interferon (IFN)-γ, IL-1 and IL-6. In the very beginning of the infection, lung immune responses are able to kill schistosomula [11]. However, Trottein and colleagues [18] showed that schistosomula reduces lung vascular cell adhesion molecule (VCAM)-1 expression and leukocyte recruitment, suggesting that at this stage of the disease endothelial cells are driven to an anti-inflammatory phenotype. As the worms mature, mate and start egg deposition, a largely Th2 response emerges with the production of cytokines such as IL-4, IL-5 and IL-13, as well as eosinophilia and intestinal and mesenteric mastocytosis, and high circulating levels of IgE [8]. IL-17 production is also tightly regulated by IFN-γ and IL-4. The capacity of limiting the pro-inflammatory Th1 response is essential for host survival. In murine model, the infection of IL-4 knockout mice (a model of polarized Th1 response) resulted in tissue damage and mortality [8], [19], [20], [21], [22]. Natural regulatory T cell (Treg) also modulates Th cell responses during schistosomiasis [23].
Macrophages are important cells for host defense that may be primed by signals from the extracellular milieu. Each subset of macrophage phenotype has distinct patterns of gene expression producing pro- and anti-inflammatory mediators [24]. The different subsets of macrophage phenotypes include the classically [cMФ (classically activated macrophage); M1 macrophages] or alternatively activated phenotypes [aaMФ (alternatively activated macrophage); M2 macrophages]. Furthermore, macrophages may express M1 and M2 markers resulting in intermediate polarization subsets [24].
Concerning schistosomiasis, aaMФ are typically found in the vicinity of granuloma and Th2 cytokines, and express the enzyme arginase 1 (Arg-1) that limits Th2-driven fibrosis [21]. Infected mice with polarized Th1 responses exhibit reduced aaMФ and granuloma formation, high inducible nitric oxide synthase (iNOS) responses and mortality due to severe intestinal and liver pathology [25], [26], [27], [28], [29].
Eggs lodged in tissues may cause necrosis and host defenses form a granuloma around the eggs in order to contain the insult, although the granuloma may also be deleterious to host physiology due to fibrosis and portal hypertension [12], [27]. The morbidity of the chronic disease takes into account the level of fibrosis and inflammation [8], [22]. According to previous data using a murine model, IL-13 plays an important role in liver fibrosis and Th1 response mediators limit such action [30], [31]. Patients with severe fibrosis were found to have high levels of TNF-α, IL-5, IL-10 and IL-13; patients with mild fibrosis showed high IFN-γ levels [27]. Interesting, although it is believed that Th1 and Th2 responses are mutually exclusive phenotypes of CD4+ helper T cell, Deaton and colleagues [32] identified IFN-γ+ IL-4+ cells in S. mansoni-infected mice. It is possible that a balance between Th1 and Th2 cytokines may counter-regulate the excessive fibrosis related to a prolonged Th2 immune response [32] and determine the outcome of the disease. Furthermore, extraimmunological effectors might also modulate immune responses during schistosomiasis [27].
Schistosoma biology: evidence of the worm purinergic system
Considering the three main species of schistosomes, molecular biology data referred initially to S. mansoni and S. japonicum; complete genome sequences of these species were published in 2009 [33], [34]. Data pertaining to the genome of S. hematobium were published some years later [35]. By relying on proteomic data and bioinformatics, it has been possible to construct schistosome phylogenetic trees of proteins encoded by the genome, the so-called phylome [4]. According to current knowledge, S. mansoni is more closely related to S. hematobium (89.4%) than to S. japonicum (67%) [4]. The genomes were revised and are available in a database (SchistoDB; [36]). Additionally, the number of known proteins is higher for S. mansoni than for the other two species [37].
According to the genome of S. mansoni, the worm has at least four genes related to P2X receptors subunits [33]. Previously, the gene of a P2X-like receptor (schP2X) was cloned and heterologously expressed in Xenopus oocytes. Electrophysiological studies have revealed that both ATP and the ATP analog benzoyl-ATP (BzATP) evoked inward currents at these recombinant schP2X receptors that were blocked completely by suramin and partially by pyridoxalphosphate-6-azophenyl-20,40-disulfonic acid (PPADS) (100 μM) [38]. Furthermore, high agonist concentrations desensitized the receptor [39]. A comparison of the amino acid sequence between schP2X and human P2X(1-7) receptors revealed an identity of 25.8% for P2X7 and 36.6% for P2X4 receptors [38] and 36% for P2X5 [39]; the latter authors referred to the receptor as SmP2X. This level of identity is remarkable since an acoelomate primitive platyhelminth has been considered to be the organism from which many other phyla have evolved [40], and some Schistosoma genes may be ancestors of mammalian genes [41]. Moreover, S. mansoni expresses other enzymes and channels involved in intracellular Ca2+ homeostasis that were initially identified by functional assays including ATPases and Ca2+ channels [42], [43], [44], [45], [46] as well as receptors for important neurotransmitters such as GABA, glutamate and acetylcholine [47], [48], [49]. These functional data have been corroborated by the genomic description of both S. mansoni [33] and S. japonicum [34].
Purines are considered to be one of the most primitive chemical messengers in the animal kingdom [50]. Schistosomes take advantage of host signaling pathways. Unlike mammalian cells, schistosomes are unable to synthesize purine nucleotides de novo. Therefore, they depend on host-preformed purines and salvage pathways for the conversion of bases and nucleosides back into nucleotides [51].
It has been suggested that nucleotide hydrolysis occurs in the tegument (external face) of the worms near the site of the uptake of the products of such hydrolysis [52], [53].
The tegumental nucleotide-metabolizing ecto-enzymes are alkaline phosphatase (smAP), ecto-phosphodiesterase (smPDE) and ecto-ATP diphosphohydrolase (also known as apyrase or E-NTPDase) (smATPDase) [54], [55]. smATPDase1 is expressed in schistosomula, female and male worms and seems to hydrolyze ATP and ADP equally (i.e., the enzyme has the same affinity for both substrates) [55], [56]. On the other hand, the homolog smATPDase2 is not present in the tegument but is highly expressed in eggs (compared with worms), and the evidence obtained so far has not shown that this enzyme is responsible for the hydrolysis of exogenous (host-derived) ATP or ADP [55]. Nevertheless, a synthetic peptide belonging to smATPDase2 was shown to be immunogenic when injected into mice [57]. Moreover, an IgG antibody from schistosomiasis patients showed cross-immunoreactivity with a domain from smATPDase2 [58], which could be useful for diagnostic purposes or vaccine development. Furthermore, adenosine monophosphate (AMP) seems to be hydrolyzed by smAP [55].
On the other hand, intact schistosomes are able to deaminate adenosine to inosine and convert adenosine to adenine, which suggests that the worms also possess adenosine deaminase (ADA) and adenosine phosphorylase [51], [59]. However, the conversion of adenosine to AMP by adenosine kinase seems to be minor [50]. Moreover, schistosomes are also able to convert the adenine analog 2-fluoroadenine into 2-fluoroadenine nucleotides such as 2-fluoro ATP [60]. Therefore, schistosomes also have adenine phosphoribosyltransferase and nucleoside kinases [51], [61], and they incorporate adenine into ATP in a higher rate than that of mammalian cells [62], [63]. Overall, schistosomes are able to convert adenosine, adenine and inosine to AMP and ultimately to ATP. In addition, inosine may also be converted to inosine monophostate (IMP) and ultimately to GTP [51].
Since schistosomes have a purine salvage network, it has been proposed that purine analogs could be potential antischistosomal drugs. However, so far no such drug has been shown to be both effective and safe for humans. A unique antischistosomal drug in clinical use, praziquantel (at micromolar concentrations), is able to reduce both [3H]-adenosine and [3H]-uridine worm uptake [64], but other mechanisms such as the influx of Ca2+, worm muscle paralysis, inhibition of P-glycoprotein-like protein [a member of ATP-binding-cassette (ABC) superfamily of proteins] and tegument rupture have been noted as relevant for the pharmacological effect [65], [66], [67]. However, there have been reports of resistance to praziquantel, and the development of new drugs for this neglected tropical disease is most welcome. In this context, the worm purine nucleoside phosphorylase has been considered to be a putative new target for new antischistosomal drugs [37].
Purinergic signaling
Cellular ATP was identified at the end of the 1920s [68]. Concomitantly, some initial evidence of a purine acting as a chemical transmitter was also unveiled by the demonstration that an organic compound isolated from animal tissues extracts exerted depressant effects on cardiac rhythm, blood pressure and intestinal movements [69]. A large number of independent studies contributed to the discovery and definition of the purinergic system, and its identification in invertebrates (including S. mansoni) implied an earlier onset of evolution [70], [71], [72]. In this context, it is possible that the co-evolution of hosts and parasites has enabled the establishment of a chronic infection.
The purinergic system is an important modulator of innate immune response and inflammation [73]. In addition, this system also regulates neurotransmission and the cardiovascular system. The complexity of the purinergic system encompasses a great variety of agonists, receptors and enzymes [74].
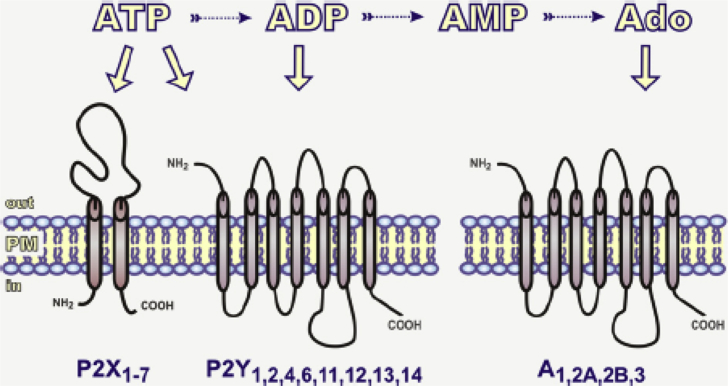
Adenosine and the nucleotides ATP, ADP, uridine 5′-triphosphate (UTP), uridine 5′-diphosphate (UDP) and UDP-glucose are key messenger molecules that mediate a wide diversity of biological actions of purinergic signaling. Moreover, nucleotide-transforming enzymes and cell-surface receptors are obligatory partners in this purinergic signaling. The International Union of Pharmacology (IUPHAR) Committee on Receptor Nomenclature and Drug Classification recognizes distinct receptors pertaining to the purinergic system. Receptors for extracellular adenosine and nucleotides can be divided into three subfamilies: metabotropic purinergic P1 receptor (or A receptor; activated by adenosine), metabotropic P2Y receptor (activated by ATP and other nucleotides) and ionotropic ATP-gated P2X receptor. According to molecular structure and functional data, adenosine P1 receptors can be divided into four subgroups: A1, A2A, A2B and A3. On the other hand, P2Y receptors can be divided into eight subgroups (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14) and P2X receptors can be divided into seven (P2X1-7) subtypes [75], [76], [77], [78] [Fig. 2]. Furthermore, dinucleotide polyphosphates (NpnNs) also modulate the purinergic system and consist of two nucleotides linked by a polyphosphate bridge containing 2–7 phosphate groups. One of most studied classes of these compounds is diadenosine polyphosphate (ApnA). P1, P4 diadenosine tetraphosphate (Ap4A) was the first ApnA described in mammalian tissues and, once in the extracellular milieu, is also a modulator of some P2 receptors. Later, other ApnA compounds were identified with distinct physiologic actions [79], [80]. Recently, a new family of adenine G protein-coupled receptor has been characterized in rodents; this family has been named P0-receptors [81].
Fig. 2.
Purinergic receptors. Extracellular nucleotides mediate intracellular signaling through cell surface ionotropic ATP-gated receptors (P2X) and metabotropic P2Y receptors. On the other hand, adenosine receptors (A) are activated by adenosine (Ado). The conversion of ATP to other nucleotides and adenosine is mediated by ecto-enzymes [76].
Reproduced from Ref. [122] with permission.
Mammalian cells contain high concentrations of ATP (mM) that can be released into the extracellular milieu. However, given the diversity of cell types, different physiologic processes of ATP release have been described such as regulated vesicular exocytose and conductive/diffusional release (via pore-forming connexin, pannexin and P2X7 receptor) or ABC cassette transporters [74], [82]. Other mechanisms have also been described such as anion channel-mediated ATP release. Moreover, ATP can be synthesized via plasma membrane F(1)/F(0)-ATP synthase. Alternatively, ATP may be released under pathologic conditions causing tissue damage, cell death or stress [74]. In such cases, ATP is recognized as a “damage-associated molecular pattern” (DAMP). Uridine 5′-triphosphate release may share some of these mechanisms [82], [83], [84], [85].
Besides interacting with cell-surface purinergic P2 receptors, ATP (and other nucleotides) is also a substrate for catabolizing ecto-enzymes (ecto-nucleotidases), thereby controlling the availability of extracellular nucleotides and purinergic P2 receptor signaling. Ecto-nucleotidases hydrolyze nucleoside mono-, di-, or triphosphates, dinucleoside polyphosphates and produce nucleoside diphosphates, nucleoside monophosphates, nucleosides, phosphate and inorganic pyrophosphate (PPi) [76], [85].
The major groups of ecto-nucleotidases are ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases; apyrase or CD39), ecto-5′-nucleotidase (eN; CD73), ecto-nucleotide phosphodiesterases/pyrophosphatase (E-NPP) and alkaline phosphatases (APs). Thus far, E-NTPDases have been considered to be the major ecto-nucleotidase of purinergic signaling. They hydrolyze selectively nucleoside triphosphates and diphosphates, generating nucleoside monophosphate. In the case of AMP, it is a specific substrate for eN, another important enzyme of the purinergic signaling, which in turn generates adenosine. Eventually, NTPDases and eN are co-expressed in the same cell [76].
NTPDases are expressed ubiquitously in eukaryotes. In mammals, researchers have identified eight paralogs of NTPDases; four of them (NTPDase1, NTPDase2, NTPDase3 and NTPDase8) are cell surface enzymes. NTPDases4-7 are intracellularly located; however, NTPDase5 and NTPDase6 may eventually be secreted [76]. NTPDases1-3 and NTPDase8 hydrolyze nucleoside triphosphates and diphosphates, but the affinity for the substrate varies according to enzyme subtype and species. Human and murine NTPDase1 have equal affinities for ATP and ADP; NTPDase2 has higher affinity for ATP than for ADP, and NTPDase3 hydrolyzes ATP to AMP [76], [86]. S. mansoni seems to express a protein closely related to NTPDase1 and NTPDase5 [76]. Of note, S. mansoni outer tegumental smATPDase I, smAP and smPDE are considered to be candidate virulence factors with the potential to modulate the host's immune system [12].
Host purinergic signaling during schistosomal infection
Besides the salvage nucleotide metabolism, it is possible that schistosomes may modulate host immune responses and platelet aggregation mediated by purinergic signaling [55]. The presence of intravascular parasites may damage endothelial cells and induce nucleotide release (DAMPs). The worm capacity to reduce ATP and ADP concentrations around themselves may limit host inflammation and platelet aggregation; adenosine formation may favor vasodilation and worm migration [54], and eventually worm's metabolism [52], [53]. Human and mice schistosomiasis is related to thrombocytopenia. Additionally the tegumental smATPDase1 possibly contribute to inhibit ADP-mediated platelet aggregation [55], [87].
A large amount of data about schistosomiasis has been obtained using S. mansoni-infected mice. Important evidence for the alteration of purinergic signaling during schistosomal infection came from work by De Man and colleagues [88]. In control mice, both adenosine and ATP attenuated the ileum contraction induced by neural-released acetylcholine, but not in response to carbachol (exogenous agonist). The adenosine effect was blocked by the antagonist 8-phenyltheophylline and mimicked by the agonist N(6)-cyclohexyladenosine, implying the participation of the presynaptic A1 receptor. Since the ATP effect was also blocked by the A1 receptor antagonist, the authors suggested that ATP was converted to adenosine to exert the inhibitory effect. According to the data, the purinergic control of cholinergic neurotransmission by A1 receptor was compromised during infection with S. mansoni, and increased ileum contraction was observed. During intestinal inflammation, there is an increased number of mast cells close to the myoenteric neurons, and the continuous exposure of A1 receptors to adenosine may lead to receptor desensitization. Chronic intestinal inflammation is related to alterations of intestinal motility, and it is accordingly possible that the enteric dysfunctional purinergic signaling contributes to schistosomal morbidity [3].
Tissue insult recruits eosinophils as inflammatory effector cells. The intracellular eosinophil granules contain preformed cytokines, eosinophil peroxidase and cationic proteins that may be released by degranulation and exocytosis. Alternatively, the presence of intact eosinophil cell-free granules in tissues is evidence of eosinophil necrosis [89]. However, such extracellular granules are secretion-competent organelles responsive to external stimuli [90].
Eosinophilia is a key feature of human schistosomiasis [10], and some lines of evidence point to a capacity of eliminating helminths. For instance, it has been shown that eosinophils are able to invade dying schistosomes, and eosinophil granule proteins may contribute to killing [91]. Using a reporter animal model characterized by an eosinophil peroxidase-luciferase (EPX-luc) transgenic mice, Davies and colleagues [91] showed that there is increased eosinophilopoese in the bone marrow and eosinophilia in the liver and intestine in response to both worms and eggs during schistosomiasis.
Human and murine eosinophils (and other immune cells) express mRNA encoding several P2X and P2Y receptors [92]. In addition, immunoreactivity against the P2Y12 receptor protein has also been identified in cells from both species. Functional data additionally suggest the expression of human P2Y12 receptors since stimulation with the agonist ADP induced the secretion of eosinophil peroxidase, which was selectively reduced by the P2Y12 receptor antagonist MRS2395 [93]. In the murine model, treatment of S. mansoni-infected mice with the P2Y12 receptor antagonist clopidogrel reduced the size of liver granuloma, collagen deposition, the number of infiltrated eosinophils, IL-4 and IL-13 levels in liver homogenates compared with infected, untreated animals. However, clopidogrel did not interfere with Th2 polarization during schistosomiasis since the plasma levels of IL-13 were only slightly reduced, and the IL-4 levels were not changed [93]. These data suggest that pro-inflammatory P2Y12 receptor signaling takes part in eosinophil migration to liver granulomas and may contribute to fibrosis.
Co-infections with Schistosoma and bacteria, virus, protozoa or other helminths are known [94], [95]. The association among Salmonella infections and eventually bacteremia and schistosomiasis has also been reported [95]. It is possible that Th2 polarization plays a role in such co-infections [96].
Macrophages are resident phagocytic cells of the innate immune system. They act as the first line of the host defense against pathogens in non-adaptive responses and may also contribute to adaptive immune responses, ultimately leading to pathogen killing [97], [98]. Moreover, during schistosomiasis the degree of differentiation between cMФ and aaMФ contributes to the level of inflammation and fibrosis [25].
Purinergic P2X7 receptors are expressed on monocytes and macrophages, regulate cytokine production, apoptosis, and take part in inflammasome. These receptors are localized in lipid rafts and therefore they interact with caveolin and also modulate the activity of phospholipases A2, C and D [99]. P2X7 receptors activation by agonists such as ATP (mM) and the analog BzATP induces a cation-specific channel opening along with Ca2+ influx and K+ efflux; moreover pore dilation allows for the permeation of large molecules such as ethidium bromide [99]. The activation of macrophage P2X7 receptors stimulates the secretion of cytokines such as TNF-α and IL-1β, but a disproportionate production may be detrimental in chronic inflammation [100]. Moreover, macrophage P2X7 receptors may function as scavenger receptors for bacteria and apoptotic cells [101]. Previous data with mice infected with S. mansoni pointed to a reduced phagocytic and bactericidal capacity of peritoneal macrophages [102].
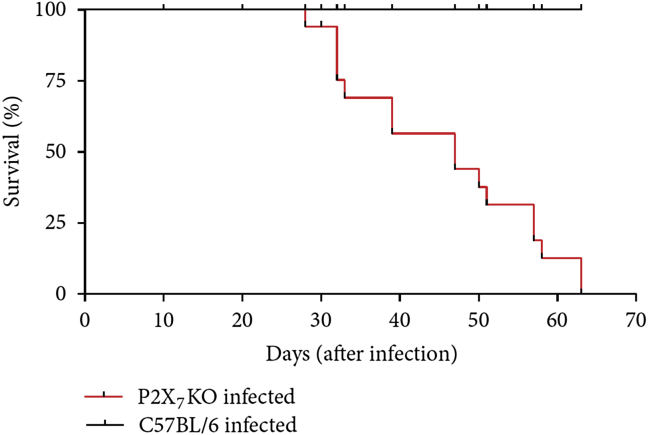
Using F4/80+ peritoneal macrophages from S. mansoni-infected mice (in the beginning of the chronic phase), we observed that both Ca2+ influx and cell permeabilization in response to ATP and BzATP were reduced compared with cells from control mice. Infected animals also exhibited increased levels of transforming growth factor (TGF)-β1 [103]. While IFN-γ increases P2X7 receptor expression [104], treatment of peritoneal macrophages with TGF-β1 reduced cell surface P2X7 receptor expression [103]. These data point to reduced P2X7 receptor signaling in macrophages during schistosomiasis. The infection of P2X7 receptor knockout mice resulted in a reduced survival curve [Fig. 3], a finding that suggests that these receptors are important to host defense.
Fig. 3.
Survival curves of S. mansoni-infected mice (black line: C57BL/6 wild type; Red line: P2X7 receptor knockout mice (P2X7RKO)). Newborn mice were infected and observed for 9 weeks.
Reproduced from Ref. [103] with permission.
In view of the reduced P2X7 receptor function it could be expected a failure of host IL-1β expression in response to pathogen. However, Ritter et al. [105] showed that egg-derived soluble antigens (SEA) (i.e., “pathogen-associated molecular patterns” (PAMPs)) stimulate IL-1β expression by dendritic cells through a dectin-2 pathway. Moreover, the stimulation of dendritic cells from P2X7 receptor knockout mice with SEA also induced IL-1β expression suggesting that this signaling does not depend largely on P2X7 receptor function. Thereby this finding implicates that the infection influences directly inflammasome activation [105].
The lumen of blood vessels and lymphatics is covered by endothelial cells that are considered important regulators of leukocyte adhesion, vascular permeability and mechanisms of vascular contraction and dilation [106], [107], [108]. The intravascular location of Schistosoma makes endothelial cells as first target of the disease. Endothelial cells show remarkable phenotypic heterogeneity and undergo epigenetic regulation [106]. According to previous data with infected mice, endothelial cells primed by schistosomiasis keep in culture the acquired phenotype [109].
Under physiologic conditions, endothelial cells show an anti-inflammatory phenotype. The constitutive production of endothelial-derived NO plays an important role inhibiting leukocyte adhesion, smooth muscle cell proliferation and controlling vascular tone. Conversely, under pathologic conditions endothelial cells become dysfunctional showing a proadhesive phenotype and expressing adhesion molecules important for leukocyte rolling, adhesion and transmigration [108], [110]. Of note, some cytokines found to be increased in schistosomal infection, such as TNF-α and IL-13, have a deleterious role on endothelial cell function favoring a pro-inflammatory phenotype [110]. Accordingly, a reduced expression of endothelial NOS (eNOS) during schistosomal infection has been described characterizing an endothelial dysfunction [109].
Purinergic system is present on endothelial cells. These cells release ATP, express purinergic receptors and ecto-nucleotidases [73], [75], [84]. Endothelial cells from different vascular beds express several subtypes of purinergic P2Y receptors, including P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptors [73], [111], [112]. Moreover, P2X4 and P2X7 receptors [73], [113], [114] and NTPDases 1, 2 and 3 are also expressed [85], [115].
Vascular P2X7 receptors activation induces endothelium-dependent vasodilation [116] and constitutive NO production in cultured endothelial cells [113]. However, excessive P2X7 receptor activation may lead to apoptosis [99]. Mesenteric endothelial cells from S. mansoni-infected mice exhibited a reduced Ca2+ influx and ethidium bromide uptake in response to the agonist BzATP [113]. We found that the downregulation of endothelial P2X7 receptor signaling was related to a reduced protein expression, which also reduced NO production in response to ATP or BzATP. So far there is no evidence of a putative protection against endothelial cell apoptosis. Nevertheless, previously we showed that ATP-induced production of NO was compromised in cells from S. mansoni-infected mice, and this endothelial dysfunction contributed to an increased leukocyte adhesion, vascular inflammation and infiltration of leukocytes in the peritoneal cavity [109] and portal vein [117].
The endothelial expressions of NTPDases 2 and 3 are increased by schistosomal infection along with a higher hydrolysis of ATP, and ADP generation [115]. ADP is the endogenous agonist of P2Y1 receptor, which is widely expressed through the vascular system [73], [81], [111], [112]. P2Y1 receptor induces the expression of adhesion molecules such as intercellular adhesion molecule (ICAM)-1 thereby having a pro-inflammatory effect [118], and ICAM-1 has been noted as the most relevant adhesion molecule for schistosomiasis-related portal inflammation [119]. In the infected group, the increased extracellular concentration of ADP was accompanied by an increased basal leukocyte adhesion to endothelial cells as compared to control group. The P2Y1 receptor agonist 2-methylthioATP (2-MeSATP) also increased leukocyte adhesion. However, the selective P2Y1 receptor antagonist MRS2179 blocked 2-MeSATP effect, and also returned basal leukocyte adhesion to control levels suggesting an upregulation of basal P2Y1 receptor signaling during this stage of schistosomiasis [115].
Data from another model of S. mansoni infection (hamster) showed a reduced content of ATP and an increased content of ADP in liver from infected animals compared with controls [120]. The altered ATP/ADP ratio could also reflect alterations of the purine metabolism during the disease.
Chronic mansonic schistosomiasis is related to a repertoire of worm- and host-derived immunomodulators culminating in intestinal and hepatosplenic alterations [3], [8], [22]. According to murine model, ADP seems to control liver and mesenteric schistosomal inflammation [92], [115]. Although the expression of P2X7 receptor and NTPDases 2 and 3 were differently altered by this stage of schistosomiasis, the expressions of P2Y1 receptor, NTPDase 1 and 5′ecto-nucleotidase were not altered in the same model [115]. Therefore, these data suggest that the disease affects in different ways the receptors and enzymes of the purinergic system.
If translated to the clinics, the purinergic signaling alterations observed during murine schistosomiasis could contribute to schistosomal morbidity. Moreover, current data suggest that P2Y1 and P2Y12 receptors could be pharmacologic targets to reduce chronic schistosomal inflammation and morbidity. Actually, P2Y receptors have been considered as potential pharmacologic targets in several other chronic inflammatory conditions [121].
Conclusion
Schistosomiasis-related chronic inflammation promotes host intestinal and liver alterations. Current data obtained with experimental models support the notion that host purinergic system is altered by schistosomiasis. The dysfunction of adenosine receptors, metabotropic P2Y receptors, ionotropic P2X7 receptors, and NTPDases likely contributes to some disease morbidity.
Conflict of interests
The author declares that there are no conflicts of interest regarding the publication of this paper.
Acknowledgements
Silva CLM is a senior fellow of CNPq (Brazil). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the paper.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.King C.H. Health metrics for helminth infections. Acta Trop. 2015;141:150–160. doi: 10.1016/j.actatropica.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nascimento G.L., de Oliveira M.R. Severe forms of schistosomiasis mansoni: epidemiologic and economic impact in Brazil, 2010. Trans R Soc Trop Med Hyg. 2014;108:29–36. doi: 10.1093/trstmh/trt109. [DOI] [PubMed] [Google Scholar]
- 3.Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 4.Silva L.L., Marcet-Houben M., Nahum L.A., Zerlotini A., Gabaldón T., Oliveira G. The Schistosoma mansoni phylome: using evolutionary genomics to gain insight into a parasite's biology. BMC Genomics. 2012;13:617. doi: 10.1186/1471-2164-13-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y.X., Salafsky B., Ramaswamy K. Comparison of skin invasion among three major species of Schistosoma. Trends Parasitol. 2005;21:201–203. doi: 10.1016/j.pt.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Grabe K., Haas W. Navigation within host tissues: Schistosoma mansoni and Trichobilharzia ocellata schistosomula respond to chemical gradients. Int J Parasitol. 2004;34:927–934. doi: 10.1016/j.ijpara.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Wolowczuk I., Roye O., Nutten S., Delacre M., Trottein F., Auriault C. Role of interleukin-7 in the relation between Schistosoma mansoni and its definitive vertebrate host. Microbes Infect. 1999;1:545–551. doi: 10.1016/s1286-4579(99)80094-x. [DOI] [PubMed] [Google Scholar]
- 8.Colley D.G., Secor W.E. Immunology of human schistosomiasis. Parasite Immunol. 2014;36:347–357. doi: 10.1111/pim.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barsoum R.S., Esmat G., El-Baz T. Human schistosomiasis: clinical perspective: review. J Adv Res. 2013;4:433–444. doi: 10.1016/j.jare.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenzi H.L., Pacheco R.G., Pelajo-Machado M., Panasco M.S., Romanha W.S., Lenzi J.A. Immunological system and Schistosoma mansoni: co-evolutionary immunobiology. What is the eosinophil role in parasite-host relationship? Mem Inst Oswaldo Cruz. 1997;92:19–32. doi: 10.1590/s0074-02761997000800005. [DOI] [PubMed] [Google Scholar]
- 11.Silva C.L.M. Endothelial cells as targets of the intravascular parasitic disease schistosomiasis. In: Gavins F.N.E., Stokes K.Y., editors. Vascular responses to pathogens. Academic Press; London: 2015. pp. 195–208. [Google Scholar]
- 12.Wilson R.A. Virulence factors of schistosomes. Microbes Infect. 2012;14:1442–1450. doi: 10.1016/j.micinf.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Schramm G., Haas H. Th2 immune response against Schistosoma mansoni infection. Microbes Infect. 2010;12:881–888. doi: 10.1016/j.micinf.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Wilson R.A., Coulson P.S. Immune effector mechanisms against schistosomiasis: looking for a chink in the parasite's armour. Trends Parasitol. 2009;25:423–431. doi: 10.1016/j.pt.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs W., van Dam G., Bogers J., Deelder A., Van Marck E. Schistosomal granuloma modulation. I. Schistosoma mansoni worm antigens CAA and CCA prime egg-antigen-induced hepatic granuloma formation. Parasitol Res. 1999;85:7–13. doi: 10.1007/s004360050499. [DOI] [PubMed] [Google Scholar]
- 16.Cutts L., Wilson R.A. The protein antigens secreted in vivo by adult male Schistosoma mansoni. Parasitology. 1997;114:245–255. doi: 10.1017/s0031182096008438. [DOI] [PubMed] [Google Scholar]
- 17.al-Sherbiny M., el Ridi R., Guirguis N.I., Dean D.A. Identification and characterization of Schistosoma mansoni antigens recognized by T and B lymphocytes of humans with early active intestinal and/or urinary schistosomiasis. Int J Parasitol. 1995;25:113–121. doi: 10.1016/0020-7519(94)e0067-w. [DOI] [PubMed] [Google Scholar]
- 18.Trottein F., Nutten S., Angeli V., Delerive P., Teissier E., Capron A. Schistosoma mansoni schistosomula reduce E-selectin and VCAM-1 expression in TNF-alpha-stimulated lung microvascular endothelial cells by interfering with the NF-kappaB pathway. Eur J Immunol. 1999;29:3691–3701. doi: 10.1002/(SICI)1521-4141(199911)29:11<3691::AID-IMMU3691>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.La Flamme A.C., Patton E.A., Bauman B., Pearce E.J. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J Immunol. 2001;166:1903–1911. doi: 10.4049/jimmunol.166.3.1903. [DOI] [PubMed] [Google Scholar]
- 20.Patton E.A., La Flamme A.C., Pedras-Vasoncelos J.A., Pearce E.J. Central role for interleukin-4 in regulating nitric oxide-mediated inhibition of T-cell proliferation and gamma interferon production in schistosomiasis. Infect Immun. 2002;70:177–184. doi: 10.1128/IAI.70.1.177-184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pesce J.T., Ramalingam T.R., Mentink-Kane M.M., Wilson M.S., El Kasmi K.C., Smith A.M. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce E.J., MacDonald A.S. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 23.Taylor J.J., Mohrs M., Pearce E.J. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Castejón G., Baroja-Mazo A., Pelegrín P. Novel macrophage polarization model: from gene expression to identification of new anti-inflammatory molecules. Cell Mol Life Sci. 2011;68:3095–3107. doi: 10.1007/s00018-010-0609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barron L., Wynn T.A. Macrophage activation governs schistosomiasis-induced inflammation and fibrosis. Eur J Immunol. 2011;41:2509–2514. doi: 10.1002/eji.201141869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maizels R.M., Pearce E.J., Artis D., Yazdanbakhsh M., Wynn T.A. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206:2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson M.S., Mentink-Kane M.M., Pesce J.T., Ramalingam T.R., Thompson R., Wynn T.A. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutitzky L.I., Hernandez H.J., Stadecker M.J. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc Natl Acad Sci U S A. 2001;98:13243–13248. doi: 10.1073/pnas.231258498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbert D.R., Hölscher C., Mohrs M., Arendse B., Schwegmann A., Radwanska M. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 30.Fallon P.G., Richardson E.J., McKenzie G.J., McKenzie A.N. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 31.de Jesus A.R., Magalhães A., Miranda D.G., Miranda R.G., Araújo M.I., de Jesus A.A. Association of type 2 cytokines with hepatic fibrosis in human Schistosoma mansoni infection. Infect Immun. 2004;72:3391–3397. doi: 10.1128/IAI.72.6.3391-3397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deaton A.M., Cook P.C., De Sousa D., Phythian-Adams A.T., Bird A., MacDonald A.S. A unique DNA methylation signature defines a population of IFN-γ/IL-4 double-positive T cells during helminth infection. Eur J Immunol. 2014;44:1835–1841. doi: 10.1002/eji.201344098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berriman M., Haas B.J., LoVerde P.T., Wilson R.A., Dillon G.P., Cerqueira G.C. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y., Zheng H., Chen Y., Zhang L., Wang K., Guo J. The Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young N.D., Jex A.R., Li B., Liu S., Yang L., Xiong Z. Whole-genome sequence of Schistosoma haematobium. Nat Genet. 2012;44:221–225. doi: 10.1038/ng.1065. [DOI] [PubMed] [Google Scholar]
- 36.Zerlotini A., Aguiar E.R., Yu F., Xu H., Li Y., Young N.D. SchistoDB: an updated genome resource for the three key schistosomes of humans. Nucleic Acids Res. 2013;41(Database issue):D728–D731. doi: 10.1093/nar/gks1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferreira L.G., Oliva G., Andricopulo A.D. Target-based molecular modeling strategies for schistosomiasis drug discovery. Future Med Chem. 2015;7:753–764. doi: 10.4155/fmc.15.21. [DOI] [PubMed] [Google Scholar]
- 38.Agboh K.C., Webb T.E., Evans R.J., Ennion S.J. Functional characterization of a P2X receptor from Schistosoma mansoni. J Biol Chem. 2004;279:41650–41657. doi: 10.1074/jbc.M408203200. [DOI] [PubMed] [Google Scholar]
- 39.Raouf R., Blais D., Séguéla P. High zinc sensitivity and pore formation in an invertebrate P2X receptor. Biochim Biophys Acta. 2005;1669:135–141. doi: 10.1016/j.bbamem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira Z.S., Silva C.L. Comparative aspects of purinergic receptors in the phylogenetic scale. In: Lazari M.F.M., Yamanouye N., editors. G protein-coupled receptors in vertebrates: comparative prespectives. Research Signpost; Kerala: 2009. pp. 73–91. [Google Scholar]
- 41.Verjovski-Almeida S., Leite L.C., Dias-Neto E., Menck C.F., Wilson R.A. Schistosome transcriptome: insights and perspectives for functional genomics. Trends Parasitol. 2004;20:304–308. doi: 10.1016/j.pt.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Pax R., Bennett J.L., Fetterer R. A benzodiazepine derivative and praziquantel: effects on musculature of Schistosoma mansoni and Schistosoma japonicum. Naunyn Schmiedeb Arch Pharmacol. 1978;304:309–315. doi: 10.1007/BF00507974. [DOI] [PubMed] [Google Scholar]
- 43.Noel F., Pardon R.S. Vanadate sensitivity of Na+, K+-ATPase from Schistosoma mansoni and its modulation by Na+, K+ and Mg2+ Life Sci. 1989;44:1677–1683. doi: 10.1016/0024-3205(89)90484-0. [DOI] [PubMed] [Google Scholar]
- 44.Cunha V.M., Meyer-Fernandes J.R., Noël F. A (Ca(2+)-Mg2+)ATPase from Schistosoma mansoni is coupled to an active transport of calcium. Mol Biochem Parasitol. 1992;52:167–173. doi: 10.1016/0166-6851(92)90049-p. [DOI] [PubMed] [Google Scholar]
- 45.Silva C.L., Cunha V.M., Mendonça-Silva D.L., Noël F. Evidence for ryanodine receptors in Schistosoma mansoni. Biochem Pharmacol. 1998;56:997–1003. doi: 10.1016/s0006-2952(98)00219-6. [DOI] [PubMed] [Google Scholar]
- 46.Mendonça-Silva D.L., Novozhilova E., Cobbett P.J., Silva C.L., Noël F., Totten M.I. Role of calcium influx through voltage-operated calcium channels and of calcium mobilization in the physiology of Schistosoma mansoni muscle contractions. Parasitology. 2006;133:67–74. doi: 10.1017/S0031182006000023. [DOI] [PubMed] [Google Scholar]
- 47.Mendonça-Silva D.L., Pessôa R.F., Noël F. Evidence for the presence of glutamatergic receptors in adult Schistosoma mansoni. Biochem Pharmacol. 2002;64:1337–1344. doi: 10.1016/s0006-2952(02)01358-8. [DOI] [PubMed] [Google Scholar]
- 48.Mendonça-Silva D.L., Gardino P.F., Kubrusly R.C., De Mello F.G., Noël F. Characterization of a GABAergic neurotransmission in adult Schistosoma mansoni. Parasitology. 2004;129:137–146. doi: 10.1017/s0031182004005554. [DOI] [PubMed] [Google Scholar]
- 49.Pessôa R.F., Castro N.G., Noël F. Binding of [3H]MK-801 in subcellular fractions of Schistosoma mansoni: evidence for interaction with nicotinic receptors. Biochem Pharmacol. 2005;69:1509–1516. doi: 10.1016/j.bcp.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Burnstock G., Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf) 2009;195:415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 51.Senft A.W., Crabtree G.W. Purine metabolism in the schistosomes: potential targets for chemotherapy. Pharmacol Ther. 1983;20:341–356. doi: 10.1016/0163-7258(83)90031-1. [DOI] [PubMed] [Google Scholar]
- 52.Levy M.G., Read C.P. Relation of tegumentary phosphohydrolase to purine and pyrimidine transport in Schistosoma mansoni. J Parasitol. 1975;61:648–656. [PubMed] [Google Scholar]
- 53.Levy M.G., Read C.P. Purine and pyrimidine transport in Schistosoma mansoni. J Parasitol. 1975;61:627–632. [PubMed] [Google Scholar]
- 54.Bhardwaj R., Skelly P.J. Purinergic signaling and immune modulation at the schistosome surface? Trends Parasitol. 2009;25:256–260. doi: 10.1016/j.pt.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Da'dara A.A., Bhardwaj R., Skelly P.J. Schistosome apyrase SmATPDase1, but not SmATPDase2, hydrolyses exogenous ATP and ADP. Purinergic Signal. 2014;10:573–580. doi: 10.1007/s11302-014-9416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vasconcelos E.G., Nascimento P.S., Meirelles M.N., Verjovski-Almeida S., Ferreira S.T. Characterization and localization of an ATP-diphosphohydrolase on the external surface of the tegument of Schistosoma mansoni. Mol Biochem Parasitol. 1993;58:205–214. doi: 10.1016/0166-6851(93)90042-v. [DOI] [PubMed] [Google Scholar]
- 57.Mendes R.G., Gusmão M.A., Maia A.C., Detoni Mde L., Porcino G.N., Soares T.V. Immunostimulatory property of a synthetic peptide belonging to the soluble ATP diphosphohydrolase isoform (SmATPDase 2) and immunolocalisation of this protein in the Schistosoma mansoni egg. Mem Inst Oswaldo Cruz. 2011;106:808–813. doi: 10.1590/s0074-02762011000700005. [DOI] [PubMed] [Google Scholar]
- 58.Maia A.C., Detoni M.L., Porcino G.N., Soares T.V., do Nascimento Gusmão M.A., Fessel M.R. Occurrence of a conserved domain in ATP diphosphohydrolases from pathogenic organisms associated to antigenicity in human parasitic diseases. Dev Comp Immunol. 2011;35:1059–1067. doi: 10.1016/j.dci.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 59.Crabtree G.W., Senft A.W. Pathways of nucleotide metabolism in Schistosoma mansoni. V. Adenosine cleavage enzyme and effects of purine analogues on adenosine metabolism in vitro. Biochem Pharmacol. 1974;23:649–660. doi: 10.1016/0006-2952(74)90630-3. [DOI] [PubMed] [Google Scholar]
- 60.Stegman R.J., Senft A.W., Brown P.R., Parks R.E., Jr. Pathways of nucleotide metabolism in Schistosoma mansoni. IV. Incorporation of adenosine analogs in vitro. Biochem Pharmacol. 1973;22:459–468. doi: 10.1016/0006-2952(73)90287-6. [DOI] [PubMed] [Google Scholar]
- 61.Romanello L., Bachega J.F., Cassago A., Brandão-Neto J., DeMarco R., Garratt R.C. Adenosine kinase from Schistosoma mansoni: structural basis for the differential incorporation of nucleoside analogues. Acta Crystallogr D Biol Crystallogr. 2013;69:126–136. doi: 10.1107/S0907444912044800. [DOI] [PubMed] [Google Scholar]
- 62.Dovey H.F., McKerrow J.H., Wang C.C. Purine salvage in Schistosoma mansoni schistosomules. Mol Biochem Parasitol. 1984;11:157–167. doi: 10.1016/0166-6851(84)90062-8. [DOI] [PubMed] [Google Scholar]
- 63.Senft A.W., Miech R.P., Brown P.R., Senft D.G. Purine metabolism in Schistosoma mansoni. Int J Parasitol. 1972;2:249–260. doi: 10.1016/0020-7519(72)90013-6. [DOI] [PubMed] [Google Scholar]
- 64.Angelucci F., Basso A., Bellelli A., Brunori M., Pica Mattoccia L., Valle C. The anti-schistosomal drug praziquantel is an adenosine antagonist. Parasitology. 2007;134:1215–1221. doi: 10.1017/S0031182007002600. [DOI] [PubMed] [Google Scholar]
- 65.da Silva S.P., Noël F. Time course of the effect of praziquantel on Schistosoma mansoni attachment in vitro: comparison with its effects on worm length and motility. Parasitol Res. 1995;81:543–548. doi: 10.1007/BF00932019. [DOI] [PubMed] [Google Scholar]
- 66.Kohn A.B., Anderson P.A., Roberts-Misterly J.M., Greenberg R.M. Schistosome calcium channel beta subunits. Unusual modulatory effects and potential role in the action of the antischistosomal drug praziquantel. J Biol Chem. 2001;276:36873–36876. doi: 10.1074/jbc.C100273200. [DOI] [PubMed] [Google Scholar]
- 67.Messerli S.M., Kasinathan R.S., Morgan W., Spranger S., Greenberg R.M. Schistosoma mansoni P-glycoprotein levels increase in response to praziquantel exposure and correlate with reduced praziquantel susceptibility. Mol Biochem Parasitol. 2009;167:54–59. doi: 10.1016/j.molbiopara.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiske C.H., Subbarow Y. Phosphorous compounds of muscle and liver. Science. 1929;70:381–382. doi: 10.1126/science.70.1816.381.b. [DOI] [PubMed] [Google Scholar]
- 69.Drury A.N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verkhratsky A., Burnstock G. Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. Bioessays. 2014;36:697–705. doi: 10.1002/bies.201400024. [DOI] [PubMed] [Google Scholar]
- 71.Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99:16–34. doi: 10.1113/expphysiol.2013.071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burnstock G. Purinoceptors: ontogeny and phylogeny. Drug Dev Res. 1996;39:204–242. [Google Scholar]
- 73.la Sala A., Ferrari D., Di Virgilio F., Idzko M., Norgauer J., Girolomoni G. Alerting and tuning the immune response by extracellular nucleotides. J Leukoc Biol. 2003;73:339–343. doi: 10.1189/jlb.0802418. [DOI] [PubMed] [Google Scholar]
- 74.Burnstock G., Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2013;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 75.Ijzerman AP, Fredholm B, Jacobson KA, Linden J, Müeller C, Frenguelli B, et al. Adenosine receptors. IUPHAR/BPS Guide to PHARMACOLOGY. http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=3 [accessed on June 2016].
- 76.Zimmermann H., Zebisch M., Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burnstock G, Abbracchio M-P, Boeynaems J-M, Boyer JL, Ceruti S, Fumagalli M, et al. IUPHAR/BPS Guide to PHARMACOLOGY. http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=52 [accessed on June 2016].
- 78.Volonté C., Amadio S., D'Ambrosi N., Colpi M., Burnstock G. P2 receptor web: complexity and fine-tuning. Pharmacol Ther. 2006;112:264–280. doi: 10.1016/j.pharmthera.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 79.Jankowski V., van der Giet M., Mischak H., Morgan M., Zidek W., Jankowski J. Dinucleoside polyphosphates: strong endogenous agonists of the purinergic system. Br J Pharmacol. 2009;157:1142–1153. doi: 10.1111/j.1476-5381.2009.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fraga H., Fontes R. Enzymatic synthesis of mono and dinucleoside polyphosphates. Biochim Biophys Acta. 2011;1810:1195–1204. doi: 10.1016/j.bbagen.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Thimm D., Knospe M., Abdelrahman A., Moutinho M., Alsdorf B.B., von Kügelgen I. Characterization of new G protein-coupled adenine receptors in mouse and hamster. Purinergic Signal. 2013;9:415–426. doi: 10.1007/s11302-013-9360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lohman A.W., Billaud M., Isakson B.E. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95:269–280. doi: 10.1093/cvr/cvs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lazarowski E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8:359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lazarowski E.R., Boucher R.C., Harden T.K. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 85.Eltzschig H.K., Sitkovsky M.V., Robson S.C. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robson S.C., Sévigny J., Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mebius M.M., van Genderen P.J., Urbanus R.T., Tielens A.G., de Groot P.G., van Hellemond J.J. Interference with the host haemostatic system by schistosomes. PLoS Pathog. 2013;9:e1003781. doi: 10.1371/journal.ppat.1003781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Man J.G., Seerden T.C., De Winter B.Y., Van Marck E.A., Herman A.G., Pelckmans P.A. Alteration of the purinergic modulation of enteric neurotransmission in the mouse ileum during chronic intestinal inflammation. Br J Pharmacol. 2003;139:172–184. doi: 10.1038/sj.bjp.0705218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spencer L.A., Bonjour K., Melo R.C., Weller P.F. Eosinophil secretion of granule-derived cytokines. Front Immunol. 2014;5:496. doi: 10.3389/fimmu.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neves J.S., Perez S.A., Spencer L.A., Melo R.C., Reynolds L., Ghiran I. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci U S A. 2008;105:18478–18483. doi: 10.1073/pnas.0804547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davies S.J., Smith S.J., Lim K.C., Zhang H., Purchio A.F., McKerrow J.H. In vivo imaging of tissue eosinophilia and eosinopoietic responses to schistosome worms and eggs. Int J Parasitol. 2005;35:851–859. doi: 10.1016/j.ijpara.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jacob F., Pérez Novo C., Bachert C., Van Crombruggen K. Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses. Purinergic Signal. 2013;9:285–306. doi: 10.1007/s11302-013-9357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muniz V.S., Baptista-Dos-Reis R., Benjamim C.F., Mata-Santos H.A., Pyrrho A.S., Strauch M.A. Purinergic P2Y12 receptor activation in eosinophils and the Schistosomal host response. PLoS One. 2015;10:e0139805. doi: 10.1371/journal.pone.0139805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abruzzi A., Fried B. Coinfection of Schistosoma (Trematoda) with bacteria, protozoa and helminths. Adv Parasitol. 2011;77:1–85. doi: 10.1016/B978-0-12-391429-3.00005-8. [DOI] [PubMed] [Google Scholar]
- 95.Lambertucci J.R., Rayes A.A., Serufo J.C., Gerspacher-Lara R., Brasileiro Filho G., Teixeira R. Schistosomiasis and associated infections. Mem Inst Oswaldo Cruz. 1998;93(Suppl 1):135–139. doi: 10.1590/s0074-02761998000700019. [DOI] [PubMed] [Google Scholar]
- 96.Damania B., Dittmer D.P. What lies within: coinfections and immunity. Cell Host Microbe. 2014;16:145–147. doi: 10.1016/j.chom.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geissmann F., Manz M.G., Jung S., Sieweke M.H., Merad M., Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naito M. Macrophage differentiation and function in health and disease. Pathol Int. 2008;58:143–155. doi: 10.1111/j.1440-1827.2007.02203.x. [DOI] [PubMed] [Google Scholar]
- 99.Coddou C., Yan Z., Obsil T., Huidobro-Toro J.P., Stojilkovic S.S. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lister M.F., Sharkey J., Sawatzky D.A., Hodgkiss J.P., Davidson D.J., Rossi A.G. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 2007;4:5. doi: 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wiley J.S., Gu B.J. A new role for the P2X7 receptor: a scavenger receptor for bacteria and apoptotic cells in the absence of serum and extracellular ATP. Purinergic Signal. 2012;8:579–586. doi: 10.1007/s11302-012-9308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muniz-Junqueira M.I., Prata A., Tosta C.E. Phagocytic and bactericidal function of mouse macrophages to Salmonella typhimurium in schistosomiasis mansoni. Am J Trop Med Hyg. 1992;46:132–136. doi: 10.4269/ajtmh.1992.46.132. [DOI] [PubMed] [Google Scholar]
- 103.Oliveira S.D., Nanini H.F., Savio L.E., Waghabi M.C., Silva C.L., Coutinho-Silva R. Macrophage P2X7 receptor function is reduced during schistosomiasis: putative role of TGF- β1. Mediat Inflamm. 2014;2014:134974. doi: 10.1155/2014/134974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Welter-Stahl L., da Silva C.M., Schachter J., Persechini P.M., Souza H.S., Ojcius D.M. Expression of purinergic receptors and modulation of P2X7 function by the inflammatory cytokine IFNgamma in human epithelial cells. Biochim Biophys Acta. 2009;1788:1176–1187. doi: 10.1016/j.bbamem.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 105.Ritter M., Gross O., Kays S., Ruland J., Nimmerjahn F., Saijo S. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci U S A. 2010;107:20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aird W.C. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–143. [PubMed] [Google Scholar]
- 107.Rothermel A.L., Wang Y., Schechner J., Mook-Kanamori B., Aird W.C., Pober J.S. Endothelial cells present antigens in vivo. BMC Immunol. 2004;5:5. doi: 10.1186/1471-2172-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 109.Oliveira S.D., Quintas L.E., Amaral L.S., Noël F., Farsky S.H., Silva C.L. Increased endothelial cell-leukocyte interaction in murine schistosomiasis: possible priming of endothelial cells by the disease. PLoS One. 2011;6:e23547. doi: 10.1371/journal.pone.0023547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vila E., Salaices M. Cytokines and vascular reactivity in resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1016–H1021. doi: 10.1152/ajpheart.00779.2004. [DOI] [PubMed] [Google Scholar]
- 111.Wang L., Karlsson L., Moses S., Hultgårdh-Nilsson A., Andersson M., Borna C. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 112.Lyubchenko T., Woodward H., Veo K.D., Burns N., Nijmeh H., Liubchenko G.A. P2Y1 and P2Y13 purinergic receptors mediate Ca2+ signaling and proliferative responses in pulmonary artery vasa vasorum endothelial cells. Am J Physiol Cell Physiol. 2011;300:C266–C275. doi: 10.1152/ajpcell.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oliveira S.D., Coutinho-Silva R., Silva C.L. Endothelial P2X7 receptors' expression is reduced by schistosomiasis. Purinergic Signal. 2013;9:81–89. doi: 10.1007/s11302-012-9332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yamamoto K., Korenaga R., Kamiya A., Qi Z., Sokabe M., Ando J. P2X(4) receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
- 115.Oliveira S.D., Oliveira N.F., Meyer-Fernandes J.R., Savio L.E., Ornelas F.G., Ferreira Z.S. Increased expression of NTPDases 2 and 3 in mesenteric endothelial cells during schistosomiasis favors leukocyte adhesion through P2Y1 receptors. Vasc Pharmacol. 2016;82:66–72. doi: 10.1016/j.vph.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 116.Liu C., Mather S., Huang Y., Garland C.J., Yao X. Extracellular ATP facilitates flow-induced vasodilatation in rat small mesenteric arteries. Am J Physiol Heart Circ Physiol. 2004;286:H1688–H1695. doi: 10.1152/ajpheart.00576.2003. [DOI] [PubMed] [Google Scholar]
- 117.Silva C.L., Morel N., Lenzi H.L., Noël F. Increased reactivity to 5-hydroxytryptamine of portal veins from mice infected with Schistosoma mansoni. Comp Biochem Physiol A Mol Integr Physiol. 1998;120:417–423. doi: 10.1016/s1095-6433(98)10041-7. [DOI] [PubMed] [Google Scholar]
- 118.Zerr M., Hechler B., Freund M., Magnenat S., Lanois I., Cazenave J.P. Major contribution of the P2Y₁ receptor in purinergic regulation of TNFα-induced vascular inflammation. Circulation. 2011;123:2404–2413. doi: 10.1161/CIRCULATIONAHA.110.002139. [DOI] [PubMed] [Google Scholar]
- 119.Ritter D.M., McKerrow J.H. Intercellular adhesion molecule 1 is the major adhesion molecule expressed during schistosome granuloma formation. Infect Immun. 1996;64:4706–4713. doi: 10.1128/iai.64.11.4706-4713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Soliman K., El-Ansary A., Mohamed A.M. Effect of carnosine administration on metabolic parameters in bilharzia-infected hamsters. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:157–164. doi: 10.1016/s1096-4959(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 121.Schuchardt M., Tölle M., van der Giet M. P2Y purinoceptors as potential emerging therapeutical target in vascular disease. Curr Pharm Des. 2012;18:6169–6180. doi: 10.2174/138161212803582504. [DOI] [PubMed] [Google Scholar]
- 122.Yegutkin G.G. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]