Abstract
Background
Existence of coronary endothelial dysfunction has been demonstrated in patients with cardiac syndrome X (CSX). In addition, Helicobacter pylorus (H. pylori) has been associated with CSX. We aimed to assess the possible association of endothelial dysfunction and cytotoxin-associated gene A-positive H. pylori (CagA+) infection in CSX patients.
Methods
Fifty-six patients with CSX (23 male/33 female; age: 51.25 ± 8.86 years) who were anti-H. pylori IgG-positive [H. pylori(+)] and 24 CSX patients (7 male/17 female; age: 52.79 ± 9.88 years) who were H. pylori(−) were included. Also, anti-H. pylori IgG-positive patients were determined by the presence of IgG antibody to CagA. Levels of endothelin-1 (ET-1), E-selectin and intercellular adhesion molecule-1 (ICAM-1) were measured.
Results
Endothelial dysfunction biomarkers were higher in H. pylori(+) than in H. pylori(−) patients (ET-1: 54.60 ± 25.39 vs. 42.59 ± 18.37 pg/ml, p = 0.04; E-selectin: 42.68 ± 14.26 vs. 31.72 ± 8.26 ng/ml, p = 0.001; ICAM-1: 339.68 ± 135.8 vs. 266.51 ± 125.1 ng/ml, p = 0.02). Among H. pylori(+) subjects, 28 cases were CagA(+) and 28 cases were CagA(−). There were significant differences in measured levels of E-selectin between CagA(+) and CagA(−) groups (48.00 ± 16.37 vs. 37.37 ± 9.37 ng/ml, p = 0.004). For ET-1 and ICAM-1 levels, the difference between CagA(+) and CagA(−) was insignificant (p = 0.174 and p = 0.07, respectively).
Conclusion
High levels of endothelial dysfunction biomarkers are found in CSX patients with anti-CagA(+). These findings suggest the infection with CagA(+) H. pylori strain may play a role as a risk factor in development of CSX through provocation of endothelial dysfunction. Therefore, a long term follow up to investigate the outcomes of these patients is proposed.
Keywords: Cardiac syndrome X, Endothelial dysfunction, Helicobacter pylori, Cytotoxin-associated gene A
At a glance commentary
Scientific background on the subject
Cardiac syndrome X (CSX) describes patients with angina-like chest pain, a positive exercise stress test result, and angiographically normal epicardial coronary arteries. Endothelial dysfunction is among the most commonly suggested pathogenic mechanisms responsible for CSX. Previous studies have revealed an association between Helicobacter pylori (H. pylori) infection with vascular discomforts. Specifically, strains bearing the cytotoxin-associated gene A [CagA(+)] exacerbate a heightened inflammatory response in vivo. In this study we would like to see whether infection with CagA(+) bearing strain of H. pylori is associated with higher endothelial dysfunction.
What this study adds to the field
Study results show the infection with CagA(+) H. pylori weather play a role as a risk factor in development of CSX through provocation of endothelial dysfunction.
Cardiac syndrome X (CSX) describes patients with angina-like chest pain, a positive exercise stress test result, and angiographically normal epicardial coronary arteries [1]. More than 40 years after the first description of the disease, the debate continues to the CSX mechanisms. Inflammation and microvascular dysfunction are among the most commonly suggested pathogenic mechanisms responsible for CSX [1], [2], [3]. The endothelial function has been studied mainly by invasive methods and by measuring humoral factors [4]. Increased levels of plasma adhesion molecules like soluble intercellular adhesion molecule-1 (sICAM-1) and soluble E-selectin (sE-selectin) have been considered as markers of endothelial injury [4], [5]. For example, Senen et al. [6] found increased plasma concentrations of ICAM-1 and sE-selectin in CSX patients. Moreover, it is known that damaged or activated endothelial cells can secrete vasoconstrictor factors such as endothelin-1(ET-1). Kaski et al. [7] suggested the association between high plasma concentrations of ET-1 and genesis of chest pain in patients with CSX.
On the other hand, previous studies have revealed an association between Helicobacter pylori (H. pylori) infection with vascular discomforts [8], [9]. H. pylori is a microaerophilic spiral shaped gram negative bacterium that colonizes the gastric lumen of humans and other primates [10]. It may cause extra-intestinal expressions such as functional ischemic heart disease [11], [12] and it has recently been associated with CSX [9], [13]. There is genetic diversity between H. pylori strains that affects virulence [14]. Specifically, strains bearing the cytotoxin-associated gene A [CagA(+)] exacerbate a heightened inflammatory response in vivo [15]. The virulent CagA(+) may induce a more consistent release of cytokines with vasoactive properties, which might be the basis of systemic extradigestive effects that led to cardiac microvascular dysfunction [8]. We aimed to evaluate the possible association of chronic CagA(+) infection and endothelial dysfunction in CSX patients.
Methods
Patient characteristics
The present study included 80 patients (30 male/50 female; mean age: 51.71 ± 9.2 years) who had been diagnosed as CSX. The entry criteria of CSX were recurrent typical angina chest pain at rest and on effort, a normal 12-lead electrocardiogram at rest, positive exercise ECG stress test response and normal coronary angiogram. Patients with evidence of myocardial infarction, valvular heart disease, left and right ventricular dysfunction, concomitant acute and chronic disease were excluded from the study. Also, patients with diabetes mellitus were not included, as endothelial dysfunction markers increase in diabetes mellitus. Non-cardiac causes of chest pain such as gastrointestinal and musculoskeletal disorders were also investigated and ruled out as appropriate. All subjects gave their informed consent prior to their inclusion in the study. The study protocol approved by the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the guidelines of the Medical Ethics Committee, Ministry of Health, Iran by our university of Medical Research Ethics committee (approved number: 481). A questionnaire was administrated to obtain general information regarding age, sex, body mass index (BMI), systolic and diastolic blood pressures.
Measurement of parameters
EDTA-anticoagulated peripheral blood sample was taken from each subject in resting on the same day that clinical data were recorded and the plasma was obtained after a centrifugation of 3000 rpm for 10 min. Collected plasma for determination of biomarkers of endothelial function (sICAM-1 and sE-selectin and ET-1) were stored at −80 °C before laboratory testing.
Specific anti-H. pylori immunoglobulin-G (IgG) positivity was determined with a commercial enzyme-linked immunosorbent assay (ELISA) kit (H. pylori-IgG and CagA–IgG, Enzyme Immunoassay; Dia pro, Italy) according to the manufacturer's instructions.
Also, plasma positivity to the antigen CagA was assessed by ELISA (Dia.Pro, Milan, Italy; sensitivity and specificity >98%) in anti-H. pylori positive (anti-H. pylori+) samples.
Endothelial function markers were measured using commercially available kits for measurement of ICAM-1 and sE-selectin levels (Platinum ELISA, Bender Med Systems, Austria). In addition, plasma levels of ET-1 were measured using immunosorbent assay method (Human ET-1, USCN LIFE, USA).
Laboratory glucose and lipid profile results used for comparison of baseline characteristics. These routine tests are performed for any patient admitted to hospital.
Statistical analysis
Data analysis was conducted using Statistical Package of the Social Sciences (IBM SPSS Statistics 19) software.
Chi square and independent sample T tests were carried out for statistic analysis. Age, systolic blood pressure, diastolic blood pressure, glucose, lipid profile and body mass index (BMI) were shown as mean ± standard deviation (SD). One-Way ANOVA with Tukey HSD test used to compare amount of endothelial function markers among three groups. Statistical significance was defined as a p-value <0.05.
Results
All individuals diagnosed as CSX and there were no significant differences in the medication status. Baseline characteristics are not different among the groups in respect to age, sex, BMI, smoking, systolic and diastolic blood pressure, glucose, lipids and medications [p > 0.05, Table 1].
Table 1.
Demographic and baseline clinical characteristics of patients with cardiac syndrome X.
| Variable | H. pylori(−) (n = 24) | H. pylori(+) (n = 56) | p-value |
H. pylori status |
p-value | |
|---|---|---|---|---|---|---|
| CagA(−) (n = 28) | CagA(+) (n = 28) | |||||
| Age (years) | 52.79 ± 9.88 | 51.25 ± 8.86 | p = 0.493 | 52.18 ± 11.11 | 50.32 ± 5.91 | p = 0.438 |
| Sex (M/F) | 7/17 | 23/33 | p = 0.450 | 9/19 | 14/14 | p = 0.139 |
| (BMI) (Kg/m2) | 26.48 ± 2.51 | 26.56 ± 5.90 | p = 0.949 | 27.82 ± 6.04 | 25.31 ± 5.60 | p = 0.113 |
| Smokers, n(%) | 2 (8.3%) | 11 (19.6%) | p = 0.324 | 5 (17.9%) | 6 (21.4%) | p = 0.500 |
| SBP (mmHg) | 113.75 ± 11.35 | 115.18 ± 10.44 | p = 0.586 | 117.50 ± 13.23 | 112.86 ± 6.00 | p = 0.197 |
| DBP (mmHg) | 71.04 ± 4.42 | 74.20 ± 8.67 | p = 0.096 | 72.86 ± 10.50 | 75.54 ± 6.29 | p = 0.252 |
| FBS (mg/dl) | 91.24 ± 6.83 | 94.52 ± 5.72 | p = 0.454 | 92.24 ± 7.33 | 96.01 ± 8.02 | p = 0.383 |
| TC (mg/dl) | 163.50 ± 11.43 | 159.33 ± 14.02 | p = 0.332 | 158 ± 13.56 | 164.44 ± 11.66 | p = 0.298 |
| LDL (mg/dl) | 86.4 ± 8.9 | 92.06 ± 83 | p = 0.139 | 94.1 ± 6.22 | 89.62 ± 5.7 | p = 0.212 |
| HDL (mg/dl) | 44.52 ± 10.85 | 46.22 ± 10.93 | p = 0.789 | 46.5 ± 11.8 | 39.12 ± 12.86 | p = 0.286 |
| TG (mg/dl) | 148.14 ± 22.8 | 154.30 ± 18.54 | p = 0.632 | 158.32 ± 20.22 | 144.30 ± 24.45 | p = 0.294 |
All values are means ± SD. Abbreviations: H. pylori: Helicobacter pylori; CagA: cytotoxin-associated gene A; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; FBS: fasting blood sugar; TC: total cholesterol; LDL: low density lipoprotein; HDL: high density lipoprotein; TG: triglyceride.
At first, we divided CSX patients into two groups according to the presence (23 male/33 female, mean age: 51.25 ± 8.86) or absence (7 male/17 female, mean age: 52.79 ± 9.88) of anti-H. pylori IgG antibody (H. pylori(+) and H. pylori(−), respectively). The measured plasma concentrations of ET-1 were significantly greater in H. pylori(+) than in H. pylori(−) patients (54.60 ± 25.39 vs. 42.59 ± 18.37 pg/ml, p = 0.040). Also the plasma E-selectin levels were higher in H. pylori(+) than in H. pylori(−) patients (42.68 ± 14.26 vs. 31.72 ± 8.26 ng/ml, p = 0.001). These patterns also were seen in plasma levels of ICAM-1. The levels of ICAM-1 concentration in H. pylori(+) were higher than H. pylori(−) patients (339.68 ± 135.8 vs. 266.51 ± 125.1 ng/ml, p = 0.02).
In the second step, the H. pylori(+) group divided into two sub-groups according to presence (14 male/14 female, mean age: 50.32 ± 5.91 years) or absence (9 male/19 female, mean age: 52.18 ± 11.11 years) of anti-CagA IgG antibody (CagA(+) and CagA(−), respectively). Differences in plasma levels of ET-1 in CagA(+) and CagA(−) groups was insignificant (60.21 ± 25.12 vs. 49.00 ± 24.83 pg/ml, p = 0.174). Furthermore, levels of plasma E-selectin levels in CagA(+) patients were much greater than CagA(−) patients (48.00 ± 16.37 vs. 37.37 ± 9.37 ng/ml, p = 0.004; [Table 2]). Greater concentrations of ICAM-1 are also seen in CagA(+) patients than CagA(−) patients (388.34 ± 149.38 vs. 308.87 ± 120.11 ng/ml, p = 0.070).
Table 2.
Multiple comparisons.
| Dependent variable | (I) HP_CagA | (J) HP_CagA | Mean difference (I−J) | Std. error | Sig. | 95% Confidence interval |
||
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| Endothelin | Tukey HSD | HPpCAGp | HPpCAGn | 11.20567 | 6.20094 | 0.174 | −3.6137 | 26.0251 |
| HPnCAGn | 17.61515a | 6.45415 | 0.021 | 2.1906 | 33.0397 | |||
| HPpCAGn | HPpCAGp | −11.20567 | 6.20094 | 0.174 | −26.0251 | 3.6137 | ||
| HPnCAGn | 6.40948 | 6.45415 | 0.583 | −9.0150 | 21.8340 | |||
| HPnCAGn | HPpCAGp | −17.61515a | 6.45415 | 0.021 | −33.0397 | −2.1906 | ||
| HPpCAGn | −6.40948 | 6.45415 | 0.583 | −21.8340 | 9.0150 | |||
| LSD | HPpCAGp | HPpCAGn | 11.20567 | 6.20094 | 0.075 | −1.1420 | 23.5533 | |
| HPnCAGn | 17.61515a | 6.45415 | 0.008 | 4.7633 | 30.4670 | |||
| HPpCAGn | HPpCAGp | −11.20567 | 6.20094 | 0.075 | −23.5533 | 1.1420 | ||
| HPnCAGn | 6.40948 | 6.45415 | 0.324 | −6.4424 | 19.2613 | |||
| HPnCAGn | HPpCAGp | −17.61515a | 6.45415 | 0.008 | −30.4670 | −4.7633 | ||
| HPpCAGn | −6.40948 | 6.45415 | 0.324 | −19.2613 | 6.4424 | |||
| sEselectin | Tukey HSD | HPpCAGp | HPpCAGn | 10.62429a | 3.21998 | 0.004 | 2.9290 | 18.3196 |
| HPnCAGn | 16.27393a | 3.35146 | 0.000 | 8.2644 | 24.2835 | |||
| HPpCAGn | HPpCAGp | −10.62429a | 3.21998 | 0.004 | −18.3196 | −2.9290 | ||
| HPnCAGn | 5.64964 | 3.35146 | 0.217 | −2.3599 | 13.6592 | |||
| HPnCAGn | HPpCAGp | −16.27393a | 3.35146 | 0.000 | −24.2835 | −8.2644 | ||
| HPpCAGn | −5.64964 | 3.35146 | 0.217 | −13.6592 | 2.3599 | |||
| LSD | HPpCAGp | HPpCAGn | 10.62429a | 3.21998 | 0.001 | 4.2125 | 17.0361 | |
| HPnCAGn | 16.27393a | 3.35146 | 0.000 | 9.6003 | 22.9475 | |||
| HPpCAGn | HPpCAGp | −10.62429a | 3.21998 | 0.001 | −17.0361 | −4.2125 | ||
| HPnCAGn | 5.64964 | 3.35146 | 0.096 | −1.0240 | 12.3233 | |||
| HPnCAGn | HPpCAGp | −16.27393a | 3.35146 | 0.000 | −22.9475 | −9.6003 | ||
| HPpCAGn | −5.64964 | 3.35146 | 0.096 | −12.3233 | 1.0240 | |||
| ICAM1 | Tukey HSD | HPpCAGp | HPpCAGn | 79.47500 | 35.41326 | 0.070 | −5.1578 | 164.1078 |
| HPnCAGn | 121.83464a | 36.85929 | 0.004 | 33.7460 | 209.9233 | |||
| HPpCAGn | HPpCAGp | −79.47500 | 35.41326 | 0.070 | −164.1078 | 5.1578 | ||
| HPnCAGn | 42.35964 | 36.85929 | 0.487 | −45.7290 | 130.4483 | |||
| HPnCAGn | HPpCAGp | −121.83464a | 36.85929 | 0.004 | −209.9233 | −33.7460 | ||
| HPpCAGn | −42.35964 | 36.85929 | 0.487 | −130.4483 | 45.7290 | |||
| LSD | HPpCAGp | HPpCAGn | 79.47500a | 35.41326 | 0.028 | 8.9582 | 149.9918 | |
| HPnCAGn | 121.83464a | 36.85929 | 0.001 | 48.4384 | 195.2309 | |||
| HPpCAGn | HPpCAGp | −79.47500a | 35.41326 | 0.028 | −149.9918 | −8.9582 | ||
| HPnCAGn | 42.35964 | 36.85929 | 0.254 | −31.0366 | 115.7559 | |||
| HPnCAGn | HPpCAGp | −121.83464a | 36.85929 | 0.001 | −195.2309 | −48.4384 | ||
| HPpCAGn | −42.35964 | 36.85929 | 0.254 | −115.7559 | 31.0366 | |||
The mean difference is significant at the 0.05 level.
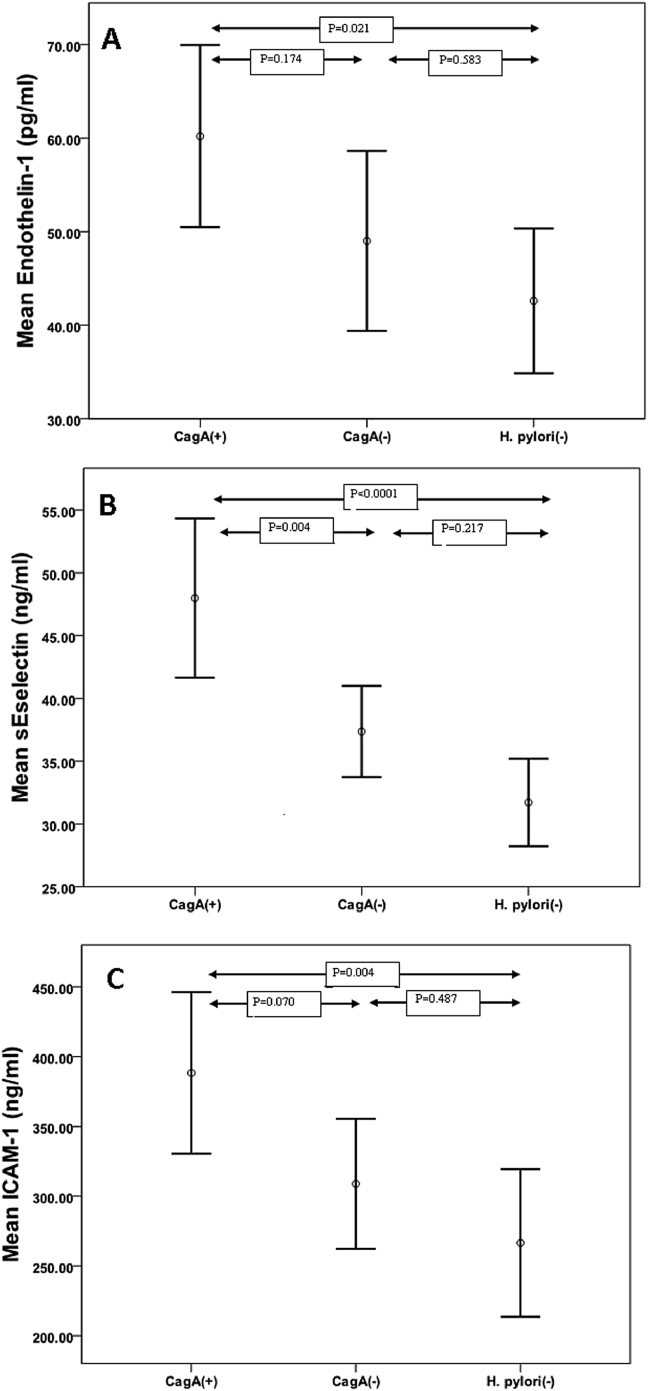
According to multiple comparison procedures, the increased levels of plasma ET-1 concentrations in CagA(+) group than H. pylori(−), were significant (p = 0.021). The mean of plasma E-selectin in CagA(+) group were higher than H. pylori(−) group (p < 0.0001). This pattern also seen for ICAM-1; the mean in CagA(+) group was greater than in H. pylori(−) group (p = 0.004), [Fig. 1].
Fig. 1.
Plasma levels of measured endothelial dysfunction markers in patients with CSX. The means of plasma levels of ET-1, E-selectin and ICAM-1 compared among three groups. The groups consisted of CagA(+) [CagA IgG(+) H. pylori IgG(+)], CagA(−) [CagA IgG(−) H. pylori IgG(+)] and H. pylori(−) [H. pylori IgG(−)]. Abbreviations used: H. pylori: Helicobacter pylori; CagA: cytotoxin-associated gene A; ICAM-1: intercellular adhesion molecule-1.
Discussion
CSX is a heterogeneous condition that encompasses several possible causal mechanisms. Cardiac and non-cardiac mechanisms have been proposed, among which endothelial dysfunction of the coronary microcirculation features prominently [3]. Current research suggests that coronary endothelial dysfunction and subsequent microvascular ischemia is the likely pathophysiologic mechanism for patients with CSX, which results in their angina-like chest pain [1]. H. pylori recently has been associated with CSX. In a previous case-control study we showed the high frequency of H. pylori in CSX [16]. Eskandarian et al. [17], showed that 95% of CSX patients were H. pylori(+), while only 47.5% of healthy control group were infected. Recent findings suggest that there are relations between chronic infection of H. pylori and endothelial dysfunction. Innocenti et al. [18] showed that H. pylori induced activation of human endothelial cells. Also, Oshima et al. [19] studied the association of H. pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. They reported that chronic infection of H. pylori involved in the development of the atherosclerosis via endothelial dysfunction. These studies are consistent with our findings which all three endothelial dysfunction markers were significantly higher in H. pylori(+) than H. pylori(−) groups. We speculated that H. pylori may also cause endothelial dysfunction directly by affecting the structure and function of vascular endothelial cells via inflammation in CSX [20].
On the other hand, a number of virulence factors of H. pylori are associated with disease outcome, including the CagA [21]. CagA is a 128-kDa H. pylori antigen, associated with enhanced virulence and cytotoxin production [22]. Recently researchers have revealed an association between CagA(+) strains and rigorous forms of gastrointestinal diseases including peptic ulcer and gastric cancer [15], [23], [24]. CagA recently has been associated with CSX [17], [25].
Although prevalence of CagA has been studied widely in gastrointestinal diseases, but no previous study has investigated the possible association of these more virulent H. pylori strains in CSX patients with involvement of endothelial dysfunction. In this study, we compared the levels of three plasma endothelial dysfunction markers, ICAM-1, sE-selectin and ET-1 among three CSX patient groups including CagA(+) H. pylori, CagA(−) H. pylori and H. pylori(−) that were statistically similar for age, sex, BMI, blood pressure, lipids, glucose, smoking and medications. We showed that the CSX patients with CagA bearing strain of H. pylori chronic infection have significantly higher levels of plasma soluble endothelial dysfunction markers when compared to CagA(−), or even H. pylori(−) groups. Therefore, finding the “High” levels of plasma soluble adhesion molecules; ICAM-1 and sE-selectin in CagA(+) group than CagA(−) and H. pylori(−) groups in the present study suggests that CagA(+) strain of this bacterium may be more associated with endothelial activation. Also, in this study, the measured levels of ET-1 in CagA(+) were higher than H. pylori(−) group and tended to be greater than CagA(−) patients. This also may indicate that the main association between H. pylori infection and endothelial dysfunction is due to CagA(+) H. pylori infection.
Recent researches suggest a role of inflammation in the pathogenesis of endothelial dysfunction and correlation of CRP-concentration (C-Reactive Protein, a sensitive marker of inflammation) with severity of symptoms in patients with CSX [26], [27]. Chronic inflammation leads to an increase in the generation of pro-inflammatory cytokines, cell adhesion molecules and growth factors that can elicit inflammatory and proliferative changes in the vessel walls, resulting in endothelial dysfunction [25]. In a previous case-control study, we investigated the association of inflammation and CagA(+) strains of H. pylori in CSX using inflammation markers such as Interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). We found that the CagA positive strain of H. pylori can not only be a trigger, and may also have a role via chronic inflammation in the pathogenesis of CSX [28]. Previous studies showed the contribution of endothelium to the inflammatory response with regard to production of cytokine and chemokine after infection with pathogenic bacteria like H. pylori [29]. During inflammatory responses, the transcription factor nuclear factor kappa B (NF-κB) plays a key role in the regulation of participating genes [30]. The expression of ICAM-1 and E-Selectin is under NF-κB control in endothelial cells [31] and NF-κB activation has been shown during infection with H. pylori [32]. Giving these information together, it is possible that CagA(+) may induce upregulation of special expression factors that led to higher levels of endothelial dysfunction markers in plasma of CagA(+) group in this study.
In this study, we had some limitations. Our criteria for being diagnosed as infection were IgG positivity to H. pylori. Because positivity of IgG to H. pylori can provide evidence of chronic infection even following eradication of H. pylori [33], [34], it is possible some of our patients not to have current active infection and probably they have been infected in the past. Therefore, it should be considered that infection to H. pylori in this research means patients who their plasma is currently positive for anti-H. pylori IgG antibody. The mean age of the CSX patients in this study were 51 which nearly consist of 2/3 female individuals. As CAD prevalence in middle age is low, resulting in high pseudopositive exercise stress test results and this was another limitation in our study.
After all, it should be added that well designed clinical trial studies might to be needed to further confirm these results. Although all patients in this study were CSX patients, studies with adjoining normal controls by regarding H. pylori and CagA status and by using non-humoral endothelial dysfunction evaluation methods like FMD is proposed. In addition, a study with H. pylori eradication and following up the angina symptoms and endothelial function quality propose to future investigation. Currently we can not prove the eradication of H. pylori infection can either improve angina symptom or progression of atherosclerosis.
Conclusion
The conclusion drawn from the results is that high levels of markers of endothelial dysfunction are related with CagA(+) H. pylori infection in patients with CSX, given contributors to endothelial dysfunction including age, sex, obesity, blood pressure, lipid, glucose, smoking and medications are statistically controlled. Consequently, the possible role of CagA(+) infection in the pathogenesis of CSX with involvement of endothelial dysfunction is suggested. This study revealed that patients with chronic CagA(+) H. pylori infection have high degree of endothelial dysfunction.
Conflicts of interest
The authors declare that there are no conflicts of interest regarding this manuscript.
Acknowledgments
This article was extracted from the thesis prepared by Hadi Rouhrazi to fulfill the requirements required for earning the MSc of Clinical Biochemistry degree. The authors wish to thank the members of Department of Cardiology, Urmia University of Medical Sciences including the staff and nurses for their tremendous cooperation and support, and the Research Deputy for the financial support of the research.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Hurst T., Olson T.H., Olson L.E., Appleton C.P. Cardiac syndrome X and endothelial dysfunction: new concepts in prognosis and treatment. Am J Med. 2006;119:560–566. doi: 10.1016/j.amjmed.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Li J.J., Li Y.S., Zhang Y., Gao Z., Li Z., Qian H.Y. Inflammation: a possible pathogenic link to cardiac syndrome X. Med Hypotheses. 2006;66:87–91. doi: 10.1016/j.mehy.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo-Espliguero R., Kaski J.C. Microvascular dysfunction in cardiac syndrome X: the role of inflammation. CMAJ. 2006;174:1833. doi: 10.1503/cmaj.051331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barac A., Campia U., Panza J.A. Methods for evaluating endothelial function in humans. Hypertension. 2007;49:748–760. doi: 10.1161/01.HYP.0000259601.38807.a6. [DOI] [PubMed] [Google Scholar]
- 5.Turhan H., Saydam G.S., Erbay A.R., Ayaz S., Yasar A.S., Aksoy Y. Increased plasma soluble adhesion molecules; ICAM-1, VCAM-1, and E-selectin levels in patients with slow coronary flow. Int J Cardiol. 2006;108:224–230. doi: 10.1016/j.ijcard.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Senen K., Ileri M., Alper A., Yetkin F., Atak R., Hisar I. Increased levels of soluble adhesion molecules E-selectin and P-selectin in patients with cardiac syndrome X. Angiology. 2005;56:273–277. doi: 10.1177/000331970505600306. [DOI] [PubMed] [Google Scholar]
- 7.Kaski J.C., Elliott P.M., Salomone O., Dickinson K., Gordon D., Hann C. Concentration of circulating plasma endothelin in patients with angina and normal coronary angiograms. Br Heart J. 1995;74:620–624. doi: 10.1136/hrt.74.6.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nocente R., Gentiloni N., Cremonini F., Giorgi A., Serricchio M., Santoliquido A. Resolution of syndrome X after eradication of virulent CagA-positive Helicobacter pylori. Southern Med J. 2000;93:1022–1023. [PubMed] [Google Scholar]
- 9.Rasmi Y., Zeynalzadeh J., Shirpoor A., Seyedmohammadzad M.H., Hajhosseini R. Lipid profile in cardiac syndrome X: association with Helicobacter pylori. J Clin Diagn Res. 2016;10 doi: 10.7860/JCDR/2016/18048.8185. BC07-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beier D., Frank R. Molecular characterization of two-component systems of Helicobacter pylori. J Bacteriol. 2000;182:2068–2076. doi: 10.1128/jb.182.8.2068-2076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanbay M., Kanbay A., Boyacioglu S. Helicobacter pylori infection as a possible risk factor for respiratory system disease: a review of the literature. Respir Med. 2007;101:203–209. doi: 10.1016/j.rmed.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Izadi M., Fazel M., Sharubandi S.H., Saadat S.H., Farahani M.M., Nasseri M.H. Helicobacter species in the atherosclerotic plaques of patients with coronary artery disease. Cardiovasc Pathol. 2012;21:307–311. doi: 10.1016/j.carpath.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Eskandarian R., Malek M., Mousavi S.H., Babaei M. Association of Helicobacter pylori infection with cardiac syndrome X. Singap Med J. 2006;47:704–706. [PubMed] [Google Scholar]
- 14.Atherton J.C. H. pylori virulence factors. Br Med Bull. 1998;54:105–120. doi: 10.1093/oxfordjournals.bmb.a011662. [DOI] [PubMed] [Google Scholar]
- 15.Peek R.M., Jr., Miller G.G., Tham K.T., Perez-Perez G.I., Zhao X., Atherton J.C. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;73:760–770. [PubMed] [Google Scholar]
- 16.Rasmi Y., Seyyed-Mohammadzad M.H. Frequency of Helicobacter pylori and cytotoxine associated gene A antibodies in patients with cardiac syndrome X. J Cardiovasc Dis Res. 2012;3:19–21. doi: 10.4103/0975-3583.91597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskandarian R.M.M., Mousavi S.H., Babaei M. Association of Helicobacter pylori infection with cardiac syndrome X. Singap Med J. 2006;47:704–706. [PubMed] [Google Scholar]
- 18.Innocenti M., Thoreson A.C., Ferrero R.L., Stromberg E., Bolin I., Eriksson L. Helicobacter pylori-induced activation of human endothelial cells. Infect Immun. 2002;70:4581–4590. doi: 10.1128/IAI.70.8.4581-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshima T., Ozono R., Yano Y., Oishi Y., Teragawa H., Higashi Y. Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J Am Coll Cardiol. 2005;45:1219–1222. doi: 10.1016/j.jacc.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Rasmi Y., Raeisi S. Possible role of Helicobacter pylori infection via microvascular dysfunction in cardiac syndrome X. Cardiol J. 2009;16:585–587. [PubMed] [Google Scholar]
- 21.Argent R.H., Thomas R.J., Letley D.P., Rittig M.G., Hardie K.R., Atherton J.C. Functional association between the Helicobacter pylori virulence factors VacA and CagA. J Med Microbiol. 2008;57(Pt 2):145–150. doi: 10.1099/jmm.0.47465-0. [DOI] [PubMed] [Google Scholar]
- 22.Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blaser M.J., Perez-Perez G.I., Kleanthous H., Cover T.L., Peek R.M., Chyou P.H. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 24.Ching C.K., Wong B.C., Kwok E., Ong L., Covacci A., Lam S.K. Prevalence of CagA-bearing Helicobacter pylori strains detected by the anti-CagA assay in patients with peptic ulcer disease and in controls. Am J Gastroenterol. 1996;91:949–953. [PubMed] [Google Scholar]
- 25.Majidinia M., Rasmi Y., Khadem Ansari M.H., Seyed-Mohammadzad M.H., Saboory E., Shirpoor A. Metoprolol improves endothelial function in patients with cardiac syndrome X. Iran J Pharm Res. 2016;15:561–566. [PMC free article] [PubMed] [Google Scholar]
- 26.Cosin-Sales J., Pizzi C., Brown S., Kaski J. C-reactive protein,clinical presentation, and ischemic activity in patients with chest pain and normal coronary artery. J Am Coll Cardiol. 2003;41:1468–1474. doi: 10.1016/s0735-1097(03)00243-2. [DOI] [PubMed] [Google Scholar]
- 27.Lanza G.A. Cardiac syndrome X: a critical overview and future perspectives. Heart. 2007;93:159–166. doi: 10.1136/hrt.2005.067330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmi Y., Raeisi S., Seyyed Mohammadzad M.H. Association of inflammation and cytotoxin-associated gene A positive strains of Helicobacter pylori in cardiac syndrome X. Helicobacter. 2012;17:116–120. doi: 10.1111/j.1523-5378.2011.00923.x. [DOI] [PubMed] [Google Scholar]
- 29.Hatz R.A., Rieder G., Stolte M., Bayerdorffer E., Meimarakis G., Schildberg F.W. Pattern of adhesion molecule expression on vascular endothelium in Helicobacter pylori-associated antral gastritis. Gastroenterology. 1997;112:1908–1919. doi: 10.1053/gast.1997.v112.pm9178683. [DOI] [PubMed] [Google Scholar]
- 30.Baeuerle P.A., Baichwal V.R. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111–137. [PubMed] [Google Scholar]
- 31.Collins T., Read M.A., Neish A.S., Whitley M.Z., Thanos D., Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 32.Isomoto H., Miyazaki M., Mizuta Y., Takeshima F., Murase K., Inoue K. Expression of nuclear factor-kappaB in Helicobacter pylori-infected gastric mucosa detected with southwestern histochemistry. Scand J Gastroenterol. 2000;35:247–254. doi: 10.1080/003655200750024092. [DOI] [PubMed] [Google Scholar]
- 33.Koizumi W., Tanabe S., Imaizumi H., Hibi K., Kida M., Ohida M. Effect of anti-Helicobacter pylori IgG antibody titer following eradication of Helicobacter pylori infection. Hepatogastroenterology. 2003;50:293–296. [PubMed] [Google Scholar]
- 34.Andersen L.P., Rosenstock S.J., Bonnevie O., Jorgensen T. Seroprevalence of immunoglobulin G, M, and A antibodies to Helicobacter pylori in an unselected Danish population. Am J Epidemiol. 1996;143:1157–1164. doi: 10.1093/oxfordjournals.aje.a008694. [DOI] [PubMed] [Google Scholar]