Abstract
Insulin resistance is a prevalent syndrome in developed as well as developing countries. It is the predisposing factor for type 2 diabetes mellitus, the most common end stage development of metabolic syndrome in the United States. Previously, studies investigating type 2 diabetes have focused on beta cell dysfunction in the pancreas and insulin resistance, and developing ways to correct these dysfunctions. However, in recent years, there has been a profound interest in the role that oxidative stress in the peripheral tissues plays to induce insulin resistance. The objective of this review is to focus on the mechanism of oxidative species generation and its direct correlation to insulin resistance, to discuss the role of obesity in the pathophysiology of this phenomenon, and to explore the potential of antioxidants as treatments for metabolic dysfunction.
Keywords: Insulin resistance, Oxidative stress, Type 2 diabetes, Obesity, Antioxidants, Adipokines
Insulin resistance is a pandemic problem that does not discriminate between demographic factors, including socioeconomics and ethnicity [1]. Type 2 diabetes mellitus, a consequence of insulin resistance, is a metabolic disease that, according to the latest data for the World Health Organization in 2014, affects 9% of the world's population, both in developed and developing countries, and directly caused 1.5 million deaths in that year alone [2]. Prediabetes, or metabolic syndrome, was found in nearly 35% of cases in adults in the United States alone between 2003 and 2012 [3]. The predisposing factor and best indicator of future diabetes development is insulin resistance. This condition is defined by a decrease in insulin sensitivity in the peripheral tissues. Insulin resistance is characterized by a decline in cellular response to insulin stimulation at the peripheral tissues. Thus, it is important to investigate the mechanisms of this peripheral response to insulin in order to understand the pathogenesis of later metabolic complications.
Oxidative stress has been recently recognized as a key mechanism in insulin resistance. Oxidative stress is defined by excess endogenous oxidative species, which both damage cells and manipulate signal pathway [4]. Reactive species, especially reactive oxygen species (ROS) like superoxide, hydrogen peroxide, and hydroxyl radical ions [5], are the agents of oxidative stress and are produced at low physiological levels mostly in the mitochondria and peroxisomes. ROS are produced endogenously and have physiological significance at low levels, especially in signaling pathways [6], although these mechanisms are not yet clear because of the dual role ROS plays as both a signal and as a damaging agent [7]. Among these signaling roles are transcriptional control [8] and cell cycle regulation [5]. There is cross talk between endogenous mitochondrial ROS and redox enzymes ranging from NADPH oxidases to angiotensin I/II receptors [9]. H2O2 has been specifically investigated in these studies as one of these “redox switches” which can lead to vicious cycles of redox stimulation. It has been accepted for some time that ROS becomes damaging to cells beyond low physiological levels. Recent studies have concluded that ROS damage has direct roles in the development and progression of many chronic diseases, including the pathogenesis of insulin resistance and type 2 diabetes.
The link between oxidative stress and insulin resistance has drawn attention. Research in this area has revealed that there is a strong correlation between the state of oxidative stress in the body and the incidence of insulin resistance and even late stage diabetes cases. The understanding of this correlation has been unclear or incomplete, often focusing on an individual detail of a specific pathway or pathogenesis. In this review, we intend to clarify the molecular pathway behind the pathogenesis of insulin resistance, explore the link between insulin resistance and obesity, and discuss antioxidants as potential treatments for diabetic and pre-diabetic patients.
Insulin receptor signaling pathway
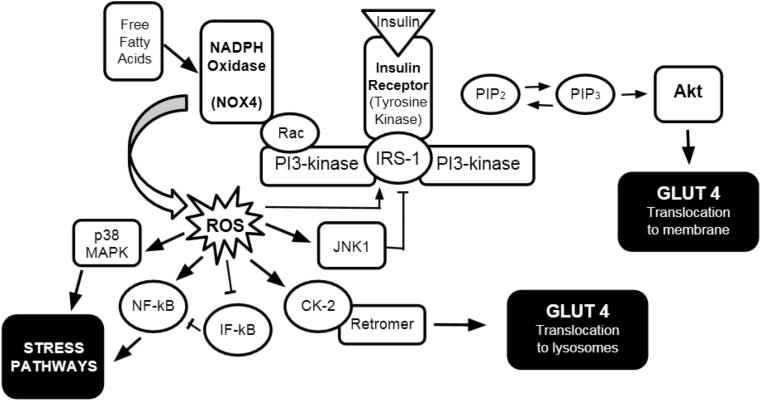
It is important to describe the insulin receptor signaling pathway when discussing insulin resistance. Insulin receptor signal transduction involves many proteins and is transected by several other pathways. A schematic of this pathway along with the pathogenic, oxidative pathway are shown in Fig. 1.
Fig. 1.
Insulin receptor signaling pathway including ROS influence. The normal pathway begins with insulin binding insulin tyrosine kinase receptor. The insulin receptor phosphorylates insulin receptor substrate-1 (IRS-1) [10] which in turn phosphorylates PI3-kinase. PI3-kinase then phosphorylates PIP2 which then activates Akt [11], eventually leading to glucose transporter 4 (GLUT4) translocation to the plasma membrane of skeletal muscle cells and adipocytes, thus allowing the cell to absorb extracellular glucose, lowering interstitial glucose levels and thus plasma glucose concentration. Abbreviations used: PIP2 and PIP3: phosphatidylinositol species; Rac: Rac GTPase; ROS: Reactive oxygen species; JNK1: c-Jun N-terminal kinase 1; CK-2: Casein kinase 2; NF-kB: nuclear factor kB; IF-kB: Inhibitory factor kB; p38 MAPK: p38 mitogen activated protein kinase; Akt: protein kinase B.
Glucose transporter 4 (GLUT4) is the main glucose transporter in the peripheral insulin sensitive tissues, namely skeletal muscle and adipose tissue [12], and it has been the focus of much research related to insulin resistance. The cell responds to insulin by increasing GLUT4 expression in the plasma membrane, thereby increasing cellular uptake of glucose from the bloodstream. However, high levels of insulin signaling produce a negative effect on GLUT4 presence in the membrane [13]. Up to what is considered optimal insulin concentration, the cell increases its utilization of the normal insulin transduction pathway. Beyond optimal concentrations, the cell shows a downward shift in GLUT4 expression. Thus, blood glucose levels remain high and the pancreas secretes more insulin in response, leading to a positive feedback loop that drives up intravascular levels of insulin and desensitizing the peripheral tissues to the insulin. The biomolecular consequence of this cycle is the continued downregulated expression of GLUT4 in the cell membrane, exacerbating the problem [13]. The systemic result of this dysfunction is hyperglycemia, hyperinsulinemia, and increased oxidative stress in tissues.
The mechanism behind this regulation involves different proteins in transduction than the normal pathway. NADPH oxidase 4 (NOX4) is a powerful oxidizing enzyme that produces ROS. Above optimal insulin concentrations, a shift in the signaling pathway happens at PI3-kinase. PI3-kinase phosphorylates Rac instead of PIP2, which amplifies the activity of NOX4 [14]. ROS levels increase as a result. The increased ROS activates casein kinase-2 (CK2) which in turn activates the retromer [13]. The retromer then signals the trans-Golgi network downstream, and GLUT4 is transported to lysosomes for degradation instead of the plasma membrane. Therefore, intravascular glucose levels remain elevated in the oxidative environment.
Mitochondria also contribute to the oxidation in the cell due to high nutrient environments. Due to an increased supply of glucose in high sugar diets, mitochondria have more substrate available to make ATP. Thus the mitochondria are hyperactive and produce more of their natural byproduct, ROS [15]. This increased ROS damages the infrastructure of the cell, and induces stress responses the mitochondria are responsible for. ROS directly stimulate NF-kB [11], JNK [16], and p38 MAPK [17] resulting in mitochondria-induced stress responses. Increased ROS levels induce mitochondrial fission resulting in actions on both the insulin receptor pathway and stress proteins [18]. Mitochondrial fission has been directly linked to insulin resistance in skeletal muscle [19]. The stress pathways and proteins induced by mitochondrial dysfunction have been extensively studied for their roles in apoptosis and the cell cycle, although they induce other stress responses [20]. A study in cell culture showed that insulin resistance can be prevented by restricting mitochondrial over-activation [21].
Relationship between obesity and insulin resistance
Obesity has become a health issue of pandemic proportions. It is linked to many different chronic diseases including cardiovascular disease, stroke, and type 2 diabetes as well as other variable illnesses among different demographics [22], [23]. It has been generally accepted for years that there is a link between obesity and insulin resistance or type 2 diabetes with much research on the relationship being devoted to inflammation [24]. This link has recently been clarified by a variety of studies.
Free fatty acids (FFAs) are abundant in obesity [25] and cause cellular dysfunction, especially in the mitochondria [7]. A study done in adipocytes showed increased levels of mitochondrial fission when these cells were exposed to either high glucose or FFA levels. This is accomplished through endogenous ROS production and release as well as the manipulation of the insulin transduction pathway. Mitochondrial fission increases p38 MAPK expression and downregulates IRS-1 and Akt function [18]. Furthermore, this mitochondrial dysfunction appears to be affected on the transcriptional level in obese persons, according to a study done in human subjects of varying degrees of obesity [26]. As adipose tissue increases, mitochondrial transcription levels in adipose tissue decrease, resulting in impaired glucose utilization in this tissue.
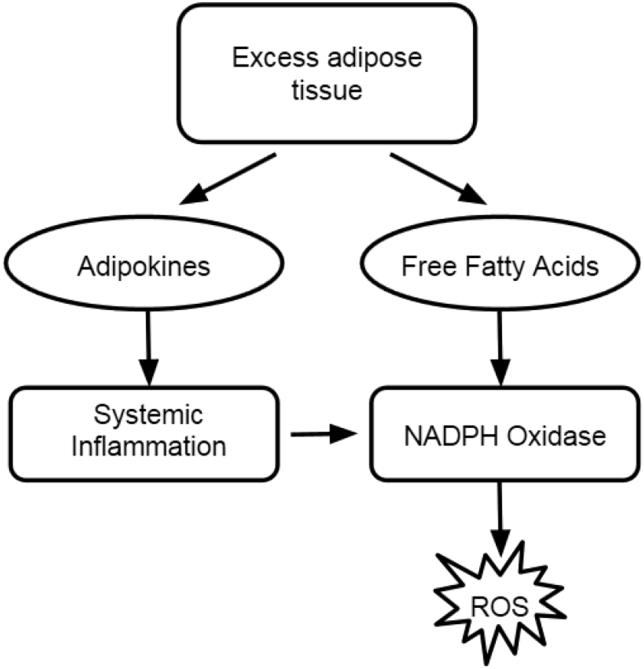
Another study done independently showed an additional mechanism by which excess adipose tissue manipulates systemic oxidation. This is accomplished through inflammatory response [27].
Excess adipose tissue secretes a special group of cytokines, appropriately named adipokines [28]. These proinflammatory molecules induce chronic inflammation [Fig. 2]. Proinflammatory cytokines induce chemotactic invasion of the target tissue by immune cells which further propagate the problem [24]. The immune system attacks accumulated adipose tissue because of this and causes a steady state of inflammation. Chronic inflammation is known as a cause of many degenerative diseases including neurodegenerative diseases and heart disease [29]. These adipokines also induce pro-inflammatory cytokine production in macrophages, furthering systemic inflammation [30].
Fig. 2.
Link between obesity and insulin resistance.
There is one adipokine that has been found to be beneficial in the study of oxidative stress and insulin resistance. Adiponectin works to correct oxidative conditions [31]. Contrary to the action of other adipokines, adiponectin levels decrease as adipose tissue accumulates [32]. The exact regulation of this inverse relationship is unknown, but it could be regulated by post-translational methods. Adiponectin is known to antagonize insulin resistance by enhancing hepatic IRS-2 expression [33], potentially mediated by FGF-21 [34]. Excess adipose tissue is therefore a dynamic organ that can either enhance or heal the effects of oxidative stress in the body [25].
Because excess fat is the cause of these inflammatory problems, it is important to maintain a healthy weight. Visceral fat is of particular importance, as it has the strongest correlation with metabolic syndromes and oxidative stress [35]. It is the main site of adipokine imbalance and FFA action [36]. Unfortunately, the most common clinical measures of fat composition – waist size and body mass index – do not always give an accurate depiction of fat distribution or content. The measureable cross-sectional area of visceral fat is a far better indicator of inflammation and oxidative potential in the body [37]. Decreasing visceral fat has substantial benefits on metabolism and decrease the likelihood of insulin resistance and the effects of established insulin resistance.
Antioxidants in the control of type 2 diabetes
The method of resolving ROS in the body is to electronically pair the radicals to each other, thereby resolving the radical electrons without propagation [38]. This “radical scavenging” is done by several mechanisms but most importantly by superoxide dismutase (SOD) and other enzymes [39]. It should be noted that the redox product of SOD and NOX4 is the potent oxidative species H2O2, and this product is finally resolved by peroxidases such as catalase and glutathione peroxidase [39]. There are also specific enzymes to metabolize ROS from mitochondria, including glutathione peroxidases and peroxiredoxin-3 [39]. Proteins damaged by ROS are also processed to prevent radical propagation and mutated proteins from causing further damage [40]. Protein turnover in the cell dictates that proteins are degraded constantly, decreasing the effective levels of the proteins and disabling the processes they are driving [41]. Excessive oxidative stress forces an imbalance in protein turnover, favoring damaged protein accumulation over degradation [20].
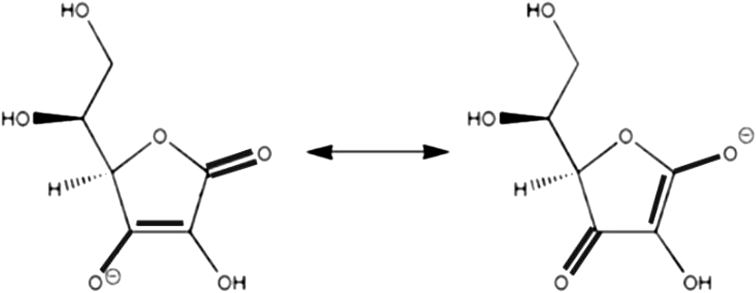
There have been several studies in which antioxidants have improved insulin sensitivity. Antioxidant vitamins such as ascorbic acid (vitamin C) and tocopherols (vitamin E) have therapeutic potential [39]. Their conjugate bonds can stabilize a radical electron by electronic resonance which is subsequently resolved by a scavenging protein [Fig. 3]. This neutralizes the oxidation being done by the radical.
Fig. 3.
Radical stability in ascorbic acid.
Ascorbic acid, was investigated in several studies for its antioxidant function. One study was done in patients with coronary artery disease and coinciding insulin resistance associated with oxidative stress [42]. Not only did the subjects' vascular flow improve, but their insulin sensitivity also increased. Another study done in a randomized patient population specifically targeting skeletal muscle insulin response to ascorbic acid showed significant amelioration of oxidative stress and improvement in insulin sensitivity in the muscle tissue [43].
Tocopherols also exhibit antioxidant properties that improve oxidative deficiencies that could be vital for disease prevention. Successful in vivo studies in rats investigated their protective effects against oxidation, preventing accumulation of oxidation in the tissues [44]. The study asserts that tocopherols are potent scavengers of peroxyl radicals made by NADPH oxidase. Another study done independently in human tissue models showed that they can even increase the expression of adiponectin receptors [45]. Therefore, tocopherols could both lower radical presence in the body and lower adipokine inflammation, preventing oxidative stress and subsequent insulin resistance.
Other antioxidative compounds have also been studied. Flavonoids are an exceptional example of some of these compounds. Diabetic patients given anthocyanin supplements, a water soluble flavonoid pigment, made improvements in metabolic function [46]. These metabolic markers included decreased fasting glucose and serum lipids, decreased inflammatory markers, and increased antioxidant potential. The flavonoids in green tea also positively impact metabolic effects in obese patients [47] and glucose-challenged rats [48]. Each model showed increases in insulin sensitivity and lower systemic oxidation.
Several studies have revealed problems associated with antioxidant therapy as it relates to oxidative stress. These issues appear to be dosage dependent and pharmacokinetic in nature. They have sometimes failed to show any results in clinical settings. Tocopherols, for instance, have been shown to be ineffective in protecting patients, including diabetic patients, from cardiovascular complications [49]. Antioxidants have also been shown to have harmful effects in patients due potentially to redox cycling reactions. A study was done in postmenopausal women with type 2 diabetes taking ascorbic acid supplements [50]. The results showed an increased risk in mortality from cardiovascular disease for higher dosages of ascorbic acid. Ascorbic acid has emerged as a prooxidant at high concentrations and has even been used as an oxidative cancer therapy as it increases H2O2 production [51]. These issues must be resolved in future investigations but should not detract from the benefits shown early in this study. The most immediate benefits of these studies could be low dose preventative treatments before a safe and effective treatment is developed. These preventative treatments can be effectively accomplished through oral administration. Ascorbic acid has a predictable bioavailability of 60–100 μM [52], and high concentrations can only be reached with intravenous administration. Tocopherols have variable bioavailability among individuals, but they are considered safe to administer at a variety of doses [53]. Intravenous administration should again be used for future aggressive therapy. Other studies have been done in rats using other antioxidants such as α-lipoic acid, and they have found bioavailability [54] and therapeutic [55] doses for a variety of oxidative diseases, but these have not made it to clinical trials in humans. These along with other studies, makes it clear that antioxidants have potential as metabolic syndrome treatments, both as preventative and standard treatments.
As oxidation is a key issue in insulin resistance, it is important to find specific, targeted ways to combat oxidative species accumulation in the tissues at the molecular level. Increased understanding and manipulation of antioxidants can afford this desired specificity in the correction of oxidative stress without some of the side effects seen in preliminary research. This redox correction will be beneficial as both a preventative measure for insulin resistance and other conditions as well as an additional treatment for those conditions once formed. A healthy and varied diet that is low in sugar could give exceptional protection against insulin resistance and prevent oxidative stress in the body. Fruits and vegetables in particular provide synergized nutrient delivery that the body can use most effectively to combat oxidation [56]. Ascorbic acid, tocopherols, and flavonoids are all found in these foods. By and large, eating a variety of fruits and vegetables conveys protective amounts of antioxidants to decrease the likelihood of systemic oxidation and insulin resistance. Investigating more potent forms of antioxidants would also be important.
Future perspectives
Combination therapy could lead to the best outcomes for patients with insulin resistance. Antioxidants show promise as effective treatments for oxidative stress. Ascorbic acid and tocopherols have been successful in rehabilitating peripheral tissues and increasing insulin sensitivity [42], [43], [44], [45]. Flavonoids and α-lipoic acid have shown similar qualities [46], [47], [48]. Future use of antioxidants could make it to clinical practice with increased understanding of the mechanisms they employ. A varied and nutritious diet can be employed as both a preventative measure for metabolic health and as a companion to metabolic treatment [56].
Maintaining a healthy weight is also important for treating insulin resistance. Limiting adipose tissue, especially visceral fat, can combat systemic inflammation and decrease the incidence of metabolic syndromes as a consequence [27]. Better methods should be developed to increase the accuracy of measuring visceral fat, as body mass index can be falsely influenced by a variety of conditions. A cross-sectional abdominal scan gives more accurate data [39].
Conclusions
Oxidative stress is a fundamental problem of metabolic syndromes including type 2 diabetes. ROS cause insulin resistance in the peripheral tissues by affecting various points in insulin receptor signal transduction, ultimately resulting in decreased expression of GLUT4 transporter in the cellular membrane [57]. Mitochondria contribute to ROS levels in nutrient rich environments and induce stress pathways in the cell [13]. The systemic result is positive feedback hyperinsulinemia and cell desensitization to glucose. Obesity is a contributing factor to metabolic disturbances and contributes to oxidative stress propagation [25]. Free fatty acids accelerate mitochondrial fission and promote ROS production [7]. Adipokines are released from accumulated adipose tissue and induce systemic inflammation [25]. Antioxidants show promise as treatments for metabolic disease, supported by in vivo and in vitro studies.
Conflicts of interest
The authors have no conflict of interest to declare regarding this manuscript.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Centers for Disease Control and Prevention . HHS; 2014. National diabetes statistics report: estimates of diabetes and its burden in the United States. [Google Scholar]
- 2.World Health Organization Media Centre . WHO Press; 2016. Global report on diabetes. [Google Scholar]
- 3.Aguilar M., Bhuket T., Torres S., Liu B., Wong R. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 4.Sies H. The concept of oxidative stress after 30 years. In: Gelpi R., Boveris A., Poderoso J., editors. Advances in biochemistry in health and disease. Springer; New York: 2016. pp. 3–11. [Google Scholar]
- 5.Schrieber M., Chandel N. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:453–462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brand M. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Gao C., Zhu C., Zhao Y., Chen X., Ji C., Zhang C. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol Cell Endocrinol. 2010;320:25–33. doi: 10.1016/j.mce.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Marinho S., Real C., Cyrne L., Soares H., Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Rad Biol Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancer N., Qiu W., Cherella C., Li Y., Copps K., White M. Insulin and metabolic stress stimulate multisite serine/threonine phosphorylation of insulin receptor substrate 1 and inhibit tyrosine phosphorylation. J Biol Chem. 2014;29:12467–12484. doi: 10.1074/jbc.M114.554162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper G. 7th ed. Sinauer; Massachusetts: 2013. The cell: a molecular approach. [Google Scholar]
- 12.Blanco C., McGill-Vargas L., Gastaldelli A., Seidner S., McCurnin D., Leland M. Peripheral insulin resistance and impaired insulin signaling contribute to abnormal glucose metabolism in preterm baboons. Endocrinology. 2015;156:813–823. doi: 10.1210/en.2014-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J., Nakagawa Y., Kojima I., Shibata H. Prolonged insulin stimulation down-regulates GLUT4 through oxidative stress-mediated retromer inhibition by a protein kinase CK2-dependent mechanism in 3T3-L1 adipocytes. J Biol Chem. 2013;298:133–142. doi: 10.1074/jbc.M113.533240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campa C., Ciraolo E., Ghigo A., Germena G., Hirsch E. Crossroads of PI3K and rac pathways. Small GTPases. 2015;6:71–80. doi: 10.4161/21541248.2014.989789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henriksen E., Diamond-Stanic M., Marchionne E. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51:993–999. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai H., Wang W., Lin C., Pai P., Lai T., Tsai C. NADPH oxidase-derived superoxide anion-induced apoptosis is mediated via the JNK dependent activation of NF-kB in cardiomyocytes exposed to high glucose. J Cell Physiol. 2012;227:1347–1357. doi: 10.1002/jcp.22847. [DOI] [PubMed] [Google Scholar]
- 17.Al-Lahham R., Deford J., Papaconstantinou J. Mitochondrial-generated ROS down regulates insulin signaling via activation of p38 MAPK stress response pathway. Mol Cell Endocrinol. 2015;419:1–11. doi: 10.1016/j.mce.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Boucher J., Kleinridders A., Kahn C. Insulin receptor signalling in normal and insulin resistant states. Cold Spring Harb Perspect Biol. 2014;6:1–23. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jheng H., Tsai P., Guo S., Kuo L., Chang C., Su I. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32:309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paio M., Kang K., Lee I., Kim H., Kim S., Choi J. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett. 2011;201:92–100. doi: 10.1016/j.toxlet.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M., Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:330–341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Cohen D., LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr Relat Cancer. 2012;19:F27–F45. doi: 10.1530/ERC-11-0374. [DOI] [PubMed] [Google Scholar]
- 23.Gaggini M., Morelli M., Buzzigoli E., DeFronzo R., Bugianesi E., Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544–1560. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esser N., Legrand-Poels S., Piette J., Scheen A., Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Boden G. Obesity, insulin resistance, and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinonen S., Muniandy M., Buzkova J., Mardinoglu A., Rodriguez A., Fruhbeck G. Mitochondria-related transcriptional signature is downregulated in adipocytes in obesity: a study of young healthy MZ twins. Diabetologia. 2017;60:169–181. doi: 10.1007/s00125-016-4121-2. [DOI] [PubMed] [Google Scholar]
- 27.Salzano S., Checconi P., Hanschmann E., Lillig C., Bowler L., Chan P. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci U S A. 2014;111:12157–12162. doi: 10.1073/pnas.1401712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee M., Wu Y., Fried S. Adipose tissue heterogeneity: implications of depot differences in adipose tissue for obesity complications. Mol Asp Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franceschi C., Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol. 2014;69:4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 30.Barnes M., Carson M., Nair M. Non-traditional cytokines: how catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system. Cytokine. 2015;72:210–219. doi: 10.1016/j.cyto.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kizer J. A tangled threesome: adiponectin, insulin sensitivity, and adiposity. Diabetes. 2013;62:1007–1009. doi: 10.2337/db12-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shehzad A., Iqbal W., Shehzad O., Lee Y. Adiponectin: regulation of its production and its role in human disease. Hormones. 2012;1:8–20. doi: 10.1007/BF03401534. [DOI] [PubMed] [Google Scholar]
- 33.Awazawa M., Ueki K., Inabe K., Yamauchi T., Kubota N., Kaneko K. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 2011;13:401–412. doi: 10.1016/j.cmet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Adams A., Kharitonenkov A. FGF21 drives a shift in adipocytokine tone to restore metabolic health. Aging. 2013;15:386–387. doi: 10.18632/aging.100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heredia F., Gomez-Martinez S., Marcos A. Obesity, inflammation, and the immune system. Proc Nutr Soc. 2012;71:332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 36.Matsuzawa Y. The metabolic syndrome and adipocytokines. Expert Rev Clin Immunol. 2014;3:39–46. doi: 10.1586/1744666X.3.1.39. [DOI] [PubMed] [Google Scholar]
- 37.Shuster A., Patlas M., Pinthus J., Mourtzakis M. The clinical importance of visceral adiposity: a critical review for methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reczek C., Chandel N. ROS-dependent signal transduction. Curr Opin Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halliwell B., Gutteridge J. 5th ed. Oxford; United Kingdom: 2015. Free radicals in biology and medicine. [Google Scholar]
- 40.Reece J., Campbell N., Cain M., Urry L., Wasserman S., Minorsky P. 10th ed. Cummings/Pearson; Massachusetts: 2014. Campbell biology. [Google Scholar]
- 41.Pratt C., Cornely K. 3rd ed. Wiley; New Jersey: 2014. Essential biochemistry. [Google Scholar]
- 42.Sleiman D., Al-Badri M., Azar S. Effect of Mediterranean diet in diabetes control and cardiovascular risk modification: a systematic review. Front Public Health. 2015;3:69. doi: 10.3389/fpubh.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mason S., Della Gatta P., Snow R., Russell A., Wadley G. Ascorbic acid supplementation improves skeletal muscle oxidative stress and insulin sensitivity in people with diabetes: findings of a randomized controlled study. Free Radic Biol Med. 2016;93:227–238. doi: 10.1016/j.freeradbiomed.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Etsuo N. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic Biol Med. 2014;66:3–12. doi: 10.1016/j.freeradbiomed.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 45.Ramezani A., Djazayeri A., Koohdani F., Nematipour E., Javanbakht M., Keshavarz S. Omega-3 fatty acids/vitamin E behave synergistically on adiponectin receptor-1 and adiponectin receptor-2 gene expressions in peripheral blood mononuclear cell of coronary artery disease patients. Curr Top Nutraceutical Res. 2015;13:23–32. [Google Scholar]
- 46.Li D., Zhang Y., Liu Y., Sun R., Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr. 2015;145:742–748. doi: 10.3945/jn.114.205674. [DOI] [PubMed] [Google Scholar]
- 47.Suliburska J., Bogdanski P., Szulinska M., Stepien M., Pupek-Musialik D., Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res. 2012;149:315–322. doi: 10.1007/s12011-012-9448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ihm S., Jang S., Kim O., Chang K., Oak M., Lee J. Decaffeinated green tea extract improves hypertension and insulin resistance in a rat model of metabolic syndrome. Atherosclerosis. 2012;224:377–383. doi: 10.1016/j.atherosclerosis.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Mann J., Lonn E., Yi Q., Gerstein H., Hoogwerf B., Pogue J. Effects of vitamin E on cardiovascular outcomes in people with mild-to-moderate renal insufficiency: results of the HOPE study. Kidney Int. 2004;65:1375–1380. doi: 10.1111/j.1523-1755.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 50.Lee D., Folsom A., Harnack L., Halliwell B., Jacobs D. Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr. 2004;80:1194–1200. doi: 10.1093/ajcn/80.5.1194. [DOI] [PubMed] [Google Scholar]
- 51.Parrow N., Leshin J., Levine M. Parenteral ascorbate as a cancer therapeutic: a reassessment based on pharmacokinetics. Antioxid Redox Signal. 2013;19:2141–2156. doi: 10.1089/ars.2013.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padayatty S., Sun H., Wang Y., Riordan H., Hewitt S., Katz A. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 53.Borel P., Desmarchelier C., Nowicki M., Bott R., Tourniaire F. Can genetic variability in α-tocopherol bioavailability explain the heterogenous response to a-tocopherol supplements? Antioxid Redox Signal. 2014;22:669–678. doi: 10.1089/ars.2014.6144. [DOI] [PubMed] [Google Scholar]
- 54.Deng C., Sun Z., Tong G., Yi W., Ma L., Zhao B. α-Lipoic acid reduces infarct size and preserves cardiac function in rat myocardial ischemia/reperfusion injury through activation of PI3K/Akt/Nrf2 pathway. PLoS One. 2013;8:e58371. doi: 10.1371/journal.pone.0058371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inman D., Lambert W., Calkins D., Horner P. α-Lipoic acid antioxidant treatment limits glaucoma retinal ganglion cell death and dysfunction. PLoS One. 2013;8:e65389. doi: 10.1371/journal.pone.0065389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu R. Health promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;14:3845–3925. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ismail H., Scapozza L., Ruegg U., Dorchies O. Diapocynin, a dimer of NADPH oxidase inhibitor apocynin, reduces ROS production and prevents force loss in eccentrically contracting dystrophic muscle. PLoS Med. 2014;9:1–8. doi: 10.1371/journal.pone.0110708. [DOI] [PMC free article] [PubMed] [Google Scholar]