Abstract
Purinergic signalling plays a crucial role in immunity and autoimmunity. Among purinergic receptors, the P2X7 receptor (P2X7R) has an undisputed role as it is expressed to high level by immune cells, triggers cytokine release and modulates immune cell differentiation. In this review, we focus on evidence supporting a possible role of the P2X7R in the pathogenesis of systemic lupus erythematosus (SLE).
Keywords: Extracellular ATP, P2X7R, Systemic lupus erythematosus, NLRP3 inflammasome, Interleukin-1β
Rat mast cells were the first inflammatory cells in which the effects of extracellular ATP where described, but with little insight into the possible pathophysiological meaning [1], [2]. Later experiments suggested that the potent Ca2+-dependent histamine-release activity due to extracellular ATP involved an unusual increase in cation permeability of the plasma membrane [3], but it was not until the key experiments by Gomperts that it was formally shown that, difficult to believe as it was, extracellular ATP caused the opening of a non-selective plasma membrane pore [4]. Over thirty-six years later, we can now appreciate in full the profound implications of this ATP-dependent response in virtually all pathophysiological processes, immunity in the first place. Plasma membrane receptors for extracellular ATP, intracellular transduction mechanisms, coupling factors and even crystal structure (for some P2 receptors) have been resolved. The challenge is now to take all this knowledge to the patient's bed. This review aims to fill, at least in part, the gap to the clinics.
ATP and purinergic signalling
Adenosine triphosphate (ATP) has been for long time considered only involved in cell metabolism as the main source of energy, any possible role in extracellular signalling being virtually unthinkable. Thus, the proposal of the purinergic hypothesis for neurotransmission put forward by Geoffrey Burnstock in the 1970's after having identified ATP as a transmitter between non-adrenergic neurons and muscle [5] was received with surprise and even with outright scepticism. Scientific community was somewhat schizophrenic about the “purinergic hypothesis” since on the one hand pharmacologists and physiologists accumulated countless data supporting the presence and functional relevance of cell receptors for extracellular ATP, while on the other biochemists, cell biologists and immunologists simply ignored the whole of these experimental observations, and when some attention was paid to the theme, they were often referred to as artifacts. With the molecular cloning of the first P2 receptor (P2R), P2Y1 (P2Y1R) [6], ATP-receptors gained a novel dignity in the realm of cell receptors and started to attract the attention of scientists from outside the pharmacology and physiology communities. Nowadays, “purinergic signalling” is a well-established concept in biomedical science and represents an important and expanding subject of research in many different areas, including immunology, oncology, developmental biology, physiology, neurobiology, not to say of pharmacology and medicinal chemistry [7], [8]. Although the first evidence of a role for purinergic receptors was in neurotransmission, it became clear soon that extracellular ATP and adenosine play a crucial role in basically all processes requiring cell-to-cell communication. It is now thought that release of ATP and other purine or pyrimidine nucleotides represents a widespread mean of cell-to-cell communication highly conserved throughout evolution, as suggested by the discovery of ATP-receptors in invertebrates [9], slime moulds [10], fishes [11], and obviously mammals [12].
It was initially thought (and many investigators still hold this view) that ATP is mainly if not exclusively released as a consequence of cell death or plasma membrane injury. This view has radically changed over the latest few years with the discovery that virtually all cells are capable of non-lytic ATP release. Several pathways have been identified: secretory vesicles [13], [14], ABC transporters [15], pannexins [16], connexins [17] and possibly the P2X7 receptor (P2X7R) [18], [19].
Once in the extracellular milieu, ATP is converted to adenosine through the enzymatic activity of two membrane-bound nucleotidases, CD39 and CD73. Adenosine extracellular concentration is generally constant in most tissues but can rapidly undergo a 100-fold increase in hypoxic or inflamed sites exerting multiple immunosuppressive effects virtually on all immune cell types.
Purinergic receptors are classified into P1 (P1Rs) and P2 (P2Rs) receptors. P1Rs, adenosine selective, are comprised of four subtypes, A1, A2A, A2B, and A3. P2Rs, nucleotide selective, include two sub-families characterized by distinct pharmacological profiles: metabotropic P2YRs and ionotropic P2XRs. P2YRs are subdivided into eight subtypes: five Gq/G11-coupled subtypes, associated to phospholipase C (PLC) activation and inositol triphosphate (IP3) generation (P2Y1R, P2Y2R, P2Y4R, P2Y6R and P2Y11R), and three Gi/o-coupled subtypes associated to adenylate cyclase inhibition and modulation of ion channels (P2Y12R, P2Y13R and P2Y14R) [20]. P2Y11R also couples to Gs to promote adenylate cyclase stimulation, thus its activation causes an intracellular Ca2+ rise as well as an increase in cAMP levels. P2YRs are activated by low (nanomolar) nucleotide concentrations, and since signal transduction requires generation of soluble intracellular second-messengers, cellular responses triggered by these receptors are rather slow. Different nucleotides are active at P2YRs, P2Y11R being the only P2YR at which ATP is the preferred agonist. The other P2YRs recognize as preferred ligands ADP (P2Y12R and P2Y13R), UTP (P2Y4R), UDP (P2Y6R), UDP-glucose or UDP-galactose (P2Y14R), and with equal potency ATP and ADP (P2Y1R) or ATP and UTP (P2Y2R) [21].
P2XRs are trimeric ion channels permeable to monovalent (Na+, K+) and divalent (Ca2+) cations. ATP is the only known physiologic agonist. Functional P2XR channels may be formed by the assembly of the same P2X subunit (homomeric channels), or different P2X subunits (heteromeric channels) [22]. Six homomeric (P2X1R-P2X5R and P2X7R) and six heteromeric (P2X1/2R, P2X1/4R, P2X1/5R, P2X2/3R, P2X2/6R and P2X4/6R) receptors have been described so far [23]. Among P2X subunits, P2X7 is generally thought not to assemble with the others, and thus form only P2X7 homomeric channels. An early report evidence suggested that P2X7 subunits may also associate with other subunits (P2X4) to form heteromeric channels [24], but later experiments were unable to confirm these findings [25], thus enforcing the view that P2X7Rs “stand alone” in the plasma membrane.
P2X subunits length ranges from 379 (P2X6) to 595 (P2X7) amino acids. Each subunit consists of two membrane spanning segments (TM1 and TM2) separated by an extracellular loop containing ten conserved cysteine residues [26], thought to form disulfide bonds, and lysine and phenylalanine residues involved in activation by ATP [27]. Both C and N termini are intracellular. The carboxyl-terminal tail of the various P2X subunits varies in length from 25 amino acids (P2X6) to 240 amino acids (P2X7) and plays a key role in setting the distinct functional features of each receptor [28], [29]. Electrophysiology data show that P2X channel activation requires binding of three ATP molecules to the extracellular domain [30]. Sensitivity of ATP-binding widely varies within the family, ranging from low nM levels required for P2X1R activation, to high μM or even mM concentrations necessary to switch on the P2X7R [31]. Cations and anions in extracellular medium also modulate P2XR activity [28], [32].
Brief exposure of all P2XRs to ATP causes a fast (milliseconds) opening of a channel that renders the plasma membrane permeable to positively charged ions, causing increase in intracellular Ca2+ and Na+ and a simultaneous decrease in intracellular K+ concentrations. Significant Ca2+ permeability of P2X receptors implies a selective filter involving TM1 and TM2 [33], [34]. Channel opening leads to cell membrane depolarisation and initiation of downstream Ca2+ signalling events [31]. On the contrary, prolonged exposure to ATP has different effects depending on the receptor sub-type: P2X1R and P2X3R undergo fast desensitisation and channel closing, whereas P2X2R, P2XR4, P2X5R and the P2X7R are slowly desensitizing. No data are available on the P2X6R since this receptor is basically unable to form a functional channel. It was originally shown that sustained activation of the P2X7R caused opening of a large transmembrane pore permeable to hydrophilic molecules of MW up to 900 Da [28], [35], [36], [37], [38], [39], [40]. Later experiments suggested that other P2XR subtypes such as P2X2R and P2X4R were also endowed with the ability to generate a large plasma membrane pore upon sustained activation [41], [42]. However, it is now an established fact that, although in some instances also P2X2R or P2X4R may undergo a channel-to-pore transition, this process is far easier to observe and is much more easily reproduced and analysed in cell expressing the P2X7R. In most P2X7R-expressing cells extracellular ATP promotes a true process of reversible plasma membrane permeabilization that, upon ATP removal and plasma membrane resealing, leaves intact most cellular physiological responses [37]. Recent electrophysiology data cast doubts on the P2X2R-dependent increases in plasma membrane permeability previously documented [43]. Therefore, it is fair to say that the channel-to-pore transition leading to ATP-stimulated reversible plasma membrane permeabilization is indeed a signature of the P2X7R.
Strong evidence supports of a key role of P2Rs in multiple different physiological and pathophysiological responses such as neuromuscular transmission [44], neuron-to-neuron communication [45], bladder function [46], inflammation [47], arthritis [48], neurodegeneration [49], cancer [50], chronic and inflammatory pain [51], [52], [53], bone formation and resorption [54], male fertility [44], [55] blood pressure regulation [56], blood coagulation and thrombosis [57]. Moreover, P2Rs are up-regulated following tissue damage, as for example in peripheral nerve injury [58], spinal cord injury [59], brain ischaemia [60], and more in general in inflammation and in cancer [61], [62]. Thus, P2Rs are involved in a wide spectrum of diseases, a finding not surprising in view of the ubiquitous distribution of these receptors.
There is no doubt that the P2R for which disease relevance is more compelling is P2Y12R. Its high level of expression in platelets and the crucial role in blood coagulation has led to the development of very effective anti-thrombotic drugs that for years have been block-busters in the world drug market [63]. Among P2XRs, the most likely candidate target for successful drug development is the P2X7R. This receptor is thought to have an important immunomodulatory function not just because of its well-known ability to activate the NLRP3 inflammasome (and therefore IL-1β release), but also for its crucial role in T lymphocyte growth and differentiation. Participation of the P2X7R in many chronic inflammatory diseases, ranging from arthritis [64] to gout [65], from tuberculosis [66] to chlamydia infection [67] has been postulated. In this review we will explore the main features and functions of the P2X7R receptor together with its possible role in systemic lupus erythematosus (SLE), one of the human pathologies more strongly characterized by immune-mediated tissue damage and inflammation.
The P2X7R receptor
The P2X7R is widely distributed in human tissues with the highest expression in cells of the immune and inflammatory systems, especially of the monocyte-macrophage lineage [68]. The P2X7R gene is located on human chromosome 12q24 (locus 12q24.31), close to the P2X4R locus (12q24.32). Both full length P2X7R (P2X7RA) and the carboxyl-terminal truncated splice variant P2X7RB show high sequence homology with the P2X4R (41% identity, 71% similarity), a finding suggestive of a common origin. Therefore, information about P2X7R structure and ligand binding might be deducted from the crystal structure solved for the homologous zebrafish P2X4R [69], [70].
The P2X7R is an oligomer made of three subunits [22] which binds three ATP molecules at sites at the interface between subunits. Activation of the receptor by ATP triggers a sigmoid dose response curve with a Hill coefficient value between of 2.0 and 2.4, suggesting an allosteric effect facilitating sequential binding of the three ATP molecules [71], [72]. Hill coefficient is about 3 with benzoyl ATP as an agonist [72]. By analogy with the P2X4R data, it can be inferred that ATP binds at an inter-subunit binding pocket lined with several positively charged residues (R298, K316, N296, K70, K193, T189, K72) located on a protein fold not found in other conventional ATP-binding sites [70].
The P2X7R is the P2XR in which the ion channel-to-large pore transition has been more thoroughly described and in which is best reproducible [36], [37], [38]. Stimulation with low ATP concentrations triggers opening of the typical cation-selective channel, whereas challenge with higher agonist concentrations (μM-mM) causes formation of the large non-selective pore [29], [36], [73]. The molecular mechanism of channel-to-pore transition has long been a matter of controversy. Most credited hypotheses hold that the large conductance pore results from either 1) dilation of the cation channel, or 2) recruitment of an accessory molecule, e.g. pannexin-1 [74], [75]. Data from pannexin-1 KO mice however indicate that pannexin-1 is not an absolute requirement for large conductance pore formation (Cavagna and Di Virgilio, unpublished), thus suggesting that either recruitment of another plasma membrane molecule occurs, or that it is the cation channel itself that dilates to generate the non-selective pore. In fact, pore shaping was supposed to depend on agonist-induced movements of TM1 and TM2 helices, hence implying dilatation of the intrinsic channel. The stoichiometry of the P2X7R channel/pore might in principle help to discriminate between these two mechanistic alternatives. Current consensus holds that the P2X7R channel is made by the assembly of three identical subunits (homotrimer), however anecdotal evidence from past studies raises the possibility that higher assembly states might also be present. Surprenant's group initially showed that in BN-PAGE specific anti-P2X7R Abs stain a 400 kDa band both in HEK293 cells transfected with the rat P2X7R and in rat peritoneal macrophages, suggesting that both heterologously expressed and native rat P2X7R, may include 5–6 subunits [76]. As a warning of caution, these authors admit that the 400 kDa band both might include some yet-to-identify interacting proteins. Several years ago, we determined receptor stoichiometry in the absence and presence of the antibiotic polymixin B in human macrophages and in HEK293 cells stably expressing the human P2X7R [77]. Polymixin B potentiates P2X7R responses because it likely acts as a positive allosteric modulator [78]. P2X7R analysis on denaturing gel revealed a 440 kDa band that was strongly enhanced in cells treated with polymixin B. These data from both Surprenant's and our laboratory suggest that while a trimeric stoichiometry is the prevalent state of assembly, it cannot be excluded that higher molecular weight states also occur. Summarizing, two distinct pathways are thought to be implicated in agonist-stimulated pore formation, the first directly depending on conformational changes intrinsic to P2X7R [12], [75], whereas the second presumably involving P2X7R-dependent recruitment of plasma membrane hemi-channels [74], [79].
A number of polymorphisms and splicing variants confer an intriguing plasticity to P2X7R [80], [81], [82]. P2X7R polymorphisms include both gain- and loss-of-function variants, some of them presumably involved in different pathologies, such as cancer [83], [84], osteoporosis [85], [86], [87] and increased susceptibility to tuberculosis [88], [89]. Alternative splicing is responsible for 23 P2X7R mRNA transcripts listed in NCBI database. Eighteen of them are predicted to produce P2X7R protein variants, including the full length P2X7RA, and the human P2X7RB isoform that lacks the cytoplasmic carboxyl tail (GenBank accession No. AY847298.1) [81]. Heterologous expression in HEK293 cells showed that the P2X7RB isoform generates a cation-selective channel, but not the large conductance pore [90]. P2X7RB has also been shown to form heterotrimers with P2X7RA resulting in stabilization of the receptor and potentiation of the associated responses, including channel and pore formation [91], [92].
P2X7R is involved in many different cell functions. This is witnessed by proteomic studies showing a link of P2X7R with different intracellular proteins, among which cytoskeletal (β-actin) and signalling proteins (protein tyrosine phosphatase, phosphatidylinositol kinases) as well as chaperones (HSP70 and HSP90). Association of P2X7R with different intracellular partners might thus be responsible for cell type-specific responses. In addition to the plasma membrane, P2X7R has also been localized to intracellular membranes, i.e. endoplasmic reticulum and nuclear membrane [93], [94].
Participation of P2X7R in several relevant pathophysiological processes has been demonstrated. First of all, P2X7R is a key trigger for maturation and release of the pro-inflammatory cytokines interleukin-1β (IL-1β) and interleukin 18 (IL-18) via activation of the NOD-like receptor (NLR)P3 inflammasome, the cytoplasmic organelle responsible for the conversion of pro-caspase-1 into active caspase-1 [95], [96], [97], [98]. While the mechanism of NLRP3 inflammasome activation by P2X7R is as yet incompletely understood, a key role is thought to be played by K+ efflux (see Di Virgilio et al. [99] and Munoz-Planillo et al. [100]). Other evidence suggests that the NLRP3 inflammasome can also be activated by reactive oxygen species, which incidentally are also produced following P2X7R stimulation [101], [102]. In addition, direct P2X7R interaction with NLRP3 cannot be excluded [103]. The intracellular K+ drop triggers a cascade of events leading to NLRP3 activation and pro-caspase-1 cleavage. The P2X7R also plays a major role in the mechanism of secretion of IL-1β. As it is well known, this cytokine lacks a leader sequence necessary for its targeting to the conventional cellular secretion pathway, thus alternative release pathways have been investigated. Such unconventional pathways include exosome (30–80 nm), and/or microvesicle (100 nm–1 μm) release [104].
Several signalling pathways are activated following P2X7R stimulation such as changes in intracellular Ca2+ and activation of transcription factors including nuclear factor kappa B (NF-κB) [105], [106], [107], hypoxia inducible factor 1α (HIF-1α) [108] and the nuclear factor of activated T cells complex 1 (NFATc1) [90], [109]. NFATc1, a key transcription factor in normal and neoplastic cell growth, plays a central role in P2X7R-mediated proliferation, as demonstrated on one hand by its strong up-regulation in HEK293 cells expressing the A and B P2X7R isoforms [91], [92], and on the other by abrogation of P2X7R-dependent cell growth following its blockade by selective inhibitors, such as cyclosporine or VIVIT [90]. Additional intracellular signalling pathways are also P2X7R-associated, such as the MAP-kinase [110] and the PI3K/Akt pathways [111]. Finally, P2X7R has a central role also in carcinogenesis. In fact, its expression supports tumour growth, both in allogenic (nude/nude host) and syngeneic mice models [112], as well as tumour associated angiogenesis [112]. P2X7R expression enhances invasiveness and metastatization, as shown in vitro, by matrigel-infiltration experiments [91], and in vivo, in a zebrafish model of metastasis [113], [114]. Several tumours overexpress P2X7R, e.g. chronic lymphocytic leukaemia, melanoma, neuroblastoma, prostate, breast, skin, thyroid cancers and osteosarcoma [92], [115]. Quite interestingly, host-P2X7R as opposed to tumour-P2X7R, is on the contrary essential to restrict tumour growth, as transplanted syngeneic tumours undergo accelerated growth and metastatic dissemination in the P2X7R-KO host [116].
The P2X7R participates in defense against pathogens since P2X7R-mediated phospholipase D (PLD) activation facilitates phagosome-lysosome fusion and therefore intraphagosomal killing of different microorganisms such as Mycobacterium tuberculosis and chlamydia [67], [117]. An as yet poorly understood P2X7R-dependent process is membrane blebbing followed by microvesicle shedding [118], [119], [120]. Increasing evidence suggest that this might be a novel avenue for dissemination of biologically active factors (e.g. IL-1β or NLRP3 inflammasome components) [121]. In support to an important role played by P2X7R in chronic inflammation, we showed that this receptor is involved in multinucleated giant cell (MGC) formation, whether in a model of heterologous P2X7R expression [122] or in a more physiological model of spontaneous or GM-CSF-stimulated fusion of human or mouse macrophages [123]. This function was shown to be strictly dependent on pore-forming activity, since cells transfected with a P2X7R receptor lacking the C-terminal domain, which were devoid of pore-forming activity, formed lower number of MGC respect to cells transfected with the full length P2X7R receptor [123]. Moreover, P2X7R-dependent ATP release and its metabolism to adenosine have also been shown to be necessary for MGC formation and osteoclast fusion [124], [125].
The P2X7R is a receptor endowed with a dual role: on one hand pharmacological stimulation by high ATP doses triggers cell death, whether by necrosis, apoptosis or pyroptosis, while on the other hand, tonic, low level of stimulation by endogenously released ATP has a trophic effect [109], [126], [127], [128]. The key intracellular target sustaining cytotoxic as well as trophic P2X7R effects is the mitochondria, as P2X7R overstimulation triggers a “mitochondrial catastrophe” witnessed by mitochondrial swelling, fragmentation and uncoupling of oxidative phosphorylation [109], while tonic P2X7R activation stabilizes the mitochondrial network, increases mitochondrial potential and enhances the efficiency of oxidative phosphorylation [109].
Based on the whole of this evidence, P2X7R has become a promising target for treatment of inflammation and pain [129], [130].
P2X7R in the pathogenesis of systemic lupus erythematosus (SLE)
The P2X7R and related molecules have been implicated in the pathogenesis of several autoimmune diseases, systemic lupus erythematosus (SLE) included [131].
SLE is a systemic autoimmune syndrome characterized by increased type I interferon (IFN) signature, and dysregulation of both innate and adaptive immune responses [132]. All SLE patients typically produce autoantibodies to components of the cell nucleus (anti-nuclear antibodies or ANA), especially against nucleosomal constituents. Autoantibody binding to self-antigens leads to formation of a large quantity of immune complexes whose clearance is in many cases reduced because of defects in the complement cascade [133], [134]. Immune-complex deposition within several tissues (e.g. skin, kidney, brain, bone marrow and lungs) is responsible of the immune-mediated organ damage characteristic of SLE. In addition, immune complexes can bind to receptors for advanced glycation end products (RAGE) on endothelial cells, thus causing immune-mediated vasculitis [135]. Severe clinical manifestations, included cardiovascular events due to accelerated atherosclerosis, are frequent in SLE, with an overall increase in mortality. Release of normally segregated nuclear components in SLE has been traditionally put down to defects in the apoptotic pathway, in particular to the frequent occurrence of secondary necrosis, a late post-apoptotic phase characterized by membrane break-down and release of intracellular content. In alternative to apoptosis, i.e. programmed cell death, and necrosis, i.e. accidental cell death, two more recently discovered cell death types, pyroptosis and NETosis, can also be responsible of release of nuclear content, and thus play a role in SLE. Pyroptosis, defined as a regulated death specific of macrophages and dendritic cells [136], is thought to accelerate immune response against pathogens and to facilitate their clearance. Pyroptosis requires inflammasome recruitment and caspase-1 activation, and combines the release of nuclear elements, such as DNA and HMGB1 (e.g. high mobility group box 1) protein, and cytoplasmic components (e.g. ATP) with that of pro-inflammatory cytokines (e.g. IL-1β). On the other hand, NETosis occurs primarily in neutrophils as another form of controlled cell death, leading to release of NETs (neutrophil extracellular trap-associated proteins). NETs are meshworks of chromatin, anti-microbial peptides and enzymes that play an important role in host defense [137], [138]. Since DNA, histones and HMGB1 are involved in the pathogenesis of SLE [139], pyroptosis is suggested to be a main mechanism responsible for release of HGMB1 in SLE, and accordingly HMGB1 a possible biomarker of the disease [140].
Different mouse models mimicking human SLE are available [141]: a) the NZB/W F1 strain, in which especially females are affected by a lupus-like disease [142]; b) the MLR/lpr strain, characterized by the lpr mutation that is known to impair transcription of the Fas receptor [143]; c) the BXSB/Yaa strain, in which Yaa is an element termed Y-linked autoimmune accelerator due to a translocation resulting in duplication of at least 16 genes, among which TLR7 [144]. Induced models are also available that provide insight mainly into the role of environmental factors in SLE pathogenesis. The most commonly used is the pristane-induced lupus model, obtained by intraperitoneal injections of pristane, an isoprenol alkane present in mineral oil, that triggers autoantibody formation and glomerulonephritis at level comparable to that found in MLR/lpr mice [145].
Several observations link SLE to P2X7R. In the first place, P2X7R inhibition, by either the semi-selective antagonist BBG or small interfering RNA (siRNA) was shown to reduce nephritis in MLR/lpr mice models [146]. A substantial up-regulation of molecules involved in P2X7R-NLRP3 inflammasome signalling, namely P2X7R, NLRP3 and ASC, was found in the kidneys of MLR/lpr mice compared to control mice. BBG treatment reduced NLRP3/caspase-1 assembly and IL-1β release, and significantly diminished both the severity of nephritis and levels of circulating anti-dsDNA antibodies. BBG also reduced serum levels of both IL-1β and IL-17, and decreased the Th17:Treg ratio. Genetic deletion of P2X7R (P2X7R-KO mice) conferred significant protection against antibody-mediated glomerulonephritis [147]. Moreover, T lymphocytes from MRL/lpr mice become with age more resistant to ATP-induced apoptosis [148], possibly because of P2X7R down-regulation. In humans, increased glomerular and tubular expression of P2X7R was detected in renal biopsies from patients with autoimmune-related glomerulonephritis [149].
Caspase-1 has also been suggested to be involved in SLE pathogenesis in a model of pristane-induced lupus nephritis. Caspase-1−/− mice show reduced autoantibodies, decreased type I IFN signature, lower renal inflammation (correlated to IL-18 levels) and fewer cardiovascular lesions compared to caspase-1+/+ mice [150]. In the pathogenesis of pristane-dependent lupus, caspase-1 might be needed to preserve anti-DNA-producing Ab B cells in the marginal zone of the spleen via an IL-18-dependent mechanism [151].
The main product of P2X7R and inflammasome activation, i.e. IL-1β, is thought to have a major role in SLE pathogenesis. Significant increase of IL-1β levels in sera from SLE patients and a correlation with disease activity has been reported [152]. Moreover, IL-1β−/− mice are resistant to development of SLE triggered by injection of anti-DNA Abs, while IL-1α−/− mice are not [153]. IL-1β, together with IL-6 and IL-23, drives the differentiation of T helper 17 (Th17) cells [154] that produce IL-17 and have a relevant role in organ specific autoimmunity. IL-17-producing T cells are increased in peripheral blood from SLE patients, this cytokine being involved in tissue injury characteristic of glomerulonephritis [155]. Another member of the IL-1 family, IL-33, has been recently implicated in SLE. MLR/lpr mice treated with anti-IL-33 Abs showed a reduction in all hallmarks and symptoms of the disease. Following anti-IL-33 treatment Tregs and MDSCs were increased whereas Th17 cells as well as IL-1β, IL-6 and IL-17, were significantly reduced. A correlation between the expansion of Tregs and MDSCs and the reduction of pro-inflammatory cytokines is suggested [156].
IL-18 dysregulation was also observed in SLE. Firstly, elevated IL-18 serum levels were found in SLE patients. IL-18 serum levels correlated with disease activity, auto Ab profiles and the presence of nephritis [157], [158], [159]. In addition, the IL-18 inhibitor, IL-18BP, was also found increased in sera from SLE patients. Despite higher IL-18P levels, free IL-18 was still significantly higher than in controls and its serum level was considered a possible marker of disease activity [160]. Increased IL-18 expression was present in biopsies from cutaneous lupus lesions. Elevated IL-18 levels might be a trigger for increased TNF-α expression typical of lupus subacute cutaneous lesions. TNF-α is known to increase keratinocyte sensitivity to apoptosis, with the result of increasing exposure of modified self-antigens [161]. Interleukin-18 might also cause dysfunction of endothelial progenitor cells, thus hindering vascular repair [162]. Finally, IL-18 is reported to significantly enhance production of NETs, a crucial factor in inflammasome activation via P2X7R [163].
P2X7R also acts as a receptor for the LL-37 cathelicidin [164]. LL-37 is a cationic peptide synthesized by neutrophils, monocytes, keratinocytes and macrophages, active against a wide range of pathogens. LL-37 appears to play a relevant role in innate immunity as it promotes chemotaxis [165], M1 macrophage differentiation [166] and enhanced TLR3 signalling in response to viral dsRNA [167]. LL-37 can form complexes with dsDNA thus stimulating a large type I IFN release by plasmacytoid dendritic cells (pDCs) [168]. LL-37 is a component of NETs, on which it can be externalized. NETs, that are a combination of chromatin and defense-related proteins, are increased in patients with SLE and likely contribute to its pathogenesis [168], [169]. P2X7R activation represents a fundamental step in LL-37-mediated release of IL-1β from peripheral blood monocytes [164], [170]. NETs and LL-37-mediated activation of the inflammasome via P2X7R is increased in macrophages from lupus patients [163]. It has been proposed that a feature of SLE is an imbalance between NETs formation and clearance, thus leading to endothelium damage, exposure of immune-stimulatory molecules and tolerance break-down [137], [169], [171]. Indeed a distinct sub-set of pro-inflammatory low-density granulocytes (LDGs) showed enhanced capacity to form NETs in lupus patients [169], [172]. Thus, enhanced NET release in lupus patients may lead to increased P2X7R and NLRP3 inflammasome activation and enhanced release of IL-1β and IL-18. The result is an auto-stimulatory loop leading to further stimulation of NETosis and amplification of the inflammatory response potentially responsible for disease flares and organ damage [163]. Dysregulated expression and/or activity of P2X7R in lupus patients might further fuel this pro-inflammatory mechanism.
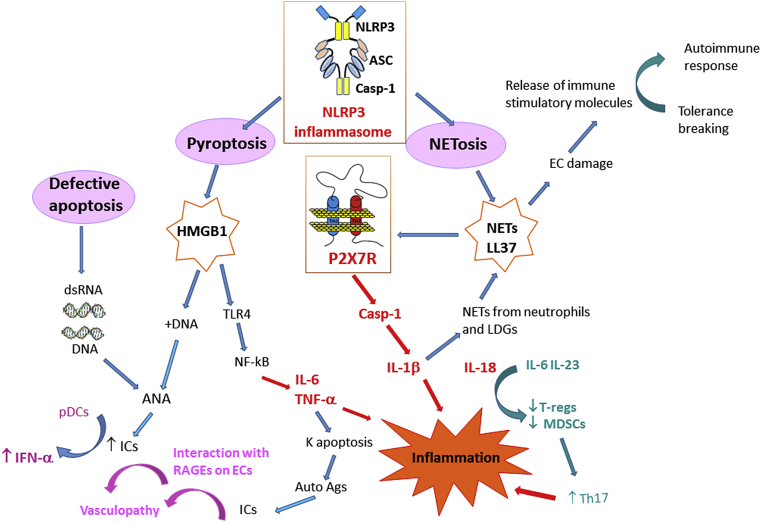
The P2X7R ligand, ATP, can be released from the cells in different conditions ranging from necrotic cell death to active extrusion via specific transport systems, among which P2X7R itself. Of relevance in LES, complement, especially the C3a component, is a stimulus for ATP release, thus acting as inflammasome activator [173]. Extracellular ATP via P2X7R induces release of another alarm molecule, i.e. HMGB1 [121], [174], [175], [176]. HMGB1 is a highly conserved non-histone nuclear protein whose function is to bind structural nuclear elements. In analogy to ATP, HMGB1 is both passively, i.e. following cell death, and actively released. All the three programmed cell death types, i.e. apoptosis, pyroptosis and NETosis, are accompanied by HMGB1 release. Once in the extracellular environment HMGB1 acts as a DAMP signal regulating, in a very complicated way, a wealth of immune responses. Indeed, depending on the redox state of three cysteine residues, it can induce immune tolerance, chemotaxis or inflammation [177], [178]. HMGB1 is released from apoptotic cells in a redox form that induces immune tolerance. On the contrary, the HMGB1 redox form secreted from cells stimulated via TLRs or undergoing pyroptosis, has pro-inflammatory activity since it activates NF-kB via TLR4 binding [179]. HMGB1, besides being released by pyroptotic cells, can also induce macrophage pyroptosis by causing cathepsin-B activation, lysosome disrupture, and consequent caspase-1 activation [180]. Extracellularly, HMGB1 forms complexes with different molecules, such as self-DNA, LPS and IL-1β, thus increasing their immunogenicity and eventually leading to generation of autoantibodies and immunecomplexes typical of lupus. HMGB1 can be also a component of NETs [181]. Therefore, P2X7R activation by ATP or by extracellular complexes, such as NETs, might have a dual pathogenetic role in promoting inflammation in lupus: on one hand, it directly triggers inflammation by stimulating the NLRP3 inflammasome, and on the other it has an indirect pro-inflammatory effect by inducing pyroptotic cell death. In genetically predisposed subjects, pyroptosis can contribute to autoimmune responses by increasing the release of nuclear and cellular autoantigens, DAMPs (ATP and HGMB1) and inflammatory cytokines (IL-1β and IL-18). A schematic rendition of the hypothetical contribution of P2X7R, NLRP3 and associated molecules is shown in [Fig. 1].
Fig. 1.
Suggested central role of P2X7R and NLRP3 inflammasome in the pathways leading to autoimmunity and tissue damage in SLE. The different types of cell death can generate molecules that contribute to inflammation and tissue damage in SLE. NETosis, which is dependent on NLRP3 inflammasome activation, produces NETs and the cathelicidin LL-37. Pyroptosis, also dependent on NLRP3 inflammasome activation, causes HMGB1 and DNA release. Finally, defective apoptosis is responsible for nucleic acid release. NETs/LL-37 activate the P2X7R leading to production and release of IL-1β and IL-18, thus promoting inflammation. IL-1β is in turn responsible of increased NETs formation from neutrophils and LDGs. Interleukin-18 works in combination with IL-6 and IL-23 to reduce T-reg and MDSC activity, to enhance Th17 activity and inflammation. NETs are also responsible for EC damage with consequent release of immune stimulatory molecules and tolerance breaking. HMGB1 interaction with TLR4 contributes to inflammation through activation of NF-kB and release of the inflammatory cytokines TNF-α and IL-6. TNF-α is responsible for keratinocyte apoptosis, a process that generates autoantigens. The alarmin HMGB1, is released upon P2X7R/NLRP3 activation and in cooperation with DNA contributes to ANA formation. Nucleic acids are responsible for production of ANAs that participate in formation of ICs. ICs on one hand, interact with RAGEs on endothelial cells, thus causing vasculopathy, whereas on the other stimulate pDCs to produce IFN-α. Abbreviations: NETs: neutrophil extracellular trap-associated proteins; HMGB1: high molecular group box-1; ANA: anti-nuclear antibodies; MDSC: myeloid derived suppressor cells; pDCs: plasmacytoid dendritic cells; K: keratinocytes; LDGs: low-density granulocytes; TLR4: Toll-like receptor 4; ICs: immune complexes; RAGEs: receptor for advanced glycation end products; NLRP3: NOD-like receptor family pyrin domain-containing 3.
Lupus is a polygenic disorder with a strong hereditary component. Clinical data show a remarkable sex and ethnic variability in disease severity, prevalence and incidence. A large number of susceptibility genes have been identified in spontaneous lupus mouse models [182], [183]. They include the lpr mutation in the Fas receptor, that in MRL/lpr mice causes a lymphoproliferative syndrome. The P2X7R has been suggested as a candidate susceptibility gene [184]. The P2X7R locus, i.e. 12q24, has been identified as SLE susceptibility locus (SLEB4) in Hispanic and European American families [185] [186]. P2X7R polymorphisms have been recently reported to associate with susceptibility to SLE and lupus nephritis in a Chinese population [187], whereas previous investigations had not detected significant differences in the distribution of the 1513 AC polymorphism in SLE patients respect to controls in Caucasian populations [188], [189]. Nevertheless, in SLE patients the 1513 AC SNP was associated with low P2X7R expression, reduced induction of apoptosis of peripheral mononuclear cells and decreased IL-1β release following stimulation by ATP, suggesting an impaired elimination of self-reactive immune cells [189].
Nowadays, only few drugs are available for SLE treatment, some of them biologics [190]. Belimumab, a mAb targeting B lymphocyte stimulating (BLyS) protein, thus preventing its binding to B cell activating factor (BAFF) receptor, is the biologic most widely used. Type I IFNs, are another potential therapeutic target and Sifalimumab, a mAb that binds IFN-α thus preventing IFN-α signalling, is currently in Phase I clinical trial. Targeting inflammasome components and related molecules is another promising strategy for the treatment of SLE [191]. In this perspective, P2X7R antagonists, currently in clinical trials for the treatment of inflammatory diseases [130], might also find applications for the treatment of SLE.
Conclusion
The P2X7R is a main player in immunity and inflammation. Its key role in IL-1β processing and release is an established fact, but accruing evidence support its participation in many additional immune responses such as T lymphocyte differentiation, Ag presentation and granuloma formation. Transition of P2X7R knowledge from the laboratory to the clinics has not been fast so far, despite efforts by Pharma Industry to develop potent and selective P2X7R drug-like antagonists. We think that this gap is in part due to less than optimal selection of candidate human diseases for clinical studies. In this review we offered an appraisal of literature evidence supporting a possible contribution of P2X7R to the pathogenesis of systemic lupus erythematosus, one of the most relevant pathologies characterized by immune-mediated tissue damage and inflammation. The challenge is now to take all this knowledge to the patient's bed.
Conflicts of interest
None.
Acknowledgement
This work was supported by grants from AIRC (no. IG 13025), Telethon (no. GGP 11014), ERA-NET Neuron “Nanostroke”, EU COST Program no. BM1406, the Ministry of Health of Italy (no. RF-2011-02348435), the Italian Ministry of Education, University and Research (no. RBAP11FXBC_001) and funds from the University of Ferrara. FDV was also supported by the 7th Framework Program HEALTH-F2-2007-202231 “ATPBone.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Sugiyama K., Yamasaki H. Calcium-dependent histamine release by ATP from isolated rat mast cells. Jpn J Pharmacol. 1969;19:175–176. doi: 10.1254/jjp.19.175. [DOI] [PubMed] [Google Scholar]
- 2.Dahlquist R., Diamant B. Further observations on ATP-induced histamine release from rat mast cells. Acta Pharmacol Toxicol (Copenh) 1970;28:43. [PubMed] [Google Scholar]
- 3.Dahlquist R., Diamant B., Kruger P.G. Increased permeability of the rat mast cell membrane to sodium and potassium caused by extracellular ATP and its relationship to histamine release. Int Arch Allergy Appl Immunol. 1974;46:655–675. doi: 10.1159/000231167. [DOI] [PubMed] [Google Scholar]
- 4.Cockcroft S., Gomperts B.D. ATP induces nucleotide permeability in rat mast cells. Nature. 1979;279:541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G., Satchell D.G., Smythe A. A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle preparations from different vertebrate species. Br J Pharmacol. 1972;46:234–242. doi: 10.1111/j.1476-5381.1972.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webb T.E., Simon J., Krishek B.J., Bateson A.N., Smart T.G., King B.F. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 8.Khakh B.S., Burnstock G. The double life of ATP. Sci Am. 2009;301 doi: 10.1038/scientificamerican1209-84. 84–90, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agboh K.C., Webb T.E., Evans R.J., Ennion S.J. Functional characterization of a P2X receptor from Schistosoma mansoni. J Biol Chem. 2004;279:41650–41657. doi: 10.1074/jbc.M408203200. [DOI] [PubMed] [Google Scholar]
- 10.Fountain S.J., Parkinson K., Young M.T., Cao L., Thompson C.R., North R.A. An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum. Nature. 2007;448:200–203. doi: 10.1038/nature05926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucenas S., Li Z., Cox J.A., Egan T.M., Voigt M.M. Molecular characterization of the zebrafish P2X receptor subunit gene family. Neuroscience. 2003;121:935–945. doi: 10.1016/s0306-4522(03)00566-9. [DOI] [PubMed] [Google Scholar]
- 12.North R.A. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Martins I., Ma Y., Kepp O., Galluzzi L., Kroemer G. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy. 2013;9:1624–1625. doi: 10.4161/auto.25873. [DOI] [PubMed] [Google Scholar]
- 14.Sneddon P., Westfall D.P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the Guinea-pig vas deferens. J Physiol. 1984;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantiello H.F. Electrodiffusional ATP movement through CFTR and other ABC transporters. Pflugers Arch. 2001;443(Suppl. 1):S22–S27. doi: 10.1007/s004240100639. [DOI] [PubMed] [Google Scholar]
- 16.Dahl G. ATP release through pannexon channels. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140191. doi: 10.1098/rstb.2014.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans W.H., De Vuyst E., Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellegatti P., Falzoni S., Pinton P., Rizzuto R., Di Virgilio F. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell. 2005;16:3659–3665. doi: 10.1091/mbc.E05-03-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suadicani S.O., Brosnan C.F., Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbracchio M.P., Burnstock G., Verkhratsky A., Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson K.A., Paoletta S., Katritch V., Wu B., Gao Z.G., Zhao Q. Nucleotides acting at P2Y receptors: connecting structure and function. Mol Pharmacol. 2015;88:220–230. doi: 10.1124/mol.114.095711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaczmarek-Hajek K., Lorinczi E., Hausmann R., Nicke A. Molecular and functional properties of P2X receptors–recent progress and persisting challenges. Purinergic Signal. 2012;8:375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubyak G.R. Go it alone no more–P2X7 joins the society of heteromeric ATP-gated receptor channels. Mol Pharmacol. 2007;72:1402–1405. doi: 10.1124/mol.107.042077. [DOI] [PubMed] [Google Scholar]
- 24.Guo C., Masin M., Qureshi O.S., Murrell-Lagnado R.D. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007;72:1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- 25.Nicke A. Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem Biophys Res Commun. 2008;377:803–808. doi: 10.1016/j.bbrc.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 26.Clyne J.D., Wang L.F., Hume R.I. Mutational analysis of the conserved cysteines of the rat P2X2 purinoceptor. J Neurosci. 2002;22:3873–3880. doi: 10.1523/JNEUROSCI.22-10-03873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X., Ma W., Surprenant A., Jiang L.H. Identification of the amino acid residues in the extracellular domain of rat P2X(7) receptor involved in functional inhibition by acidic pH. Br J Pharmacol. 2009;156:135–142. doi: 10.1111/j.1476-5381.2008.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarvis M.F., Khakh B.S. ATP-gated P2X cation-channels. Neuropharmacology. 2009;56:208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 29.Khakh B.S., North R.A. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 30.Jiang L.H., Kim M., Spelta V., Bo X., Surprenant A., North R.A. Subunit arrangement in P2X receptors. J Neurosci. 2003;23:8903–8910. doi: 10.1523/JNEUROSCI.23-26-08903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young M.T. P2X receptors: dawn of the post-structure era. Trends Biochem Sci. 2010;35:83–90. doi: 10.1016/j.tibs.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubick C., Schmalzing G., Markwardt F. The effect of anions on the human P2X7 receptor. Biochim Biophys Acta. 2011;1808:2913–2922. doi: 10.1016/j.bbamem.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Egan T.M., Khakh B.S. Contribution of calcium ions to P2X channel responses. J Neurosci. 2004;24:3413–3420. doi: 10.1523/JNEUROSCI.5429-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samways D.S., Egan T.M. Acidic amino acids impart enhanced Ca2+ permeability and flux in two members of the ATP-gated P2X receptor family. J Gen Physiol. 2007;129:245–256. doi: 10.1085/jgp.200609677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cockcroft S., Gomperts B.D. The ATP4- receptor of rat mast cells. Biochem J. 1980;188:789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinberg T.H., Swanson J.A., Silverstein S.C. A prelysosomal compartment sequesters membrane-impermeant fluorescent dyes from the cytoplasmic matrix of J774 macrophages. J Cell Biol. 1988;107:887–896. doi: 10.1083/jcb.107.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Virgilio F., Fasolato C., Steinberg T.H. Inhibitors of membrane transport system for organic anions block fura-2 excretion from PC12 and N2A cells. Biochem J. 1988;256:959–963. doi: 10.1042/bj2560959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Surprenant A., Rassendren F., Kawashima E., North R.A., Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 39.Wiley J.S., Chen R., Wiley M.J., Jamieson G.P. The ATP4- receptor-operated ion channel of human lymphocytes: inhibition of ion fluxes by amiloride analogs and by extracellular sodium ions. Arch Biochem Biophys. 1992;292:411–418. doi: 10.1016/0003-9861(92)90010-t. [DOI] [PubMed] [Google Scholar]
- 40.Faria R.X., Cascabulho C.M., Reis R.A., Alves L.A. Large-conductance channel formation mediated by P2X7 receptor activation is regulated through distinct intracellular signaling pathways in peritoneal macrophages and 2BH4 cells. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:73–87. doi: 10.1007/s00210-010-0523-8. [DOI] [PubMed] [Google Scholar]
- 41.Khakh B.S., Bao X.R., Labarca C., Lester H.A. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci. 1999;2:322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- 42.Virginio C., MacKenzie A., Rassendren F.A., North R.A., Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 43.Li M., Toombes G.E., Silberberg S.D., Swartz K.J. Physical basis of apparent pore dilation of ATP-activated P2X receptor channels. Nat Neurosci. 2015;18:1577–1583. doi: 10.1038/nn.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulryan K., Gitterman D.P., Lewis C.J., Vial C., Leckie B.J., Cobb A.L. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- 45.Khakh B.S., Gittermann D., Cockayne D.A., Jones A. ATP modulation of excitatory synapses onto interneurons. J Neurosci. 2003;23:7426–7437. doi: 10.1523/JNEUROSCI.23-19-07426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong Y., Banning A.S., Cockayne D.A., Ford A.P., Burnstock G., McMahon S.B. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120:667–675. doi: 10.1016/s0306-4522(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 47.Bours M.J., Swennen E.L., Di Virgilio F., Cronstein B.N., Dagnelie P.C. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 48.Labasi J.M., Petrushova N., Donovan C., McCurdy S., Lira P., Payette M.M. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 49.Woods L.T., Ajit D., Camden J.M., Erb L., Weisman G.A. Purinergic receptors as potential therapeutic targets in Alzheimer's disease. Neuropharmacology. 2016;104:169–179. doi: 10.1016/j.neuropharm.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnstock G., Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal. 2013;9:491–540. doi: 10.1007/s11302-013-9372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toulme E., Tsuda M., Khakh B.S., Inoue K. On the role of ATP-gated P2X receptors in acute, inflammatory and neuropathic pain. In: Kruger L., Light A.R., editors. Translational pain research: from mouse to man. Frontiers in Neuroscience; Boca Raton, FL: 2010. [Google Scholar]
- 52.Barclay J., Patel S., Dorn G., Wotherspoon G., Moffatt S., Eunson L. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J Neurosci. 2002;22:8139–8147. doi: 10.1523/JNEUROSCI.22-18-08139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cockayne D.A., Dunn P.M., Zhong Y., Rong W., Hamilton S.G., Knight G.E. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567(Pt 2):621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ke H.Z., Qi H., Weidema A.F., Zhang Q., Panupinthu N., Crawford D.T. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 55.Rossato M., Merico M., Bettella A., Bordon P., Foresta C. Extracellular ATP stimulates estradiol secretion in rat Sertoli cells in vitro: modulation by external sodium. Mol Cell Endocrinol. 2001;178:181–187. doi: 10.1016/s0303-7207(01)00426-9. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto K., Sokabe T., Matsumoto T., Yoshimura K., Shibata M., Ohura N. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12:133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 57.Hechler B., Gachet C. Purinergic receptors in thrombosis and inflammation. Arterioscler Thromb Vasc Biol. 2015;35:2307–2315. doi: 10.1161/ATVBAHA.115.303395. [DOI] [PubMed] [Google Scholar]
- 58.Tsuda M., Shigemoto-Mogami Y., Koizumi S., Mizokoshi A., Kohsaka S., Salter M.W. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 59.Schwab J.M., Guo L., Schluesener H.J. Spinal cord injury induces early and persistent lesional P2X4 receptor expression. J Neuroimmunol. 2005;163:185–189. doi: 10.1016/j.jneuroim.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 60.Cavaliere F., Florenzano F., Amadio S., Fusco F.R., Viscomi M.T., D'Ambrosi N. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120:85–98. doi: 10.1016/s0306-4522(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 61.Guo L.H., Schluesener H.J. Lesional accumulation of P2X(4) receptor(+) macrophages in rat CNS during experimental autoimmune encephalomyelitis. Neuroscience. 2005;134:199–205. doi: 10.1016/j.neuroscience.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 62.Di Virgilio F., Vuerich M. Purinergic signaling in the immune system. Auton Neurosci. 2015;191:117–123. doi: 10.1016/j.autneu.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 63.Cattaneo M. P2Y12 receptors: structure and function. J Thromb Haemost. 2015;13(Suppl. 1):S10–S16. doi: 10.1111/jth.12952. [DOI] [PubMed] [Google Scholar]
- 64.McInnes I.B., Cruwys S., Bowers K., Braddock M. Targeting the P2X7 receptor in rheumatoid arthritis: biological rationale for P2X7 antagonism. Clin Exp Rheumatol. 2014;32:878–882. [PubMed] [Google Scholar]
- 65.Marques-da-Silva C., Chaves M.M., Castro N.G., Coutinho-Silva R., Guimaraes M.Z. Colchicine inhibits cationic dye uptake induced by ATP in P2X2 and P2X7 receptor-expressing cells: implications for its therapeutic action. Br J Pharmacol. 2011;163:912–926. doi: 10.1111/j.1476-5381.2011.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amaral E.P., Ribeiro S.C., Lanes V.R., Almeida F.M., de Andrade M.R., Bomfim C.C. Pulmonary infection with hypervirulent Mycobacteria reveals a crucial role for the P2X7 receptor in aggressive forms of tuberculosis. PLoS Pathog. 2014;10:e1004188. doi: 10.1371/journal.ppat.1004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coutinho-Silva R., Stahl L., Raymond M.N., Jungas T., Verbeke P., Burnstock G. Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity. 2003;19:403–412. doi: 10.1016/s1074-7613(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 68.Surprenant A., North R.A. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 69.Kawate T., Michel J.C., Birdsong W.T., Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hattori M., Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485:207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pizzo P., Zanovello P., Bronte V., Di Virgilio F. Extracellular ATP causes lysis of mouse thymocytes and activates a plasma membrane ion channel. Biochem J. 1991;274(Pt 1):139–144. doi: 10.1042/bj2740139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gargett C.E., Cornish J.E., Wiley J.S. ATP, a partial agonist for the P2Z receptor of human lymphocytes. Br J Pharmacol. 1997;122:911–917. doi: 10.1038/sj.bjp.0701447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gomperts B.D., Cockcroft S., Bennett J.P., Fewtrell C.M. Early events in the activation of Ca2+ dependent secretion: studies with rat peritoneal mast cells. J Physiol (Paris) 1980;76:383–393. [PubMed] [Google Scholar]
- 74.Pelegrin P. Many ways to dilate the P2X7 receptor pore. Br J Pharmacol. 2011;163:908–911. doi: 10.1111/j.1476-5381.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coddou C., Yan Z., Obsil T., Huidobro-Toro J.P., Stojilkovic S.S. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim M., Spelta V., Sim J., North R.A., Surprenant A. Differential assembly of rat purinergic P2X7 receptor in immune cells of the brain and periphery. J Biol Chem. 2001;276:23262–23267. doi: 10.1074/jbc.M102253200. [DOI] [PubMed] [Google Scholar]
- 77.Ferrari D., Pizzirani C., Gulinelli S., Callegari G., Chiozzi P., Idzko M. Modulation of P2X7 receptor functions by polymyxin B: crucial role of the hydrophobic tail of the antibiotic molecule. Br J Pharmacol. 2007;150:445–454. doi: 10.1038/sj.bjp.0706994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferrari D., Pizzirani C., Adinolfi E., Forchap S., Sitta B., Turchet L. The antibiotic polymyxin B modulates P2X7 receptor function. J Immunol. 2004;173:4652–4660. doi: 10.4049/jimmunol.173.7.4652. [DOI] [PubMed] [Google Scholar]
- 79.Baroja-Mazo A., Barbera-Cremades M., Pelegrin P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta. 2013;1828:79–93. doi: 10.1016/j.bbamem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Bartlett R., Stokes L., Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev. 2014;66:638–675. doi: 10.1124/pr.113.008003. [DOI] [PubMed] [Google Scholar]
- 81.Cheewatrakoolpong B., Gilchrest H., Anthes J.C., Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun. 2005;332:17–27. doi: 10.1016/j.bbrc.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 82.Wiley J.S., Sluyter R., Gu B.J., Stokes L., Fuller S.J. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 2011;78:321–332. doi: 10.1111/j.1399-0039.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 83.Cabrini G., Falzoni S., Forchap S.L., Pellegatti P., Balboni A., Agostini P. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol. 2005;175:82–89. doi: 10.4049/jimmunol.175.1.82. [DOI] [PubMed] [Google Scholar]
- 84.Dardano A., Falzoni S., Caraccio N., Polini A., Tognini S., Solini A. 1513A>C polymorphism in the P2X7 receptor gene in patients with papillary thyroid cancer: correlation with histological variants and clinical parameters. J Clin Endocrinol Metab. 2009;94:695–698. doi: 10.1210/jc.2008-1322. [DOI] [PubMed] [Google Scholar]
- 85.Ohlendorff S.D., Tofteng C.L., Jensen J.E., Petersen S., Civitelli R., Fenger M. Single nucleotide polymorphisms in the P2X7 gene are associated to fracture risk and to effect of estrogen treatment. Pharmacogenet Genomics. 2007;17:555–567. doi: 10.1097/FPC.0b013e3280951625. [DOI] [PubMed] [Google Scholar]
- 86.Mrazek F., Gallo J., Stahelova A., Petrek M. Functional variants of the P2RX7 gene, aseptic osteolysis, and revision of the total hip arthroplasty: a preliminary study. Hum Immunol. 2010;71:201–205. doi: 10.1016/j.humimm.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 87.Jorgensen N.R., Syberg S., Ellegaard M. The role of P2X receptors in bone biology. Curr Med Chem. 2015;22:902–914. doi: 10.2174/0929867321666141215094749. [DOI] [PubMed] [Google Scholar]
- 88.Shemon A.N., Sluyter R., Fernando S.L., Clarke A.L., Dao-Ung L.P., Skarratt K.K. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem. 2006;281:2079–2086. doi: 10.1074/jbc.M507816200. [DOI] [PubMed] [Google Scholar]
- 89.Xiao J., Sun L., Yan H., Jiao W., Miao Q., Feng W. Metaanalysis of P2X7 gene polymorphisms and tuberculosis susceptibility. FEMS Immunol Med Microbiol. 2010;60:165–170. doi: 10.1111/j.1574-695X.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- 90.Adinolfi E., Callegari M.G., Cirillo M., Pinton P., Giorgi C., Cavagna D. Expression of the P2X7 receptor increases the Ca2+ content of the endoplasmic reticulum, activates NFATc1, and protects from apoptosis. J Biol Chem. 2009;284:10120–10128. doi: 10.1074/jbc.M805805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adinolfi E., Cirillo M., Woltersdorf R., Falzoni S., Chiozzi P., Pellegatti P. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010;24:3393–3404. doi: 10.1096/fj.09-153601. [DOI] [PubMed] [Google Scholar]
- 92.Giuliani A.L., Colognesi D., Ricco T., Roncato C., Capece M., Amoroso F. Trophic activity of human P2X7 receptor isoforms A and B in osteosarcoma. PLoS One. 2014;9:e107224. doi: 10.1371/journal.pone.0107224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atkinson L., Milligan C.J., Buckley N.J., Deuchars J. An ATP-gated ion channel at the cell nucleus. Nature. 2002;420:42. doi: 10.1038/420042a. [DOI] [PubMed] [Google Scholar]
- 94.Gonnord P., Delarasse C., Auger R., Benihoud K., Prigent M., Cuif M.H. Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J. 2009;23:795–805. doi: 10.1096/fj.08-114637. [DOI] [PubMed] [Google Scholar]
- 95.Ferrari D., Chiozzi P., Falzoni S., Dal Susino M., Melchiorri L., Baricordi O.R. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 96.Mariathasan S., Weiss D.S., Newton K., McBride J., O'Rourke K., Roose-Girma M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 97.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 98.Piccini A., Carta S., Tassi S., Lasiglie D., Fossati G., Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Virgilio F., Ferrari D., Falzoni S., Chiozzi P., Munerati M., Steinberg T.H. P2 purinoceptors in the immune system. Ciba Found Symp. 1996;198:290–302. doi: 10.1002/9780470514900.ch17. discussion -5. [DOI] [PubMed] [Google Scholar]
- 100.Munoz-Planillo R., Kuffa P., Martinez-Colon G., Smith B.L., Rajendiran T.M., Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cruz C.M., Rinna A., Forman H.J., Ventura A.L., Persechini P.M., Ojcius D.M. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sperlagh B., Hasko G., Nemeth Z., Vizi E.S. ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int. 1998;33:209–215. doi: 10.1016/s0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 103.Franceschini A., Capece M., Chiozzi P., Falzoni S., Sanz J.M., Sarti A.C. The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J. 2015;29:2450–2461. doi: 10.1096/fj.14-268714. [DOI] [PubMed] [Google Scholar]
- 104.Ferrari D., Pizzirani C., Adinolfi E., Lemoli R.M., Curti A., Idzko M. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 105.Ferrari D., Wesselborg S., Bauer M.K., Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y., Xiao Y., Li Z. P2X7 receptor positively regulates MyD88-dependent NF-kappaB activation. Cytokine. 2011;55:229–236. doi: 10.1016/j.cyto.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 107.Chang X., He H., Zhu L., Gao J., Wei T., Ma Z. Protective effect of apigenin on Freund's complete adjuvant-induced arthritis in rats via inhibiting P2X7/NF-kappaB pathway. Chem Biol Interact. 2015;236:41–46. doi: 10.1016/j.cbi.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 108.Tafani M., De Santis E., Coppola L., Perrone G.A., Carnevale I., Russo A. Bridging hypoxia, inflammation and estrogen receptors in thyroid cancer progression. Biomed Pharmacother. 2014;68:1–5. doi: 10.1016/j.biopha.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 109.Adinolfi E., Callegari M.G., Ferrari D., Bolognesi C., Minelli M., Wieckowski M.R. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell. 2005;16:3260–3272. doi: 10.1091/mbc.E04-11-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Budagian V., Bulanova E., Brovko L., Orinska Z., Fayad R., Paus R. Signaling through P2X7 receptor in human T cells involves p56lck, MAP kinases, and transcription factors AP-1 and NF-kappa B. J Biol Chem. 2003;278:1549–1560. doi: 10.1074/jbc.M206383200. [DOI] [PubMed] [Google Scholar]
- 111.Amoroso F., Capece M., Rotondo A., Cangelosi D., Ferracin M., Franceschini A. The P2X7 receptor is a key modulator of the PI3K/GSK3beta/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene. 2015;34:5240–5251. doi: 10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- 112.Adinolfi E., Raffaghello L., Giuliani A.L., Cavazzini L., Capece M., Chiozzi P. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 113.Jelassi B., Chantome A., Alcaraz-Perez F., Baroja-Mazo A., Cayuela M.L., Pelegrin P. P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene. 2011;30:2108–2122. doi: 10.1038/onc.2010.593. [DOI] [PubMed] [Google Scholar]
- 114.Jelassi B., Anchelin M., Chamouton J., Cayuela M.L., Clarysse L., Li J. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis. 2013;34:1487–1496. doi: 10.1093/carcin/bgt099. [DOI] [PubMed] [Google Scholar]
- 115.Di Virgilio F., Ferrari D., Adinolfi E. P2X(7): a growth-promoting receptor-implications for cancer. Purinergic Signal. 2009;5:251–256. doi: 10.1007/s11302-009-9145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adinolfi E., Capece M., Franceschini A., Falzoni S., Giuliani A.L., Rotondo A. Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Res. 2015;75:635–644. doi: 10.1158/0008-5472.CAN-14-1259. [DOI] [PubMed] [Google Scholar]
- 117.Kusner D.J., Barton J.A. ATP stimulates human macrophages to kill intracellular virulent Mycobacterium tuberculosis via calcium-dependent phagosome-lysosome fusion. J Immunol. 2001;167:3308–3315. doi: 10.4049/jimmunol.167.6.3308. [DOI] [PubMed] [Google Scholar]
- 118.Pizzirani C., Ferrari D., Chiozzi P., Adinolfi E., Sandona D., Savaglio E. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood. 2007;109:3856–3864. doi: 10.1182/blood-2005-06-031377. [DOI] [PubMed] [Google Scholar]
- 119.Bianco F., Pravettoni E., Colombo A., Schenk U., Moller T., Matteoli M. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 120.Baroja-Mazo A., Martin-Sanchez F., Gomez A.I., Martinez C.M., Amores-Iniesta J., Compan V. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014;15:738–748. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- 121.Thomas L.M., Salter R.D. Activation of macrophages by P2X7-induced microvesicles from myeloid cells is mediated by phospholipids and is partially dependent on TLR4. J Immunol. 2010;185:3740–3749. doi: 10.4049/jimmunol.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chiozzi P., Sanz J.M., Ferrari D., Falzoni S., Aleotti A., Buell G.N. Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol. 1997;138:697–706. doi: 10.1083/jcb.138.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lemaire I., Falzoni S., Leduc N., Zhang B., Pellegatti P., Adinolfi E. Involvement of the purinergic P2X7 receptor in the formation of multinucleated giant cells. J Immunol. 2006;177:7257–7265. doi: 10.4049/jimmunol.177.10.7257. [DOI] [PubMed] [Google Scholar]
- 124.Lemaire I., Falzoni S., Zhang B., Pellegatti P., Di Virgilio F. The P2X7 receptor and Pannexin-1 are both required for the promotion of multinucleated macrophages by the inflammatory cytokine GM-CSF. J Immunol. 2011;187:3878–3887. doi: 10.4049/jimmunol.1002780. [DOI] [PubMed] [Google Scholar]
- 125.Pellegatti P., Falzoni S., Donvito G., Lemaire I., Di Virgilio F. P2X7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J. 2011;25:1264–1274. doi: 10.1096/fj.10-169854. [DOI] [PubMed] [Google Scholar]
- 126.Di Virgilio F., Bronte V., Collavo D., Zanovello P. Responses of mouse lymphocytes to extracellular adenosine 5′-triphosphate (ATP). Lymphocytes with cytotoxic activity are resistant to the permeabilizing effects of ATP. J Immunol. 1989;143:1955–1960. [PubMed] [Google Scholar]
- 127.Zanovello P., Bronte V., Rosato A., Pizzo P., Di Virgilio F. Responses of mouse lymphocytes to extracellular ATP. II. Extracellular ATP causes cell type-dependent lysis and DNA fragmentation. J Immunol. 1990;145:1545–1550. [PubMed] [Google Scholar]
- 128.Baricordi O.R., Ferrari D., Melchiorri L., Chiozzi P., Hanau S., Chiari E. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood. 1996;87(2):682–690. [PubMed] [Google Scholar]
- 129.Romagnoli R., Baraldi P.G., Cruz-Lopez O., Lopez-Cara C., Preti D., Borea P.A. The P2X7 receptor as a therapeutic target. Expert Opin Ther Targets. 2008;12:647–661. doi: 10.1517/14728222.12.5.647. [DOI] [PubMed] [Google Scholar]
- 130.Arulkumaran N., Unwin R.J., Tam F.W. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opin Investig Drugs. 2011;20:897–915. doi: 10.1517/13543784.2011.578068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang C.A., Chiang B.L. Inflammasomes and human autoimmunity: a comprehensive review. J Autoimmun. 2015;61:1–8. doi: 10.1016/j.jaut.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 132.Kirou K.A., Lee C., George S., Louca K., Peterson M.G., Crow M.K. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 133.Nagata S., Hanayama R., Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 134.Kruse K., Janko C., Urbonaviciute V., Mierke C.T., Winkler T.H., Voll R.E. Inefficient clearance of dying cells in patients with SLE: anti-dsDNA autoantibodies, MFG-E8, HMGB-1 and other players. Apoptosis. 2010;15:1098–1113. doi: 10.1007/s10495-010-0478-8. [DOI] [PubMed] [Google Scholar]
- 135.Sun W., Jiao Y., Cui B., Gao X., Xia Y., Zhao Y. Immune complexes activate human endothelium involving the cell-signaling HMGB1-RAGE axis in the pathogenesis of lupus vasculitis. Lab Invest. 2013;93:626–638. doi: 10.1038/labinvest.2013.61. [DOI] [PubMed] [Google Scholar]
- 136.Miao E.A., Rajan J.V., Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Garcia-Romo G.S., Caielli S., Vega B., Connolly J., Allantaz F., Xu Z. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brinkmann V., Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. 2012;198:773–783. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pisetsky D.S. The complex role of DNA, histones and HMGB1 in the pathogenesis of SLE. Autoimmunity. 2014;47:487–493. doi: 10.3109/08916934.2014.921811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Magna M., Pisetsky D.S. The role of cell death in the pathogenesis of SLE: is pyroptosis the missing link? Scand J Immunol. 2015;82:218–224. doi: 10.1111/sji.12335. [DOI] [PubMed] [Google Scholar]
- 141.Perry D., Sang A., Yin Y., Zheng Y.Y., Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Theofilopoulos A.N., Dixon F.J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 143.Watson M.L., Rao J.K., Gilkeson G.S., Ruiz P., Eicher E.M., Pisetsky D.S. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med. 1992;176:1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Subramanian S., Tus K., Li Q.Z., Wang A., Tian X.H., Zhou J. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Satoh M., Kumar A., Kanwar Y.S., Reeves W.H. Anti-nuclear antibody production and immune-complex glomerulonephritis in BALB/c mice treated with pristane. Proc Natl Acad Sci U S A. 1995;92:10934–10938. doi: 10.1073/pnas.92.24.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao J., Wang H., Dai C., Wang H., Zhang H., Huang Y. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 2013;65:3176–3185. doi: 10.1002/art.38174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taylor S.R., Turner C.M., Elliott J.I., McDaid J., Hewitt R., Smith J. P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J Am Soc Nephrol. 2009;20:1275–1281. doi: 10.1681/ASN.2008060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Le Gall S.M., Legrand J., Benbijja M., Safya H., Benihoud K., Kanellopoulos J.M. Loss of P2X7 receptor plasma membrane expression and function in pathogenic B220+ double-negative T lymphocytes of autoimmune MRL/lpr mice. PLoS One. 2012;7:e52161. doi: 10.1371/journal.pone.0052161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Turner C.M., Tam F.W., Lai P.C., Tarzi R.M., Burnstock G., Pusey C.D. Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant. 2007;22:386–395. doi: 10.1093/ndt/gfl589. [DOI] [PubMed] [Google Scholar]
- 150.Kahlenberg J.M., Yalavarthi S., Zhao W., Hodgin J.B., Reed T.J., Tsuji N.M. An essential role of caspase 1 in the induction of murine lupus and its associated vascular damage. Arthritis Rheumatol. 2014;66:152–162. doi: 10.1002/art.38225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morse M.D., Clark K.L., Cascalho M., Kahlenberg J.M. Caspase-1 is required for maintenance of marginal zone B cells in pristane-induced lupus. Lupus. 2016;25:81–87. doi: 10.1177/0961203315606982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cigni A., Pileri P.V., Faedda R., Gallo P., Sini A., Satta A.E. Interleukin 1, interleukin 6, interleukin 10, and tumor necrosis factor alpha in active and quiescent systemic lupus erythematosus. J Investig Med. 2014;62:825–829. doi: 10.2310/JIM.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 153.Voronov E., Dayan M., Zinger H., Gayvoronsky L., Lin J.P., Iwakura Y. IL-1 beta-deficient mice are resistant to induction of experimental SLE. Eur Cytokine Netw. 2006;17:109–116. [PubMed] [Google Scholar]
- 154.Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen D.Y., Chen Y.M., Wen M.C., Hsieh T.Y., Hung W.T., Lan J.L. The potential role of Th17 cells and Th17-related cytokines in the pathogenesis of lupus nephritis. Lupus. 2012;21:1385–1396. doi: 10.1177/0961203312457718. [DOI] [PubMed] [Google Scholar]
- 156.Li P., Lin W., Zheng X. IL-33 neutralization suppresses lupus disease in lupus-prone mice. Inflammation. 2014;37:824–832. doi: 10.1007/s10753-013-9802-0. [DOI] [PubMed] [Google Scholar]
- 157.Tucci M., Quatraro C., Lombardi L., Pellegrino C., Dammacco F., Silvestris F. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 2008;58:251–262. doi: 10.1002/art.23186. [DOI] [PubMed] [Google Scholar]
- 158.Hu D., Liu X., Chen S., Bao C. Expressions of IL-18 and its binding protein in peripheral blood leukocytes and kidney tissues of lupus nephritis patients. Clin Rheumatol. 2010;29:717–721. doi: 10.1007/s10067-010-1386-6. [DOI] [PubMed] [Google Scholar]
- 159.Calvani N., Richards H.B., Tucci M., Pannarale G., Silvestris F. Up-regulation of IL-18 and predominance of a Th1 immune response is a hallmark of lupus nephritis. Clin Exp Immunol. 2004;138:171–178. doi: 10.1111/j.1365-2249.2004.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Migliorini P., Anzilotti C., Pratesi F., Quattroni P., Bargagna M., Dinarello C.A. Serum and urinary levels of IL-18 and its inhibitor IL-18BP in systemic lupus erythematosus. Eur Cytokine Netw. 2010;21:264–271. doi: 10.1684/ecn.2010.0210. [DOI] [PubMed] [Google Scholar]
- 161.Wang D., Drenker M., Eiz-Vesper B., Werfel T., Wittmann M. Evidence for a pathogenetic role of interleukin-18 in cutaneous lupus erythematosus. Arthritis Rheum. 2008;58:3205–3215. doi: 10.1002/art.23868. [DOI] [PubMed] [Google Scholar]
- 162.Kahlenberg J.M., Thacker S.G., Berthier C.C., Cohen C.D., Kretzler M., Kaplan M.J. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J Immunol. 2011;187:6143–6156. doi: 10.4049/jimmunol.1101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kahlenberg J.M., Carmona-Rivera C., Smith C.K., Kaplan M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;190:1217–1226. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Elssner A., Duncan M., Gavrilin M., Wewers M.D. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 165.De Y., Chen Q., Schmidt A.P., Anderson G.M., Wang J.M., Wooters J. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.van der Does A.M., Beekhuizen H., Ravensbergen B., Vos T., Ottenhoff T.H., van Dissel J.T. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J Immunol. 2010;185:1442–1449. doi: 10.4049/jimmunol.1000376. [DOI] [PubMed] [Google Scholar]
- 167.Lai Y., Adhikarakunnathu S., Bhardwaj K., Ranjith-Kumar C.T., Wen Y., Jordan J.L. LL37 and cationic peptides enhance TLR3 signaling by viral double-stranded RNAs. PLoS One. 2011;6:e26632. doi: 10.1371/journal.pone.0026632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lande R., Ganguly D., Facchinetti V., Frasca L., Conrad C., Gregorio J. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Villanueva E., Yalavarthi S., Berthier C.C., Hodgin J.B., Khandpur R., Lin A.M. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tomasinsig L., Pizzirani C., Skerlavaj B., Pellegatti P., Gulinelli S., Tossi A. The human cathelicidin LL-37 modulates the activities of the P2X7 receptor in a structure-dependent manner. J Biol Chem. 2008;283:30471–30481. doi: 10.1074/jbc.M802185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hakkim A., Furnrohr B.G., Amann K., Laube B., Abed U.A., Brinkmann V. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Denny M.F., Yalavarthi S., Zhao W., Thacker S.G., Anderson M., Sandy A.R. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Asgari E., Le Friec G., Yamamoto H., Perucha E., Sacks S.S., Kohl J. C3a modulates IL-1beta secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122:3473–3481. doi: 10.1182/blood-2013-05-502229. [DOI] [PubMed] [Google Scholar]
- 174.Auger R., Motta I., Benihoud K., Ojcius D.M., Kanellopoulos J.M. A role for mitogen-activated protein kinase(Erk1/2) activation and non-selective pore formation in P2X7 receptor-mediated thymocyte death. J Biol Chem. 2005;280:28142–28151. doi: 10.1074/jbc.M501290200. [DOI] [PubMed] [Google Scholar]
- 175.Kawano A., Tsukimoto M., Mori D., Noguchi T., Harada H., Takenouchi T. Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem Biophys Res Commun. 2012;420:102–107. doi: 10.1016/j.bbrc.2012.02.122. [DOI] [PubMed] [Google Scholar]
- 176.Toki Y., Takenouchi T., Harada H., Tanuma S., Kitani H., Kojima S. Extracellular ATP induces P2X7 receptor activation in mouse Kupffer cells, leading to release of IL-1beta, HMGB1, and PGE2, decreased MHC class I expression and necrotic cell death. Biochem Biophys Res Commun. 2015;458:771–776. doi: 10.1016/j.bbrc.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 177.Magna M., Pisetsky D.S. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol Med. 2014;20:138–146. doi: 10.2119/molmed.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Antoine D.J., Harris H.E., Andersson U., Tracey K.J., Bianchi M.E. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol Med. 2014;20:135–137. doi: 10.2119/molmed.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Park J.S., Svetkauskaite D., He Q., Kim J.Y., Strassheim D., Ishizaka A. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 180.Xu J., Jiang Y., Wang J., Shi X., Liu Q., Liu Z. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 2014;21:1229–1239. doi: 10.1038/cdd.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mitroulis I., Kambas K., Chrysanthopoulou A., Skendros P., Apostolidou E., Kourtzelis I. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One. 2011;6:e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Benihoud K., Bonardelle D., Soual-Hoebeke E., Durand-Gasselin I., Emilie D., Kiger N. Unusual expression of LINE-1 transposable element in the MRL autoimmune lymphoproliferative syndrome-prone strain. Oncogene. 2002;21:5593–5600. doi: 10.1038/sj.onc.1205730. [DOI] [PubMed] [Google Scholar]
- 183.Morel L. Genetics of SLE: evidence from mouse models. Nat Rev Rheumatol. 2010;6:348–357. doi: 10.1038/nrrheum.2010.63. [DOI] [PubMed] [Google Scholar]
- 184.Elliott J.I., McVey J.H., Higgins C.F. The P2X7 receptor is a candidate product of murine and human lupus susceptibility loci: a hypothesis and comparison of murine allelic products. Arthritis Res Ther. 2005;7(3):R468–R475. doi: 10.1186/ar1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Buell G.N., Talabot F., Gos A., Lorenz J., Lai E., Morris M.A. Gene structure and chromosomal localization of the human P2X7 receptor. Recept Channels. 1998;5(6):347–354. [PubMed] [Google Scholar]
- 186.Nath S.K., Quintero-Del-Rio A.I., Kilpatrick J., Feo L., Ballesteros M., Harley J.B. Linkage at 12q24 with systemic lupus erythematosus (SLE) is established and confirmed in Hispanic and European American families. Am J Hum Genet. 2004;74:73–82. doi: 10.1086/380913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen G.M., Feng C.C., Ye Q.L., Tao J.H., Li R., Peng H. Association of P2X7R gene polymorphisms with systemic lupus erythematosus in a Chinese population. Mutagenesis. 2013;28:351–355. doi: 10.1093/mutage/get007. [DOI] [PubMed] [Google Scholar]
- 188.Forchap S.L., Anandacoomarasamy A., Wicks J., Di Virgilio F., Baricordi O.R., Rubbini M. P2X7 gene polymorphisms do not appear to be a susceptibility gene locus in sporadic cases of systemic lupus erythematosus. Tissue Antigens. 2008;72:487–490. doi: 10.1111/j.1399-0039.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 189.Portales-Cervantes L., Nino-Moreno P., Doniz-Padilla L., Baranda-Candido L., Garcia-Hernandez M., Salgado-Bustamante M. Expression and function of the P2X(7) purinergic receptor in patients with systemic lupus erythematosus and rheumatoid arthritis. Hum Immunol. 2010;71:818–825. doi: 10.1016/j.humimm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 190.Conti F., Ceccarelli F., Massaro L., Cipriano E., Di Franco M., Alessandri C. Biological therapies in rheumatic diseases. Clin Ter. 2013;164:e413–e428. doi: 10.7417/CT.2013.1622. [DOI] [PubMed] [Google Scholar]
- 191.McCoy S.S., Stannard J., Kahlenberg J.M. Targeting the inflammasome in rheumatic diseases. Transl Res. 2016;167:125–137. doi: 10.1016/j.trsl.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]