Abstract
Introduction:
Chronic hepatitis C virus (HCV) infection is a leading cause of cirrhosis, hepatocellular carcinoma and liver failure. Moreover, chronic HCV infection is associated with liver steatosis and metabolic disorders. With 130–150 million people chronically infected in the world, HCV infection represents a major public health problem. One hallmark on the virus is its close link with hepatic lipid and lipoprotein metabolism.
Areas covered:
HCV is associated with lipoprotein components such as apolipoproteins. These interactions play a key role in the viral life cycle, viral persistence and pathogenesis of liver disease. This review introduces first the role of apolipoproteins in lipoprotein metabolism, then highlights the molecular mechanisms of HCV-lipoprotein interactions and finally discusses their clinical impact.
Expert commentary:
While the study of virus-host interactions has resulted in a improvement of the understanding of the viral life cycle and the development of highly efficient therapies, major challenges remain: access to therapy is limited and an urgently needed HCV vaccine remains still elusive. Furthermore, the pathogenesis of disease biology is still only partially understood. The investigation of HCV-lipoproteins interactions offers new perspectives for novel therapeutic approaches, contribute to HCV vaccine design and understand virus-induced liver disease and cancer.
Keywords: apolipoproteins, hepatitis C virus, lipid metabolism, liver pathophysiology, steatosis
1. Introduction
Hepatitis C virus (HCV) infection is a major cause of chronic hepatitis, liver cirrhosis and hepatocellular carcinoma worldwide, which are major indications for liver transplantation [1]. As long as a prophylactic vaccine is not available, the HCV pandemic has to be controlled by drug treatments. Within the last decade, direct-acting antivirals (DAA) had a major impact on the management of chronic hepatitis C, which has become a curable disease for more than 90% of the DAA-treated patients [2]. Nevertheless, while a new era of HCV treatment is on the horizon, several challenges remain: high costs limit access to therapy, emergence of viral resistance occurs and certain difficult-to-treat patients may need adjunctive therapeutic approaches [1].
Several studies strongly indicated that there is a close connection between HCV and lipid metabolism [3]. Notably, HCV was found to be associated to host lipoproteins. These infectious hybrid particle complexes have been termed lipo-viro-particles (LVP). In addition, each steps of the HCV lifecycle is linked to lipoprotein and apolipoprotein metabolism. Furthermore, HCV infection has been shown to be associated with non-alcoholic steato-hepatitis (NASH) and metabolic disorders [4]. Thus, understanding the relationships between the virus and lipid metabolism is of utmost importance to understand the physiopathology of the infection and to find potential targets for therapies.
Therefore, in this review, we will first provide a concise overview of the lipoprotein metabolism, the importance of apolipoproteins and the central role of the liver in this physiological process. Then, we will briefly describe the life cycle of HCV, focusing on the interaction between the virus and lipid metabolism. And finally, we will summarize the role of apolipoproteins at each step of HCV lifecycle in the prospect of therapeutic targets as well as pathogenesis.
2. Focus on lipoproteins metabolism
2.1. Lipoproteins structure and properties
Diet derived exogenous lipids as well as endogenous lipids produced by the organism must be transported to various cells and organs for utilization or storage. However, the insolubility of lipids in aqueous plasma requires their association with amphipathic proteins in spheroidal watermiscible macromolecules named lipoproteins [5, 6]. Lipoproteins constitute a wide family of particles, which differ in size, density, composition and function. They are usually classified into five main classes in humans, based on their buoyant density by ultracentrifugation: chylomicrons (CM), very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL). While CM and VLDL are triglyceride-rich lipoproteins (TRL), LDL and HDL contain high cholesterol levels. Lipoprotein density depends on their lipid and protein composition. Thus, CM are the biggest particles but have the lowest buoyant density since they contain less than 2% of proteins. Conversely, HDL are the smallest particles but have the heaviest buoyant density because they are protein-rich. Their physicochemical properties are summarized in Table 1[6–8].
Table 1: Physicochemical properties of the main classes of lipoproteins.
CM: chylomicrons, VLDL: very-low-density lipoproteins, IDL: intermediate-density lipoproteins, LDL: low-density lipoproteins, HDL: high-density lipoproteins.
| Exogenous pathway | Endogenous pathway | Reverse cholesterol transport | |||
|---|---|---|---|---|---|
| Lipoprotein class | CM | VLDL | IDL | LDL | HDL |
| Density (g/mL) | <0.95 | 0.950–1.006 | 1.006–1.019 | 1.019–1.063 | 1.063–1.210 |
| Diameter (nm) | 100–1000 | 30–80 | 25–50 | 18–28 | 5–15 |
| Protein (% by weight) | <2 | 7–10 | 10–18 | 20–25 | 33–57 |
| Main lipids | Triglycerides | Triglycerides | Triglycerides, cholesterol | Cholesterol | Cholesterol, phospholipids |
| Main apolipoproteins | A-I, A-IV, A-V, B-48, C-I, C-II, C-III, E | B-100, E, C-I, C-II, C-III | B-100, E | B-100 | A-I, A-II, E, C-I, C-II, C-III |
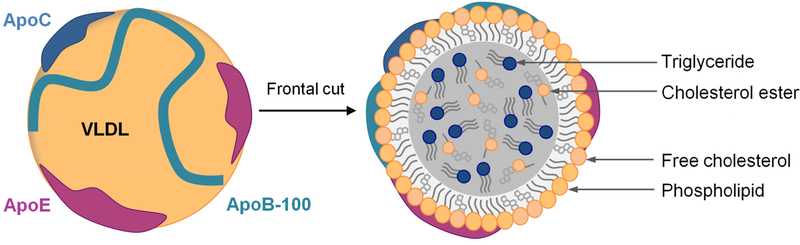
Lipoproteins all have a common structure, consisting of a single outer layer of phospholipids, free cholesterol and amphipathic proteins that surrounds a non-polar core composed of triglycerides, cholesterol esters and fat-soluble components such as vitamins [5, 7]. The structure of a VLDL is illustrated in Figure 1.
Figure 1: Structure of a lipoprotein, example of the very low-density lipoproteins (VLDL).
VLDL consist of a single outer layer of phospholipids and free cholesterol and are stabilized by apolipoproteins (Apo) such as ApoB-100, ApoC (I, II and III) and ApoE. A single molecule of ApoB-100 is present per VLDL. ApoB-100 is an integral protein compared to ApoC and ApoE that are exchangeable Apo. VLDL possess a hydrophobic core essentially composed of triglycerides and to a lesser extent of cholesterol esters.
Apolipoproteins (Apo) represent the protein moiety of lipoproteins, constituting 1 to nearly 60% of lipoprotein weight. They can be divided into two groups based on their biophysical properties, namely exchangeable and non-exchangeable apolipoproteins. For example, ApoB are nonexchangeable proteins irreversibly associated with CM, VLDL and LDL, whereas ApoA, ApoC and ApoE can be easily exchanged between the different classes of lipoproteins [9]. Apolipoproteins are critical regulators of lipid transport and lipoprotein metabolism. They carry out three major functions. First, they have a structural role and help stabilize the lipoprotein structure. Secondly, they act as ligands for lipoprotein receptors and are involved in lipoprotein clearance. Finally, they serve as activators or inhibitors of key enzymes involved in lipoprotein metabolism [10]. Their main functions will be described in the following paragraphs and are summarized in Table 2.
Table 2: Main functions of major apolipoproteins.
ABCA1: ATP-binding cassette sub-family A member 1, CETP: cholesteryl ester transfer protein, HL: hepatic lipase, HSPG: heparan sulfate proteoglycans, LCAT: lecithin cholesterol acyltransferase, LDLR: low-density lipoproteins receptor, LPL: lipoprotein lipase, LRP1: LDLR related protein 1, SR-BI: scavenger receptor class B type I, TRL: triglyceride-rich lipoproteins.
| Name | Primary source | Lipoprotein association |
Main function | Ref |
|---|---|---|---|---|
| ApoA-I | Liver, intestine | HDL >> CM | Structural role (major protein of HDL), LCAT activator, SR- Bl ligand, ABCA1 ligand (cholesterol efflux) | 11, 12 |
| ApoA-II | Liver, intestine | HDL | Structural role, LCAT and HL regulator | 10, 11 |
| ApoA-IV | Intestine | CM | Synthetized during fat absorption, involved in TRL metabolism, regulates food intake and satiety | 5, 11, 13 |
| ApoA-V | Liver | CM, VLDL, HDL | LPL activator, involved in TRL metabolism | 15, 16 |
| ApoB-100 | Hepatocyte | VLDL, IDL, LDL | Structural role (major component of VLDL, IDL and LDL), involved in VLDL synthesis, secretion, and clearance, LDLR ligand | 14, 17 |
| ApoB-48 | Intestine (splicing variant of ApoB-100) | CM | Structural role, involved in CM synthesis and secretion | 14, 17, 18 |
| ApoC-I | Liver | CM, VLDL, HDL | CETP inhibitor, LCAT activator | 19–21 |
| ApoC-II | Liver | CM, VLDL, HDL | LPL activator | 19, 20 |
| ApoC-III | Liver | CM, VLDL, HDL | LPL inhibitor, inhibits the interaction of TRL with their receptors | 19–21 |
| ApoE | Liver, intestine, macrophage | CM, VLDL, IDL, HDL | HSPG, LRP1 and LDLR ligand, involved in TRL metabolism, involved in reverse cholesterol transport | 22–24 |
2.2. Overview of lipoprotein metabolism
Lipoprotein metabolism is a complex homeostatic network that ensures the strict maintenance of tissues cholesterol and triglycerides levels [10]. It consists of three main pathways: the exogenous pathway, the endogenous pathway and the reverse cholesterol transport. An overview is depicted in Figure 2 and Figure 3. The role of apolipoproteins in these pathways will be highlighted in the following paragraphs.
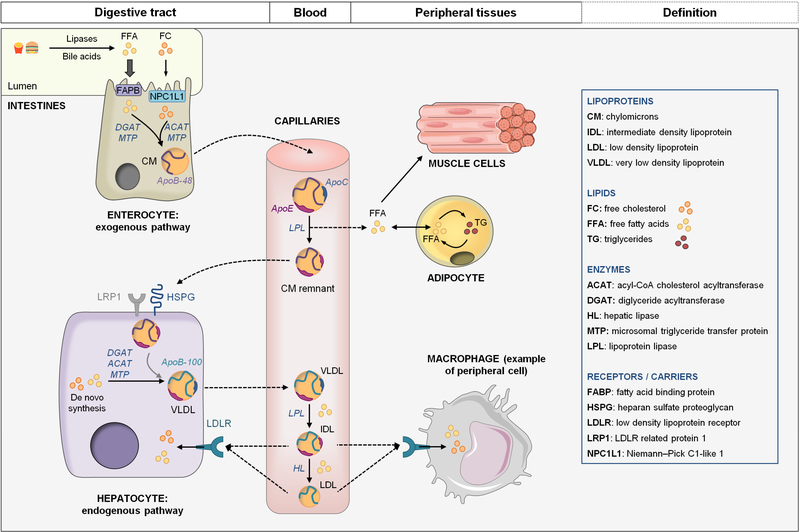
Figure 2: TG-rich lipoproteins (TRL) metabolism, summary of the exogenous and the endogenous lipid transport pathways.
During digestion, FFA and FC are absorbed by enterocytes through FABP and NPC1L1 respectively and are assembled with ApoB-48 to form CM. CM are secreted and maturated in lymphatic and blood circulation by acquiring exchangeable apolipoproteins such as ApoE and ApoC. CM are metabolized by LPL to release FFA and CM remnants. FFA are taken up by muscle cells for energy production or by adipocytes for storage. CM remnants are captured by hepatocytes through interaction of ApoE with remnant receptors (LRP1 and HSPG). Dietary lipids can be stored in hepatocytes. Both stored and de novo synthetized lipids can be mobilized to produce VLDL. VLDL production requires ApoB-100 and MTP. They are secreted in blood circulation to transport lipids, mainly TG, to peripheral tissues. TG are hydrolyzed by LPL to release FFA and VLDL remnants also named IDL. IDL can be captured by the liver or further metabolized into LDL. LDL are enriched in cholesterol and ensure its transport to the peripheral tissues and the liver.
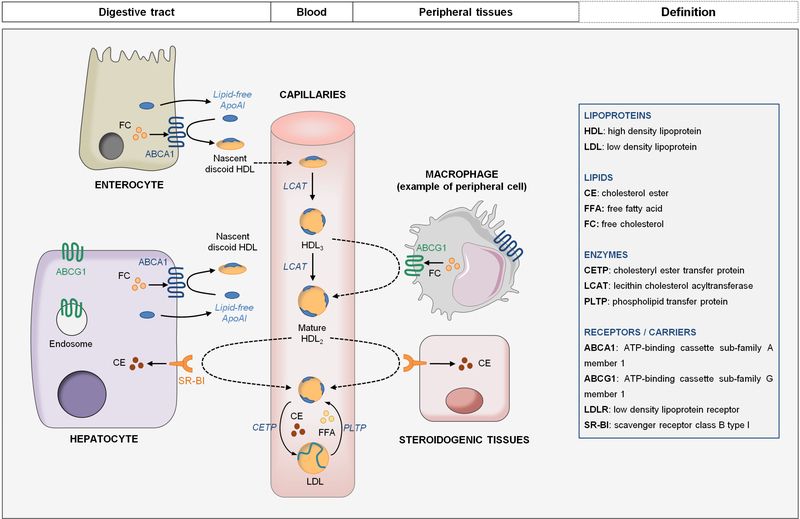
Figure 3: HDL metabolism and reverse cholesterol transport.
ApoA-I is mainly produced by hepatocytes and enterocytes. HDL synthesis begins with the interaction of ApoA-I with ABCA1 that mediates cholesterol efflux to produce nascent discoid HDL. Discoid HDL are converted into spherical HDL3 by LCAT. HDL3 are then enriched in cholesterol through their interaction with ABCG1 in peripheral tissues to transform into mature HDL2. HDL2 are ligands for SR-BI that ensures cholesterol influx into hepatocytes or into steroidogenic tissues. Alternatively, cholesterol returns to the liver via the CETP pathway: CETP mediates the transfer of CE on Apo-B-containing lipoproteins that are captured by hepatocytes through LDLR (see figure 2).
2.2.1. Exogenous pathway: from the intestine to the liver
After food intake, dietary fats are absorbed in the small intestine by enterocytes to be packaged into CM. Triglycerides (TG) are the predominant lipids in the diet and represent ≈ 90% of the lipid content of CM [10]. During digestion, TG are hydrolyzed by pancreatic lipase to generate free fatty acids (FFA). These FFA are taken up by enterocytes through the fatty acid binding protein (FABPB). In cells, they are used to resynthesize TG by the combined action of the monoacylglycerol acyltransferase (MGAT) and the diglyceride acyltransferase (DGAT), before being packaged into CM [18]. Dietary free cholesterol (FC) is also absorbed by enterocytes through the Niemann-Pick C1-like 1 protein (NPC1L1) and must be esterified by acyl-CoAcholesterol acyltransferase (ACAT) to be efficiently incorporated into CM. However, only 25% of the cholesterol absorbed by enterocytes derives from the diet, the major source being the reabsorption from bile salts [25].
After absorption, dietary lipids are assembled with ApoB-48 to form CM. ApoB-48 is exclusively found in the small intestine. It results from a post-transcriptional modification of the ApoB mRNA by the ApoB mRNA editing complex (APOBEC1), leading to a truncated form of the protein lacking the LDL receptor (LDLR) binding-domain [17]. During its synthesis, ApoB-48 is cotranslationally lipidated in the endoplasmic reticulum (ER) by the microsomal transfer protein (MTP). Nascent CM are then enriched in TG and acquire exchangeable apolipoproteins such as ApoA-I and ApoA-IV prior to their secretion into lymphatic vessels. They reach the systemic circulation through the thoracic duct where they are maturated by addition of ApoC-I, ApoC-II, ApoC-III and ApoE originating from HDL [10]. Acquisition of ApoC-II on CM surface enables the activation of lipoprotein lipase (LPL). This enzyme is located on vascular endothelial cells and mediates the hydrolysis of TG to release FFA that are used by muscle cells for energy production or by adipocytes for storage [20]. LPL mediated TG hydrolysis is accompanied by a reduction of CM size and a transfer of cholesterol, ApoC-II and ApoC-III back to HDL. The remnant particles are then cleared from the circulation by hepatocytes [10]. Due to the absence of LDLR-binding domain in ApoB-48, the remnant clearance is mediated by the interaction of ApoE with heparan sulfate proteoglycan (HSPG) and LDLR related protein 1 (LRP1) [17].
2.2.2. Endogenous pathway: from liver to peripheral tissues
The small intestine has a strong capacity to rapidly respond to fat ingestion but cannot store lipids for long periods. The liver, on the other hand, is the gatekeeper of ingested and de novo synthesized lipids, with a strong capacity for storage and maintenance of lipid homeostasis [14]. Hepatocytes produce VLDL, another class of TRL. While CM mediate the transport of dietary lipids, VLDL deliver mainly endogenous TG, derived from both lipid storage pool and the de novo synthesis, to peripheral tissues [10].
VLDL assembly starts with the lipidation of ApoB-100 in hepatocytes by MTP after their translocation across the ER into the lumen. Nascent VLDL are then enriched in TG and cholesterol ester (CE) and acquire ApoE and ApoC prior to their secretion into blood circulation [10, 14]. In the plasma, VLDL are hydrolyzed by LPL to generate FFA that are delivered to the peripheral tissues, and smaller particles named IDL or VLDL remnants. During the lipolysis, IDL are depleted of ApoC-II, the LPL activator and are enriched in HDL-derived ApoE. Approximately 50% of IDL are cleared from the circulation through their interaction with hepatic remnant receptors and LDLR, while the remaining IDL can undergo further catabolism by the hepatic lipase (HL) to become LDL through the loss of ApoE. LDL have a high cholesterol content and provide cholesterol to peripheral tissues or liver cells via interaction of ApoB-100 with LDLR [7, 8]
2.2.3. Reverse transport cholesterol: from peripheral tissues to liver
Cholesterol is an important biological molecule since it is a constituent of cell membranes, a precursor of vitamin D, steroid hormones and bile acids, and of utmost importance for neurotransmission. However, the biosynthesis and transport of cholesterol must be tightly regulated in order to prevent toxic cellular accumulation and pathologies such as atherosclerosis. Reverse cholesterol transport (RCT) contributes to cholesterol homeostasis by ensuring cholesterol transport through HDL from peripheral tissues to the liver for excretion [25]. In human plasma, HDL are a highly heterogeneous lipoprotein family consisting of several subclasses with different density, size and composition [26]. Although HDL contain a variety of proteins, ApoA-I represents 70% of the total HDL protein content. The first step of the HDL biogenesis is the secretion of ApoA-I by hepatocytes and enterocytes into the circulation as a lipid-free or a lipidpoor protein. ApoA-I then interacts with the cellular ATP-binding cassette transporter A1 (ABCA1). ABCA1 mediates the transfer of cellular phospholipids and unesterified cholesterol to ApoA-I to produce discoidal particles [11]. FC esterification by the lecithin cholesterol acyltransferase (LCAT), converts the discoidal HDL to spherical HDL3 particles. HDL3 are then enriched in cholesterol through their interaction with the ATP-binding cassette transporter G1 (ABCG1) in peripheral tissues, to transform into mature HDL2. ABCG1 cannot mediate cholesterol efflux on lipid-poor ApoA-I, implying a synergistic relationship between ABCA1 and ABCG1. Thus, cellular cholesterol excess can be removed and transported by HDL2 to the liver for excretion through the RCT. Indeed, mature HDL2 are ligands for the scavenger receptor BI (SR-BI) that provides cholesterol influx into hepatocytes. Alternatively, SR-BI can deliver cholesterol to steroidogenic tissues to produce hormone precursors. Interestingly, HDL and ApoB-containing lipoprotein can exchange CE for TG through the action of the CE transport protein (CETP). By this mean, CE is also sent back CE to the hepatocytes through LDLR [10, 12].
3. HCV lifecycle, apolipoproteins and lipoprotein metabolism
Each step of the HCV life cycle is intimately connected to the hepatic lipid metabolism [3]. Notably, HCV infectious particles are closely associated with lipoprotein components such as apolipoproteins, which affect many aspects of viral life cycle. In the following paragraph, we will present the link between HCV viral particle and lipoprotein metabolism and an will summarize the role of the different apolipoproteins involved in HCV life cycle steps, from HCV entry to virion maturation.
3.1. The complex structure of HCV particle.
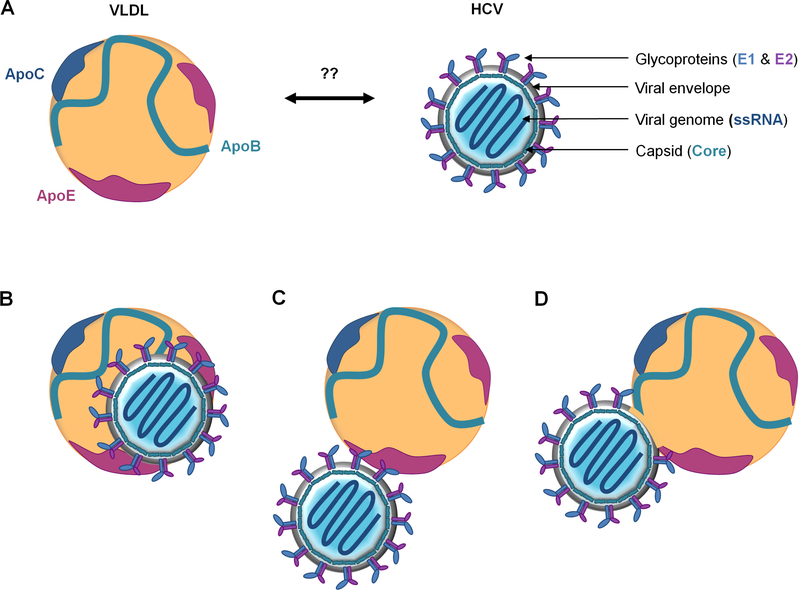
HCV is an enveloped positive-stranded RNA virus that belongs to the Flaviviridae family. The putative HCV particle consists of a broad nucleocaspid containing the viral genome, surrounded by an ER-derived membrane where E1 and E2 glycoproteins are anchored as heterodimers [23] (Figure 4A). Before the advent of an HCV cell culture system (HCVcc), knowledge on virion properties were based on studies performed on serum-derived HCV particles isolated from chronically infected patients or from experimentally infected chimpanzees. These studies have revealed that HCV particles display a complex broad density profile ranging from 1.03 to 1.20 g/mL. The higher density fractions are less infectious and can often be precipitated by anti-human IgG, suggesting the presence of immune complexes in serum. In contrast, the highly infectious HCV particles are present in the lower density fractions, ranging from 1.03 to 1.10 g/mL. This atypically low buoyant density is due to the interaction of viral particles with serum lipoproteins, earning it the name of lipoviroparticle (LVP) [28–30]. Consistent with this, apolipoproteins such as ApoB-100, ApoE, ApoA-I, ApoC-I and ApoC-III are associated with HCV particles [31–33]. Furthermore, HCV particles contain lipids and cholesteryl ester contents similar to VLDL and LDL [34]. The complexity of the HCV particle was confirmed with the development of the HCVcc system. Indeed, HCVcc-derived virions have a buoyant density peak close to 1.15 g/mL and are also found in association with lipoprotein components [35–37]. Nonetheless, it is important to note that the physical properties of HCVcc-derived particles differ from those of patient-derived HCV particles, likely due to defects in VLDL production by human hepatocarcinoma-derived cell lines (Huh-7) used to produce HCV in vitro [38].
Figure 4: Interaction between VLDL and HCV particle.
(A) The putative HCV particle consists of a broad nucleocapsid, formed by the capsid protein core, containing the viral RNA genome. The nucleocapsid is surrounded by an ER-derived membrane decorated with the viral E1-E2 glycoproteins. (B-D) Illustrations of different models of infectious LVP. “True LVP” corresponds to a hybrid particle composed of viral and lipoprotein components sharing the same envelop (B). “Transient LVP” corresponds to a putative HCV particle interacting with ApoB-containing lipoproteins (VLDL/CM) through Apo-glycoprotein interactions (C) or through the lipid moiety of the viral envelop (D).
It is assumed that the association of HCV particles with lipoprotein components provides an efficient escape mechanism from neutralizing antibodies [29]. However, the exact nature of HCVlipoprotein interactions remains unclear. A widely accepted model of the LVP organization has been described, depicting it as a hybrid particle composed of viral and lipoprotein components sharing the same envelop (Figure 4B). Alternatively, another model hypothesizes that lipoproteins could transiently associate with the HCV virion through the interaction of apolipoproteins with HCV glycoproteins (Figure 4C) or with the lipid moiety of the viral enveloppe (Figure 4D) [7, 39, 40]. This model is supported by a report showing that serum-derived HCV particles are associated with ApoB-48, an apolipoprotein exclusively synthetized by the intestine and associated with CM [41]. Moreover it was observed that the buoyant density of LVP in patient serum rapidly shifts to 1.025 g/mL after oral intake of TG, suggesting that HCV can be transferred onto CM similarly to exchangeable apolipoproteins [42]. Very recently, Piver and collaborators provided new findings about the precise morphology of HCV particle. By transmission electron microscopy, they confirmed the ultrastructure of LVP predicted by several authors [43]. Interestingly, their results also indicate that HCV is present in patient serum as a mixed population including true-HCV-LVP and LVP-like particles. The latter contain neither Core protein nor viral RNA, but express E1 and E2 glycoproteins on the surface. The LVP-like particles are predominant in patient’s serum and could contribute to the viral escape strategy from host immunity [43].
Whatever the mode of interaction of HCV with lipoproteins, it is clear that the virus has the capacity to take advantage from TRL production pathway for its own production to be easily delivered to the liver while escaping host immunity.
3.2. The HCV life cycle
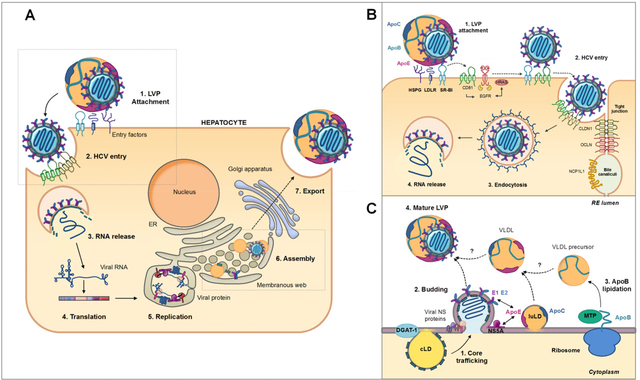
HCV takes advantage of many aspects of lipid metabolism for an efficient replication and propagation in hepatocytes. The different steps of HCV life cycle are depicted in Figure 5A. Briefly, HCV enters the cells via a clathrin-dependent endocytosis that requires plethora of host factors including attachment factors (HSPG and LDLR), entry receptors (SR-BI, CD81, claudin 1 (CLDN1), and occludin (OCLN)) and co-receptors (e.g. epidermal growth factor receptor (EGFR), and NPC1L1) [44]. After acidic endosomal fusion and uncoating, viral RNA is released in the cell cytoplasm. The internal ribosome entry site (IRES) located in the 5’ non-translated region (NTR) of viral RNA mediates the translation of a precursor protein. The polyprotein is maturated by viral and host proteases into three structural (Core, E1 and E2) and seven nonstructural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B) [45]. Viral proteins associate with the so-called “membranous web” (MW), which consists of double-membrane vesicles containing HCV RNA and non-structural proteins, ER derived-membranes and lipid droplets (LD) [46]. Viral replication occurs in this web under the control of viral RNA polymerase NS5B. This process is highly dependent on cholesterol metabolism [3]. HCV Core protein is, on the other hand, recruited to the surface of LD by two enzymes involved in lipid metabolism, DGAT-1 and the cytosolic phospholipase A2 group IVA (cPLA2G4A) [47–49]. Core-LD association appears to be critical in viral assembly for the recruitment of the other viral proteins and viral RNA to assembly sites [47–51]. Then, viral RNA and Core protein form the HCV nucleocapsid that acquires the envelop proteins embedded in the ER membrane, through budding into the ER lumen [40]. The VLDL secretion pathway is closely related to virion assembly and release. Many reports have shown that VLDL-associated proteins including ApoB, ApoE and MTP play crucial roles in the formation and maturation of infectious LVP [42–46]. However, the detailed mechanism has not been elucidated yet.
Figure 5: Overview of HCV life cycle and role of apolipoproteins in HCV entry and morphogenesis. (A) HCV life cycle is divided into 7 main steps.
(1) LVP are captured by attachment factors at the hepatocyte surface. (2) Then, HCV enters the cells by interacting with several host factors. (3) After clathrin mediated-endocytosis and membrane fusion, viral RNA is release in cytoplasm. (4) Viral polyprotein is generated by an IRES-mediated translation. This polyprotein is maturated by viral and host proteases into 7 viral proteins. (5) Non-structural viral proteins form RNA replication complex in a particular web of ER- derived membranes named “membranous web” (MW). (6) After RNA replication, viral particles are assembled in MW in close proximity with lipid droplet (LD). (7) Finally, viral particles interact with VLDL to form an infectious LVP that is maturated and excreted through the VLDL secretion pathway. (B) HCV entry pathway. (1) Viral particle attachment is mediated by LVP-associated ApoE through interaction with HSPG, LDLR and SR-BI. (2) Then HCV interacts with CD81 and SR-BI through E2. The lipid-transfer activity of SR-BI seems to destabilize the VLDL-HCV interaction to induce E2 binding on cell receptors and HCV entry. ApoC-I facilitates this step (not illustrated). Binding on CD81 induces EGFR signaling pathway activation and interaction between CD81 and CLDN1 that triggers HCV entry. (3) Viral particle is internalized through a clathrin-dependent endocytosis. (4) Viral envelope and vesicle membrane fuse to induce viral RNA release in cytoplasm. OCLDN and NPC1L1 illustrated in this picture are co-factors involved in HCV entry. (C) HCV assembly pathway. (1) HCV particle assembly is triggered by Core protein trafficking from cytosolic LD (cLD), to assembly site. Core then associated with viral RNA to form nuleocapsids. These two steps are facilitated by non-structural proteins (illustrated on the scheme) and several host factors. (2) The nascent HCV particles bud at ER-surface where E1 and E2 proteins are anchored. (3) In parallel, ApoB is translocated into ER lumen and lipidated by MTP to generate VLDL precursor. VLDL precursor, luminal LD (luLD) associated with ApoE and ApoC and nascent HCV particles are then associated through an unknown mechanism to generate mature LVP (4). Interaction between NS5A and LD-associated ApoE but also between ApoE and viral glycoproteins are involved in this process.
Apolipoproteins are key actors of lipoprotein metabolism, but are also crucial for LVP formation and maturation. In this review, we propose to summarize the roles of apolipoproteins in the HCV life cycle.
3.3. Apo are key determinants of HCV infectivity
HCV entry in hepatocytes is a complex process divided into three main steps: viral attachment, receptor-mediated endocytosis and membrane fusion. Actors of the lipoprotein metabolism, and in particular apolipoproteins, play crucial roles at all these steps, depicted in Figure 5B [57].
Several studies have demonstrated that ApoE mediates cell attachment of LPV through interactions with the cell surface heparan sulfate (HS) [54, 58, 59]. HS is covalently attached to core proteins embedded in the cell membrane to form HSPG. The HSPG core proteins include syndecans (SDC), glypicans, perlecans and agrin. Interestingly, two groups have shown that SDC1 and SDC4, two receptors involved in VLDL remnant clearance, are also the major attachment factors for HCV LVP [60, 61]. ApoE appears to play a critical role in LVP propagation in hepatocytes. Indeed HCV production and infectivity are drastically suppressed by siRNAmediated knockdown of ApoE expression, whereas ectopic ApoE expression restores virus assembly and release [61–63]. Furthermore, HCV infectivity can be efficiently neutralized by ApoE-specific monoclonal antibodies [34, 62]. Consistent with this, human ApoE-derived peptides specifically block the binding of both HCVcc and patient serum-derived HCV to target cells, by competing with HSPG binding [58, 61].
LDLR has also been proposed to be a receptor for HCV entry [64, 65]. In 2009, Owen and collaborators described the LDLR as a cooperative HCV co-receptor mediating viral entry through interaction with ApoE. By competition experiments between LVP and human lipoproteins, they showed that VLDL and LDL efficiently inhibit HCV binding and entry. Moreover, they demonstrated that the presence of ApoE within VLDL confers higher affinity for LDLR binding than for LDL and an increased ability of VLDL to block HCV infection [66]. However, the exact role of LDLR in HCV infection still remains controversial. It is not clear whether LDLR acts as a true HCV receptor or could instead act as a facilitator of initial LVP attachment to the hepatocyte surface. In 2012, a study notably showed that LDLR is not essential for infectious HCV particle entry, whereas the physiological function of this receptor is important for optimal replication of the HCV genome [67].
In 2002, SR-BI has been identified as a main HCV receptor, due to its capacity to directly interact with the viral glycoprotein E2 [68]. SR-BI is the major HDL receptor but can also bind ApoBcontaining lipoproteins such as VLDL/LDL [10]. Interestingly, during the first steps of HCV entry, interaction between LVP and SR-BI is not sensitive to anti-E2 antibodies but is efficiently inhibited by antibodies specific for ApoB-containing lipoproteins [69]. Moreover, LVP interaction with SR-BI is out-competed by both VLDL and purified ApoE [69–70]. Together, these data indicate that the initial attachment of LVP to SR-BI is independent of E2 but is rather mediated by ApoB and ApoE. However, the binding of HCV E2 to SR-BI is required for the subsequent SR-BI function at a postattachment step [70]. This interaction is thought to trigger the dissociation of lipoproteins from LVP through the SR-BI-mediated cholesterol transfer activity, and to expose the CD81 interaction domain of the E2 glycoprotein [71].
In addition to its interaction with lipoproteins and viral components, it was further demonstrated that the lipid transfer function of SR-BI must be intact to mediate HCV entry [71, 72]. Interestingly, it was shown that HDL facilitates HCV entry through the SR-BI cholesterol-transfer activity. Nevertheless, no direct interaction between HDL and HCV was reported and it remains therefore unclear how HDL enhance HCV infectivity [73–75]. SR-BI localizes to cholesterol-enriched plasma membrane microdomains where it forms the HCV receptor complex together with CD81. Therefore, cholesterol enrichment of the plasma membrane mediated by SR-BI/HDL interaction could increase the efficiency of co-receptor complex formation and thus, of HCV entry [76, 77]. A role of ApoC-I located on HDL, has also been suggested [32, 78, 79]. Indeed, the group of Cosset has reported that ApoC-I recruitment on LVP specifically enhances HCV infectivity by increasing the fusion rates between LVP and cellular plasma membrane. Furthermore, they showed that HDL-associated ApoC-I could be swapped from HDL to LVP during a complex interplay between HCV E2, HDL and SR-BI [79].
Changes in the nature of the HCV-associated lipoproteins have also been demonstrated to influence HCV entry. In particular, LPL can disrupt the structure of serum LVP [80]. Physiologically, LPL has two main functions: (i) it hydrolyzes TG in ApoB-containing lipoproteins to generate FFA and remnants; (ii) it facilitates the uptake of these remnants by creating a “bridge” between the lipoprotein and HSPG/LDLR. Interestingly, it was shown that LVP treatment with ApoC-II activated LPL leads to a shift of particles to higher-densities and to a lower amount of virion-associated ApoE, crucially reducing HCV infectivity [80, 81]. Furthermore, as is the case for TRL, LPL is able to mediate binding and internalization of treated-HCV particles on hepatocytes, acting as a bridge between LVP and HSPG/LDLR. However, it was observed that LPL treatment inhibits HCV infectivity, in spite of a higher yield of particle internalization, indicating that LPL promotes a non-productive virus uptake [82]. Consistent with this finding, Maillard et al revealed that LPL blocks HCV entry by immobilizing viral particles at the cell surface through its bridging function [83]. Thus LPL and its activator ApoC-II are physiological anti-HCV factors.
In 2013, the group of Young corroborated these studies by showing that LPL activity is inversely correlated to HCV viral loads in patients [84]. This study also revealed that VLDL- and LDLassociated LVP extracted from the serum of HCV-infected patients, as well as normal VLDL extracted from healthy donors are able to inhibit LPL lipolytic activity in vitro. Conversely normal LDL did not exhibit an inhibitory effect, suggesting that a common component of normal VLDL, VLDL-LVP and LDL-LVP that is not present on normal LDL could be a LPL inhibitor. This component was identified as ApoC-III, the natural inhibitor of LPL. In physiological conditions, ApoC-III is not present at the surface of LDL, but it is found on the LDL-associated HCV surface. Thus, VLDL-LVP and LDL-LVP could inhibit LPL activity through its ApoC-III moiety and facilitate the propagation and the persistence of HCV [84].
3.4. Apolipoproteins, lipid metabolism and HCV replication
Viral RNA replication occurs in the MW, in close proximity to lipid rafts, which consist of cholesterol-enriched microdomains. Thus HCV replication is highly dependent on cholesterol metabolism [3, 46, 85, 86]. A study performed in our laboratory notably demonstrated that the lipid form of ApoE, when added to cell culture media of hepatic cells, is able to inhibit HCV RNA replication by regulating cholesterol metabolism in hepatocytes [87]. As is the case for ApoA-I, ApoE can be secreted in a lipid-free form and induce cholesterol efflux from different cell types to generate HDL [22, 88, 89]. In our study, we demonstrated that extracellular lipid-free ApoE promotes HDL formation in hepatocytes, which in turn becomes available for cholesterol loading by ABCG1. This mechanism is responsible for the inhibition of HCV replication as a consequence of cellular cholesterol depletion [87]. In the case of HCV infection, where ApoE appears to play a critical role in LVP propagation in hepatocytes, the lipid-free ApoE-induced inhibition of replication might appear paradoxical. But on the one hand, we demonstrated that lipid-free ApoE acts on HCV replication independently of the classical ApoE receptors also involved in HCV entry, which points to a different mechanism of action. And on the other hand, one explanation could be that HCV might take advantage of the lipid-free-dependent regulation of cholesterol metabolism to fine-tune its replication level.
ApoAI also appears to be involved in the viral RNA replication. Indeed, siRNA-mediated downregulation of ApoA-I expression induces a significant decrease of viral RNA levels [90]. Interestingly, another study found that one of the transcriptional effects of a histone deacetylase inhibitor treatment (SAHA) was the down-regulation of ApoA-I, which induced the inhibition of HCV RNA replication [91]. To date, the role of ApoA-I in HCV replication is unclear, but could be linked to its crucial role in lipoprotein and cholesterol metabolism.
3.5. Controversial role of apolipoproteins in HCV morphogenesis
After HCV entry in its target cell, viral RNA is released into the cytoplasm, translated and replicated [45, 92]. Then HCV particles are assembled in close proximity to LD. Viral assembly appears to share numerous features with VLDL assembly, as illustrated in Figure 5C. VLDL biosynthesis is divided into two main steps. Fist, ApoB-100 is co-translationally lipidated as it is translocated into the ER by MTP to form a VLDL precursor [14, 17]. In the second step, VLDL precursors are further enriched in lipids to form mature VLDL, which are secreted through the Golgi apparatus [93, 94]. This mechanism is not well understood. A model proposes that VLDL precursors could acquire lipids via fusion with ER-resident ApoE and ApoC-containing LD [17, 95, 96].
Although widely studied, the precise role of apolipoproteins in the HCV morphogenesis and infectivity is still controversial, notably the functional importance of ApoB. Some groups reported that virus production could be blocked by MTP inhibitors or siRNA-mediated knockdown of ApoB expression [31, 52]. Moreover, Icard et al. demonstrated that secretion of HCV envelope glycoproteins depends on MTP and on the assembly of ApoB-containing lipoproteins [97]. By contrast, Jiang and Luo showed that HCV assembly was not dependent on ApoB expression but was highly dependent on ApoE [63]. Accordingly, Coller and collaborators observed that the majority of HCV core proteins traffic through the secretory pathway in association with ApoE, but not with ApoB [98]. The observed differences might be due, in part, to the use of different models. It is important to remind that Huh-7 cells, which robustly support HCV replication, do not produce authentic VLDL but rather underlipidated VLDL-like particles [36, 37]. The group of Majano raised a novel hypothesis. In a study published in 2015, they demonstrated that ApoB is required for the assembly of cell-free infective HCV particles whereas ApoE, but not ApoB is essential for efficient cell-to-cell transmission of HCV. Thus, the authors suggest the existence of mechanisms involving VLDL components that differentially regulate cell-free and cell-to-cell HCV transmission [99].
ApoE appears to play a critical role in LVP assembly [61–63]. However, how ApoE contributes to virion formation and how it remains associated with HCV particles is still unclear. In 2010, the interaction of ApoE with the viral protein NS5A was identified as a crucial interplay for viral assembly, suggesting a potential role of NS5A in the recruitment of ApoE to the viral assembly sites [53, 100]. Moreover, in 2014, two studies evidenced the initial contact between HCV components and ApoE during HCV morphogenesis. The authors demonstrated that viral glycoproteins E1 and most notably E2 associate with ApoE in the ER. This complex may initiate LVP morphogenesis. Nascent LVP might then be lipidated along the VLDL pathway in the ER to generate mature infectious LVP [55, 56]. The ApoE-E2 complex is detected at the surface of extracellular mature LVP. A study performed in our lab showed that this association helps the virus avoid neutralization by anti-HCV antibodies isolated from chronically infected patients. Indeed, we demonstrated that E2 conformational epitopes are exposed after ApoE deletion and are more sensitive to patients neutralizing antibodies. This finding could be relevant for vaccine design: immunogens that mimic epitopes at the E2/ApoE interface might help to achieve an efficient humoral immune response [101]. To summarize, ApoE is a key actor in HCV morphogenesis and in viral escape of host immunity.
3.6. Redundant role of exchangeable apolipoproteins in LVP morphogenesis
Previously, we described the importance of ApoE in HCV morphogenesis. However, it was reported that ApoA and ApoC also participate in infectious LVP production, suggesting that several apolipoproteins are involved in this process [32, 33, 79, 84, 90].
ApoA, ApoC and ApoE belong to the family of exchangeable apolipoproteins that are able to dissociate from one lipoprotein and reassociate with another in the circulation [9]. The exchangeability of apolipoproteins is attributable to their high content in α-helical structure, which confers the ability to switch easily from a lipid-free to a lipid-bound form [102]. Accumulating experimental evidence suggests that ApoA, ApoC and ApoE have an important intracellular impact on lipoprotein assembly and secretion. Moreover, three-dimensional structural analyses revealed similarities in exchangeable apolipoproteins structure suggesting that they could redundantly participate to lipoprotein formation [9].
Based on this knowledge, Fukuhara and collaborators investigated the role of these apolipoproteins in LVP morphogenesis. For this, they used Huh7 cells knocked down for both APOB and APOE genes. According to previous studies, they observed that infectious HCV particle formation in these cells was severely impaired. Very interestingly, HCV particle formation was partially restored not only by ApoE expression but also by other exchangeable apolipoproteins including ApoA-I, ApoA-II, ApoC-I, ApoC-II and ApoC-III. Moreover, they showed that the exogenous expression of only short sequences containing amphipathic α-helices from these apolipoproteins is sufficient to restore the formation of infectious HCV particles. Consistent with this finding, ApoM, ApoH and ApoD, other apolipoproteins lacking amphipathic α-helices, were not able to rescue infectious virion production [103]. In 2015, Hueging et al. corroborated this study by demonstrating that all ApoE-related exchangeable apolipoproteins belonging to the ApoA and ApoC family could partially take over the role of ApoE in HCV morphogenesis [104]. Finally, a study performed by the group Matsuuraa supported the importance of α-helix domains for HCV particle assembly. Indeed, they demonstrated that the human cathelicidin antimicrobial peptide (CAMP), a protein containing amphipathic α-helices, is able to rescue HCV infectious particle formation, raising the possibility of extra-hepatic propagation of HCV in cells with low-level or no expression of apolipoproteins [105]. To summarize, exchangeable apolipoproteins possess redundant roles in the assembly of HCV through the interaction of the amphipathic α-helices with viral particles.
3.7. Role of apolipoproteins in LVP maturation
Indubitably, maturation and release of HCV particles, the late assembly stage, appears to be strictly linked to the VLDL pathway [31]. However, it is still enigmatic how apolipoproteins are incorporated into mature LVP. Bartenschlager et al. propose the following model, illustrated in Figure 5C. During its translation, nascent ApoB is translocated into the ER lumen and lipidated by MTP to generate VLDL precursor. In parallel, luminal LD (luLD) associated with resident ApoE and ApoC are formed from the ER membrane also through an MTP dependent pathway. Viral glycoproteins E1 and E2, retained at the ER membrane, then associate with these luLD. The nucleocapsid would be inserted into the lipid core of the luLD due to its hydrophobic properties. Then, lipidated and enveloped HCV particles could fuse with VLDL precursors to generate LVP. Alternatively, in Huh7 cell where the VLDL pathway is impaired, HCVcc could be secreted predominantly as lipidated particles lacking ApoB [39]. Consistent with this model, Hueging et al showed that ApoE plays a role in HCV assembly in a post-envelopment step [104]. This would imply an ApoE-HCV particle interaction in a post-budding step possibly by direct envelope-ApoE interaction as it was previously reported [55, 56].
Mature LVP are considerably enriched in ApoE as compared to normal VLDL. Indeed, purified VLDL-LVP bear approximately 300 molecules of ApoE, whereas normal VLDL contains only five to seven molecules [34, 106]. Moreover, it was reported that the ApoE content in LVP positively correlates with HCV infectivity [62]. How can LVP be enriched in ApoE? As is the case for lipoprotein remnants in blood circulation, LVP can acquire exchangeable ApoE extracellularly. A recent study showed that ApoE exchange occurs between LVP and lipoproteins. This process is important to maintain the high ApoE level on LVP required for an efficient attachment to cell surface HSPG [107]. These data highlight the role of extracellular ApoE in HCV infection and suggest an active exchange of apolipoproteins between LVP and lipoproteins, which could explain the heterogeneity in LVP composition.
4. Clinical implications
4.1. Chronic hepatitis C disturbed lipoprotein metabolism
The abnormal accumulation of fat in the liver, also named steatosis, is a common feature of chronic HCV infection. It appears to be a direct consequence of viral protein expression in HCV genotype 3 infections whereas it is associated with lipid metabolism disorders in other genotype infections. Disturbed lipid metabolism in chronically infected patients leads to liver functions deterioration. Indeed, steatosis associates with chronic inflammation and may contribute to cirrhosis and hepatocellular carcinoma development [4]. Therefore, correction of disturbed lipid metabolism is important for preventing the progression of HCV-related liver diseases. HCV modulates lipid homeostasis by increasing lipogenesis and reducing lipid oxidation and export, leading to lipid accumulation in cells. Moreover, HCV infection is associated with low LDLcholesterol level and reduced ApoB-containing lipoproteins level [4, 108]. In addition, it was reported that HCV infection disturbs serum apolipoprotein levels, depending of the genotype. Genotype 1b infections were found to be associated with higher levels of ApoA-II and ApoE and lower levels of ApoC-II and ApoC-III, while genotype 2 infections were associated only with lower levels of ApoC-II and ApoC-III [109]. As explained previously, ApoC-II is a LPL activator that mediates inhibition of HCV infection by inducing hydrolysis of LVP [80–83]. As a consequence, a decline in serum ApoC-II in infected patients alone promotes HCV infection and was also correlated to advanced liver fibrosis [109]. Together, these data show that the HCV-induced deregulation of lipoprotein and apolipoprotein metabolism is involved in progression of liver diseases.
The severity of altered lipid metabolism also depends on host IL28B gene polymorphism. In 2009, two studies have reported that a single nucleotide polymorphism (SNP) (rs12979860) upstream of the IL28B gene encoding IFN-λ3, is strongly associated with response to IFN in patients. The IL28B non-responder genotype has been associated with an increased ISG gene expression, which explains IFNα insensitivity [110, 111]. Moreover, Li et al. observed that the rs12979860 CC responder genotype was significantly associated with higher levels of ApoB-containing lipoproteins, suggesting that these lipoproteins might regulate IFN response [112]. Indeed, retrospective studies have showed that higher ApoB-containing lipoproteins (VLDL/LDL) level is positively associated with treatment outcome in chronic hepatitis C patients receiving antiviral therapy [113, 114]. Interestingly, another study implicated LVP-associated ApoE in IFN sensitivity. It showed that a complete sustained virological response (SVR) in HCV-infected patients was associated with a lower serum ApoE [115]. These studies suggest that ApoB-containing lipoprotein and ApoE affect ISG expression and thus improve the response to antiviral-therapy. Modulation of lipid metabolism could be used to restore IFN sensitivity, to limit HCV propagation and to minimize resistance to DAA in a combination therapy.
4.2. Target lipoproteins/apolipoproteins metabolism to improve HCV-related liver diseases progression
Over the past years, therapy for HCV has undergone a revolution. With the approval of novel DAA, such as Sofosbuvir (Gilead), chronic hepatitis C has become a curable disease in the majority of treated patients. However several challenges remain: high costs limit access to therapy, and certain difficult-to-treat patients may need adjunctive therapeutic approaches [1, 116]. Moreover, despite the high SVR rates obtained with DAA, drug resistance has recently emerged [117]. Host-targeting agents (HTA) offer a prospective option to prevent viral resistance. Indeed, by targeting host proteins that contribute to the HCV life cycle, HTA have pangenotypic antiviral activity and confer a high barrier to resistance [2]. As described herein, HCV co-opts the VLDL pathway for assembly, secretion and entry into hepatocytes. Moreover, apolipoproteins play a crucial role at all these steps, in viral escape of host immunity. The close relationship between apolipoproteins and the HCV life cycle generates opportunities for new therapeutic options and vaccine design.
An antisense inhibitor of ApoB synthesis, named Mipomersen, has been shown to be effective and safe as an agent to lower LDL in patients with familial hypercholesterolaemia. Mipomersen is a sequence of nucleotides complementary to a section of ApoB mRNA, which inhibits the translation into a mature protein. This results in a strong inhibition of VLDL synthesis [118, 119]. Recently, it was shown that inhibition of ApoB with Mipomersen blocks LVP morphogenesis in vitro. Targeting ApoB represents a novel host targeted strategy to inhibit HCV [120]. Accordingly, another study demonstrated the ability of Amiodarone, a cationic amphipathic drug that inhibits MTP activity and thus ApoB lipidation, to down-regulate HCV assembly and release in cell culture [121]. However, whether Mipomersen and Amiodarone display an antiviral effect in vivo remains to be determined. Furthermore, a recent study reports that Amiodarone could induce bradycardia in patients when this drug is administered in combination with DAA, indicating that the potential drug-drug interactions must be carefully evaluated before Amiodarone administration [122].
ApoE appears to have multiple functions in the HCV life cycle. ApoE facilitates HCV entry by interacting with several receptors (HSPG, LDLR, SR-BI), participates to HCV morphogenesis and LVP maturation, and helps the virus escape immune responses. A human ApoE derived-peptide, was shown to specifically block both HCVcc and patients-derived HCV binding on hepatoma cells and primary human hepatocytes [58, 61]. The use of such a peptide could be an excellent strategy to block HCV propagation. Moreover, Crouchet et al. demonstrated that lipid-free ApoE inhibits HCV replication in cell culture by regulating lipid metabolism [87]. The use of an ApoEderived peptide that mimics the effect of lipid-free ApoE on hepatic cells could be envisaged. However, it was also demonstrated that lipid-free ApoE facilitates HCV entry by interacting with LVP [107]. Thus, much remains to be done before using ApoE against the virus itself.
Finally, several other apolipoproteins have been shown to participate in the formation of LVP. Molecules targeting these apolipoproteins are all potential inhibitors of HCV. However, since it has been reported that exchangeable apolipoproteins play redundant roles in HCV particle formation [103, 104], the suppression of only some apolipoproteins may be insufficient to have an efficient antiviral activity. Because exchangeable apolipoproteins are suggested to stabilize LVP through their interaction with amphipathic α-helices, the dissociation of apolipoproteins from LVP by targeting these domains might be an alternative strategy. Indeed, Cheng et al. have designed an amphipathic -helical peptide derived from the membrane anchor domain of the HCV NS5A protein that suppress HCV infectivity by destabilizing the viral envelope, offering a potential therapeutic approach against HCV [121].
5. Conclusion
The hallmark of HCV is its association with lipoproteins and apolipoproteins, which affects the way HCV interacts with the host cell from the entry step to the generation of infectious particles. Indeed, HCV co-opts the VLDL pathway for assembly, secretion and entry into hepatocytes. Moreover, the unique interaction of HCV with apolipoproteins helps the virus escape innate (IFN) and acquired (neutralizing antibodies) immune responses and propagates into hepatocytes to establish persistent infection. Thus, apolipoproteins remain targets for the development of new antivirals and vaccines. However, further studies are needed to deeply investigate the role of apolipoproteins in HCV life cycle.
6. Expert commentary
Within the past years, therapy for HCV has undergone a revolution with the development of DAA. However, despite effective cure in the large majority of infected patients several challenges remain. Notably, high costs of DAA limit access to therapy and an urgently needed HCV vaccine remains still elusive. Moreover, drug resistance is a challenge in fraction of patients including genotype 3 [116]. HCV exists as a heterogenous population of genetic variants in infected patients. Due to the high error rate of HCV polymerase and high rate of virion production, viral variants resistant to DAA can exist naturally at low levels prior to treatment and may get selected upon drug exposure. Thus, the clinical implication is resistance to DAA and treatment failure [123]. In contrast to DAA that target viral proteins, host-targeting agents interfere with cellular factors involved in the viral life cycle. Thus, HTA offer a promising option to prevent and treat viral resistance by exhibiting a high barrier to resistance and a pangenotypic antiviral activity. Used in combination, HTA and DAA could act synergistically to reduce viral loads [2]. As described previously, HCV coopts VLDL pathway for, assembly, secretion and entry into hepatocytes.
Moreover, the HCV-induced deregulation of lipoprotein metabolism play a role in the development of liver diseases such as NASH and liver cancer. Thus, the close relationship between apolipoproteins and the HCV life cycle generates opportunities of new therapeutic options and vaccine design. IFN-free regimens are likely to dominate the HCV therapeutic landscape within the next 5 years. Several HTA targeting apolipoproteins and lipid metabolism are currently in development in clinical trials. These include drugs already in use or in development for other indications (e.g. statins, niacin, CETP inhibitors, ApoB antisense, miRNA-122 inhibitors) but also entry inhibitors (e.g. SR-BI antagonists) and novel targets (e.g. DGAT inhibitors) [2]. The main challenge will be to identify the right combination of HTA/DAA with the highest efficacy, the highest barrier to resistance and with lowest side effects.
To conclude, the investigation of HCV-lipoproteins interactions offers new perspectives for novel therapeutic approaches, contribute to HCV vaccine design and understand virus-induced liver disease and cancer. However important questions remain. Notably, how are apolipoproteins involved in the exchange of the lipidic part of the LVP in the different stages of the viral entry? How apolipoproteins are precisely involved in regulation of HCV replication? How LVP are assembled and how apolipoproteins participate to LVP morphogenesis? What is exactly the role of apolipoproteins in disease progression? Further studies are needed to deeply investigate the role of apolipoproteins in HCV life cycle.
7. Five-year view
Since 2015, HCV therapy has evolved in multiple aspects. DAA development has led to cure rates of 90–95% with shorter duration and low toxicity regimens. Currently, researchers aim to improve the available treatments. New generations of DAA and HTA will enter clinical development within the next 2–5 years to increase treatment potency, pangenotypic antiviral activity and barriers to resistance. Moreover, all-oral, IFN-free strategies based on the combination of several drugs are now investigated in phase III trials. Thus, IFN-free regimens are likely to dominate the HCV therapeutic landscape within the next 5 years. However, despite promising results, the cost of these therapies represents a limiting factor to their use. Most HCVinfected patients live in areas with limited resources and where new therapies are not available. Manufacturers have to make more effort to produce low-cost drugs available for all. To summarize, the ideal oral drug combination for HCV cure has not been found yet, but can be expected over the next 5–10 years.
8. Key issues.
HCV life cycle is closely linked to hepatic lipid and lipoprotein metabolisms.
The infectious viral particle consists in a hybrid complex between putative HCV particleand lipoproteins, such as VLDL and LDL, named lipoviroparticle (LVP) Therefore, HCV isstrongly associated with apolipoproteins.
Apolipoproteins represent the protein moiety of lipoproteins. They are critical regulators of lipoprotein metabolism, by carrying out three major functions: (i) they stabilize the lipoprotein structure; (ii) they act as ligands for lipoprotein receptors and are involved in lipoproteins clearance, (iii) they serve as regulators of key enzymes involved in lipoprotein metabolism.
Apolipoproteins also play crucial role in HCV life cycle. Notably, ApoE is involved in HCV attachment and entry in hepatocytes through interaction with specific apolipoprotein receptors. ApoA-I, ApoC-I and ApoC-III facilitates HCV infection, whereas ApoC-II is a natural inhibitor of HCV entry. Similarly, ApoB and ApoE are important for LVP morphogenesis and maturation. ApoE also play a role in HCV escape from host immunity. Finally, ApoE and ApoA-I regulates HCV replication by regulating hepatic lipid metabolism.
HCV disturbs lipoprotein metabolism to promote its propagation in hepatocytes. This deregulation is involved in development of HCV-related liver diseases such as NASH and liver cancer.
The unique link between HCV and apolipoproteins offers new perspectives to develop new therapeutic options. Indeed, host-targeting agents directed against apolipoproteins may widen the therapeutic arsenal against chronic HCV infection.
Acknowledgments
Funding
This paper was not funded.
List of abbreviations:
- ABCA1
ATP-binding cassette transporter A1
- ABCG1
ATP-binding cassette transporter G1
- ACAT
acyl-CoA-cholesterol acyltransferase
- Apo
apolipoproteins
- APOBEC1
ApoB mRNA editing complex 1
- CAMP
cathelicidin antimicrobial peptide
- CE
cholesterol ester
- CETP
CE transport protein
- CLDN1
claudin 1
- CM
chylomicrons (CM)
- cPLA2G4A
cytosolic phospholipase A2 group IVA
- DAA
direct-acting antivirals
- DGAT
diglyceride acyltransferase
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- FABP
fatty acid binding protein
- FC
free cholesterol
- FFA
free fatty acids
- HCV
hepatitis C virus (HCV)
- HCVcc
HCV cell culture system
- HDL
high-density lipoproteins
- HL
hepatic lipase
- HS
heparan sulfate
- HSPG
heparan sulfate proteoglycan
- HTA
host-targeting agents
- Huh
hepatocarcinoma
- IDL
intermediate-density lipoproteins
- IRES
internal ribosome entry site
- LCAT
lecithin cholesterol acyltransferase
- LD
lipid droplets
- LDL
low-density lipoproteins
- LDLR
LDL receptor
- LPL
lipoprotein lipase
- LRP1
LDLR related protein 1
- luLD
luminal LD
- LVP
lipo-viro-particles
- MGAT
monoacylglycerol acyltransferase
- MTP
microsomal transfer protein
- MW
membranous web
- NASH
nonalcoholic steato-hepatitis
- NPC1L1
Niemann-Pick C1-like 1 protein
- NTR
non-translated region
- OLCN
occludin
- PEG-IFNα
pegylated interferon alpha
- RBV
ribavirin
- RCT
reverse cholesterol transport
- SDC
syndecans
- SNP
single nucleotide polymorphism
- SR-BI
scavenger receptor BI
- SVR
sustained virological response
- TG
triglycerides
- TRL
triglyceride-rich lipoproteins
- VLDL
very low-density lipoproteins
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- [1].Chung RT, Baumert TF. Curing Chronic Hepatitis C — The Arc of a Medical Triumph. New England Journal of Medicine. 2014;370:1576–1578. [DOI] [PubMed] [Google Scholar]
- [2].Zeisel MB, Crouchet E, Baumert TF, et al. Host-Targeting Agents to Prevent and Cure Hepatitis C Virus Infection. Viruses. 2015;7:5659–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[3].Felmlee DJ, Hafirassou ML, Lefevre M, et al. Hepatitis C Virus, Cholesterol and Lipoproteins — Impact for the Viral Life Cycle and Pathogenesis of Liver Disease. Viruses. 2013;5:1292–1324. (Summarizes the relationships between lipoproteins, cholesterol metabolism and the viral cycle of HCV) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Negro F, Sanyal AJ. Hepatitis C virus, steatosis and lipid abnormalities: clinical and pathogenic data. Liver International. 2009;29:26–37. [DOI] [PubMed] [Google Scholar]
- [5].Wasan KM, Brocks DR, Lee SD, et al. Impact of lipoproteins on the biological activity and disposition of hydrophobic drugs: implications for drug discovery. Nat Rev Drug Discov. 2008;7:84–99. [DOI] [PubMed] [Google Scholar]
- [6].Hegele RA. Plasma lipoproteins: genetic influences and clinical implications. Nat. Rev. Genet 2009;10:109–121. [DOI] [PubMed] [Google Scholar]
- [7].Aizawa Y, Seki N, Nagano T, et al. Chronic hepatitis C virus infection and lipoprotein metabolism. World J. Gastroenterol 2015;21:10299–10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Feingold KR, Grunfeld C. Introduction to Lipids and Lipoproteins. In: De Groot LJ, BeckPeccoz P, Chrousos G, et al. , editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc; 2000. [cited 2016 Jul 4]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK305896/. [Google Scholar]
- [9].Sundaram M, Yao Z. Intrahepatic role of exchangeable apolipoproteins in lipoprotein assembly and secretion. Arterioscler. Thromb. Vasc. Biol 2012;32:1073–1078. [DOI] [PubMed] [Google Scholar]
- [10].Ramasamy I Recent advances in physiological lipoprotein metabolism. Clin. Chem. Lab. Med 2014;52:1695–1727. [DOI] [PubMed] [Google Scholar]
- [11].Wang S, Smith JD. ABCA1 and nascent HDL biogenesis. Biofactors. 2014;40:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zannis VI, Fotakis P, Koukos G, et al. HDL biogenesis, remodeling, and catabolism. Handb Exp Pharmacol. 2015;224:53–111. [DOI] [PubMed] [Google Scholar]
- [13].Kohan AB, Wang F, Lo C-M, et al. ApoA-IV: current and emerging roles in intestinal lipid metabolism, glucose homeostasis, and satiety. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2015;308:G472–G481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xiao C, Hsieh J, Adeli K, et al. Gut-liver interaction in triglyceride-rich lipoprotein metabolism. Am. J. Physiol. Endocrinol. Metab 2011;301:E429–E446. [DOI] [PubMed] [Google Scholar]
- [15].Merkel M, Heeren J. Give me A5 for lipoprotein hydrolysis J Clin Invest. 2005;115:2694–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Blade AM, Fabritius MA, Hou L, et al. Biogenesis of apolipoprotein A-V and its impact on VLDL triglyceride secretion. J Lipid Res. 2011;52:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Davidson NO, Shelness GS. APOLIPOPROTEIN B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu. Rev. Nutr 2000;20:169–193. [DOI] [PubMed] [Google Scholar]
- [18].Giammanco A, Cefalù AB, Noto D, et al. The pathophysiology of intestinal lipoprotein production. Front Physiol [Internet]. 2015. [cited 2017 Feb 18];6. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4367171/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jong MC, Hofker MH, Havekes LM. Role of ApoCs in Lipoprotein Metabolism. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:472–484. [DOI] [PubMed] [Google Scholar]
- [20].Metabolism Morita S. and Modification of Apolipoprotein B-Containing Lipoproteins Involved in Dyslipidemia and Atherosclerosis. Biol. Pharm. Bull 2016;39:1–24. [DOI] [PubMed] [Google Scholar]
- [21].Shachter NS. Apolipoproteins C-I and C-III as important modulators of lipoprotein metabolism. Curr. Opin. Lipidol 2001;12:297–304. [DOI] [PubMed] [Google Scholar]
- [22].Getz GS, Reardon CA. Apoprotein E as a lipid transport and signaling protein in the blood, liver, and artery wall. J Lipid Res. 2009;50:S156–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen J, Li Q, Wang J. Topology of human apolipoprotein E3 uniquely regulates its diverse biological functions. Proc Natl Acad Sci U S A. 2011;108:14813–14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J. Mol. Med 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gylling H Cholesterol metabolism and its implications for therapeutic interventions in patients with hypercholesterolaemia. International Journal of Clinical Practice. 2004;58:859–866. [DOI] [PubMed] [Google Scholar]
- [26].Kontush A, Lindahl M, Lhomme M, et al. Structure of HDL: particle subclasses and molecular components. Handb Exp Pharmacol. 2015;224:3–51. [DOI] [PubMed] [Google Scholar]
- [27].Moradpour D, Penin F. Hepatitis C virus proteins: from structure to function. Curr. Top. Microbiol. Immunol 2013;369:113–142. [DOI] [PubMed] [Google Scholar]
- [28].Thomssen R, Bonk S, Propfe C, et al. Association of hepatitis C virus in human sera with beta-lipoprotein. Med. Microbiol. Immunol 1992;181:293–300. [DOI] [PubMed] [Google Scholar]
- **[29].André P, Komurian-Pradel F, Deforges S, et al. Characterization of low- and very-lowdensity hepatitis C virus RNA-containing particles. J. Virol 2002;76:6919–6928. (This article demonstrated that the infectivity of LVP is mediated by hosts proteins rather than by viral components and provided a mechanism of escape from the humoral immune response). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nielsen SU, Bassendine MF, Burt AD, et al. Association between hepatitis C virus and verylow-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J. Virol 2006;80:2418–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[31].Gastaminza P, Cheng G, Wieland S, et al. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J. Virol 2008;82:2120–2129. (This study shows that HCV coopts the VLDL assembly, maturation, degradation, and secretory machinery.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Meunier J-C, Russell RS, Engle RE, et al. Apolipoprotein C1 Association with Hepatitis C Virus. J. Virol 2008;82:9647–9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Catanese MT, Uryu K, Kopp M, et al. Ultrastructural analysis of hepatitis C virus particles. Proc. Natl. Acad. Sci. U.S.A 2013;110:9505–9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Merz A, Long G, Hiet M-S, et al. Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. J. Biol. Chem 2011;286:3018–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[35].Gastaminza P, Kapadia SB, Chisari FV. Differential biophysical properties of infectious intracellular and secreted hepatitis C virus particles. J. Virol 2006;80:11074–11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lindenbach BD, Meuleman P, Ploss A, et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc. Natl. Acad. Sci. U.S.A 2006;103:3805–3809. (These two studies demonstrated that HCV-derived patients and HCVcc have different properties). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Podevin P, Carpentier A, Pène V, et al. Production of infectious hepatitis C virus in primary cultures of human adult hepatocytes. Gastroenterology. 2010;139:1355–1364. [DOI] [PubMed] [Google Scholar]
- [38].Jammart B, Michelet M, Pécheur E-I, et al. Very-low-density lipoprotein (VLDL)-producing and hepatitis C virus-replicating HepG2 cells secrete no more lipoviroparticles than VLDLdeficient Huh7.5 cells. J. Virol 2013;87:5065–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bartenschlager R, Penin F, Lohmann V, et al. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 2011;19:95–103. [DOI] [PubMed] [Google Scholar]
- [40].Lindenbach BD. Virion Assembly and Release. Curr Top Microbiol Immunol. 2013;369:199–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Diaz O, Delers F, Maynard M, et al. Preferential association of Hepatitis C virus with apolipoprotein B48-containing lipoproteins. J Gen Virol. 2006;87:2983–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[42].Felmlee DJ, Sheridan DA, Bridge SH, et al. Intravascular Transfer Contributes to Postprandial Increase in Numbers of Very-Low-Density Hepatitis C Virus Particles. Gastroenterology. 2010;139:1774–1783.e6 (This article suggests that HCV could be transiently associated with lipoproteins). [DOI] [PubMed] [Google Scholar]
- **[43].Piver E, Boyer A, Gaillard J, et al. Ultrastructural organisation of HCV from the bloodstream of infected patients revealed by electron microscopy after specific immunocapture. Gut. 2016. (This study provides information about the precise morphological organisation of viral particles) [DOI] [PubMed] [Google Scholar]
- [44].Zeisel MB, Felmlee DJ, Baumert TF. Hepatitis C virus entry. Curr. Top. Microbiol. Immunol 2013;369:87–112. [DOI] [PubMed] [Google Scholar]
- [45].Niepmann M Hepatitis C virus RNA translation. Curr. Top. Microbiol. Immunol 2013;369:143–166. [DOI] [PubMed] [Google Scholar]
- [46].Paul D, Madan V, Bartenschlager R. Hepatitis C Virus RNA Replication and Assembly: Living on the Fat of the Land. Cell Host & Microbe. 2014;16:569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[47].Miyanari Y, Atsuzawa K, Usuda N, et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol 2007;9:1089–1097. (First evidences indicating that HCV assembly is dependent of hepatic lipid droplets). [DOI] [PubMed] [Google Scholar]
- [48].Herker E, Harris C, Hernandez C, et al. Efficient Hepatitis C Virus Particle Formation Requires Diacylglycerol Acyltransferase 1 (DGAT1). Nat Med. 2010;16:1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Menzel N, Fischl W, Hueging K, et al. MAP-Kinase Regulated Cytosolic Phospholipase A2 Activity Is Essential for Production of Infectious Hepatitis C Virus Particles. PLOS Pathog. 2012;8:e1002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Boulant S, Targett-Adams P, McLauchlan J. Disrupting the association of hepatitis C virus core protein with lipid droplets correlates with a loss in production of infectious virus. J. Gen. Virol 2007;88:2204–2213. [DOI] [PubMed] [Google Scholar]
- [51].Counihan NA, Rawlinson SM, Lindenbach BD. Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly. PLOS Pathog. 2011;7:e1002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Huang H, Sun F, Owen DM, et al. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc. Natl. Acad. Sci. U.S.A 2007;104:5848–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Benga WJA, Krieger SE, Dimitrova M, et al. Apolipoprotein E interacts with hepatitis C virus nonstructural protein 5A and determines assembly of infectious particles. Hepatology. 2010;51:43–53. [DOI] [PubMed] [Google Scholar]
- [54].Jiang J, Cun W, Wu X, et al. Hepatitis C Virus Attachment Mediated by Apolipoprotein E Binding to Cell Surface Heparan Sulfate. J Virol. 2012;86:7256–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Boyer A, Dumans A, Beaumont E, et al. The Association of Hepatitis C Virus Glycoproteins with Apolipoproteins E and B Early in Assembly Is Conserved in Lipoviral Particles. J. Biol. Chem 2014;289:18904–18913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lee J-Y, Acosta EG, Stoeck IK, et al. Apolipoprotein E likely contributes to a maturation step of infectious hepatitis C virus particles and interacts with viral envelope glycoproteins. J. Virol 2014;88:12422–12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Grassi G, Di Caprio G, Fimia GM, et al. Hepatitis C virus relies on lipoproteins for its life cycle. World J Gastroenterol. 2016;22:1953–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu S, McCormick KD, Zhao W, et al. Human apolipoprotein E peptides inhibit hepatitis C virus entry by blocking virus binding. Hepatology. 2012;56:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xu Y, Martinez P, Séron K, et al. Characterization of hepatitis C virus interaction with heparan sulfate proteoglycans. J. Virol 2015;89:3846–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shi Q, Jiang J, Luo G. Syndecan-1 Serves as the Major Receptor for Attachment of Hepatitis C Virus to the Surfaces of Hepatocytes. J Virol. 2013;87:6866–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lefèvre M, Felmlee DJ, Parnot M, et al. Syndecan 4 is involved in mediating HCV entry through interaction with lipoviral particle-associated apolipoprotein E. PLoS ONE. 2014;9:e95550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[62].Chang K-S, Jiang J, Cai Z, et al. Human apolipoprotein e is required for infectivity and production of hepatitis C virus in cell culture. J. Virol 2007;81:13783–13793. (Identification of apoE as a novel host factor involved in HCV morphogenesis) [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[63].Jiang J, Luo G. Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J. Virol 2009;83:12680–12691. (Controversial study demonstrating that ApoB is not required for infectious LVP formation). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Agnello V, Ábel G, Elfahal M, et al. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci U S A 1999;96:12766–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Molina S, Castet V, Fournier-Wirth C, et al. The low-density lipoprotein receptor plays a role in the infection of primary human hepatocytes by hepatitis C virus. J. Hepatol 2007;46:411–419. [DOI] [PubMed] [Google Scholar]
- [66].Owen DM, Huang H, Ye J, et al. Apolipoprotein E on Hepatitis C Virion Facilitates Infection through Interaction with Low Density Lipoprotein Receptor. Virology. 2009;394:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Albecka A, Belouzard S, Op de Beeck A, et al. Role of low-density lipoprotein receptor in the hepatitis C virus life cycle. Hepatology. 2012;55:998–1007. [DOI] [PubMed] [Google Scholar]
- [68].Scarselli E, Ansuini H, Cerino R, et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Maillard P, Huby T, Andréo U, et al. The interaction of natural hepatitis C virus with human scavenger receptor SR-BI/Cla1 is mediated by ApoB-containing lipoproteins. FASEB J [Internet]. 2006. [cited 2017 Mar 2]; Available from: http://www.fasebj.org/content/early/2006/04/01/fj.054728fje [DOI] [PubMed] [Google Scholar]
- [70].Dao Thi VL, Granier C, Zeisel MB, et al. Characterization of hepatitis C virus particle subpopulations reveals multiple usage of the scavenger receptor BI for entry steps. J. Biol. Chem 2012;287:31242–31257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zahid MN, Turek M, Xiao F, et al. The postbinding activity of scavenger receptor class B type I mediates initiation of hepatitis C virus infection and viral dissemination. Hepatology. 2013;57:492–504. [DOI] [PubMed] [Google Scholar]
- [72].Dreux M, Thi VLD, Fresquet J, et al. Receptor Complementation and Mutagenesis Reveal SR-BI as an Essential HCV Entry Factor and Functionally Imply Its Intra- and Extra-Cellular Domains. PLOS Pathogens. 2009;5:e1000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bartosch B, Verney G, Dreux M, et al. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J. Virol 2005;79:8217–8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Voisset C, Callens N, Blanchard E, et al. High density lipoproteins facilitate hepatitis C virus entry through the scavenger receptor class B type I. J. Biol. Chem 2005;280:7793–7799. [DOI] [PubMed] [Google Scholar]
- [75].Dreux M, Boson B, Ricard-Blum S, et al. The exchangeable apolipoprotein ApoC-I promotes membrane fusion of hepatitis C virus. J. Biol. Chem 2007;282:32357–32369. [DOI] [PubMed] [Google Scholar]
- [76].Kapadia SB, Barth H, Baumert T, et al. Initiation of Hepatitis C Virus Infection Is Dependent on Cholesterol and Cooperativity between CD81 and Scavenger Receptor B Type I. J. Virol 2007;81:374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zeisel MB, Koutsoudakis G, Schnober EK, et al. Scavenger receptor class B type I is a key host factor for hepatitis C virus infection required for an entry step closely linked to CD81. Hepatology. 2007;46:1722–1731. [DOI] [PubMed] [Google Scholar]
- [78].Meunier J-C, Engle RE, Faulk K, et al. Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1. PNAS. 2005;102:4560–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Dreux M, Pietschmann T, Granier C, et al. High density lipoprotein inhibits hepatitis C virusneutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J. Biol. Chem 2006;281:18285–18295. [DOI] [PubMed] [Google Scholar]
- [80].Thomssen R, Bonk S. Virolytic action of lipoprotein lipase on hepatitis C virus in human sera. Med. Microbiol. Immunol 2002;191:17–24. [DOI] [PubMed] [Google Scholar]
- [81].Shimizu Y, Hishiki T, Sugiyama K, et al. Lipoprotein lipase and hepatic triglyceride lipase reduce the infectivity of hepatitis C virus (HCV) through their catalytic activities on HCVassociated lipoproteins. Virology. 2010;407:152–159. [DOI] [PubMed] [Google Scholar]
- [82].Andréo U, Maillard P, Kalinina O, et al. Lipoprotein lipase mediates hepatitis C virus (HCV) cell entry and inhibits HCV infection. Cell. Microbiol 2007;9:2445–2456. [DOI] [PubMed] [Google Scholar]
- [83].Maillard P, Walic M, Meuleman P, et al. Lipoprotein Lipase Inhibits Hepatitis C Virus (HCV) Infection by Blocking Virus Cell Entry. PLOS ONE. 2011;6:e26637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sun H-Y, Lin C-C, Lee J-C, et al. Very low-density lipoprotein/lipo-viro particles reverse lipoprotein lipase-mediated inhibition of hepatitis C virus infection via apolipoprotein C-III. Gut. 2013;62:1193–1203. [DOI] [PubMed] [Google Scholar]
- [85].Shi ST, Lee K- J, Aizaki H, et al. Hepatitis C Virus RNA Replication Occurs on a DetergentResistant Membrane That Cofractionates with Caveolin-2. J. Virol 2003;77:4160–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sagan SM, Rouleau Y, Leggiadro C, et al. The influence of cholesterol and lipid metabolism on host cell structure and hepatitis C virus replication. Biochem. Cell Biol 2006;84:67–79. [DOI] [PubMed] [Google Scholar]
- *[87].Crouchet E, Lefèvre M, Verrier ER, et al. Extracellular lipid-free apolipoprotein E inhibits HCV replication and induces ABCG1-dependent cholesterol efflux. Gut. 2016. (Highlights a new role of ApoE in HCV life cycle). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Vedhachalam C, Narayanaswami V, Neto N, et al. The C-terminal lipid-binding domain of apolipoprotein E is a highly efficient mediator of ABCA1-dependent cholesterol efflux that promotes the assembly of high-density lipoproteins. Biochemistry. 2007;46:2583–2593. [DOI] [PubMed] [Google Scholar]
- [89].Yancey PG, Yu H, Linton MF, et al. A pathway-dependent on apoE, ApoAI, and ABCA1 determines formation of buoyant high-density lipoprotein by macrophage foam cells. Arterioscler. Thromb. Vasc. Biol 2007;27:1123–1131. [DOI] [PubMed] [Google Scholar]
- [90].Mancone C, Steindler C, Santangelo L, et al. Hepatitis C virus production requires apolipoprotein A-I and affects its association with nascent low-density lipoproteins. Gut. 2011;60:378–386. [DOI] [PubMed] [Google Scholar]
- [91].Sato A, Saito Y, Sugiyama K, et al. Suppressive effect of the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) on hepatitis C virus replication. J. Cell. Biochem 2013;114:1987–1996. [DOI] [PubMed] [Google Scholar]
- [92].Lohmann V Hepatitis C virus RNA replication. Curr. Top. Microbiol. Immunol 2013;369:167–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gusarova V, Brodsky JL, Fisher EA. Apolipoprotein B100 Exit from the Endoplasmic Reticulum (ER) Is COPII-dependent, and Its Lipidation to Very Low Density Lipoprotein Occurs Post-ER. J. Biol. Chem. 2003;278:48051–48058. [DOI] [PubMed] [Google Scholar]
- [94].Siddiqi SA. VLDL exits from the endoplasmic reticulum in a specialized vesicle, the VLDL transport vesicle, in rat primary hepatocytes. Biochem. J 2008;413:333–342. [DOI] [PubMed] [Google Scholar]
- [95].Olofsson S-O, Boström P, Andersson L, et al. Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochim. Biophys. Acta 2009;1791:448–458. [DOI] [PubMed] [Google Scholar]
- [96].Ye J, Li JZ, Liu Y, et al. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 2009;9:177–190. [DOI] [PubMed] [Google Scholar]
- [97].Icard V, Diaz O, Scholtes C, et al. Secretion of hepatitis C virus envelope glycoproteins depends on assembly of apolipoprotein B positive lipoproteins. PLoS ONE. 2009;4:e4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Coller KE, Heaton NS, Berger KL, et al. Molecular Determinants and Dynamics of Hepatitis C Virus Secretion. PLOS Pathog. 2012;8:e1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[99].Gondar V, Molina-Jiménez F, Hishiki T, et al. Apolipoprotein E, but Not Apolipoprotein B, Is Essential for Efficient Cell-to-Cell Transmission of Hepatitis C Virus. J. Virol 2015;89:9962–9973. (This study suggest the existence of mechanisms involving VLDL components that differentially regulate cell-free and cell-to-cell HCV transmission) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Cun W, Jiang J, Luo G. The C-terminal alpha-helix domain of apolipoprotein E is required for interaction with nonstructural protein 5A and assembly of hepatitis C virus. J. Virol 2010;84:11532–11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[101].Fauvelle C, Felmlee DJ, Crouchet E, et al. Apolipoprotein E Mediates Evasion From Hepatitis C Virus Neutralizing Antibodies. Gastroenterology. 2016;150:206–217.e4 (Highlighs the role of ApoE and lipoproteins in evasion from host immunity). [DOI] [PubMed] [Google Scholar]
- [102].Saito H, Lund-Katz S, Phillips MC. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Progress in Lipid Research. 2004;43:350–380. [DOI] [PubMed] [Google Scholar]
- **[103].Fukuhara T, Wada M, Nakamura S, et al. Amphipathic α-helices in apolipoproteins are crucial to the formation of infectious hepatitis C virus particles. PLoS Pathog. 2014;10:e1004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[104].Hueging K, Weller R, Doepke M, et al. Several Human Liver Cell Expressed Apolipoproteins Complement HCV Virus Production with Varying Efficacy Conferring Differential Specific Infectivity to Released Viruses. PLoS ONE. 2015;10:e0134529 (These two studies demonstrated the redundant role of exchangeable Apolipoproteins in HCV morphogenesis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Puig-Basagoiti F, Fukuhara T, Tamura T, et al. Human Cathelicidin Compensates for the Role of Apolipoproteins in Hepatitis C Virus Infectious Particle Formation. J. Virol 2016;90:8464–8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Tomiyasu K, Walsh BW, Ikewaki K, et al. Differential metabolism of human VLDL according to content of ApoE and ApoC-III. Arterioscler. Thromb. Vasc. Biol 2001;21:1494–1500. [DOI] [PubMed] [Google Scholar]
- [107].Yang Z, Wang X, Chi X, et al. Neglected but Important Role of Apolipoprotein E Exchange in Hepatitis C Virus Infection. J. Virol 2016;90:9632–9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hofer H, Bankl HC, Wrba F, et al. Hepatocellular fat accumulation and low serum cholesterol in patients infected with HCV-3a. Am. J. Gastroenterol 2002;97:2880–2885. [DOI] [PubMed] [Google Scholar]
- [109].Seki N, Sugita T, Aida Y, et al. Assessment of the features of serum apolipoprotein profiles in chronic HCV infection: difference between HCV genotypes 1b and 2. Hepatol Int. 2014;8:550–559. [DOI] [PubMed] [Google Scholar]
- [110].Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. [DOI] [PubMed] [Google Scholar]
- [111].Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Li JH, Lao XQ, Tillmann HL, et al. Interferon-lambda genotype and low serum low-density lipoprotein cholesterol levels in patients with chronic hepatitis C infection. Hepatology. 2010;51:1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gopal K, Johnson TC, Gopal S, et al. Correlation between beta-lipoprotein levels and outcome of hepatitis C treatment. Hepatology. 2006;44:335–340. [DOI] [PubMed] [Google Scholar]
- [114].Sheridan DA, Price DA, Schmid ML, et al. Apolipoprotein B-associated cholesterol is a determinant of treatment outcome in patients with chronic hepatitis C virus infection receiving anti-viral agents interferon-alpha and ribavirin. Aliment. Pharmacol. Ther 2009;29:1282–1290. [DOI] [PubMed] [Google Scholar]
- *[115].Sheridan DA, Bridge SH, Felmlee DJ, et al. Apolipoprotein-E and hepatitis C lipoviral particles in genotype 1 infection: Evidence for an association with interferon sensitivity. Journal of Hepatology. 2012;57:32–38. (This article shows that ApoE is associated with IFN response) [DOI] [PubMed] [Google Scholar]
- [116].Pawlotsky J-M. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176–1192. [DOI] [PubMed] [Google Scholar]
- [117].Esposito I, Trinks J, Soriano V. Hepatitis C virus resistance to the new direct-acting antivirals. Expert Opin Drug Metab Toxicol 2016. [DOI] [PubMed] [Google Scholar]
- [118].Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. [DOI] [PubMed] [Google Scholar]
- [119].Visser ME, Kastelein JJP, Stroes ESG. Apolipoprotein B synthesis inhibition: results from clinical trials. Curr. Opin. Lipidol 2010;21:319–323. [DOI] [PubMed] [Google Scholar]
- [120].Schaefer EAK, Meixiong J, Mark C, et al. Apolipoprotein B100 is required for hepatitis C infectivity and Mipomersen inhibits hepatitis C. World J. Gastroenterol 2016;22:9954–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Cheng Y-L, Lan K-H, Lee W-P, et al. Amiodarone inhibits the entry and assembly steps of hepatitis C virus life cycle. Clin. Sci 2013;125:439–448. [DOI] [PubMed] [Google Scholar]
- [122].Renet S, Chaumais M- C, Antonini T, et al. Extreme bradycardia after first doses of sofosbuvir and daclatasvir in patients receiving amiodarone: 2 cases including a rechallenge. Gastroenterology. 2015;149:1378–1380.e1. [DOI] [PubMed] [Google Scholar]
- [123].Ahmed A, Felmlee DJ. Mechanisms of Hepatitis C Viral Resistance to Direct Acting Antivirals. Viruses. 2015;7:6716–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]