Abstract
Acinetobacter baumannii is an opportunistic pathogen that poses an increasing threat in the health-care community. Colistin is one of the promising options for treatment of multidrug-resistant A. baumannii. The current study investigated the emergence of colistin resistance among carbapenem-resistant strains of A. baumannii in Egypt. It involved identification of clinically recovered A. baumannii isolates using the VITEK-2 system, and screening of their antimicrobial susceptibilities using broth microdilution techniques. Characterizations of carbapenemase and 16S rRNA methyltransferase genes were performed using PCR. Colistin-resistance determinants were characterized by sequencing. Carbapenem-resistant A. baumannii isolates (n = 40) showed resistance to amoxicillin-clavulanic acid, cefotaxime, gentamicin and amikacin. Most isolates revealed resistance to ciprofloxacin (95%; n = 38) and co-trimoxazole (92.5%; n = 37). Resistance to tobramycin and doxycycline was 80% (n = 32) and 62.5% (n = 25), respectively. Only two A. baumannii isolates demonstrated colistin resistance. Carbapenemase activity was tested by modified Hodge test and 78% of isolates were positive. All isolates carried blaOXA-51-like genes whereas bla-OXA-23 was detected in 80% (n = 32) of isolates. Among 16S rRNA methylase genes, armA was detected in 22.5% (n = 9) of the isolates. Analyses of lpxA, lpxC, lpxD and pmrCAB genetic sequences suggest that colistin resistance could be attributed to mutations in pmrCAB genes. Alarmingly, colistin resistance was associated with high levels of resistance to other antimicrobials. The current findings represent a serious health-care problem capable of restraining future therapeutic options.
Keywords: Acinetobacter baumannii, armA, blaOXA-23, colistin resistance, pmrABCgenes
Introduction
Infections with multiple drug-resistant Acinetobacter baumannii are of increasing concern [22]. Acinetobacter is the main source of various infections including pneumonia, septicaemia, wound sepsis, urinary tract infection and meningitis [22]. Multidrug-resistant (MDR) A. baumannii strains are shown to be responsible for several worldwide outbreaks. Acinetobacter baumannii has the ability to survive harsh conditions, also their resistance to disinfectants allows them to persist in the hospital environment [9], [20]. Carbapenems have been the most appropriate option for treatment of A. baumannii infections, but the development of carbapenem-resistant A. baumannii (CRAB) significantly limits their use. Several mechanisms are involved in carbapenem resistance, including production of carbapenem-hydrolysing β-lactamas (carbapenemase), reduced permeability and active efflux. Carbapenemase is the most common resistance mechanism, including the intrinsic blaOXA-51-like and the acquired blaOXA-23-like, blaOXA-24-like, and blaOXA-58-like forms [34].
Nowadays, colistin (polymyxin E), is one of the last therapeutic options for CRAB strains. Colistin was recently reintroduced as the last resort for treatment of MDR A. baumannii infections [26], [15] and colistin therapy with a new dosing regimen has lowered its toxic effects [15]. Nevertheless, some strains are now reported to be colistin resistant, as well as extensively drug resistant. MDR A. baumannii isolates have been recovered from intensive care units in Mediterranean hospitals and various countries [17], [9].
A prominent mechanism involved in A. baumannii resistance to colistin is the mutation within the lipid A biosynthetic pathway that consequently causes a loss of outer lipopolysaccharide (LPS) and elimination of colistin target site [6]. Another potential mechanism is the alteration of lipid A components of LPS through mutations in the pmrA and pmrB genes of the regulatory system and pmrC that encodes a lipid A phosphoethanolamine transferase enzyme [30], [32], [4], [29]. To the best of our knowledge, this is the first report addressing the emergence of colistin resistance, as well as its potential underlying mechanisms, among clinical isolates of CRAB in Egypt.
Materials and methods
Clinical isolates
Forty non-duplicated carbapenem-resistant Acinetobacter baumannii isolates were recovered from different clinical specimens (pus, sputum and urine) of inpatients admitted to El-Kasr El-Aini hospital (Cairo, Egypt) from January 2015 to July 2015.
Identification and susceptibility testing
Identification and antimicrobial susceptibility testing of clinical isolates were carried out using the VITEK-2 system (bioMérieux, Marcy l’Étoile, France). MICs were determined according to The CLSI guidelines using the broth microdilution method. Escherichia coli ATCC 25922 and A. baumannii ATCC17978 were used as control strains [8].
β-Lactamase assays
Clinical isolates of A. baumannii were screened for carbapenemase and metallo-β-lactamase (MBL) production using the modified Hodge test (MHT) and imipenem-EDTA double-disc synergy test, respectively. The two methods were performed as previously described [40], [19].
Molecular characterization of carbapenemase and 16S rRNA methyltransferase genes
DNA extraction was performed using DNeasy Blood & Tissue Kits (Qiagen, Hilden, Germany). Carbapenemase-encoding genes (blaOXA-23-like, blaOXA-24-like, blaOXA-51-like and blaOXA-58-like) were detected by PCR for the tested isolates. The amplification conditions involved, initial denaturation at 94°C for 5 min, 30 cycles of 94°C for 25 s, 52°C for 40 s, and 72°C for 50 s, and a final elongation at 72°C for 7 min, as described previously [39].
Furthermore, all A. baumannii isolates were subjected to multiplex-PCR for amplification of 16S rRNA methyltransferase genes (armA, rmtB and rmtC genes) using the primers and PCR conditions that were previously described by Doi et al. [11]. Specific primers used in this study are described in Table 1.
Table 1.
List of primers used in this study
| Gene | Primer sequences | Product size | References | |
|---|---|---|---|---|
| blaOXA-23 | Forward | 5ʹ-GATCGGATTGGAGAACCAGA-3ʹ | 501 bp | 16 |
| Reverse | 5ʹ-ATTTCTGACCGCATTTCCAT-3ʹ | |||
| blaOXA-24 | Forward | 5ʹ-TTCCCCTAACATGAATTTGT-3ʹ | 1024 bp | 16 |
| Reverse | 5ʹ-GTACTAATCAAAGTTGTGAA-3ʹ | |||
| blaOXA-51 | Forward | 5ʹ-TAATGCTTTGATCGGCCTTG-3ʹ | 353 bp | 16 |
| Reverse | 5ʹ-TGGATTGCACTTCATCTTGG-3ʹ | |||
| blaOXA-58 | Forward | 5ʹ-TGGCACGCATTTAGACCG-3ʹ | 507 bp | 16 |
| Reverse | 5ʹ-AAACCCACATACCAACCC-3ʹ | |||
| armA | Forward | 5ʹ-ATT CTG CCT ATC CTA ATT GG-3ʹ | 315 bp | 17 |
| Reverse | 5ʹ-ACC TAT ACT TTA TCG TCG TC-3ʹ | |||
| rmtB | Forward | 5ʹ-GCT TTCTGCGGG CGA TGTAA-3ʹ | 173 bp | 17 |
| Reverse | 5ʹ-ATG CAA TGC CGC GCT CGT AT-3ʹ | |||
| rmtC | Forward | 5ʹ-CGA AGA AGT AAC AGC CAA AG-3ʹ | 711 bp | 17 |
| Reverse | 5ʹ-ATC CCA ACA TCT CTC CCA CT-3ʹ | |||
Investigation of colistin-resistant determinants
The colistin-resistant strains were screened for the presence of mutations in the lpxA, lpxC, lpxD and pmrCAB genes. The genes were amplified by PCR as described previously [4], [30], [23]. The PCR amplicons were sequenced on both DNA strands using ABI 310 Genetic analyser sequencing (Applied Biosystems, Foster City, CA, USA). Nucleotide sequences of lpxA, lpxC, lpxD and pmrCAB genes from colistin-resistant isolates were compared with the respective sequences obtained from colistin-susceptible and A. baumannii ATCC17978 strains.
Ethical approval
All experiments were carried out in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Throughout the study period, 40 CRAB isolates were recovered from male (n = 28; 70%) and female (n = 12; 30%) patients in the El-Kasr El-Aini hospital (Cairo, Egypt). Almost half of the A. baumannii isolates were recovered from sputum samples (n = 19; 47.5%) followed by urine (n = 13; 32.5%) and pus (n = 8; 20%). All isolates (n = 40; 100%) were resistant to amoxicillin-clavulanic acid, cefotaxime, imipenem, meropenem, gentamicin and amikacin. Most isolates showed resistance to ciprofloxacin (95%; n = 38) and co-trimoxazole (92.5%; n = 37). Resistance to tobramycin and doxycycline was found in 80% (n = 32) and 62.5% (n = 25) of the tested clinical isolates, respectively. Only two A. baumannii isolates (A119 and A140) demonstrated colistin resistance and they were selected for further study (Table 2).
Table 2.
Phenotypic and genotypic characterization of Acinetobacter baumannii isolates
| Sample | MIC (mg/L) (antibiotic susceptibility patterns)a |
Type | Gender | MHT | Genes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMC | CTX | IPM | MEM | CIP | CN | AK | Tob | DO | SXT | CO | |||||
| A101 | 512(R) | 256(R) | 64(R) | 64(R) | 128(R) | 256(R) | 512(R) | 256(R) | 32(R) | 4/76(R) | 0.25(S) | Sputum | Male | + | blaOXA-51, blaOXA-23, armA |
| A102 | 512(R) | 128(R) | 64(R) | 64(R) | 64(R) | 256(R) | 256(R) | 1(S) | 4(S) | 16/304(R) | 0.25(S) | Sputum | Female | + | blaOXA-51, blaOXA-23 |
| A103 | 256(R) | 256(R) | 64(R) | 32(R) | 64(R) | 256(R) | 256(R) | 512(R) | 2(S) | 32/608(R) | 0.25(S) | Sputum | Male | – | blaOXA-51, armA |
| A104 | 512(R) | 256(R) | 128(R) | 64(R) | 64(R) | 256(R) | 128(R) | 128(R) | 2(S) | 64/1216(R) | 0.5(S) | Sputum | Female | – | blaOXA-51 |
| A105 | 512(R) | 256(R) | 64(R) | 64(R) | 32(R) | 256(R) | 32(R) | 1(S) | 1(S) | 2/38(S) | 0.25(S) | Sputum | Female | + | blaOXA-51, blaOXA-23 |
| A106 | 512(R) | 256(R) | 64(R) | 32(R) | 128(R) | 128(R) | 256(R) | 512(R) | 64(R) | 32/608 (R) | 0.25(S) | Urine | Male | + | blaOXA-51, blaOXA-23, armA |
| A107 | 256(R) | 256(R) | 128(R) | 64(R) | 32(R) | 128(R) | 256(R) | 256(R) | 32(R) | 16/304(R) | 0.5(S) | Urine | Male | + | blaOXA-51, blaOXA-23, armA |
| A108 | 512(R) | 256(R) | 64(R) | 32(R) | 32(R) | 256(R) | 256(R) | 1(S) | 64(R) | 32/608(R) | 0.25(S) | Sputum | Female | + | blaOXA-51, blaOXA-23 |
| A109 | 512(R) | 256(R) | 64(R) | 64(R) | 64(R) | 512(R) | 256(R) | 512(R) | 128(R) | 16/304(R) | 0.25(S) | Sputum | Male | + | blaOXA-51, blaOXA-23, armA |
| A110 | 512(R) | 256(R) | 64(R) | 64(R) | 32(R) | 256(R) | 256(R) | 64(R) | 2(S) | 32/608(R) | 0.5(S) | Urine | Male | + | blaOXA-51, blaOXA-23 |
| A111 | 256(R) | 256(R) | 32(R) | 32(R) | 16(R) | 128(R) | 256(R) | 512(R) | 1(S) | 16/304 (R) | 0.25(S) | Urine | Male | – | blaOXA-51, armA |
| A112 | 512(R) | 256(R) | 128(R) | 64(R) | 64(R) | 256(R) | 512(R) | 512(R) | 1(S) | 32/608(R) | 0.5(S) | Urine | Female | + | blaOXA-51, blaOXA-23, armA |
| A113 | 512(R) | 256(R) | 32(R) | 64(R) | 32(R) | 128(R) | 128(R) | 128(R) | 128(R) | 64/1216(R) | 0.5(S) | Pus | Male | + | blaOXA-51, blaOXA-23 |
| A114 | 512(R) | 256(R) | 64(R) | 64(R) | 128(R) | 256(R) | 512(R) | 512(R) | 2(S) | 16/304 (R) | 0.25(S) | Sputum | Male | + | blaOXA-51, blaOXA-23, armA |
| A115 | 512(R) | 256(R) | 32(R) | 32(R) | 64(R) | 256(R) | 128(R) | 1(S) | 64(R) | 1/19(S) | 0.25(S) | Sputum | Female | + | blaOXA-51, blaOXA-23 |
| A116 | 512(R) | 512(R) | 64(R) | 64(R) | 16(R) | 256(R) | 256(R) | 1(S) | 128(R) | 32/608 (R) | 0.5(S) | Urine | Female | + | blaOXA-51, blaOXA-23 |
| A117 | 512(R) | 128(R) | 64(R) | 32(R) | 32(R) | 128(R) | 256(R) | 64(R) | 32(R) | 32/608 (R) | 0.25(S) | Urine | Male | + | blaOXA-51, blaOXA-23 |
| A118 | 512(R) | 256(R) | 32(R) | 32(R) | 16(R) | 128(R) | 128(R) | 128(R) | 2(S) | 64/1216 (R) | 0.25(S) | Urine | Male | + | blaOXA-51 |
| A119 | 256(R) | 128(R) | 32(R) | 64(R) | 16(R) | 64(R) | 256(R) | 64(R) | 64(R) | 32/608(R) | 32(R) | Urine | Male | + | blaOXA-51, blaOXA-23 |
| A120 | 512(R) | 256(R) | 64(R) | 128(R) | 64(R) | 128(R) | 256(R) | 1(R) | 32(R) | 32/608(R) | 0.25(S) | Sputum | Female | + | blaOXA-51, blaOXA-23 |
| A121 | 512(R) | 256(R) | 32(R) | 64(R) | 64(R) | 256(R) | 256(R) | 1(S) | 64(R) | 4/76(R) | 1(S) | Sputum | Male | + | blaOXA-51, blaOXA-23 |
| A122 | 512(R) | 256(R) | 32(R) | 64(R) | 32(R) | 128(R) | 128(R) | 1(S) | 128(R) | 32/608 (R) | 0.25(S) | Urine | Female | + | blaOXA-51, blaOXA-23 |
| A123 | 512(R) | 256(R) | 32(R) | 32(R) | 32(R) | 128(R) | 256(R) | 64(R) | 32(R) | 32/608 (R) | 0.25(S) | Pus | Male | – | blaOXA-51 |
| A124 | 512(R) | 128(R) | 64(R) | 64(R) | 1(S) | 256(R) | 256(R) | 128, R | 64(R) | 32/608 (R) | 1(S) | Sputum | Male | + | blaOXA-51, blaOXA-23 |
| A125 | 512(R) | 512(R) | 128(R) | 64(R) | 32(R) | 512(R) | 256(R) | 64(R) | 2(S) | 64/1216 (R) | 0.25(S) | Urine | Male | + | blaOXA-51, blaOXA-23 |
| A126 | 512(R) | 256(R) | 32(R) | 128(R) | 64(R) | 512(R) | 256(R) | 64(R) | 16(R) | 16/304(R) | 1(S) | Pus | Male | + | blaOXA-51, blaOXA-23 |
| A127 | 512(R) | 512(R) | 32(R) | 32(R) | 128(R) | 512(R) | 512(R) | 64(R) | 4(S) | 64/1216 (R) | 0.25(S) | Sputum | Male | – | blaOXA-51, blaOXA-23 |
| A128 | 512(R) | 512(R) | 64(R) | 64(R) | 128(R) | 256(R) | 256(R) | 128(R) | 32(R) | 32/608(R) | 0.25(S) | Sputum | Male | – | blaOXA-51 |
| A129 | 512(R) | 256(R) | 32(R) | 64(R) | 1(S) | 128(R) | 512(R) | 64(R) | 1(S) | 32/608(R) | 0.25(S) | Sputum | Male | + | blaOXA-51, blaOXA-23 |
| A130 | 512(R) | 512(R) | 32(R) | 32(R) | 32(R) | 256(R) | 512(R) | 128(R) | 32(R) | 64/1216(R) | 0.25(S) | Sputum | Female | – | blaOXA-51, blaOXA-23 |
| A131 | 512(R) | 128(R) | 32(R) | 32(R) | 64(R) | 512(R) | 512(R) | 64(R) | 1(S) | 16/304(R) | 0.25(S) | Sputum | Male | + | blaOXA-51, blaOXA-23 |
| A132 | 512(R) | 512(R) | 128(R) | 64(R) | 32(R) | 256(R) | 256(R) | 128(R) | 64(R) | 32/608(R) | 2(S) | Pus | Male | – | blaOXA-51 |
| A133 | 512(R) | 256(R) | 64(R) | 64(R) | 32(R) | 256(R) | 256(R) | 64(R) | 16(R) | 32/608 (R) | 2(S) | Sputum | Male | + | blaOXA-51, blaOXA-23 |
| A134 | 512(R) | 256(R) | 64(R) | 64(R) | 16(R) | 256(R) | 128(R) | 64(R) | 32(R) | 4/76(R) | 1(S) | Pus | Male | + | blaOXA-51, blaOXA-23 |
| A135 | 512(R) | 256(R) | 32(R) | 64(R) | 128(R) | 256(R) | 512(R) | 128(R) | 64(R) | 4/76(R) | 2(S) | Urine | Female | + | blaOXA-51, blaOXA-23 |
| A136 | 512(R) | 128(R) | 64(R) | 32(R) | 128(R) | 512(R) | 256(R) | 512(R) | 128(R) | 64/1216(R) | 2(S) | pus | Female | + | blaOXA-51, blaOXA-23, armA |
| A137 | 512(R) | 128(R) | 32(R) | 64(R) | 128(R) | 128(R) | 128(R) | 64(R) | 16(R) | 32/608 (R) | 0.5(S) | sputum | Male | + | blaOXA-51, blaOXA-23 |
| A138 | 512(R) | 512(R) | 128(R) | 64(R) | 32(R) | 256(R) | 256(R) | 64(R) | 64(R) | (R) | 2(S) | pus | Male | – | blaOXA-51 |
| A139 | 512(R) | 128(R) | 32(R) | 128(R) | 64(R) | 256(R) | 256(R) | 128(R) | 2(S) | 2/38(S) | 0.5(S) | pus | Male | + | blaOXA-51, blaOXA-23 |
| A140 | 512(R) | 128(R) | 64(R) | 64(R) | 128(R) | 128(R) | 64(R) | 64(R) | 1(S) | 32/608 (R) | 32(R) | Urine | Male | + | blaOXA-51, blaOXA-23 |
AK, amikacin; AMC, co-amoxiclav; CIP, ciprofloxacin; CN, gentamicin; CO, colistin; CTX, cefotaxime; Do, doxycycline; IPM, imipenem; MEM, meropenem; SXT, co-trimoxazole; TOB, tobramycin; +, positive; –, negative; MHT, modified Hodge test.
Interpretive breakpoints of antibiotic susceptibility are based on the CLSI criteria.
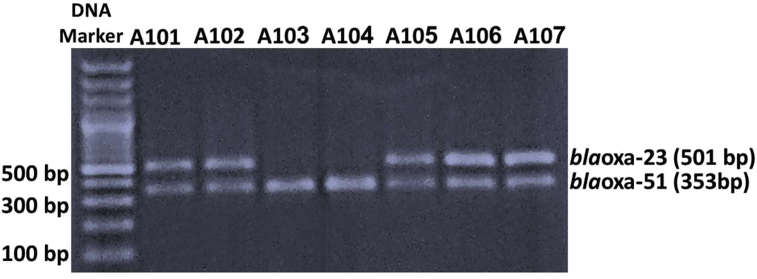
All A. baumannii isolates were negative for MBLs using an EDTA double-disc synergy test and 78% of the tested isolates showed positive carbapenemases activity by MHT (Table 2). In contrast, all isolates (100%; n = 40) harboured blaOXA-51-like genes while bla-OXA-23 was detected in 80% (n = 32) of isolates (Fig. 1). None of the tested isolates harboured blaOXA-58-like or blaOXA-24-like genes. Among 16S rRNA methyltransferase genes, armA was detected in 22.5% (n = 9) of the isolates. While rmtB and rmtC genes were not detected among the studied isolates.
Fig. 1.
Multiplex PCRs for detection of blaOXA carbapenemase genes. First lane represents DNA marker (100-bp DNA ladder). The agarose gel illustrates that Acinetobacter baumannii strains A101, A102, A105, A106 and A107 are positive for both blaOXA-23 and blaOXA-51; A. baumannii A103 and A104 are blaOXA-23 gene negative.
Colistin-resistant isolates (5%; n = 2) were genetically screened for the existence of mutations in the lpxA, lpxC, lpxD and pmrCAB genes. The obtained sequences of the tested genes were analysed using BLAST at the National Center of Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST). Analyses of sequences were also carried out through the EXPASY translate tool at the Swiss Institute of Bioinformatics website (http://web/expasy.org/translate/) as well as the ClustalW2 multiple sequence alignment program (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Sequencing of lpxA, lpxC and lpxD genes revealed the presence of five amino acid substitutions (three in the lpxA gene (A82E, H135Y and H257Q), two in the lpxC gene (D296N and V324I), and none in the lpxD gene) in sequences of both colistin-resistant and colistin-susceptible A. baumannii isolates, compared with that of A. baumannii ATCC 17978. Consequently, the aforementioned amino acid substitutions may not play a role in colistin resistance in the tested A. baumannii isolates.
Regarding pmrCAB genes, unique mutation patterns of pmrCAB genes were detected in colistin-resistant isolates as follows: pmrA (L46F), pmrB (Y125F, A140T, P174L and A456V) and pmrC (A81K, I238G, V254A and K553T) in comparison with their sequences in A. baumannii ATCC 17978. Our GenBank accession numbers are LC371058 and LC371059 for pmrC and pmrB, respectively.
Discussion
Prevalence of A. baumannii has become a critical problem that threatens health care in Egypt. The current study involved 40 carbapenem-resistant isolates. Demographic data illustrated that most of these isolates were collected from male patients. Most of the A. baumannii isolates were associated with respiratory tract infections. None of amoxicillin-clavulanic acid, cefotaxime, imipenem, meropenem, gentamicin or amikacin was effective in treatment of the recovered isolates. Out of 40 isolates, 80% and 62.5% of isolates showed resistance to tobramycin and doxycycline, respectively. The highest susceptibility rate (95%) was observed with colistin, as only two isolates (5%) were colistin resistant. Accordingly, colistin remains the most effective agent for treatment A. baumannii infection in comparison with other tested antibiotics.
During the last decade, carbapenems were the treatment of choice for management of MDR A. baumannii [3]. Nevertheless, carbapenem misuse and over-use for management of Acinetobacter infections is responsible for the emergence of CRAB [5]. The importance of colistin as one of the last therapeutic options has been noted as a result of the escalation in CRAB. Carbapenemase is considered one of the main resistance mechanisms of A. baumannii to carbapenem. We screened the production of carbapenemase and MBL phenotypically using MHT and EDTA double-disc synergy, respectively. MHT revealed that 78% of isolates showed positive carbapenemase phenotype. On the other hand, all isolates were MBL negative according to an EDTA double-disc synergy test, indicating that CRAB isolates do not produce MBLs. This finding agrees with that of Mathlouthi et al. [28].
Worldwide dissemination of MDR A. baumannii harbouring OXA-type carbapenemase has been progressively reported [33]. The OXA-type carbapenemase has a relatively lower catalytic efficiency to hydrolyse carbapenems compared with MBLs, but it is essential to consider its presence as a crucial factor because the expression of OXA-type carbapenemase can be fundamentally unregulated by the upstream existence of insertion sequence elements such as insertion sequence Aba1 [37], [35]. The impact of OXA-type carbapenemase on the resistance profile can be intensified when other mechanisms of resistance are present, such as increased expression of efflux pumps and/or loss of some porins [38], [27], [31].
In the current work, a blaOXA-51-like gene was detected in all isolates whereas blaOXA-23 was harboured by 80% of the isolates. The blaOXA-51-like genes are intrinsic genes in A. baumannii species. In this study, the results revealed the presence of A. baumannii isolates (20%) carrying only blaoxa-51 carbapenemase, and showing a high level of resistance to carbapenem. This may be attributed to the existence of other carbapenem-hydrolysing enzymes. Furthermore, carbapenem resistance can possibly be mediated by a combination of blaoxa-51 and efflux, as reported previously [18].
The noticeable prevalence of blaOXA-23-carrying Acinetobacter in Egypt is a crucial health-care concern that necessitates strict interventions to eliminate such infections [13], [12]. Neither blaOXA-24 nor blaOXA-58 was found among the tested isolates. This finding is consistent with that of Ghaith et al. [16].
The 16S rRNA methylation mechanism has been found to confer high aminoglycoside resistance levels on A. baumannii [11]. The armA gene was reported in A. baumannii isolates recovered from Korea [24], China [41] and North America [11]. In the current study, the armA gene of 16S rRNA methyltransferase could be detected in 22.5% of the tested isolates. All the armA-positive isolates showed high aminoglycosides resistance rates with amikacin, gentamicin and tobramycin MICs of >256 mg/L. Many reports have addressed the coexistence of blaOXA-23 and armA among A. baumannii in China [36], [42] and India [42], [21]. In the current work, blaOXA-23 and armA coexisted in 17.5% of A. baumannii isolates. Some investigators have documented the emergence of colistin resistance among A. baumannii in China after colistin was reintroduced to treat infections with CRAB [7]. In this study, two A. baumannii isolates (5%) revealed colistin resistance, with a MIC value of 32 mg/L. This is the first report indicating the emergence of colistin resistance among clinical isolates of A. baumannii in Egypt. Many factors have been implicated in A. baumannii resistance to colistin. Alterations of the lipid A portion of LPS [2] or LPS biosynthetic alterations [29] were defined as the primary resistance mechanisms. Also, reduction of negative charges on the outer membrane reduces its affinity for positively charged molecules and may lead to colistin insensitivity [2], [4].
It is worth noting that the emergence of colistin resistance was not linked with higher susceptibility to other antimicrobial agents. In accordance with previous reports, colistin resistance due to pmrCAB mutations is not associated with increased sensitivity to other antimicrobials, in contrast to Lpx variations [30], [25].
Surveillance reports in US hospitals revealed that colistin resistance was significantly higher among imipenem non-susceptible strains compared with imipenem-sensitive strains [14]. Other reports from Bulgaria and Spain mentioned higher colistin resistance rates of 16.7% and 19.1%, respectively [7], [9], [10], [1], compared with that in the current study.
Our findings suggest that colistin resistance was mostly attributed to mutations in pmrCAB genes rather than lpx genes. To the best of our knowledge, this is the first report addressing the emergence of colistin resistance as well as its potential underlying mechanisms among clinical isolates of CRAB in Egypt. More rigorous regulation of antibiotic prescription and administration, as well as antibiotic stewardship programmes are required in Egyptian hospitals to hinder the dissemination of CRAB and colistin-resistant A. baumannii.
Conclusions
Emergence of colistin-resistant/carbapenem-resistant A. baumannii in our health-care setting is an alarming issue. Colistin resistance was associated with mutations in pmrABC genes and OXA-23-like carbapenem-hydrolysing class was the most predominant carbapenemase. In addition, armA was the main methyltransferase gene among the clinical isolates of A. baumannii. Our findings revealed that colistin resistance was associated with a high resistance level to other antimicrobials. The current findings represent a serious health-care problem capable of restraining future therapeutic options. Strict regulation of antibiotic usage is needed in Egyptian hospitals to prohibit the spread of CRAB and colistin-resistant A. baumannii in clinical settings.
Transparency declaration
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank the medical staff as well as the microbiologists and technicians for collection of clinical specimens and recovery of A. baumannii.
Contributor Information
Mahmoud A.F. Khalil, Email: mahmouadfouad@gmail.com.
Walid F. Elkhatib, Email: walid-elkhatib@pharma.asu.edu.eg.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Arroyo L.A., Garcia-Curiel A., Pachon-Ibanez M.E., Llanos A.C., Ruiz M., Pachon J. Reliability of the E-test method for detection of colistin resistance in clinical isolates of Acinetobacter baumannii. J Clin Microbiol. 2005;43:903–905. doi: 10.1128/JCM.43.2.903-905.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arroyo L.A., Herrera C.M., Fernandez L., Hankins J.V., Trent M.S., Hancock R.E. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother. 2011;55:3743–3751. doi: 10.1128/AAC.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassetti M., Righi E., Esposito S., Petrosillo N., Nicolini L. Drug treatment for multidrug-resistant Acinetobacter baumannii infections. Future Microbiol. 2008;3:649–660. doi: 10.2217/17460913.3.6.649. [DOI] [PubMed] [Google Scholar]
- 4.Beceiro A., Llobet E., Aranda J., Bengoechea J.A., Doumith M., Hornsey M. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother. 2011;55:3370–3379. doi: 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonomo R.A., Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis. 2006;43(Suppl. 2):S49–S56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 6.Cai X.F., Sun J.M., Bao L.S., Li W.B. Risk factors and antibiotic resistance of pneumonia caused by multidrug resistant Acinetobacter baumannii in pediatric intensive care unit. World J Emerg Med. 2012;3:202–207. doi: 10.5847/wjem.j.issn.1920-8642.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Y., Chai D., Wang R., Liang B., Bai N. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother. 2012;67:1607–1615. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
- 8.CLSI. Clinical and Laboratory Standards Institute . CLSI; Wayne, PA: 2013. Performance standards for antimicrobial susceptibility testing; 23rd international supplement, CLSI document M100-S23. [Google Scholar]
- 9.Dijkshoorn L., Nemec A., Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 10.Dobrewski R., Savov E., Bernards A.T., van den Barselaar M., Nordmann P., van den Broek P.J. Genotypic diversity and antibiotic susceptibility of Acinetobacter baumannii isolates in a Bulgarian hospital. Clin Microbiol Infect. 2006;12:1135–1137. doi: 10.1111/j.1469-0691.2006.01530.x. [DOI] [PubMed] [Google Scholar]
- 11.Doi Y., Adams J.M., Yamane K., Paterson D.L. Identification of 16S rRNA methylase-producing Acinetobacter baumannii clinical strains in North America. Antimicrob Agents Chemother. 2007;51:4209–4210. doi: 10.1128/AAC.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Bannah A.M.S., Nawar N.N., Hassan R.M.M., Salem S.T.B. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in a tertiary care hospital in Egypt: clonal spread of blaOXA-23. Microb Drug Resist. 2018;24:269–277. doi: 10.1089/mdr.2017.0057. [DOI] [PubMed] [Google Scholar]
- 13.Fouad M., Attia A.S., Tawakkol W.M., Hashem A.M. Emergence of carbapenem-resistant Acinetobacter baumannii harboring the OXA-23 carbapenemase in intensive care units of Egyptian hospitals. Int J Infect Dis. 2013;17:e1252–e1254. doi: 10.1016/j.ijid.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Gales A.C., Jones R.N., Sader H.S. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09) J Antimicrob Chemother. 2011;66:2070–2074. doi: 10.1093/jac/dkr239. [DOI] [PubMed] [Google Scholar]
- 15.Garnacho-Montero J., Ortiz-Leyba C., Jimenez-Jimenez F.J., Barrero-Almodovar A.E., Garcia-Garmendia J.L., Bernabeu-Wittel I.M. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis. 2003;36:1111–1118. doi: 10.1086/374337. [DOI] [PubMed] [Google Scholar]
- 16.Ghaith D.M., Hassan R.M., Hasanin A.M. Rapid identification of nosocomial Acinetobacter baumannii isolated from a surgical intensive care unit in Egypt. Ann Saudi Med. 2015;35:440–444. doi: 10.5144/0256-4947.2015.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannouli M., Tomasone F., Agodi A., Vahaboglu H., Daoud Z., Triassi M. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii strains in intensive care units of multiple Mediterranean hospitals. J Antimicrob Chemother. 2009;63:828–830. doi: 10.1093/jac/dkp032. [DOI] [PubMed] [Google Scholar]
- 18.Hu W.S., Yao S.M., Fung C.P., Hsieh Y.P., Liu C.P., Lin J.F. An OXA-66/OXA-51-like carbapenemase and possibly an efflux pump are associated with resistance to imipenem in Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3844–3852. doi: 10.1128/AAC.01512-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong S.H., Bae I.K., Park K.O., An Y.J., Sohn S.G., Jang S.J. Outbreaks of imipenem-resistant Acinetobacter baumannii producing carbapenemases in Korea. J Microbiol. 2006;44:423–431. [PubMed] [Google Scholar]
- 20.Karah N., Sundsfjord A., Towner K., Samuelsen O. Insights into the global molecular epidemiology of carbapenem non-susceptible clones of Acinetobacter baumannii. Drug Resist Updates. 2012;15:237–247. doi: 10.1016/j.drup.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Karthikeyan K., Thirunarayan M.A., Krishnan P. Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii from India. J Antimicrob Chemother. 2010;65:2253–2254. doi: 10.1093/jac/dkq273. [DOI] [PubMed] [Google Scholar]
- 22.Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lean S.S., Suhaili Z., Ismail S., Rahman N.I., Othman N., Abdullah F.H. Prevalence and genetic characterization of carbapenem- and polymyxin-resistant Acinetobacter baumannii isolated from a tertiary hospital in Terengganu, Malaysia. ISRN Microbiol. 2014;2014:953417. doi: 10.1155/2014/953417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H., Yong D., Yum J.H., Roh K.H., Lee K., Yamane K. Dissemination of 16S rRNA methylase-mediated highly amikacin-resistant isolates of Klebsiella pneumoniae and Acinetobacter baumannii in Korea. Diagn Microbiol Infect Dis. 2006;56:305–312. doi: 10.1016/j.diagmicrobio.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Lesho E., Yoon E.J., McGann P., Snesrud E., Kwak Y., Milillo M. Emergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infections. J Infect Dis. 2013;208:1142–1151. doi: 10.1093/infdis/jit293. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Nation R.L., Milne R.W., Turnidge J.D., Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005;25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Luo L., Jiang X., Wu Q., Wei L., Li J., Ying C. Efflux pump overexpression in conjunction with alternation of outer membrane protein may induce Acinetobacter baumannii resistant to imipenem. Chemotherapy. 2011;57:77–84. doi: 10.1159/000323620. [DOI] [PubMed] [Google Scholar]
- 28.Mathlouthi N., Ben Lamine Y., Somai R., Bouhalila-Besbes S., Bakour S., Rolain J.M. Incidence of OXA-23 and OXA-58 carbapenemases coexpressed in clinical isolates of Acinetobacter baumannii in Tunisia. Microb Drug Resist. 2018;24:136–141. doi: 10.1089/mdr.2016.0306. [DOI] [PubMed] [Google Scholar]
- 29.Moffatt J.H., Harper M., Adler B., Nation R.L., Li J., Boyce J.D. Insertion sequence ISAba11 is involved in colistin resistance and loss of lipopolysaccharide in Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55:3022–3024. doi: 10.1128/AAC.01732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moffatt J.H., Harper M., Harrison P., Hale J.D., Vinogradov E., Seemann T. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother. 2010;54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opazo A.C., Mella S.M., Dominguez M.Y., Bello H.T., Gonzalez G.R. Multi-drug efflux pumps and antibiotic resistance in Acinetobacter baumannii. Rev Chilena Infectol. 2009;26:499–503. [PubMed] [Google Scholar]
- 32.Park Y.K., Lee J.Y., Ko K.S. Transcriptomic analysis of colistin-susceptible and colistin-resistant isolates identifies genes associated with colistin resistance in Acinetobacter baumannii. Clin Microbiol Infect Dis. 2015;21 doi: 10.1016/j.cmi.2015.04.009. 765 e1–7. [DOI] [PubMed] [Google Scholar]
- 33.Pogue J.M., Mann T., Barber K.E., Kaye K.S. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Exp Rev Anti infect Ther. 2013;11:383–393. doi: 10.1586/eri.13.14. [DOI] [PubMed] [Google Scholar]
- 34.Poirel L., Marque S., Heritier C., Segonds C., Chabanon G., Nordmann P. OXA-58, a novel class D {beta}-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother. 2005;49:202–208. doi: 10.1128/AAC.49.1.202-208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segal H., Garny S., Elisha B.G. Is IS(ABA-1) customized for Acinetobacter? FEMS Microbiol Lett. 2005;243:425–429. doi: 10.1016/j.femsle.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Shen M., Luan G., Wang Y., Chang Y., Zhang C., Yang J. Coexistence of blaOXA-23 with armA in quinolone-resistant Acinetobacter baumannii from a Chinese university hospital. Diagn Microbiol Infect Dis. 2016;84:230–231. doi: 10.1016/j.diagmicrobio.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Turton J.F., Ward M.E., Woodford N., Kaufmann M.E., Pike R., Livermore D.M. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett. 2006;258:72–77. doi: 10.1111/j.1574-6968.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 38.Vila J., Marti S., Sanchez-Cespedes J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2007;59:1210–1215. doi: 10.1093/jac/dkl509. [DOI] [PubMed] [Google Scholar]
- 39.Woodford N., Ellington M.J., Coelho J.M., Turton J.F., Ward M.E., Brown S. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27:351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Yong D., Lee K., Yum J.H., Shin H.B., Rossolini G.M., Chong Y. Imipenem-EDTA disk method for differentiation of metallo-beta-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002;40:3798–3801. doi: 10.1128/JCM.40.10.3798-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Y.S., Zhou H., Yang Q., Chen Y.G., Li L.J. Widespread occurrence of aminoglycoside resistance due to ArmA methylase in imipenem-resistant Acinetobacter baumannii isolates in China. J Antimicrob Chemother. 2007;60:454–455. doi: 10.1093/jac/dkm208. [DOI] [PubMed] [Google Scholar]
- 42.Zhao W.S., Liu G.Y., Mi Z.H., Zhang F. Coexistence of blaOXA-23 with armA and novel gyrA mutation in a pandrug-resistant Acinetobacter baumannii isolate from the blood of a patient with haematological disease in China. J Hosp Infect. 2011;77:278–279. doi: 10.1016/j.jhin.2010.11.006. [DOI] [PubMed] [Google Scholar]