Abstract
The emergence of Middle East respiratory syndrome coronavirus (MERS-CoV) in September 2012 in Saudi Arabia had attracted the attention of the global health community. In 2017 the Saudi Ministry of Health released a visual triage system with scoring to alert healthcare workers in emergency departments (EDs) and haemodialysis units for the possibility of occurrence of MERS-CoV infection. We performed a retrospective analysis of this visual score to determine its sensitivity and specificity. The study included all cases from 2014 to 2017 in a MERS-CoV referral centre in Riyadh, Saudi Arabia. During the study period there were a total of 2435 suspected MERS cases. Of these, 1823 (75%) tested negative and the remaining 25% tested positive for MERS-CoV by PCR assay. The application of the visual triage score found a similar percentage of MERS-CoV and non–MERS-CoV patients, with each score from 0 to 11. The percentage of patients with a cutoff score of ≥4 was 75% in patients with MERS-CoV infection and 85% in patients without MERS-CoV infection (p 0.0001). The sensitivity and specificity of this cutoff score for MERS-CoV infection were 74.1% and 18.6%, respectively. The sensitivity and specificity of the scoring system were low, and further refinement of the score is needed for better prediction of MERS-CoV infection.
Keywords: Middle East respiratory syndrome coronavirus (MERS-CoV), Saudi Arabia, Scoring, Visual triage
Introduction
The first case of Middle East respiratory syndrome coronavirus (MERS-CoV) was described in 2012 from a hospitalized patient in a private hospital in the Kingdom of Saudi Arabia [1]. The disease has attracted the attention of the global heath community because it carries a high fatality rate of 40% to 60% [2], [3], [4], [5]. The high case fatality rate could be an overestimate because the exact numbers of asymptomatic and mild cases were not well defined. Recent estimates indicate an overall fatality rate of 35%. Over the 4 years since the virus's discovery, there have been multiple healthcare-associated outbreaks [2], [3], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. The main reason for these outbreaks was the difficulties in early identification of MERS-CoV confirmed cases from influenza-like illness cases, leading to inappropriate application of infection control standards and quarantine. In an effort to facilitate this task, differentiation between MERS-CoV and non–MERS-CoV cases based on epidemiologic and clinical indicators was evaluated in few studies, but with no helpful findings [16], [17], [18]. A case–control analysis identified some significant predictors in univariate but not in multivariate analysis [16]. In other studies, presenting symptoms were not specific for MERS-CoV infection [17], [18].
The Saudi Ministry of Health (MoH) developed and released a visual triage system to be used by all hospitals for the early identification of patients with acute respiratory illness in EDs, dialysis units and clinics [19]. In this study, we retrospectively evaluated the performance of this visual acute respiratory illness triage system for the prediction of MERS-CoV infection.
Patients and methods
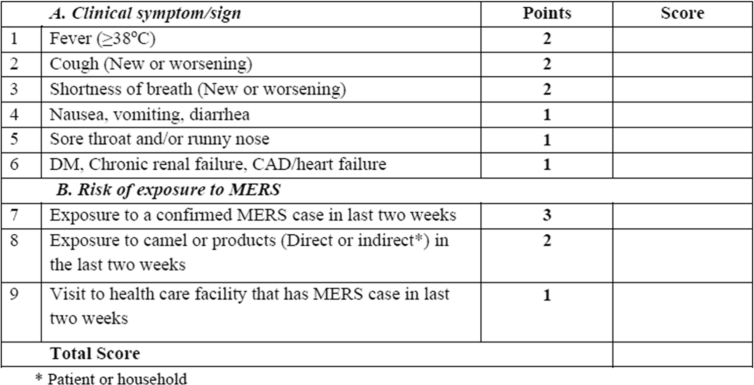
Prince Mohammed bin Abdulaziz Hospital is a referral centre for MERS-CoV patients diagnosed in the central region based in Riyadh, Saudi Arabia. The visual triage form documents the institution, unit, healthcare worker involved in the triage with name and signature, and patient contact details (Fig. 1). It comprises nine items classified into two sections, with one related to the patient's symptoms and signs and presentation and the other section related to the patient's potential risk of exposure to MERS-CoV, each with a defined predetermined score (Fig. 1). The patient's symptoms include fever, cough, shortness of breath, nausea, vomiting or diarrhea, sore throat or runny nose and the presence of underlying conditions including diabetes mellitus, chronic renal failure, coronary artery disease or heart failure. Any patient scoring ≥4 will need isolation and assessment by a physician before ruling out MERS-CoV. All admitted patients from 1 April 2014 to December 2017 who were tested for MERS-CoV were included in this study. MERS-CoV testing was done using nasopharyngeal swabs as described previously [4]. MERS-CoV diagnosis was based on positive real-time reverse-transcriptase PCR as described previously [4], [16], [20].
Fig. 1.
Visual triage form showing two sections, one related to patient presenting symptoms and signs and one related to risk of exposure to MERS-CoV. CAD, coronary artery disease; DM, diabetes mellitus; MERS-CoV, Middle East respiratory syndrome coronavirus.
We calculated the triage score for each patient on the basis of the scores from Saudi MoH [19] using the identified signs and symptoms. The percentage of patients with the specified score was calculated and compared between those with and without MERS-CoV infection using a chi-square test.
We then calculated the sensitivity and specificity of the scoring scale adopted for the identification of positive cases in relation to virus detection by real-time PCR. Sensitivity and specificity were calculated, for an original cutoff value of 4. We subsequently challenged the triage scoring by increasing the values of MERS-CoV potential exposure specific items 7 to 9 by giving 6 points for factor 7 instead of 3, 4 points for factor 8 instead of 2 and 2 for factor 9 instead of 1. We then calculated sensitivity and specificity in the same manner.
Results
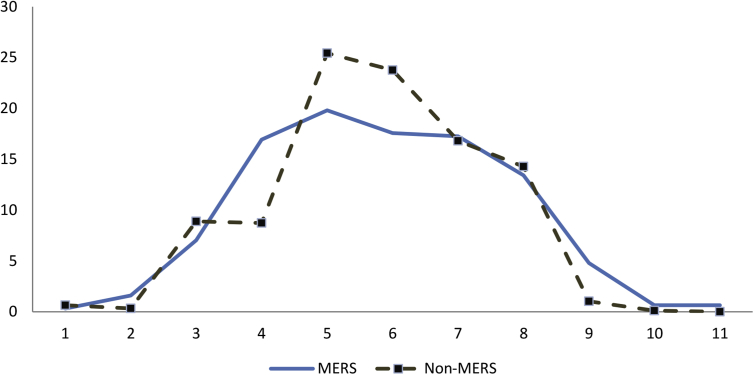
During the study period from 2014 to 2017, there was a total of 2435 suspected MERS-CoV cases. Of these, 1823 cases (75%) tested negative and the remaining 25% tested positive for MERS-CoV by PCR. The application of the visual triage score resulted in a similar percentage of MERS-CoV and non–MERS-CoV patients with each score from 0 to 11 (Fig. 2). The percentage of patients with a cutoff score of ≥4 was 75% in patients with MERS and 85% in patients without MERS (p 0.0001). The sensitivity and specificity of this cutoff score for MERS-CoV infection were 74.1% and 18.6%, respectively. Increasing the values of items 7 to 9 and recalculating the score, as indicated above, was not discriminative as well.
Fig. 2.
Percentage of patients (y-axis) with specified score (x-axis) in those with (solid line) and without (dashed line) MERS-CoV infection. MERS-CoV, Middle East respiratory syndrome coronavirus.
Discussion
We retrospectively evaluated the proposed Saudi MoH triage scoring system for the screening of MERS-CoV patients. During the outbreak of severe acute respiratory syndrome, one hospital expanded the isolation criteria to include all patients with undifferentiated fever with or without respiratory symptoms or chest X-ray changes [21]. Differentiating patients with MERS-CoV from other non–MERS-CoV patients has been a challenge for the healthcare workers in Saudi Arabia. There are no signs, symptoms, physical findings, specific laboratory or radiologic findings that can help healthcare workers identify MERS-CoV patients [17], [18], [19]. This was made clear from recent MoH data, which showed that every year more than 50,000 patients suspected to be infected with MERS-CoV were tested kingdomwide, with a yield of 0.7% positivity [22]. This calls for regular review of MERS-CoV case definition and a reevaluation of the visual triage scoring to ensure its accuracy and to avoid clogging isolation rooms and exhausting MoH financial resources by excessive testing of suspected MERS-CoV cases. In addition, it is important to have healthcare organizations ready to identify and isolate patients with suspected MERS-CoV. In a recent study evaluating the readiness of hospitals in the United States, 42 specific drills for patients with possible MERS-CoV infection were conducted in New York City [23]. The drills revealed variable identification of potentially infectious patients and implementation of appropriate infection control measures [23].
Rever-transcriptase PCR testing for MERS-CoV is the reference standard test for MERS-CoV, and in the Kingdom of Saudi Arabia, the MoH assigned five regional laboratories for testing all samples (Makkah, Madinah, Jeddah, Riyadh and Dammam). When samples are collected from the same city where a laboratory is located, it takes an average of a full day for the result, while samples from outside the city will take 1.5 days, and from one region to another, it will take 2 days. In the setting of crowded EDs, limited numbers of isolation rooms and the need to rapidly decide on contact tracing and intervention for contacts of positive patients, this time frame is potentially long.
To address this concern and to avoid a huge backlog of patients in the ED, two strategies are needed. First, the case definition needs to be revised after careful review of available data to the MoH for 5 years. Second, we must invest in developing highly sensitive and specific point-of-care testing in EDs and haemodialysis units where the results will be available in 1 to 2 hours [24], [25], [26]. Emerging diseases with regional and international implications need to be addressed in a consultative format involving all stakeholders locally, regionally and internationally.
The current study, conducted on a large number of patients, showed that clinical scoring is not predictive of MERS infection. Our results are robust and confirm that MERS cannot be distinguished from other respiratory infections on the basis of risk factors and clinical features. Thus, all patients with nonspecific symptoms in a MERS-endemic area will have to be isolated until MERS can be ruled out by PCR testing. This strategy has important consequences in terms of public health, notably in order to limit the transmission of MERS in the healthcare setting. This is particularly relevant in countries where access to rapid diagnosis is scarce. Meanwhile, until point-of-care MERS-CoV testing is made available, triage of patients on the basis of the current MoH triage score should be revised to a robust scoring system.
The current study has few limitations. This is a retrospective study, and thus the exact application of the findings on new cases might not be generalizable. The study was performed in a MERS-CoV referral centre, and the applicability of the findings to patients being seen in the ED therefore might not be accurate. Thus, further refinement of the score is needed for better prediction of MERS-CoV infection in patients seeking care in the ED.
Conflict of interest
None declared.
References
- 1.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus: epidemiology and disease control measures. Infect Drug Resist. 2014;7:281–287. doi: 10.2147/IDR.S51283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A.T. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penttinen P.M., Kaasik-Aaslav K., Friaux A., Donachie A., Sudre B., Amato-Gauci A.J. Taking stock of the first 133 mers coronavirus cases globally—is the epidemic changing? Euro Surveill. 2013;18:20596. doi: 10.2807/1560-7917.es2013.18.39.20596. [DOI] [PubMed] [Google Scholar]
- 6.Oboho I.K., Tomczyk S.M., Al-Asmari A.M., Banjar A.A., Al-Mugti H., Aloraini M.S. 2014 MERS-CoV outbreak in Jeddah—a link to health care facilities. N Engl J Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drosten C., Muth D., Corman V.M., Hussain R., Al Masri M., HajOmar W. An observational, laboratory-based study of outbreaks of Middle East respiratory syndrome coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia, 2014. Clin Infect Dis. 2015;60:369–377. doi: 10.1093/cid/ciu812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Tawfiq J.A., Memish Z.A. An update on Middle East respiratory syndrome: 2 years later. Expert Rev Respir Med. 2015;9:327–335. doi: 10.1586/17476348.2015.1027689. [DOI] [PubMed] [Google Scholar]
- 9.Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus: transmission and phylogenetic evolution. Trends Microbiol. 2014;22:573–579. doi: 10.1016/j.tim.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hijawi B., Abdallat M., Sayaydeh A., Alqasrawi S., Haddadin A., Jaarour N. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Heal J. 2013;19(Suppl. 1):S12–S18. [PubMed] [Google Scholar]
- 11.Kim Y., Lee S., Chu C., Choe S., Hong S., Shin Y. The characteristics of Middle Eastern respiratory syndrome coronavirus transmission dynamics in South Korea. Osong Public Health Res Perspect. 2016;7:49–55. doi: 10.1016/j.phrp.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seong M.W., Kim S.Y., Corman V.M., Kim T.S., Cho S.I., Kim M.J. Microevolution of outbreak-associated Middle East respiratory syndrome coronavirus, South Korea, 2015. Emerg Infect Dis. 2016;22:327–330. doi: 10.3201/eid2202.151700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Memish Z.A., Zumla A.I., Al-Hakeem R.F., Al-Rabeeah A.A., Stephens G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 14.Omrani A.S., Matin M.A., Haddad Q., Al-Nakhli D., Memish Z.A., Albarrak A.M. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17:e668–e672. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Memish Z.A., Cotten M., Watson S.J., Kellam P., Zumla A., Alhakeem R.F. Community case clusters of Middle East respiratory syndrome coronavirus in Hafr Al-Batin, Kingdom of Saudi Arabia: a descriptive genomic study. Int J Infect Dis. 2014;23:63–68. doi: 10.1016/j.ijid.2014.03.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Tawfiq J.A., Hinedi K., Ghandour J., Khairalla H., Musleh S., Ujayli A. Middle East respiratory syndrome–coronavirus (MERS-CoV): a case–control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohd H.A., Memish Z.A., Alfaraj S.H., McClish D., Altuwaijri T., Alanazi M.S. Predictors of MERS-CoV infection: a large case control study of patients presenting with ILI at a MERS-CoV referral hospital in Saudi Arabia. Travel Med Infect Dis. 2016;14:464–470. doi: 10.1016/j.tmaid.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garbati M.A., Fagbo S.F., Fang V.J., Skakni L., Joseph M., Wani T.A. A comparative study of clinical presentation and risk factors for adverse outcome in patients hospitalised with acute respiratory disease due to MERS coronavirus or other causes. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Command and Control Center Ministry of Health Kingdom of Saudi Arabia Scientific Advisory Board . 4th ed. 2017. Infection prevention and control guidelines for the Middle East respiratory syndrome coronavirus (MERS-CoV) infection.http://www.moh.gov.sa/endepts/Infection/Documents/Guidelines-for-MERS-CoV.PDF Available at: [Google Scholar]
- 20.Al-Tawfiq J.A., Hinedi K., Abbasi S., Babiker M., Sunji A., Eltigani M. Hematologic, hepatic, and renal function changes in hospitalized patients with Middle East respiratory syndrome coronavirus. Int J Lab Hematol. 2017;39:272–278. doi: 10.1111/ijlh.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh K., Hsu L.Y., Villacian J.S., Habib A., Fisher D., Tambyah P.A. Severe acute respiratory syndrome: lessons from Singapore. Emerg Infect Dis. 2003;9:1294–1298. doi: 10.3201/eid0910.030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bin Saeed A.A., Abedi G.R., Alzahrani A.G., Salameh I., Abdirizak F., Alhakeem R. Surveillance and testing for Middle East respiratory syndrome coronavirus, Saudi Arabia, April 2015–February 2016. Emerg Infect Dis. 2017;23:682–685. doi: 10.3201/eid2304.161793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foote M.M.K., Styles T.S., Quinn C.L. Assessment of hospital emergency department response to potentially infectious diseases using unannounced mystery patient drills—New York City, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:945–949. doi: 10.15585/mmwr.mm6636a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huh H.J., Kim J.Y., Kwon H.J., Yun S.A., Lee M.K., Ki C.S. Performance evaluation of the PowerChek MERS (upE & ORF1a) real-time PCR kit for the detection of Middle East respiratory syndrome coronavirus RNA. Ann Lab Med. 2017;37:494–498. doi: 10.3343/alm.2017.37.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S.H., Baek Y.H., Kim Y.H., Choi Y.K., Song M.S., Ahn J.Y. One-pot reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) for cetecting MERS-CoV. Front Microbiol. 2017:2166. doi: 10.3389/fmicb.2016.02166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim K.H., Tandi T.E., Choi J.W., Moon J.M., Kim M.S. Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, 2015: epidemiology, characteristics and public health implications. J Hosp Infect. 2017;95:207–213. doi: 10.1016/j.jhin.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]