Abstract
Cervical cancer is the third most common cancer among women worldwide and is usually managed with chemoradiation in advanced disease. This case presents a 41-year-old female with locally advanced cervical cancer who underwent combination intracavitary/interstitial brachytherapy after chemoradiation for local disease control. At her fifth brachytherapy session, one of the interstitial needles was malpositioned and lead to vascular injury with significant blood loss. She subsequently underwent emergent embolization of a branch of the right obturator artery with immediate clinical improvement and no complications. This is the first reported case of vascular injury from an interstitial brachytherapy needle that required arterial embolization for hemostasis.
Keywords: Interstitial brachytherapy, Cervical cancer, Bleeding, Embolization
Introduction
Cervical cancer is the third most common cancer among women worldwide, but improvements in screening methods and the development of the human papilloma virus vaccine have decreased its incidence by 70% over 50 years [1]. Early stage cervical cancer, defined as stage IA and IB1 based on the International Federation for Gynecology and Obstetrics staging classification, is primarily treated with modified radical hysterectomy rather than primary radiation therapy (RT) [2], [3]. For patient with advanced disease, defined stage IB2 to IVA, the primary treatment is RT with concurrent cisplatin chemotherapy. For patients with para-aortic nodal disease, treatment is usually extended field radiation therapy with concurrent cisplatin therapy [4], [5], [6].
Brachytherapy allows for delivery of internal radiation therapy localized to the cervix with relative sparing of the surrounding tissues, and has been shown to significantly increase cancer-specific and overall survival rates in patients with advanced disease [7]. There are 2 main techniques for delivering brachytherapy that may be used concurrently for cervical cancer: intracavitary and interstitial. Intracavitary brachytherapy is delivered using a intrauterine tandem with either vaginal ovoids, vaginal cylinders, or vaginal rings [8]. Interstitial brachytherapy provides targeted therapy for larger masses that may exhibit significant local spread or lower vaginal involvement, and is performed by placing needles using freehand or a template [8]. We present the first reported case of vascular injury from interstitial needle placement during a brachytherapy session requiring embolization in a patient with locally advanced cervical cancer.
Case presentation
Institutional Review Board exemption was obtained for this case report. A 41-year-old female presented with several months of progressive menorrhagia as well as pelvic and back pain. On physical exam, she was noted to have a 7 cm circumferential necrotic cervical lesion with pathology showing squamous cell carcinoma. Positron emitted tomography-computed tomography (CT) demonstrated a 6.3 × 5.5 cm hypermetabolic mass with necrotic center and numerous bilateral pelvic and peri-aortic hypermetabolic lymph nodes. She was diagnosed with FIGO IIB pelvic and para-aortic lymph node positive squamous cell carcinoma of the cervix. She subsequently underwent extended field external beam radiation therapy with concurrent cisplatin. Postchemoradiation magnetic resonance imaging showed persistent but decreased size of the tumor with bilateral parametrial involvement. It was recommended that she undergo 5 sessions of high dose rate (HDR) brachytherapy.
Brachytherapy was delivered with a tandem and ovoid/interstitial hybrid technique utilizing the Utrecht applicator (Elekta, Stockholm, Sweden) and between 8 and 10 interstitial needles per treatment. Prior to each brachytherapy treatment, she was taken to the operating room for placement of the brachytherapy applicator and interstitial needles under anesthesia, then transferred to the radiation oncology department for CT-based 3D treatment delivery planning. The first 4 sessions were tolerated without any complications. During the fifth session, the applicator with interstitial needles was placed in the operating room in standard fashion without immediate complications. On routine CT-based 3D treatment planning, it was noted that the brachytherapy applicator geometry was inadequate for treatment, and thus the applicator was removed. Upon removal of the applicator, brisk vaginal bleeding was immediately encountered. Multiple attempts to control the bleeding with vaginal packing and tamponade were unsuccessful. There was an estimated blood loss of 700 ml and she was transfused with 4 units of packed RBCs but continued to experience vaginal bleeding. She then underwent emergent angiography to identify and embolize the source of bleeding.
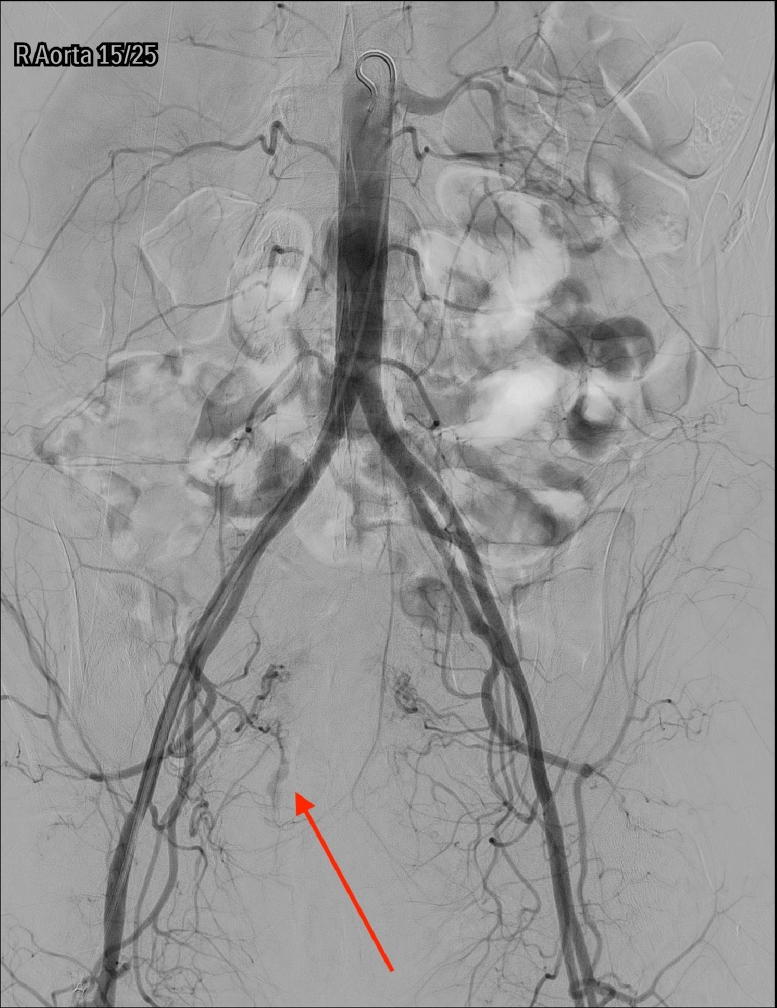
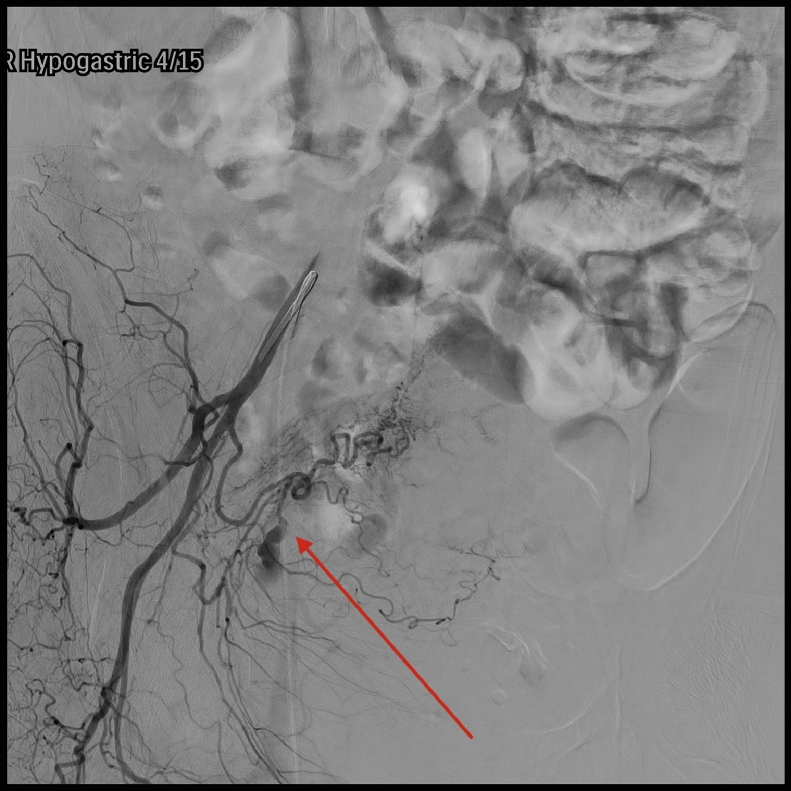
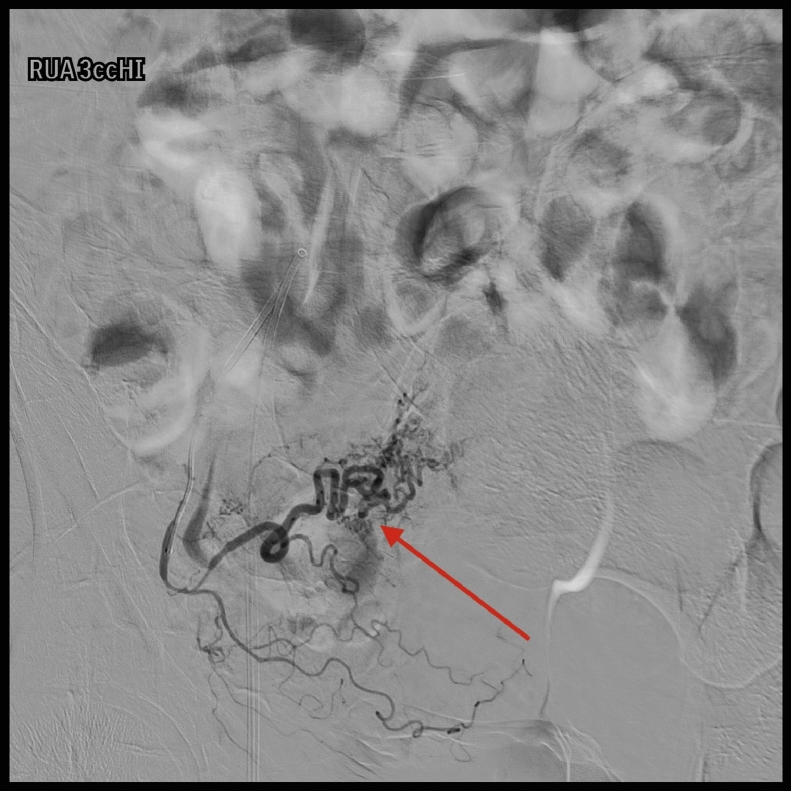
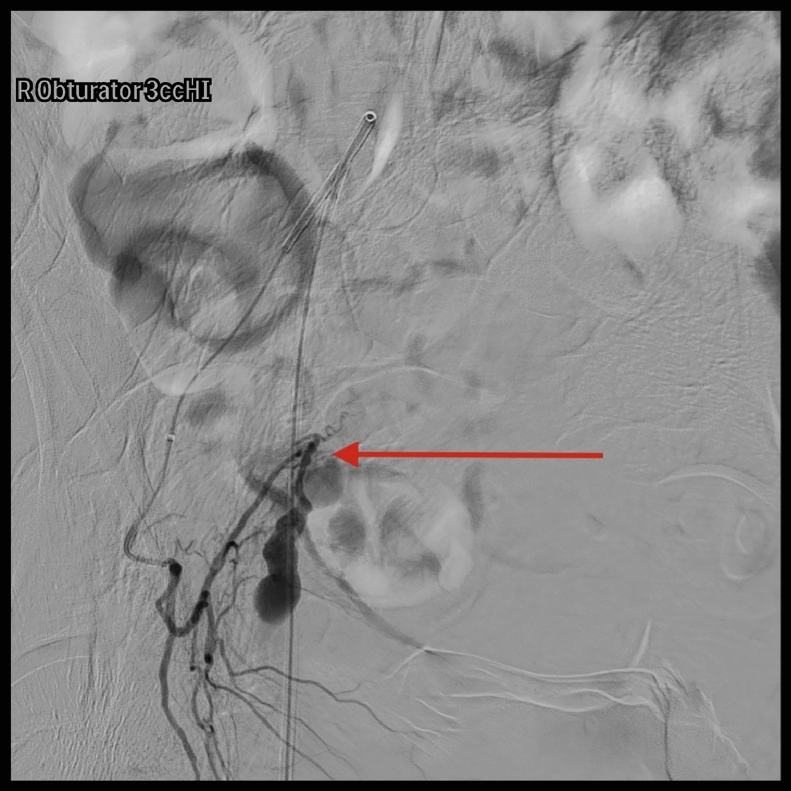
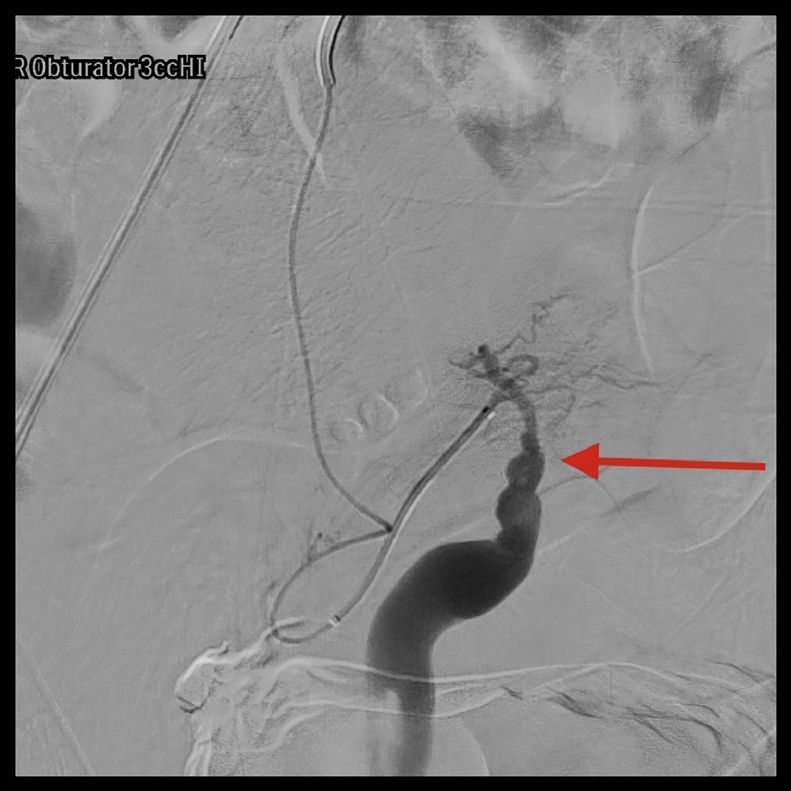
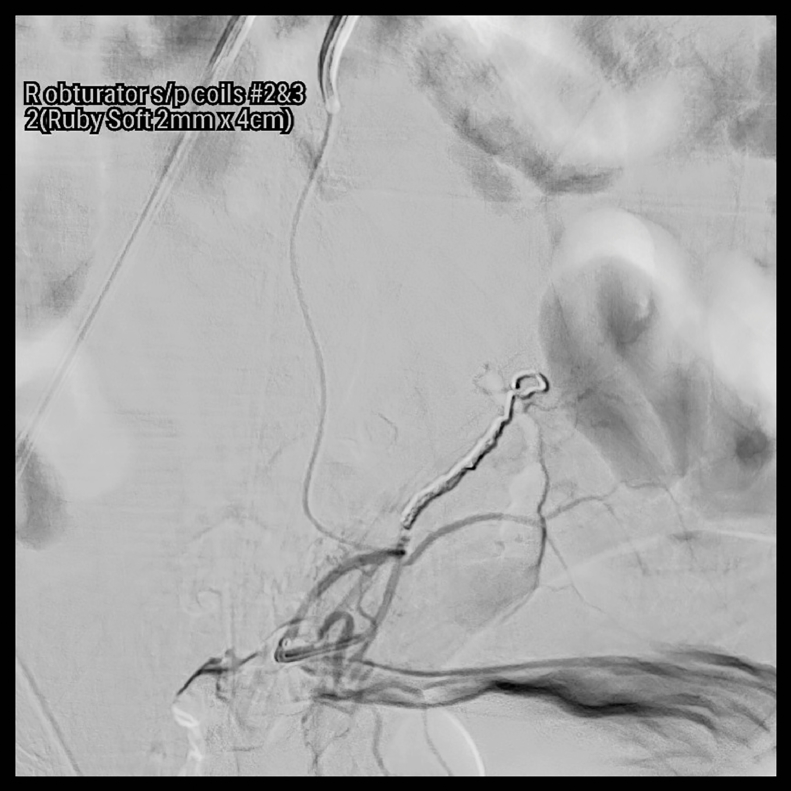
A pelvic arteriogram showed extraluminal contrast extravasation in the right pelvis (Fig. 1). Right hypogastric angiogram demonstrated extraluminal contrast extravasation originating from one of the branches of the right hypogastric artery (Fig. 2). Selective catheterization and angiogram of the right uterine artery revealed no contrast extravasation (Fig. 3). Selective catheterization and angiogram of the third anterior division of the right hypogastric, the right obturator artery, showed a cephalad branch in communication with the extravasated contrast accumulation (Fig. 4). This branch was selectively catheterized (Fig. 5) and embolized with a series of 2 mm and 3 mm Ruby coils (Penumbra Inc., Alameda, CA; Fig. 6). Coils were also placed in a more proximal branch in this territory to complete occlusion of the distal aspect of the territory. Postembolization angiography showed lack of filling of the embolized territory and no further contrast extravasation (Fig. 7).
Fig. 1.
Pelvic arteriogram with arrow pointing to extraluminal contrast extravasation in the right pelvis.
Fig. 2.
Right hypogastric angiogram with arrow pointing to extraluminal contrast extravasation originating from one of the branches of the right hypogastric artery.
Fig. 3.
Selective catheterization of the right uterine artery with subsequent angiogram revealing no contrast extravasation.
Fig. 4.
Selective catheterization with subsequent angiogram of the third anterior division of the right hypogastric, likely the right obturator artery, with arrow pointing at cephalad branch in communication with the extravasated contrast accumulation.
Fig. 5.
Selective catheterization with subsequent angiogram of a branch of the right obturator artery showing contrast extravasation.
Fig. 6.
Postembolization angiogram showing successful target embolization.
Fig. 7.
Postembolization angiography showed lack of filling of the embolized territory and no further contrast extravasation.
Postembolization, she was admitted to the intensive care unit for intensive monitoring. Her hemoglobin continued to stabilize over the next 48 hours and vaginal bleeding slowed significantly. On hospital day 2, her vitals signs were within normal limits, she was ambulating, and vaginal bleeding was noted to be light enough for packing to be removed. She was discharged in stable condition to home on hospital day 3. Upon her next visit 10 days after discharge to reattempt her fifth and final HDR, she denied further bleeding and her only complaints were mild diarrhea and urinary frequency.
She proceeded to receive her final fraction of HDR with the tandem and ovoid applicator implant without interstitial needles to complete her treatment. Total dose delivered was 30 Gy in 5 fractions to the high risk clinical target volume.
Discussion
Given the tendency of cervical carcinoma to extend into local contiguous tissue, risk assessment of targeted therapy should utilize imaging to determine the degree of cancer spread and possibility of collateral damage to healthy tissue [1]. There are many techniques available to guide the placement of interstitial brachytherapy needles under fluoroscopic, ultrasound, MRI, CT, and laparoscopic guidance to minimize the risk of damage to surrounding structures [1]. One study also used 3D MRI preplanning to reduce the risk of complications by virtually planning needle positions before the procedure was performed [12]. CT preplanning was used in this case, but due to distorted anatomy likely secondary to reduction in tumor burden as a result of prior chemoradiation, applicator placement at the fifth brachytherapy session was challenging and complicated.
Injury to the uterus, vagina, bladder, small bowel, sigmoid colon, ureters, and vasculature and infection are known complications of brachytherapy with interstitial needle placement for cervical carcinoma [8], [9]. Thus, needle insertions increase the risks of complications from brachytherapy, with vascular injury reportedly occuring in up to 5.2% of instances of applicator removal in patients receiving interstitial brachytherapy for cervical carcinoma. These vascular injuries were reported to have been successfully managed with vaginal tamponade and/or stitch, blood transfusions, inpatient stays for close monitoring or endoscopic intervention [9], [10], [11], [12]. In this case, the patient had 10 needles taken out upon applicator removal, with ensuing hemodynamic instability unresponsive to tamponade and repeat blood transfusions. Thus, interventional radiology was consulted for a more creative solution.
Injury to a major blood vessel is perhaps one of the most time-sensitive causes of procedure-associated morbidity and mortality, but also one of the most manageable. Thus, our goal was to share our finding that the obturator artery is a bleeding risk with interstitial brachytherapy for cervical carcinoma, and that proximal bleeding control may be achieved with embolization, when more conservative measures are unyielding.
Footnotes
Conflicts of Interest: None.
Contributor Information
Vanya Aggarwal, Email: vanya.aggarwal5@gmail.com.
Anthony Chuprin, Email: chupria@evms.edu.
Abhimanyu Aggarwal, Email: abhiaggarwalmd@gmail.com.
Harlan Vingan, Email: vinganmd@gmail.com.
Edwin Crandley, Email: crandlef@evms.edu.
References
- 1.Banerjee R, Kamrava M. Brachytherapy in the treatment of cervical cancer: a review. Int J Womens Health. 2014;6(1):555–564. doi: 10.2147/IJWH.S46247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landoni F, Maneo A, Colombo A. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet (London, England) 1997;350(9077):535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 3.Bansal N, Herzog TJ, Shaw RE, Burke WM, Deutsch I, Wright JD. Primary therapy for early-stage cervical cancer: radical hysterectomy vs radiation. Am J Obstet Gynecol. 2009;201(5):485. doi: 10.1016/j.ajog.2009.06.015. e1-9. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita H, Okuma K, Kawana K. Comparison between conventional surgery plus postoperative adjuvant radiotherapy and concurrent chemoradiation for FIGO stage iib cervical carcinoma. Am J Clin Oncol. 2010;33(6):583–586. doi: 10.1097/COC.0b013e3181cae5b7. [DOI] [PubMed] [Google Scholar]
- 5.Peters WA, Liu PY, Barrett RJ. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 6.Varia MA, Bundy BN, Deppe G. Cervical carcinoma metastatic to para-aortic nodes: extended field radiation therapy with concomitant 5-fluorouracil and cisplatin chemotherapy: a Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1998;42(5):1015–1023. doi: 10.1016/s0360-3016(98)00267-3. http://www.ncbi.nlm.nih.gov/pubmed/9869224 Accessed February 19, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Han K, Milosevic M, Fyles A, Pintilie M, Viswanathan AN. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;87(1):111–119. doi: 10.1016/j.ijrobp.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan AN, Thomadsen B. American Brachytherapy Society Consensus guidelines for locally advanced carcinoma of the cervix. Part I: general principles. Brachytherapy. 2012;11(1):33–46. doi: 10.1016/j.brachy.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Leung E, Mendez L, Lang P. Acute catheter complications from perineal interstitial brachytherapy (ISBT) in gynecological cancer patients: a prospective analysis of organ injury, infections and radiological needle intrusions. Brachytherapy. 2017;16(3):S74. [Google Scholar]
- 10.Bernstein M, Mehta KJ, Yaparpalvi R, Kuo H, Kalnicki S. PO-290 results of the hybrid interstitial-intracavitary utrecht applicator for cervical cancer in an outpatient setting. 2012. doi: 10.1016/S0167-8140(12)72257-0. [DOI]
- 11.Celada Alvarez F, Burgos J, Roldán S, et al. Dosimetric outcome and perioperative toxicity using Utrecht applicator in cervical brachytherapy. ESTRO 35, 29 April - 3 May 2016, Turin, Italy. 2016;119:S466-S467. doi: 10.1016/S0167-8140(16)32209-5. [DOI]
- 12.Fokdal L, Tanderup K, Hokland SB. Clinical feasibility of combined intracavitary/interstitial brachytherapy in locally advanced cervical cancer employing MRI with a tandem/ring applicator in situ and virtual preplanning of the interstitial component. Radiother Oncol. 2013;107(1):63–68. doi: 10.1016/j.radonc.2013.01.010. [DOI] [PubMed] [Google Scholar]