Abstract
Purpose:
Colorectal cancer (CRC) continues to demonstrate racial disparities in incidence and survival in the United States. This study investigates the role of neighborhood concentrated disadvantage in racial disparities in CRC incidence in Louisiana.
Methods:
Louisiana Tumor Registry and U.S. Census data were used to assess the incidence of CRC diagnosed in individuals 35 years and older between 2008 and 2012. Neighborhood concentrated disadvantage index (CDI) was calculated based on the PhenX Toolkit protocol. The incidence of CRC was modeled using multilevel binomial regression with individuals nested within neighborhoods.
Results:
Our study included 10,198 cases of CRC. Adjusting for age and sex, CRC risk was 28% higher for blacks than whites (risk ratio [RR] = 1.28; 95% confidence interval [CI] = 1.22–1.33). One SD increase in CDI was associated with 14% increase in risk for whites (RR = 1.14; 95% CI = 1.10–1.18) and 5% increase for blacks (RR = 1.05; 95% CI = 1.02–1.09). After controlling for differential effects of CDI by race, racial disparities were not observed in disadvantaged areas.
Conclusion:
CRC incidence increased with neighborhood disadvantage and racial disparities diminished with mounting disadvantage. Our results suggest additional dimensions to racial disparities in CRC outside of neighborhood disadvantage that warrants further research.
Keywords: Colorectal cancer, Concentrated disadvantage, Disparities, Multilevel modeling, Social epidemiology
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States [1]. CRC incidence is significantly higher in Louisiana than in the United States, for all race-sex groups [1]. In the past 2 decades, CRC incidence has decreased overall, but racial disparities remain, with blacks having greater incidence of CRC and poorer survival than non-Hispanic whites [2].
The World Health Organization’s Commission on the Social Determinants of Health recognizes that health disparities arise from the conditions in which people are born, grow, live, work, and age [3]. Social determinants of health encompass the social, economic, political, cultural, and environmental factors that affect a person’s health. Social determinants potentially play a principal role in the development and progression of certain cancers. This is partially attributable to the increasing recognition that social stress represents an environmental exposure that affects individual health through metabolic pathways leading to obesity [4–9]. Obesity has been identified as a risk factor for a variety of chronic disease outcomes, including cancer [10].
Neighborhood environment is an important indicator of individual health, independent of individual characteristics, such as income or education [4,11–13]. Adverse neighborhood living environments are associated with conditions that contribute to metabolic dysfunction either directly through social stressors(i.e., lack of resources and crime) or indirectly through neighborhood conditions that promote unhealthy behaviors (i.e., unhealthy diet and physical inactivity) [14]. With regard to social stressors, studies have found that chronic exposure to disadvantaged neighborhoods has a direct effect on individual health through prolonged activation of the hypothalamic-pituitary-adrenal and sympathetic adrenal axes in a process described as allostasis [15–18]. Allostatic changes are associated with a chronic inflammatory state and metabolic dysfunction. With regard to neighborhood conditions, disadvantaged neighborhoods lack physical characteristics that can promote healthy behavior (e.g., walkability and access to fresh foods), a constraint that also leads to metabolic dysfunction [19–22].
Conventionally, incidence rates for cancer are reported for geographic units larger than the census tract to increase the reliability of calculated rates. Thus, most studies regarding the incidence of cancer are conducted at the county level. However, it is recognized that smaller units are more ideal for characterizing exposures to social determinants in the neighborhood environment(i.e., census tract and block group) because of socioeconomic heterogeneity within counties [23]. Although some researchers use small unit area–based social measures as a proxy for individual status in traditional single-level models, research has shown that this approach can lead to model misspecification, particularly in the realm of health disparities, and that multilevel models are superior [24,25]. However, a common limitation of multilevel studies of social determinants of health is that many data sources lack a sufficient number of individuals nested within neighborhoods to produce a strong multilevel data structure.
Using methods initially proposed by Subramanian et al. [26], we designed a population-based study using cancer registry and U.S. Census population data to investigate the role of neighborhood concentrated disadvantage in the incidence of CRC and the degree this appears to contribute to racial disparities. Two previous studies have used similar methods to examine racial disparities in mortality. In both studies, differential exposure to neighborhoods by race resulted in the misspecification of race in a single-level model. In addition, area poverty explained a significant portion of neighborhood variation in all-cause mortality, which evidenced area poverty as a key contributor to mortality and to racial disparities overall [25,26].
The aim of this study is to investigate potential risk of CRC associated with living in a local environment characterized by concentrated disadvantage and if differential exposure to neighborhood environments contributes to racial disparities. We hypothesize that differential exposure to adverse community environments leads to increased incidence of CRC in disadvantaged and minority residents. Results from this study will shed light on the role of neighborhood environment in disparities in CRC.
Methods
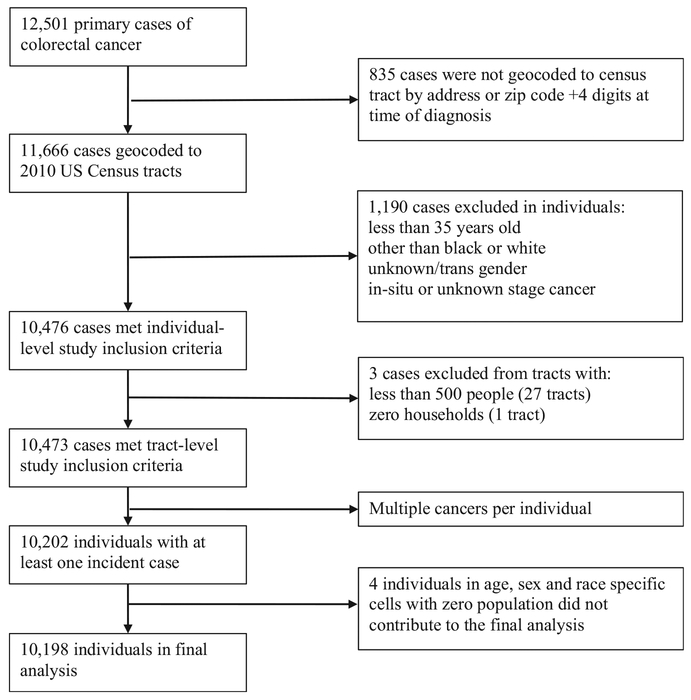
This study involves secondary data analysis, using data from the Louisiana Tumor Registry, a participant of the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, and U.S. Census data. The Louisiana State University Health Sciences Center Institutional Review Board approved this research. Primary invasive colon and rectum cancer cases diagnosed from January 2008 to December 2012 were identified by International Class-ification of Diseases for Oncology, Third Edition, site codes C180–C189, C199, C209, and C260. Cases with International Classification of Diseases for Oncology, Third Edition, morphology codes 9050–9055, 9140, and 9590–9992 were excluded. Cases of CRC from the Louisiana Tumor Registry were geocoded to 2010 census tracts using the Automated Geospatial Geocoding Interface Environment system, which was developed through a partnership between the North American Association of Central Cancer Registries, Texas A&M University, and the National Cancer Institute as a single, uniform geocoding platform for open use by cancer registries [27]. The geocoding process used street address or zip code +4 at the time of diagnosis, with a success rate above 93%. The population at risk was determined from the 2010 U.S. Census.
In assessing cancer incidence, we address adults 35 years and older, of black or white race alone, diagnosed with at least one primary invasive colorectal tumor during the study period. In situ or unknown stage tumors were not included. Age was categorized into four age groups (35–44, 45–54, 55–64, and 65 years and older). Sex was categorized as male or female. Race was grouped as black or white, as defined by the U.S. Census; other races were not included in these analyses due to small group numbers. The total number of possible individual-level demographic risk factor combinations was 16.
An individual’s neighborhood environment was measured in terms of socioeconomic disadvantage. Concentrated disadvantage index (CDI) scores for neighborhoods (i.e., census tracts) were calculated based on the PhenX Toolkit protocol, established by a collaboration of the Research Triangle Institute and the National Human Genome Research Institute for the development of consensus measures of phenotypes and exposures across research studies [28]. CDI is a sample-based composite score derived from a principle components analysis of six measures at the census tract level (given as percentages): (1) individuals below the poverty line;(2) households receiving public assistance income; (3) female-headed families; (4) individuals who are unemployed; (5) individuals below the age of 18 years; and (6) individuals who are black. The construct operationalizes urban theory regarding the overconcentration of blacks, children, and female-headed families in economically disadvantaged neighborhoods [29]. In addition to associations with adverse neighborhood conditions such as increased crime, the construct has been linked to metabolic conditions and racial disparities in cancer survival [7,30,31]. We derived CDI using 2008–2012 5-year estimates from the American Community Survey, to align with the study period. The resulting factor explained 58% of the variance present in the original six variables for the state of Louisiana during the study period. Factor loadings for the original variables are provided as supplemental material (Table S1). Factor scores for study census tracts follow a standard normal distribution with a mean of zero and SD of 1.
Louisiana has one thousand one hundred forty-eight 2010 census tracts; there were 19 census tracts with no population, which did not contribute to the analysis. Standard U.S. census tracts typically contain between 2500 and 8000 residents [32]. We excluded eight nonstandard census tracts that had a population less than 500. These tracts had a total of 1190 residents and three incident cases. Because census tracts are designed to be relatively homogenous in terms of socioeconomic characteristics [32], we did not feel it was appropriate to merge the population into other tracts. Finally, we excluded the census tract occupied by the Orleans Parish Prison. The prison had a population of 3089 in 2010 and no incident cases of CRC. After these exclusions, individuals from 1120 Louisiana census tracts remained eligible for the study.
Based on the study design, cases of CRC were aggregated for 16 demographic risk combinations (cells) for each of the 1120 census tracts, which yielded 17,920 possible data cells. The population at risk for age-, sex-, and race-specific cells within each census tract was determined from 2010 U.S. Census population counts, multiplied by 5 to represent person-years at risk. For each data cell, cancer cases and population-years were used to construct a binomial random variable, where the response was given as the number of incident cases out of the person-years at risk. Less than 2% of cells had no at-risk population (person-years) and did not contribute to the analysis. There were 4 incident cases of CRC recorded for these cells but due to lack of population at risk, these cases were not included in the analysis. A total of 10,198 cases of CRC were included in the analysis; a case inclusion summary has been provided in the supplemental materials (Figure S1).
Statistical methods
Multilevel binomial regression models were used to model the incidence of CRC in Louisiana. The data structure consisted of two levels, with individuals (level 1) nested within census tracts (level2). The binomial response was number of incident cases over population-years at risk. Models were executed as generalized mixed linear models with fixed effects for age, sex, race, and CDI. To account for correlation among individuals within census tracts, or clustering, all models included a random intercept at the census tract level. We used a log link to directly estimate relative risk in the form of adjusted risk ratios (RRs). All statistical analyses were performed in SAS, version 9.4, software (SAS Institute, Cary, NC). Generalized mixed linear models were executed using the GLIMMIX procedure, with maximum likelihood estimation via adaptive quadrature method. Model fit was assessed through the Pearson Х2 goodness-of-fit statistic [33].
Our analyses focused on estimating racial disparities in CRC incidence and how neighborhood CDI relates to the disparities. A null model with no covariates was used to determine overall neighborhood variation, or clustering, in incidence. Our initial model (model1) was used to estimate racial disparities after adjusting for age and sex. A second model (model 2) included CDI to evaluate whether neighborhood disadvantage explained census tract–level variation in CRC incidence and how CDI affected observed racial differences. A final model (model 3) included an interaction between race and CDI to evaluate effect modification. P-values smaller than .05 were considered statistically significant.
Results
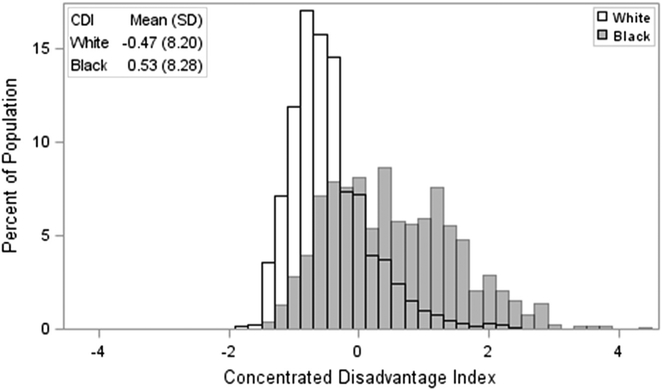
The study included 2,217,949 (Table 1) Louisiana residents aged 35 years and older. Black residents comprised 29.0% of the study population. Hispanic individuals were included based on race and not identified separately. In the study population, 96.4% of white residents and 99.3% of black residents were non-Hispanic. The majority of the study population was female (52.8%). There was a notable difference in the exposure of CDI for the study population by race. Black residents were disproportionately represented in more disadvantaged areas, as evidenced by higher CDI scores overall. The average CDI score for black residents in the study was 0.53 (SD = 8.20), contrasted with 0.47 (SD = 8.28) for white residents (Figure 1).
Table 1.
Louisiana study population characteristics by race in 2010 (n = 2,217,949)
| Total (n = 2,217,949) | White (n = 1,575,338) | Black (n = 642,611) | |
|---|---|---|---|
| Total (%) | 71.03 | 28.97 | |
| Age (%) | |||
| 35–44 y | 23.94 | 22.78 | 26.78 |
| 45–54 y | 28.25 | 27.28 | 30.62 |
| 55–64 y | 23.29 | 23.37 | 23.08 |
| 65+ y | 24.53 | 26.57 | 19.51 |
| Sex (%) | |||
| Female | 52.78 | 51.93 | 54.87 |
| Male | 47.22 | 48.07 | 45.13 |
| CDI | |||
| Mean | −0.18 | −0.47 | 0.53 |
Fig. 1.
Distribution of concentrated disadvantage index scores by race.
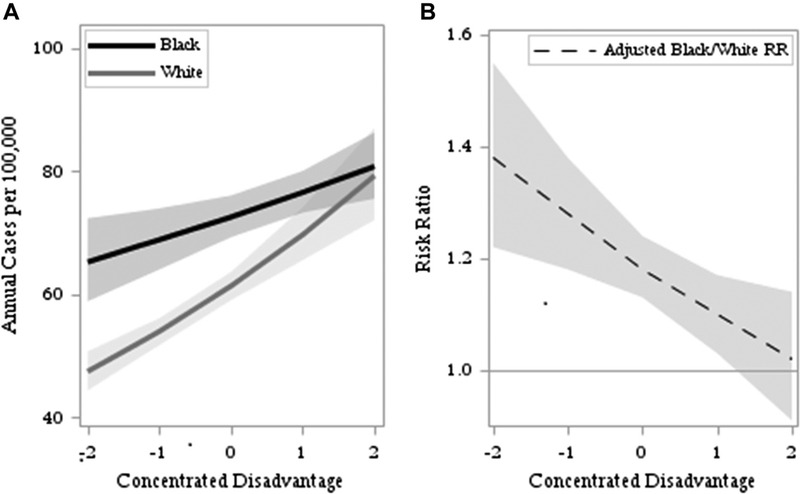
Age-, sex-, and race-specific cell CRC incidences are provided in Table 2. Age was categorized into 10-year groups; thus, rates do not reflect conventional 5-year group age-adjusted rates. Our study included 11,089,745 person-years of exposure with 10,198 incident cases of CRC, for a crude average annual incidence of 92 per 100,000. Across all age-sex groups, black residents had higher crude CRC incidence, which increased with advancing age, ranging from 17 to 286 cases per 100,000. Men had higher rates than women, except for the age group 35–44 years. Results from the null model and three adjusted multilevel binomial regression models are provided in Table 3. There was significant tract-level variation in the incidence of CRC in a null model. Adjusting for age and sex (model 1), black residents had 28% greater risk of CRC compared with whites. Unexplained tract-level variation decreased but remained significant after accounting for the demographic composition. Adding interactions between the individual-level demographic variables did not significantly improve the fit of the model, which was supported by the overall patterns in incidence by age, race, and sex in Table 2. Results from model 2 show that CDI was positively associated with CRC incidence overall. However, there was a significant interaction with race, indicating the magnitude of the effect of CDI was greater for the white population (model 3). One SD increase in CDI was associated with an estimated 14% increase in risk of CRC for whites and 5% increase for blacks. CDI and race interacted in such a way that estimated racial disparities largely disappeared in the most disadvantaged populations, as shown in Figure 2. At the midpoint CDI score of 0, the adjusted black/white RR indicated that blacks had an 18% greater risk of CRC incidence as compared with whites. The estimated disparity was greater in areas of less disadvantage, while the adjusted RR decreased toward 1 (no disparity) in more disadvantaged areas.
Table 2.
Age-, sex-, and race-specific average annual incidence of colon and rectal cancer diagnosed in Louisiana, 2008–2012
| Age | Sex | Race | Tracts | Cases | Population-years | Average annual cell incidence* |
|---|---|---|---|---|---|---|
| 35–44 y | Female | White | 1105 | 131 | 888,960 | 15 |
| Black | 1104 | 86 | 458,660 | 19 | ||
| Male | White | 1102 | 127 | 905,230 | 14 | |
| Black | 1107 | 70 | 401,940 | 17 | ||
| 45–54 y | Female | White | 1106 | 458 | 1,079,910 | 42 |
| Black | 1109 | 288 | 524,530 | 55 | ||
| Male | White | 1109 | 532 | 1,068,900 | 50 | |
| Black | 1107 | 298 | 459,275 | 65 | ||
| 55–64 y | Female | White | 1098 | 634 | 935,365 | 68 |
| Black | 1092 | 436 | 401,205 | 109 | ||
| Male | White | 1107 | 955 | 905,590 | 105 | |
| Black | 1096 | 518 | 340,420 | 152 | ||
| 65+ y | Female | White | 1100 | 1999 | 1,185,750 | 169 |
| Black | 1077 | 764 | 378,550 | 202 | ||
| Male | White | 1096 | 2192 | 906,985 | 242 | |
| Black | 1068 | 710 | 248,475 | 286 | ||
| All | 10,198 | 11,089,745 | 92 |
Average annual cell incidences are per 100,000 persons.
Table 3.
Adjusted RRs and 95% CIs from multilevel log-binomial models of colon and rectal cancer incidence in Louisiana
| Null model | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| RR (95% confidence interval) | ||||
| Black/White | 1.28 (1.22,1.33) | 1.17 (1.12,1.23) | 1.18 (1.13,1.24) | |
| Male/Female | 1.38 (1.33,1.44) | 1.38 (1.33,1.44) | 1.38 (1.33,1.44) | |
| 45–54/35–44 y | 3.24 (2.90,3.61) | 3.23 (2.90,3.60) | 3.23 (2.90,3.60) | |
| 55–64/35–44 y | 6.39 (5.76,7.09) | 6.37 (5.74,7.07) | 6.38 (5.75,7.07) | |
| 65+/35–44 y | 13.96 (12.63,15.43) | 13.87 (12.55,15.33) | 13.88 (12.56,15.34) | |
| CDI | 1.09 (1.07,1.12) | |||
| White | 1.14(1.10,1.18) | |||
| Black* | 1.05 (1.02,1.09) | |||
| Tract Variance | 0.046 | 0.021 | 0.016 | 0.016 |
| X2/df | 1.48 | 1.05 | 1.04 | 1.02 |
Race-specific relative risks reflect estimates based on main effects and interaction term from the model.
Fig. 2.
Results from the final multilevel model: (A) predicted annual incidence for black and white residents in a hypothetical age- and sex-balanced population aged over 35 years and (B) adjusted black/white risk ratio as a function of concentrated disadvantage.
To confirm the relationship between CDI and CRC was linear in nature, we performed the analyses with CDI defined as a categorical variable. The categorical analysis supported a linear relationship, with CDI positively associated with CRC. We have chosen to keep the measure as continuous in the interest of statistical power and reproducibility of results.
Results in Table 3 reflect colon and rectal cancers combined. However, there is evidence that the relationship between socioeconomic status and CRC differs by tumor site [34,35]. Racial disparities may also differ by anatomic site [36]. Thus, we performed the analysis separately for cancers of the proximal colon (n = 4319), distal colon (n = 2642), and rectum (n = 2970). We found that while the magnitude of the CDI effect differed among the tumor sites, neighborhood disadvantage was significantly associated with incidence of cancer and appeared to contribute to racial disparities for all sites. The interaction between neighborhood CDI and cancer incidence was only significant for distal colon cancer. Results for the site-stratified analysis have been provided as supplemental material (Table S2).
We ran sensitivity analyses to investigate alternative calculations of neighborhood CDI. PhenX does propose the construct be calculated without including a measure of percent black in the event race is a key factor being studied [28]. Therefore, we computed an alternative measure of CDI using a principle components analysis without percent black. Results were consistent regardless of which CDI measure was used.
Discussion
This study assessed the impact of neighborhood disadvantage on racial disparities in CRC incidence using cancer registry and U.S. Census data from a state with a large proportion of minority black and economically disadvantage residents. We found a significant association of area disadvantage and CRC incidence, which supports the hypothesis that CRC is more likely to affect disadvantaged populations. Controlling for area disadvantage partially explained racial disparities in CRC incidence in Louisiana. Specifically, significant racial disparities in CRC incidence were not observed among residents in highly disadvantaged areas. However, among residents of average or low disadvantage, blacks still had greater risk of incident CRC compared with whites.
Screening for CRC is recommended for all individuals beginning at 50 years of age and can include fecal occult blood tests, sigmoidoscopy, or colonoscopy. Colonoscopy used to detect and remove precancerous polyps can significantly reduce CRC incidence [37]. Recent national surveys report socioeconomic status and health care access as key correlates of CRC screening that contribute to disparities in screening rates between black and white populations [38–40]. We anticipate that lack of screening does contribute to the effect of CDI on CRC incidence overall. However, trends in screening do not seem to explain the differential effect of CDI by race or the racial disparity found in more advantaged populations. It may be that other uncontrolled social factors or comorbidities may be contributing to excess CRC incidence in blacks in more advantaged areas. The national Centers for Disease Control and Prevention’s CRC control program has recently migrated from a broad objective of achieving an 80% screening rate overall to a more targeted objective of improving screening among certain populations through community-based interventions [41]. Our research supports the new shift in targeted community-level control programs and underscores the need to identify contributing risks in minority populations that can be addressed through targeted prevention strategies.
With regard to limitations of the present study, the study data set does not include many individual risk factors that could have affected results, including family history, screening, and overall access to health care as well as individual socioeconomic status and health-related behaviors. We have assessed the effects of neighborhood living environment based on a census-defined spatial unit (tract), which is designed to be relatively homogenous in terms of social characteristics but does lack a subjective definition of “neighborhood.” This study does not attempt to characterize other potentially influential social environments such as the family unit/household or the workplace. Furthermore, the design is cross-sectional in nature so the data does not capture duration of exposure or life course–type risk associated with CDI.
A person’s environment including the community and social contexts in which people are born and live is of high importance for shaping health and gives rise to health disparities across a number of chronic conditions including cancer. Previous studies have evidenced a link between socioeconomic status and CRC incidence [34,35,42]. This study identified a significant association between neighborhood concentrated disadvantage and CRC incidence. Many known behavioral risks of CRC such as diet and physical activity are associated with neighborhood environment [19–21]. Thus, there is a need for further research into the mediating mechanisms through which neighborhood social environment affects individual CRC risk. While neighborhood disadvantage appeared to contribute to racial disparities, our results suggest an additional dimension to racial disparities in CRC outside of neighborhood environment that warrants further research. Identifying modifiable social and behavioral risk factors with the greatest relative contribution to CRC is essential for developing public health inventions or public policy aimed at prevention and control.
Acknowledgments
This work was supported by National Institutes of Health, National Institute on Minority Health and Health Disparities (NIMHD; 5R24MD008121–03 and U54MD008176), the Louisiana Tumor Registry, Centers for Disease Control–National Patient-Centered Research, and the National Cancer Institute–Surveillance, Epidemiology, and End Results Program.
Appendix
Fig. S1. Case inclusion summary.
Table S1: Factor loadings for individual census tract measures used in the principle components analysis for CDI, Louisiana, 2008–2012
| Variable | Factor loading |
|---|---|
| Poverty | 0.83 |
| Public assistance | 0.61 |
| Female-headed families | 0.84 |
| Unemployed | 0.77 |
| Under 18 | 0.43 |
| Black | 0.86 |
Table S2: Adjusted RRs and 95% confidence intervals from multilevel log-binomial models of colon and rectal cancer incidence in Louisiana, by anatomical site of tumor
| N Cases | Null Model | Model 1 | Model 2 | Model 3 | |
| RR (95% confidence interval) | |||||
|---|---|---|---|---|---|
| Proximal | 4319 | ||||
| Black/White | 1.32 (1.24,1.41) | 1.23 (1.14,1.33) | 1.24(1.15,1.34) | ||
| Male/Female | 1.16(1.09,1.23) | 1.16(1.09,1.23) | 1.16(1.09,1.23) | ||
| 45–54/35–44 y | 3.17 (2.59,3.87) | 3.16 (2.59,3.87) | 3.16 (2.59,3.87) | ||
| 55–64/35–44 y | 7.85 (6.49,9.49) | 7.82 (6.47,9.46) | 7.83 (6.48,9.47) | ||
| 65+/35–44 y | 23.71 (19.76,28.45) | 23.57 (19.64,28.28) | 23.59 (19.66,28.30) | ||
| CDI | 1.07 (1.03,1.12) | ||||
| White | 1.10(1.04,1.17) | ||||
| Black | 1.04 (0.99,1.10) | ||||
| σv | 0.060 | 0.021 | 0.018 | 0.011 | |
| X2/df | 1.42 | 1.17 | 1.16 | 1.14 | |
| Distal | 2642 | ||||
| Black/White | 1.34 (1.23,1.45) | 1.20(1.09,1.32) | 1.22 (1.11,1.34) | ||
| Male/Female | 1.49(1.38,1.61) | 1.50(1.39,1.62) | 1.50(1.39,1.62) | ||
| 45–54/35–44 y | 3.36 (2.74,4.13) | 3.35 (2.73,4.12) | 3.36 (2.73,4.12) | ||
| 55–64/35–44 y | 6.50 (5.33,7.92) | 6.47 (5.31,7.88) | 6.48 (5.32,7.90) | ||
| 65+/35–44 y | 12.38 (10.22,14.99) | 12.27 (10.13,14.85) | 12.28 (10.14,14.88) | ||
| CDI | 1.11 (1.06,1.17) | ||||
| White | 1.19(1.11,1.28) | ||||
| Black | 1.04(0.97,1.11) | ||||
| σv | 0.038 | 0.015 | 0.008 | 0.006 | |
| X2/df | 1.18 | 1.02 | 1.01 | 0.97 | |
| Rectal | 2970 | ||||
| Black/White | 1.16(1.07,1.26) | 1.04 (0.95,1.15) | 1.05 (0.96,1.16) | ||
| Male/Female | 1.72 (1.60,1.85) | 1.72 (1.60,1.85) | 1.72 (1.60,1.85) | ||
| 45–54/35–44 y | 3.41 (2.86,4.06) | 3.40 (2.85,4.05) | 3.40 (2.85,4.05) | ||
| 55–64/35–44 y | 5.64 (4.75,6.69) | 5.61 (4.73,6.66) | 5.62 (4.74,6.67) | ||
| 65+/35–44 y | 8.84 (7.49,10.44) | 8.77 (7.42,10.35) | 8.77 (7.43,10.36) | ||
| CDI | 1.12 (1.07,1.18) | ||||
| White | 1.16(1.08,1.24) | ||||
| Black | 1.08 (1.01,1.16) | ||||
| Tract Variance | 0.072 | 0.061 | 0.053 | 0.017 | |
| X2/df | 1.14 | 0.98 | 0.98 | 0.96 | |
Footnotes
Publisher's Disclaimer: Disclaimer: All statements and views expressed in the submitted article are of the authors’ own and not an official position of the institution or funder.
The authors do not have any conflicts of interest to disclose.
References
- [1].Maniscalco L, Lefante C, Hsieh M, Andrews P, Pareti L, Mumphrey B, et al. Cancer in Louisiana, 2009–2013 New Orleans: Louisiana Tumor Registry; 2015. (Cancer in Louisiana; Vol. 31). [Google Scholar]
- [2].American Cancer Society. Cancer Facts & Figures for African Americans 2013–2014 Atlanta: American Cancer Society; 2013. [Google Scholar]
- [3].World Health Organization. Commission on Social Determinants of Health–Final Report Copenhagen: WHO; 2008. [Google Scholar]
- [4].Diez Roux A, Merkin S, Arnett D, Chambless L, Massing M, Nieto F, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345(2):99–106. [DOI] [PubMed] [Google Scholar]
- [5].King K, Morenoff J, House J. Neighborhood context and social disparities in cumulative biological risk factors. Psychosom Med 2011;73(7):572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan G, Katz L, et al. Neighborhoods, Obesity, and Diabetesda randomized social experiment. N Engl J Med 2011;365(16):1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Coulon S, Velasco-Gonzalez C, Scribner R, Park C, Gomez R, Vargas A, et al. Racial differences in neighborhood disadvantage, inflammation and metabolic control in black and white pediatric type 1 diabetes patients. Pediatr Diabetes 2017;18(2):120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Greenberg A, Obin M. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006;83(2):461s22125s. [DOI] [PubMed] [Google Scholar]
- [9].Peters A, McEwen B. Stress habituation, body shape and cardiovascular mortality. Neurosci Biobehav Rev 2015;56:139–50. [DOI] [PubMed] [Google Scholar]
- [10].Nimptsch K, Pischon T. Obesity biomarkers, metabolism and risk of cancer: an epidemiological perspective. Recent Results Cancer Res 2016;208:199–217. [DOI] [PubMed] [Google Scholar]
- [11].Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health 2001;91(11):1783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yen IH, Syme SL. The social environment and health: a discussion of the epidemiologic literature. Annu Rev Public Health 1999;20:287–308. [DOI] [PubMed] [Google Scholar]
- [13].Gomez SL, Shariff-Marco S, DeRouen M, Keegan T, Yen I, et al. The impact of neighborhood social and built environment factors across the cancer continuum: current research, methodological considerations, and future directions. Cancer 2015;121(14):2314–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McEwen B, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med 1993;153(18):2093–101. [PubMed] [Google Scholar]
- [15].Stress McEwen B., adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- [16].Geronimus A, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health 2006;96:826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen B. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann N Y Acad Sci 2010;1186:223–39. [DOI] [PubMed] [Google Scholar]
- [18].Schulz A, Mentz G, Lachance L, Johnson J, Gaines C, Israel B. Associations between socioeconomic status and allostatic load: effects of neighborhood poverty and tests of mediating pathways. Am J Public Health 2012;102: 1706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Franco M, Diez Roux A, Glass T, Caballero B, Brancati F. Neighborhood characteristics and availability of healthy foods in Baltimore. Am J Prev Med 2008;35(6):561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Larson N, Story M, Nelson M. Neighborhood environments: disparities in access to healthy foods in the US. Am J Prev Med 2009;36(1):74–81. [DOI] [PubMed] [Google Scholar]
- [21].King K Neighborhood walkable urban form and C-reactive protein. Prev Med 2013;57(6):850–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Silverman J, Krieger J, Kiefer M, Hebert P, Robinson J, Nelson K. The relationship between food insecurity and depression, diabetes distress and medication adherence among low-income patients with poorly-controlled diabetes. J Gen Intern Med 2015;30(10):1476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krieger N, Chen J, Waterman P, Rehkopf D, Subramanian S. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures–the public health disparities geocoding project. Am J Public Health 2003;93(10):1655–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].LaVeist T, Thorpe R, Bowen-Reid T, Jackson J, Gary T, Gaskin D, et al. Exploring health disparities in integrated communities: overview of the EHDIC Study. J Urban Health 2008;85:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Scribner RA, Theall KP, Simonsen NR, Mason KE, Yu Q. Misspecification of the effect of race in fixed effects models of health inequalities. Soc Sci Med 2009;69(11):1584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Subramanian S, Chen J, Rehkopf D, Waterman P, Krieger N. Racial disparities in context: a multilevel analysis of neighborhood variations in poverty and excess mortality among black populations in Massachusetts. Am J Public Health 2005;95(2):260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].NAACCR. GIS Resources. https://www.naaccr.org/gis-resources/; 2018. [Accessed 23 February 2017].
- [28].Toolkit PhenX. Protocol Overview - Neighborhood Concentrated Disadvantage. https://www.phenxtoolkit.org/index.php?pageLink=browse.protocoldetails&id=211302; 2016. [Accessed 23 February 2017]
- [29].Sampson R, Raudenbush S, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science 1997;277(5328):918–24. [DOI] [PubMed] [Google Scholar]
- [30].Peterson C, Rauscher G, Johnson T, Kirschner C, Freels S, Barret R, et al. The effect of neighborhood disadvantage on the racial disparity in ovarian cancer-specific survival in a large hospital-based study in cook county, illinois. Front Public Health 2015;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kepper M, Sothern M, Zabaleta J, Ravussin E, Velasco-Gonzalez C, Leonardi C, et al. Prepubertal children exposed to concentrated disadvantage: An exploratory analysis of inflammation and metabolic dysfunction. Obesity (Silver Spring) 2016;24(5):1148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Geography UCB. Geographic Areas Reference Manual. https://www.census. gov/geo/reference/garm.html; 2012. [Accessed 31 March 2017].
- [33].Blizzard L, Hosmer D. Parameter estimation and goodness-of-fit in log binomial regression. Biom J 2006;48(1):5–22. [DOI] [PubMed] [Google Scholar]
- [34].Wu X, Cokkinides V, Chen V, Nadel M, Ren Y, Martin J, et al. Associations of subsite-specific colorectal cancer incidence rates and stage of disease at diagnosis with county-level poverty, by race and sex. Cancer 2006;107(5 Suppl):1121–7. [DOI] [PubMed] [Google Scholar]
- [35].Steinbrecher A, Fish K, Clarke C, West D, Gomez S, Cheng I. Examining the association between socioeconomic status and invasive colorectal cancer incidence and mortality in California. Cancer Epidemiol Biomarkers Prev 2012;21(10):1814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rim S, Seeff L, Ahmed F, King J, Coughlin S. Colorectal cancer incidence in the United States, 1999–2004: an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer 2009;115(9):1967–76. [DOI] [PubMed] [Google Scholar]
- [37].Ananthakrishnan A, Cagan A, Cai T, Gainer V, Shaw S, Churchill S, et al. Colonoscopy is associated with a reduced risk for colon cancer and mortality in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2015;13(2):322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liss D, Baker D. Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. Am J Prev Med 2014;46(3):228–36. [DOI] [PubMed] [Google Scholar]
- [39].Shapiro J, Klabunde C, Thompson T, Nadel M, Seeff L, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev 2012;21(6): 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Soneji S, Armstrong K, Asch D. Socioeconomic and physician supply determinants of racial disparities in colorectal cancer screening. J Oncol Pract 2012;8(5):e125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].CDC. About the CRCCP. https://www.cdc.gov/cancer/crccp/about.htm; 2017. [Accessed 31 March 2017].
- [42].Doubeni C, Laiyemo A, Major J, Schootman M, Lian M, Park Y, et al. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer 2012;118(14):3636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]