Abstract
Background
Functional evaluation is a cornerstone of multidimensional geriatric assessment; however, little is known of the clinical value of standardized performance-based assessment in the acute care setting. The aim of this study was to evaluate the clinical correlates and short-term predictive value of the Short Physical Performance Battery (SPPB) in older patients admitted to the hospital for an acute medical event.
Methods
We enrolled 92 women and men 65 years old or older who were able to walk, who had a Mini-Mental State Examination (MMSE) score ≥18, and who were admitted to the hospital with a clinical diagnosis of congestive heart failure, pneumonia, chronic obstructive pulmonary disease (COPD), or minor stroke. The SPPB was assessed at hospital admission and discharge. Self-report functional assessment included basic activities of daily living (ADL) and instrumental activities of daily living (IADL). Spearman’s rank correlation coefficients and multivariable linear regression analyses were used to study the association of SPPB score and functional and clinical characteristics, including length of hospital stay.
Results
The mean age was 77.7 years (range 65–94 years), 49% were female, 64.1% had congestive heart failure, 16% COPD, 13.1% pneumonia, and 6.5% minor stroke. At hospital admission the mean SPPB score was 6.0 ± 2.7. SPPB scores were inversely correlated with age, the severity of the index disease, and IADL and ADL difficulty 2 weeks before hospital admission (p < .01), and were directly correlated with MMSE score (p = .002). On average, SPPB score increased 1 point (+0.97, standard error of the mean=0.2; p for paired t test < .001) from baseline to hospital discharge assessment. After adjustment for potential confounders, baseline SPPB score was significantly associated with the length of hospital stay (p < .007).
Conclusion
In older acute care inpatients, SPPB is a valid indicator of functional and clinical status. SPPB score at hospital admission is an independent predictor of the length of hospital stay.
Keywords: Short Physical Performance Battery, Functional assessment, Hospital, Feasibility, Prognosis, Aging
Older persons are at high risk for worsening health and functional decline. Older patients admitted to the hospital for medical events represent a subgroup at higher risk (1). In hospitalized patients, poor functional status has important consequences as it has been associated with the risk of longer hospital stay, home care placement, and mortality. Furthermore, hospitalization has been associated with risk of incident disability 6 and 18 months later (2).
Identification of patients at high risk of functional decline is of paramount importance for the prevention of this common negative outcome. However, medical diagnoses and traditional clinical assessment have limited capacity to discriminate high- and low-risk groups (3). Objective measures of physical performance may prove to be an additional and useful clinical tool for risk stratification.
In the Established Populations for the Epidemiologic Study of the Elderly it was demonstrated that the Short Physical Performance Battery (SPPB), a set of objective measures of lower extremity physical performance, was highly predictive of subsequent disability, institutionalization, and mortality (4). The risk gradient for these outcomes was seen across the full spectrum of baseline functional performance, indicating that increased risk can be documented even for persons in the mid-range of performance. An important extension to the research use of these measures is their application in the clinical setting. Studenski and colleagues (5) demonstrated that the SPPB could be performed safely and in a short time in outpatient clinics, and that it predicted adverse outcomes similarly to what were found in epidemiological research cohorts. The authors proposed the physical performance measures as “simple and accessible vital signs” for the screening of older adults in the clinical setting. This finding supports the premise that standardized performance measures of physical functioning may be valuable when used in routine clinical practice as early warning signs of impending problems. Functional evaluation is a cornerstone of multidimensional geriatric assessment. Nevertheless, performance-based measures have not been incorporated in routine evaluation of older inpatients so far (6).
To investigate the feasibility and clinical utility of physical performance assessment in the geriatric acute setting, we conducted a 1-year observational study of acutely ill older inpatients. The primary objective of this data analysis was to describe the clinical correlates and short-term predictive value of SPPB assessed in a sample of older patients admitted to the hospital for an acute medical event.
Methods
Study Design and Data Collection
Between October 1, 2004 and December 31, 2006, patients admitted to the Section of Internal Medicine and Geriatrics (University of Ferrara, Italy) were screened for eligibility for a 1-year longitudinal observational study. Inclusion criteria for the study were as follows: (a) age 65 years or older; (b) ability to stand and walk for a few meters; (c) a clinical diagnosis of one of the following conditions: congestive heart failure, chronic obstructive pulmonary disease (COPD), pneumonia, and minor stroke. These conditions were selected because they are common causes of hospitalizations in older patients and because they are conditions that strongly affect physical function in older frail persons. Patients were considered ineligible for the study if they had severe cognitive impairment (Mini-Mental State Examination [MMSE] score < 18) or acute coronary syndrome, if they were living more than 25 km (15.5 miles) from the medical center, or if they refused to participate in the study. Every day, staff physicians informed three trained research physicians of all newly admitted patients potentially eligible. In addition, the research physicians reviewed all patients’ charts for other potentially eligible patients. Eligibility was finally verified by the trained research physicians by means of chart review and physical examination when appropriate. After the screening process, 125 patients fulfilled all the inclusion and exclusion criteria and therefore were considered eligible for the study. Of those eligible, 92 (74%) agreed to participate, signed the informed consent, and were enrolled in the study. Participants were evaluated by means of a comprehensive geriatric assessment at hospital admission, then were reevaluated the day of hospital discharge, then 1 week and 1 month after hospital discharge by in-home visits and, subsequently, every 3 months by telephone interviews.
The performance-based measures of physical function were assessed at hospital admission (within 56 hours after actual hospital admission), within 24 hours before hospital discharge, and during the home visits 1 week and 1 month after hospital discharge. By daily meetings with the department referent-physicians (the physician in charge of patient’s care), the second performance-based assessment was performed for all patients the day of hospital discharge. The local Institutional Review Board reviewed and approved the study protocol.
Measures
Demographics
Sociodemographic information, including gender, marital status, living arrangement, educational level, and smoking habits were collected at baseline by standardized interview.
Performance-based measures of physical function
Performance-based measures of physical function included the SPPB and handgrip strength. The trained research physicians tested all performance-based measures. The SPPB includes usual walking speed over 4 meters; five chair stands test, and balance test. A score (scale, 0–4) was assigned to performance on time to rise five times from a seated position, standing balance, and 4-meter walking velocity. Individuals received a score of 0 for each task they were unable to complete. Participants were coded as “unable to perform” when (a) they tried but were unable; or (b) the interviewer or subject felt it was unsafe. Scores of 1–4 for each task were assigned based on quartiles of performance for more than 6000 participants in the Established Populations for the Epidemiologic Study of the Elderly (4,7). Summing the three individual categorical scores, a summary performance score was created for each participant (range 0–12), with higher scores indicating better lower body function. The SPPB score is a global measure of lower extremity functioning that predicts mobility loss, nursing home placement, and mortality among community-dwelling elderly individuals. The SPPB has been shown to be reliable, valid, and sensitive to change. Intraclass correlation coefficients ranged from 0.88 to 0.92 for measures made 1-week apart, with a 6-month average correlation coefficient of 0.78 (8). Handgrip strength was measured using a standardized protocol (9). The assessment of grip strength using a handheld dynamometer has been previously shown to be reliable and valid among hospitalized older patients (10).
Self-report measure of physical function
Information on six instrumental activities of daily living (IADL) was obtained using a modified version of the Lawton and Brody scale (11). The six activities included were as follows: using the telephone, traveling via car or public transportation, shopping, housecleaning, handling money, and taking medications. Participants were asked if they had any difficulty performing each task without help from another person or special equipment. If they said they did have difficulty, they were then asked how much difficulty, with response options of “some difficulty, a lot of difficulty, or unable to do without help.” At hospital admission the patients were asked if they had any difficulty performing each task during the preceding 2 weeks. The same information was collected at home visits and during the four telephone interviews.
Walking performance was assessed inquiring the patient about level of difficulty walking 400 meters (a quarter of a mile) and walking up 10 steps without resting. Disability in basic ADL (ADL) was measured according to the participants’ self-reported difficulty in performing each of six activities: getting in and out of a bed, bathing, dressing, eating, personal hygiene, and using the toilet (12). For mobility and basic ADL the format was the same used for IADLs. Based on the results of previous works (1,3) showing a high rate of decline in ADL function in the weeks preceding hospital admission, information on basic ADL was queried for both hospital admission and during the preceding 2 weeks.
Cognitive and affective function
Cognitive functioning was assessed using the MMSE (13). Patients with scores <24 were considered to have mild cognitive impairment. Depressive symptoms were measured using the Center for Epidemiological Studies–Depression (CES-D) Scale (14) (range from 0 to 60, with higher scores indicating more depressive symptomatology). Patients with scores >16 were considered to have depressive symptomatology (15).
Comorbidity and indicators of disease severity
Comorbidity was assessed by using the Cumulative Illness Rating Scale (CIRS; 16) a validated (17) physician-rated index derived by means of patient history as well as physical examination and laboratory findings. The CIRS is divided into 14 categories or disorders. This index measures the chronic medical illness burden while taking into consideration the severity of chronic diseases. The final score of the CIRS is the sum of each of the 14 individual system scores, with higher values indicating greater disease burden severity. In addition, congestive heart failure, COPD, pneumonia, and stroke severity were assessed by the New York Heart Association cardiac function classification, the GOLD classification (18), the Fine prognostic score (19), and the Canadian Neurological scale (20), respectively.
Analysis
Data for this article are from the in-hospital evaluation, including admission and discharge assessments. Variables are reported as mean values ± standard deviation, median and interquartile range (Q1–Q3), or percentages. The associations of SPPB score with clinical and self-reported functional characteristics were evaluated by using Spearman’s rank correlation tests. Difference between the SPPB score at admission and discharge was analyzed using the t test for paired data. To explore the short-term predictive value of SPPB, we estimated hospital length of stay, a proxy measure of health status and length of recovery (21), as a function of SPPB score evaluated at hospital admission. This analysis was performed using multivariable linear regression models adjusting for age, gender, comorbidity, and basic ADL disability. SBBP was analyzed both as three-group categorical variable and as continuous variable. All analyses were performed using STATA statistical software (release 9; College Station, TX).
Results
Patient Characteristics
The study sample consisted of 92 patients; the mean age was 77.7 years (range 65–94 years), 49% were female, 64.1% had congestive heart failure, 16% COPD, 13.1% pneumonia, and 6.5% minor stroke. According to inclusion criteria, the mean MMSE score was 25.6, and 21.7% of patients had mild cognitive impairment (MMSE score between 18 and 23.9). At baseline interview, 56.6% reported having difficulties in performing at least one IADL, and 31.5% had difficulties with at least one basic ADL during the 2 weeks before hospital admission, whereas 53.3% of the patients reported difficulties in performing at least one basic ADL at the time of hospital admission. The mean length of hospital stay was 9.8 days (Table 1).
Table 1.
Selected General and Clinical Characteristics of the Study Sample at Baseline (N = 92)
| Characteristic | Mean or N | SD or Range |
|---|---|---|
| Age, y [mean (SD), range] | 77.7 (6.4) | 65–94 |
| Women, (n; %) | 45 | 48.9 |
| Educational level [y, mean (SD), range] | 5.5 (2.9) | 1–13 |
| Smoking, n; % | ||
| Former | 46 | 53.5 |
| Current | 11 | 12.8 |
| Body mass index [kg/m2, mean (SD), range] | 27.6 (6.1) | 17.3–49.0 |
| MMSE score [mean (SD), range] | 25.6 (2.6) | 18.4–30 |
| Mild cognitive impairment [MMSE < 24, (n; %)] | 20 | 21.7 |
| CES-D [mean (SD), range] | 16.4 (8.3) | 1–39 |
| Depressive symptoms [CES-D ≥ 16, (n; %)] | 49 | 53.9 |
| Admission diagnosis (n; %) | ||
| Congestive heart failure | 59 | 64.1 |
| COPD | 15 | 16.3 |
| Pneumonia | 12 | 13.1 |
| Minor stroke | 6 | 6.5 |
| Total CIRS score [mean (SD), range] | 8.9 (3.2) | 3–20 |
| CIRS: Number of severe ratings [median (Q1–Q3 range), range] | 0 (0–4) | 0–9 |
| Length of hospital stay [days, mean (SD), range] | 9.8 (4.8) | (2–30) |
| IADL difficulty 2 weeks before admission (n; %) | 52 | 56.6 |
| Basic ADL difficulty 2 weeks before admission (n; %) | 29 | 31.5 |
| Basic ADL difficulty at admission (n; %) | 49 | 53.3 |
| Short Physical Performance Battery [mean (SD), range] | 6.0 (2.7) | 0–11 |
Note: SD = standard deviation; MMSE = Mini-Mental State Examination; CES-D = Center for Epidemiological Studies–Depression scale; COPD = chronic obstructive pulmonary disease; CIRS = Cumulative Illness Rating Scale; IADL = instrumental activity of daily living; ADL = activity of daily living.
SPPB Results
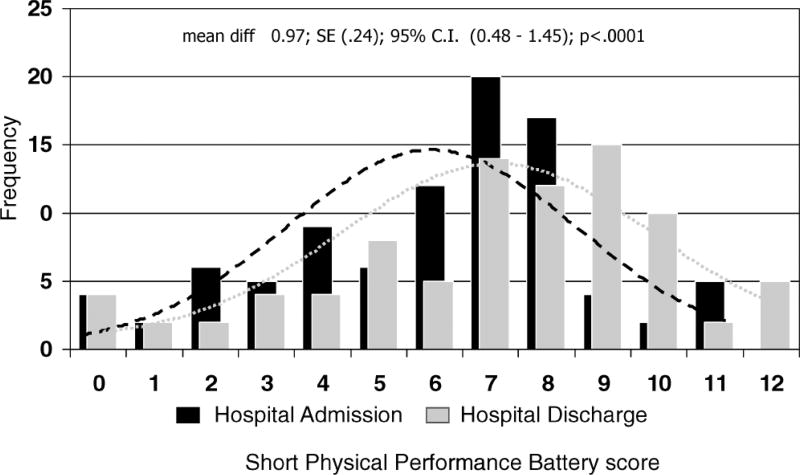
At hospital admission, SPPB scores ranged from 0 to 11, with a mean score of 6.0 ± 2.7, with men having better performance compared to women (6.7 vs 5.4, p=.019). There were not significant differences in SPPB score at hospital admission according to admission diagnosis (analysis of variance: F = 1.1; p = .380). Compared to admission assessment, 63.2% of patients had better SPPB performance at hospital discharge, 17.3% remained stable, and 19.5% had worse performance. On average, SPPB score difference between admission and discharge evaluation was +0.97 (standard error of the mean [SEM] = 0.2), (p for paired t test < .001) (Figure 1). There was no significant difference in SPPB score at discharge according to admission diagnosis (analysis of covariance adjusted for age and gender: F=0.53; p=.663).
Figure 1.
Short Physical Performance Battery score distribution at hospital admission (black bars) and hospital discharge (gray bars). The lines are appropriately scaled normal density curves (black for hospital admission and gray for hospital discharge). SE = standard error; diff = difference; C. I. = confidence interval.
SPPB scores and measures of clinical and functional status
Table 2 displays Spearman correlation coefficients for SPPB scores at hospital admission and discharge evaluations with selected clinical and functional characteristics. Both SPPB scores were inversely and significantly correlated with age, the severity of the main disease, IADL, and basic ADL difficulty during the 2 weeks before hospital admission, and directly correlated with MMSE score; overall, the strength of these associations ranged from moderate to good. SPPB score at hospital admission, but not at hospital discharge, was also significantly correlated with decline in basic ADL function during the last 2 weeks before hospital admission and with the length of hospital stay.
Table 2.
Spearman Correlation Coefficients Between SPPB Score and Selected Clinical and Functional Characteristics
| SPPB at Admission | SPPB at Discharge | |||
|---|---|---|---|---|
|
|
|
|||
| Characteristics | r | p | r | p |
| Age | −.29 | .0045 | −.40 | .001 |
| BMI | .06 | .610 | .13 | .208 |
| MMSE | .32 | .002 | .36 | .0007 |
| CES-D | −.08 | .476 | −.09 | .432 |
| Total CIRS score | −.27 | .009 | −.28 | .009 |
| CIRS: No. of severe ratings | −.25 | .015 | −.11 | .327 |
| Severity of index disease | −.33 | .002 | −.28 | .010 |
| Length of stay | −.24 | .022 | −.13 | .221 |
| Number of IADLs with difficulty 2 wk before admission | −.51 | <.0001 | −.51 | <.0001 |
| Number of basic ADLs 2 wk before admission | −.45 | <.0001 | −.54 | <.0001 |
| Number of basic ADLs with difficulty at admission | −.45 | <.0001 | −.33 | .002 |
| Basic ADL change at admission | .23 | .027 | .05 | .673 |
Note: SPPB = Short Physical Performance Battery; BMI = body mass index; CES-D = Center for Epidemiological Studies–Depression scale; CIRS = Cumulative Illness Rating Scale; IADL = instrumental activity of daily living; ADL = activity of daily living.
SPPB scores predictive of hospital length of stay
Table 3 displays the results of the multivariate regression analyses predicting length of hospital stay. After adjustment for age and gender, patients with better SPPB scores (8–12) compared to patients with the poorest SPPB scores (0–4) had a shorter hospital stay, with an average difference of 4 days. This difference was attenuated after controlling for level of comorbidity and basic ADL status evaluated at hospital admission. Nevertheless, after full adjustment, the difference was still clinically and statistically significant (2.5 days; p < .036). Conversely, basic ADL status and level of comorbidity were no longer significant predictors of length of stay. When SPPB score was analyzed as a continuous variable, a 1-point increase of the SPPB score was associated with a 0.5-day reduction in hospital stay (p < .007). Because gait speed is easy to measure and may be done quickly in the clinical setting, it is useful to evaluate whether measuring gait speed alone may capture the predictive power of the more comprehensive and demanding full battery. To do this we compared the variance (adjusted r2) explained by the SPPB with the variance explained by the three subtasks in age- and gender-adjusted models predicting hospital length of stay. Of the three SPPB components, walking speed had the highest predicting value (r2=0.16) that was also slightly higher than that of the whole battery (r2 = 0.13)
Table 3.
Multiple Linear Regression Models Predicting Hospital Length of Stay According to SPPB Score
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Parameters | β Coefficient (SE) | p | β Coefficient (SE) | p | β Coefficient (SE) | p |
| SBBP categories | ||||||
| 0–4 (n = 25) | Reference* | Reference* | Reference* | |||
| 5–7 (n = 37) | −2.2 (1.5) | .151 | −1.9 (1.6) | .240 | −1.3 (1.4) | .359 |
| 8–12 (n = 28) | −3.9 (1.4) | .005 | −3.2 (1.4) | .026 | −2.5 (1.2) | .036 |
| SBBP continuous score† | −0.72 (0.21) | .001 | −0.62 (0.22) | .005 | −0.54 (0.20) | .007 |
Notes: Model 1: adjusted for age and gender; Model 2: adjusted for age, gender, and Cumulative Illness Rating Scale (number of severe ratings); Model 3: adjusted for age, gender, Cumulative Illness Rating Scale (number of severe ratings), and basic activities of daily living disability at admission.
For 0–4 category, the adjusted means (SE) are: Model 1 [11.9 (1.2)]; Model 2 [11.6 (1.2)]; Model 3 [11.1 (1.0)].
Tested as separate models.
SPPB = Short Physical Performance Battery; SE = standard error.
Feasibility and Acceptability
The full SPPB testing took, on average, 12 minutes to complete and in less than 10% of the tests took more than 15 minutes. Participants were asked about the acceptability of the SPPB. None felt it was less than acceptable, but five complained of excessive fatigue in performing the five chair stands test. With regard to adverse events after more than 177 tests were performed, only one syncope episode, without subsequent trauma, was registered.
Discussion
In older acute medical inpatients, the SPPB, a simple battery of lower extremity physical function measures, correlates with pre-admission and baseline self-report measures of functional status and with common clinical indicators of comorbidity and disease severity. SPPB score assessed within 48 hours after hospital admission is significantly associated with the length of hospital stay independent of traditional prognostic indicators including age, ADL status, cognitive status, and level of comorbidity. Our findings demonstrate that the SPPB is a tool that can be feasibly and safely administered in the acute-care geriatric ward and can provide initial evidence for its validity and utility as a clinical instrument for in-depth functional evaluation and prognostic stratification.
Despite a large body of epidemiological evidence demonstrating the predictive value of different mobility performance tests in terms of various adverse outcomes in community-dwelling elders (7,22–25), the use of physical performance measures in the acute-care clinical setting has received little attention so far. Our results confirm and extend, at a clinical level, the findings of previous epidemiological studies. In particular, our findings are in good agreement with the study of Purser and colleagues (6) who demonstrated, in a sample of frail male veterans, that walking speed assessed during hospitalization provides useful information for the functional and prognostic assessment of acutely ill older adults. Our study demonstrated that SPPB score has good concurrent validity with baseline clinical indicators and self-report measures of disability; nevertheless, the values of the correlation coefficients for the association between SPPB and ADL disability (0.45–0.53) suggested that, although statistically correlated, these two tools explore different and only partially overlapping domains of physical function. Furthermore, SPPB score evaluated at hospital admission was linearly and directly associated with length of hospital stay, with the relationship remaining significant after adjustment for comorbidity and level of self-reported disability, whereas the association between ADL disability and length of hospital stay was statistically significant in the univariate analysis but was attenuated and no longer statistically significant in the multivariate analysis. From this point of view, our findings reinforce the concept that self-reported and performance-based measurements provide complementary information and that performance-based assessment might add prognostic information in patients with or with no or moderate ADL disability (26). Indeed, we excluded patients who were unable to walk, and only five patients reported being unable to perform one or more basic ADLs before hospital admission.
In older inpatients, change in functional status during hospitalization has been related to important short- and long-term outcomes, including nursing home admission, disability, and mortality (27). Previous studies in which functional status was assessed by means of self-report measures reported a 37% rate of change in ADL status between hospital admission and hospital discharge. In our study, 83.7% of the patients experienced a clinically relevant change in lower extremity function (±1 point in SPPB score) over hospitalization (28), suggesting that also in this care setting SPPB might be a more sensitive tool for functional status monitoring than self-report.
Objective measures of physical performance are likely to capture the integrated and multisystemic effect of aging, comorbidity, disease severity, malnutrition, motivation, and cognition (29–32) on the health status of older persons. Like other biomarkers, including cholesterol and albumin levels (33), SPPB might be considered to be a nonspecific but highly sensitive indicator of global health status reflecting several underlying physiological impairments (34). This hypothesis would explain why SPPB had better prognostic value compared to other individual indicators.
Our data might have important implications for the safety and feasibility with which the SPPB can be assessed in hospitalized patients. Trained research physicians conducted all the 4-meter gait speed, balance, and chair rise tests for this study of acutely ill older patients within 48 hours after hospital admission, and there was no report of any major adverse event. To the best of our knowledge, this is the first time in which three different and complementary time-based tests of lower extremity function were administered to older patients admitted to the hospital. This research also demonstrated that assessing gait speed alone is as good as performing the full battery of performance tests in the prediction of length of hospital stay. If confirmed with the longitudinal data and with more specific functional outcomes, this result might have important practical applications in facilitating the widespread use of objective performance assessment in the acute care setting.
The limitations of the study include the small sample size that reduced the statistical power of our analysis and the enrolment of patients admitted to the hospital with selected medical conditions (i.e., congestive heart failure, COPD, pneumonia, and minor stroke). Although these medical conditions are among the most common reasons for hospital admission in older persons (1,3), these restricted inclusion criteria might have reduced the external validity of our findings.
Summary
This study presented the first evidence of the feasibility, validity, and preliminary prognostic utility of objective lower extremity performance assessment in the acute geriatric setting. Although our findings need to be replicated and extended in long-term longitudinal analysis, taken together these results support the hypothesis that, if incorporated into the routine in-hospital geriatric assessment, SPPB score could lead to better functional and prognostic evaluation of older acutely ill patients.
Acknowledgments
This research was supported in part by contracts from the National Institute on Aging, Intramural Research Program, National Institutes of Health.
References
- 1.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 2.Boyd CM, Xue QL, Guralnik JM, Fried LP. Hospitalization and development of dependence in activities of daily living in a cohort of disabled older women: the Women’s Health and Aging Study I. J Gerontol A Biol Sci Med Sci. 2005;60:888–893. doi: 10.1093/gerona/60.7.888. [DOI] [PubMed] [Google Scholar]
- 3.Volpato S, Onder G, Cavalieri M, et al. Characteristics of nondisabled older patients developing new disability associated with medical illnesses and hospitalization. J Gen Intern Med. 2007;22:668–674. doi: 10.1007/s11606-007-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 5.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 6.Purser JL, Weinberger M, Cohen HJ, et al. Walking speed predicts health status and hospital costs for frail elderly male veterans. J Rehabil Res Dev. 2005;42:535–546. doi: 10.1682/jrrd.2004.07.0087. [DOI] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55A:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women’s Health and Aging Study. J Clin Epidemiol. 2002;55:916–921. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 9.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 10.Bautmans I, Mets T. A fatigue resistance test for elderly persons based on grip strength: reliability and comparison with healthy young subjects. Aging Clin Exp Res. 2005;17:217–222. doi: 10.1007/BF03324600. [DOI] [PubMed] [Google Scholar]
- 11.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 12.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137:1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- 15.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: A validation study. Am J Epidemiol. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 16.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 17.Conwell Y, Forbes NT, Cox C, Caine ED. Validation of a measure of physical illness burden at autopsy: the cumulative illness rating scale. J Am Geriatr Soc. 1993;41:38–41. doi: 10.1111/j.1532-5415.1993.tb05945.x. [DOI] [PubMed] [Google Scholar]
- 18.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global initiative for chronic obstructive lung disease (gold) workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 19.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 20.Cote R, Battista RN, Wolfson C, Boucher J, Adam J, Hachinski V. The Canadian Neurological Scale: validation and reliability assessment. Neurology. 1989;39:638–643. doi: 10.1212/wnl.39.5.638. [DOI] [PubMed] [Google Scholar]
- 21.Maguire PA, Taylor IC, Stout RW. Elderly patients in acute medical wards: factors predicting length of stay in hospital. Br Med J (Clin Res Ed) 1986;292:1251–1253. doi: 10.1136/bmj.292.6530.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55A:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 24.Newman AB, Haggerty CL, Kritchevsky SB, Nevitt MC, Simonsick EM. Walking performance and cardiovascular response: associations with age and morbidity–the Health, Aging and Body Composition study. J Gerontol A Biol Sci Med Sci. 2003;58:715–720. doi: 10.1093/gerona/58.8.m715. [DOI] [PubMed] [Google Scholar]
- 25.Sherman SE, Reuben D. Measures of functional status in community-dwelling elders. J Gen Intern Med. 1998;13:817–823. doi: 10.1046/j.1525-1497.1998.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reuben DB, Seeman TE, Keeler E, et al. Refining the categorization of physical functional status: the added value of combining self-reported and performance-based measures. J Gerontol A Biol Sci Med Sci. 2004;59:1056–1061. doi: 10.1093/gerona/59.10.m1056. [DOI] [PubMed] [Google Scholar]
- 27.Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263–1270. doi: 10.1111/j.1532-5415.2004.52354.x. [DOI] [PubMed] [Google Scholar]
- 28.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 29.Karlamangla A, Tinetti M, Guralnik J, Studenski S, Wetle T, Reuben D. Comorbidity in older adults: Nosology of impairment, diseases, and conditions. J Gerontol A Biol Sci Med Sci. 2007;62:296–300. doi: 10.1093/gerona/62.3.296. [DOI] [PubMed] [Google Scholar]
- 30.Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: the InChianti study. J Gerontol A Biol Sci Med Sci. 2007;62:440–446. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the Health, Aging and Body Composition study. J Gerontol A Biol Sci Med Sci. 2007;62:844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 32.Forrest KY, Zmuda JM, Cauley JA. Correlates of decline in lower extremity performance in older women: a 10-year follow-up study. J Gerontol A Biol Sci Med Sci. 2006;61:1194–1200. doi: 10.1093/gerona/61.11.1194. [DOI] [PubMed] [Google Scholar]
- 33.Volpato S, Leveille SG, Corti MC, Harris TB, Guralnik JM. The value of serum albumin and high-density lipoprotein cholesterol in defining mortality risk in older persons with low serum cholesterol. J Am Geriatr Soc. 2001;49:1142–1147. doi: 10.1046/j.1532-5415.2001.49229.x. [DOI] [PubMed] [Google Scholar]
- 34.Morley JE. Mobility performance: a high-tech test for geriatricians. J Gerontol A Biol Sci Med Sci. 2003;58:712–714. doi: 10.1093/gerona/58.8.m712. [DOI] [PubMed] [Google Scholar]