Abstract
Benign metastasizing leiomyoma (BML) is a rare tumor comprising histologically benign smooth muscle cells and exhibits the same histological findings as a uterine myoma although in an extra-uterine location. Most BMLs occur several years after surgery for uterine myoma in women of reproductive age. Here, we report a case of pulmonary BML in a 54-year-old postmenopausal woman with no previous history of myomectomy or hysterectomy. The patient presented with a rapid increase in abdominal girth over the past 3 months and a cough lasting for 2 months. Chest computed tomography (CT) revealed multiple pulmonary nodules, ranging in diameter from a few millimeters to 1.5 cm. Abdominal CT revealed a well-defined heterogeneous hypervascular uterine mass measuring 25 cm at the widest diameter. In addition to the uterine mass, imaging studies identified no other origin of the metastatic lung nodules. Total abdominal hysterectomy and bilateral salpingo-oophorectomy were performed followed by video-assisted thoracoscopy. The histological findings of the lungs and uterus suggested myoma. The patient remains asymptomatic and disease-free at 7 years after surgery without adjuvant treatment.
Keywords: Benign metastasizing leiomyomas, Postmenopause, Myoma, Cough
Highlights
-
•
Benign metastasizing leiomyoma (BML) can occur in postmenopausal woman without uterine surgery.
-
•
BMLs in postmenopausal woman can cause respiratory and gynecologic symptoms.
-
•
Total abdominal hysterectomy and bilateral salpingo-oophorectomy could reduce lung BML in postmenopausal woman.
1. Introduction
Uterine myomas are the most common benign tumors of the genital organs in women of reproductive age (Sparic et al., 2016). These neoplasms are clinically benign with little metastatic capability. Benign metastasizing leiomyoma (BML) is a benign-appearing smooth muscle tumor similar to a uterine myoma, which occurs at extra-uterine sites, being most common in the lung (Jautzke et al., 1996). The term BML was introduced by Steiner in 1939 (Steiner, 1939). Since then, approximately 160 cases have been reported (Barnas et al., 2017). Although the pathophysiology of BML metastasis has not been clarified, it has been suggested that BML is produced by the metastasis of a uterine lesion through lymphovascular spread or through the hormone-sensitive in situ proliferation of smooth muscle cells (Awonuga et al., 2010). The majority of women diagnosed with a BML have a history of uterine myoma surgery since there have been only 10 reported cases of BMLs in women without a history of gynecological surgery (Barnas et al., 2017). This further raises the question of the possibility of iatrogenic lymphovascular spread due to former surgery. Fewer than 10 cases of BML have been reported in postmenopausal woman (Awonuga et al., 2010; Barnas et al., 2017). To the best of our knowledge, this is the first reported case of simultaneous uterine myoma and pulmonary BML in a postmenopausal woman with respiratory and gynecological symptoms but no history of gynecological surgery.
2. Case report
A 54-year-old woman, gravida 2 para 1, presented with a rapid increase in abdominal girth over the past 3 months and a 2-month history of a mild, non-productive cough, but no other respiratory symptoms. She underwent menopause at the age of 50 years. Her past menstrual cycle was regular and uneventful. She had no relevant surgical or medical history. There was no abnormal finding on a chest X-ray performed 2 years before for routine health screening.
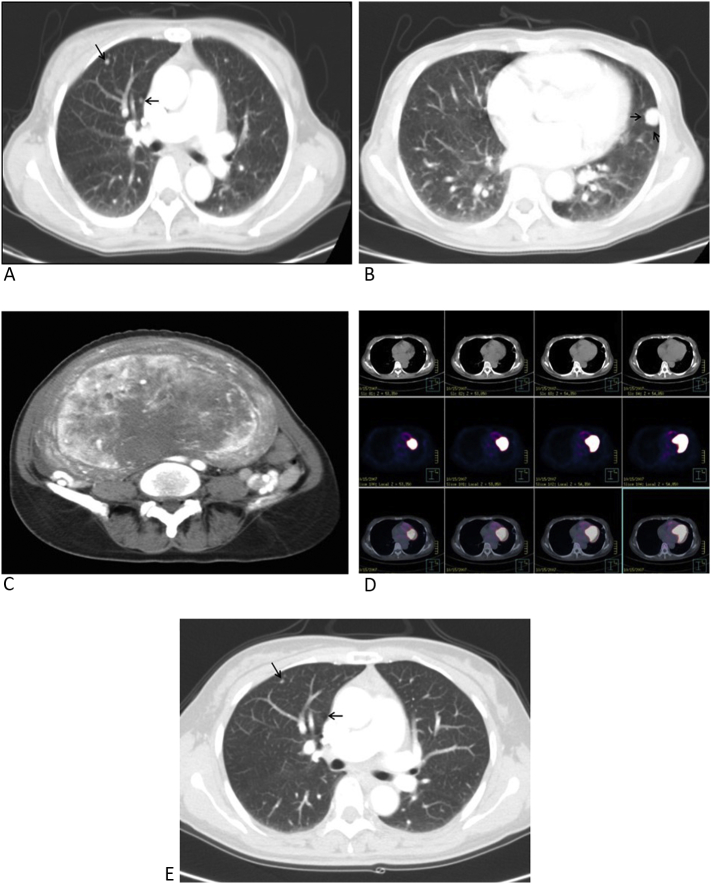
Routine blood chemistry and inflammatory reaction test results were within normal ranges. A serum tumor marker study showed all within normal ranges. The patient's chest radiograph revealed multiple tiny nodules in both lungs, suggesting metastases (Fig. 1). Chest CT was performed for further evaluation, which revealed multiple (Fig. 2), well-circumscribed pulmonary nodules with dense enhancement patterns that ranged in diameter from a few millimeters to 1.5 cm (Fig. 2B).
Fig. 1.
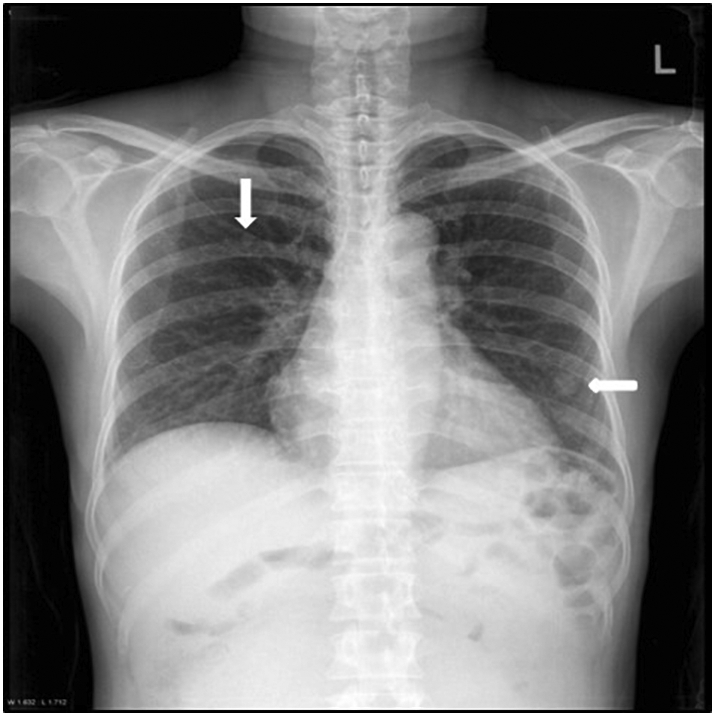
Chest radiograph showing multiple tiny nodules (white arrow) in both lung fields.
Fig. 2.
A. Chest CT scan showing well-defined, round tiny nodules of variable sizes (black arrow) in the right lung field. B. The largest nodule was approximately 1.5 × 1.5 cm in diameter. C. Abdominal CT showing a well-defined heterogeneous hypervascular uterine mass lesion in the arterial phase. D. 18F-FDG PET-CT showed no significant metabolic activity of the nodules. E. Chest CT at a 12-month follow-up showed a slight size reduction of the metastatic lung nodules (comparing Fig. 2A with Fig. 2E).
Abdominal CT revealed a well-defined heterogeneous hypervascular mass in the uterus that measured 25 cm at its widest dimension (Fig. 2C). The clinical diagnosis was suggestive of uterine sarcoma with multiple lung metastases. However, there was no specific tomographic feature of a uterine sarcoma to aid in the differential diagnosis. Even though a huge uterine mass was present, hematogenous metastasis from an unknown malignancy was considered. A whole body bone scan, whole body positron emission tomography (PET)-CT, gastroscopy, colonoscopy, and breast sonography were also performed to search for the primary origin of the suspicious metastatic lung nodules, but revealed no abnormal findings. 18F-fluorodeoxyglucose (18F-FDG) PET-CT revealed no metastatic disease (Fig. 2D).
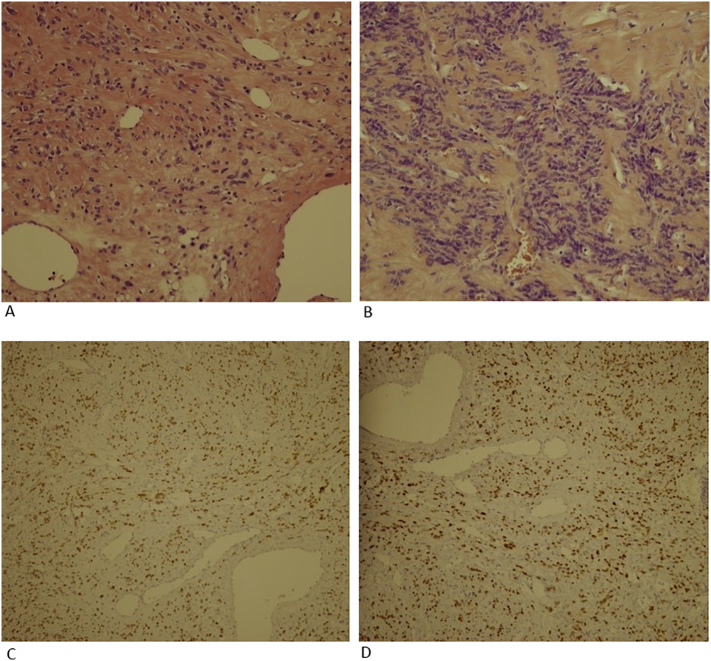
The patient underwent both hysterectomy and salpingo-oophorectomy. Owing to the concern that the lung lesions might be a metastatic uterine sarcoma from a primary pelvic mass, the decision was made to explore the patient's abdomen surgically. On gross examination, the removed uterine mass measured 28 cm × 21 cm × 11 cm and weighed 3620 g. On sectioning, the myometrium contained a huge solid grayish mass that was histopathologically diagnosed as a benign leiomyoma with no atypical or malignant features (Fig. 3A).
Fig. 3.
A. Uterine leiomyoma. The mass comprised smooth muscle, featuring short fascicles of spindle-shaped cells with indistinct borders and abundant, often fibrillar, eosinophilic cytoplasm (hematoxylin & eosin stain; original magnification, ×200). B. Lung leiomyoma. Tumor cells showed no nuclear atypia, mitotic figures, or tumor cell necrosis (hematoxylin & eosin stain; original magnification, × 200). C. Immunohistochemical analysis was positive for ER (original magnification, ×100). D. Immunohistochemical analysis was positive for PR (original magnification, ×100).
Approximately 2 weeks after abdominal surgery, the patient underwent video-assisted thoracoscopy (VATS) and wedge resection of the left lingular segment was attempted. The largest mass of the lingular segment was excised. Because the residual nodule was too small and too deep in the pulmonary parenchyma from the pleural surface, the residual nodules were not completely removed.
A histopathological examination of this pulmonary nodule revealed the presence of lesions composed of benign-appearing bundles of smooth muscle cells with no nuclear atypia (Fig. 3B). These tumor cells were strongly positive for SMA, desmin, estrogen receptor (ER) (Fig. 3C), and progesterone receptor (PR) (Fig. 3D), while negative for cytokeratin, HMB45, S-100, and c-kit. The histology of the resected lung specimens was similar to that of a uterine leiomyoma. The pulmonary lesion was diagnosed as a BML.
Although hormonal treatment was recommended, the patient refused adjuvant medical treatment, thus close observation was chosen. The residual small nodules in the right upper lobe were slightly reduced in size at a 12-month follow-up examination (Fig. 2E), which included serial CT scans of the chest, abdomen, and pelvis. She remains asymptomatic with stable residual disease on imaging at 7 years after hysterectomy and VATS.
3. Discussion
A uterine leiomyoma is the most common type of benign smooth muscle tumor among women of reproductive age (Sparic et al., 2016). Leiomyomas are typically benign and rarely metastasize to extra-uterine sites. Other smooth muscle tumors, such as diffuse leiomyomatosis, intravenous leiomyomatosis, metastasizing leiomyoma, and disseminated peritoneal leiomyomatosis, have unusual growth patterns, locations, and distribution (Bell et al., 1994).
In contrast to the general belief that benign tumors do not metastasize, Steiner reported the first case of a metastasizing fibroleiomyoma of the uterus in 1939 (Steiner, 1939). The term of BML is now used to describe the presence of histologically benign smooth muscle tumors in extra-uterine sites with the coexistence or previous removal of a uterine leiomyoma (Awonuga et al., 2010). BMLs most commonly occur in the lung, but have been reported in the lymph nodes, mediastinum, heart, retroperitoneum, brain, and bones (Consamus et al., 2014; Jo et al., 2006).
At present, more than 160 cases of BML have been reported in the literature. Almost all BMLs occur in premenopausal women, but some cases have been reported in postmenopausal woman (Barnas et al., 2017; Moon et al., 2009). In the literature, most cases of BML have been reported in patients with a history of uterine leiomyoma. Mean ages (±standard deviation) in myoma surgery and diagnosis of BML patients were 38.5 ± 8.9 and 47.3 ± 10.0 years, respectively. The mean time from the uterine surgery to BML diagnosis was 8.8 years (Barnas et al., 2017).
Unlike other extra-uterine smooth muscle tumors, BMLs are positive for ER and PR (Jautzke et al., 1996). The results of an X chromosome inactivation assay showed that BMLs are monoclonal in origin with a shared cytogenetic profile (Nucci et al., 2007). These results suggest that a leiomyoma could be the source of a BML.
Although the pathogenesis of the tumor has not been clarified, current hypotheses include lymphatic and vascular spread, peritoneal seeding, and coelomic metaplasia (Awonuga et al., 2010). Among these hypotheses, surgically induced hematogenous spread is most commonly accepted because metastasis of a BML most often occurs in patients with a history of uterine surgery and lung metastasis. However, BMLs have been reported in women with no history of gynecological surgery (Barnas et al., 2017). BMLs have also been reported in men and children (Pacheco-Rodriguez et al., 2016). which raises the possibility of surgically induced hematogenous spread. However, the peritoneal seeding theory has limitations in explaining metastasis to the lung (Awonuga et al., 2010). The lung, which is the most frequent metastatic site, has no communication with the peritoneal cavity. The coelomic metaplasia theory assumes that subcoelomic mesenchymal cells undergo metaplasia under the influence of hormones and then differentiate into myofibroblast (Awonuga et al., 2010). The pathogenesis of a BML is likely multifactorial, thus further multidirectional research is required.
Pulmonary BMLs are usually asymptomatic and incidentally discovered, although some patients present with symptoms of cough, chest pain, hemoptysis, and shortness of breath (Schneider et al., 2001). The growth of a BML is dependent on responsiveness to estrogen and progesterone. BMLs in postmenopausal women do not generally cause respiratory symptoms (Moon et al., 2009). Interestingly, the present case complained of cough. It is thought that factors other than hormone stimulation are involved in the growth and clinical course of a BML.
Typical radiological findings of BMLs show numerous well-defined solid pulmonary nodules of various sizes that are scattered within the bilateral normal interstitium, but with no calcification or contrast enhancement. Less frequently, a BML may present with military, solitary, or cystic/cavitary nodular patterns, or a combination thereof (Choe et al., 2017). In our case, enhanced images of the lung nodules were produced after administration of contrast medium. PET-CT cannot be used to rule out the malignancy of pulmonary nodules, as this examination is helpful only with the lack of nodule uptake (Sawai et al., 2017).
Potential options for BML treatment include careful observation, surgical resection of pulmonary nodules, hysterectomy and bilateral salpingo-oophorectomy, as well as the administration of progesterone, selective ER modulators, aromatase inhibitor (AI), and gonadotropin-releasing hormone (GnRH) agonists (Lewis et al., 2013). There is a lack of evidence regarding the efficacy of chemotherapy for BMLs. Premenopausal patients may be amendable to hormone treatment. Adjuvant treatment should be individualized and long-term surveillance is important to prevent disease progression.
The number of reported cases BML after 2010 has surpassed the sum of all cases published before (Barnas et al., 2017). It is difficult to know the exact cause of the increase in the number of reported cases. Although the incidence of BML is low, it is important to keep in mind that the likelihood of BML cases.
Although this report describes a single case, BML can occur in postmenopausal women with respiratory and gynecologic symptoms but no history of uterine surgery. In postmenopausal women, a BML could be mistaken for metastatic cancer. Hence, accurate diagnosis is important to determine appropriate individualized treatment without overtreatment. Our case suggested that other factors may be involved in the pathogenies of a BML. Further investigations on possible etiological factors based on clinical, biological, and genetic perspectives are essential.
Ethical consent
Consent was obtained from the patient for publication of this case report.
Disclosure
The authors have no conflict of interest to disclose.
Author contributions
Hyun Chul JO wrote the paper and prepared figures.
Jong Chul Baek reviewed drafts of the paper.
Both authors contributed equally to this work.
References
- Awonuga A.O., Shavell V.I., Imudia A.N., Rotas M., Diamond M.P., Puscheck E.E. Pathogenesis of benign metastasizing leiomyoma: a review. Obstet Gynecol Surv. 2010;65(3):189–195. doi: 10.1097/OGX.0b013e3181d60f93. [DOI] [PubMed] [Google Scholar]
- Barnas E., Ksiazek M., Ras R., Skret A., Skret-Magierlo J., Dmoch-Gajzlerska E. Benign metastasizing leiomyoma: a review of current literature in respect to the time and type of previous gynecological surgery. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0175875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S.W., Kempson R.L., Hendrickson M.R. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am. J. Surg. Pathol. 1994;18(6):535–558. [PubMed] [Google Scholar]
- Choe Y.H., Jeon S.Y., Lee Y.C., Chung M.J., Park S.Y., Lee Y.C., Kim S.R. Benign metastasizing leiomyoma presenting as multiple cystic pulmonary nodules: a case report. BMC Womens Health. 2017;17(1):81. doi: 10.1186/s12905-017-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consamus E.N., Reardon M.J., Ayala A.G., Schwartz M.R., Ro J.Y. Metastasizing leiomyoma to heart. Methodist Debakey Cardiovasc J. 2014;10(4):251–254. doi: 10.14797/mdcj-10-4-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jautzke G., Muller-Ruchholtz E., Thalmann U. Immunohistological detection of estrogen and progesterone receptors in multiple and well differentiated leiomyomatous lung tumors in women with uterine leiomyomas (so-called benign metastasizing leiomyomas). A report on 5 cases. Pathol. Res. Pract. 1996;192(3):215–223. doi: 10.1016/S0344-0338(96)80224-X. [DOI] [PubMed] [Google Scholar]
- Jo J.H., Lee J.H., Kim D.C., Kim S.H., Kwon H.C., Kim J.S., Kim H.J. A case of benign metastasizing leiomyoma with multiple metastasis to the soft tissue, skeletal muscle, lung and breast. Korean J Intern Med. 2006;21(3):199–201. doi: 10.3904/kjim.2006.21.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E.I., Chason R.J., Decherney A.H., Armstrong A., Elkas J., Venkatesan A.M. Novel hormone treatment of benign metastasizing leiomyoma: an analysis of five cases and literature review. Fertil. Steril. 2013;99(7):2017–2024. doi: 10.1016/j.fertnstert.2013.01.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H., Park S.J., Lee H.B., Kim S.R., Choe Y.H., Chung M.J.…Lee Y.C. Pulmonary benign metastasizing leiomyoma in a postmenopausal woman. Am J Med Sci. 2009;338(1):72–74. doi: 10.1097/MAJ.0b013e31819c7160. [DOI] [PubMed] [Google Scholar]
- Nucci M.R., Drapkin R., Dal Cin P., Fletcher C.D., Fletcher J.A. Distinctive cytogenetic profile in benign metastasizing leiomyoma: pathogenetic implications. Am. J. Surg. Pathol. 2007;31(5):737–743. doi: 10.1097/01.pas.0000213414.15633.4e. [DOI] [PubMed] [Google Scholar]
- Pacheco-Rodriguez G., Taveira-Dasilva A.M., Moss J. Benign Metastasizing Leiomyoma. Clin. Chest Med. 2016;37(3):589–595. doi: 10.1016/j.ccm.2016.04.019. [DOI] [PubMed] [Google Scholar]
- Sawai Y., Shimizu T., Yamanaka Y., Niki M., Nomura S. Benign metastasizing leiomyoma and 18-FDG-PET/CT: a case report and literature review. Oncol. Lett. 2017;14(3):3641–3646. doi: 10.3892/ol.2017.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T., Kugler C., Kayser K., Dienemann H. Chirurg. 2001;72(3):308–311. doi: 10.1007/s001040051311. Benign, pulmonary metastatic leiomyoma of the uterus. [DOI] [PubMed] [Google Scholar]
- Sparic R., Mirkovic L., Malvasi A., Tinelli A. Epidemiology of uterine myomas: a review. Int J Fertil Steril. 2016;9(4):424–435. doi: 10.22074/ijfs.2015.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner P.E. Metastasizing fibroleiomyoma of the uterus: report of a case and review of the literature. Am. J. Pathol. 1939;15(1):89–110 117. [PMC free article] [PubMed] [Google Scholar]