Abstract
Primary coenzyme Q10 deficiency-7 is caused by homozygous or compound heterozygous mutations in the COQ4 gene. Until now 12 patients have been reported, most presenting with a lethal infantile phenotype with encephalopathy, epilepsy and cardiomyopathy. We report on a new phenotype of COQ4 deficiency: a childhood onset spinocerebellar ataxia with stroke-like episodes.
List of abbreviations
| CoQ10 | Coenzyme Q10 |
1. Introduction
Coenzyme Q10 (ubiquinone; CoQ10, EC 206–147-9) is essential for mitochondrial respiratory chain electron transport [1,2]. Primary CoQ10 deficiency is caused by mutations in genes of the COQ10 biosynthetic pathway. Primary coenzyme Q10 deficiency-7 (COQ10D7, MIM 616276) is caused by homozygous or compound heterozygous mutation in the COQ4 gene (MIM 612898). Symptoms include early onset encephalomyopathy, cerebellar ataxia and nephropathy [3]. A first patient with CoQ10 deficiency due to COQ4 haploinsufficiency was published in 2012 [4]. So far a total of 12 patients with compound heterozygous or homozygous COQ4 variants have been reported, most (11/12) patients presented with a lethal infantile phenotype with encephalopathy, epilepsy and cardiomyopathy [[5], [6], [7]]. We report on a new phenotype of COQ4 deficiency: a childhood onset spinocerebellar ataxia with stroke-like episodes.
2. Patients and methods
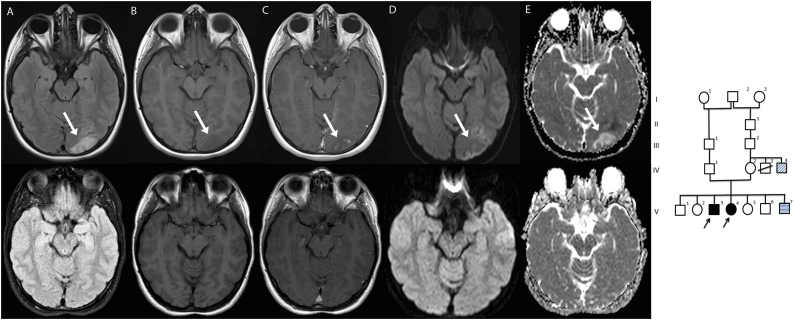
The family pedigree is shown in Fig. 1: V-3 is patient 1 and V-4 is patient 2. Patient V-3 and V-4 have four healthy siblings and one younger brother with a complex constellation of congenital abnormalities not caused by mutations in the COQ4 gene.
Fig. 1.
On the right side of the figure is the family pedigree. On the left side the MRI scan from patient 2 (top row MRI at the time of the first stroke like episode, bottom row 3 months later). There is a lesion in the left occipital lobe (indicated by the white arrow) with increased signal on FLAIR (A) and low signal on T1-weighted images (B). There is patchy enhancement after gadolinium administration (C) and is abnormal signal intensity on DWI (D) and ADC (E). There is complete normalization on a follow-up scan about 3 months later.
Patient 1 is the 15 year old third child of consanguineous parents. His early psychomotor development was abnormal with delayed speech development. He developed a “tremor” at the age of four years. Physical examination was otherwise unremarkable. There were no dysmorphic features, no skin abnormalities and no hepatosplenomegaly. Brain MRI at age 5 revealed a suspected tectal glioma. He was treated with radiotherapy and the lesion has been stable since age 10. However, ambulation progressively deteriorated, and he became wheelchair dependent around age 12. On neurological examination there was dysarthria, spastic tetraparesis and prominent ataxia of upper and lower limbs. He developed tonic-clonic seizures treated with carbamazepine at age 12. Cognitive development was abnormal (TIQ 60), necessitating special education.
Patient 2 is the 14 year old sister of patient 1. After a normal early development she developed tonic clonic seizures at age 9, treated with carbamazepine, and a “tremor” of the hands at age 10. Her IQ was around 55–60. Walking became difficult, with frequent falls. On examination she had a spinocerebellar syndrome like her brother, with a normal general physical examination. MRI of the brain revealed a cavernoma in de left parietal lobe, but no other abnormalities. General laboratory tests were normal, specifically creatinine. At age 13 years she was admitted to our hospital because of severe headache, vomiting, and a decreased consciousness. On examination there was a right sided hemi-anopsia. An MRI scan identified a lesion in the left occipital lobe on T2 and FLAIR images, with clear diffusion restriction (Fig. 1). Within 24 h she made a full recovery and follow-up imaging at 3 months showed normalization on MRI.
Since these siblings both developed a progressive spinocerebellar syndrome, a genetic cause was suspected. Whole exome sequencing (specifics can be requested from the authors) revealed a homozygous variant in the COQ4 gene (NM_016035.3: c.230C > T (p.Thr77Ile)) in patient 2 which was also identified in patient 1 by targeted sequencing. Thr77 concerns an evolutionary conserved amino acid located in a conserved region in orthologues (up to Baker's yeast) and this variant is not reported in healthy individuals (ExAC databasePMID: 27535533). The segregation pattern in this family, with both parents being carriers and the identification of the same homozygous COQ4 variants in the affected sibling, supports pathogenicity of this variant. Functional studies in cultured skin fibroblasts of patient 2 demonstrated decreased levels of CoQ10 (0.26 nmol/U CS; reference range 0.77–1.61 (mean 1.19); n = 30), as detected by nanoHPLC-ECD. This proves that the patient has a coenzyme Q10 deficiency, supporting the notion that the variant in the COQ4 gene is pathogenic.
After the diagnosis at age 13 and 11 respectively, CoQ10 supplementation in a dose of 1000 mg once daily was initiated for both siblings. The 6 min walk test was stable over the period of a year. Patient 2 developed a second stroke-like episode at age 14.
3. Discussion
This is the first report of a childhood-onset spinocerebellar syndrome with stroke-like episodes caused by COQ4-deficiency and is different from the lethal infantile presentation described earlier [[5], [6], [7]]. Patient S5 reported by Brea-Calvo [5] is most comparable to our patients with progressive motor deterioration from age 10 months, spastic ataxic gait at age 3, wheelchair dependency, epilepsy and progressive deterioration. However, other primary CoQ10 deficiencies have been associated with a late-onset ataxic phenotype, like ARCA2 (due to mutations in the ACDK3 gene) [8]. Stroke-like episodes were also reported in patients with ADCK3 mutations and in patients with infantile encephalomyopathy and nephropathy with CoQ10 deficiency [8,9]. This further supports that these symptoms are related to CoQ10 deficiency.
The majority of previously reported patients with CoQ10 deficiency demonstrated cerebellar atrophy on imaging [8] but this was not seen in our patients. It is possible that this feature will develop later in life. Imaging did reveal a tectal glioma in patient 1 but this seems unrelated to the COQ4 mutation. Other primary CoQ10 deficiencies were associated with cardiac failure, but echocardiography demonstrated no cardiomyopathy in our patients. Except for the stroke-like episodes our patients did not deteriorate rapidly, it is unclear if this is due to CoQ10 supplementation. In other patients, a beneficial effect of CoQ10 suppletion was reported [8,9]. However, follow-up on our patients is not yet long enough to establish that there is no disease progression. Also, patient 2 developed a new stroke like episode while being treated with CoQ10. It seems therefore that CoQ10 suppletion does not prevent (all) stroke-like episodes in this disorder.
With whole exome sequencing becoming routine in the evaluation of neurodegenerative disorders, more cases will be diagnosed in the near future and the clinical spectrum of COQ4 deficiency will be more clearly defined. Functional studies in cultured fibroblasts from a skin biopsy can provide strong supportive evidence for pathogenicity of unknown variants in genes encoding enzymes of the coenzyme Q10 biosynthesis pathway, as illustrated in these cases. Treatment with CoQ10 can be considered, though clear evidence of efficacy in these slowly progressive ataxic phenotypes is still lacking.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Annet M. Bosch, Email: a.m.bosch@amc.uva.nl.
Erik-Jan Kamsteeg, Email: Erik-Jan.Kamsteeg@radboudumc.nl.
Richard J. Rodenburg, Email: Richard.Rodenburg@radboudumc.nl.
Arend W. van Deutekom, Email: a.w.vandeutekom@amc.uva.nl.
Dennis R. Buis, Email: d.r.buis@amc.uva.nl.
Marc Engelen, Email: m.engelen@amc.uva.nl.
Jan-Maarten Cobben, Email: j.m.cobben@amc.uva.nl.
References
- 1.Crane F.L., Hatefi Y., Lester R.L., Widmer C. Biochim. Biophys. Acta. 1957 Jul;25(1):220–221. doi: 10.1016/0006-3002(57)90457-2. [DOI] [PubMed] [Google Scholar]
- 2.Turunen M., Olsson J., Dallner G. Biochim. Biophys. Acta. 2004 Jan 28;1660(1–2):171–199. doi: 10.1016/j.bbamem.2003.11.012. (Review) [DOI] [PubMed] [Google Scholar]
- 3.Desbats M.A., Lunardi G., Doimo M., Trevisson E., Salviati L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ 10) deficiency. J. Inherit. Metab. Dis. 2015;38(1):145–156. doi: 10.1007/s10545-014-9749-9. [DOI] [PubMed] [Google Scholar]
- 4.Salviati L., Trevissin E., Rodriguez Hernandez M.A. Haploinsufficiency of COQ4 causes coenzyme Q10 deficiency. J. Med. Genet. 2012;49(3):187–191. doi: 10.1136/jmedgenet-2011-100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brea-Calvo G., Haack T.B., Karall D. CoQ4 mutations cause a broad spectrum of mitochondrial disorders associated with CoQ10 deficiency. Am. J. Hum. Genet. 2015;96:309–317. doi: 10.1016/j.ajhg.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung W.K., Martin K., Jalas C. Mutations in COQ4 , an essential component of coenzyme Q biosynthesis, cause lethal neonatal mitochondrial encephalomyopathy. J. Med. Genet. 2015;52:627–635. doi: 10.1136/jmedgenet-2015-103140. [DOI] [PubMed] [Google Scholar]
- 7.Sondheimer N., Hewson S., Cameron j.M. Novel recessive mutations in COQ4 cause severe infantile cardiomyopathy and encephalopathy associated with CoQ10 deficiency. Mol Genet Metab Reports. 2017;12:23–27. doi: 10.1016/j.ymgmr.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mignot C., Apartis E., Durr A. Orphanet J Rare Dis. 2013 Oct 28;8:173. doi: 10.1186/1750-1172-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salviati L., Sacconi S., Murer L. Neurology. 2005; Aug 23;65(4):606–608. doi: 10.1212/01.wnl.0000172859.55579.a7. [DOI] [PubMed] [Google Scholar]