Abstract
Background
Data on the pathogens responsible for nosocomial bloodstream infection (BSI) and their antimicrobial resistance (AMR) in Hubei province are limited. This study was conducted to determine the major pathogens causing BSI and to characterize their AMR.
Methods
Data from the China Antimicrobial Resistance Surveillance System (CARSS) from 2014 to 2016 were analyzed retrospectively.
Results
Escherichia coli, Staphylococcus aureus and Klebsiella pneumoniae were the most common pathogens responsible for nosocomial BSI. Individuals aged 0–5 years and ≥ 40 years old were the major demographics at risk of infection by E. coli, K. pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii and Enterobacter cloacae, while individuals aged 0–5 years were the major demographic at risk of infection by S. aureus, Enterococcus faecalis, E. faecium, Streptococcus pneumoniae and Stenotrophomonas maltophilia. The frequencies of E. coli and K. pneumoniae isolates resistant to cefotaxime were 59.1% and 24.3%, respectively, and the frequencies of resistant isolates to ceftazidime were 42.9% and 27.2%, respectively. From 2014 to 2016, the frequency of extended-spectrum β-lactamase (ESBL)-positive E. coli declined from 29.07 to 24.5%, and the frequency of ESBL-positive K. pneumoniae declined from 18.64 to 12.33%. The frequency of carbapenem-resistant (CR) E. coli was below 0.5%, but 1–10% of K. pneumoniae isolates were CR.
Conclusions
The emergence of methicillin-resistant S. aureus and the expansion of ESBL and fluoroquinolone resistance among Gram-negative Enterobacteriaceae increased AMR severity. Carbapenemase-producing K. pneumoniae isolates responsible for nosocomial BSI increased year over year and effective infection control measures should be taken to prevent them from spreading.
Keywords: Nosocomial bloodstream infection, Antimicrobial resistance, MRSA, ESBL
Background
Bloodstream infection (BSI) is very common and is associated with high morbidity and mortality worldwide. The etiology of BSI and the antimicrobial resistance (AMR) of the pathogens causing it differ significantly between developed and developing countries [1]. Data from the Finnish Hospital Infection Program during 1999–2001 and 2005–2010 demonstrated that the most common pathogens responsible for BSI were coagulase-negative Staphylococcus, S. aureus and Escherichia coli [2]. By contrast, BSI surveillance in Malawi from 1998 to 2016 indicated that non-typhoidal Salmonella, S. enterica Typhi and Streptococcus pneumoniae were the most common causative agents [3]. In China, the pathogens responsible for BSI vary from region to region. A study of the clinical features of nosocomial BSI in neonates from two prefectures, Henan and Chongqing, indicated that E. coli was the most prevalent BSI pathogen in Henan while Klebsiella pneumoniae was the predominant BSI pathogen in Chongqing [4]. Reports on the causes of BSI in Hubei province have been limited.
Surveillance and analysis of AMR are crucial in preventing BSI. The Hubei Province bacterial resistance surveillance network was founded in 1998 and includes surveillance data on hospital-acquired and community-acquired infections. By the end of 2016, a total of 11 secondary care hospitals and 41 tertiary care hospitals had joined the network, covering all prefecture-level cities in Hubei Province (Fig. 1). Here, in order to provide a basis for empirical treatment and policy making for the Department of Health Administration, we reported BSI surveillance data over the last three years, from 2014 to 2016.
Fig. 1.
Location distribution of the members of the monitoring network
Methods
Study design and data collection
We undertook a retrospective surveillance study of nosocomial BSI in Hubei province. The data were derived from the China Antimicrobial Resistance Surveillance System (CARSS). Hospital-acquired, nosocomial, and health care-associated infections were defined according to the World Health Organization (WHO) as infections occurring in a patient residing in a hospital or another health care facility, in whom the infection was not present nor incubating at the time of admission [5]. An infection occurring 48 h or later after admission to hospital was considered nosocomial [5]. An infection occurring within 48 h of admission to hospital or not associated with healthcare interventions within the previous 12 months was defined as community-acquired [6]. If the isolates were identified as coagulase-negative Staphylococcus, Corynebacterium, Bacillus, Propionibacterium or other potential skin contaminants, two or more separate blood cultures were required for diagnosis [7].
Strain identification and antibiotic sensitivity testing
Blood samples (8–10 mL for adult patients and 2–5 mL for pediatric patients) were collected into special blood culture bottles. Various automated blood culture instruments were applied. Manual biochemical tests, automatic bacteria identification instruments and/or an IVD-MALDI Biotyper (Bruker, Germany) were used for identification of bacterial species. The Kirby-Bauer method and/or the minimum inhibitory concentration (MIC) method were used for antimicrobial susceptibility testing according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. The results of antimicrobial sensitivity tests were interpreted according to the CLSI 2016 standards [8]. Isolation and identification of strains and antibiotic sensitivity testing were performed by members of the bacterial resistance monitoring network. Each member of the monitoring network reported data regarding bacterial identification and antibiotic sensitivity to the CARSS each quarter.
Statistical analysis
Antibiotic susceptibility data was analyzed using WHONET 5.6 software. Only the first isolate of a given species from a patient was analyzed according to CLSI M-39 [9]. When antibiotic resistance tests were carried out by the Kirby-Bauer method, the MIC method and the E-test method simultaneously, the results of E-tests were preferred, followed by the MIC method and the Kirby-Bauer method.
Results
Distribution of common pathogens responsible for BSI
In this investigation, only strains isolated more than 100 times per year were analyzed. E. coli was the most common pathogen responsible for nosocomial BSI during 2014–2016, followed by S. aureus and K. pneumonia. Each of these pathogens was isolated more than 500 times per year (Fig. 2a). Enterococcus faecium, E. faecalis, Pseudomonas aeruginosa and Acinetobacter Baumannii isolated were each isolated between 250 and 500 times per year (Fig. 2b). Enterobacter cloacae, S. pneumoniae and Stenotrophomonas maltophilia were each isolated between 100 and 250 times per year (Fig. 2c).
Fig. 2.
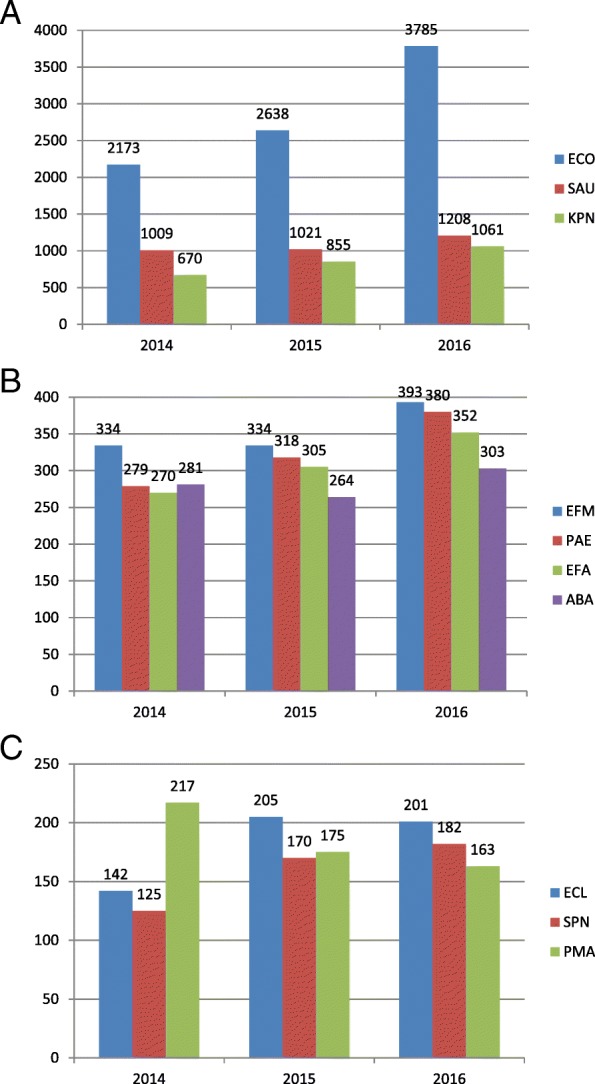
Pathogens in bloodstream infection, 2014~ 2016. a Pathogens isolated at high frequency (N > 500/year). b Pathogens isolated at intermediate frequency (250/year<N < 500/year). c Pathogens isolated at low frequency (100/year<N < 250/year)
Age demographics affected by BSI
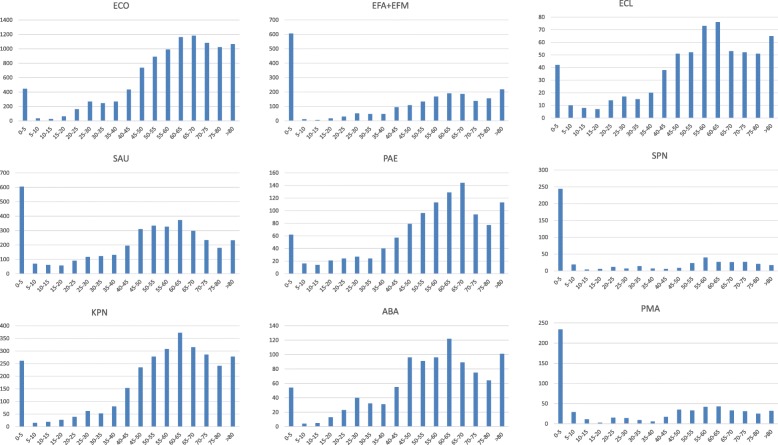
Individuals aged 0–5 years and ≥ 40 years old were the major demographics at risk of infection by E. coli, K. pneumoniae, P. aeruginosa, A. baumannii and E. cloacae, while individuals aged 0–5 years were the major demographic at risk of infection by S. aureus, E. faecalis, E. faecium, S. pneumoniae and S. maltophilia (Fig. 3).
Fig. 3.
The main pathogens isolated from bloodstream infection stratified by age
Seasonal distribution of BSI pathogens
Most E. coli isolates were identified from October to December (10–12) and from July to September (7–9), with the lowest number identified from January to March (1–3). Isolates of S. aureus, E. faecalis and E. faecium were distributed roughly evenly across all four seasons. The highest number of K. pneumoniae, P. aeruginosa, A. baumannii and E. cloacae isolates identified in a quarter was 7–9 and the lowest number of these species was 1–3. Most S. maltophilia isolates were identified from April to June (4–6) and the lowest number observed in a quarter was 1–3 (Fig. 4).
Fig. 4.
The distribution proportion in the four seasons of the main pathogens isolated from BSI
Antimicrobial susceptibility
For E. coli isolates, resistance rates to ampicillin (AMP), piperacillin (PIP), cefazolin (CZO), cefuroxime (CXM), cefotaxime (CTX) and trimethoprim sulfamethoxazole (SXT) were > 50%, but more than 89% of isolates were sensitive to imipenem (IPM), meropenem (MEM), piperacillin-tazobactam (TZP), amikacin (AMK) and cefoxitin (FOX) (Fig. 5a). K. pneumoniae isolates were naturally resistant to AMP, and resistance rates to all other antibiotics except for PIP were > 50% (Fig. 5b). E. cloacae isolates were naturally resistant to AMP, PIP, CZO, CXM, FOX and ampicillin-sulbactam (SAM), but > 50% of isolates were sensitive to the other antibiotics (Fig. 5c). P. aeruginosa isolates were naturally resistant to SXT, and the resistance rate to minocycline (MNO) was 80.4%. However, > 60% of isolates were sensitive the other antibiotics except for ticarcillin-clavulanate Acid (TCC) (Fig. 5d). More than 65% of A. baumannii isolates were resistant to common antibiotics except for MNO (Fig. 5e). Sensitivity rates of S. maltophilia isolates to SXT, MNO, levofloxacin (LVX) and chloramphenicol (CHL) exceeded 80% (Fig. 5f). More than 50% of S. aureus isolates were resistant to penicillin (PEN), erythromycin (ERY), CZO and CXM (Fig. 5g). More than 60% of E. faecalis isolates were sensitive to common antibiotics except for ERY and ciprofloxacin (CIP) (Fig. 5h). For E. faecium isolates, sensitivity rates to vancomycin (VAN), teicoplanin (TEC) and linezolid (LNZ) exceeded 95%, but resistance rates to other antibiotics exceeded 60% (Fig. 5i). For S. pneumoniae isolates, resistance rates to ERY and clindamycin (CLI) exceeded 89%, but sensitivity rates to other antibiotics exceeded 80% (Fig. 5j).
Fig. 5.
Susceptibility rates and resistance rates to common antibiotics. a (Escherichia coli). b (Klebsiella pneumoniae). c (Enterobacter cloacae). d (Pseudomonas aeruginosa). e (Acinetobacter Bauman). f (Stenotrophomonas maltophilia). g (Staphylococcus aureus). h (Enterococcus faecalis). i (Enterococcus faecium). j (Streptococcus pneumonia)
Trends of multi-drug resistant (MDR) strains
The frequency of methicillin-resistant S. aureus (MRSA), extended-spectrum β-lactamase (ESBL)-positive E. coli and K. pneumoniae, and carbapenem-resistant (CR) E. coli and K. pneumoniae were analyzed during 2014–2016. The proportion of MRSA isolates ranged from 30 to 40%, ESBL-positive E. coli isolates ranged from 20 to 30%, and ESBL-positive K. pneumoniae isolates ranged from 10 to 20%. The frequency of CR-E. coli was below 0.5%, but CR-K. pneumoniae isolates were detected at a frequency of 1–10% (Fig. 6).
Fig. 6.

Trends of common multi-drug resistant strains from 2014 to 2016
Discussion
Our surveillance data indicated E. coli, S. aureus and K. pneumoniae were the most common pathogens responsible for nosocomial BSI in Hubei province from 2014 to 2016. For common gram-positive bacteria and S. maltophilia, individuals < 5 years of age were the major demographic at risk of nosocomial BSI. By contrast, for common gram-negative bacteria except S. maltophilia, individuals < 5 years of age and ≥ 40 years of age were the main demographics at risk of BSI. Resistance of E. coli isolates to common antibiotics was more severe than K. pneumoniae isolates, especially to third-generation cephalosporins and fluoroquinolones. ESBL production by E. coli isolates was more common than in K. pneumoniae isolates. Most P. aeruginosa isolates (> 60%) were sensitive to antibiotics, while most A. baumannii isolates (> 60%) were antibiotic-resistant.
E. coli, S. aureus and K. pneumoniae were the most common pathogens responsible for BSI according to our surveillance data. These results differed from reports in European and African populations, in which coagulase-negative Staphylococcus and non-typhoidal Salmonella were the predominant causative agents of BSI (in Finland and Malawi, respectively) [2, 3]. This may be due to the distinct social, economic and environmental factors in these regions. The spectrum of pathogens responsible for BSI is highly variable both geographically and temporally [10]. Timely analysis and reporting of local AMR resistant isolates is important for infection control.
The most common pathogens responsible for BSI are different in people of different ages [3]. Our study found that individuals aged 0–5 years old were an important demographic at high risk of BSI. It has been well established that children under 5 years of age are susceptible to infection by Salmonella and other intestinal pathogens [11]. Our findings indicated that children under 5 years of age were also the main demographic susceptible to BSI. For Gram-positive bacteria (S. aureus, E. faecalis, E. faecium, and S. pneumoniae) and S. maltophilia, individuals under 5 years of age were the major demographic at risk, while individuals above 40 years of age or under 5 years of age were the primary population at risk of infection by Gram-negative bacteria (E. coli, K. pneumoniae, E cloacae, P. aeruginosa and A. baumanni). Our findings indicated that the incidence of E. coli and K. pneumoniae BSIs was highest in elderly patients older than 40 years, consistent with global data [12]. The prolongation of human life and the aging of populations in society maybe have something to do with increasing rates of BSI caused by E. coli and K. pneumoniae.
It was reported that BSI by Bacillus cereus had a seasonal tendency with increased risk of infection during the summer [13]. Our data showed that S. pneumoniae was more often isolated in winter while K. pneumoniae, P. aeruginosa, A. baumanni, and E. cloacae were most prevalent in autumn. No obvious seasonal trend was observed for S. aureus, E. faecalis and E. faecium. Whether BSIs have seasonal trends like food-borne diseases is worth further study.
The frequency of ESBL-producing E. coli and K. pneumoniae isolates has been increasing worldwide, resulting in prolonged hospital stays, increased costs, reduced rates of clinical and microbiological responses, and poor outcomes for patients and healthcare systems [14–20]. Previous studies have analyzed the relationship between mortality rate and ESBL-producing pathogens and observed an association with KP-BSIs, although this finding remains controversial [16, 21–23]. The frequency of ESBL-producing E. coli and K. pneumoniae isolates decreased year by year from 2014 to 2016 according to our surveillance data. This finding may be related to the strategies implemented by the USA CDC for controlling the transmission of MDR bacteria such as quarantine of patients with MDR infections, strict hand disinfection by medical staff, routine disinfection of medical devices and limited use of specific antibiotics. For E. coli, resistances rate to the third-generation cephalosporins CTX and CAZ were 59.1% and 24.3%, respectively; ESBL may be the main cause of resistance, especially for bla(CTX-M) isolates. The molecular epidemiology of E. coli isolates causing BSI in Shanghai during 2011–2013 demonstrated that CTX-14, CTX-55 and CTX-15 were the most common β-lactamases [24]. By contrast, bla(SHV) and bla(CTX-M) were the most common β-lactamases in K. pneumoniae isolates [25]. MDR and the increase in infections caused by Gram-negative bacilli producing ESBL have contributed to intensive use of carbapenems [26]. Data from the European Survey on Carbapenemase-Producing Enterobacteriaceae in Europe (EuSCAPE) indicated that the incidence of carbapenemase-producing (CP) E. coli and K. pneumoniae (CP-E/K) isolates in hospitals increased from 0.124 per 1000 admissions in 2013 to 0.223 per 1000 admissions in 2014 [27]. Our data showed that CP-E/K isolates are a growing risk, especially CP-K. pneumoniae.
In our surveillance data, S. aureus was the most frequently observed Gram-positive pathogen responsible for BSI. MRSA is a worldwide issue associated with significant morbidity and mortality [28]. Our data showed that the frequency of methicillin resistance among S. aureus isolates was 30–40%,consistent with a study conducted in 26 Hong Kong public hospitals between January 2010 and December 2012 [29], In the United States and Europe, multiple infection control approaches have been tested to control the spread of MRSA. These approaches included strict contact precautions including single rooms for MRSA-colonized or -infected patients, targeted admission screening for high-risk patients and healthcare workers at risk for infection, molecular typing of all MRSA strains, and routine decolonization of MRSA carriers including healthcare workers [28, 30, 31]. Measures to control MRSA transmission should be carried out strictly according to the principles of infection control.
This study had several limitations. In our surveillance data, only nosocomial BSI was analyzed and community-acquired BSI was not included. In Hubei province, the characteristics of community-acquired BSI are mostly unknown. The molecular mechanisms of MDR, especially ESBLs and carbapenemases, have not been tested. Molecular epidemiological studies need be carried out in the future.
Conclusions
Our findings indicated that E. coli, S. aureus and K. pneumoniae were the most common pathogens responsible for nosocomial BSI in Hubei province. Individuals aged 0–5 years were the major demographic at risk of infection by S. aureus, E. faecalis, E. faecium, S. pneumoniae and S. maltophilia, while individuals < 5 years of age or ≥ 40 years of age were primarily at risk for E. coli, K. pneumoniae, P. aeruginosa, A. baumanni and E. cloacae infection. Antibiotic resistance testing showed that MDR isolates such as MRSA and ESBL-produced E. coli and K. pneumoniae species were still a serious concern. It was particularly noteworthy that the frequency of CR-KPN isolates was increasing significantly over time.
Acknowledgments
We thank all of the members of HuBei Province bacterial resistance surveillance network. We thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This work was supported by research grants from the National Mega Project on Major Infectious Disease Prevention (2017ZX10103005–007). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABA
Acinetobacter Bauman
- AMK
Amikacin
- AMP
Ampicillin
- AMR
Antimicrobial resistance
- BSI
Bloodstream infection
- CARSS
China Antimicrobial Resistance Surveillance System
- CHL
Chloramphenicol
- CIP
Ciprofloxacin
- CLI
Clindamycin
- CLSI
Clinical and Laboratory Standards Institute
- CP
carbapenemase-producing
- CP-E/K
Carbapenemase-producing E. coli and K. pneumoniae
- CR
Carbapenem-resistant
- CTX
Cefotaxime
- CXM
Cefuroxime
- CZO
Cefazolin
- ECL
Enterobacter cloacae
- ECO
Escherichia coli
- EFA
Enterococcus faecalis
- EFM
Enterococcus faecium
- ERY
Erythromycin
- ESBL
Extended-spectrum β-lactamase
- EuSCAPE
Carbapenemase-Producing Enterobacteriaceae in Europe
- FOX
Cefoxitin
- IPM
Imipenem
- KP-BSIs
Bloodstream infections caused by klebsiella pneumonia
- KPN
Klebsiella pneumonia
- LNZ
Linezolid
- LVX
Levofloxacin
- MDR
Multi-drug resistant
- MEM
Meropenem
- MIC
Minimum inhibitory concentration
- MNO
Minocycline
- MRSA
Methicillin-resistant S. aureus
- PAE
Pseudomonas aeruginosa
- PEN
Penicillin
- PIP
Piperacillin
- PMA
Stenotrophomonas maltophilia
- SAM
Ampicillin-sulbactam
- SAU
Staphylococcus aureus
- SCN
Coagulase negative staphylococci
- SPN
Streptococcus pneumonia
- SXT
Trimethoprim Sulfamethoxazole
- TCC
Ticarcillin-Clavulanate Acid
- TEC
Teicoplanin
- TZP
Piperacillin-tazobactam
- VAN
Vancomycin
- WHO
World Health Organization
Authors’ contributions
LT designed the study. ZZ did the statistical analysis. LT wrote the manuscript. ZS were in charge of the laboratory work. All authors read and approved the final manuscript.
Authors’ information
Lei Tian and Ziyong Sun were from Department of Clinical Laboratory, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan,Hubei Province, China. Zhen Zhang was from Department of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan,Hubei Province, China.
Ethics approval and consent to participate
The study protocol was approved by the Tongji Hospital ethics committee for research in health. The Tongji Hospital ethics committee also approved the waiver of informed consent to participate in this study due to its retrospective design. All patient data were anonymised prior to the analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lei Tian, Email: iso15189@126.com.
Ziyong Sun, Email: zysun@tjh.tjmu.edu.cn.
Zhen Zhang, Email: 258452160@qq.com.
References
- 1.Dat VQ, Vu HN, Nguyen The H, Nguyen HT, Hoang LB, Vu Tien Viet D, Bui CL, Van Nguyen K, Nguyen TV, Trinh DT, et al. Bacterial bloodstream infections in a tertiary infectious diseases hospital in Northern Vietnam: aetiology, drug resistance, and treatment outcome. BMC Infect Dis. 2017;17(1):493. doi: 10.1186/s12879-017-2582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huttunen R, Attman E, Aittoniemi J, Outinen T, Syrjanen J, Karki T, Lyytikainen O. Nosocomial bloodstream infections in a Finnish tertiary care hospital: a retrospective cohort study of 2175 episodes during the years 1999–2001 and 2005–2010. Infect Dis. 2015;47(1):20–26. doi: 10.3109/00365548.2014.956791. [DOI] [PubMed] [Google Scholar]
- 3.Musicha P, Cornick JE, Bar-Zeev N, French N, Masesa C, Denis B, Kennedy N, Mallewa J, Gordon MA, Msefula CL, et al. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998–2016): a surveillance study. Lancet Infect Dis. 2017;17(10):1042–1052. doi: 10.1016/S1473-3099(17)30394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Shanmei, Chen Sheng, Feng Wei, Sun Fengjun, Wang Qian, Zhu Ke, Song Jie. Clinical Characteristics of Nosocomial Bloodstream Infections in Neonates in Two Hospitals, China. Journal of Tropical Pediatrics. 2017;64(3):231–236. doi: 10.1093/tropej/fmx054. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Department of Communicable Disease, Surveillance and Response, Prevention of Hospital-acquired Infections. Geneva: World Health Organization; 2002. [Google Scholar]
- 6.Levesque S, Bourgault AM, Galarneau LA, Moisan D, Doualla-Bell F, Tremblay C. Molecular epidemiology and antimicrobial susceptibility profiles of methicillin-resistant Staphylococcus aureus blood culture isolates: results of the Quebec Provincial Surveillance Programme. Epidemiol Infect. 2015;143(7):1511–1518. doi: 10.1017/S095026881400209X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein MP, Murphy JR, Reller LB, Lichtenstein KA. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. II. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis. 1983;5(1):54–70. doi: 10.1093/clinids/5.1.54. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing, Twenty-sixth Informational Supplement, M100-S26. Wayne, PA: Clin Lab Stand Institute; 2016. [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Analysis and presentation of cumulative antimicrobial susceptibility test data, M39-A4. Wayne, PA: Clinical Lab Stand Institute; 2014.
- 10.Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M, Group SP Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(Suppl 2):S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 11.Tian L, Zhu X, Chen Z, Liu W, Li S, Yu W, Zhang W, Xiang X, Sun Z. Characteristics of bacterial pathogens associated with acute diarrhea in children under 5 years of age: a hospital-based cross-sectional study. BMC Infect Dis. 2016;16:253. doi: 10.1186/s12879-016-1603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laupland KB, Gregson DB, Church DL, Ross T, Pitout JD. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin Microbiol Infect. 2008;14(11):1041–1047. doi: 10.1111/j.1469-0691.2008.02089.x. [DOI] [PubMed] [Google Scholar]
- 13.Kato K, Matsumura Y, Yamamoto M, Nagao M, Ito Y, Takakura S, Ichiyama S. Erratum to: Seasonal trend and clinical presentation of Bacillus cereus bloodstream infection: association with summer and indwelling catheter. Eur J Clin Microbiol Infect Dis. 2016;35(5):875–883. doi: 10.1007/s10096-016-2618-8. [DOI] [PubMed] [Google Scholar]
- 14.Gagliotti C, Balode A, Baquero F, Degener J, Grundmann H, Gür D, Jarlier V, Kahlmeter G, Monen J, Monnet DL, et al. Escherichia coli and Staphylococcus aureus: bad news and good news from the European Antimicrobial Resistance Surveillance Network (EARS-Net, formerly EARSS), 2002 to 2009. Euro Surveill. 2011;16(14):pii/19834. [DOI] [PubMed]
- 15.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. National Healthcare Safety Network T, Participating National Healthcare Safety Network F: NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 16.Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;60(5):913–920. doi: 10.1093/jac/dkm318. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Bano J, Picon E, Gijon P, Hernandez JR, Cisneros JM, Pena C, Almela M, Almirante B, Grill F, Colomina J, et al. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-beta-lactamase-producing Escherichia coli. J Clin Microbiol. 2010;48(5):1726–1731. doi: 10.1128/JCM.02353-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Kraker Marlieke E. A., Davey Peter G., Grundmann Hajo. Mortality and Hospital Stay Associated with Resistant Staphylococcus aureus and Escherichia coli Bacteremia: Estimating the Burden of Antibiotic Resistance in Europe. PLoS Medicine. 2011;8(10):e1001104. doi: 10.1371/journal.pmed.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leistner R, Gurntke S, Sakellariou C, Denkel LA, Bloch A, Gastmeier P, Schwab F. Bloodstream infection due to extended-spectrum beta-lactamase (ESBL)-positive K. pneumoniae and E. coli: an analysis of the disease burden in a large cohort. Infection. 2014;42(6):991–997. doi: 10.1007/s15010-014-0670-9. [DOI] [PubMed] [Google Scholar]
- 20.Stewardson Andrew, Fankhauser Carolina, Augelis Giulia De, Rohner Peter, Safran Edith, Schrenzel Jacques, Pittet Didier, Harbarth Stephan. Burden of Bloodstream Infection Caused by Extended-Spectrum β-Lactamase–Producing Enterobacteriaceae Determined Using Multistate Modeling at a Swiss University Hospital and a Nationwide Predictive Model. Infection Control & Hospital Epidemiology. 2013;34(02):133–143. doi: 10.1086/669086. [DOI] [PubMed] [Google Scholar]
- 21.Lin YT, Liu CJ, Fung CP, Tzeng CH. Nosocomial Klebsiella pneumoniae bacteraemia in adult cancer patients--characteristics of neutropenic and non-neutropenic patients. Scand J Infect Dis. 2011;43(8):603–608. doi: 10.3109/00365548.2011.577800. [DOI] [PubMed] [Google Scholar]
- 22.Nasa P, Juneja D, Singh O, Dang R, Singh A. An observational study on bloodstream extended-spectrum beta-lactamase infection in critical care unit: incidence, risk factors and its impact on outcome. Euro J Int Med. 2012;23(2):192–195. doi: 10.1016/j.ejim.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, Maor Y, Rahav G. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18(1):54–60. doi: 10.1111/j.1469-0691.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhao SY, Wang YC, Xiao SZ, Jiang XF, Guo XK, Ni YX, Han LZ. Drug susceptibility and molecular epidemiology of Escherichia coli in bloodstream infections in Shanghai, China, 2011–2013. Infect Dis. 2015;47(5):310–318. doi: 10.3109/00365548.2014.990509. [DOI] [PubMed] [Google Scholar]
- 25.Xiao SZ, Wang S, Wu WM, Zhao SY, Gu FF, Ni YX, Guo XK, Qu JM, Han LZ. The Resistance Phenotype and Molecular Epidemiology of Klebsiella pneumoniae in Bloodstream Infections in Shanghai, China, 2012–2015. Front Microbiol. 2017;8:250. doi: 10.3389/fmicb.2017.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durdu B, Hakyemez IN, Bolukcu S, Okay G, Gultepe B, Aslan T. Mortality markers in nosocomial Klebsiella pneumoniae bloodstream infection. SpringerPlus. 2016;5(1):1892. doi: 10.1186/s40064-016-3580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Laveleye M, Huang TD, Bogaerts P, Berhin C, Bauraing C, Sacre P, Noel A, Glupczynski Y. Multicenter study g: Increasing incidence of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in Belgian hospitals. Eur J Clin Microbiol Infect Dis. 2017;36(1):139–146. doi: 10.1007/s10096-016-2782-x. [DOI] [PubMed] [Google Scholar]
- 28.Widmer AF, Lakatos B, Frei R. Strict infection control leads to low incidence of methicillin-resistant Staphylococcus aureus bloodstream infection over 20 years. Infect Control Hosp Epidemiol. 2015;36(6):702–709. doi: 10.1017/ice.2015.28. [DOI] [PubMed] [Google Scholar]
- 29.You JHS, Choi KW, Wong TY, Ip M, Ming WK, Wong RY, Chan SN, Tse HT, Chau CTS, Lee NLS. Disease Burden, Characteristics, and Outcomes of Methicillin-Resistant Staphylococcus aureus Bloodstream Infection in Hong Kong. Asia Pac J Public Health. 2017;29(5):451–461. doi: 10.1177/1010539517717365. [DOI] [PubMed] [Google Scholar]
- 30.Landrum ML, Neumann C, Cook C, Chukwuma U, Ellis MW, Hospenthal DR, Murray CK. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA. 2012;308(1):50–59. doi: 10.1001/jama.2012.7139. [DOI] [PubMed] [Google Scholar]
- 31.de Kraker ME, Jarlier V, Monen JC, Heuer OE, van de Sande N, Grundmann H: The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect 2013, 19(9):860–868. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.