Abstract
Introduction
This study examined the feasibility of aortic valve planimetry at 7 T ultrahigh field MRI in intraindividual comparison to 3 T and 1.5 T MRI.
Material and methods
Aortic valves of eleven healthy volunteers (mean age, 26.4 years) were examined on a 7 T, 3 T, and 1.5 T MR system using FLASH and TrueFISP sequences. Two experienced radiologists evaluated overall image quality, the presence of artefacts, tissue contrast ratios, identifiability, and image details of the aortic valve opening area (AVOA). Furthermore, AVOA was quantified twice by reader 1 and once by reader 2. Correlation analysis between artefact severity and employed magnetic field strength was performed by modified Fisher’s exact-test. Paired t-test was used to analyse for AVOA differences, and Bland-Altman plots were used to analyse AVOA intra-rater and inter-rater variability.
Results
Aortic valve imaging at 7 T, 3 T, and 1.5 T with using FLASH was less hampered by artefacts than TrueFISP imaging at 3 T and 1.5 T. Tissue contrast and image details were rated best at 7 T. AVOA was measured slightly smaller at 7 T compared to 3 T (TrueFISP, p-value = 0.057; FLASH, p-value = 0.016) and 1.5 T (TrueFISP, p-value = 0.029; FLASH, p-value = 0.018). Intra-rater and inter-rater variability of AVOA tended to be slightly smaller at 7 T than at 3 T and 1.5 T.
Conclusion
Aortic valve planimetry at 7 T ultrahigh field MRI is technically feasible and in healthy volunteers offers an improved tissue contrast and a slightly better reproducibility than MR planimetry at 1.5 T and 3 T.
Abbreviations: SAR, specific absorption rate; UHF, ultrahigh field
Keywords: Aortic valve imaging, Planimetry, Aortic valve opening area, 7 T ultrahigh field MRI, Cardiovascular magnetic resonance imaging
1. Introduction
With a prevalence of 33.9%, valvular aortic stenosis is the most frequent type of valvular heart disease in Europe [1]. Diagnostic aortic valve imaging is possible by echocardiography, catheterization, computed tomography, or cardiac magnetic resonance imaging (CMR) [2]. Choosing CMR, aortic valve imaging can be performed either by phase contrast imaging or by time-resolved cine imaging which allows planimetry of the maximum aortic valve opening area [2].
In the last decade, magnetic resonance systems with increased static magnetic field strengths have been developed and introduced in clinical routine. While 1.5 T could be considered the established standard magnetic field strength for clinical MRI systems, a significant market share of clinical MRI systems today operates at 3 T. Since about a decade, ultrahigh field (UHF) MRI systems operating at 7 T field strength have been introduced for research applications [3,4]. The increased field strength provides inherent advantages and disadvantages. On the one hand, the higher field strength inherently provides higher signal-to-noise ratios allowing for a higher spatial and/ or temporal resolution. Additionally, due to higher tissue susceptibility, higher magnetic field strength may improve tissue contrast [[5], [6], [7], [8]]. On the other hand, use of clinical established cardiac imaging sequences may be limited, consequently True Fast Imaging balanced Steady-state free Precession (TrueFISP) may be hampered by artefacts at higher field strength MRI [9]. Furthermore, increased susceptibility and chemical shift artefacts as well as inhomogeneities of the B1 excitation field can interfere with ultrahigh field MR imaging [6]. The aforementioned factors render homogenization of the static magnetic field (B0 shimming) and homogenization of the excitation field (B1 shimming) crucial, especially in the cardiac region. Multi-channel B1 shimming of the transmit RF field at 7 T MRI is mandatory to provide homogenization of the signal excitation across the cardiac volume [10,11]. Simultaneously an increased power deposition (specific absorption rate, SAR) in the examined human tissue has to be monitored and, if necessary, limited.
Performing CMR at 7 T UHF is even more challenging. The associated higher resonance frequency (300 MHz) and shorter radiofrequency (RF) excitation wavelength may lead to increased excitation field (B1) inhomogeneities [3,4]. This is a limiting factor and requires the development of appropriate RF shimming hardware and RF transmit/receive coils [3,4]. In addition, correct cardiac triggering for CMR at 7 T is challenging. Due to an increasing magneto-hydrodynamic effect the T-wave of the recorded electrocardiogram (ECG) is elevated, leading to a mix-up of the elevated T-wave and R-wave, resulting in decreased reliability of ECG gating in 7 T CMR studies [12,13]. Because the pulse oximetry, as an alternative for cardiac triggering at 7 T, is less precise in dynamic CMR, a new acoustic option for cardiac triggering, the phonocardiogram, had been developed and successfully tested at 1.5 T and 7 T [[13], [14], [15], [16]]. So far, feasibility of cine cardiac imaging for global cardiac function assessment, feasibility of cardiac T2* mapping and feasibility of right coronary MR angiography at 7 T could be demonstrated [[17], [18], [19], [20], [21]].
Reflecting the inherently high signal to noise ratio and tissue contrast at 7 T UHF MRI, we hypothesized that high spatial resolution and improved contrast at 7 T might result in better aortic valve delineation, improved planimetry, and consequently in increased diagnostic certainty in aortic valve imaging when compared to clinical field strength MRI at 3 T and 1.5 T. Thus, the aim of this study was to examine the technical feasibility and systematic evaluation of aortic valve planimetry at 7 T.
2. Material and methods
2.1. Volunteers
Eleven healthy volunteers (five females, six males) were examined. Mean age was 26.4 ± 4.7 years (range, 22–38 years). Mean weight was 68.4 ± 10.6 kg (range, 52–85 kg) and mean height 173 ± 7 cm (range, 162–184 cm). Informed consent for the CMR examination and trial participation was given by all participating volunteers. Prospective analysis and use of data was approved by the local ethic committee.
2.2. Cardiovascular magnetic resonance imaging
CMR examinations were performed on a 1.5-Tesla system (MAGNETOM Aera, Siemens Healthcare GmbH, Erlangen, Germany), a 3-Tesla system (MAGNETOM Skyra, Siemens Healthcare GmbH), and a 7-Tesla whole-body research MR system (MAGNETOM 7 T, Siemens Healthcare GmbH). At 1.5 T and 3 T, using the auto coil select mode (Siemens Healthcare GmbH) an 18-channel RF receive body coil (Body 18, Siemens Healthcare GmbH) and a 32-channel RF receive spine array coil (Spine 32, Siemens Healthcare GmbH) were combined for image acquisition. At 7.0 T UHF MRI, an in-house developed 8-channel RF transmit/receive coil consisting of two arrays with each four elements placed ventrally and dorsally on the chest was used [11]. Additionally, static 8-channel RF shimming was applied to increase B1 transmit homogeneity at 7 T MRI. The 8-channel static RF shimming setup allows for individual manipulation of RF amplitude and phase for each of the RF channels. A graphic user interface enables patient-specific optimization of RF shimming parameters to provide improved RF homogenization across the cardiac volume [10,11]. Stacks of short axis cine images covering the aortic valve and the adjacent left ventricular outflow tract/ ascending aorta were acquired using a TrueFISP sequence (at 1.5 T and 3 T) and a Fast Low-Angle Shot (FLASH) sequence (at 1.5 T, 3 T, and 7 T) in breath-hold technique on all three MRI systems. The detailed sequence parameters are provided in Table 1. At 1.5 T and 3 T ECG-gating was used for sequence triggering. At 7 T, a finger pulse sensor was used for cardiac triggering and synchronization of the CMR sequences.
Table 1.
Detailed sequence parameters of the acquired cine CMR sequences.
| 1.5 T |
3 T |
7 T | |||
|---|---|---|---|---|---|
| FLASH | TrueFISP | FLASH | TrueFISP | FLASH | |
| TR [ms] | 49.14 | 39.39 | 50.76 | 29.1 | 40.9 |
| TE [ms] | 3.45 | 1.36 | 2.89 | 1.27 | 4.76 |
| Matrix [pixel] | 208*136 | 208*170 | 208*141 | 208*144 | 240*196 |
| Field of View [mm2] | 340*278 | 340*278 | 340*278 | 340*285 | 360*294 |
| Flip angle [°] | 15 | 54 | 12 | 38 | 70 |
| Segments | 7 | 13 | 9 | 10 | 5 |
| Calculated phases | 25 | 25 | 25 | 25 | 25 |
| Spatial resolution [mm3] | 1.6 × 1.97 × 4 | 1.6 × 1.6 × 4 | 1.6 × 1.97 × 4 | 1.6 × 1.98 × 4 | 1.5 × 1.5 × 3 |
| Voxel Volume [mm3] | 12.6 | 10.2 | 12.6 | 12.7 | 6.8 |
| Temporal resolution [ms] (given a mean heart rate of 70 bpm) |
2.8 | 2.8 | 2.8 | 2.8 | 2.8 |
| Bandwidth [Hz/px] | 253 | 925 | 445 | 1502 | 992 |
| pMRI Grappa | R = 1 | R = 2 | R = 1 | R = 3 | R = 2 |
| Number of active RF coil channels | 20-32 Rxa | 20-32 Rxa | 20-32 Rxa | 20-32 Rxa | 8 Tx/Rx |
FLASH, Fast Low-Angle Shot sequence; TrueFISP, True Fast Imaging balanced Steady-state free Precession; TR, repetition time; TE, echo time; pMRI, parallel MRI; RF, radiofrequency; Rx, receive; Tx, transmit;
Using auto coil select mode. In TrueFISP relatively low flip angle had to been chosen due to SAR-restrictions.
2.3. Image assessement
Two radiologists with CMR experience (>6 years and >3 years) independently evaluated the images. The following parameters were assessed for evaluation:
(1) Presence of interfering artefacts:
-
•
Qualitative severity of artefacts was rated using a 5-point Likert-type scale (0, no artefacts; 1, slight artefacts/ image evaluation hardly hampered; 2, considerable artefacts; 3, severe artefacts/ image evaluation still possible; 4, image analysis not feasible due to artefacts).
(2) Tissue contrast:
-
•
Qualitative rating of the tissue contrast comparing 1.5 T and 3 T to 7 T using a 3-point Likert-type scale (1, superiority of 7 T compared to 1.5 T or 3 T; 2, equality of 7 T and 1.5 T or 3 T; 3, inferiority of 7 T compared to 1.5 T or 3 T).
-
•
Quantitative image assessment was performed by comparison of the contrast ratios (CR = (signal of blood pool – signal of aortic valve rim) / (signal of blood pool + signal of aortic valve rim)) between 7 T, 3 T, and 1.5 T [22].
(3) Aortic valve opening area:
-
•
Qualitative rating of the identifiability of the aortic valve using a 4-point Likert-type scale (1, poorly definable; 2, moderately definable; 3, well definable; 4, excellently definable).
-
•
Qualitative rating of the image details comparing 1.5 T and 3 T to 7 T using a 3-point Likert-type scale (1, superiority of 7 T compared to 1.5 T or 3 T; 2, equality of 7 T and 1.5 T or 3 T; 3, inferiority of 7 T compared to 1.5 T or 3 T).
-
•
Quantitative measurement of the aortic valve opening area by manual drawing of regions of interest. This was performed twice by reader #1 (for intra-reader variability assessment) and once by reader #2 (for inter-reader variability assessment).
2.4. Statistical analysis
MedCalc (version 12.3.0.0, MedCalc Software, Belgium) was used for statistical analysis. Testing for normal distribution was done by D’Agostino-Pearson test. Normally distributed continuous data are given as mean ± standard deviation, ordinal or non-normally distributed data as median and interquartile range. Modified Fisher’s exact-test was performed to analyse for a statistical correlation between artefact severity and employed MRI sequence/ field strength. Wilcoxon test was used to compare CR between employed MRI sequences/ field strengths. Intra-rater and inter-rater variability of the aortic valve opening area measurements was analysed using Bland-Altman plots [23]. Paired t-test was used to analyse for mean aortic valve opening area differences between employed MRI sequences/ field strengths.
3. Results
All eleven volunteers were successfully investigated at all field strengths, 1.5 T, 3 T, and 7 T. Initial tests comparing TrueFISP and FLASH at 7 T showed that TrueFisp images were much more hampered by artefacts and that the contrast between blood pool and aortic valve rim was high in FLASH images (Fig. 1). Therefore, further aortic valve imaging at 7 T was performed only using FLASH. Aortic valve imaging using FLASH at 7 T, 3 T, and 1.5 T was less hampered by artefacts than was aortic valve imaging using TrueFISP at 3 T and 1.5 T in both readers (Table 2, reader #1: p-value <0.001, reader #2: p-value = 0.003, Fig. 2). Qualitative tissue contrast of the aortic valve was better at 7 T compared to 1.5 T or 3 T (Table 3, Fig. 3, Fig. 4). This was supported by the quantitative tissue contrast comparison using CR, which was significantly higher in 7 T FLASH (median = 0.46) compared to 3 T TrueFISP (median = 0.22, p-value = 0.024), 3 T FLASH (median = 0.24, p-value = 0.014), 1.5 T TrueFISP (median = 0.19, p-value = 0.005), or 1.5 T FLASH (median = 0.20, p-value = 0.027). Median rating of aortic valve identifiability at 7 T was excellent according to reader #1 (rating of reader #1 ranging from moderately definable to excellently definable) and well according to reader #2 (rating of reader #2 ranging from well definable to excellently definable). Qualitative image detail in aortic valve imaging was rated higher in 7 T FLASH than in 1.5 T TrueFISP, 1.5 T FLASH, 3 T TrueFISP, or 3 T FLASH by both readers (Table 4).
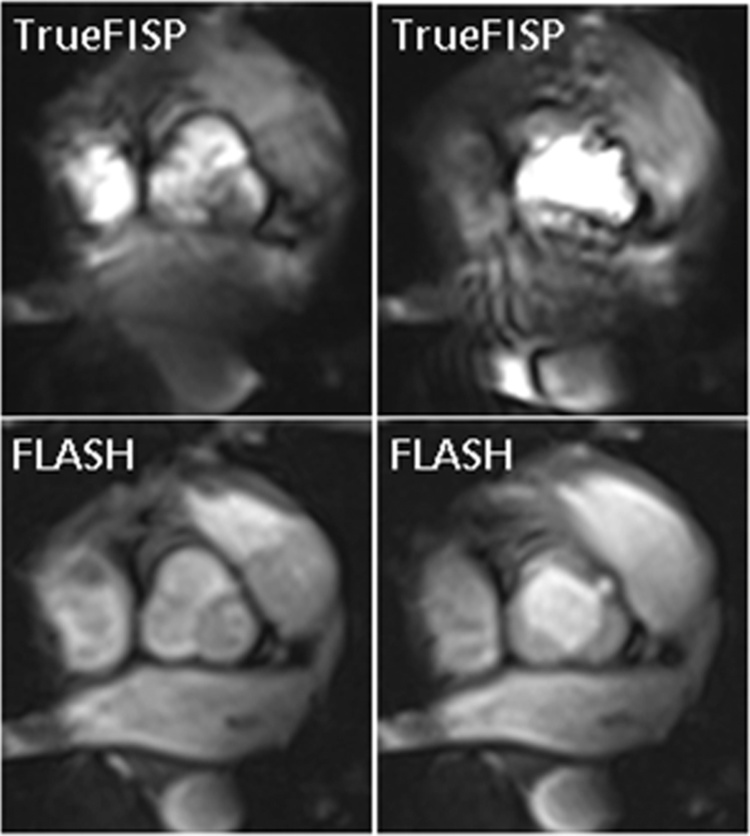
Fig. 1.
Aortic valve imaging in a 29-years-old female at 7 T. On the left side the aortic valve is displayed in the closed state, while on the right side it is displayed opened. TrueFISP images were considerably hampered by artefacts, while FLASH images were less hampered by artefacts and provided sufficiently high contrast between blood pool and aortic valve rim.
Table 2.
Qualitative artefact severity (given as median (range)).
| Sequence/ field strength | reader #1 | reader #2 |
|---|---|---|
| 7 T FLASH | 0 (0–1) | 1 (0–1) |
| 3 T FLASH | 1 (0–1) | 1 (0–1) |
| 1.5 T FLASH | 1 (0–1) | 1 (1–2) |
| 3 T TrueFISP | 2 (1–4) | 2 (1–3) |
| 1.5 T TrueFISP | 2 (0–4) | 2 (0–3) |
0, no artefacts; 1, slight artefacts; 2, considerable artefacts; 3, severe artefacts; 4, image analysis not feasible.
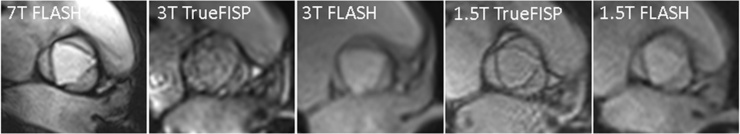
Fig. 2.
Aortic valve imaging in a 25-years-old male at 7 T, 3 T, and 1.5 T. TrueFISP images in general were more hampered by artefacts than were FLASH images.
Table 3.
Qualitative tissue contrast of the aortic valve (given as median (range)).
| Sequence/ field strength | reader #1 | reader #2 |
|---|---|---|
| 7 T FLASH vs. 1.5 T FLASH | 1 (1–3) | 1 (1–2) |
| 7 T FLASH vs. 3 T FLASH | 1 (1–2) | 1 (1–2) |
| 7 T FLASH vs. 1.5 T TrueFISP | 1 (1–2) | 1 (1–2) |
| 7 T FLASH vs. 3 T TrueFISP | 1 (1–2) | 1 (1–2) |
Vs., versus; 1, superiority of 7 T compared to 1.5 T/3 T; 2, equality of 7 T and 1.5 T/3 T; 3, inferiority of 7 T compared to 1.5 T/3 T.
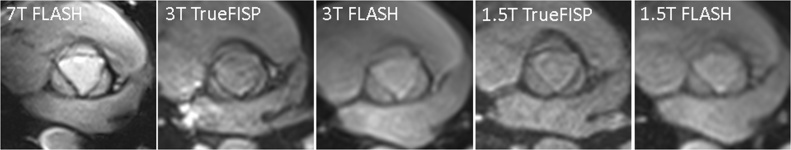
Fig. 3.
Aortic valve imaging in a 24-years-old female at 7 T, 3 T, and 1.5 T. Qualitative tissue contrast of the aortic valve was better at 7 T than at 3 T or 1.5 T.
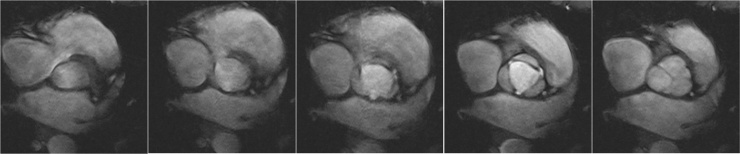
Fig. 4.
Aortic valve imaging in a 22-years-old female at 7 T. Five cine FLASH images of the aortic valve nicely demonstrate the aortic valve movement over time.
Table 4.
Qualitative image detail in aortic valve imaging (given as median (range)).
| Sequence/ field strength | reader #1 | reader #2 |
|---|---|---|
| 7 T FLASH vs. 1.5 T FLASH | 1 (1–2) | 1 (1–1) |
| 7 T FLASH vs. 3 T FLASH | 1 (1–1) | 1 (1–2) |
| 7 T FLASH vs. 1.5 T TrueFISP | 1 (1–2) | 1 (1–2) |
| 7 T FLASH vs. 3 T TrueFISP | 1 (1–2) | 1 (1–2) |
Vs., versus; 1, superiority of 7 T compared to 1.5 T/3 T; 2, equality of 7 T and 1.5 T/3 T; 3, inferiority of 7 T compared to 1.5 T/3 T.
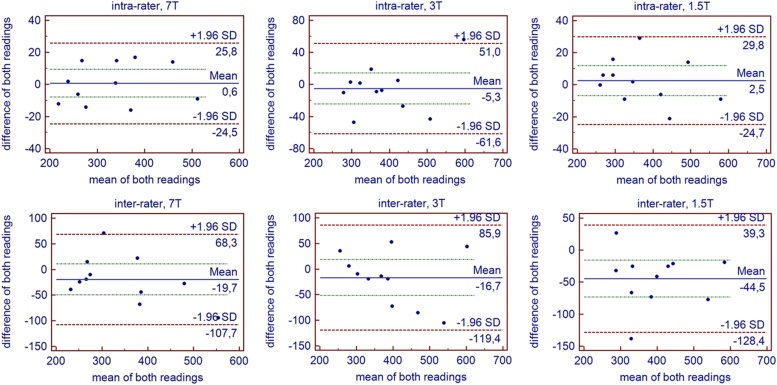
The aortic valve opening area was measured slightly smaller at 7 T compared to 3 T (Table 5, 3 T TrueFISP: p-value = 0.057, 3 T FLASH: p-value = 0.016) and 1.5 T (1.5 T TrueFISP: p-value = 0.029, 1.5 T FLASH: p-value = 0.018). Intra-rater and inter-rater variability of the aortic valve opening area measurements tended to be slightly smaller at 7 T compared to 3 T and 1.5 T (Table 6, Fig. 5).
Table 5.
Results of the quantitative aortic valve opening area measurement.
| mean ± standard deviation [mm²] | range [mm²] | ||
|---|---|---|---|
| 7 T FLASH | #1, reading 1 | 333 ± 94 | 212–507 |
| #1, reading 2 | 332 ± 92 | 224–516 | |
| #2 | 353 ± 116 | 251–601 | |
| #1, reading 1 | 368 ± 102 | 238–588 | |
| #1, reading 2 | 375 ± 105 | 198–569 | |
| #2 | 388 ± 111 | 235–580 | |
| 3 T FLASH | #1, reading 1 | 387 ± 108 | 273–624 |
| #1, reading 2 | 392 ± 98 | 283–568 | |
| #2 | 404 ± 123 | 237–591 | |
| 1.5 T TrueFISP | #1, reading 1 | 359 ± 87 | 267–543 |
| #1, reading 2 | 355 ± 92 | 234–543 | |
| #2 | 386 ± 128 | 210–602 | |
| 1.5 T FLASH | #1, reading 1 | 383 ± 99 | 260–574 |
| #1, reading 2 | 381 ± 103 | 260–583 | |
| #2 | 429 ± 98 | 275–593 | |
#1, reader #1; #2, reader #2.
Table 6.
Intra-rater and inter-rater variability of aortic valve opening area measurements (given in mm²).
| bias | SD | 95%-confidence interval | ||
|---|---|---|---|---|
| Intra-rater | 7 T FLASH | 1 | 13 | −25 to 26 |
| 3 T TrueFISP | −7 | 25 | −55 to 42 | |
| 3 T FLASH | −5 | 30 | −64 to 54 | |
| 1.5 T TrueFISP | 4 | 31 | −58 to 65 | |
| 1.5 T FLASH | 2 | 15 | −27 to 31 | |
| Inter-rater | 7 T FLASH | −20 | 45 | −108 to 68 |
| 3 T TrueFISP | −20 | 53 | −125 to 85 | |
| 3 T FLASH | −17 | 55 | −125 to 91 | |
| 1.5 T TrueFISP | −27 | 66 | −156 to 102 | |
| 1.5 T FLASH | −46 | 45 | −134 to 42 | |
SD, standard deviation.
Fig. 5.
Intra-rater and inter-rater variability of the aortic valve opening area measurements. Both, intra- and inter-rater variability (visualized by Bland-Altman plots), tended to be slightly smaller at 7 T than at 3 T or 1.5 T (used sequence: FLASH).
4. Discussion
This study demonstrated that aortic valve imaging at 7 T despite significant technical challenges is feasible. Aortic valve images at 7 T exhibited a better qualitative tissue contrast than aortic valve images at 1.5 T or 3 T and the intra-rater and inter-rater variability of the aortic valve opening area tended to be slightly smaller at 7 T.
For correct diagnosis and treatment planning in patients suffering from aortic valve stenosis, an exact aortic valve opening area planimetry is crucial. As demonstrated by Debl et al., the aortic valve opening area quantified by 1.5 T CMR planimetry correlates highly with the aortic valve opening area quantified by transesophageal echocardiography or catheterization [2]. In addition, Debl et al. showed that CMR planimetry at 1.5 T overestimated the aortic valve opening area measured by transesophageal echocardiography by 15% and the aortic valve opening area measured by catheterization by 27%. Thus, our finding, that the aortic valve opening area was slightly smaller measured at 7 T than at 1.5 T or 3 T might be interpreted as a hint that aortic valve measurement at 7 T is more precise. This interpretation is underpinned by the slightly smaller intra-rater and inter-rater aortic opening area variability at 7 T. Hence, aortic valve planimetry seems to benefit from imaging at higher magnetic field strength as it seems to be more accurate at 7 T. From our results we conclude, that the increased spatial resolution and the increased contrast between blood pool and aortic valve rim in 7 T measurements contributed to the better depictability of the aortic valve opening area when compared to examinations acquired at 3 T and 1.5 T field strength.
Performing CMR and especially CMR of the aortic valve with UHF MRI at a field strength of 7 T is still associated with numerous and significant technical challenges [3,4]. The resonance frequency at 7 T is about 300 MHz. This is associated with a rather short excitation RF wavelength, leading to B1 signal homogeneities and signal dropouts when imaging the human body [3,4]. Multi-channel RF transmit/receive technology and B1-shimming strategies are thus technical preconditions to perform CMR at 7 T [3,4]. Against this background, researchers in the UHF MRI community rely on developing their own multi-channel RF equipment and transmit/receive RF coils [10,24,25]. In this context, our study demonstrates technical feasibility of aortic valve delineation with 7 T MRI and the results reflect the current state in 7 T aortic valve measurements. With further developments of multi-channel transmit/receive RF technology and RF coils we believe that the inherently high signal to noise ratio and contrast potential of 7 T UHF MRI can be even better utilized and transferred into diagnostic improvements compared to 3 T and 1.5 T MR cardiac imaging.
Another specific challenge of CMR at 7 T is exact and robust cardiac gating and triggering of MRI sequences in the 7 T UHF environment [4,13]. While conventional ECG triggering due to the magneto-hydrodynamic effect often fails in 7 T MRI, pulse triggering works mostly robust [4,12,13]. However, it fails to provide a well-defined trigger signal to synchronize the imaging sequences. Here, the use of acoustic triggering has been proposed and evaluated, providing convincing results in 7 T CMR 13,16]. Also, the use of ultrasound sensors has been suggested and tested at 1.5 T, 3 T, and 7 T CMR [15,26]. In this study, pulse triggering was used for cardiac gating. Due to the inherently limited precision of this gating method, this may have introduced slight motion artefacts and image blurring in the 7 T acquisitions.
Another aspect worth discussing is the potential transfer from our results to clinical applicability of 7 T UHF MRI on cardiac patients. Cardiac patients often come with passive or even active metallic implants. Accordingly, MRI safety aspects have to be clarified for each individual case before 7 T MRI can be considered [3]. Aside from the safety aspects, the general volunteer and patient compliance of 7 T UHF MRI is high and the observed side effects such as temporary dizziness have been reported as only minor in our own institution [27,28] and other UHF MRI institutions [29].
4.1. Conclusions
Aortic valve imaging at 7 T UHF MRI is technically feasible and in healthy volunteers offers an improved tissue contrast, improved spatial resolution, and slightly better reproducibility compared to aortic valve imaging at 1.5 T and 3 T.
Competing interests
The authors have no competing interests to declare.
Acknowledgements
The authors thank Lena Schaefer, RT, for technical assistance in 7 T MRI data acquisition.
References
- 1.Iung B., Baron G., Butchart E.G. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003;24(13):1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 2.Debl K., Djavidani B., Seitz J. Planimetry of aortic valve area in aortic stenosis by magnetic resonance imaging. Invest. Radiol. 2005;40(10):631–636. doi: 10.1097/01.rli.0000178362.67085.fd. [DOI] [PubMed] [Google Scholar]
- 3.Kraff O., Quick H.H. 7T: physics, safety, and potential clinical applications. J. Magn. Reson. Imaging. 2017;46(6):1573–1589. doi: 10.1002/jmri.25723. [DOI] [PubMed] [Google Scholar]
- 4.Ladd M.E. High-field-strength magnetic resonance: potential and limits. Top. Magn. Reson. Imaging. 2007;18(2):139–152. doi: 10.1097/RMR.0b013e3180f612b3. [DOI] [PubMed] [Google Scholar]
- 5.Umutlu L., Ladd M.E., Forsting M. 7 Tesla MR imaging: opportunities and challenges. Rofo. 2014;186(2):121–129. doi: 10.1055/s-0033-1350406. [DOI] [PubMed] [Google Scholar]
- 6.Barth M.M., Smith M.P., Pedrosa I. Body MR imaging at 3.0 T: understanding the opportunities and challenges. Radiographics. 2007;27(5):1445–1462. doi: 10.1148/rg.275065204. discussion 62-4. [DOI] [PubMed] [Google Scholar]
- 7.Norris D.G. High field human imaging. J. Magn. Reson. Imaging. 2003;18(5):519–529. doi: 10.1002/jmri.10390. [DOI] [PubMed] [Google Scholar]
- 8.Ugurbil K., Adriany G., Andersen P. Ultrahigh field magnetic resonance imaging and spectroscopy. Magn. Reson. Imaging. 2003;21(10):1263–1281. doi: 10.1016/j.mri.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Ladd M.E., Bock M. Problems and chances of high field magnetic resonance imaging. Radiologe. 2013;53(5):401–410. doi: 10.1007/s00117-012-2344-x. [DOI] [PubMed] [Google Scholar]
- 10.Maderwald S., Schäfer L., Bitz A.K. 7T Human in vivo cardiac imaging with an 8-channel transmit/receive array. Proc. Intl. Soc. Mag. Reson. Med. 2009;17:822. [Google Scholar]
- 11.Bitz A.K., Orzada S., Kraff O. Comparison of simulation-based and measurement-based RF shimming for whole-body MRI at 7 Tesla. Proc. Intl. Soc. Mag. Reson. Med. 2010;18:4720. [Google Scholar]
- 12.Schenck J.F. Physical interactions of static magnetic fields with living tissues. Prog. Biophys. Mol. Biol. 2005;87(2-3):185–204. doi: 10.1016/j.pbiomolbio.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Frauenrath T., Hezel F., Heinrichs U. Feasibility of cardiac gating free of interference with electro-magnetic fields at 1.5 Tesla, 3.0 Tesla and 7.0 Tesla using an MR-stethoscope. Invest. Radiol. 2009;44(9):539–547. doi: 10.1097/RLI.0b013e3181b4c15e. [DOI] [PubMed] [Google Scholar]
- 14.Becker M., Frauenrath T., Hezel F. Comparison of left ventricular function assessment using phonocardiogram- and electrocardiogram-triggered 2D SSFP CINE MR imaging at 1.5 T and 3.0 T. Eur. Radiol. 2018;20(6):1344–1355. doi: 10.1007/s00330-009-1676-z. [DOI] [PubMed] [Google Scholar]
- 15.Nassenstein K., Orzada S., Haering L. Cardiac MRI: evaluation of phonocardiogram-gated cine imaging for the assessment of global and regional left ventricular function in clinical routine. Eur. Radiol. 2012;22(3):559–568. doi: 10.1007/s00330-011-2279-z. [DOI] [PubMed] [Google Scholar]
- 16.Frauenrath T., Hezel F., Renz W. Acoustic cardiac triggering: a practical solution for synchronization and gating of cardiovascular magnetic resonance at 7 Tesla. J. Cardiovasc. Magn. Reson. 2010;12 doi: 10.1186/1532-429X-12-67. 67-429X-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Knobelsdorff-Brenkenhoff F., Frauenrath T., Prothmann M. Cardiac chamber quantification using magnetic resonance imaging at 7 Tesla–a pilot study. Eur. Radiol. 2010;20(12):2844–2852. doi: 10.1007/s00330-010-1888-2. [DOI] [PubMed] [Google Scholar]
- 18.Suttie J.J., Delabarre L., Pitcher A. 7 Tesla (T) human cardiovascular magnetic resonance imaging using FLASH and SSFP to assess cardiac function: validation against 1.5 T and 3 T. NMR Biomed. 2012;25(1):27–34. doi: 10.1002/nbm.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hezel F., Thalhammer C., Waiczies S. High spatial resolution and temporally resolved T2* mapping of normal human myocardium at 7.0 Tesla: an ultrahigh field magnetic resonance feasibility study. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Elderen S.G., Versluis M.J., Westenberg J.J. Right coronary MR angiography at 7 T: a direct quantitative and qualitative comparison with 3 T in young healthy volunteers. Radiology. 2010;257(1):254–259. doi: 10.1148/radiol.100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Knobelsdorff-Brenkenhoff F., Tkachenko V., Winter L. Assessment of the right ventricle with cardiovascular magnetic resonance at 7 Tesla. J. Cardiovasc. Magn. Reson. 2013;15 doi: 10.1186/1532-429X-15-23. 23-429X-15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraff O., Theysohn J.M., Maderwald S. MRI of the knee at 7.0 Tesla. Rofo. 2007;179(12):1231–1235. doi: 10.1055/s-2007-963607. [DOI] [PubMed] [Google Scholar]
- 23.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 24.Winter L., Kellman P., Renz W. Comparison of three multichannel transmit/receive radiofrequency coil configurations for anatomic and functional cardiac MRI at 7.0T: implications for clinical imaging. Eur. Radiol. 2012;22(10):2211–2220. doi: 10.1007/s00330-012-2487-1. [DOI] [PubMed] [Google Scholar]
- 25.Graessl A., Renz W., Hezel F. Modular 32-channel transceiver coil array for cardiac MRI at 7.0T. Magn. Reson. Med. 2014;72(1):276–290. doi: 10.1002/mrm.24903. [DOI] [PubMed] [Google Scholar]
- 26.Kording F., Yamamura J., Lund G. Doppler ultrasound triggering for cardiovascular MRI at 3T in a healthy volunteer study. Magn. Reson. Med. Sci. 2017;16(2):98–108. doi: 10.2463/mrms.mp.2015-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heilmaier C., Theysohn J.M., Maderwald S. A large-scale study on subjective perception of discomfort during 7 and 1.5 T MRI examinations. Bioelectromagnetics. 2011;32(8):610–619. doi: 10.1002/bem.20680. [DOI] [PubMed] [Google Scholar]
- 28.Theysohn J.M., Kraff O., Eilers K. Vestibular effects of a 7 Tesla MRI examination compared to 1.5 T and 0 T in healthy volunteers. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0092104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauschenberg J., Nagel A.M., Ladd S.C. Multicenter study of subjective acceptance during magnetic resonance imaging at 7 and 9.4 T. Invest. Radiol. 2014;49(5):249–259. doi: 10.1097/RLI.0000000000000035. [DOI] [PubMed] [Google Scholar]