Abstract
Stress-related substance use is a major challenge for treating substance use disorders. This selective review focuses on emerging pharmacotherapies with potential for reducing stress-potentiated seeking and consumption of nicotine, alcohol, marijuana, cocaine, and opioids (i.e., key phenotypes for the most commonly abused substances). I evaluate neuropharmacological mechanisms in experimental models of drug-maintenance and relapse, which translate more readily to individuals presenting for treatment (who have initiated and progressed). An affective/motivational systems model (three dimensions: valence, arousal, control) is mapped onto a systems biology of addiction approach for addressing this problem. Based on quality of evidence to date, promising first-tier neurochemical receptor targets include: noradrenergic (α1 and β antagonist, α2 agonist), kappa-opioid antagonist, nociceptin antagonist, orexin-1 antagonist, and endocannabinoid modulation (e.g., cannabidiol, FAAH inhibition); second-tier candidates may include corticotropin releasing factor-1 antagonists, serotonergic agents (e.g., 5-HT reuptake inhibitors, 5-HT3 antagonists), glutamatergic agents (e.g., mGluR2/3 agonist/positive allosteric modulator, mGluR5 antagonist/negative allosteric modulator), GABA-promoters (e.g., pregabalin, tiagabine), vasopressin 1b antagonist, NK-1 antagonist, and PPAR-γ agonist (e.g., pioglitazone). To address affective/motivational mechanisms of stress-related substance use, it may be advisable to combine agents with actions at complementary targets for greater efficacy but systematic studies are lacking except for interactions with the noradrenergic system. I note clinically-relevant factors that could mediate/moderate the efficacy of anti-stress therapeutics and identify research gaps that should be pursued. Finally, progress in developing anti-stress medications will depend on use of reliable CNS biomarkers to validate exposure-response relationships.
Keywords: Stress, Addiction, Drug seeking, Self-administration, Mechanisms, Medications
Highlights
-
•
Selective review of stress-potentiated seeking/use of nicotine, alcohol, opioids, cocaine, and cannabis.
-
•
Affective/motivational theoretical model is mapped onto a systems biology approach.
-
•
Neurochemical targets are discussed, and potential combination approaches are identified.
-
•
Clinically-relevant factors that could influence efficacy of anti-stress therapeutics are discussed.
-
•
Research gaps are identified and future avenues of investigation are suggested.
1. Introduction
1.1. Scope of review
Stress-related substance use poses a critical challenge for treating all substance use disorders (SUDs), yet this scientific field is at an early investigational stage. Candidate therapies for attenuating stress-potentiated drug-seeking/use include medications, neuro-stimulation, cognitive-behavioral, exercise/physical activity, and other approaches. Due to that panoramic breadth, this selective review focuses on neuropharmacological strategies grounded in preclinical and clinical evidence that offer translational promise for reducing stress-induced seeking and consumption of nicotine, alcohol, marijuana, cocaine, and opioids. This review does not discuss effects of medications on attenuating drug-abstinence signs/symptoms (which are stressful); although withdrawal effects can lead to drug seeking, that outcome is not inevitable, mostly relates to physical dependence on specific substances, and retaining that topic would greatly lengthen this review. In short, this restricted scope highlights neurochemical mechanisms for reducing stress-induced seeking/self-administration for the most prevalent abused substances. Literature gaps are identified as a means to advance this field of inquiry.
1.2. Neurobehavioral mechanisms underlying drug-seeking/self-administration
Mechanisms that link stress reactivity to psychoactive substance use/abuse are complex and incompletely understood. Stressors modulate the neurobehavioral effects of abused substances in stressor-, procedure- and drug-class-specific ways, but also activate some common brain pathways (Armario, 2010; Lopez et al., 1999). Excellent reviews at a more granular level than this translational review indicate numerous neurochemical systems modulate stress-induced substance-seeking/use including noradrenaline (NA), hypothalamic-pituitary-adrenal (HPA) axis including corticotrophin-releasing factor (CRF) and glucocorticoids, opioid, endocannabinoid (eCB), serotonin (5-HT), orexin/hypocretin, dopamine (DA), glutamate, and γ-amino-butyric acid (GABA) (Briand and Blendy, 2010; Dunn and Swiergiel, 2008; Groeneweg et al., 2011; Herman and Cullinan, 1997; Koob, 2010; Mantsch et al., 2016; Plaza-Zabala et al., 2012; Shalev et al., 2000, 2010; Sinha, 2008; Sinha et al., 2011; Viveros et al., 2005), and other systems that are less well studied (see section 2.10). Due to this “neuro-symphony of stress” (Jöels and Baram, 2009), it is only feasible to study a limited number of mechanisms at the same time. When relevant, I mention research that has examined pairwise interactions of transmitter systems.
A motivational systems model (Fig. 1), based on affective neuroscience theories (Alcaro and Panksepp, 2011; Baker et al., 2004; Davidson et al., 2000; Diekhof et al., 2008) suggests that three empirically separable dimensions may underlie stress-induced drug seeking/use, being greatest at the nexus of negative-hedonic or dysphoric (avoidance-punishment), high-arousal (activation), and low-control (disinhibition) states. Based on research in the systems biology of addiction (Aston-Jones and Harris, 2004; Briand and Blendy, 2010; Hester and Garavan, 2004; Koob, 2008; Shalev et al., 2000; Sinha, 2008; Spanagel et al., 1992; Waselus et al., 2011; Zhou et al., 2010), this review adopts the approach that stress-related drug-seeking/use is a function of dysregulated neural (particularly limbic) systems underlying these affective/motivational dimensions. Throughout this review, I link candidate anti-stress pharmacological approaches to these motivational dimensions (to the extent that current evidence allows).
Fig. 1.
Motivational Systems: Stress-induced substance use behaviors are a function of three motivational dimensions: hedonic valence (approach/avoidance), arousal/activation, and self-control (inhibition/disinhibition). Cone depicts the motivational sector (negative hedonic, high activation, and disinhibition) in which stressors are predicted to amplify drug seeking.
1.3. Experimental models of stress-induced drug-seeking/use
Experimental approaches to studying stress-related drug-seeking/use can be classified with regard to: (a) type of stressor, e.g., physical, environmental, and pharmacological, (b) stage in the behavioral cycle of addiction (initiation, progression, maintenance, relapse), and (c) drug-seeking outcome measure (e.g., operant responding for drug, conditioned place preference). This literature review focuses on models of maintenance and relapse stages, which more closely translate to individuals presenting for SUD treatment (who have already initiated and progressed). Most studies with laboratory animals to date have measured reinstatement of operant responding for drug as the outcome measure; fewer studies have measured conditioned place preference (CPP) but these are included in this review for comprehensiveness. The CPP model has limitations, particularly because drug is experimenter-administered and drug exposure is minimal.
As summarized in Table 1, drug seeking/consumption is enhanced by a wide range of stressors including: social factors (defeat, isolation), physical factors (restraint, cold swim, food deprivation, footshock), and pharmacological probes (e.g., α2-adrenoceptor antagonist yohimbine [section 2.1], kappa-opioid agonist, and neuropeptides [sections 2.10, 2.2.1, 2.7]). Each stressor type has strengths and weaknesses, and translation of these models to clinical treatment is limited by several moderating factors, briefly discussed next.
Table 1.
Studies demonstrating a significant direct effect of stressor on reinstatement of (or increase in) seeking behavior, by type of abused substance.
CPP paradigm.
Clinical study.
1.4. Translation of experimental models to clinical application: moderator variables
Experimental models of stress-related drug seeking/use have not routinely included clinically-relevant factors that might mediate/moderate the efficacy of anti-stress therapeutic agents. Next, I briefly discuss selected factors worthy of investigation in developing anti-stress pharmacotherapies in these models. Detailed analysis of these factors exceeds the scope of this review, but the purpose is to prime the reader to consider these factors in the context of the discussion that follows in section 2.
1.4.1. History of substance exposure/use
The extent to which acute stressors augment the reinforcing effects of psychoactive substances may partly depend on the subject's history of substance exposure (e.g., in utero) or use (i.e., voluntary intake). Chronic and/or escalating substance intake has been shown to produce stress-like responses that, in turn, sensitize animals – via CRF, glucocorticoid, and glutamatergic mechanisms – to acute stress-potentiation of the reinforcing effects of cocaine (Ahmed et al., 2000; Graf et al., 2011; Mantsch et al., 2008; Sorge and Stewart, 2005) and alcohol (Liu and Weiss, 2002; Sidhpura et al., 2010). These data suggest that agents capable of desensitizing these neurochemical systems could be therapeutically useful, especially for subjects with extensive chronic exposure or high levels of tolerance/dependence.
1.4.2. Environmental factors
Research into effects of stressors on drug-motivated behavior has not adequately investigated the environmental context, particularly non-drug choice alternatives. Higher magnitude or probability of non-drug positive reinforcers in the environment during stress-exposure may bias animals away from habitual drug seeking, i.e., serve a stress-protective function. This phenomenon has been modeled in rats using environmental enrichment (Chauvet et al., 2009). Future studies should examine whether anti-stress pharmacotherapies are more effective in combination with non-drug positive reinforcers. In contrast, economic insecurities among humans (i.e., dearth of positive reinforcement in a person's environment) are chronic stressors that contribute to substance use. Furthermore, magnitude and/or probability of punishers in the subject's environment may play an important role in modulating stress-induced drug seeking, although this has rarely been investigated. For instance, early social isolation of rats enhances cocaine motivation, but isolation did not affect extinction or stress-reinstatement of cocaine seeking (Baarendse et al., 2014). Future studies should investigate whether drug intake that is suppressed by punishment (cf. Pelloux et al., 2007) is more easily reinstated by stress than drug intake that is suppressed by non-drug positive reinforcers, and whether other factors such as drug history moderate these effects (see Smith and Laiks, 2017).
1.4.3. Sleep loss
Sleep loss (and/or circadian disruption) is a biological stressor but has rarely been investigated in studies of drug seeking/self-administration. This is noteworthy because insomnia is a predictor of relapse to substance abuse (Roehrs and Roth, 2015; Kaplan et al., 2014). Also, sleep loss causes hyperalgesia (Alexandre et al., 2017; Roehrs et al., 2006; Vanini, 2016) that, in turn, may increase misuse of opioids and other substances (Edwards et al., 2011; Hipólito et al., 2015; Wachholtz et al., 2015). Recognizing the role of insomnia as a stressor leads to the idea of treating insomnia as one cause of substance use. Non-addictive sleep-promoting agents might be useful in this regard (see section 2.7).
1.4.4. Chronic pain
Stress can increase nociceptive signaling and contribute to chronic pain states (Ghitza, 2016; Imbe et al., 2006; Jennings et al., 2014; Johnson and Greenwood-Van Meerveld, 2014). This overlap is important because it suggests (1) stress-exposure could cross-sensitize subjects – presumably via shared neurobiological pathways – to drug seeking, and (2) therapeutics used to attenuate stress-reactivity could have dual actions on analgesia and drug seeking/use. On the other hand, it is well established that stress exposure can sometimes produce analgesia (Butler and Finn, 2009; Parikh et al., 2011). Thus, parametric studies are needed to examine basic interactions between (acute and chronic) stressors and (acute and chronic) nociceptive stimulation, and to translate this knowledge toward the development of therapeutic approaches that could potentially address both conditions.
1.4.5. Chronic stress and mental health conditions
Early-life or chronic stress as well as psychiatric conditions (e.g., anxiety, depression) are highly prevalent among individuals with SUDs and may increase risk of relapse to substance use especially under stressful conditions (Chida and Hamer, 2008; Lijffijt et al., 2014). Experimental animal models have only infrequently created such a behavioral history (e.g., repeatedly exposing animals to anxiety-provoking or helplessness-provoking conditions) or exposed adolescent animals to the biological stressor corticosterone (Al-Hasani et al., 2013a; Bertholomey et al., 2016) to determine its effects on stress-induced drug seeking/use. Further systematic research would be valuable for understanding a complex yet important set of clinically relevant influences.
1.4.6. Trait factors
Impulsivity is an example of one trait variable that may influence stress-induced drug seeking behavior; furthermore, this trait maps onto a key motivational dimension, disinhibition/control, proposed in this review (Fig. 1). Specifically, high trait impulsivity (associated with less prefrontal cortical inhibitory control) could, in the presence of a stressor, result in less resistance to drugs and related cues, making the individual more prone to substance use. We have found that the relationship between trait impulsivity on current mood state (e.g., Beck Depression Inventory scores) is mediated by effects of cumulative adverse drug-use consequences for both cocaine and heroin users (Lister et al., 2015; Reid et al., 2018). Other inter-related trait variables such as anxiety sensitivity (Lejuez et al., 2008), distress tolerance (Brown et al., 2005), emotion regulation (Naragon-Gainey et al., 2017), and resilience or coping ability (Belding et al., 1996; Hyman et al., 2009) are stress-mediator/moderator variables that are not routinely assessed in human studies, but these might potentially predict drug abstinence during addiction treatment (Strong et al., 2012).
1.4.7. Biological sex
Chronic manipulation of HPA axis (CORT) during adolescence increases sensitivity to YOH-induced alcohol seeking in female more than male adult rats (Bertholomey et al., 2016). Neonatal isolation stress increased cocaine seeking behavior in adult rats, however, effects did not differ for males and females (Lynch et al., 2005). Some effects of acute stressors on relapse-like behavior are sex-dependent, with females typically more sensitive especially during high-estradiol levels (Anker and Carroll, 2010; Back et al., 2005; Chartoff and Mavrikaki, 2015; Feltenstein et al., 2011; Swalve et al., 2016; Verplaetse et al., 2015) although not all studies observe clear sex differences in stress-induced drug seeking (Buffalari et al., 2012; Zhou et al., 2012; also, see reviews by Becker et al., 2012; Bobzean et al., 2014; Fox and Sinha, 2009; Hudson and Stamp, 2011). Consistent with current NIH policy, studies should be adequately powered to detect sex differences in stress-induced drug seeking/use as well as candidate pharmacotherapies that may attenuate these effects.
1.4.8. Neurotransmission-related genetic factors
Few studies have addressed the role of genetic variation in stress-related substance use and relapse, in contrast to genetic association studies of initial addiction vulnerability. We reported that, in heroin-dependent volunteers undergoing buprenorphine dose tapering with an abstinence incentive, variations in genes encoding the kappa-receptor (OPRK1 rs3802281; Greenwald et al., 2012) and glucocorticoid receptor (NR3C1 rs6877893; Greenwald and Burmeister, 2018) predicted opioid relapse potential. Variation in OPRK1 rs6989250 is also associated with risk of cocaine relapse (Xu et al., 2013). Although CRH-binding protein (CRHBP) genotype variation moderated the association between stress-induced negative affect and negative consequences of alcohol intake in heavy-drinking subjects (Tartter and Ray, 2012), effects of this genotype on stress-induced drinking were not studied. Nonetheless, CRF is a promising target, as CRHR1 knockout animals are less sensitive to stress-induced alcohol intake (Hansson et al., 2006; Molander et al., 2012; Pastor et al., 2011). CRF-R1 knockdown mice are also less sensitive to stress-reinstatement of cocaine seeking (Chen et al., 2014).
2. Neuropharmacological targets
This section reviews evidence from studies related to various neurochemical systems that offer anti-stress therapeutic potential. To promote translational studies, each section indicates positron emission tomography (PET) imaging radiotracers that could be used to investigate proof-of-targeting in future prospective studies.
2.1. Noradrenergic system
The NA system has been the most commonly studied neurochemical domain for stress-related substance use, alone or in combination with other systems (see below). Discontinuation of chronic exposure to nicotine (Bruijnzeel et al., 2010; Sofuoglu et al., 2003), alcohol (Muzyk et al., 2011), cocaine (McDougle et al., 1994; Sofuoglu and Sewell, 2009), and opioids (Maldonado, 1997; Van Bockstaele et al., 2001) is a functional stressor associated with increased NA neurotransmission. It has been hypothesized that elevated NA release in the extended amygdala, and altered DA-mediated plasticity in the ventral tegmental area (VTA), alter hedonic processing of drug-related stimuli and are common substrates in withdrawal-associated relapse to drug seeking (Aston-Jones and Harris, 2004; Espana et al., 2016; Fitzgerald, 2013; Smith and Aston-Jones, 2008; Weinshenker and Schroeder, 2007).
Yohimbine (YOH) is an α2-adrenoceptor antagonist that increases NA neurotransmission by blocking feedback at presynaptic autoreceptors (Doxey et al., 1984; Goldberg and Robertson, 1983) and has become an important tool for investigating stress-related drug seeking/use. YOH-mediated increases in NA release and synaptic levels regulate HPA axis activity (Armario, 2010; Banihashemi and Rinaman, 2006; Grunhaus et al., 1989; Leri et al., 2002; Smythe et al., 1983), as well as 5-HT and DA neurotransmission (Brannan et al., 1991; Cheng et al., 1993; Hopwood and Stamford, 2001; Maura et al., 1982; McCall et al., 1991; Millan et al., 2000; Mongeau et al., 1993; Raiteri et al., 1990; Söderpalm et al., 1995a, b; Winter and Rabin, 1992). In a PET neuroimaging study of rhesus monkeys, YOH increased [11C]-flumazenil binding potential (Matsunaga et al., 2001) indicating YOH actions at GABA-A receptors that might correlate with its anxiogenic (negative-hedonic, arousing) and/or disinhibiting motivational effects (Fig. 1).
YOH has been used extensively as an experimental stressor in animal and human laboratory models. It produces anxiogenic effects in animals, healthy subjects, patients with panic disorder and opioid use disorder, which can be blocked by the α2-adrenoceptor agonist clonidine (Albus et al., 1992; Bremner et al., 1996; Cameron et al., 2000; Charney et al., 1983, 1992; Gurguis et al., 1997; Mattila et al., 1988; Pellow et al., 1987; Stine et al., 2002). These anxiogenic effects are presumed to mediate the effects of YOH on the reinforcing effects of drugs and drug-related stimuli. Reviews have concluded that YOH is a reliable and potent inducer of drug seeking with translational value (Bossert et al., 2005; Figlewicz et al., 2014; See and Waters, 2011; Shaham et al., 2000a). On the other hand, Chen et al. (2015) found that the effect of YOH on food-reinforced operant behavior may partly depend on factors unrelated to stress-induction; namely, YOH did not induce conditioned place aversion, YOH increased responding independent of the rat's history of contingent self-administration of food reward, and YOH increased medial prefrontal cortex (but not nucleus accumbens) dopamine levels. As a result, Chen et al. proposed that YOH may invigorate responding for cues that possess weaker rewarding effects in rodents (also see Box 2 in Mantsch et al., 2016). Given the extensive use of YOH in experimental stress studies, such findings warrant caution in relying only on YOH as an experimental model, yet encourage further investigation to determine the precise conditions under which YOH exerts its response-enhancing effects. For instance, YOH effects could differ for food vs. drug reinforcers, for paradigms involving drug-paired cues, for single-operant vs. choice scenarios, the subject's behavioral history, or other methodological factors.
In animal studies, YOH administration delays extinction of cocaine seeking (Kupferschmidt et al., 2009) and – as summarized in Table 1 – YOH robustly reinstates previously extinguished responding for nicotine, alcohol, cocaine, and opioids. Reinstatement of alcohol seeking by YOH (previously established to be a 5-HT1A partial agonist; Millan et al., 2000; Winter and Rabin, 1992) was blocked not only by the α2 agonist clonidine but also by a selective 5-HT-1A antagonist (WAY-100,635), suggesting an important role for 5-HT in mediating YOH-induced alcohol seeking (Lê et al., 2009; see section 2.3). Although YOH did not increase heroin seeking in opioid-dependent rats (Minhas and Leri, 2014), our group was the first to find that YOH increased drug seeking in humans, i.e., the stressor increased opioid-maintained responding in a sample of buprenorphine-maintained heroin-dependent human subjects (Greenwald et al., 2013) and we recently extended this to cigarette puff-maintained responding in tobacco users (Woodcock et al., under review). Tests of YOH to reinstate Δ9−THC- responding have not been reported (due to limitations of animal models in demonstrating reinforcing effects of Δ9-THC), although chronic Δ9−THC exposure during adolescence augmented YOH reinstatement of heroin seeking (Stopponi et al., 2014).
Furthermore, YOH augments cue-induced reinstatement for nicotine (Costello et al., 2014), heroin (Banna et al., 2010) and cocaine (Buffalari and See, 2011; Feltenstein and See, 2006; Fletcher et al., 2008) in animals, and enhances cocaine cue-reactivity in cocaine users (Moran-Santa Maria et al., 2014), suggesting this primarily NA stressor can enhance the conditioned reinforcing properties of drug cues to elevate relapse risk. Moreover, across animal and human studies (Adams et al., 2017; Bari and Robbins, 2013; Ma et al., 2003; Sanger, 1988; Schippers et al., 2016; Sun et al., 2010; Swann et al., 2005), YOH and other α2-antagonists increase impulsive responding (i.e., decrease self-control; see Fig. 1), suggesting another mechanism by which YOH could increase substance use (see Table 2). Interestingly, NA binding to α2 receptors in prefrontal cortex regulates attentional set, response inhibition and behavioral flexibility (Arnsten, 1998; Aston-Jones and Cohen, 2005; De Martino et al., 2008; Ramos and Arnsten, 2007; Robbins and Roberts, 2007). YOH also causes perseverative responding (Caetano et al., 2013), which predicts that YOH should promote drug-maintained responding particularly habitual substance use.
Table 2.
Candidate therapeutic targets, neuropharmacological agents, and related motivational mechanisms for attenuating stress-induced drug seeking/self-administration.
Based on broad, non-exhaustive assessment of empirical literature (using serial PubMed searches that crossed each CNS target label [or closely related terms, e.g., “anxiety” instead of “dysphoria”, “impulsivity” instead of “disinhibition”] with each stress-motivational dimension label in this table, as well as studies reviewed herein), the author's subjective designation of “+” indicates that the CNS target is likely involved in counteracting the stress-induced motivation-dimensional outcome. Designation of “?” indicates mixed evidence or uncertainty, and blank cells indicate minimal or no supporting evidence. The designation of “–“ indicates possible worsening of this motivational feature (particularly with chronic dosing). This global summary is simply intended for guidance in mapping these theoretical dimensions to neurochemical mechanisms and, more importantly, for designing studies to formally test effects of these CNS targets against motivational phenotypes, ultimately moving toward combinations of medications for pan-efficacy (see Table 3).
CPP paradigm.
Clinical study.
The foregoing data suggest agents that reduce NA neurotransmission are first-line candidates as anti-stress medications for at least some SUDs; this proposition has been tested in animal studies. As indicated in Table 2, the α2-agonists clonidine, lofexidine and guanfacine block stress-reinstated responding for nicotine, alcohol, heroin, cocaine, and heroin/cocaine “speedball” (Highfield et al., 2001) at doses that blocked footshock-induced NA release in prefrontal cortex and amygdala. We recently proposed (Woodcock et al., under review) that the occupancy ratio of NA receptors (higher-affinity α2-receptors vs. lower-affinity α1-and β-receptors) may underlie stress-induced drug use. Specifically, we hypothesized that during acute stress-exposure, NA released into the synapse may exceed capacity of higher-affinity α2 receptors, possibly leading to spillover binding at lower-affinity α1-and β-receptors; and, in turn, this altered receptor occupancy ratio could potentiate drug seeking. If this theory were confirmed, it could suggest that α1-and β-receptor antagonists may also attenuate drug seeking, especially at higher stress intensities (when NA transmission is greatest). In fact, the α1 antagonists, prazosin and doxazosin, and the α2 agonist guanfacine blocked several types of stress-reinstatement of alcohol-seeking in rodents (Funk et al., 2016; Lê et al., 2011; Riga et al., 2014), as well as YOH-induced motoric impulsivity (Adams et al., 2017). Stress-reinstatement of cocaine seeking is attenuated by the β-receptor antagonist propranolol (Mantsch et al., 2010) and the NA synthesis inhibitor nepicastat (Schroeder et al., 2013). However, when injected directly into the amygdala central nucleus, the α1-antagonist prazosin and the β1/2-antagonist propanolol did not attenuate footshock-reinstatement of nicotine seeking, whereas the α2 agonist clonidine did (Yamada and Bruijnzeel, 2011), highlighting different effects of site-specific versus systemic drug administration across studies. One study found a double dissociation in NA receptor subtypes mediating rewarding and somatic withdrawal effects during mecamylamine-precipitated nicotine withdrawal: only prazosin attenuated brain reward threshold deficits during nicotine withdrawal, whereas only clonidine and propanolol attenuated somatic signs (Bruijnzeel et al., 2010), suggesting that these NA agents address different motivational mechanisms.
In clinical practice, α2-receptor agonists are typically used as antihypertensive agents and for improving cognitive control in attention-deficit/hyperactivity disorder. Clonidine is a second-line treatment for smoking cessation (Gómez-Coronado et al., 2018) and guanfacine exhibited a promising signal in a pilot-test as a anti-stress/cognitive-control agent in tobacco smokers (McKee et al., 2015). Regarding cannabis use disorder, one study found that lofexidine reduced marijuana relapse-like behavior in a human laboratory model (Haney et al., 2008) but was not generally effective, when combined with oral THC, for reducing outpatient cannabis use (Levin et al., 2016). For cocaine use disorder, during early cocaine abstinence, guanfacine attenuated stress reactivity and craving (Fox et al., 2012) and enhanced inhibitory control (Fox et al., 2015), which might treat dysphoric and impaired self-control dimensions in the drug-motivational space (Fig. 1). Another study found guanfacine did not attenuate stress responses, prompting the authors to question its value for cocaine use disorder (Moran-Santa Moria et al., 2015). Furthermore, these human studies focused on drug craving rather than drug seeking/use, which may limit their clinical utility. Another caveat is that a study in rhesus monkeys found that, in the absence of stress, chronic lofexidine treatment produced a leftward shift in the cocaine self-administration dose-response curve; accordingly, the authors suggested α2 agonist maintenance may not be generally useful for cocaine use disorder (Kohut et al., 2013). In opioid-dependent humans, where YOH produced anxiety and opioid withdrawal-like effects (Greenwald et al., 2013; Oliveto et al., 2003; Stine et al., 2002), α2 agonists suppress opioid withdrawal symptoms (Gowing et al., 2014) and stress-induced craving (Jobes et al., 2011; Sinha et al., 2007; but see Moran-Santa Maria et al., 2015). Although maintenance on clonidine (vs. placebo) significantly lengthened duration of opioid abstinence among methadone-maintained patients, survival curve analysis revealed no significant clonidine vs. placebo group difference in time to opioid relapse, suggesting circumscribed efficacy (Kowalczyk et al., 2015). Some data suggest α-2A receptors primarily mediate anti-stress therapeutic effects of α2 agonists (e.g., guanfacine is a α-2A-specific partial agonist), whereas uncertainty remains regarding the roles of α-2B and α-2C subtypes, as well as whether these effects occur via pre-synaptic autoreceptors or heteromers with non-NA neurons such as glutamate (Shields et al., 2009).
α1 antagonists have been investigated for efficacy in alcohol use disorder, with mixed results. In a recent, placebo-controlled, phase 2 trial, six-week treatment with prazosin (up to 16 mg/day) did not exhibit overall efficacy (Wilcox et al., 2018). Interestingly, pretreatment blood pressure – possibly reflecting chronic stress levels – has been found to moderate the efficacy of prazosin and doxazosin in reducing alcohol intake in two clinical trials (Haass-Koffler et al., 2017; Wilcox et al., 2018), such that these medications reduced alcohol intake to a greater degree among patients with higher pretreatment blood pressure.
This review emphasizes a system biology approach to the influence of stress on substance use. Notably, the NA system has been the most extensively studied on its own and interacting with other neurochemical systems. Accordingly, Table 3 summarizes studies that have experimentally analyzed effects of manipulating NA signaling in combination with other systems, specifically for an effect on stress-related substance use. This table is intended to advance the idea that multi-modal medication (polypharmacy or “cocktail”) approaches may be useful, and perhaps preferable (i.e., safer and more effective than unimodal intervention), for treating stress-related substance use. Table 3 also illustrates the current research landscape: alcohol and cocaine have most often been studied using this systems biology approach, but there are substantial research gaps for other abused drugs. From a clinical perspective, it is notable that the use of other (non-NA) medications in combination with NA agents might minimize incidence of hypotension (i.e., produce a dose-sparing effect for the NA medication), which is the primary side effect of the NA medication class. Finally, prescribing NA medications for substance abusers who have hypertension could help treat this common comorbid condition.
Table 3.
Studies investigating potential interactions between noradrenergic and other neurochemical systems in stress-related substance use.
| Neurochemical System | Nicotine | Alcohol | Cocaine | Opioid |
|---|---|---|---|---|
| CRF-1 | Ayanwuyi et al., 2013; Lê et al., 2013; Marinelli et al., 2007 | Brown et al., 2009, 2012; McReynolds et al., 2014,a | ||
| CORT | Bertholomey et al., 2016; Simms et al., 2012 | Greenwald et al. (in prep.)b | ||
| Orexin-1 | Richards et al., 2008; Kastman et al., 2016 | Boutrel et al., 2005; Schmeichel et al., 2017 | ||
| 5-HT | Lê et al., 2009 | Land et al., 2009,a | ||
| Kappa-opioid | Grella et al., 2014; Nygard et al., 2016 | Funk et al., 2014 | Al-Hasani et al., 2013ba; Valdez et al., 2007 | Zhou et al., 2013 |
| Delta-opioid | Nielsen et al., 2012 | |||
| Nociceptin | Rorick-Kehn et al., 2016 | |||
| eCB | Gueye et al., 2016 | Cippitelli et al., 2008 | Vaughn et al., 2012 | |
| GABA-B | Williams et al., 2016 | |||
| GABA-A | de Guglielmo et al., 2013 | |||
| α3β4 nAChR | Cippitelli et al., 2015; Yuan et al., 2017 | |||
| PPAR-γ | Stopponi et al., 2011, 2013 | de Guglielmo et al., 2017 | ||
| Neurokinin-1 | Schank et al., 2014 | Schank et al., 2014 | ||
| Neuropeptide S | Schmoutz et al., 2012 | |||
| Neuropeptide Y | Cippitelli et al., 2010 |
CPP paradigm.
Clinical study.
For proof-of-target studies, PET radiotracers exist for measuring occupancy of α2C receptors ([11C]-ORM-13070; Arponen et al., 2014) and noradrenaline transporter ([11C]-MENET; Adhikarla et al., 2016), but none are available for α1-or β-adrenergic receptors.
2.2. HPA axis
2.2.1. Corticotropin-releasing factor (CRF)
As noted in section 1.3, infusion of CRF (which mimics supra-physiologic endogenous release from the hypothalamus) reinstates previously extinguished drug seeking in animal models. Notably, CRF-1 receptor knockout animals are less sensitive to stress-induced cocaine seeking (Chen et al., 2014). Thus, CRF-1 antagonists have been explored for blocking stress-induced reinstatement. Early studies found that ICV infusion of non-selective CRF-1/2 receptor antagonists decreased footshock reinstatement of responding for heroin (Shaham et al., 1997), cocaine (Erb et al., 1998), alcohol (Lê et al., 2000; Liu and Weiss, 2002) and nicotine (Zislis et al., 2007). As summarized in Table 2, selective CRF-1 antagonism attenuated stress-reinstatement of responding for nicotine, alcohol, cocaine and heroin.
Although these preclinical studies suggest CRF-1 antagonism might be a useful approach for reducing stress-induced drug seeking, this apparent promise must be tempered by contradictory results from recent clinical studies. Two CRF-1 antagonists, pexacerfont and verucerfont, failed to demonstrate anti-craving effects in patients with alcohol use disorder (Kwako et al., 2015b; Schwandt et al., 2016). A third CRF-1 antagonist, GSK561679, failed to show anxiolytic effects in a human laboratory model (Grillon et al., 2015) and lacked efficacy in a clinical trial with posttraumatic stress disorder patients (Dunlop et al., 2017). Given this lack of translational efficacy, considerable caution is warranted for similar congeners in this pharmacological class. Several methodological factors could have led to poor translation (Pomrenze et al., 2017; Shaham and de Wit, 2016); if these are properly addressed, there could still be a path forward for these agents but, in all likelihood, therapeutic application may be narrower than originally hoped (Spierling and Zorrilla, 2017). Alternative study designs may be needed that measure not only direct effects of the medication on substance use, but also intermediate stress-related phenotypes, e.g., coping or resilience (Contoreggi et al., 2013). One potential moderating factor is that some clinical studies were conducted exclusively with female volunteers, which could influence the generalizability of findings.
Contribution of CRF-2 receptors to stress-induced drug seeking is unclear, due to mixed methods and findings in the literature (Blacktop et al., 2011; Bruijnzeel et al., 2009; Wang et al., 2007; Zorrilla et al., 2014). CRF-2 receptors mediate anxiolysis (Bale et al., 2000; Kishimoto et al., 2000; Risbrough et al., 2004), suggesting CRF-2 agonists (not antagonists) are more appropriate anti-stress medication candidates but, presently, there are no published studies on this topic. Currently, there are no viable CRF-1 or CRF-2 receptor PET ligands for use in studies.
Brown et al. (2009) found that ICV infusion of NA reinstated cocaine seeking, which was blocked by pretreatment with the non-selective CRF antagonist D-Phe CRF12-41. In contrast, ICV CRF reinstated cocaine seeking but this was not blocked by clonidine pretreatment. Pretreating with the CRF antagonist or clonidine failed to block YOH-induced reinstatement. These findings suggest a functional interaction between NA and CRF systems in mediating stress-reinstatement of cocaine seeking, such that CRF receptor activation occurs downstream from NA sites of action (i.e., at extra-hypothalamic sites, independent of the HPA axis).
McReynolds et al. (2014) demonstrated that reinstatement of cocaine CPP using forced swim or systemic injection of the β2-receptor agonist clenbuterol was blocked by antalarmin or the β2-antagonist ICI-118,551; whereas clenbuterol reinstatement of cocaine CPP was only blocked by antalarmin but not the β2-antagonist. The authors hypothesized that stress-induced liberation of NA activates, via β2 receptors, CRF neurons that lead to drug-motivated responding.
2.2.2. Glucocorticoids
Several early studies demonstrated that footshock stress, while increasing corticosterone (CORT) levels in rats, did not reinstate responding for heroin (Shaham et al., 1997), cocaine (Erb et al., 1998), or alcohol (Lê et al., 2000). Although acute pretreatment with the corticosteroid synthesis inhibitor, metyrapone, itself reinstated heroin-seeking behavior, neither acute nor chronic metyrapone exposure, nor adrenalectomy, attenuated footshock-reinstatement of heroin seeking (Shaham et al., 1997). These data implied that CORT does not specifically mediate reinstatement. Although one study found that acute pretreatment with the corticosteroid synthesis inhibitor, ketoconazole, blocked footshock reinstatement of cocaine seeking (Mantsch and Goeders, 1999), ketoconazole has other pharmacological actions, making it unclear whether this effect was HPA axis-specific. Furthermore, sustained ketoconazole treatment in a clinical study led to increased use of cocaine and heroin among methadone-maintained patients (Kosten et al., 2002).
Despite the important role of the HPA axis in addiction (Goeders, 2003), preclinical studies have found that glucocorticoid manipulations, rather than directly influencing drug seeking (given the negative findings above), may instead sensitize the animal to respond more in the presence of drug-related stimuli. Graf et al. (2013) found that exposing rats to footshock failed to reinstate cocaine seeking, but footshock did facilitate reinstatement to a subthreshold cocaine priming dose. Further, CORT administered to adrenalectomized rats also reinstated cocaine seeking with a subthreshold cocaine priming dose. Interestingly, CORT infused into the nucleus accumbens (NAc) was found to decrease DA clearance, suggesting CORT potentiation of NAc DA signaling may partly underlie stress-induced reinstatement. This research group also found that acute CORT alone did not reinstate extinguished cocaine CPP (McReynolds et al., 2017b) or self-administration (McReynolds et al., 2017a); however, CORT enhanced efficacy of a sub-threshold cocaine priming dose to reinstate cocaine CPP and self-administration (McReynolds et al., 2017a, b); thus, the stressor (CORT) “set the stage” for acute cocaine exposure to reinvigorate drug- or drug-paired responding. Also, these investigators found CORT and footshock-stress effects on cocaine-primed reinstatement of self-administration that were mediated by eCB signaling via CB1 receptors and the enzyme monoacylglycerol (McReynolds et al., 2016, 2017a). This research illustrates an interaction between the HPA axis and eCB system in the control of stress-occasioned relapse-like behaviors and suggests a role for using eCB modulators to attenuate the direct and indirect effects of stressors on drug-maintained behaviors (see section 2.6).
Given the complexities of the HPA axis (e.g., negative feedback loops within the HPA axis; see Fig. 2), and the findings above that adrenalectomy or inhibition of corticosteroid synthesis did not block drug seeking, the potential utility of glucocorticoid receptor (GR) antagonists as anti-stress therapeutics must be seriously questioned. Also, nicotine abstinence is associated with HPA axis hypo-sensitivity (al’Absi et al., 2005; Semba et al., 2004), so it is unclear whether GR antagonists would be useful. On the other hand, a recent study found that the dual GR/progesterone antagonist mifepristone and the GR-specific antagonist CORT113176 each dose-dependently reduced escalated alcohol intake in rats and, in a companion clinical study, maintenance on mifepristone 600 mg/day (vs. placebo) reduced alcohol drinking among individuals with alcohol use disorder (Vendruscolo et al., 2015). Further investigation of these agents is warranted to shed light on the specific mechanisms through which this effect may occur, and whether it may be unique to alcohol or apply to other abused drugs.
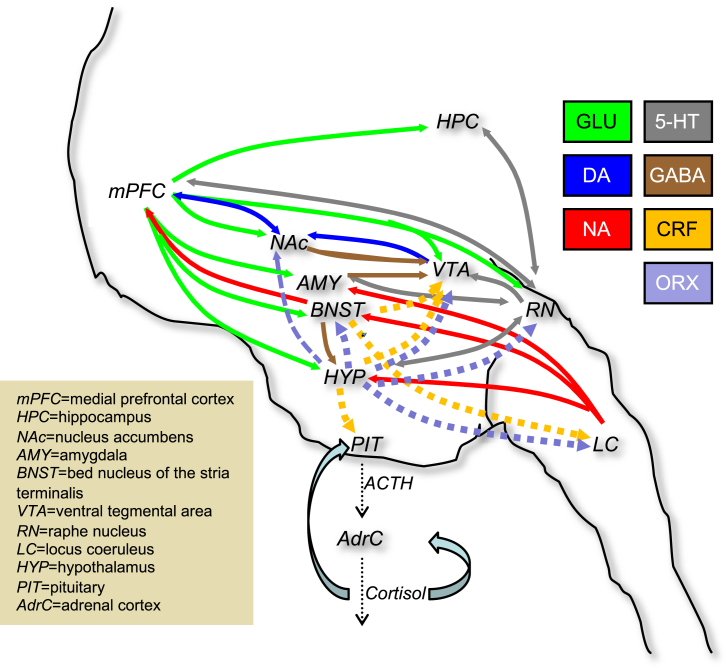
Fig. 2.
Systems Biology: Illustration of major neurochemical pathways that integrate and underlie stress-potentiated drug-seeking/use behaviors. See text for discussion of some of these inter-connections, as well as Table 3 relating NA function to other neurochemical systems.
Consistent with the systems biology focus in this review, NA and glucocorticoid systems produce co-operative effects in multiple paradigms (Dunn and Swiergiel, 2008; Forray and Gysling, 2004; Koob, 2008; Schwabe et al., 2010, 2012; Torregrossa et al., 2011; Valentino and Van Bockstaele, 2008; van Stegeren et al., 2010; Vasa et al., 2009). This prompted our recent study to co-activate these systems using separate and combined challenges with YOH and hydrocortisone (synthetic CORT) (Greenwald et al., in preparation). In a within-subject randomized crossover design, heroin-dependent buprenorphine-maintained volunteers were pretreated with oral YOH (0-, 27- and 54-mg) and CORT (0-, 20- and 40-mg) alone and combined. YOH/CORT increased opioid (hydromorphone) choices, relative to placebo pretreatment, while reducing money choices (i.e., stress increased opioid preference) but this effect was modulated by individual's pre-experimental variation in daily nicotine use. This pharmacological stress model was recently applied to cigarette smokers. Relative to placebo, YOH-54mg/CORT-10mg pretreatment induced similar physiological stress responses as above. Controlling for nicotine-dependence level, acute stress increased nicotine seeking (Woodcock et al., under review).
2.3. Serotonin system
Several studies implicate 5-HT neurotransmission in stress-induced reinstatement of alcohol seeking (whereas similar studies with abused drugs are lacking). In an initial study, alcohol seeking was reinstated by footshock and reversed by the 5-HT reuptake inhibitor fluoxetine (Lê et al., 1999). Subsequently, the 5-HT-1A agonist 8-OH-DPAT (which decreases 5-HT cell firing and transmitter release) infused in the median raphe nucleus (MRN) reinstated alcohol seeking; furthermore, intra-MRN infusion of CRF reinstated alcohol seeking (Lê et al., 2002). In a follow-up study, CRF antagonist infusion in the MRN attenuated YOH-reinstatement of alcohol seeking (Lê et al., 2013). Together, these findings point to 5-HT/CRF interaction within the MRN as mediating this stress effect. In addition to its primary α2-antagonist action, YOH also acts as a 5HT-1A partial agonist, which could partly explain its stress-reinstating efficacy. Furthermore, intra-MRN infusion of muscimol (GABA-A receptor agonist) reinstated alcohol seeking, implicating a 5-HT/GABA-A interaction in the MRN (Lê et al., 2008).
The 5-HT3 receptor antagonists, ondansetron and tropisetron (FDA-approved for treating nausea and vomiting), have been demonstrated to attenuate footshock-reinstatement of alcohol seeking in rats (Lê et al., 2006). However, these anti-stress effects have not yet been explored for other abused substances.
In summary, limited data suggest agents that promote 5-HT release, prevent reuptake, or block 5-HT-3 receptors could be useful therapeutics, although such effects may depend on raphe-mediated interactions; thus, systemic delivery could produce mixed effects. Given widespread clinical use of SSRIs and 5-HT-3 antagonists, systematic research across classes of abused drugs is warranted. Target-selective PET tracers are available that measure 5-HT transporter occupancy using [11C]DASB (for review, Spies et al., 2015); 5-HT synthesis using α-[11C]-methyl-L-tryptophan (Kumar et al., 2011); and 5-HT1A receptor binding using [carbonyl-11C]-WAY100635 or possibly [11C]-CUMI-101, but none for 5-HT-3 receptors (for review, Kumar and Mann, 2014).
2.4. Opioid system
Although the non-selective opioid receptor antagonist naltrexone (NTX) is used to treat alcohol and opioid use disorders, NTX does not block stress-induced alcohol or opioid use (e.g., Hyman et al., 2007; Lê et al., 1999). Notably, NTX can increase YOH reactivity (Rosen et al., 1999), and a clinical trial found that adding the α2-agonist guanfacine to NTX did not improve opioid abstinence over NTX alone (Krupitsky et al., 2013).
It is presently unclear whether opioid agonist medications might (in addition to their withdrawal-suppressing and opioid blockade properties; see Greenwald et al., 2014) attenuate stress-reactivity among individuals with opioid use disorder. One animal study found that maintenance on the mu-agonist methadone (MTD) did not block stress-reinstatement of heroin seeking (Leri et al., 2004). Results from cross-sectional clinical studies suggest MTD might blunt stress-reactivity (Kakko et al., 2008; Kreek et al., 1984; Schluger et al., 2001, 2003) but those studies have methodological flaws, e.g., variable maintenance doses, potential pre-morbid differences between patients and controls, and failure to match controls on other substance use. Buprenorphine (BUP) is a partial mu-receptor agonist/kappa-receptor antagonist with rapid-onset antidepressant and anxiolytic effects in animal models (Falcon et al., 2015) and human studies (Bershad et al., 2015; Bodkin et al., 1995; Karp et al., 2014; Nyhuis et al., 2008). In the clinical dosing range for opioid use disorder, BUP may be stress-protective via one or both of these mechanisms; our lab is presently investigating this issue.
2.4.1. Kappa-opioid receptor (KOR) antagonists
Stressors have been shown to release dynorphin and activate KORs located on DA and 5-HT neurons, thereby reducing DA and 5-HT levels (Carlezon et al., 2006; Ebner et al., 2010; Gehrke et al., 2008; Land et al., 2008; Zhang et al., 2007) that, in turn, are associated with negative-hedonic effects of stressors (Fig. 1). Furthermore, KOR agonist administration reliably reinstates responding for cocaine (see Table 1).
Accordingly, KOR antagonists are being evaluated as potential anti-depressants (Carr et al., 2010; Filho et al., 2013; Grimwood et al., 2011; Zhang et al., 2007) and anti-stress/drug-relapse prevention agents. As summarized in Table 2, KOR antagonists reliably attenuate stress-reinstated responding for nicotine, alcohol (but see Schank et al., 2012), cocaine, and heroin. Although no tests of KOR antagonism of stress-related substance abuse have been conducted in humans to date, PET ligands for measuring KOR occupancy are available including [11C]-LY2795050 (Naganawa et al., 2014) and [18F]-LY2459989 (Li et al., 2018).
Evidence also suggests dynorphin projections to NA and GABA neurons in the locus coeruleus (Kreibich et al., 2008; Reyes et al., 2007) modulate stress-related drug reinforcement. Studies in rats have identified KOR/noradrenergic interactions in the locus coeruleus that mediated reinstatement of cocaine lever-responding (Al-Hasani et al., 2013b), and KOR/serotonergic projections from the dorsal raphe to the NAc that mediated reinstatement of cocaine conditioned place preference (CPP) (Land et al., 2009). Finally, interactions between dynorphin/KORs and CRF have been proposed as one mechanism by which stressors lead to changes in allostatic load, altering the motivational valence of the abused substance (Bruchas et al., 2010; Koob, 2015).
2.4.2. Delta-opioid receptor (DOR) compounds
DORs are a potential anti-stress target, given their established role in anxiety-like behaviors in animal models; however, their pharmacology is incompletely understood, including differing functions of DOR subtypes, stress-induced translocation of DORs from cytoplasm to cell surface, and potential for DORs to form heteromers with mu-receptors (van Rijn et al., 2013). In the only relevant study to date, YOH-reinstatement of alcohol seeking was attenuated by a DOR-1 antagonist, SoRI-9409 (Nielsen et al., 2012). Further research with DOR subtype-specific agonist and antagonists is needed to determine whether such agents could be useful anti-stress therapeutics. The PET ligand [11C]methyl-naltrindole is clinically available (Madar et al., 1996; Smith et al., 1999) and has been used to measure DOR binding potential in alcohol-dependent individuals (Wand et al., 2013; Weerts et al., 2008, 2011).
2.5. Nociceptin receptor (NOP) compounds
Although the NOP system is involved in stress-reactivity and addictive behaviors, historically less clear is whether NOP agonists or antagonists should be effective anti-stress agents. On the one hand, NOP agonists have shown therapeutic promise against stress-reinstatement of alcohol seeking. Footshock-reinstatement of alcohol seeking was attenuated by ICV administration of nociceptin (Martin-Fardon et al., 2000) and systemic administration of the non-peptide, brain-penetrant NOP agonist MT-7716 in post-dependent rats but not alcohol-naïve rats (de Guglielmo et al., 2014). The latter findings suggest a history of alcohol dependence dysregulates the nociceptin system, which can be reversed with NOP agonist treatment. A different small-molecule NOP agonist, SR-8993, blocked YOH-reinstatement of alcohol seeking (Aziz et al., 2016). Conversely, other studies have found that NOP antagonists and NOP knockout mice produce anti-obesity and anti-depressant effects (review by Witkin et al., 2014), and the orally bioavailable, brain-penetrant, NOP antagonist LY2940094 blocked YOH-reinstatement of alcohol seeking in alcohol-preferring rats (Rorick-Kehn et al., 2016).
Witkin et al. (2014) proposed a hypothesis to reconcile these differences; specifically, nociceptin serves as part of a stress-coping negative-feedback mechanism, eliciting a stress-like response within the HPA axis but anti-stress effects at extra-hypothalamic sites. For future studies, the PET ligand [11C]NOP-1A (Lohith et al., 2012) is available to measure NOP binding potential in human subjects (Narendran et al., 2017).
2.6. Endocannabinoid (eCB) system
Initial studies of the eCB system as an anti-stress target focused on CB1 receptor antagonists. Administration of the CB1 antagonist rimonabant to rats failed to attenuate footshock-reinstatement of alcohol seeking (Economidou et al., 2006; 2007) and cocaine seeking (De Vries et al., 2001). Moreover, side effects of rimonabant in clinical trials led to discontinuation of its therapeutic development. The neutral CB1 antagonist AM4113 dose-dependently reduced YOH-reinstatement of nicotine seeking without altering inactive-lever or food-maintained responding acutely, although chronic treatment led to weight loss (Gueye et al., 2016). In contrast, the CB1 antagonist AM251 blocked forced swim-induced cocaine reinstatement (Vaughn et al., 2010) and AM251 blocked CRF- but not footshock-reinstatement of cocaine seeking (Kupferschmidt et al., 2012). Given these mixed effects of CB1 antagonists on stress-reinstatement, it is unclear whether findings may depend on interactions between the CB1 antagonist, stressor, and abused substance.
An alternative strategy is to modulate signaling indirectly within the eCB system. One promising approach is to promote eCB transmission by facilitating actions of the endogenous ligand anandamide (AEA). Although acute administration of the FAAH inhibitor URB597 (which increases AEA levels by inhibiting its catabolism) did not attenuate footshock- or YOH-reinstatement of alcohol seeking (Cippitelli et al., 2008), Chauvet et al. (2014) argued that chronic administration of anti-stress medication is a preferable model, because (1) this directly pertains to the human condition, (2) chronic exposure controls for possible tolerance development, and (3) medication-induced neuroadaptations may be required for a therapeutic effect. Using this approach, they demonstrated that URB597 significantly attenuated YOH-reinstatement of cocaine seeking (Chauvet et al., 2014). Targeting fatty acid binding proteins, which transport AEA intracellularly to FAAH for degradation, is a related strategy to interfere with stress-induced drug seeking (Hamilton et al., 2018).
The eCB cannabidiol (CBD) has diverse neuropharmacological actions but salient among these includes enhancing AEA levels with weak antagonist actions at CB1 and CB2 receptors (Campos et al., 2012; Izzo et al., 2009; Kathman et al., 2006; McPartland et al., 2015; Mijangos-Moreno et al., 2014; Pertwee, 2008; Petitet et al., 1998; Russo et al., 2005; Thomas et al., 2007). Transdermal CBD administration was recently found to attenuate acute and repeated YOH-reinstatement of cocaine and alcohol seeking and produced anxiolytic and anti-impulsive effects without disrupting other reward behavior or locomotion, and this profile of effects endured long after CBD was biologically detectable (Gonzalez-Cuevas et al., 2018). Further studies are needed, but CBD represents a novel anti-stress therapeutic approach.
PET radiotracers are clinically available that measure binding potential in the eCB system including [11C]-CURB (Boileau et al., 2015; Rusjan et al., 2013) and [11C]-MK3168 (Postnov et al., 2018) for FAAH; [11C]-OMAR (Normandin et al., 2015) and [11C]-MePPEP and [18F]-FMPEP-d (Terry et al., 2010) for CB1 receptors; and [11C]-NE40 for CB2 receptors (Ahmad et al., 2013). Measurement of eCB levels in biological matrices is promising and undergoing refinement (Dlugos et al., 2012; Feuerecker et al., 2012; Vaughn et al., 2010; Walter et al., 2013; Witkamp and Balvers, 2016).
2.7. Orexin system
Orexin fibers (originating mostly in the lateral hypothalamus) project widely in the brain, including to VTA, NAc and extended amygdala and, thus, are well-positioned to influence motivated behavior. The orexin system has been demonstrated to influence stress-induced seeking for several abused substances. ICV administration of orexin-1 but not orexin-2 peptide reinstates drug seeking (Boutrel et al., 2005; Harris et al., 2005; Plaza-Zabala et al., 2010; Wang et al., 2009), consistent with a broader role for orexin-1 in compulsive behavior (Pich and Melotto, 2014). Notably, the orexin-1 receptor-selective antagonist SB-334867 dose-dependently reduced footshock- and YOH-reinstatement of cocaine responding, particularly in rats given long-access to cocaine, a model of escalated drug use (Boutrel et al., 2005; Schmeichel et al., 2017; Wang et al., 2009). A set of studies found that restraint stress initially activated hypothalamic orexin neurons to release orexins into the VTA, activating postsynaptic VTA DA neurons, causing retrograde eCB inhibition of GABA release, leading to VTA DA disinhibition that resulted in reinstatement of cocaine CPP (Tung et al., 2016).
Systemic pretreatment with SB-334867 attenuated YOH-reinstatement of alcohol seeking, without altering locomotion (Richards et al., 2008). However, a later study found that only micro-injection of an orexin-2 receptor antagonist (but not SB-334867) into the pontine nucleus incertus attenuated YOH-reinstatement of alcohol seeking (Kastman et al., 2016). These mixed results could be due to different methods. A human study found that plasma concentrations of orexin – which may reflect diffusion of the peptide between CNS and peripheral compartments (Kastin and Akerstrom, 1999) – during alcohol abstinence positively correlated with psychological distress levels (von der Goltz et al., 2011); thus, it is plausible that orexin modulates stress- or abstinence-related alcohol use. However, there are also contrary data in cigarette smokers, showing a negative relationship between peripheral orexin-1 levels and nicotine craving (von der Goltz et al., 2010).
For nicotine, SB-334867 blocked reinstating effects of ICV orexin and footshock on nicotine seeking; in contrast, SB-334867 did not block CRF-induced nicotine reinstatement, and the CRF-1 antagonist antalarmin did not block ICV orexin-induced nicotine reinstatement, indicating autonomous control of stress-related drug-seeking across these two systems (Plaza-Zabala et al., 2010).
For opioids, pretreatment with either orexin-1 or orexin-2 receptor antagonists in the NAc shell (which processes positive and negative affectively-valenced behaviors) attenuated reinstatement of morphine CPP (Qi et al., 2013).
Suvorexant is a non-selective orexin-1/2 receptor antagonist approved for treating insomnia. Evidence indicates that orexin-2 antagonism is responsible for the sleep-inducing effect (Andrews et al., 2016). Consistent with findings above, orexin-1 antagonists such as GSK1059865 (Gozzi et al., 2011) are being developed as anti-stress and anti-addiction pharmacological tools, although none are clinically available at this time. Although there are emerging orexin-2 ligands for PET imaging such as [11C]-MK-1064 (Gao et al., 2016) and [11C]-CW4 (Wang et al., 2013), at this time there are no viable orexin-1 radiotracers.
2.8. Glutamatergic system
Multiple signals converge on glutamate signaling to alter stress-induced drug seeking. The effect of footshock stress on cocaine responding was shown to depend on glutamate via dorsal PFC/NAc core interaction (McFarland et al., 2004) and VTA CRF signaling (Wang et al., 2005; Williams et al., 2014), ultimately leading to increase NAc DA transmission (Wise, 2009). Cold-water forced-swim stress was shown to alter AMPA/NMDA receptor ratio in the NAc shell, an effect that was reversed with the glucocorticoid receptor antagonist RU486 (Campioni et al., 2009). NA signaling through α2 and β2 receptors in the dorsal BNST, can bidirectionally modulate glutamate transmission (Egli et al., 2005). Although these examples are not exhaustive, they illustrate that glutamate signaling in stress-related behavior is mostly secondary to other systems. This implies that a direct pharmacological approach to modulating glutamate function may be challenging for treating stress-related substance use.
Two agents with glutamate transmission-dampening properties, a mGluR2/3 agonist (LY379268) and mGluR5 antagonist (MTEP), each dose-dependently reduced footshock-reinstatement of cocaine seeking (Martin-Fardon and Weiss, 2012) and alcohol seeking (Sidhpura et al., 2010; Zhao et al., 2006). These findings suggest mGluR2/3 and mGluR5 targets (primarily located in pre-/peri- and post-synaptic spaces, respectively) should be studied further, given the established anxiolytic effects of LY379268 and MTEP in animal models (e.g., Kenny and Markou, 2004).
On the other hand, a review of medications for treating cocaine addiction (not specific to stress-induced drug seeking) suggested that allosteric modulators could be pursued (Kalivas and Volkow, 2011). Given the evidence above, reasonable candidates to be considered would be positive allosteric modulators of mGluR2/3 receptors and negative allosteric modulators of mGluR5 receptors. Given similar neuroadaptations from chronic alcohol (relative to cocaine) use, including a hypersensitive glutamate system (Vengeliene et al., 2008), it may be fruitful to investigate these allosteric modulators for stress-induced alcohol use. Yet, an initial test of this hypothesis found that the mGluR2-selective positive allosteric modulator AZD8529 attenuated cue- but not footshock-reinstatement of alcohol seeking in rats (Augier et al., 2016). Those authors note that this difference in findings across studies could imply that mGluR3 rather than mGluR2 receptors are distinctly involved in stress-related drug responding, or that orthosteric agonists and positive allosteric modulators could modulate glutamate function in distinct ways.
Several biomarkers are available to measure glutamate function in human subjects. Emerging PET radiotracers for measurement of brain glutamate targets include [18F]-FIMX for mGluR1 receptors (Zanotti-Fregonara et al., 2016), and [11C]-ABP688 (Kågedal et al., 2013) and [18F]-FPEB (Sullivan et al., 2013) for mGluR5 receptors. High-field proton magnetic resonance spectroscopy (1H-MRS) has proved sensitive to pharmacotherapy-related repeated-measures change in brain-regional glutamate concentrations in substance abusers (Greenwald et al., 2015; Umhau et al., 2010).
2.9. GABAergic system
The GABA-A agonist muscimol, when injected into the median raphe nucleus, reinstated alcohol seeking (Lê et al., 2008). However, intra-VTA modulation of GABA-A receptors using the GABA-A antagonist bicuculline did not block footshock- or intra-VTA CRF-induced reinstatement of cocaine seeking (Blacktop et al., 2016). However, it is possible that a GABA-A positive allosteric modulator could offer a useful alternative; one such compound is in development (Martinez Botella et al., 2017).
In contrast, the GABA-B agonist baclofen was found to block YOH-induced alcohol seeking (Williams et al., 2016) and to attenuate forced swim stress-reinstatement of morphine CPP (Meng et al., 2014). However, these encouraging results must be reconciled with conflicting findings that intra-VTA infusion of a GABA-B antagonist, 2-hydroxysaclofen, blocked reinstatement of cocaine seeking by footshock and intra-VTA CRF administration (Blacktop et al., 2016). Thus, it is unclear whether using a GABA-B agonist or antagonist would be preferable against stress-induced drug seeking, or whether this might vary by the abused substance or route of delivery.
GABA modulation with pregabalin (a clinically available medication) was found to block YOH-reinstatement of alcohol seeking (Stopponi et al., 2012) and cocaine seeking (de Guglielmo et al., 2013). The GAT-1 inhibitor tiagabine has been demonstrated to reduce anxiety- and depressive-like behaviors in animal models (Thoeringer et al., 2010) but has not yet been tested in experimental models of stress-induced drug seeking/use.
Biomarkers for GABA system targets include the PET radiotracer [11C]-flumazenil for GABA-A receptors (Jucaite et al., 2017; Lingford-Hughes et al., 2005), and 1H MRS imaging for cortical GABA concentrations (Mason et al., 2006; Streeter et al., 2005).
2.10. Other candidates
For completeness of coverage, this section briefly notes promising alternative candidates with relatively less empirical evidence.
2.10.1. Neuropeptides
Oxytocin is synthesized in the hypothalamus and binds to oxytocin and vasopressin receptors in brain regions implicated in regulating stress-reactivity, emotional and social behaviors (Heinrichs and Domes, 2008; Neumann and Landgraf, 2012; Windle et al., 2004). Studies of oxytocin suggest its clinical potential for several psychiatric conditions (Keech et al., 2018; Meyer-Lindenberg et al., 2011; Naja and Aoun, 2017; Quintana et al., 2017) although some therapeutic effects are likely to be sex-dependent (Bisagno and Cadet, 2014). Overall, few studies relate to substance abuse, and oxytocin might have greater promise for treating stress-related psychiatric conditions comorbid with SUDs (Zanos et al., 2017). An oxytocin analogue, carbetocin, attenuated forced-swim stress-reinstatement of morphine CPP, while attenuating stress-induced plasma CORT response (Zanos et al., 2014). Amygdala arginine vasopressin (AVP)1b receptors have been implicated in footshock-reinstatement of heroin seeking (Zhou et al., 2008, 2015), suggesting V1b receptor antagonism might be a viable target for blocking stress-related relapse. One V1b antagonist (ABT-436) is in clinical trials for treatment of alcohol use disorder (Ryan et al., 2017).
Neuropeptide S (NPS) acts at the NPS receptor to increase arousal and locomotion while decreasing anxiety-like behavior (Leonard et al., 2008; Rizzi et al., 2008; Xu et al., 2004). This paradoxical profile – anxiolysis but increased locomotion (often associated with reinforcing effects) – resembles that of nicotine, creating uncertainty as to whether agonists or antagonists of this system would be optimal anti-stress candidates. Schmoutz et al. (2012) adopted an NPS receptor antagonist approach, based on the idea that NPS may promote addictive behaviors. Using RTI-118, they found it decreased YOH- as well as cue- and cocaine priming-induced reinstatement of cocaine seeking, indicating a broad efficacy profile (Schmoutz et al., 2012). NPS receptors are co-localized with other hypothalamic-originating neuropeptides discussed in this review, including orexin and CRF receptors. Hypothalamic NPS infusion has been shown to reinstate alcohol and cocaine seeking, effects that are reversed by the orexin-1 antagonist SB-334867 (Cannella et al., 2009; Kallupi et al., 2010; Ubaldi et al., 2016) and the CRF-1 antagonist antalarmin (Paneda et al., 2009).
Neuropeptide Y binds multiple receptor subtypes; however, most studies have investigated Y1 (mostly postsynaptic) and Y2 (mostly presynaptic) receptors with the latter negatively modulating NPY release (Colmers et al., 1991; Eva et al., 2006; King et al., 1999) to produce numerous biobehavioral effects including anxiolysis (Heilig, 2004). The actions of NPY counteract CRF-induced anxiogenesis (Heilig et al., 1994; Sajdyk et al., 2004; Valdez and Koob, 2004). Central NPY infusion blocked YOH-reinstatement of alcohol seeking (Cippitelli et al., 2010). Systemic pretreatment with the Y2-selective antagonist, JNJ-31020028, failed to block footshock-reinstatement of alcohol responding (Cippitelli et al., 2011). A similar stress-reinstatement blocking test has not been reported for a Y1-selective agonist. Experimental results from studies of this system could be complicated by findings that Y1 and Y2 receptors (which have opposite functions regarding anxiety-like behavior) can form homo- and hetero-dimers whose net effect might negate the effects of receptor-selective stimulation (Dinger et al., 2003; Silva et al., 2003).
Substance P is a tachykinin-family neuropeptide selective for the neurokinin-1 (NK-1) receptor. The NK-1 receptor antagonist aprepitant is FDA-approved for treating chemotherapy-induced nausea and biosimilars are in the pipeline. Clinical development of NK-1 antagonists has met with difficulties perhaps, not surprisingly, because these agents share functional features with CRF-1 antagonists that have associated negative findings (see section 2.2.1; Schank and Heilig, 2017). Preclinical studies have shown that the NK-1 antagonist L822429 blocks YOH- or footshock-reinstatement of alcohol and cocaine seeking (Schank et al., 2011, 2014, 2015). Although a phase 2 clinical study found that the NK-1 antagonist LY686017 decreased spontaneous and psychological stress-induced alcohol craving and cortisol responses in high trait-anxious, alcohol-dependent volunteers (George et al., 2008), a subsequent placebo-controlled study of aprepitant administered to alcohol-dependent patients with co-occurring PTSD failed to replicate these findings (Kwako et al., 2015a). Although it remains unclear whether NK-1 antagonists could reduce acute stress-induced effects among individuals who are not highly anxious, this would considerably restrict the clinical utility of this class of medication. The PET ligand [11C]-GR205171 is available for measuring NK-1 binding potential in humans (Frick et al., 2015; Ridler et al., 2014; Spinelli et al., 2014).
Relaxin-3 is a peptide acting at its cognate receptor RXFP3 and, similar to orexin, relaxin-3 agonists increase stress-reactivity, food intake and arousal. In contrast, relaxin-3 antagonism, at least partly mediated via the BNST, blocked YOH-reinstatement of alcohol- but not sucrose-seeking (Ryan et al., 2013); furthermore, RXFP3 deletion blocked physical (repeated restraint followed by swim) stress-reinstatement of alcohol seeking without disrupting baseline alcohol or saccharin intake, or hepatic metabolism of alcohol (Walker et al., 2015). Thus, relaxin-3 antagonists may be reasonable anti-stress agents, although efficacy with other abused drugs has not been tested to date.
2.10.2. Non-peptides
FDA-approved nicotine replacement treatments (NRTs) do not attenuate stress-induced drug seeking or use (Kotlyar et al., 2006; Ray et al., 2013). Interestingly, the α3β4 nAChR partial agonist AT-1001 dose-dependently attenuated YOH-reinstatement of nicotine seeking (Yuan et al., 2017). Furthermore, AT-1001 selectively blocked YOH- but not cue-reinstatement of alcohol seeking without affecting baseline alcohol or food intake (Cippitelli et al., 2015). This novel approach could potentially address some limitation of current NRTs and assist the many individuals who concurrently use tobacco and alcohol.
Pioglitazone, agonist at the peroxisome proliferator-activated receptor-gamma (PPARγ) subtype, was shown to attenuate YOH-reinstatement of alcohol seeking (Stopponi et al., 2011, 2013) and heroin seeking (de Guglielmo et al., 2017).
3. Discussion
The development of anti-stress medications is critical for the advancement of treating all SUDs. The impact of stressors extends across abused substances (which contrasts with the effects of drug-priming and drug-cue exposure, which tend to be specific to drug classes). Therefore, progress in this field has significant potential to apply scientifically across all drug classes and to improve treatment of all patients with SUDs. This review describes available evidence on promising neuropharmacological approaches, using mechanistic studies based on chemical and non-chemical probes (Table 1). Although studies of Δ9-THC/marijuana were surveyed for this review, there is a paucity of studies in this area.
Based on the quality of evidence to date, promising first-tier neurochemical targets include: NA (α1-and β-antagonist, α2 agonist), kappa-opioid antagonist, NOP antagonist, orexin-1 antagonist, and eCB modulation (e.g., cannabidiol, FAAH inhibition); second-tier candidates may include CRF-1 antagonists, serotonergic agents (reuptake inhibitors, 5-HT-3 antagonists), glutamatergic agents (mGluR2/3 agonist/positive allosteric modulator, mGluR5 antagonist/negative allosteric modulator), GABA-signaling promoters (e.g., pregabalin, tiagabine), vasopressin 1b antagonist, NK-1 antagonist, and PPAR-γ agonist (e.g., pioglitazone). DA antagonists were excluded from this review because, although brain site-specific studies in animals support a role for DA in mediating stress-induced drug seeking (McFarland et al., 2004), when DA antagonists are administered systemically (and especially chronically) they produce side effects that do not translate well into clinical practice.
Table 2 complements this review by adding a layer of theoretical analysis pertaining to affective/motivational mechanisms that may be modulated while intervening in these neurochemical systems. I propose the overarching hypothesis that, to be effective, anti-stress medications for substance use disorders must alleviate the multidimensional burden of stressors that can lead to behavioral deficits including avoidance (valence dimension), hyperactivation (arousal dimension), and impulsivity (control dimension), that can perpetuate substance use and its adverse consequences. Table 2 illustrates that few candidate pharmacotherapeutic approaches are likely to tackle all these problems. Accordingly, to address these affective/motivational mechanisms of stress-related substance use, it seems advisable to combine agents with actions at complementary targets for greater efficacy; however, it should be noted that systematic studies are lacking except for interactions with the NA system (Table 3). Future research could be directed at whether some agents may function to desensitize subjects from chronic stress-induced effects (e.g., glucocorticoid receptor antagonist), whereas other agents might help to block acute stress-induced effects (e.g., α2 agonist or α1 antagonist). It is plausible that both types of agents together may be more effective than either alone. Some agents may also have additional effects beyond anti-stress, such as to block cue-induced drug seeking (e.g., eCB modulators, orexin-1 antagonist).
A key methodological factor concerns whether medication development studies employ acute vs. chronic administration. Although acute medication administration is the dominant paradigm to date, partly due to its efficiency, Chauvet et al. (2014) effectively argued that chronic exposure is desirable for applied clinical relevance, to avoid false negative results attributable to development of tolerance, and to enable within- and/or between-system neuroadaptations (including epigenetic changes) to occur. Future studies should carefully weigh these issues, e.g., whether to screen medications acutely (but perhaps also chronically) during early-stage development but move to chronic dosing in phase 2 studies.
3.1. Biomarkers
Programmatic studies should include biomarkers of stress-reactivity and medication targeting to confirm mediation of effects on drug-seeking behavior. Section 2 mentioned clinically available PET ligands for measuring occupancy of molecular targets and use of 1H-MRS for measuring glutamate and GABA brain-regional concentrations. These CNS biomarkers should be paired with measurement of medication plasma concentrations, and target occupation/stimulation and plasma pharmacokinetic data should be correlated with one another and with pharmacodynamic effects (clinical endpoints) to generate exposure-response functions. We successfully applied a similar PK/PD modeling strategy for optimizing sublingual and depot buprenorphine treatment of opioid use disorder (Greenwald et al., 2014; Nasser et al., 2014, 2016) and this approach was recently applied in the development of the NOP antagonist LY2940094 (Rorick-Kehn et al., 2014, 2016).
3.2. Heterogeneity of effect
As indicated in Table 2, which maps neurobiological and motivational foundations of anti-stress medications, it seems unlikely that any single medication will be highly effective for attenuating stress-mediated negative-hedonic, arousal/activating, and disinhibiting/loss-of-control effects on drug-maintained behaviors. Furthermore, chronic dosing may lead to between-system adaptations resulting in loss of efficacy over time, so it may be advantageous to employ a redundant approach (e.g., use two medications that each target one motivational dimension, but via different pharmacological stimulation). This approach emphasizes using one or more anti-stress medications that exert actions that address multiple motivational features of stress-reactive drug use. Using the model proposed here, a predictable consequence of deploying highly targeted compounds would be restricted efficacy. Individual difference factors need to be considered in the context of medication development; inattention to these variables will also limit efficacy (section 1.4).
3.3. Summary
This review has identified promising, evidence-based neurochemical mechanisms and potential pharmacotherapeutic leads to combat stress-induced substance seeking/use, a major problem for all SUDs. Medications that prove safe and effective could be added to existing SUD pharmacotherapies (e.g., agonist therapies for opioid or nicotine use disorder) to augment treatment efficacy, as most FDA-approved agonist treatments were not designed as anti-stress agents. To address motivational dimensions of stress-related substance use, it will be theoretically and pragmatically valuable to test agents with complementary actions alone and in combination to improve efficacy. As these agents are developed, it will be important to recognize numerous clinically-relevant factors (some of which could be modeled in animal studies) that could mediate/moderate the efficacy of anti-stress agents. Finally, progress in developing anti-stress medications will also depend on use of reliable CNS biomarkers to validate exposure-response relationships.
Author disclosures
Role of funding source
NIH 2 R01 DA015462 from the National Institute on Drug Abuse, Helene Lycaki/Joe Young Sr. funds (State of Michigan), and the Detroit Wayne Mental Health Authority supported preparation of this manuscript.
Contributors
The author is responsible for the content of this manuscript, but gratefully acknowledges the many valuable contributions offered by two anonymous reviewers.
Conflicts of interest
The author has received compensation as a scientific consultant to Indivior, Inc., which manufactures and markets buprenorphine products, and he has co-authored publications without compensation regarding RBP-6000 (Sublocade™).
References
- Adams W.K., Barrus M.M., Zeeb F.D., Cocker P.J., Benoit J., Winstanley C.A. Dissociable effects of systemic and orbitofrontal administration of adrenoceptor antagonists on yohimbine-induced motor impulsivity. Behav. Brain Res. 2017;328:19–27. doi: 10.1016/j.bbr.2017.03.034. [DOI] [PubMed] [Google Scholar]
- Adhikarla V., Zeng F., Votaw J.R., Goodman M.M., Nye J.A. Compartmental modeling of [11C]MENET binding to the norepinephrine transporter in healthy human brain. Nucl. Med. Biol. 2016;43:318–323. doi: 10.1016/j.nucmedbio.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad R., Koole M., Evens N. Whole-body distribution and radiation dosimetry of the cannabinoid type 2 receptor ligand [11C]-NE40 in healthy subjects. Mol. Imag. 2013;15:384–390. doi: 10.1007/s11307-013-0626-y. [DOI] [PubMed] [Google Scholar]
- Ahmed S.H., Koob G.F. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132:289–296. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Ahmed S.H., Walker J.R., Koob G.F. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- al'Absi M., Hatsukami D.K., Davis G. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berlin) 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- Albus M., Zahn T.P., Breier A. Anxiogenic properties of yohimbine. I. Behaviour, physiological and biochemical measures. Eur. Arch. Psychiatr. Clin. Neurosci. 1992;241:337–344. doi: 10.1007/BF02191958. [DOI] [PubMed] [Google Scholar]
- Alcaro A., Panksepp J. The SEEKING mind: primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci. Biobehav. Rev. 2011;35:1805–1820. doi: 10.1016/j.neubiorev.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Alexander B.K., Coambs R.B., Hadaway P.F. The effect of housing and gender on morphine self-administration in rats. Psychopharmacology. 1978;58:175–179. doi: 10.1007/BF00426903. [DOI] [PubMed] [Google Scholar]
- Alexandre C., Latremoliere A., Ferreira A., Miracca G., Yamamoto M., Scammel T.E., Woolf C.J. Decreased alertness due to sleep loss increases pain sensitivity in mice. Nat. Med. 2017;23:768–774. doi: 10.1038/nm.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R., McCall J.G., Bruchas M.R. Exposure to chronic mild stress prevents kappa opioid-mediated reinstatement of cocaine and nicotine place preference. Front. Pharmacol. 2013;4:96. doi: 10.3389/fphar.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R., McCall J.G., Foshage A.M., Bruchas M.R. Locus coeruleus kappa-opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mechanism. Neuropsychopharmacology. 2013;38:2484–2497. doi: 10.1038/npp.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S.P., Aves S.J., Christopher J.A., Nonoo R. Orexin receptor antagonists: historical perspectives and future opportunities. Curr. Top. Med. Chem. 2016;16:3438–3469. doi: 10.2174/1568026616666150929111607. [DOI] [PubMed] [Google Scholar]
- Anker J.J., Carroll M.E. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A. Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: different pathways, common outcome. Trends Pharmacol. Sci. 2010;31:318–325. doi: 10.1016/j.tips.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cognit. Sci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Arponen E., Helin S., Marjamäki P. A PET tracer for brain a2C adrenoceptors, (11)C-ORM-13070: radiosynthesis and preclinical evaluation in rats and knockout mice. J. Nucl. Med. 2014;55:1171–1177. doi: 10.2967/jnumed.113.135574. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Harris G.C. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl. 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Augier E., Dulman R.S., Rauffenbart C., Augier G., Cross A.J., Heilig M. The mGluR2 positive allosteric modulator, AZD8529, and cue-induced relapse to alcohol seeking in rats. Neuropsychopharmacology. 2016;41:2932–2940. doi: 10.1038/npp.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayanwuyi L.O., Carvajal F., Lerma-Cabrera J.M. Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcoholic drinking and seeking behaviors of Marchigian Sardinian alcohol-preferring rats. Front. Psychiatr. 2013;4:23. doi: 10.3389/fpsyt.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz AMA Brothers S., Sartor G., Holm L., Heilig M., Wahlestedt C., Thorsell A. The nociception/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: validation in rat models. Psychopharmacology. 2016;233:3553–3563. doi: 10.1007/s00213-016-4385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse P.J.J., Limpens J.H.W., Vanderschuren L.J.M.J. Disrupted social development enhances the motivation for cocaine in rats. Psychopharmacology. 2014;231:1695–1704. doi: 10.1007/s00213-013-3362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.E., Brady K.T., Jackson J.L., Salstrom S., Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berlin) 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Baker T.B., Piper M.E., McCarthy D.E. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol. Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bale T.L., Contarino A., Smith G.W. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Banihashemi L., Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J. Neurosci. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banna K.M., Back S.E., Do P., See R.E. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav. Brain Res. 2010;208:144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A., Robbins T.W. Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task. Psychopharmacology. 2013;230:89–111. doi: 10.1007/s00213-013-3141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley P.M., Howard J.L., Shelton K.L., Carroll F.I. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berlin) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Beardsley P.M., Pollard G.T., Howard J.L., Carroll F.I. Effectiveness of analogs of the kappa opioid receptor antagonist (3R)-7-hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl] methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic) to reduce U50,488-induced diuresis and stress-induced cocaine reinstatement in rats. Psychopharmacology. 2010;210:189–198. doi: 10.1007/s00213-010-1846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Perry A.N., Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Diffs. 2012;3:14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belding M.A., Iguchi M.Y., Lamb R., Lakin M., Terry R. Coping strategies and continued drug use among methadone maintenance patients. Addict. Behav. 1996;21:389–401. doi: 10.1016/0306-4603(95)00069-0. [DOI] [PubMed] [Google Scholar]
- Bershad A.K., Jaffe J.H., Childs E., de Wit H. Opioid partial agonist buprenorphine dampens responses to psychosocial stress in humans. Psychoneuroendocrinology. 2015;52:281–288. doi: 10.1016/j.psyneuen.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey M.L., Nagarajan V., Torregrossa M.M. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology. 2016;233:2277–2287. doi: 10.1007/s00213-016-4278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey M.L., Verplaetse T.L., Czachowski C.L. Alterations in ethanol seeking and self- administration following yohimbine in selectively bred alcohol-preferring (P) and high alcohol drinking (HAD-2) rats. Behav. Brain Res. 2013;238:252–258. doi: 10.1016/j.bbr.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisagno V., Cadet J.L. Stress, sex, and addiction: potential roles of corticotropin-releasing factor, oxytocin, and arginine vasopressin. Behav. Pharmacol. 2014;25:445–457. doi: 10.1097/FBP.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop J.M., Seubert C., Baker D.A., Ferda N., Lee G., Graf E.N., Mantsch J.R. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J. Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop J.M., Vranjkovic O., Mayer M., Van Hoof M., Baker D.A., Mantsch J.R. Antagonism of GABA-B but not GABA-A receptors in the VTA prevents stress- and intra-VTA CRF-induced reinstatement of extinguished cocaine seeking in rats. Neuropharmacology. 2016;102:197–206. doi: 10.1016/j.neuropharm.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobzean S.A.M., DeNobrega A.K., Perriotti L.I. Sex differences in the neurobiology of drug addiction. Exp. Neurol. 2014;259:64–74. doi: 10.1016/j.expneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Bodkin J.A., Zomberg G.L., Lukas S.E., Cole J.O. Buprenorphine treatment of refractory depression. J. Clin. Psychopharmacol. 1995;15:49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Boileau I., Tyndale R.F., Williams B. The fatty acid amide hydrolase C385A variant affects brain binding of the positron emission tomography tracer [11C]CURB. J. Cerebr. Blood Flow Metabol. 2015;35:1237–1240. doi: 10.1038/jcbfm.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert J.M., Ghitza U.E., Lu L., Epstein D.H., Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur. J. Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Boutrel B., Kenny P.J., Specio S.E., Martin-Fardon R., Markou A., Koob G.F. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc. Natl. Acad. Sci. Unit. States Am. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth M.A., Murray A., Wise R.A. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol. Biochem. Behav. 1989;33:903–907. doi: 10.1016/0091-3057(89)90490-5. [DOI] [PubMed] [Google Scholar]
- Brannan T., Martinez-Tica J., Yahr M.D. Effect of yohimbine on brain monoamines an in vivo study. J. Neural Transm. Parkinson’s Dis. Dementia Sect. 1991;3:81–87. doi: 10.1007/BF02260883. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Krystal J.H., Southwick S.M., Charney D.S. Noradrenergic mechanisms in stress and anxiety: II. clinical studies. Synapse. 1996;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Briand L.A., Blendy J.A. Molecular and genetic substrates linking stress and addiction. Brain Res. 2010;1314:219–234. doi: 10.1016/j.brainres.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.A., Lejuez C.W., Kahler C.W., Strong D.R., Zvolensky M.J. Distress tolerance and early smoking lapse. Clin. Psychol. Rev. 2005;25:713–733. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Z.J., Kupferschmidt D.A., Erb S. Reinstatement of cocaine seeking in rats by the pharmacological stressors, corticotropin-releasing factor and yohimbine: role for D1/5 dopamine receptors. Psychopharmacology. 2012;224:431–440. doi: 10.1007/s00213-012-2772-3. [DOI] [PubMed] [Google Scholar]
- Brown Z.J., Tribe E., D'Souza N.A., Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berlin) 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Bruchas M.R., Land B.B., Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel A.W., Bishnoi M., van Yujil I.A. Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology. 2010;212:485–499. doi: 10.1007/s00213-010-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel A.W., Prado M., Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit brain reward function and stress-induced relapse. Biol. Psychiatr. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczek Y., Wang A., Stewart J., Shaham Y. Stress reinstates nicotine seeking, but not sucrose solution seeking rats. Psychopharmacology. 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Buffalari D.M., Baldwin C.K., Feltenstein M.W., See R.E. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol. Behav. 2012;105:209–214. doi: 10.1016/j.physbeh.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari D.M., See R.E. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berlin) 2011;213:19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R.K., Finn D.P. Stress-induced analgesia. Prog. Neurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Caetano M.S., Jin L.E., Harenberg L., Stachenfeld K.L., Arnsten A.F.T., Laubach M. Noradrenergic control of error perseveration in medial prefrontal cortex. Front. Integr. Neurosci. 2013;6:125. doi: 10.3389/fnint.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron O.G., Zubieta J.K., Grunhaus L., Minoshima S. Effects of yohimbine on cerebral blood flow, symptoms, and physiological functions in humans. Psychosom. Med. 2000;62:549–559. doi: 10.1097/00006842-200007000-00014. [DOI] [PubMed] [Google Scholar]
- Campioni M.R., Xu M., McGehee D.S. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J. Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos A.C., Moreira F.A., Gomes F.V., Del Bel E.A., Guimaraes F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Phil. Trans. R Soc. B. 2012;367:3364–3378. doi: 10.1098/rstb.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N., Economidou D., Kallupi M., Stopponi S., Heilig M., Massi M., Ciccocioppo R. Persistent increase of alcohol-seeking evoked by neuropeptide S: an effect mediated by the hypothalamic hypocretin system. Neuropsychopharmacology. 2009;34:2125–2134. doi: 10.1038/npp.2009.37. [DOI] [PubMed] [Google Scholar]
- Carey A., Borozny K., Aldrich J., McLaughlin J. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur. J. Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon W.A., Jr., Béguin C., DiNieri J.A. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J. Pharmacol. Exp. Therapeut. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carr G.V., Bangasser D.A., Bethea T., Young M., Valentino R.J., Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar-Kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M.E., Meisch R.A. Increased drug-reinforced behavior due to food deprivation. In: Thompson T., Barrett J.E., editors. vol. 4. Academic Press; NY: 1984. pp. 47–88. (Advances in Behavioral Pharmacology). [Google Scholar]
- Charney D.S., Heninger G.R., Redmond D.E. Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- Charney D.S., Woods S.W., Krystal J.H., Nagy L.M., Heninger G.R. Noradrenergic neuronal dysregulation in panic disorder: the effects of intravenous yohimbine and clonidine in panic disorder patients. Acta Psychiatr. Scand. 1992;86:273–282. doi: 10.1111/j.1600-0447.1992.tb03266.x. [DOI] [PubMed] [Google Scholar]
- Chartoff E., Mavrikaki M. Sex differences in kappa opioid receptor function and their potential impact on addiction. Front. Neurosci. 2015;9:466. doi: 10.3389/fnins.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C., Lardeux V., Goldberg S.R., Jaber M., Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C., Nicolas C., Lafay-Chebassier C., Jaber M., Thiriet N., Solinas M. Statins reduce the risks of relapse to addiction in rats. Neuropsychopharmacology. 2016;41:1588–1597. doi: 10.1038/npp.2015.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C., Nicolas C., Thiriet N., Lardeux V., Duranti A., Solinas M. Chronic stimulation of the tone of endogenous anandamide reduces cue- and stress-induced relapse in rats. Int. J. Neuropsychopharmacol. 2014;18:1–5. doi: 10.1093/ijnp/pyu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N.A., Jupp B., Sztainberg Y. Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaine seeking in mice. J. Neurosci. 2014;34:11560–11570. doi: 10.1523/JNEUROSCI.4763-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.W., Fiscella K.A., Bacharach S.Z., Tanda G., Shaham Y., Calu D.J. Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. Addiction Biol. 2015;20:690–700. doi: 10.1111/adb.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.H., Costall B., Ge J., Naylor R.J. The profiles of interaction of yohimbine with anxiolytic and putative anxiolytic agents to modify 5-HT release in the frontal cortex of freely-moving rats. Br. J. Pharmacol. 1993;110:1079–1084. doi: 10.1111/j.1476-5381.1993.tb13924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y., Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol. Bull. 2008;134:829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R., Stopponi S., Economidou D. Chronic treatment with novel brain-penetrating selective NOP receptor agonist MTT-7716 reduces alcohol drinking and seeking in the rat. Neuropsychopharmacology. 2014;39:2601–2610. doi: 10.1038/npp.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A., Brunori G., Gaiolini K.A., Zaveri N.T. Pharmacological stress is required for the anti-alcohol effects of the α3β4* nAChR partial agonist AT-1001. Neuropharmacology. 2015;93:229–236. doi: 10.1016/j.neuropharm.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A., Cannella N., Braconi S. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology (Berlin) 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Cippitelli A., Damadzic R., Hansson A.C. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology. 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Cippitelli A., Rezvani A.H., Robinson J.E. The novel, selective, brain-penetrant neuropeptide Y Y2 receptor antagonist, JNJ-31020028, tested in animal models of alcohol consumption, relapse, and anxiety. Alcohol. 2011;45:567–576. doi: 10.1016/j.alcohol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Colmers W.F., Klapstein G.J., Fournier A., St-Pierre S., Treherne K.A. Presynaptic inhibition by neuropeptide Y in rat hippocampal slice in vitro is mediated by a Y2 receptor. Br. J. Pharmacol. 1991;102:41–44. doi: 10.1111/j.1476-5381.1991.tb12129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad K.L., McCutcheon J.E., Cotterly L.M., Ford K.A., Beales M., Marinelli M. Persistent increases in cocaine-seeking behavior after acute exposure to cold swim stress. Biol. Psychiatr. 2010;68:303–305. doi: 10.1016/j.biopsych.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contoreggi C., Lee M.R., Chrousos G. Addiction and corticotropin-releasing hormone type 1 receptor antagonist medications. Ann. N. Y. Acad. Sci. 2013;1282:107–118. doi: 10.1111/nyas.12007. [DOI] [PubMed] [Google Scholar]
- Costello M.R., Reynaga D.D., Mojica C.Y., Zaveri N.T., Belluzzi J.D., Leslie F.M. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39:1843–1851. doi: 10.1038/npp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J., Jackson D.C., Kalin N.H. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol. Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G., Cippitelli A., Somaini L. Pregabalin reduces cocaine self-administration and relapse to cocaine seeking in the rat. Addiction Biol. 2013;18:644–653. doi: 10.1111/j.1369-1600.2012.00468.x. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G., Kallupi M., Scuppa G., Demopulos G., Gaitanaris G., Ciccocioppo R. Pioglitazone attenuates the opioid withdrawal and vulnerability to relapse to heroin seeking in rodents. Psychopharmacology. 2017;234:223–234. doi: 10.1007/s00213-016-4452-1. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G., Martin-Fardon R., Teshima K., Ciccocioppo R., Weiss F. MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addiction Biol. 2014;20:643–651. doi: 10.1111/adb.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B., Strange B.A., Dolan R.J. Noradrenergic neuromodulation of human attention for emotional and neutral stimuli. Psychopharmacology. 2008;197:127–136. doi: 10.1007/s00213-007-1015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries T.J., Shaham Y., Homberg J.R. A cannabinoid mechanism in relapse to cocaine seeking. Nat. Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- Diekhof E.K., Falkai P., Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res. Rev. 2008;59:164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Dinger M.C., Bader J.E., Kobor A.D., Kretzschmer A.K., Beck-Sickinger A.G. Homodimerization of neuropeptide Y receptors investigated by fluorescence residence energy transfer in living cells. J. Biol. Chem. 2003;278:10562–10571. doi: 10.1074/jbc.M205747200. [DOI] [PubMed] [Google Scholar]
- Dlugos A., Childs E., Stuhr K.L., Hillard C.J., de Wit H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology. 2012;37:2416–2427. doi: 10.1038/npp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domi E., Barbier E., Augier E. Preclinical evaluation of the kappa-opioid receptor antagonist CERC-501 as a candidate therapeutic for alcohol use disorders. Neuropsychopharmacology. 2018 Feb 5 doi: 10.1038/s41386-018-0015-y. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey J.C., Lane A.C., Roach A.G., Virdee N.K. Comparison of the alpha-adrenoceptor antagonist profiles of idazoxan (RX 781094), yohimbine, rauwolscine coynanthine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1984;325:136–144. doi: 10.1007/BF00506193. [DOI] [PubMed] [Google Scholar]
- Dunlop B.W. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol. Psychiatr. 2017;82(12):866–874. doi: 10.1016/j.biopsych.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A.J., Swiergiel A.H. The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationship between the two systems. Eur. J. Pharmacol. 2008;583:186–193. doi: 10.1016/j.ejphar.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner S.R., Roitman M.F., Potter D.N., Rachlin A.B., Chartoff E.H. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berlin) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D., Mattioli L., Cifani C. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology. 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- Economidou D., Mattioli L., Ubaldi M. Role of cannabinoidergic mechanisms in ethanol self-administration and ethanol seeking in rat adult offspring following perinatal exposure to delta9-tetrahydrocannabinol. Toxicol. Appl. Pharmacol. 2007;223:73–85. doi: 10.1016/j.taap.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Edwards R.R., Wasan A.D., Michna E., Greenbaum S., Ross E., Jamison R.N. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. J. Pain. 2011;12:953–963. doi: 10.1016/j.jpain.2011.02.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli R.E., Kash T.L., Choo K., Savchenko V., Matthews R.T., Blakely R.D., Winder D.G. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Erb S., Hitchott P.K., Rajabi H., Mueller D., Shaham Y., Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S., Shaham Y., Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Erb S., Shaham Y., Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J. Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana R.A., Schmeichel B.E., Berridge C.W. Norepinephrine at the nexus of arousal, motivation and relapse. Brain Res. 2016;1641:207–216. doi: 10.1016/j.brainres.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva C., Serra M., Mele P., Panzica G., Oberto A. Physiology and gene regulation of the brain NPY Y1 receptor. Front. Neuroendocrinol. 2006;27:308–339. doi: 10.1016/j.yfrne.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Falcon E., Maier K., Robinson S.A., Hill-Smith T.E., Lucki I. Effects of buprenorphine on behavioral tests for antidepressant and anxiolytic drugs in mice. Psychopharmacology. 2015;232:907–915. doi: 10.1007/s00213-014-3723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein M.W., Ghee S.M., See R.E. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein M.W., Henderson A.R., See R.E. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and role of the estrous cycle. Psychopharmacology (Berlin) 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein M.W., See R.E. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav. Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Feuerecker M., Hauer D., Gresset T. Effect of an acute consumption of a moderate amount of ethanol on plasma endocannabinoid levels in humans. Alcohol Alcohol. 2012;47:226–232. doi: 10.1093/alcalc/agr162. [DOI] [PubMed] [Google Scholar]
- Figlewicz D.P., Hill S.R., Jay J.L., West C.H., Zavosh A.S., Sipols A.J. Effect of recurrent yohimbine on immediate and post-hoc behaviors, stress hormones, and energy homeostatic parameters. Physiol. Behav. 2014;129:186–193. doi: 10.1016/j.physbeh.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filho C.B., Del Fabbro L., de Gomes M.G., Goes A.T., Souza L.C., Boeira S.P., Jesse C.R. Kappa-opioid receptors mediate the antidepressant-like activity of hesperidin in the mouse forced swimming test. Eur. J. Pharmacol. 2013;698:286–291. doi: 10.1016/j.ejphar.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.J. Elevated norepinephrine may be a unifying etiological factor in the abuse of a broad range of substances: alcohol, nicotine, marijuana, heroin, cocaine and caffeine. Subst. Abuse Res. Treat. 2013;7:171–183. doi: 10.4137/SART.S13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.J., Rizos Z., Sinyard J., Tampakeras M., Higgins G.A. The 5-HT2C receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Forray M.I., Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res. Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Fox H.C., Seo D., Tuit K., Hansen J., Kimmerling A., Morgan P.T., Sinha R. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J. Psychopharmacol. 2012;26:958–972. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.C., Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv. Rev. Psychiatr. 2009;17:103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.C., Sofuoglu M., Sinha R. Guanfacine enhances inhibitory control and attentional set shifting in early abstinent cocaine-dependent individuals. J. Psychopharmacol. 2015;29:312–323. doi: 10.1177/0269881114562464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A., Ahs F., Linnman C. Increased neurokinin-1 receptor availability in the amygdala in social anxiety disorder: a positron emission tomography study with [11C]-GR205171. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D., Coen K., Lê A.D. The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain Behav. 2014;4:356–367. doi: 10.1002/brb3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D., Coen K., Tamadon S., Li Z., Loughlin A., Lê A.D. Effects of prazosin and doxazosin on yohimbine-induced reinstatement of alcohol seeking in rats. Psychopharmacology. 2016;233:2197–2207. doi: 10.1007/s00213-016-4273-2. [DOI] [PubMed] [Google Scholar]
- Funk D., Harding S., Juzytsch W., Lê A.D. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology. 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Gao M., Wang M., Zheng Q.H. Synthesis of [(11)C]MK-1064 as a new PET radioligand for imaging of orexin-2 receptor. Bioorg. Med. Chem. Lett. 2016;26:3694–3699. doi: 10.1016/j.bmcl.2016.05.083. [DOI] [PubMed] [Google Scholar]
- Gass J.T., Olive F. Reinstatement of ethanol-seeking behavior following intravenous self-administration in Wistar rats. Alcohol Clin. Exp. Res. 2007;31:1–5. doi: 10.1111/j.1530-0277.2007.00480.x. [DOI] [PubMed] [Google Scholar]
- Gehrke B.J., Chefer V.I., Shippenberg T.S. Effects of acute and repeated administration of salvinorin A on dopamine function in the rat dorsal striatum. Psychopharmacology (Berlin) 2008;197:509–517. doi: 10.1007/s00213-007-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D.T., Gilman J., Hersh J. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Ghitza U.E. Overlapping mechanisms of stress-induced relapse to opioid use disorder and chronic pain: clinical implications. Front. Psychiatr. 2016;7:80. doi: 10.3389/fpsyt.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders N.E. The impact of stress on addiction. Eur. Neuropsychopharmacol. 2003;13:435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Goldberg M.R., Robertson D. Yohimbine: a pharmacological probe for study of the α2-adrenoceptor. Pharmacol. Rev. 1983;35:143–180. [PubMed] [Google Scholar]
- Gómez-Coronado N., Walker A.J., Berk M., Dodd S. Current and emerging pharmacotherapies for cessation of tobacco smoking. Pharmacotherapy. 2018;38:235–258. doi: 10.1002/phar.2073. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cuevas G., Martin-Fardon R., Kerr T.M. Unique treatment potential of cannabidiol for the prevention of relapse to drug use: pre-clinical proof of principle. Neuropsychopharmacology. 2018 March 14 doi: 10.1038/s41386-018-0050-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L.R., Farrell M., Ali R.L., White J.M. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst. Rev. Mar. 2014;31(3) [Google Scholar]
- Gozzi A., Turrini G., Piccoli L. Functional magnetic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E.N., Hoks M.A., Baumgardner J. Adrenal activity during repeated long-access cocaine self-administration is required for later CRF-induced and CRF-dependent stressor-induced reinstatement in rats. Neuropsychopharmacology. 2011;36:1444–1454. doi: 10.1038/npp.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E.N., Wheeler R.A., Baker D.A. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J. Neurosci. 2013;33:11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziane N.M., Polter A.M., Briand L.A., Pierce R.C., Kauer J.A. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77:942–954. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald M.K., Burmeister M. Oral Presentation at the 2018 College on Problems of Drug Dependence Annual Meeting. 2018. Variants in stress-signaling genes NR3C1 and FKBP5 modulate opioid abstinence during buprenorphine (BUP) stabilization and dose tapering. [Google Scholar]
- Greenwald M.K., Comer S.D., Fiellin D.A. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1–11. doi: 10.1016/j.drugalcdep.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald M.K., Lundahl L.H., Steinmiller C.L. Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals. Psychopharmacology. 2013;225:811–824. doi: 10.1007/s00213-012-2868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Woodcock EA (in preparation) Dose-effects of Yohimbine And/or Hydrocortisone Pretreatment on Stress-reactivity and Opioid-seeking Behavior in Heroin-dependent, Buprenorphine-maintained Volunteers.
- Greenwald M.K., Woodcock E.A., Khatib D., Stanley J.A. Methadone maintenance dose modulates anterior cingulate glutamate levels in heroin-dependent individuals: a preliminary in vivo (1)H MRS study. Psychiatry Res. 2015;233:218–224. doi: 10.1016/j.pscychresns.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Greenwald M.K., Zohrob D., Steinmiller C.L., Sliwerska E., Burmeister M. Oral Presentation at the 2012 College on Problems of Drug Dependence Annual Meeting. 2012. Mu- and kappa-receptor single nucleotide polymorphisms (SNPs) are associated with opioid use phenotypes in heroin dependent volunteers. [Google Scholar]
- Grella S.L., Funk D., Coen K., Li Z., Lê A.D. Role of the kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats. Behav. Brain Res. 2014;265:188–197. doi: 10.1016/j.bbr.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Hale E., Lieberman L., Davis A., Pine D.S., Ernst M. The CRH1 antagonist GSK561679 increases human fear but not anxiety as assessed by startle. Neuropsychopharmacology. 2015;40:1064–1071. doi: 10.1038/npp.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood S., Lu Y., Schmidt A.W. Pharmacological characterization of 2-methyl-N-((2'-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine (PF-04455242), a high-affinity antagonist selective for κ-opioid receptors. J. Pharmacol. Exp. Therapeut. 2011;339:555–566. doi: 10.1124/jpet.111.185108. [DOI] [PubMed] [Google Scholar]
- Groeneweg F.L., Karst H., de Kloet E.R., Jöels M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Grunhaus L., Tiongco D., Zelnik T., Flegel P., Hollingsworth P.J., Smith C.B. Intravenous yohimbine. Selective enhancer of norepinephrine and cortisol secretion and systolic blood pressure in humans. Clin. Neuropharmacol. 1989;12:106–114. doi: 10.1097/00002826-198904000-00004. [DOI] [PubMed] [Google Scholar]
- Gueye A.B., Pryslawsky Y., Trigo J.M. The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss with better psychiatric tolerability. Int. J. Neuropsychopharmacol. 2016;19:1–11. doi: 10.1093/ijnp/pyw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurguis G.N., Vitton B.J., Uhde T.W. Behavioral, sympathetic and adrenocortical responses to yohimbine in panic disorder patients and normal controls. Psychiatr. Res. 1997;71:27–39. doi: 10.1016/s0165-1781(97)00041-3. [DOI] [PubMed] [Google Scholar]
- Haack A.K., Sheth C., Schwager A.L., Sinclair M.S., Tandon S., Taha S.A. Lesions of the lateral habenula increase voluntary ethanol consumption in operant self-administration, block yohimbine induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler C.L., Goodyear K., Zywiak W.H. Higher pretreatment blood pressure is associated with greater alcohol drinking reduction in alcohol-dependent individuals treated with doxazosin. Drug Alcohol Depend. 2017;177:23–28. doi: 10.1016/j.drugalcdep.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J., Marion M., Figueiredo A. Fatty acid binding protein deletion prevents stress-induced preference for cocaine and dampens stress-induced corticosterone levels. Synapse. 2018 Feb 18 doi: 10.1002/syn.22031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M., Hart C.L., Vosburg S.K., Comer S.D., Reed S.C., Foltin R.W. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berlin) 2008;197:157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson A.C., Cippitelli A., Sommer W.H. Variation at the rat CRHR1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G.C., Wimmer M., Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Heilig M., Koob G.F., Ekman R., Britton K.T. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heinrichs M., Domes G. Neuropeptides and social behavior: effects of oxytocin and vasopressin in humans. Prog. Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Cullinan W.E. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenal axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hester R., Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J. Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfield D., Yap J., Grimm J.W., Shalev U., Shaham Y. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–331. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Hipólito L., Wilson-Poe A., Campos-Jurado Y. Inflammatory pain promotes increased opioid self-administration: role of dysregulated ventral tegmental area μ opioid receptors. J. Neurosci. 2015;35:12217–12231. doi: 10.1523/JNEUROSCI.1053-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood S.E., Stamford J.A. Noradrenergic modulation of serotonin release in rat dorsal and median raphe nuclei via α1- and α2-adrenoreceptors. Neuropharmacology. 2001;41:433–442. doi: 10.1016/s0028-3908(01)00087-9. [DOI] [PubMed] [Google Scholar]
- Hudson A., Stamp J.A. Ovarian hormones and propensity to drug relapse: a review. Neurosci. Biobehav. Rev. 2011;35:427–436. doi: 10.1016/j.neubiorev.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Hyman S.M., Fox H., Hong K.I., Doebrick C., Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp. Clin. Psychopharmacol. 2007;15:134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S.M., Hong K.-I., Chaplin T.M., Dabre Z., Comegys A.D., Kimmerling A., Sinha R. A stress-coping profile of opioid dependent individuals entering naltrexone treatment: a comparison with healthy controls. Psychol. Addict. Behav. 2009;23:613–619. doi: 10.1037/a0017324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbe H., Iwai-Liao Y., Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front. Biosci. 2006;11:2179–2192. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- Izzo A.A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. TIPS (Trends Pharmacol. Sci.) 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Jackson K.J., McLaughlin J.P., Carroll F.I., Damaj M.I. Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology (Berlin) 2013;226:763–768. doi: 10.1007/s00213-012-2716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings E.M., Okine B.N., Roche M., Finn D.P. Stress-induced hyperalgesia. Prog. Neurobiol. 2014;121:1–18. doi: 10.1016/j.pneurobio.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Jobes M.L., Ghitza U., Epstein D.H., Phillips K.A., Heishman S.J., Preston K.L. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology (Berlin) 2011;218:83–88. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöels M., Baram T.Z. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A., Greenwood-Van Meerveld B. Stress-induced pain: a target for the development of novel therapeutics. J. Pharmacol. Exp. Therapeut. 2014;351:327–335. doi: 10.1124/jpet.114.218065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucaite A., Cselényi Z., Lappalainen J. GABAA receptor occupancy by subtype selective GABAα2,3 modulators: PET studies in humans. Psychopharmacology (Berlin) 2017;234:707–716. doi: 10.1007/s00213-016-4506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kågedal M., Cselényi Z., Nyberg S. Positron emission tomography study in healthy volunteers to estimate mGluR5 receptor occupancy of AZD2066 – estimating occupancy in the absence of a reference region. Neuroimage. 2013;82:160–169. doi: 10.1016/j.neuroimage.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Kakko J., von Wachenfeldt J., Svanborg K.D., Lidström J., Barr C.S., Heilig M. Mood and neuro-endocrine response to a chemical stressor, metyrapone, in buprenorphine-maintained heroin dependence. Biol. Psychiatr. 2008;63:172–177. doi: 10.1016/j.biopsych.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Kalivas P.W., Volkow N.D. New medications for drug addiction hiding in glutamatergic plasticity. Mol. Psychiatr. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M., Cannella N., Economidou D. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19567–19572. doi: 10.1073/pnas.1004100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K.A., McQuaid J., Primich C., Rosenlicht N. An evidence-based review of insomnia treatment in early recovery. J. Addiction Med. 2014;8:389–394. doi: 10.1097/ADM.0000000000000052. [DOI] [PubMed] [Google Scholar]
- Karimi S., Attarzadeh-Yazdi G., Yazdi-Ravandi S., Hesam S., Azizi P., Razavi Y., Haghparast A. Forced swim but not exogenous corticosterone could induce the reinstatement of extinguished morphine conditioned place preference in rats: the Involvement of glucocorticoid receptors in the basolateral amygdala. Behav. Brain Res. 2014;264:43–50. doi: 10.1016/j.bbr.2014.01.045. [DOI] [PubMed] [Google Scholar]
- Karp J.F., Butters A., Begley A. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in mid-life and older adults. J. Clin. Psychiatr. 2014;75:e785–793. doi: 10.4088/JCP.13m08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin A.J., Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J. Pharmacol. Exp. Therapeut. 1999;289:219–223. [PubMed] [Google Scholar]
- Kastman H.E., Blasiak A., Walker L., Siwiec M., Krstew E.V., Gundlach A.L., Lawrence A.J. Nucleus incertus orexin2 receptors mediate alcohol seeking in rats. Neuropharmacology. 2016;110:82–91. doi: 10.1016/j.neuropharm.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Kathman M., Flau K., Redmer A., Trankle C., Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2006;372:354–361. doi: 10.1007/s00210-006-0033-x. [DOI] [PubMed] [Google Scholar]
- Keech B., Crowe S., Hocking D.R. Intranasal oxytocin, social cognition and neurodevelopmental disorders: a meta-analysis. Psychoneuroendocrinology. 2018;87:9–19. doi: 10.1016/j.psyneuen.2017.09.022. [DOI] [PubMed] [Google Scholar]
- Kenny P.J., Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol. Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- King P.J., Widdowson P.S., Doods H.N., Williams G. Regulation of neuropeptide Y release by neuropeptide Y receptor ligands and calcium channel antagonists in hypothalamic slices. J. Neurochem. 1999;73:641–646. doi: 10.1046/j.1471-4159.1999.0730641.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Radulovic J., Radulovic M. Deletion of CRHR2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Kohut S.J., Fivel P.A., Mello N.K. Differential effects of acute and chronic treatment with the α2-adrenergic agonist, lofexidine, on cocaine self-administration in rhesus monkeys. Drug Alcohol Depend. 2013;133:593–599. doi: 10.1016/j.drugalcdep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. The dark side of emotion: the addiction perspective. Eur. J. Pharmacol. 2015;753:73–87. doi: 10.1016/j.ejphar.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T.R., Oliveto A., Sevarino K.A., Gonsai K., Feingold A. Ketoconazole increases cocaine and opioid use in methadone maintained patients. Drug Alcohol Depend. 2002;66:173–180. doi: 10.1016/s0376-8716(01)00198-3. [DOI] [PubMed] [Google Scholar]
- Kotlyar M., Brauer L.H., al'Absi M. Effect of bupropion on physiological measures of stress in smokers during nicotine withdrawal. Pharmacol. Biochem. Behav. 2006;83:370–379. doi: 10.1016/j.pbb.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Kowalczyk W.J., Phillips K.A., Jobes M.L. Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: a randomized controlled trial with ecological momentary assessment. Am. J. Psychiatr. 2015;172:760–767. doi: 10.1176/appi.ajp.2014.14081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek M.J., Ragunath J., Plevy S., Hamer D., Schneider B., Hartman N. ACTH, cortisol and beta-endorphin response to metyrapone testing during chronic methadone maintenance treatment in humans. Neuropeptides. 1984;5:277–278. doi: 10.1016/0143-4179(84)90081-7. [DOI] [PubMed] [Google Scholar]
- Kreibich A., Reyes B.A.S., Curtis A.L. Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J. Neurosci. 2008;28:6516–6525. doi: 10.1523/JNEUROSCI.0390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E., Zvartau E., Blokhina E. Naltrexone with or without guanfacine for preventing relapse to opiate addiction in St. Petersburg, Russia. Drug Alcohol Depend. 2013;132:674–680. doi: 10.1016/j.drugalcdep.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Asano E., Chugani H.T. α-[11C]-methyl-L-tryptophan PET for tracer localization of eptileptogenic brain regions: clinical studies. Biomarkers Med. 2011;5:577–584. doi: 10.2217/bmm.11.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J.S.D., Mann J.J. PET tracers for serotonin receptors and their applications. Cent. Nerv. Syst. Agents Med. Chem. 2014;14:96–112. doi: 10.2174/1871524914666141030124316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt D.A., Klas P.G., Erb S. Cannabinoid CB1 receptors mediate the effects of corticotropin-releasing factor on the reinstatement of cocaine seeking and expression of cocaine induced behavioural sensitization. Br. J. Pharmacol. 2012;167:196–206. doi: 10.1111/j.1476-5381.2012.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt D.A., Tribe E., Erb S. Effects of repeated yohimbine on the extinction and reinstatement of cocaine seeking. Pharmacol. Biochem. Behav. 2009;91:473–480. doi: 10.1016/j.pbb.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Kuzmin A., Semenova S., Zvartau E.E., Van Ree J.M. Enhancement of morphine self-administration in drug naive, inbred strains of mice by acute emotional stress. Eur. Neuropsychopharmacol. 1996;6:63–68. doi: 10.1016/0924-977x(95)00066-x. [DOI] [PubMed] [Google Scholar]
- Kwako L.E., George D.T., Schwandt M.L. The neurokinin-1 receptor antagonist aprepitant in co-morbid alcohol dependence and posttraumatic stress disorder: a human experimental study. Psychopharmacology (Berlin) 2015;232:295–304. doi: 10.1007/s00213-014-3665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako L.E., Spagnolo P.A., Schwandt M.L. The corticotropin releasing hormone 1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40:1053–1063. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B.B., Bruchas M.R., Lemos J.C., Xu M., Melief E.J., Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B.B., Bruchas M.R., Schattauer S. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc. Natl. Acad. Sci. U.S.A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A.D., Funk D., Coen K., Li Z., Shaham Y. Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats. Addiction Biol. 2013;18:448–451. doi: 10.1111/j.1369-1600.2011.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A.D., Funk D., Coen K., Tamadon S., Shaham Y. Role of κ-opioid receptors in the bed nucleus of stria terminalis in reinstatement of alcohol seeking. Neuropsychopharmacology. 2018;43:838–850. doi: 10.1038/npp.2017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A.D., Funk D., Harding S., Juzytsch W., Fletcher P.J. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology. 2009;204:477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A.D., Funk D., Harding S., Juzytsch W., Fletcher P.J., Shaham Y. Effects of dexfenfluramine and 5-HT3 receptor antagonists on stress-induced reinstatement of alcohol seeking in rats. Psychopharmacology. 2006;186:82–92. doi: 10.1007/s00213-006-0346-y. [DOI] [PubMed] [Google Scholar]
- Lê A.D., Funk D., Harding S., Juzytsch W., Li Z., Fletcher P.J. Intra-median raphe nucleus (MRN) infusions of muscimol, a GABA-A receptor agonist, reinstate alcohol seeking in rats: role of impulsivity and reward. Psychopharmacology. 2008;195:605–615. doi: 10.1007/s00213-007-0943-4. [DOI] [PubMed] [Google Scholar]
- Lê A.D., Funk D., Juzytsch W., Coen K., Navarre B.M., Cifani C., Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A.D., Harding S., Juzytsch W., Fletcher P.J., Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J. Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A.D., Harding S., Juzytsch W., Funk D., Shaham Y. Role of alpha-2 adrenoreceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Lê A.D., Harding S., Juzytsch W., Watchus J., Shalev U., Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berlin) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lê A.D., Poulos C.X., Harding S., Watchus W., Juzytsch W., Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress in rats. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Lê A.D., Quan B., Juzytsch W., Fletcher P.J., Joharchi N., Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Lee B., Teifenbacher S., Platt D.M., Spealman R.D. Pharmacological blockade of α2-adrenoreceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Lejuez C., Zvolensky M.J., Daughters S.B. Anxiety sensitivity: a unique predictor of dropout among inner-city heroin and crack/cocaine users in residential substance use treatment. Behav. Res. Ther. 2008;46:811–818. doi: 10.1016/j.brat.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S.K., Dwyer J.M., Sukoff Rizzo S.J. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berlin) 2008;197:601–611. doi: 10.1007/s00213-008-1080-4. [DOI] [PubMed] [Google Scholar]
- Leri F., Flores J., Rodaros D., Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J. Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F., Tremblay A., Sorge R.E., Stewart J. Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacology. 2004;29:1312–1320. doi: 10.1038/sj.npp.1300435. [DOI] [PubMed] [Google Scholar]
- Levin F.R., Mariani J.J., Pavlicova M. Dronabinol and lofexidine for cannabis use disorder: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2016;159:53–60. doi: 10.1016/j.drugalcdep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Cai Z., Zheng M.Q. Novel 18F-labeled κ-opioid receptor antagonist as PET radiotracer: synthesis and in vivo evaluation of 18F-LY2459989 in nonhuman primates. J. Nucl. Med. 2018;59:140–146. doi: 10.2967/jnumed.117.195586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M., Hu K., Swann A. Stress modulates illness-course of substance use disorders: a translational review. Front. Psychiatr. 2014;5:1–20. doi: 10.3389/fpsyt.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A.R., Wilson S.J., Cunningham V.J., Feeney A., Stevenson B., Brooks D.J., Nutt D.J. GABA-benzodiazepine receptor function in alcohol dependence: a combined 11C-flumazenil PET and pharmacodynamic study. Psychopharmacology (Berlin) 2005;180:595–606. doi: 10.1007/s00213-005-2271-x. [DOI] [PubMed] [Google Scholar]
- Lister J.J., Ledgerwood D.M., Lundahl L.H., Greenwald M.K. Causal pathways between impulsivity, cocaine use consequences, and depression. Addict. Behav. 2015;41:1–6. doi: 10.1016/j.addbeh.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J. Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohith T.G., Zoghbi S.S., Morse C.L. Brain and whole-body imaging of nociceptor/orphanin FQ peptide receptor in humans using the PET ligand 11C-NOP-1A. J. Nucl. Med. 2012;53:385–392. doi: 10.2967/jnumed.111.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J.F., Akil H., Watson S.J. Neural circuits mediating stress. Biol. Psychiatr. 1999;46:1461–1471. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- Lynch W.J., Mangini L.D., Taylor J.R. Neonatal isolation potentiates cocaine seeking behavior in adult male and female rats. Neuropsychopharmacology. 2005;30:322–329. doi: 10.1038/sj.npp.1300594. [DOI] [PubMed] [Google Scholar]
- Ma C.L., Qi X.L., Peng J.Y., Li B.M. Selective deficit in no-go performance induced by blockade of prefrontal cortical alpha 2-adrenoceptors in monkeys. Neuroreport. 2003;14:1013–1016. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- Madar I., Lever J.R., Kinter C.M. Imaging of delta opioid receptors in human brain by N1’-([11C]methyl)naltrindole and PET. Synapse. 1996;24:19–28. doi: 10.1002/(SICI)1098-2396(199609)24:1<19::AID-SYN3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- McFarland K., Davidge S.B., Lapish C.C., Kalivas P.W. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci. Biobehav. Rev. 1997;21:91–104. doi: 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Mantsch J.R., Baker D.A., Francis D.M., Katz E.S., Hoks M.A., Serge J.P. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long access cocaine self-administration by rats. Psychopharmacology. 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch J.R., Baker D.A., Funk D., Lê A.D., Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch J.R., Weyer A., Vranjkovic O., Beyer C.E., Baker D.A., Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch J.R., Goeders N.E. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology. 1999;142:399–407. doi: 10.1007/s002130050905. [DOI] [PubMed] [Google Scholar]
- Manvich D.F., Stowe T.A., Godfrey J.R., Weinshenker D. A method for psychosocial stress-induced reinstatement of cocaine seeking in rats. Biol. Psychiatr. 2016;79:940–946. doi: 10.1016/j.biopsych.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli P.W., Funk D., Juzytsch W., Harding S., Rice K.C., Shaham Y., Lê A.D. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berlin) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Martinez Botella G., Salituro F.G., Harrison B.L. Neuroactive steroids. 2. 3α-hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1’-yl)-19-nor-5β-pregnan-20-one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (γ-aminobutyric acid)A receptor. J. Med. Chem. 2017;60:7810–7819. doi: 10.1021/acs.jmedchem.7b00846. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R., Ciccocioppo R., Massi M., Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R., Weiss F. 2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid (LY379268) and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine (MTEP) similarly attenuate stress-induced reinstatement of cocaine seeking. Addiction Biol. 2012;17:557–564. doi: 10.1111/j.1369-1600.2011.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason G.F., Petrakis I.L., de Graaf R.A. Cortical gamma-aminobutyric acid levels in the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol. Psychiatr. 2006;59:85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Matsunaga T., Tsukada H., Nishiyama S., Sekine Y., Kakiuchi T., Iyo M., Mori N. Yohimbine increases the binding potential for [11C]flumazenil in the monkey brain. J. Neural. Transm. 2001;108:1375–1382. doi: 10.1007/s007020100014. [DOI] [PubMed] [Google Scholar]
- Mattila M., Seppala T., Mattila M.J. Anxiogenic effect of yohimbine in healthy subjects: comparison with caffeine and antagonism by clonidine and diazepam. Int. Clin. Psychopharmacol. 1988;3:215–229. doi: 10.1097/00004850-198807000-00003. [DOI] [PubMed] [Google Scholar]
- Maura G., Gemignani A., Raiter M. Noradrenaline inhibits central serotonin release through alpha2-adrenoceptors located on serotonergic nerve terminals. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1982;320:272–274. doi: 10.1007/BF00510140. [DOI] [PubMed] [Google Scholar]
- McCall R.B., Harris L.T., King K.A. Sympatholytic action of yohimbine mediated by 5-HT1A receptors. Eur. J. Pharmacol. 1991;199:263–265. doi: 10.1016/0014-2999(91)90468-6. [DOI] [PubMed] [Google Scholar]
- McDougle C.J., Black J.E., Malison R.T. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch. Gen. Psychiatr. 1994;51:713–719. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- McKee S.A., Potenza M.N., Kober H. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J. Psychopharmacol. 2015;29:300–311. doi: 10.1177/0269881114562091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J.P., Land B.B., Li S., Pintar J.E., Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J.P., Li S., Valdez J., Chavkin T., Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J.M., Duncan M., Di Marzo V., Pertwee R.G. Are cannabidiol and Δ9-tetrahydro-cannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015;172:737–753. doi: 10.1111/bph.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J.R., Doncheck E.M., Li Y. Stress promotes drug seeking through glucocorticoid-dependent endocannabinoid mobilization in the prelimbic cortex. Biol. Psychiatr. 2017 Oct 6 doi: 10.1016/j.biopsych.2017.09.024. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J.R., Doncheck E.M., Vranjkovic O. CB1 receptor antagonism blocks stress-potentiated reinstatement of cocaine seeking in rats. Psychopharmacology. 2016;233:99–109. doi: 10.1007/s00213-015-4092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J.R., Taylor, Vranjkovic O. Corticosterone potentiation of cocaine-induced reinstatement of conditioned place preference in mice is mediated by blockade of the organic cation transporter 3. Neuropsychopharmacology. 2017;42:757–765. doi: 10.1038/npp.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J.R., Vranjkovic O., Thao M., Baker D.A., Makky K., Lim Y., Mantsch J.R. Beta-2 adrenergic receptors mediate stress-evoked reinstatement of cocaine-induced conditioned place preference and increases in CRF mRNA in the bed nucleus of the stria terminalis in mice. Psychopharmacology. 2014;231:3953–3963. doi: 10.1007/s00213-014-3535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S., Quan W., Qi X., Su Z., Yang S. Effect of baclofen on morphine-induced conditioned place preference, extinction, and stress-induced reinstatement in chronically stressed mice. Psychopharmacology. 2014;231:27–36. doi: 10.1007/s00213-013-3204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Domes G., Kirsch P., Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mijangos-Moreno S., Poot-Aké A., Arankowsky-Sandoval G., Murillo-Rodríguez E. Intrahypothalamic injection of cannabidiol increases the extracellular levels of adenosine in nucleus accumbens in rats. Neurosci. Res. 2014;84:60–63. doi: 10.1016/j.neures.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Millan M.J., Newman-Tancredi A., Audinot V. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (ARs), serotonin (5-HT)1A, 5-HT1B, 5-HT1D and dopamine D2 and D3 receptors: significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Minhas M., Leri F. The effect of heroin dependence on resumption of heroin self-administration in rats. Drug Alcohol Depend. 2014;138:24–31. doi: 10.1016/j.drugalcdep.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Molander A., Vengeliene V., Heilig M., Wurst W., Deussing J.M., Spanagel R. Brain-specific inactivation of the CRHR1 gene inhibits post-dependent and stress-induced alcohol intake does not affect relapse-like drinking. Neuropsychopharmacology. 2012;37:1047–1056. doi: 10.1038/npp.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeau R., Blier P., de Montigny C. In vivo electrophysiological evidence for tonic activation by endogenous noradrenaline of α2-adrenoreceptors on 5-hydroxytryptamine terminals in the rat hippocampus. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1993;347:266–272. doi: 10.1007/BF00167444. [DOI] [PubMed] [Google Scholar]
- Moran-Santa Maria M.M., Baker N.L., Ramakrishnan V., Brady K.T., McRae-Clark A. Impact of acute guanfacine administration on stress and cue reactivity in cocaine-dependent individuals. Am. J. Drug Alcohol Abuse. 2015;41:146–152. doi: 10.3109/00952990.2014.945590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria M.M., McRae-Clark A., Baker N., Ramakrishnan V., Brady K.T. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology. 2014;213:4157–4165. doi: 10.1007/s00213-014-3555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyk A.J., Fowler J.A., Norwood D.K., Chilipko A. Role of α2-agonists in the treatment of acute alcohol withdrawal. Ann. Pharmacother. 2011;45:649–657. doi: 10.1345/aph.1P575. [DOI] [PubMed] [Google Scholar]
- Naganawa M., Zheng M.Q., Nabulsi N. Kinetic modeling of (11)C-LY2795050, a novel antagonist radiotracer for PET imaging of the kappa opioid receptor in humans. J. Cerebr. Blood Flow Metabol. 2014;34:1818–1825. doi: 10.1038/jcbfm.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naja W.J., Aoun M.P. Oxytocin and anxiety disorders: translational and therapeutic aspects. Curr. Psychiatr. Rep. 2017;19:67. doi: 10.1007/s11920-017-0819-1. [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K., McMahon T.P., Chacko T.P. The structure of common emotion regulation strategies: a meta-analytic examination. Psychol. Bull. 2017;143:384–427. doi: 10.1037/bul0000093. [DOI] [PubMed] [Google Scholar]
- Narendran R., Ciccocioppo R., Lopresti B., Paris J., Himes M.L., Mason N.S. Nociceptin receptors in alcohol use disorders: as positron emission tomography study using [11C]NOP-1A. Biol. Psychiatr. 2017 May 31 doi: 10.1016/j.biopsych.2017.05.019. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser A.F., Heidbreder C., Gomeni R., Fudala P.J., Zheng B., Greenwald M.K. A population pharmacokinetic and pharmacodynamic modeling approach to support the clinical development of RBP-6000, a new, subcutaneously injectable, long-acting, sustained-release formulation of buprenorphine, for the treatment of opioid dependence. Clin. Pharmacokinet. 2014;53:813–824. doi: 10.1007/s40262-014-0155-0. [DOI] [PubMed] [Google Scholar]
- Nasser A.F., Greenwald M.K., Vince B. Sustained-release buprenorphine (RBP-6000) blocks the effects of opioid challenge with hydromorphone in subjects with opioid use disorder. J. Clin. Psychopharmacol. 2016;36:18–26. doi: 10.1097/JCP.0000000000000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I.D., Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Nielsen C.K., Simms J.A., Bito-Onon J.J., Li R., Ananthan S., Bartlett S.E. The delta opioid receptor antagonist, SoRI-9409, decreases yohimbine stress-induced reinstatement of ethanol-seeking. Addiction Biol. 2012;17:224–234. doi: 10.1111/j.1369-1600.2010.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normandin M.D., Zheng M.Q., Lin K.S. Imaging the cannabinoid CB1 receptor in humans with [11C]OMAR: assessment of kinetic analysis methods, test-retest reproducibility, and gender differences. J. Cerebr. Blood Flow Metabol. 2015;35:1313–1322. doi: 10.1038/jcbfm.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhuis P.W., Gastpar M., Scherbaum N. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. J. Clin. Psychopharmacol. 2008;28:593–595. doi: 10.1097/JCP.0b013e31818638a4. [DOI] [PubMed] [Google Scholar]
- Nygard S.K., Hourguettes N.J., Sobczak G.G., Carlezon W.A., Bruchas M.R. Stress-induced reinstatement of nicotine preference requires dynorphin/kappa opioid activity in the basolateral amygdala. J. Neurosci. 2016;36:9937–9948. doi: 10.1523/JNEUROSCI.0953-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveto A., Sevarino K., McCance-Katz E., Benios T., Poling J., Feingold A. Clonidine and yohimbine in opioid-dependent humans responding under a naloxone novel-response discrimination procedure. Behav. Pharmacol. 2003;14:97–109. doi: 10.1097/00008877-200303000-00001. [DOI] [PubMed] [Google Scholar]
- Paneda C., Huitron-Resendiz S., Frago L.M. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin releasing factor receptor 1 in mice. J. Neurosci. 2009;29:4155–4161. doi: 10.1523/JNEUROSCI.5256-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh D., Hamid A., Friedman T.C., Nguyen K., Tseng A., Marquez P., Lutfy K. Stress-induced analgesia and endogenous opioid peptides: the importance of stress duration. Eur. J. Pharmacol. 2011;650:563–567. doi: 10.1016/j.ejphar.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor R., Reed C., Burkhart-Kasch S. Ethanol concentration-dependent effects and the role of stress on ethanol drinking in corticotropin-releasing factor type 1 and double type 1 and 2 receptor knockout mice. Psychopharmacology (Berlin) 2011;218:169–177. doi: 10.1007/s00213-011-2284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S., Johnston A.L., File S.E. Selective agonists and antagonists for 5-hydroxytryptamine receptor subtypes and interactions with yohimbine and FG7142 using the elevated plus-maze test in the rat. J. Pharm. Pharmacol. 1987;39:917–928. doi: 10.1111/j.2042-7158.1987.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Pelloux Y., Everitt B.J., Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Pertwee R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahyrocannabidiol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitet F., Jeantaud B., Reibaud M., Imperato A., Dubroeucq M.C. Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of delta9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998;63:PL1–6. doi: 10.1016/s0024-3205(98)00238-0. [DOI] [PubMed] [Google Scholar]
- Pich E.M., Melotto S. Orexin 1 receptor antagonists in compulsive behavior and anxiety: possible therapeutic use. Front. Neurosci. 2014;8:26. doi: 10.3389/fnins.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A., Maldonado R., Berrendero F. The hypocretin/orexin system: implications for drug reward and relapse. Mol. Neurobiol. 2012;45:424–439. doi: 10.1007/s12035-012-8255-z. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A., Martín-García E., de Lecea L., Maldonado R., Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J. Neurosci. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter A.M., Bishop R.A., Briand L.A., Graziane N.M., Pierce R.C., Kauer J.A. Poststress block of kappa opioid receptors rescues long-term potentiation of inhibitory synapses and prevents reinstatement of cocaine seeking. Biol. Psychiatr. 2014;76:785–793. doi: 10.1016/j.biopsych.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze M.B., Fetterly T.L., Winder D.G., Messing R.O. The corticotropin releasing factor receptor 1 in alcohol use disorder: still a valid drug target? Alcohol Clin. Exp. Res. 2017;41:1986–1999. doi: 10.1111/acer.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postnov A., Schmidt M.E., Pemberton D.J. Fatty acid amide hydrolase inhibition by JNJ-42165279: a multiple-ascending dose and a positron emission tomography study in healthy volunteers. Clin. Transl. Sci. 2018 Mar 25 doi: 10.1111/cts.12548. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi K., Wei C., Li Y., Sui N. Orexin receptors within the nucleus accumbens shell mediate the stress but not drug priming-induced reinstatement of morphine conditioned place preference. Front. Behav. Neurosci. 2013;144:1. doi: 10.3389/fnbeh.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana D.S., Dieset I., Elvsåshagen T., Westlye L.T., Andreassen O.A. Oxytocin system dysfunction as a common mechanism underlying metabolic syndrome and psychiatric symptoms in schizophrenia and bipolar disorders. Front. Neuroendocrinol. 2017;45:1–10. doi: 10.1016/j.yfrne.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Maura G., Folghera S. Modulation of 5-hydroxytryptamine release by presynaptic inhibitory α2-adrenoceptors in the human cerebral cortex. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1990;342:508–512. doi: 10.1007/BF00169037. [DOI] [PubMed] [Google Scholar]
- Ramos B.P., Arnsten A.F.T. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol. Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L.A., Lunny K., Bujarski S., Moallem N., Krull J.L., Miotto K. The effects of varenicline on stress-induced and cue-induced craving for cigarettes. Drug Alcohol Depend. 2013;131:136–142. doi: 10.1016/j.drugalcdep.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Redila V., Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berlin) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier P.S., Claxton A.B., Zlebnik N.E., Carroll M.E. Cocaine-, caffeine-, and stress-evoked cocaine reinstatement in high vs. low impulsive rats: treatment with allopregnanolone. Drug Alcohol Depend. 2014;143:58–64. doi: 10.1016/j.drugalcdep.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid H.H., Lundahl L.H., Lister J.J., Woodcock E.A., Greenwald M.K. Mediational pathways among trait impulsivity, heroin-use consequences, and current mood state. Addiction Res. Theor. 2018 doi: 10.1080/16066359.2018.1434513. In press [Epub 1-31-18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes B.A., Johnson A.D., Glaser J.D., Commons K.G., Van Bockstaele E.J. Dynorphin-containing axons directly innervate noradrenergic neurons in the rat nucleus locus coeruleus. Neuroscience. 2007;145:1077–1086. doi: 10.1016/j.neuroscience.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do Couto B., Aguilar M.A., Manzanedo C., Rodriguez-Arias M., Armario A., Minarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology. 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Richards J.K., Simms J.A., Bartlett S.E. Conditioned cues and yohimbine induce reinstatement of beer and near-beer seeking in Long-Evans rats. Addiction Biol. 2009;14:144–151. doi: 10.1111/j.1369-1600.2008.00139.x. [DOI] [PubMed] [Google Scholar]
- Richards J.K., Simms J.A., Steensland P., Taha S.A., Borgland S.L., Bonci A., Bartlett S.E. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridler K., Gunn R.N., Searle G.E. Characterising the plasma target occupancy relationship of the neurokinin antagonist GSK1144814 with PET. J. Psychopharmacol. 2014;28:244–253. doi: 10.1177/0269881113517953. [DOI] [PubMed] [Google Scholar]
- Riga D., Schmitz L.J.M., van der Harst J.E. A sustained depressive state promotes a guanfacine reversible susceptibility to alcohol seeking in rats. Neuropsychopharmacology. 2014;39:1115–1124. doi: 10.1038/npp.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough V.B., Hauger R.L., Roberts A.L., Vale W.W., Geyer M.A. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J. Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A., Vergura R., Marzola G. Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br. J. Pharmacol. 2008;154:471–479. doi: 10.1038/bjp.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T.W., Roberts A.C. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cerebr. Cortex. 2007;17(1):151–160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- Roehrs T.A., Hyde M., Blaisdell B., Greenwald M.K., Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- Roehrs T.A., Roth T. Sleep disturbance in substance use disorders. Psychiatr. Clin. 2015;38:793–803. doi: 10.1016/j.psc.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn L.M., Witkin J.M., Statnick M.A. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 2014;77:131–144. doi: 10.1016/j.neuropharm.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn L.M., Ciccocioppo R., Wong C.J. A novel, orally bioavailable nociceptin receptor antagonist, LY2940093, reduces ethanol self-administration and ethanol seeking in animal models. Alcohol Clin. Exp. Res. 2016;40:945–954. doi: 10.1111/acer.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M.I., Kosten T.R., Kreek M.J. The effects of naltrexone maintenance on the response to yohimbine in healthy volunteers. Biol. Psychiatr. 1999;45:1636–1645. doi: 10.1016/s0006-3223(98)00259-5. [DOI] [PubMed] [Google Scholar]
- Rusjan P.M., Wilson A.A., Mizrahi R. Mapping human brain fatty acid amide hydrolase activity with PET. J. Cerebr. Blood Flow Metabol. 2013;33:407–414. doi: 10.1038/jcbfm.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E.B., Burnett A., Hall B., Parker K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Ryan M.L., Falk D.E., Fertig J.B. A phase 2, double-blind, placebo-controlled randomized trial assessing the efficacy of ABT-436, a novel V1b receptor antagonist, for alcohol dependence. Neuropsychopharmacology. 2017;42:1012–1023. doi: 10.1038/npp.2016.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P.J., Kastman H.E., Krstew E.V. Relaxin-3/RXFP3 system regulates alcohol-seeking. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110:20789–20794. doi: 10.1073/pnas.1317807110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk T.J., Shekhar A., Gehlert D.R. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38:225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sanger D.J. The alpha 2-adrenoceptor antagonists idazoxan and yohimbine increase rates of DRL responding in rats. Psychopharmacology. 1988;95:413–417. doi: 10.1007/BF00181958. [DOI] [PubMed] [Google Scholar]
- Schank J.R., Goldstein A.L., Rowe K.E. The kappa opioid receptor antagonist JDTic attenuates alcohol seeking and withdrawal anxiety. Addiction Biol. 2012;17:634–647. doi: 10.1111/j.1369-1600.2012.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank J.R., Heilig M. Substance P and the neurokinin-1 receptor: the new CRF. Int. Rev. Neurobiol. 2017;136:151–175. doi: 10.1016/bs.irn.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Schank J.R., King C.E., Sun K. The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking. Neuropsychopharmacology. 2014;39:1093–1101. doi: 10.1038/npp.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank J.R., Nelson B.S., Damadzic R. Neurokinin-1 receptor antagonism attenuates neuronal activity triggered by stress-induced reinstatement of alcohol seeking. Neuropharmacology. 2015;99:106–114. doi: 10.1016/j.neuropharm.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank J.R., Pickens C.L., Rowe K.E. Stress-induced reinstatement of alcohol seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berlin) 2011;218:111–119. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler A.G., Messinger D.I., Smith J.S. Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral stratum to locally stimulate serotonin reuptake. J. Neurosci. 2012;32:17582–17592. doi: 10.1523/JNEUROSCI.3220-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers M.C., Schetters D., De Vries T.J., Pattij T. Differential effects of the pharmacological stressor yohimbine on impulsive decision making and response inhibition. Psychopharmacology. 2016;233:2775–2785. doi: 10.1007/s00213-016-4337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluger J.H., Borg L., Ho A., Kreek M.J. Altered HPA axis responsivity to metyrapone testing in methadone maintained former heroin addicts with ongoing cocaine addiction. Neuropsychopharmacology. 2001;24:568–575. doi: 10.1016/S0893-133X(00)00222-0. [DOI] [PubMed] [Google Scholar]
- Schluger J.H., Bart G., Green M., Ho A., Kreek M.J. Corticotropin releasing factor testing reveals a dose-dependent difference in methadone maintained versus control subjects. Neuropsychopharmacology. 2003;28:985–994. doi: 10.1038/sj.npp.1300156. [DOI] [PubMed] [Google Scholar]
- Schmeichel B.E., Herman M.A., Roberto M., Koob G.F. Hypocretin neurotransmission within the central amygdala mediates escalated cocaine self-administration and stress-induced reinstatement in rats. Biol. Psychiatr. 2017;81:606–615. doi: 10.1016/j.biopsych.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoutz C.D., Zhang Y., Runyon S.P., Goeders N.E. Antagonism of the neuropeptide S receptor with RTI-118 decreases cocaine self-administration and cocaine-seeking behavior in rats. Pharmacol. Biochem. Behav. 2012;103:332–337. doi: 10.1016/j.pbb.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J.P., Epps S.A., Grice T.W., Weinshenker D. The selective dopamine beta-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology. 2013;38:1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L., Tegenthoff M., Höffken O., Wolf O.T. Concurrent glucocorticoid and noradrenergic activity shifts instrumental behavior from goal-directed to habitual control. J. Neurosci. 2010;30:8190–8196. doi: 10.1523/JNEUROSCI.0734-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L., Tegenthoff M., Höffken O., Wolf O.T. Simultaneous glucocorticoid and noradrenergic activity disrupts the neural basis of goal-directed action in the human brain. J. Neurosci. 2012;32:10146–10155. doi: 10.1523/JNEUROSCI.1304-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt M.L., Cortes C.R., Kwako L.E. The CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effect. Neuropsychopharmacology. 2016;41:2818–2829. doi: 10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedki F., Eigenmann K., Gelinas J., Schouela N., Courchesne S., Shalev U. A role for kappa-, but not mu-opioid, receptor activation in acute food deprivation-induced reinstatement of heroin seeking in rats. Addiction Biol. 2015;20:423–432. doi: 10.1111/adb.12133. [DOI] [PubMed] [Google Scholar]
- See R.E., Waters R.P. Pharmacologically-induced stress: a cross-species probe for translational research in drug addiction and relapse. Am. J. Transl. Res. 2011;3:81–89. [PMC free article] [PubMed] [Google Scholar]
- Semba J., Wakuta M., Maeda J., Suhara T. Nicotine withdrawal induces subsensitivity of hypothalamic–pituitary–adrenal axis to stress in rats: implications for precipitation of depression during smoking cessation. Psychoneuroendocrinology. 2004;29:215–226. doi: 10.1016/s0306-4530(03)00024-6. [DOI] [PubMed] [Google Scholar]
- Shaham Y., de Wit H. Lost in translation: CRF1 receptor antagonists and addiction treatment. Neuropsychopharmacology. 2016;41:2795–2797. doi: 10.1038/npp.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., Erb S., Leung S., Buczek Y., Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berlin) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Erb S., Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Funk D., Erb S., Brown T.J., Walker C.-D., Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin seeking in rats. J. Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., Highfield D., Delfs J., Leung S., Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur. J. Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Klein L.C., Alvares K., Grunberg N.E. Effect of stress on oral fentanyl consumption in rats in an operant self-administration paradigm. Pharmacol. Biochem. Behav. 1993;46:315–322. doi: 10.1016/0091-3057(93)90359-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Rajabi H., Stewart J. Relapse to heroin-seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. J. Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology (Berlin) 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology. 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Shalev U., Erb S., Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U., Finnie P.S., Quinn T., Tobin S., Wahi P. A role for corticotropin-releasing factor, but not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats. Psychopharmacology. 2006;187:376–384. doi: 10.1007/s00213-006-0427-y. [DOI] [PubMed] [Google Scholar]
- Shalev U., Highfield D., Yap J., Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology. 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shalev U., Morales M., Hope B., Yap J., Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berlin) 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Sheth C., Furlong T.M., Keefe K.A., Taha S.A. The lateral hypothalamus to lateral habenula projection, but not the ventral pallidum to lateral habenula projection, regulates voluntary ethanol consumption. Behav. Brain Res. 2017;328:195–208. doi: 10.1016/j.bbr.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A.D., Wang Q., Winder D.G. α2A-Adrenergic receptors heterosynaptically regulate glutamate transmission in the BNST. Neuroscience. 2009;163:339–351. doi: 10.1016/j.neuroscience.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhpura N., Weiss F., Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonists MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol. Psychiatr. 2010;67:804–841. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.P., Carvalho A.O., Carvalho C.M., Malva J.O. Functional interaction between neuropeptide Y receptors and modulation of calcium channels in the rat hippocampus. Neuropharmacology. 2003;44:282–292. doi: 10.1016/s0028-3908(02)00382-9. [DOI] [PubMed] [Google Scholar]
- Simms J.A., Haass-Koffler C.L., Bito-Onon J., Li R., Bartlett S.E. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol seeking. Neuropsychopharmacology. 2012;37:906–918. doi: 10.1038/npp.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms J.A., Richards J.K., Mill D., Kanholm I., Holgate J.Y., Bartlett S.E. Induction of multiple reinstatements of ethanol- and sucrose-seeking behavior in Long-Evans rats by the α2 adrenoreceptor antagonist yohimbine. Psychopharmacology. 2011;218:101–110. doi: 10.1007/s00213-011-2451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Kimmerling A., Doebrick C., Kosten T.R. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rats: preliminary findings. Psychopharmacology. 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Sinha R., Shaham Y., Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berlin) 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.S., Zubieta J.K., Price J.C. Quantification of delta-opioid receptors in human brain with N1’-([11C]methyl) naltrindole and positron emission tomography. J. Cerebr. Blood Flow Metabol. 1999;19:956–966. doi: 10.1097/00004647-199909000-00003. [DOI] [PubMed] [Google Scholar]
- Smith R.J., Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct. Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.J., Laiks L.S. Behavioral and neural mechanisms underlying habitual and compulsive drug seeking. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017 doi: 10.1016/j.pnpbp.2017.09.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe G.A., Duncan M.W., Bradshaw J.E., Nicholson M.V. Effects of 6-methoxy-1,2,3,4-tetrahydro-beta-carboline and yohimbine on hypothalamic monoamine status and pituitary hormone release in the rat. Aust. J. Biol. Sci. 1983;36:379–386. doi: 10.1071/bi9830379. [DOI] [PubMed] [Google Scholar]
- Söderpalm A., Blomqvist O., Söderpalm B. The yohimbine-induced anticonflict effect in the rat, Part I. Involvement of noradrenergic, serotonergic and benzodiazepinergic mechanisms. J. Neural Transm. Gen. Sect. 1995;100:175–189. doi: 10.1007/BF01276457. [DOI] [PubMed] [Google Scholar]
- Söderpalm A., Ehrenström F., Söderpalm B. The yohimbine-induced anticonflict effect in the rat, Part II. Neurochemical findings. J. Neural Transm. Gen. Sect. 1995;100:191–206. doi: 10.1007/BF01276458. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M., Babb D., Hatsukami D.K. Labetalol treatment enhances the attenuation of tobacco withdrawal symptoms by nicotine in abstinent smokers. Nicotine Tob. Res. 2003;5:947–953. doi: 10.1080/14622200310001615312. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M., Sewell R.A. Norepinephrine and stimulant addition. Addiction Biol. 2009;14:119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R.E., Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology. 2005;183:210–217. doi: 10.1007/s00213-005-0160-y. [DOI] [PubMed] [Google Scholar]
- Spanagel R., Herz A., Shippenberg T.S. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R.E., Gomes S.M., Sypek E.I., Carey A.N., McLaughlin J.P. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berlin) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Spierling S.R., Zorrilla E.P. Don't stress about CRF: assessing the translational failures of CRF1 antagonists. Psychopharmacology. 2017;234:1467–1481. doi: 10.1007/s00213-017-4556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies M., Knudsen G.M., Lanzenberger R., Kasper S. The serotonin transporter in psychiatric disorders: insights from PET imaging. Lancet Psychiatr. 2015;2:743–755. doi: 10.1016/S2215-0366(15)00232-1. [DOI] [PubMed] [Google Scholar]
- Spinelli T., Calcagnile S., Giuliano C. Netupitant PET imaging and ADME studies in humans. J. Clin. Pharmacol. 2014;54:97–108. doi: 10.1002/jcph.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine S.M., Southwick S.M., Petrakis I.L., Kosten T.R., Charney D.S., Krystal J.H. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol. Psychiatr. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Stopponi S., de Guglielmo G., Somaini L. Activation of PPARγ by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol Clin. Exp. Res. 2013;37:1351–1360. doi: 10.1111/acer.12091. [DOI] [PubMed] [Google Scholar]
- Stopponi S., Somaini L., Cippitelli A. Activation of nuclear PPARγ receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol. Psychiatr. 2011;69:642–649. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Stopponi S., Somaini L., Cippitelli A. Pregabalin reduces alcohol drinking and relapse to alcohol seeking in the rat. Psychopharmacology (Berlin) 2012;220:87–96. doi: 10.1007/s00213-011-2457-3. [DOI] [PubMed] [Google Scholar]
- Stopponi S., Soverchia L., Ubaldi M., Cippitelli A., Serpelloni G., Ciccocioppo R. Chronic THC during adolescence increases the vulnerability to stress-induced relapse to heroin seeking in adult rats. Eur. Neuropsychopharmacol. 2014;24:1037–1045. doi: 10.1016/j.euroneuro.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Streeter C.C., Hennen J., Ke Y. Prefrontal GABA levels in cocaine-dependent subjects increase with pramipexole and venlafaxine treatment. Psychopharmacology (Berlin) 2005;182:516–526. doi: 10.1007/s00213-005-0121-5. [DOI] [PubMed] [Google Scholar]
- Strong D.R., Brown R.A., Sims M., Herman D.S., Anderson B.J., Stein M.D. Persistence on a stress-challenge task before initiating buprenorphine treatment was associated with successful transition from opioid use to early abstinence. J. Addiction Med. 2012;6:219–225. doi: 10.1097/ADM.0b013e31825d927f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J.M., Lim K., Labaree D. Kinetic analysis of the metabotropic glutamate subtype 5 tracer [(18)F]FPEB in bolus and bolus-plus-constant-infusion studies in humans. J. Cerebr. Blood Flow Metabol. 2013;33:532–541. doi: 10.1038/jcbfm.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Green T.A., Theobald D.E.H., Birnbaum S.G., Graham D.L., Zeeb F.D., Nestler E.J., Winstanley C.A. Yohimbine increases impulsivity through activation of cAMP response binding in the orbito-frontal cortex. Biol. Psychiatr. 2010;67:649–656. doi: 10.1016/j.biopsych.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalve N., Smethells J.R., Zlebnik N.E., Carroll M.E. Sex differences in reinstatement of cocaine-seeking with combination treatments of progesterone and atomoxetine. Pharmacol. Biochem. Behav. 2016;145:17–23. doi: 10.1016/j.pbb.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann A.C., Birnbaum D., Jagar A.A., Dougherty D.M., Moeller F.G. Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biol. Psychiatr. 2005;57:1209–1211. doi: 10.1016/j.biopsych.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Tartter M.A., Ray L.A. A prospective study of stress and alcohol craving in heavy drinkers. Pharmacol. Biochem. Behav. 2012;101:625–631. doi: 10.1016/j.pbb.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Terry G.E., Hirvonen J., Liow J.S. Biodistribution and dosimetry in humans of two inverse agonists to image cannabinoid CB1 receptors using positron emission tomography. Eur. J. Nucl. Med. Mol. Imag. 2010;37:1499–1506. doi: 10.1007/s00259-010-1411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoeringer C.K., Erhardt A., Sillaber I., Mueller M.B., Ohl F., Holsboer F., Keck M.E. Long-term anxiolytic and antidepressant-like behavioural effects of tiagabine, a selective GABA transporter-1 (GAT-1) inhibitor, coincide with a decrease in HPA system activity in C57BL/6 mice. J. Psychopharmacol. 2010;24:733–743. doi: 10.1177/0269881109103091. [DOI] [PubMed] [Google Scholar]
- Thomas A., Baillie G.L., Phillips A.M. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptors in vitro. Br. J. Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin S., Sedki F., Abbas Z., Shalev U. Antagonism of the dopamine D1-like receptor in mesocorticolimbic nuclei attenuates cute food deprivation-induced reinstatement of heroin seeking in rats. Eur. J. Neurosci. 2013;37:972–981. doi: 10.1111/ejn.12112. [DOI] [PubMed] [Google Scholar]
- Torregrossa M.M., Xie M., Taylor J.R. Chronic corticosterone exposure during adolescence reduces impulsive action but increases impulsive choice and sensitivity to yohimbine in male Sprague-Dawley rats. Neuropsychopharmacology. 2011;37:1656–1670. doi: 10.1038/npp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung L.-W., Lu G.-L., Lee Y.-H. Orexins contribute to restraint stress-induced cocaine relapse by endocannabinoid-mediated disinhibition of dopaminergic neurons. Nat. Commun. 2016;7:12199. doi: 10.1038/ncomms12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubaldi M., Giordano A., Severi I. Activation of hypocretin-1/orexin-A neurons projecting to the bed nucleus of the stria terminalis and paraventricular nucleus is critical for reinstatement of alcohol seeking by neuropeptide S. Biol. Psychiatr. 2016;79:452–462. doi: 10.1016/j.biopsych.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Umhau J.C., Momenan R., Schwandt M.L. Acamprosate suppresses magnetic resonance spectroscopy measures of central glutamate in detoxified alcoholics: a randomized controlled experimental medicine study. Arch. Gen. Psychiatr. 2010;67:1069–1077. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G.R., Koob G.F. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol. Biochem. Behav. 2004;79:671–689. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Valdez G.R., Platt D.M., Rowlett J.K., Rüedi-Bettschen D., Spealman R.D. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J. Pharmacol. Exp. Therapeut. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Valentino R.J., Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele E.J., Menko A.S., Drolet G. Neuroadaptive responses in brainstem noradrenergic nuclei following chronic morphine exposure. Mol. Neurobiol. 2001;23:155–171. doi: 10.1385/mn:23:2-3:155. [DOI] [PubMed] [Google Scholar]
- Vanini G. Sleep deprivation and recovery sleep prior to a noxious inflammatory insult influence characteristics and duration of pain. Sleep. 2016;39:133–142. doi: 10.5665/sleep.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn R.M., DeFriel J.N., Whistler J.L. Pharmacological traits of delta opioid receptors: pitfalls or opportunities? Psychopharmacology (Berlin) 2013;228:1–18. doi: 10.1007/s00213-013-3129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren A.H., Roozendaal B., Kindt M., Wolf O.T., Joëls M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol. Learn. Mem. 2010;93:56–65. doi: 10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Vasa R.A., Pine D.S., Masten C.L. Effects of yohimbine and hydrocortisone on panic symptoms, autonomic responses, and attention to threat in healthy adults. Psychopharmacology. 2009;204:445–455. doi: 10.1007/s00213-009-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn L.K., Denning G., Stuhr K.L., de Wit H., Hill M.N., Hillard C.J. Endocannabinoid signaling: has it got rhythm? Br. J. Pharmacol. 2010;160:530–543. doi: 10.1111/j.1476-5381.2010.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn L.K., Mantsch J.R., Vranjkovic O., Stroh G., LaCourt M., Kreutter M., Hillard C.J. Cannabinoid receptor involvement in stress-induced cocaine reinstatement: potential interaction with noradrenergic pathways. Neuroscience. 2012;204:117–124. doi: 10.1016/j.neuroscience.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo L.F., Estey D., Goodell V. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J. Clin. Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V., Bilbao A., Molander A., Spanagel R. Neuropharmacology of alcohol addiction. Br. J. Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse T.L., Weinberger A.H., Smith P.H. Targeting the noradrenergic system for gender-sensitive medication development for tobacco dependence. Nicotine Tob. Res. 2015;17:486–495. doi: 10.1093/ntr/ntu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros M.P., Marco E.M., File S.E. Endocannabinoid system and stress and anxiety responses. Pharmacol. Biochem. Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- von der Goltz C., Koopmann A., Dinter C. Orexin and leptin are associated with nicotine craving: a link between smoking, appetite and reward. Psychoneuroendocrinology. 2010;35:570–577. doi: 10.1016/j.psyneuen.2009.09.005. [DOI] [PubMed] [Google Scholar]
- von der Goltz C., Koopmann A., Dinter C. Involvement of orexin in the regulation of stress, depression and reward in alcohol dependence. Horm. Behav. 2011;60:644–650. doi: 10.1016/j.yhbeh.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Vranjkovic O., Gasser P.J., Gerndt C.H., Baker D.A., Mantsch J.R. Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J. Neurosci. 2014;34:12504–12514. doi: 10.1523/JNEUROSCI.0680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O., Hang S., Baker D.A., Mantsch J.R. β-adrenergic receptor mediation of stress-induced reinstatement of extinguished cocaine induced conditioned place preference in mice: roles for β1 and β2 adrenergic receptors. J. Pharmacol. Exp. Therapeut. 2012;342:541–551. doi: 10.1124/jpet.112.193615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachholtz A., Foster S., Cheatle M. Psychophysiology of pain and opioid use: implications for managing pain in patients with an opioid use disorder. Drug Alcohol Depend. 2015;146:1–6. doi: 10.1016/j.drugalcdep.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A.W., Smith C.M., Chua B.E., Krstew E.V., Zhang C., Gundlach A.L., Lawrence A.J. Relaxin-3 receptor (RXFP3) signaling mediates stress-related alcohol preference. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter C., Ferreirós N., Bishay P., Geisslinger G., Tegeder I., Lötsch J. Exogenous delta9- tetrahydrocannabinol influences circulating endogenous cannabinoids in humans. J. Clin. Psychopharmacol. 2013;33:699–705. doi: 10.1097/JCP.0b013e3182984015. [DOI] [PubMed] [Google Scholar]
- Wand G.S., Weerts E.M., Kuwabara H., Wong D.F., Xu X., McCaul M.E. The relationship between naloxone-induced cortisol and delta opioid receptor availability in mesolimbic structures is disrupted in alcohol dependent subjects. Addiction Biol. 2013;18:181–192. doi: 10.1111/j.1369-1600.2011.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Shaham Y., Zitzman D., Azari S., Wise R.A., You Z.-B. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress induced-relapse to drug seeking. J. Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., You Z.-B., Rice K.C., Wise R.A. Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology. 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang B., You Z.-B., Wise R.A. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol. Psychiatr. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Wilson C.M., Moseley C.K. Evaluation of potential PET imaging probes for the orexin 2 receptor. Nucl. Med. Biol. 2013;40:1000–1005. doi: 10.1016/j.nucmedbio.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Fang Q., Liu Z., Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology. 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Waselus M., Valentino R.J., Van Bockstaele E.J. Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J. Chem. Neuroanat. 2011;41:266–280. doi: 10.1016/j.jchemneu.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts E.M., Kim Y.K., Wand G.S., Dannals R.F., Lee J.S., Frost J.J., McCaul M.E. Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology. 2008;33:653–665. doi: 10.1038/sj.npp.1301440. [DOI] [PubMed] [Google Scholar]
- Weerts E.M., Wand G.S., Kuwabara H. Positron emission tomography imaging of mu- and delta-opioid receptor binding in alcohol-dependent and healthy control subjects. Alcohol Clin. Exp. Res. 2011;35:2162–2173. doi: 10.1111/j.1530-0277.2011.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D., Schroeder J.P. There and back again: a tale of norepinephrine and drug addiction. Psychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Wilcox C.E., Tonigan J.S., Bogenschutz M.P., Clifford J., Bigelow R., Simpson T. A randomized, placebo-controlled, clinical trial of prazosin for the treatment of alcohol use disorder. J. Addiction Med. 2018 Apr 16 doi: 10.1097/ADM.0000000000000413. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.L., Buchta W.C., Riegel A.C. CRF-R2 and the heterosynaptic regulation of VTA glutamate during reinstatement of cocaine seeking. J. Neurosci. 2014;34:10402–10414. doi: 10.1523/JNEUROSCI.0911-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K.L., Nickel M.M., Bielak J.T. Baclofen blocks yohimbine-induced increases in ethanol-reinforced responding in rats. Pharmacol. Biochem. Behav. 2016;144:20–25. doi: 10.1016/j.pbb.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Windle R.J., Kershaw Y.M., Shanks N., Wood S.A., Lightman S.L., Ingram C.D. Oxytocin attenuates stress induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J. Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J.C., Rabin R.A. Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. J. Pharmacol. Exp. Therapeut. 1992;263:682–689. [PubMed] [Google Scholar]
- Wise R.A. Ventral tegmental glutamate: a role in stress-, cue-, and cocaine-induced reinstatement of cocaine-seeking. Neuropharmacology. 2009;56(Suppl 1):174–176. doi: 10.1016/j.neuropharm.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkamp R.F., Balvers M.G. Analysis of omega-3 fatty acid derived N-acylethanolamines in biological matrices. Meth. Mol. Biol. 2016;1412:27–40. doi: 10.1007/978-1-4939-3539-0_4. [DOI] [PubMed] [Google Scholar]
- Witkin J.M., Statnick M.A., Rorick-Kehn L.M. The biology of nociceptin/orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol. Ther. 2014;141:283–299. doi: 10.1016/j.pharmthera.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Stanley JA, Diwadkar VA, Khatib D, Greenwald MK (manuscript submitted for publication) Acute Stress Impairs Prefrontal Cortex Function and Potentiates Nicotine-seeking Among Cigarette Smokers.
- Xu Y.L., Reinscheid R.K., Huitron-Resendiz S. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Xu K., Seo D., Hodgkinson C., Hu Y., Goldman D., Sinha R. A variant on the kappa opioid receptor gene (OPRK1) is associated with the stress response and related drug craving, limbic brain activation and cocaine relapse. Transl. Psychiatry. 2013;3 doi: 10.1038/tp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Bruijnzeel A.W. Stimulation of α2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology. 2011;60:303–311. doi: 10.1016/j.neuropharm.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Malagon A.M., Yasuda D., Belluzzi J.D., Leslie F.M., Zaveri N.T. The α3β4 nAChR partial agonist AT-1001 attenuates stress-induced reinstatement of nicotine seeking in a rat model of relapse and induces minimal withdrawal in dependent rats. Behav. Brain Res. 2017;333:251–257. doi: 10.1016/j.bbr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Georgiou P., Weber C., Robinson F., Kouimtsidis C., Niforooshan R., Bailey A. Oxytocin and opioid addiction revisited: old drug, new applications. Br. J. Pharmacol. 2017 April 5 doi: 10.1111/bph.13757. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Georgiou P., Wright S.R., Hourani S.M., Kitchen I., Winsky-Sommerer R., Bailey A. The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology. 2014;39:855–865. doi: 10.1038/npp.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti-Fregonara P., Xu R., Zoghbi S.S. The PET radioligand 18F-FIMX images and quantifies metabotropic glutamate receptor 1 in proportion to the regional density of its gene transcript in human brain. J. Nucl. Med. 2016;57:242–247. doi: 10.2967/jnumed.115.162461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Shi Y.G., Woods J.H., Watson S.J., Ko M.C. Central kappa-opioid receptor-mediated antidepressant-like effects of nor-binaltorphimine: behavioral and BDNF mRNA expression studies. Eur. J. Pharmacol. 2007;570:89–96. doi: 10.1016/j.ejphar.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Dayas C.V., Aujla H., Baptista M.A.S., Martin-Fardon R., Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J. Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Ghee S.M., Chan C., Lin L., Cameron M.D., Kenny P.J. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J. Pharmacol. Exp. Therapeut. 2012;340:801–809. doi: 10.1124/jpet.111.187567. See RE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Leri F., Cummins E., Hoeschele M., Kreek M.J. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Leri F., Cummins E., Kreek M.J. Individual differences in gene expression of vasopressin, D2 receptor, POMC and orexin: vulnerability to relapse to heroin-seeking in rats. Physiol. Behav. 2015;139:127–135. doi: 10.1016/j.physbeh.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Leri F., Grella S.L., Aldrich J.V., Kreek M.J. Involvement of dynorphin and kappa opioid receptor in yohimbine-induced reinstatement of heroin seeking in rats. Synapse. 2013;67:358–361. doi: 10.1002/syn.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Proudnikov D., Yuferov V., Kreek M.J. Drug-induced and genetic alterations in stress-responsive systems: implications for specific addictive diseases. Brain Res. 2010;1314:235–252. doi: 10.1016/j.brainres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zislis G., Desai T.V., Prado M., Shah H.P., Bruijnzeel A.W. Effects of the CRF receptor antagonist D-Phe CRF(12-41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla E.P., Logrip M.L., Koob G.F. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front. Neuroendocrinol. 2014;35:234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]