Abstract
The dataset for this article contains phytochemical and FTIR data for three different extracts from two indigenous medicinal plants obtained from Ogun State, Southwest Nigeria and the GC–MS characterisation data for their ethanolic extracts. To obtain this data, the leaves of Azadirachta indica and Cymbopogon citratus were collected from the premises of Covenant University, Nigeria. The plants were dried, pulverized and extracted with ethanol, distilled water and ethanol:water (50:50), before phytochemical screening (qualitative and quantitative), FTIR and GC–MS analyses were carried out. The dataset provides insight into the presence of bioactive phyto-constituents such as polyphenols and tannins as potential precursors for green-based nanoparticle synthesis.
Specifications Table
| Subject area | Chemistry, Biology |
| More specific subject area | Analytical Chemistry, Phytochemistry and Nanotechnology |
| Type of data | Table, figure, image |
| How data was acquired | Fourier Transform Infrared Spectroscopy (FTIR, AGILENT CARY 630) |
| Gas Chromatography-Mass Spectroscopy (GC–MS, AGILENT 7890A GC/5977 MS) | |
| Data format | Raw, analysed |
| Experimental factors | Phytochemicals (Fresh leaves were air-dried, pulverized, extracted with ethanol, distilled water, ethanol/water (1:1) and concentrated using rotary extractor under reduced pressure. Crude extracts were used for qualitative phytochemical analysis) |
| FTIR (Range – 4000-650 cm−1, Resolution – 8 cm−1, Microlab PC software with ATR sampling unit) | |
| GCMS (Column - 30 mm × 0.25 mm ID × 0.25 μm film, Carrier gas - Helium, flow - 1.0 ml/min, electron ionization - 70 Ev, Software - Masshunter) | |
| Experimental features | Phytochemical analysis of carbohydrates, tannins, saponins, flavonoids, alkaloids, anthocyanins, betacyanins, quinones, glycosides, cardiac glycosides, terpenoids, triterpenoids, phenols, coumarins, steroids, acids, FTIR scan of functional groups and GCMS scan of bioactive constituents. |
| Data source location | Ota, Nigeria |
| Data accessibility | Data included in this article |
| Related research article | [1] P. Dubey, P. Sharma, V. Kumar, FTIR and GC–MS spectral datasets of wax from Pinus roxburghii Sarg. needles biomass, Data Brief. 15 (2017) 615–622. doi:10.1016/j.dib.2017.09.074. |
| [2] K.M. Hammi, M. Hammami, C. Rihouey, D. Le Cerf, R. Ksouri, H. Majdoub, GC-EI-MS identification data of neutral sugars of polysaccharides extracted from Zizyphus lotus fruit, Data Brief. 18 (2018) 680–683. doi:10.1016/j.dib.2018.01.085. |
Value of the data
-
•
The dataset provides insight into the exact phyto-constituents, which are responsible for stabilization and reduction of metal ions during nanoparticles formation, thereby aiding proposition of mechanistic pathways for these reactions.
-
•
The data provides information on the most potent of the locally selected plants for biosynthesis of nanoparticles using readily available indigenous plants in Southwest Nigeria.
-
•
The methods used can be extended to other indigenous plants, forming a large database capable of informing researchers on the active plant(s) for nanoparticle synthesis.
-
•
The dataset can be used for educational purposes, drug synthesis and multidisciplinary research. Similar data articles can be found in [1], [2].
1. Data
The dataset on phytochemical screening of three extracts of Azadirachta indica and Cymbopogon citratus is presented in Table 1. FTIR spectra and data of different crude extracts of each plant are presented in Fig. 1, Fig. 2 and Table 2, respectively. GC–MS chromatogram/TIC of phyto-constituents of ethanolic extracts of plants and identification data of each constituent is provided in Fig. 3, Fig. 4 and Table 3, Table 4, respectively.
Table 1.
Phytochemical screening of ethanol, water and ethanol/water (1:1) extracts of Azadirachta indica and Cymbopogon citratus leaves.
|
Biochemicals / Inference | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHO | TAN | SAP | FLA | ALK | ANTHO | BETA | QUIN | GLY | CARD-GLY | TER | TRI-TERP | PHE | COU | STE | ACIDS | |
| Ethanol extract | ||||||||||||||||
| C. citratus | – | + | + | + | + | + | + | – | + | + | – | + | + | – | – | |
| A. indica | – | +++ | + | ++ | – | – | – | – | – | + | – | + | – | – | – | |
| Ethanol:water (1:1) extract | ||||||||||||||||
| C. citratus | – | ++ | – | – | – | – | + | – | + | ++ | – | + | + | – | – | |
| A. indica | – | +++ | – | + | + | + | – | – | – | + | – | + | + | – | – | |
| Water extract | ||||||||||||||||
| C. citratus | – | + | – | – | + | + | – | – | + | + | – | – | + | – | – | |
| A. indica | – | +++ | – | – | + | + | – | – | – | + | – | – | + | – | – | |
+ = trace amount; ++ = moderately present; +++ = highly present; - = absent.
CHO – Carbohydrates, TAN – Tannins, SAP – Saponins, FLA – Flavonoids, ALK – Alkaloids, ANTHO – Anthocyanins, BETA – Betacyanin, QUIN – Quinones, GLY – Glycosides, CARD-GLY – Cardiac.
Glycosides, TER – Terpenoids, TRI-TERP – Triterpenoids, PHE – Phenols, COU – Coumarins, STE – Steroids.
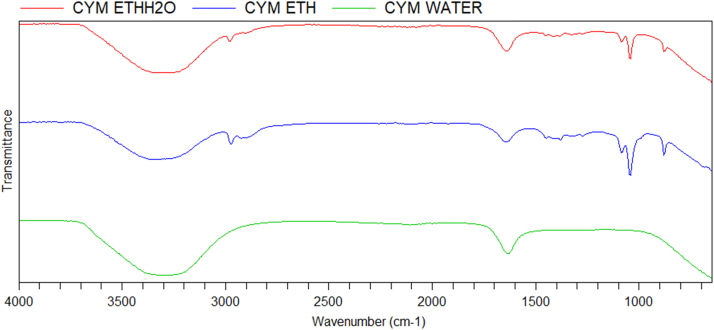
Fig. 1.
FTIR spectrum of three extracts of Cymbopogon citratus leaves.
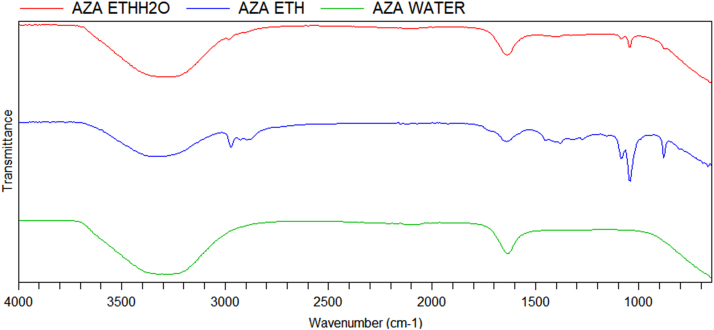
Fig. 2.
FTIR spectrum of three extracts of Azadirachta indica leaves.
Table 2.
FTIR frequency/intensity table for ethanol, water and ethanol/water extracts of Cymbopogon citratus and Azadirachta indica leaves.
| FTIR Absorption frequency (cm-1)/intensity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. citratusextracts | |||||||||||
| Ethanol | 881 (m) | 1048 (s) | 1089 (m) | 1275 (w) | 1383 (w) | 1640 (w) | 2929 (w) | 2974 (w) | 3357(m,b) | – | – |
| Ethanol/water | 881 (m) | 1048 (m) | 1089 (w) | – | – | 1640 (m) | – | 2891 (w) | 3316 (s,b) | – | – |
| Water | – | – | – | – | – | 1640 (m) | – | – | 3316 (s,b) | – | – |
| A. indicaextracts | |||||||||||
| Ethanol | 881 (m) | 1048 (s) | 1089 (m) | 1383 (w) | 1640 (w) | 2892 (w) | 2929 (w) | 2974 (w) | 3361(m,b) | – | – |
| Ethanol/water | 881 (w) | 1048 (w) | 1089 (w) | – | 1640 (m) | – | – | – | 3264(s,b) | – | – |
| Water | – | – | – | – | 1637 (m) | – | – | – | 3331(s,b) | – | – |
m – medium, s – strong, w – weak, b – broad.
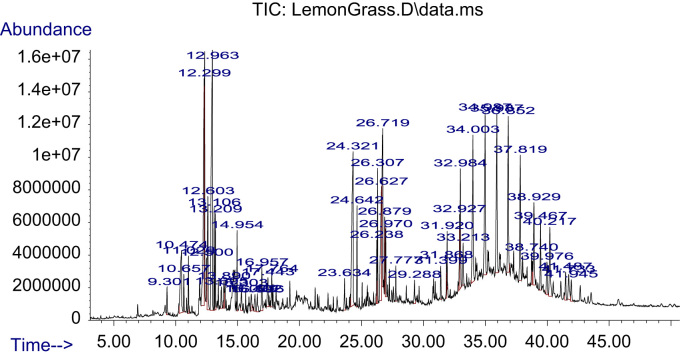
Fig. 3.
TIC of Cymbopogon citratus ethanolic extract.
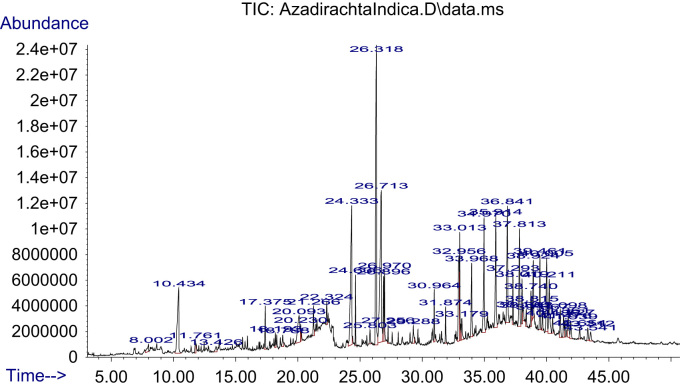
Fig. 4.
TIC of Azadirachta indica ethanolic extract.
Table 3.
Identification of phyto-constituents in ethanolic extract of C. citratus leaves using GC–MS.
| Ret. time | Area % | IUPAC name of compound | Mol formular | Mol. wt. |
|---|---|---|---|---|
| 6.92 | 0.2126 | Cyclohexane, 1,3,5-trimethyl-, (1.alpha.,3.alpha.,5.alpha.)- | C9H18 | 126.2392 |
| 9.14 | 0.2154 | 2-Acetylcyclopentanone | C7H10O2 | 126.1531 |
| 9.30 | 0.4783 | 1,6-Octadien-3-ol, 3,7-dimethyl- | C10H18O | 154.2493 |
| OR Linalool | ||||
| 10.47 | 2.6532 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144.1253 |
| 10.65 | 0.5139 | Cyclooctane, ethenyl- | C10H18 | 138.2499 |
| 10.88 | 0.2167 | Furan-2-carbohydrazide, N2-(1-methylhexylideno)- | ||
| 11.02 | 0.7758 | 7-Oxabicyclo[4.1.0]heptane, 1-methyl-4-(1-methylethenyl)- | C10H16O | 152.2334 |
| 11.87 | 0.2083 | Oxiranecarboxaldehyde, 3-methyl-3-(4-methyl-3-pentenyl)- | C10H16O2 | 168.2328 |
| 11.93 | 0.2702 | Benzofuran, 2,3-dihydro- | C8H8O | 120.1485 |
| 12.29 | 6.6959 | 2,6-Octadienal, 3,7-dimethyl-, (Z)- | C10H16O | 152.2334 |
| 12.49 | 0.6401 | Geraniol | C10H18O | 154.2493 |
| 12.60 | 2.545 | |||
| 12.96 | 9.4115 | Citral | C10H16O | 152.2334 |
| 13.10 | 1.1612 | Epoxy-linalooloxide | ||
| 13.20 | 1.1323 | |||
| 13.44 | 0.3872 | Cyclopentane, (1-methylethyl)- | C8H16 | 112.2126 |
| 13.69 | 0.4762 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.1745 |
| 13.88 | 0.5025 | Bicyclo[2.2.2]octan-1-amine | ||
| 13.98 | 0.3829 | 3-Cyclopropylcarbonyloxydodecane | ||
| 14.49 | 0.217 | Triallylsilane | C9H16Si | 152.3088 |
| 14.58 | 0.1855 | 3-Heptanol, 2-methyl- | C8H18O | 130.2279 |
| 14.64 | 0.1943 | 1,5-Heptadiene, 3,3-dimethyl-, (E)- | ||
| 14.95 | 0.8738 | Geranyl acetate | C12H20O2 | 196.2860 |
| 15.17 | 0.2231 | Cyclopropanemethanol,.alpha.,2-dimethyl-2-(4-methyl-3-pentenyl)-, [1.alpha.(R*),2.alpha.]- | ||
| 15.30 | 0.514 | Vanillin | C8H8O3 | 152.1473 |
| 15.65 | 0.3534 | 3,5-Heptadienal, 2-ethylidene-6-methyl- | C10H14O | 150.2176 |
| 16.09 | 0.4413 | Adamantane | C10H16 | 136.2340 |
| 16.40 | 0.4469 | 3-Cyclopentylpropionic acid, but-3-yn-2-yl ester | ||
| 16.59 | 0.4452 | 2-Propanol, 1,1,1-trichloro-2-methyl- | C4H7Cl3O | 177.457 |
| 16.95 | 0.9541 | 2,6-Octadienal, 3,7-dimethyl-, (Z)- | C10H16O | 152.2334 |
| 17.44 | 0.7992 | 3-Cyclohexene-1-acetaldehyde,.alpha.,4-dimethyl- | C10H16O | 152.2334 |
| 17.76 | 0.6387 | 3-n-Propyl-2-pyrazolin-5-one | C6H10N2O | 126.1564 |
| 17.85 | 0.3297 | 4-Methyl-5H-furan-2-one | C5H6O2 | 98.0999 |
| 18.11 | 0.3635 | Dodecanoic acid | C12H24O2 | 200.3178 |
| 19.00 | 0.1681 | 1-Methyl-3-n-propyl-2-pyrazolin-5-one | C7H12N2O | 140.1830 |
| 19.21 | 0.4101 | Selina-6-en-4-ol | ||
| 19.76 | 0.1779 | 2-(2-Hydroxyethylthio)propionic acid | ||
| 20.89 | 0.2064 | Phenylacetylformic acid, 4-hydroxy-3-methoxy- | ||
| 21.25 | 0.3042 | Tetradecanoic acid | C14H28O2 | 228.3709 |
| 21.45 | 0.2678 | Benzene, 1,1׳-ethylidenebis- | C14H14 | 182.2610 |
| 21.56 | 0.2124 | Pyridine, 4-[(1,1-dimethylethyl)thio]- | ||
| 22.30 | 0.3018 | p-Hydroxycinnamic acid, ethyl ester | ||
| 23.02 | 0.3948 | 2-Propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)- | C10H10O4 | 194.1840 |
| 23.63 | 0.5611 | p-Fluoroethylbenzene | C8H9F | 124.1555 |
| 24.32 | 5.9637 | n-Hexadecanoic acid | C16H32O2 | 256.4241 |
| 24.64 | 1.1441 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284.4772 |
| 25.05 | 0.3111 | Heptadecanoic acid | C17H34O2 | 270.4507 |
| 25.47 | 0.3151 | 3-Methyl-2-butenoic acid, 2-tridecyl ester | ||
| 26.23 | 0.7845 | Phytol | C20H40O | 296.5310 |
| 26.30 | 1.6582 | Diboroxane, triethyl[(4-methyl-2-pyridyl)amino]- | ||
| 26.62 | 3.8736 | 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 280.4455 |
| 26.71 | 3.6845 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- | C18H30O2 | 278.4296 |
| 26.79 | 0.1683 | Cyclooctene, 3-ethenyl- | ||
| 26.87 | 0.9543 | Linoleic acid ethyl ester | C20H36O2 | 308.4986 |
| 26.97 | 0.988 | Ethyl 9,12,15-octadecatrienoate | ||
| 27.11 | 0.2389 | p-Menth-2-en-9-ol, trans- | ||
| 27.25 | 0.3594 | Octadecanoic acid, ethyl ester | C20H40O2 | 312.5304 |
| 27.44 | 0.2377 | 5,9-Undecadien-2-one, 6,10-dimethyl-, (E)- | C13H22O | 194.3132 |
| 27.57 | 0.2752 | Naphtho[2,1-b:3,4-b׳]difuran, 2,3,8,9-tetrahydro-2,9-dimethyl- | ||
| 27.77 | 0.4932 | Cyclohexanol, 5-methyl-2-(1-methylethenyl)- | C10H18O | 154.2493 |
| 28.62 | 0.2172 | 1,6,10,14-Hexadecatetraen-3-ol, 3,7,11,15-tetramethyl-, (E,E)- | C20H34O | 290.4834 |
| 29.28 | 0.4722 | Eicosanoic acid | C20H40O2 | 312.5304 |
| 29.65 | 0.2219 | Methyl 19-methyl-eicosanoate | ||
| 30.77 | 0.3075 | 9-Tricosene, (Z)- | C23H46 | 322.6113 |
| 31.86 | 0.527 | |||
| 34.22 | 0.2424 | |||
| 30.82 | 0.2058 | Heptadecane | C17H36 | 240.4677 |
| 30.95 | 0.237 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | C19H38O4 | 330.5026 |
| 31.39 | 0.5588 | Dichloroacetic acid, heptadecyl ester | ||
| 31.91 | 0.6148 | Hexacosane | C26H54 | 366.7070 |
| 32.79 | 0.2052 | Cyclohexane, 1,1׳-[4-(3-cyclohexylpropyl)-1,7-heptanediyl]bis- | C28H52 | 388.7125 |
| 32.92 | 1.2801 | 1-Nonadecene | C19H38 | 266.5050 |
| 32.98 | 1.9114 | Tetracosane | C24H50 | 338.6538 |
| 34.00 | 3.8594 | |||
| 36.85 | 3.8693 | |||
| 37.81 | 2.8508 | |||
| 38.92 | 2.0079 | |||
| 33.21 | 0.8589 | Butane, 2,2-bis(5-acetyl-2-thienyl)- | ||
| 34.32 | 0.1678 | Squalene | C30H50 | 410.7180 |
| 34.98 | 4.4543 | Nonacosane | C29H60 | 408.7867 |
| 35.21 | 0.2288 | Nonadecyl heptafluorobutyrate | ||
| 35.26 | 0.2462 | Heptacosyl acetate | ||
| 35.93 | 4.1869 | Triacontane | C30H62 | 422.8133 |
| 36.15 | 0.2301 | Triacontyl acetate | C32H64O2 | 480.8494 |
| 37.29 | 0.4097 | dl-.alpha.-Tocopherol | C29H50O2 | 430.7061 |
| 38.01 | 0.2942 | Benzene, 1-nitro-4-(phenylthio)- | C12H9NO2S | 231.270 |
| 38.37 | 0.3256 | Campesterol | C28H48O | 400.6801 |
| 38.74 | 0.5393 | Stigmasterol | C29H48O | 412.6908 |
| 38.81 | 0.3692 | 1,2,3,4-4H-Isoquinolin-1,3-dione, 4,4,5,6,8-pentamethyl- | ||
| 39.46 | 1.3421 | .gamma.-Sitosterol | C29H50O | 414.7067 |
| 39.97 | 0.8629 | 2-Furancarboxamide, N-[3-methyl-1-(phenylmethyl)-1H-pyrazol-5-yl]- | ||
| 40.21 | 1.9876 | Tetratriacontane | C34H70 | 478.9196 |
| 40.48 | 0.2395 | 9,19-Cyclolanost-24-en-3-ol, (3.beta.)- | C30H50O | 426.7174 |
| 41.09 | 0.4235 | 4-[5-(3,4-Diethoxy-benzyl)-[1,2,4]oxadiazol-3-yl]-furazan-3-ylamine | ||
| 41.48 | 0.6734 | Cannabidiol | C21H30O2 | 314.4617 |
| 41.73 | 0.8292 | Eicosane | C20H42 | 282.5475 |
| 43.54 | 0.3269 | |||
| 41.94 | 0.5473 | Cyclopropane-1-carboxamide, 2-butyl-N-(5,6,7,8-tetrahydro-7,7-dimethyl-5-oxoquinazolin-2-yl)- | ||
| 42.67 | 0.382 | 3-Methoxy-17beta-(O-nitrobenzoyloxy)-estra-1,3,5(10)-triene | ||
| 43.30 | 0.1722 | 2-(Acetoxymethyl)-3-(methoxycarbonyl)biphenylene |
Table 4.
Identification of phyto-constituents in ethanolic extract of A. indica leaves using GC–MS.
| Ret. time | Area % | IUPAC name of compound | Mol formular | Mol weight |
|---|---|---|---|---|
| 6.85 | 0.263 | Thiazole, 4,5-dihydro-2-methyl- | C4H7NS | 101.170 |
| 8.00 | 0.4441 | 2-Hexenoic acid | C6H10O2 | 114.1424 |
| 8.67 | 0.1883 | 2-Fluoro-5-methoxypyrimidine | ||
| 10.43 | 4.0847 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144.1253 |
| 10.88 | 0.1569 | Isopropyl isothiocyanate | C4H7NS | 101.170 |
| 11.42 | 0.2128 | N-Aminopyrrolidine | C4H10N2 | 86.1356 |
| 11.76 | 0.4228 | Benzofuran, 2,3-dihydro- | C8H8O | 120.1485 |
| 11.83 | 0.2203 | D-Alanine, N-allyloxycarbonyl-, decyl ester | ||
| 12.04 | 0.1826 | 2(1H)Pyrimidinone,4-amino-1,N-dimethyl- | C6H9N3O | 139.1552 |
| 12.23 | 0.1549 | 2,6-Octadienal, 3,7-dimethyl-, (Z)- | C10H16O | 152.2334 |
| 12.47 | 0.2256 | Geraniol | C10H18O | 154.2493 |
| OR 2,6-Octadien-1-ol, 3,7-dimethyl-, (E)- | ||||
| 12.61 | 0.2274 | N-[5-(3,4-Dimethoxy-benzyl)-[1,3,4]thiadiazol-2-yl]-3-fluoro-benzamide | ||
| 13.42 | 0.3247 | Malic Acid | C4H6O5 | 134.0874 |
| 13.67 | 0.2593 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.1745 |
| 15.56 | 0.2434 | 1H-Cycloprop[e]azulene, 1a,2,3,4,4a,5,6,7b-octahydro-1,1,4,7-tetramethyl-, [1aR-(1a.alpha.,4.alpha.,4a.beta.,7b.alpha.)]- | C15H24 | 204.3511 |
| 15.75 | 0.1914 | trans-Cinnamic acid | C9H8O2 | 148.1586 |
| 15.94 | 0.2789 | .gamma.-Elemene OR γ-Elemene | C15H24 | 204.3511 |
| 17.37 | 0.8838 | 2-Hydroxy-1-(1׳-pyrrolidiyl)-1-buten-3-one | ||
| 17.96 | 0.1632 | L-Proline, 1-acetyl- | C7H10NO3 | 156.1592 |
| 18.08 | 0.1893 | Dodecanoic acid | C12H24O2 | 200.3178 |
| 18.19 | 0.3327 | Cyclohexane, 1-ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene)- | C15H24 | 204.3511 |
| 18.30 | 0.2576 | Fumaric acid, cyclobutyl ethyl ester | ||
| 18.59 | 0.2391 | Phosphine, methyl(1-methylethyl)phenyl- | ||
| 18.78 | 0.4281 | Carbamic acid, methylphenyl-, ethyl ester | C10H13NO2 | 179.2157 |
| 20.09 | 2.4879 | Ethyl.alpha.-d-glucopyranoside | ||
| 20.22 | 0.3267 | .beta.-D-Glucopyranoside, methyl | C7H14O6 | 194.1825 |
| 20.29 | 0.2465 | d-Glycero-l-gluco-heptose | ||
| 21.26 | 0.7649 | 2(1H)-Pyrimidinone, 5-methyl- | ||
| 21.54 | 0.2506 | Sorbitol | C6H14O6 | 182.1718 |
| 22.32 | 0.5687 | Piperidine, 1-(1-pentenyl)- | ||
| 22.53 | 0.2716 | Galactitol | C6H14O6 | 182.1718 |
| 22.90 | 0.2182 | Cyclohexane, 1,5-diisopropyl-2,3-dimethyl- | ||
| 23.91 | 0.2894 | Palmitoleic acid | C16H30O2 | 254.4082 |
| 24.33 | 7.424 | n-Hexadecanoic acid | C16H32O2 | 256.4241 |
| 24.40 | 0.1754 | 11-Oxa-tricyclo[4.4.1.0(1,6)]undecan-2-ol | ||
| 24.63 | 1.0398 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284.4772 |
| 25.54 | 0.1899 | Heptadecanoic acid | C17H34O2 | 270.4507 |
| 25.80 | 0.4054 | 3-Heptanol, 3,5-dimethyl- | C9H20O | 144.2545 |
| 26.31 | 11.5639 | Phytol | C20H40O | 296.5310 |
| 26.71 | 9.7212 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- | C18H30O2 | 278.4296 |
| 26.89 | 1.5401 | Octadecanoic acid | C18H36O2 | 284.4772 |
| 26.97 | 1.4276 | Ethyl 9,12,15-octadecatrienoate | ||
| 27.25 | 0.329 | Octadecanoic acid, ethyl ester | C20H40O2 | 312.5304 |
| 27.58 | 0.2923 | Naphtho[2,1-b:7,8-b׳]difuran, 1,2,9,10-tetrahydro-2,9-dimethyl- | ||
| 28.06 | 0.2169 | 1-Heneicosyl formate | C22H44O2 | 340.5836 |
| 28.40 | 0.2843 | Benzyl.beta.-d-glucoside | ||
| 29.02 | 0.213 | Z,Z-8,10-Hexadecadien-1-ol acetate | ||
| 29.28 | 0.6416 | Eicosanoic acid | C20H40O2 | 312.5304 |
| 29.65 | 0.2674 | Methyl 19-methyl-eicosanoate | ||
| 29.75 | 0.1501 | (1S,15S)-Bicyclo[13.1.0]hexadecan-2-one | ||
| 30.78 | 0.2073 | Cyclotetradecane, 1,7,11-trimethyl-4-(1-methylethyl)- | C20H40 | 280.5316 |
| 30.82 | 0.245 | Eicosane | C20H42 | 282.5475 |
| 30.96 | 1.0086 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | C19H38O4 | 330.5026 |
| 31.07 | 0.254 | Glycerol 1-palmitate | C19H38O4 | 330.5026 |
| 31.41 | 0.2199 | Bis(2-ethylhexyl) phthalate | C24H38O4 | 390.5561 |
| 31.55 | 0.2299 | Docosanoic acid | C22H44O2 | 340.5836 |
| 31.87 | 0.6983 | Nonadecanoic acid, ethyl ester | C21H42O2 | 326.5570 |
| 32.64 | 0.2932 | Cyclopentadecanone, 2-hydroxy- | C15H28O2 | 240.3816 |
| 32.70 | 0.2054 | 9,12,15-Octadecatrienoic acid, ethyl ester, (Z,Z,Z)- | C20H34O2 | 306.4828 |
| 32.95 | 3.8689 | Ethanol, 2-(octadecyloxy)- | C20H42O2 | 314.5463 |
| 33.01 | 1.9763 | Linolenic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester (Z,Z,Z)- | C21H36O4 | 352.5081 |
| 33.09 | 0.309 | |||
| 33.17 | 0.8062 | Benzene, 1,2-dimethoxy-4-nitro- | C8H9NO4 | 183.1614 |
| 33.48 | 0.1631 | Fumaric acid, pent-4-en-2-yl tridecyl ester | ||
| 33.96 | 2.3197 | Octacosane | C28H58 | 394.7601 |
| 34.31 | 0.1787 | Squalene | C30H50 | 410.7180 |
| 34.96 | 3.6131 | Nonacosane | C29H60 | 408.7867 |
| 35.19 | 0.1721 | Octacosyl acetate | C30H60O2 | 452.7962 |
| 35.25 | 0.2752 | 1-Nonadecene | C19H38 | 266.5050 |
| 35.66 | 0.1803 | |||
| 35.91 | 3.7204 | Tetracosane | C24H50 | 338.6538 |
| 36.84 | 4.1428 | |||
| 36.14 | 0.2142 | Triacontyl acetate | C32H64O2 | 480.8494 |
| 36.21 | 0.165 | |||
| 36.62 | 0.1643 | |||
| 36.50 | 0.1834 | .gamma.-Tocopherol | C28H48O2 | 416.6795 |
| 37.29 | 0.9935 | Vitamin E | C29H50O2 | 430.7061 |
| 37.81 | 3.0663 | Octadecane | C18H38 | 254.4943 |
| 38.92 | 1.5839 | |||
| 38.01 | 1.0458 | Pregn-4-ene-3,20-dione, 16-hydroxy-, (16.alpha.)- | ||
| 38.09 | 0.4445 | 2,6,10,14-Tetramethyl-7-(3-methylpent-4-enylidene) pentadecane | ||
| 38.36 | 0.4534 | Campesterol | C28H48O | 400.6801 |
| 38.74 | 0.8867 | Stigmasterol | C29H48O | 412.6908 |
| 38.81 | 0.3705 | 4-Cyclohexene-1,2-dicarboximide, N-butyl-, cis- | ||
| 39.46 | 2.0086 | .gamma.-Sitosterol | C29H50O | 414.7067 |
| 39.66 | 0.5223 | 4,4,6a,6b,8a,11,11,14b-Octamethyl-1,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-octadecahydro-2H-picen-3-one | ||
| 40.00 | 2.5958 | 2-Furancarboxamide, N-(8-methyl-2H-[1,2,4]thiadiazolo[2,3-a]pyridin-2-ylidene)- | ||
| 40.21 | 2.2065 | Eicosane | C20H42 | 282.5475 |
| 41.72 | 0.9181 | |||
| 43.54 | 0.4271 | |||
| 40.48 | 0.4548 | 4,22-Stigmastadiene-3-one | C29H46O | 410.6749 |
| 41.09 | 0.9555 | D:A-Friedoursan-3-one | ||
| 41.36 | 0.7263 | Stigmast-4-en-3-one | C29H48O | 412.6908 |
| 41.47 | 0.5 | Cyclopropane-1-carboxamide, 2-butyl-N-(5,6,7,8-tetrahydro-7,7-dimethyl-5-oxoquinazolin-2-yl)- | ||
| 41.61 | 0.1877 | Hexahydropyridine, 1-methyl-4-[4,5-dihydroxyphenyl]- | ||
| 41.93 | 0.6689 | Cannabidiol | C21H30O2 | 314.4617 |
| 42.65 | 0.3853 | 1H-1,2,4-Triazole-5(4H)-thione, 4-allyl-3-(3-furyl)- | ||
| 43.00 | 0.1593 | 1,2-Bis(trimethylsilyl)benzene | ||
| 43.31 | 0.4858 | Pyrido[2,3-d]pyrimidine, 4-phenyl- | ||
| 45.73 | 0.2579 | 2-(Acetoxymethyl)-3-(methoxycarbonyl)biphenylene |
2. Experimental design, materials and methods
2.1. Sample collection
Fresh leaves of two (2) indigenous plants namely Cymbopogon citratus and Azadirachta indica were collected in March 2018 from Covenant University, Nigeria. The leaf samples were thoroughly washed in distilled water before air-drying at room temperature for 21 days. Dried leaves were then pulverized and preserved in airtight containers until further use.
2.2. Sample preparation and characterisation
For phytochemical screening, 25 g of pulverized plant leaves was extracted with 125 mL of three solvents namely; ethanol, distilled water and ethanol/water (1:1) for 72 h. The plant extracts were filtered and concentrated using rotary evaporator under reduced pressure. Preliminary phytochemical analysis was carried out to test for the presence of tannins, saponins, flavonoids, alkaloids, anthocyanins, betacyanins, quinones, glycosides, cardiac glycosides, terpenoids, triterpenoids, phenols, coumarins, steroids and acids in all the three extracts following the standard test methods [3], [4].
Also, 10 g of each powdered plant material was extracted with ethanol, distilled water and ethanol/distilled water (1:1), respectively, for 72 h. The extracts were filtered and concentrated to 1 mL using BUCHI rotary evaporator under reduced pressure. Then, 1 mL of crude ethanolic, water and ethanol/water extracts were taken for FTIR analysis, while 1 mL ethanolic extracts were taken in amber GC vials for GC–MS analysis.
2.3. Fourier transform infrared spectroscopy analysis
The extracts were analysed using Agilent Cary 630 FTIR spectrometer equipped with Microlab PC software with ATR sampling unit with a resolution of 8 cm−1 and scan range of 4000 cm−1 to 650 cm−1.
2.4. Gas chromatography mass spectroscopy analysis
The GC–MS analysis was carried out using Agilent 7890 A gas chromatograph coupled with a 5977 A mass spectrometer. The temperature programme of the GC was maintained at an initial temperature of 50 °C with a hold for 1 min, followed by gradual increase to 300 °C at 7 °C/min for 14 min. 1 µL of each sample was injected in the split mode (split ratio 1:10). The identification of components was based on retention time on the capillary column and matching the GC mass spectra with the National Institute of Standards and Technology (NIST) library.
Acknowledgements
The authors are thankful to Covenant University, Nigeria for providing institutional and publication support.
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.08.133.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Dubey P., Sharma P., Kumar V. FTIR and GC–MS spectral datasets of wax from Pinus roxburghii Sarg. needles biomass. Data Brief. 2017;15:615–622. doi: 10.1016/j.dib.2017.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammi K.M., Hammami M., Rihouey C., Le Cerf D., Ksouri R., Majdoub H. GC-EI-MS identification data of neutral sugars of polysaccharides extracted from Zizyphus lotus fruit. Data Brief. 2018;18:680–683. doi: 10.1016/j.dib.2018.01.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varadharajan V., Janarthanan U.K., Krishnamurthy V. Physicochemical, phytochemical screening and profiling of secondary metabolites of Annona squamosa leaf extract. World J. Pharm. Res. 2012;1:1143–1164. [Google Scholar]
- 4.Ali S., Khan M.R., Irfanullah M.S., Zahra Z. Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement Altern. Med. 2018;18(43):1–15. doi: 10.1186/s12906-018-2114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material