Abstract
Unicameral bone cysts (UBC) or simple/solitary bone cysts are benign fluid filled cavities that enlarge over time, resulting in thinning of the bone. Usually these cysts are reported in the metaphyseal areas of long bones with open physes. 85% of UBCs occur almost exclusively in children and adolescents. UBCs are more aggressive in the first decade of life and correspondingly the recurrence rate for these patients is four times that for adolescents. The proximal humerus and femur account for almost 90% of these cases. UBCs are classified as active when they are within 1 cm of the physis and latent as they progress to a diaphyseal location. Differential diagnoses for UBC include aneurysmal bone cyst, fibrous dysplasia, enchondroma, and intraosseous ganglia. By the time of skeletal maturity most UBCs tend to resolve. Nonoperative treatment may be a viable option for many patients with small or symptomatic lesions. Interventions include steroid injection, open curettage and bone grafting, decompression and percutaneous injection of marrow or graft substitutes.
Keywords: Unicameral bone cyst, Current concepts
Highlights
-
•
Unicameral bone cysts (UBCs) are benign fluid filled cavities that enlarge over time, resulting in thinning of the bone.
-
•
Proximal humerus and femur account for almost 90% of these cases.
-
•
UBCs are more aggressive in the first decade of life.
-
•
By the time of skeletal maturity most UBCs tend to resolve.
-
•
Nonoperative treatment may be a viable option for many patients with small or symptomatic lesions.
-
•
Interventions: steroid injection, open curettage/bone grafting, decompression and percutaneous injection of marrow/graft substitutes.
1. Introduction
Unicameral bone cysts (UBC) or simple/solitary bone cysts were initially reported by Virchow in 1891 as “cystic structures”, thought to be at that time due to anomalies in the local circulation [1]. These cysts can also be multi-loculated. These benign fluid filled cavities enlarge over time, resulting in thinning of the bone. Usually these cysts are reported in the metaphyseal areas of long bones with open physes. Efforts to classify these lesions in a manner that predicts their natural history have not been met with success.
2. Epidemiology
85% of UBCs occur almost exclusively in children and adolescents. The reported peak is between the ages of 3 and 14 years with the mean age at diagnosis being approximately 9 years [[1], [2], [3], [4]] UBCs represent about 3% of all biopsied bone tumors and occur twice as more in boys as compared to girls [4].
3. Genetics
In a single case translocation t (16; 20) (p11.2; q13) has been found. In an 11 year old boy, complicated clonal structural rearrangement involving chromosomes 4, 6, 8, 16, 21, and both chromosomes 12 has been described.
4. Pathogenesis
The exact pathogenesis remains elusive. Many hypotheses have been suggested for the formation of a UBC. These included disturbance in bone growth locally, role of pre-existing lesions, intramedullary hemorrhages as some posttraumatic cysts have same histological features as of UBC and small nests of synovial cells trapped in an intraosseous position [48].
Blockage in the venous drainage is the most favored mechanism which occurs in a rapidly growing and remodeling portion of cancellous bone. Chiriga et al. [8] found slightly increased internal pressure of involved bone as compared to normal pressure of bone marrow lower partial pressure of oxygen of cyst fluid than arterial or venous blood suggesting a venous obstruction. Factors contributing to bone resorption are high internal pressure and fluid accumulation, venous stasis and developmental anomaly occurring in the veins [9].
The cyst fluid has been shown to contain increased levels of lysosomal enzymes than serum. Based on this observation, enzymatic role in simple bone cyst growth was postulated. Bone resorptive factors (prostaglandins, interleukin 1β, nitrate and nitrites levels, and proteolytic enzymes) in cyst fluid were measured in a study by Komiya [9]. Tumor necrosis factor ⍺, and interleukins 1β and 6.
5. Clinical presentation
The patient's age has great bearing on the presentation and clinical behavior of the bone cysts. UBCs are more aggressive in the first decade of life and correspondingly the recurrence rate for these patients is four times that for adolescents [10]. As the UBCs are painless, 80% of the patients will not have any symptoms unless a pathologic gross fracture or undisplaced stress fracture occurs. In patients where there is no history of trauma, symptoms include mild pain, local tenderness and occasionally swelling. In some patients who never develop symptoms the lesion may be an incidental finding on roentgenograms.
UBCs have been diagnosed in almost every bone. However more than 95% of the cases involve long bones [10]. The proximal humerus and femur account for almost 90% of these cases. UBCs in the proximal humerus develop more frequently where 80% of the growth occurs and tend to disappear after puberty. Conversely 50% patients with proximal femoral UBCs are older than 17 years with age range as high as 54 years [10].
UBCs are classified as active when they are within 1 cm of the physis and latent as they progress to a diaphyseal location. UBCs usually begin in the metaphysis in proximity to the physis and off the apophysis of the greater trochanter in long bones. Over time with growth the humeral UBC moves distally towards the diaphysis and can be seen at the center of the humeral diaphysis. On the other hand in the proximal femur, the UBC rarely descends below the proximal diaphysis. Around 6%–10% of UBCs are reported in adults in flat bones. It has been hypothesized that this late presentation is potentially due to location of the flat bone that protects it from trauma [10].
6. Gross features
An intact simple bone cyst is very rarely sent for histological examination. Gross evaluation of an unaltered cyst shows a large intramedullary cavity filled with clear or straw-colored fluid. The cyst is lined by a thin, smooth, greyish-white to reddish-brown membrane. The cyst is usually solitary, but occasionally partial septations divide it into several cavities. These septations develop during the process of healing. Focally peripherally located within the medullary cavity is a spongy component of smaller cysts which are attached to the wall of cyst. The wall of cyst is composed of thin fibrous tissue showing bony ridges. Secondary to trauma, the cyst may contain blood.
7. Microscopic features
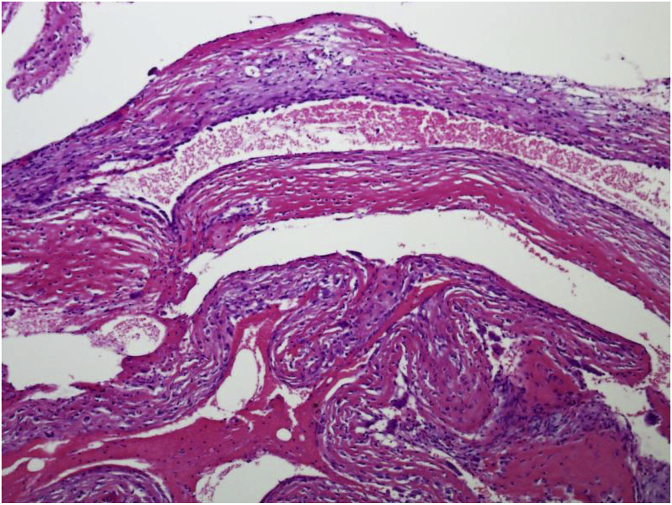
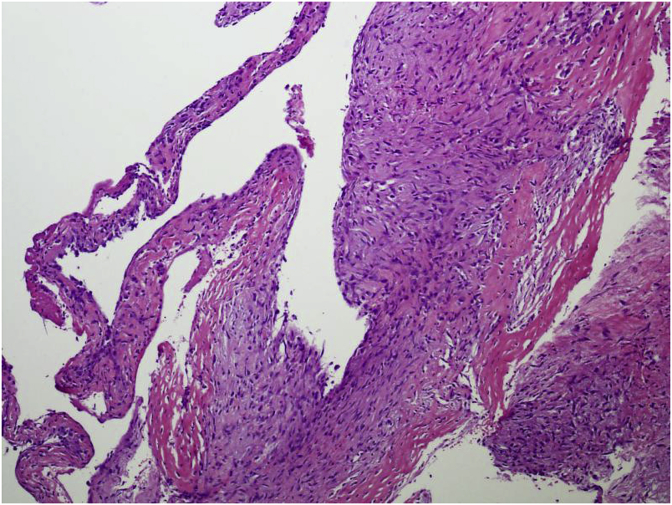
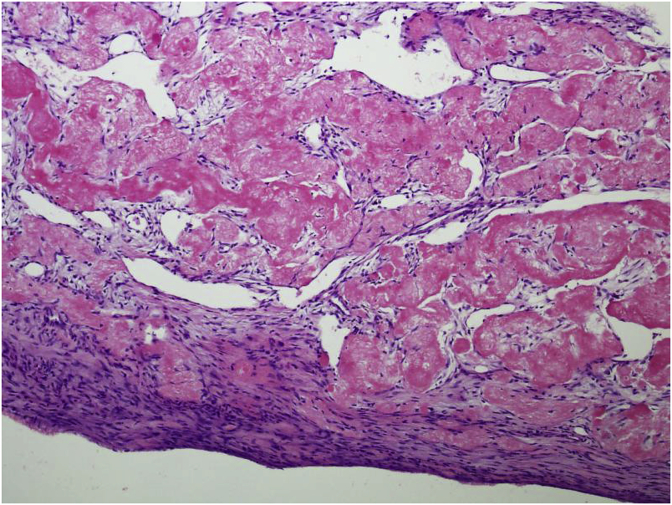
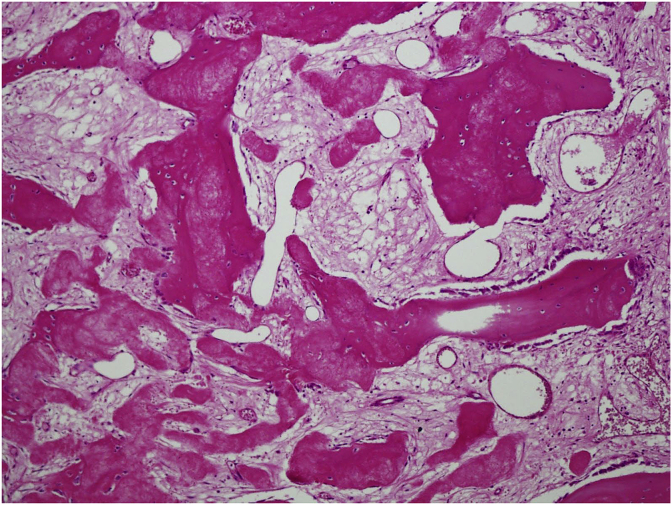
The cyst wall lacks an epithelial lining and composed of thin layer of fibrovascular stroma focally containing osteoclast type multinucleated giant cells (Fig. 4). As fracture complicates most of these cysts, secondary changes such as hemorrhage, hemosiderin and foamy histiocytes, granulation tissue, cholesterol clefts, fibrin, calcification and reactive bone are seen. Fracture thickens the cyst wall with fibroblasts, osteoclasts, hemosiderin and reactive woven bone with numerous osteoclasts (Fig. 5). The wall often contains cementum-like pink amorphous material which sometimes can look like fibrin. Jaffe [5] first report it as calcified material in the cyst wall, representing calcified fibrin and blood clots (Fig. 6). Later an ultrastructural examination of this material revealed collagen bundles and numerous matrix vesicles. The later were seen produced by osteoblasts and considered as the initial sites of calcification. Therefore, the cementum-like material probably represents a special type of bone and is not a separate entity (Fig. 7).
Fig. 4.
Thin fibrous wall of simple bone cyst focally containing multinucleated giant cells.
Fig. 5.
Focally loose fibroblasts seen in wall of cyst.
Fig. 6.
Characteristic amorphous acellular cementum-like material in wall of cyst.
Fig. 7.
Focal transformation of this cementum-like material into immature bone with osteoblastic rimming.
Baumhoer et al. [6] tested cementum-like material for various special and immunohistochemical stains. On elastic van Geison (EVG) stain, strong fuscinophilic appearance was noted. Procollagen I, collagen I, collagen III, and bone proteoglycan (decorin) were positive and fibrin and other noncollagenous proteins were negative. Immunohistochemical stains for transcription factors Runx-2 and Osterix in osteoblasts of close proximity showed positive expression supporting the concept that this material is an immature form of bone. We also observed evidence for this concept in one of our study by demonstrating transformation of cementum-like material into immature and then mature bone [7].
8. Plain radiography
Plain x-ray is modality of choice and has a high diagnostic accuracy. Cyst is not eccentric and arises centrally in medullary cavity with long axis parallel to length of bone. Because of its central location, the cortical break and soft tissue component are rare. [11, 12].
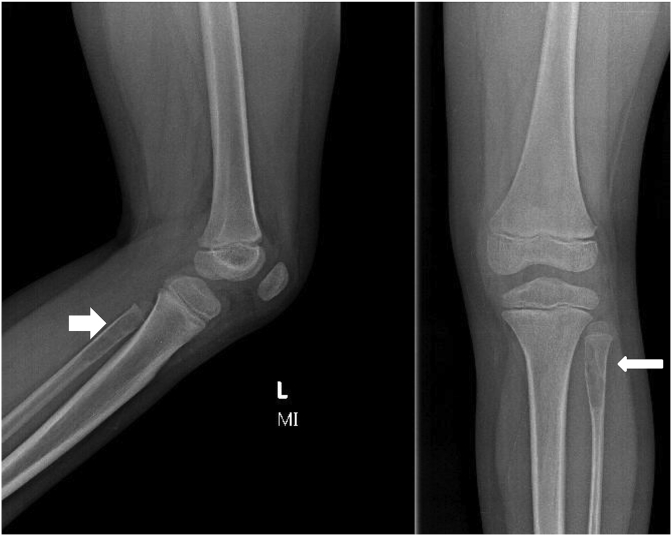
These are metaphyseal and juxtaphyseal in location and geographic in appearance with thin sclerotic margin (Fig. 1). The cortical break and periosteal reaction are usually absent nevertheless present in cases of associated fractures. Rarely, simple bone cysts may be found on the diaphysis and appear as a large, multicameral with slight expansion. In the presence of fracture, the “fallen fragment “sign is noted. The fractured fragment moves in dependent position and changes its position with change in patient's posture (Fig. 3 A).
Fig. 1.
7 years old boy with left fibula unicameral bone cyst. AP & lateral radiograph shows a central cystic cavitation, well demarcated, with cortical thinning and mild expansion.
Fig. 3.
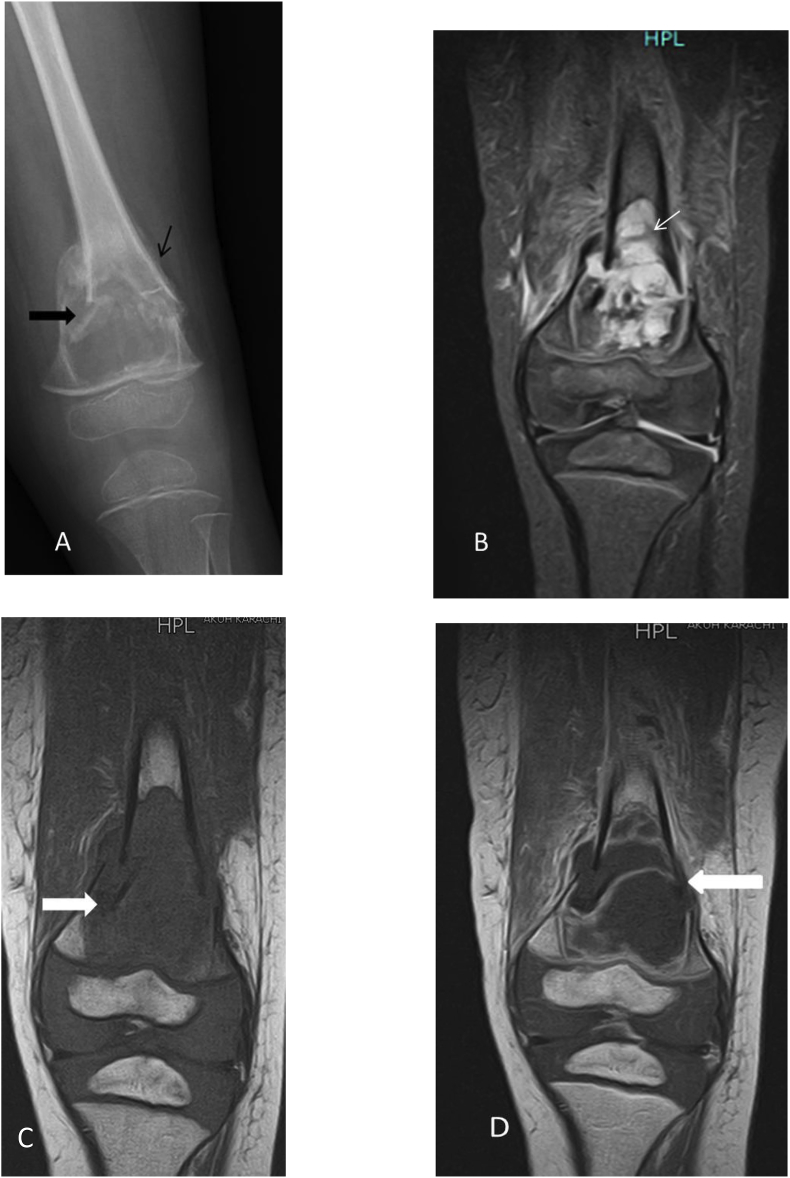
8 years old boy with unicameral bone cyst of distal femur. A) Radiograph shows a central lytic metaphyseal lesion with a thin sclerotic margin. There is a pathologic fracture as well as a “fallen bone” fragment (thick arrow) and periosteal reaction (thin arrow). B) Coronal T2 FS MR in same patient shows hyper-intense lesion with fluid levels (arrow). C) Coronal T1WI MR in the same patient shows a low signal lesion, signal nearly isointense to muscle. (Arrow). D) Coronal T1 C + MR, in same patient shows lesion pronounced rim enhancement. The entire imaging pattern is typical of a UBC.
Similarly, a gas bubble that has migrated upward (“rising bubble sign”) is also described for unicameral cyst. This sign is consider as pathognomic and if seen doesn't require any other modality for confirmation of this diagnosis. [11, 13].
In healing phase, sclerosis is noted with some residual lucency. Mal alignment of lime can be seen in fracture cases.
9. CT
The role of CT is to evaluate cyst present in areas such as pelvis and spine, areas difficult to assess on plain x-ray. CT demonstrates more accurate extent of cyst in complex areas e.g. Spine and pelvis as well as reveal complication such as fracture which are sometime subtle on plain X ray. The CT mainly evaluates cyst wall thickness and risk fracture. (12) CT also helps to differential cyst from a lipoma which is difficult to assess on plain x-ray [14].
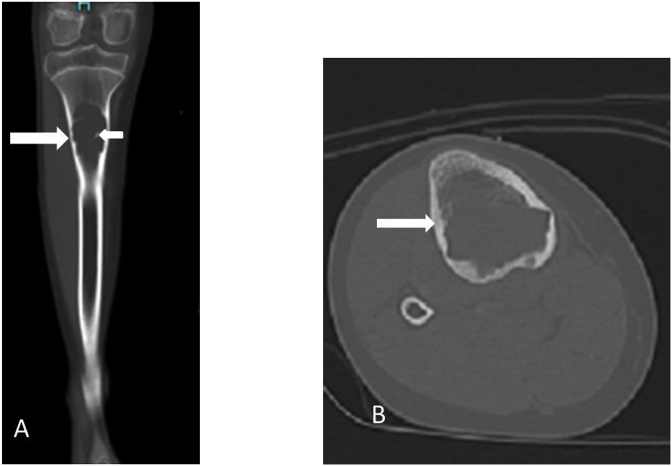
Similar radiographic feature are usually seen on plain CT. Cyst appears as centrally located lesion with surrounding cortex thinning which is intact (Fig. 2). The HU (houns field unit) on CT are between 15 and 20 HU. Occasionally fluid - fluid level are seen. The “fallen fragment” of bone is seen as floating bone in intracytic fluid in cases of fracture. The bubble gas sign, a pathognomic features to suggests presence of pathological fracture also known as “rising Bubble sign” is also seen on CT. CT also provides road Map for intervention. [2,7].
Fig. 2.
12 years old girl with unicameral bone cyst of Tibia. A, coronal and B, axial CT images show expansile lytic lesion (long arrow) containing septa (small arrow). The lesion has the typical appearance of a UBC.
10. MRI
MRI is used together with plain x-ray or CT. MRI demonstrates presence of fluid and confirms its cystic nature (Fig. 3 B). Moreover, MRI not only confirms its cystic nature and location but is the modality to further elaborates its aggressive features. The aggressive features in terms of local aggressive changes are fracture, local expansion, thinning of cortex, erosion and significant disfigurement [46,47].
The simple bone cyst shows homogenous low signal intensity on T1-weighted images, and high signal intensity on T2-weighted and fluid sensitive sequences (Fig. 3B and C). On TI-weighted images, heterogeneous scattered high signals some time observed. These high signals are related to blood products associated to fracture.
On Post contrast MRI, marginal enhancement is noted with central non enhancing area. However, central enhancement may happen in some part of lesion occasionally (Fig. 3C and D).
Rarely fluid –fluid levels are also seen if fibrous septations are present within cyst with are not seen with plain radiograph. These appear as thin, low signal with surrounding high signal on T2 weighted or STIR sequences (fluid sequences). On past contrast MRI, septations can enhance. Septa may be only partial or may have loculated regions (Fig. 3C and D).
Cyst with associated fracture has heterogeneous and complicated MRI signals on all sequences because of blood products. On T1WI, high signal are noted secondary to blood products. On T2WI and fluid sequences, high signals are notes in hematoma, periosteal reaction and surrounding edema [15, 16].
11. Bone scintigraphy/PET
Both modalities have no significant role in diagnostic work up and might be a negative scan. But may show marginal increased tracer uptake with central photopenia.
One PET increased FDG-activity is occasionally noted and can be confused with bony metastasis. Increase up take is also noted in cases of fracture [11].
12. Role of imaging in post followup/post treatment phase
X-ray is recommended to follow post treatment cases because it is effective, fast, and inexpensive way to get required information and to guide further management.
On X-ray gradually increased sclerosis at intervention site is noted from ground glass haziness to dense sclerosis. Healing on follow up cases may appear as opacification of cyst with cortical thickening. But, if lucency visualized it is suggestive of residual or recurrence [17].
According to rating system for treated unicameral bone cyst described by Neer et al. in 1966 [18], an excellent response is either complete obliteration of the cyst during the period of operation or a residual defect with one or more static cyst-like defects persisting with healthy bone seen on plain film. An enlarging cyst on follow-up examinations suggests recurrence and need surgical intervention.
The current follow up imaging recommendation in children, is plain x ray to see post treatment or post-surgical changes.
In complicated cases such pathological fractures or cases with post-operative complications like infection, incomplete healing or premature closure of growth plate. The MRI should be considered over CT to avoid unnecessary ionizing radiation [19].
13. Differential diagnosis
Differential diagnoses for UBC include aneurysmal bone cyst, fibrous dysplasia, enchondroma, eosinophlic granuloma and intraosseous ganglia. [1], [2],44,45,46. Clinical and radiologic features help to differentiate these pathologies. Aneurysmal bone cyst on roentgenograms appear as a lytic, eccentric, intramedullary bone lesion, with a transverse diameter that is wider than the epiphyseal plate. MRI of these lesions shows double-density fluid levels and septations [20]. The matrix of fibrous dysplasia reveals a ground glass appearance that differentiates it from a UBC. Enchondromas usually seen in short tubular bones of the hands and feet, are distinct radiolucent intramedullary lesions with thinning and expansion of the cortices. Intraosseous ganglia are small radiolucent lesions usually reported incidently in the epiphysis and subchondral region [2, [21], [22], [23]].
Eosinphilic granuloma frequently involves axial skeleton than appendicular skeleton. Spinal Colum involvement is mostly seen in children. These are most destructive lytic lesion with associated soft tissue component and vertebral fracture or vertebra plana [[44], [45], [46]].
14. Treatment
The treatment of UBCs has continued to evolve with time. Contemporary methods include injection, decompression and combined surgical techniques. The aim of treatment is to prevent or manage pathologic fracture, promote cyst healing, prevent cyst recurrence and re-fracture. The treatment choice is individualized for each patient based on the clinical and radiologic features. If the UBC is picked up incidently on roentgenograms in a patient who does not have any symptoms and the bone is not at risk of pathological fracture, then nonoperative management is recommended with close follow up. For patients in whom a pathological fracture has already developed in the upper extremity due to UBC, one can go with nonoperative treatment with immobilization. Mik et al. [24] described three factors to asses UBC healing which include one clinical characteristic of pain, and two roentgenographic features of cyst opacification and cortical thickening. They described complete healing as >95% opacification of the cyst with cortical thickening and no pain. Partial healing includes >80%–95% opacification of the cyst with or without cortical thickening. Incomplete healing is represented by <80% opacification of the cyst and no cortical thickening [2].
14.1. Intralesional injections
Scaglietti [25] first reported using methylprednisolone into UBCs under fluoroscopy with radiopaque dye for localization. He reported “favourable” results in 90% cases. However reports thereafter have been unable to show good results even after using multiple steroid injections and recurrence rates of 15%–88% have been shown after an average of three injections [[26], [27], [28]] Gebhart and colleagues [29] have suggested that it is the mechanical effect of the injection rather than the steroid that helps in resorption. On the other hand Chigira et al. [8] consider the multiple punctures made in the cyst normalize its local circulation and disrupt any venous obstruction. (see Fig. 4).
Surgical option should be considered for those patients who have an obvious or suspected pathologic fracture, those whose cysts continue to enlarge, or cysts located in areas of the body that have a high risk of pathologic fracture such as the proximal femur [2]. [30, 1] Mik and colleagues [24] published a series of 55 patients with UBCs of whom 91% presented with pathologic fractures. Dormans et al. [31] reported risk factors for pathologic fracture associated with UBC are a cyst with a transverse diameter that is >85% of the diameter of the affected bone and a cyst wall that is < 0.5 mm thick [32]. [33] Several different surgical options have been recommended for active lesions. Traditionally curettage and bone grafting have been the definitive procedure for UBC. Other surgical procedures that have also been described include decompression without instrumentation, decompression with instrumentation like cannulated screws, flexible intramedullary nails and combined methods [2]. (see Fig. 5).
14.2. Resection, curettage and bone grafting
With curettage and bone grafting healing rates as low as 25%–36% have been reported. These rates improved slightly from 37% to 50% even after a repeat procedure was performed [34]. [35] When calcium sulphate pellets were added at the time of grafting, healing rates improved to 66%; nevertheless, recurrence rate remained high at 25% [36]. (see Fig. 6).
14.3. Curettage, local adjuvant treatment and grafting
In order to further optimize the treatment of UBC with curettage and bone grafting, some investigators used local adjuvants to improve outcomes. Hou et al. [36] in addition to curettage, intramedullary decompression and bone grafting with calcium sulphate in 12 patients, injected a 95% ethanol solution into the cyst cavity thrice to kill the membrane lining of the active cyst. Thereafter a cannulated screw was then placed for continuous decompression. They showed a partial or complete healing in 11 patients, and one patient has a subtrochanteric femur fracture two months after the initial treatment. (see Fig. 7).
14.4. Decompression/combined techniques
Decompression can be achieved by needles, curets, or implants like cannulated screws, K-wires or flexible intramedullary nails. This decompression allows native marrow to enter the cavity thus facilitate healing. Canavese and colleagues [37] compared UBC patients undergoing percutaneous curettage, steroid injection or autologous bone marrow injection. They showed that simple cyst decompression with percutaneous curettage had better healing rates (70%) than did steroid (41%) or bone marrow injections (21%). Contemporary methods decompress and stabilize the cysts with flexible intramedullary nails alone or in combination with demineralized bone marrow. Healing rates of 73% have been reported at 2–10 year follow-up solely with flexible intramedullary nails with recurrence rates of <10% [[38], [39], [40]] Kanellopoulos et al. [41] in addition to flexible intramedullary nails used injection of demineralized bone marrow/iliac crest bone marrow and reported healing rates of 77% with no recurrence.
Some investigators have combined mechanical techniques with biologic agents to enhance outcomes. Dormans et al. [17] used percutaneous intramedullary decompression, curettage and grafting with calcium sulphate pellets. After a mean followup of 22 months complete healing was reported in 92% patients. Using the same technique, Mik and colleagues [24] published their results at mean followup of 37 months with 80% showing a partial or complete healing response after initial surgery with a cumulative healing rate of 100% after three procedures.
14.5. Pathologic proximal femur fractures
UBCs in the proximal femur can lead to pathologic fractures with serious complications including varus malunion, osteonecrosis, and growth arrest of the proximal femoral physis [2]. [31] Dormans and Pill [31] classified pathological fractures of the proximal femur with UBC into six types based on the location and size of the cyst and the presence or absence of the lateral buttress. They recommended in addition to curettage and grafting use of a pediatric hip screw and side plate in cases where the lateral buttress is compromised for skeletally immature and mature hips in addition to selective postop spica application.
14.6. Prognosis
Most UBCs will heal by the time of physeal closure. Dormans et al. [31] consider patient age as an important factor. Patients older than 10 years heal at a higher rate (90%) than younger patients (60%), irrespective of the treatment modality. On the other hand, Haidar and colleagues [42] consider a lesion located < 2 cm from the physis as a risk factor for recurrence. The risk of recurrence can also be related to the type of treatment rather than the location of the lesion [2].
14.7. Complications
The most common complication is recurrence after treatment and developing a subsequent pathological fracture. (1) Other complications are potential embolization of the injected material such as steroid, bone marrow aspirate, local reactions to the material used to fill the cyst cavity, pathologic fracture and growth disturbances [2]. MacDonald reported an exaggerated inflammatory response following the use of recombinant bone morphogenic protein in patients with recalcitrant UBCs [43].
14.8. Conclusion
Plain roentgenograms are the diagnostic modality of choice. By the time of skeletal maturity most UBCs tend to resolve. Treatment has to be balanced with the morbidity associated with different options as well as the risk of pathological fractures. Nonoperative treatment may be a viable option for many patients with small or symptomatic lesions. Interventions include steroid injection, open curettage and bone grafting, decompression and percutaneous injection of marrow or graft substitutes.
Ethical approval
Not applicable.
Sources of funding
No funding was obtained for this research.
Author contribution
Dr. Shahryar Noordin – study design, data collection, data analysis, writing.
Dr. Salim Allana – study design, data collection, data analysis, writing.
Dr. Masood Umer – study design, data analysis, writing.
Dr. Mujahid Jamil – data analysis, writing.
Dr. Kiran Hilal – data collection, data analysis, writing.
Dr. Nasir Uddin – data collection, data analysis, writing.
Conflicts of interest
No authors have any conflicts of interest to declare with respect to this manuscript.
Research registration number
Not applicable.
Guarantor
Dr. Shahryar Noordin.
Dr. Salim Allana.
Dr. Masood Umer.
Dr. Mujahid Jamil.
Dr. Kiran Hilal.
Dr. Nasir Uddin.
References
- 1.Wilkins R.M. Unicameral bone cysts. J. Am. Acad. Orthop. Surg. 2000;8(4):217–224. doi: 10.5435/00124635-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Pretell-Mazzini J., Murphy R.F., Kushare I., Dormans J.P. Unicameral bone cysts: general characteristics and management controversies. J. Am. Acad. Orthop. Surg. 2014;22(5):295–303. doi: 10.5435/JAAOS-22-05-295. [DOI] [PubMed] [Google Scholar]
- 3.Biermann J.S. Common benign lesions of bone in children and adolescents. J. Pediatr. Orthop. 2002;22(2):268–273. [PubMed] [Google Scholar]
- 4.Boseker E., Bickel W., Dahlin D. A clinicopathologic study of simple unicameral bone cysts. Surg. Gynecol. Obstet. 1968;127(3):550–560. [PubMed] [Google Scholar]
- 5.Jaffe H.L., Lichtenstein L. Solitary unicameral bone cyst: with emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch. Surg. 1942;44(6):1004–1025. [Google Scholar]
- 6.Baumhoer D., Smida J., Nathrath M., Jundt G. The nature of the characteristic cementum-like matrix deposits in the walls of simple bone cysts. Histopathology. 2011;59(3):390–396. doi: 10.1111/j.1365-2559.2011.03962.x. [DOI] [PubMed] [Google Scholar]
- 7.Tariq M.U., Din N.U., Ahmad Z., Kayani N., Ahmed R. Cementum-like matrix in solitary bone cysts: a unique and characteristic but yet underrecognized feature of promising diagnostic utility. Ann. Diagn. Pathol. 2014;18(1):1–4. doi: 10.1016/j.anndiagpath.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Chigira M., Maehara S., Arita S., Udagawa E. The aetiology and treatment of simple bone cysts. Bone & Joint Journal. 1983;65(5):633–637. doi: 10.1302/0301-620X.65B5.6643570. [DOI] [PubMed] [Google Scholar]
- 9.Komiya S., Inoue A. Development of a solitary bone cyst–a report of a case suggesting its pathogenesis. Arch. Orthop. Trauma Surg. 2000;120(7):455–457. doi: 10.1007/s004029900082. [DOI] [PubMed] [Google Scholar]
- 10.Lokiec F., Wientroub S. Simple bone cyst: etiology, classification, pathology, and treatment modalities. J. Pediatr. Orthop. B. 1998;7(4):262–273. [PubMed] [Google Scholar]
- 11.Mascard E., Gomez-Brouchet A., Lambot K. Bone cysts: unicameral and aneurysmal bone cyst. J. Orthop. Traumatol.: Surgery & Research. 2015;101(1):S119–S127. doi: 10.1016/j.otsr.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Docquier P., editor. Kyste Osseux Essentiel. Elsevier Masson SAS; 2011. EMC. [Google Scholar]
- 13.Jordanov M.I. The “rising bubble” sign: a new aid in the diagnosis of unicameral bone cysts. Skeletal Radiology. 2009;38(6):597–600. doi: 10.1007/s00256-009-0685-y. [DOI] [PubMed] [Google Scholar]
- 14.Wu J.S., Hochman M.G. Bone Tumors: Springer; 2012. Cases; pp. 251–403. [Google Scholar]
- 15.Wootton-Gorges S.L. MR imaging of primary bone tumors and tumor-like conditions in children. Magn. Reson. Imag. Clin. N. Am. 2009;17(3):469–487. doi: 10.1016/j.mric.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Sanal H.T., Chen L., Haghighi P., Trudell D.J., Resnick D.L. Carpal bone cysts: MRI, gross pathology, and histology correlation in cadavers. Diagn. Interventional Radiol. 2014;20(6):503. doi: 10.5152/dir.2014.14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dormans J.P., Sankar W.N., Moroz L., Erol B. Percutaneous intramedullary decompression, curettage, and grafting with medical-grade calcium sulfate pellets for unicameral bone cysts in children: a new minimally invasive technique. J. Pediatr. Orthop. 2005;25(6):804–811. doi: 10.1097/01.bpo.0000184647.03981.a5. [DOI] [PubMed] [Google Scholar]
- 18.Neer C.S., Francis K.C., Marcove R.C., Terz J., Carbonara P.N. Treatment of unicameral bone cyst. J Bone Joint Surg Am. 1966;48(4):731–745. [PubMed] [Google Scholar]
- 19.Weinman J., Servaes S., Anupindi S. Treated unicameral bone cysts. Clin. Radiol. 2013;68(6):636–642. doi: 10.1016/j.crad.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan R.J., Meyer J.S., Dormans J.P., Davidson R.S. Diagnosing aneurysmal and unicameral bone cysts with magnetic resonance imaging. Clin. Orthop. Relat. Res. 1999;366:186–190. doi: 10.1097/00003086-199909000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Tahririan M., Motiffard M. Unicameral bone cyst of the proximal tibia in a five year old girl. Journal of research in medical sciences. The Official Journal of Isfahan University of Medical Sciences. 2012;17(1):104. [PMC free article] [PubMed] [Google Scholar]
- 22.Remotti F., Feldman F. Nonneoplastic lesions that simulate primary tumors of bone. Arch. Pathol. Lab Med. 2012;136(7):772–788. doi: 10.5858/arpa.2011-0557-RA. [DOI] [PubMed] [Google Scholar]
- 23.Gereige R., Kumar M. Bone lesions: benign and malignant. Pediatr. Rev. 2010;31(9):355. doi: 10.1542/pir.31-9-355. [DOI] [PubMed] [Google Scholar]
- 24.Mik G., Arkader A., Manteghi A., Dormans J.P. Results of a minimally invasive technique for treatment of unicameral bone cysts. Clin. Orthop. Relat. Res. 2009;467(11):2949. doi: 10.1007/s11999-009-1008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scaglietti O. L’azione osteogenetica dell acetato di metilprednisolone. Bull. Sci. Med. 1974;146:15–17. [Google Scholar]
- 26.Oppenheim W.L., Galleno H. Operative treatment versus steroid injection in the management of unicameral bone cysts. J. Pediatr. Orthop. 1984;4(1):1–7. doi: 10.1097/01241398-198401000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Scaglietti O., Marchetti P., Bartolozzi P. Final results obtained in the treatment of bone cysts with methylprednisolone acetate (depo-medrol) and a discussion of results achieved in other bone lesions. Clin. Orthop. Relat. Res. 1982;165:33–42. [PubMed] [Google Scholar]
- 28.Goel A., Kriger J., Bronfman R., Lauf E. Unicameral bone cysts: treatment with methylprednisone acetate injections. J. Foot Ankle Surg.: Official Publication of the American College of Foot and Ankle Surgeons. 1993;33(1):6–15. [PubMed] [Google Scholar]
- 29.Gebhart M., Blaimont P. Contribution to the vascular origin of the unicameral bone cyst. Acta Orthop. Belg. 1996;62(3):137–143. [PubMed] [Google Scholar]
- 30.Virchow R. Sitzungsb d Akad Wissensch; Berlin: 1876. Ueber die Bildung von Knochenzysten; p. 369. [Google Scholar]
- 31.Dormans J., Pill S. Fractures through bone cysts: unicameral bone cysts, aneurysmal bone cysts, fibrous cortical defects, and nonossifying fibromas. Instr. Course Lect. 2002;51:457. [PubMed] [Google Scholar]
- 32.Nakamura T., Takagi K., Kitagawa T., Harada M. Microdensity of solitary bone cyst after steroid injection. J. Pediatr. Orthop. 1988;8(5):566–568. doi: 10.1097/01241398-198809000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Ahn J., Park J. Pathological fractures secondary to unicameral bone cysts. Int. Orthop. 1994;18(1):20–22. doi: 10.1007/BF00180173. [DOI] [PubMed] [Google Scholar]
- 34.Brecelj J., Suhodolcan L. Continuous decompression of unicameral bone cyst with cannulated screws: a comparative study. J. Pediatr. Orthop. B. 2007;16(5):367–372. doi: 10.1097/BPB.0b013e32826d1ad6. [DOI] [PubMed] [Google Scholar]
- 35.Sung A.D., Anderson M.E., Zurakowski D., Hornicek F.J., Gebhardt M.C. Unicameral bone cyst: a retrospective study of three surgical treatments. Clin. Orthop. Relat. Res. 2008;466(10):2519–2526. doi: 10.1007/s11999-008-0407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou H.-Y., Wu K., Wang C.-T., Chang S.-M., Lin W.-H., Yang R.-S. Treatment of unicameral bone cyst. J Bone Joint Surg Am. 2010;92(4):855–862. doi: 10.2106/JBJS.I.00607. [DOI] [PubMed] [Google Scholar]
- 37.Canavese F., Wright J.G., Cole W.G., Hopyan S. Unicameral bone cysts: comparison of percutaneous curettage, steroid, and autologous bone marrow injections. J. Pediatr. Orthop. 2011;31(1):50–55. doi: 10.1097/BPO.0b013e3181ff7510. [DOI] [PubMed] [Google Scholar]
- 38.de Sanctis N., Andreacchio A. Elastic stable intramedullary nailing is the best treatment of unicameral bone cysts of the long bones in children?: prospective long-term follow-up study. J. Pediatr. Orthop. 2006;26(4):520–525. doi: 10.1097/01.bpo.0000217729.39288.df. [DOI] [PubMed] [Google Scholar]
- 39.Masquijo J.J., Baroni E., Miscione H. Continuous decompression with intramedullary nailing for the treatment of unicameral bone cysts. Journal of children's orthopaedics. 2008;2(4):279–283. doi: 10.1007/s11832-008-0114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glanzmann M.C., Campos L. Flexible intramedullary nailing for unicameral cysts in children's long bones. Journal of children's orthopaedics. 2007;1(2):97–100. doi: 10.1007/s11832-007-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanellopoulos A.D., Mavrogenis A.F., Papagelopoulos P.J., Soucacos P.N. Elastic intramedullary nailing and DBM-bone marrow injection for the treatment of simple bone cysts. World Journal of Surgical Oncology. 2007;5(1):111. doi: 10.1186/1477-7819-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haidar S.G., Culliford D.J., Gent E.D., Clarke N.M. Distance from the growth plate and its relation to the outcome of unicameral bone cyst treatment. Journal of children's orthopaedics. 2011;5(2):151–156. doi: 10.1007/s11832-010-0323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacDonald K.M., Swanstrom M.M., McCarthy J.J., Nemeth B.A., Guliani T.A., Noonan K.J. Exaggerated inflammatory response after use of recombinant bone morphogenetic protein in recurrent unicameral bone cysts. J. Pediatr. Orthop. 2010;30(2):199–205. doi: 10.1097/BPO.0b013e3181cec35b. [DOI] [PubMed] [Google Scholar]
- 44.Huang W.D. Langerhans cell histiocytosis of spine: a comparative study of clinical, imaging features, and diagnosis in children, adolescents, and adults. Spine J. 2013;13(9):1108–1117. doi: 10.1016/j.spinee.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Docquier P.-L. Elsevier Masson SAS; 2011. Kyste Osseux Essentiel. in: EMC. [Google Scholar]
- 46.Kaelin A. Kystes essentiels des os. In: Duparc J., editor. Cahiers d’enseignementsde la Sofcot. L'Expansion scientifique franc, aise; Paris: 1995. pp. 167–179. [Google Scholar]
- 47.vol 2017. 2017. (A Simple Bone Cyst in Cervical Vertebrae of an Adolescent Patient,” Case Reports in Orthopedics). Article ID 8908216, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.CASE REPORT Unusually large and aggressive traumatic bone cysts of the jaws – a series of five cases, Bhattacharyya1, Islam1 M.N., Chehal2 H.K., Reith3 J.D., Islam4 S., Fernandes5 R., McNally6 S.J., Cohen1 D.M. Oral Surgery. 2014;7(Issue Supplement S1) Version of Record online: 1 APR. [Google Scholar]