Abstract
This article present data related to the publication entitled “Interactions of pyrene and/or 1-hydroxypyrene with bovine serum albumin based on EEM-PARAFAC combined with molecular docking” (Zhang et al., 2018) [1]. The excitation-emission matrix (EEM) fluorescence spectral parameters of pyrene, 1-hydroxypyrene, bovine serum albumin (BSA), and their mixtures were presented in this article. Combined EEM - parallel factor analysis with fluorescence quenching analysis, some data related to the binding affinity of pyrene and/or 1-hydroxypyrene with BSA in the binary and ternary systems were obtained.
Specifications Table
| Subject area | Chemistry |
| More specific subject area | Analytical chemistry |
| Type of data | Table, figure |
| How data was acquired | FLS 920 steady/transient fluorescence spectrometer (Edinburgh, UK) |
| Data format | Raw, analyzed |
| Experimental factors | For all of the excitation-emission matrix (EEM) spectroscopy measurements, different concentrations of Pyrene, 1-hydroxypyrene, and BSA were prepared in 0.05 mol L−1Tris–HCl buffer (pH = 7.40, containing 0.10 mol L−1NaCl), with each containing ethanol of no more than 0.5‰. |
| Experimental features | Combined with EEM fluorescence spectra with parallel factor analysis to investigate the binding ability of BSA with pyrene and/or 1-hydroxypyrene |
| Data source location | State Key Laboratory of Marine Environmental Sciences of China (Xiamen University) (MEL), Xiamen university, Xiamen, China |
| Data accessibility | Data are provided with this article |
| Related research article | Interactions of pyrene and/or 1-hydroxypyrene with bovine serum albumin based on EEM-PARAFAC combined with molecular docking[1] |
Value of the data
-
●
The EEM data showed that the spectra of pyrene, 1-hydroxypyrene and BSA overlap, and this work provided the way to solve it, which may provide an insight to study other similar ternary interaction systems.
-
●
The data showed the difference of the binding affinity of pyrene or 1-hydroxypyrene with BSA both in the binary and ternary systems, which was helpful for readers to compare the binding affinity of pyrene or 1-hydroxypyrene with BSA.
-
●
The data may be further helpful for understanding the combined toxicity of pyrene and 1-hydroxypyrene.
1. Data
1.1. Excitation-emission matrix (EEM) fluorescence spectral parameters of Pyrene (Pyr), 1-hydroxypyrene (1-OHPyr), bovine serum albumin (BSA), and their mixtures
Table 1 showed the EEM fluorescence spectral parameters of Pyr (6.0 × 10−7 mol L−1), 1-OHPyr (7.5 × 10−7 mol L−1), BSA (1.0 × 10−6 mol L−1), and their mixtures. The range of excitation/emission wavelengths of Pyr, 1-OHPyr, BSA, and their mixtures, and the maximum excitation/emission wavelength of each were presented. As can be seen, the excitation spectra of Pyr were in the range of 260–278 and 295–340 nm, with the emission spectra ranging from 370 to 440 nm; for 1-OHPyr, its excitation spectra ranged from 260 to 290 nm and 310 to 370 nm, with the emission spectra ranging from 380 to 440 nm; however, excitation spectra of BSA ranged from 250 to 310 nm, with the emission spectra in the range of 290–450 nm. The data indicated that the EEM spectra of the mixed system of BSA, Pyr, and/or 1-OHPyr overlap severely.
Table 1.
Fluorescence spectra properties of Pyr, 1-OHPyr, BSA, and their mixtures.a
| System | λex (nm) | λem (nm) | Peak position λex (nm) | Peak position λem (nm) |
|---|---|---|---|---|
| Pyr | 260–278, | 370–440 | 333 | 372 |
| 295–340 | ||||
| 1-OHPyr | 260–290, | 380–440 | 346 | 386 |
| 310–370 | ||||
| BSA | 250–310 | 290–450 | 280 | 341 |
| Mixtures | 250–390 | 290–460 | 335 | 388 |
CBSA = 1.0 × 10−6 mol L−1; C1-OHPyr = 7.5 × 10−7 mol L−1; CPyr = 6.0 × 10−7 mol L−1
1.2. Fluorescence quenching data of BSA by Pyr and 1-OHPyr in the binary and ternary systems using Stern–Volmer plots
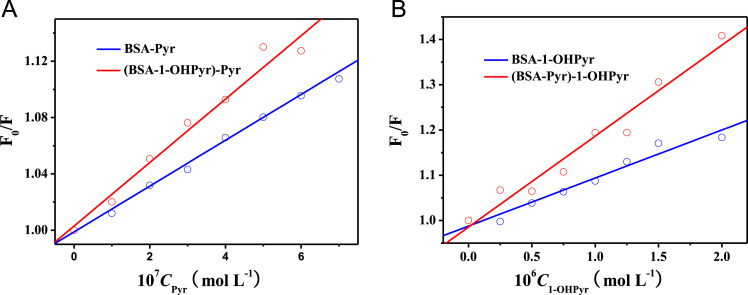
After using the parallel factor analysis to decompose the EEM spectra of complex systems of Pyr, 1-OHP and BSA, the fluorescence properties of each individual component were obtained [1]. Combined with the fluorescence quenching method, the interactions of BSA with Pyr and 1-OHPyr in binary and ternary interaction systems were further studied. The measured fluorescence quenching data of BSA by Pyr and 1-OHPyr in the binary and ternary systems were fitted by Origin 7.5 based on Stern–Volmer plots (Eq. (1)) (Fig. 1) [2], and corresponding values were listed in Table 2.
| (1) |
Fig. 1.
Stern–Volmer plots for the quenching of BSA by Pyr and 1-OHPyr in the binary and ternary systems.
Table 2.
Ksv and Kq values for Pyr - BSA and 1-OHPyr-BSA in the binary and ternary systems.
| System | 10−5Ksv (L mol−1) | 10–13Kq (L mol−1 s−1) | Ra |
|---|---|---|---|
| BSA-Pyr | 1.63 | 1.63 | 0.992 |
| (BSA-1-OHPyr)-Pyr | 2.25 | 2.25 | 0.990 |
| BSA-1-OHPyr | 1.06 | 1.06 | 0.980 |
| (BSA-Pyr)-1-OHPyr | 2.01 | 2.01 | 0.980 |
R is the correlation coefficient.
F0 and F are the relative fluorescence intensities of BSA in the absence and presence of Pyr or 1-OHPyr, respectively; [Q] is the concentration of Pyr or 1-OHPyr, Ksv is the Stern–Volmer quenching constant, and Kq is the quenching rate constant.
Fig. 2 showed the double logarithm plots for the quenching of BSA by Pyr and 1-OHPyr in binary and ternary systems. The binding constant (Kb) and the number of binding sites (n) of Pyr and 1-OHPyr with BSA in binary and ternary interaction systems could be calculated by fitting the curve based on Eq. (2) [3], [4].
| (2) |
Fig. 2.
Double logarithm plots for the quenching of BSA by Pyr and 1-OHPyr in binary and ternary systems.
2. Experimental design, materials, and methods
2.1. Materials
BSA (purity > 99.5%), Pyr (purity > 98%) and 1-OHP (purity > 99%) were purchased from Sigma Chemical Company (St. Louis, MO, USA) and were used without further purification. Stock solutions of 4.0 × 10−5 mol L−1 BSA were prepared in 0.05 mol L−1 Tris–HCl buffer (pH = 7.40, containing 0.10 mol L−1 NaCl). Stock solutions of 1-OHP and Pyr were prepared individually in ethanol at concentrations of 2.0 × 10−3 mol L−1. The stock solutions were stored at 277 K in the dark. All of the other chemicals that were used were of analytical reagent grade. Milli-Q water (18.2 MΩ cm) was used throughout the study.
2.2. Methods
2.2.1. EEM fluorescence spectra of Pyr, 1-OHPyr, BSA, and their mixtures
For all of the EEM spectroscopy measurements, 32 samples of different concentrations of Pyr, 1-OHPyr, and BSA were prepared with Tris–HCl buffer (Table 3), with each containing ethanol of no more than 0.5‰. Moreover, pure solutions of Pyr (6.0 × 10−5 mol L−1), 1-OHPyr (7.5 × 10−5 mol L−1), and BSA (1.0 × 10−6 mol L−1) were also prepared in Tris–HCl buffer as reference samples. After equilibration at 25 ± 1 °C for 20 min, EEM measurements were carried out on an FLS 920 steady/transient fluorescence spectrometer (Edinburgh, UK), which was equipped with a 150-W xenon lamp. Samples were measured in the excitation range of 250–410 nm (every 5 nm) and emission range of 280–500 nm (every 2 nm) using a 10-mm quartz cuvette. The excitation and emission slits were both set at 1 nm. Thus, the EEM dataset had the dimensions of 33 (Ex.) × 111 (Em.) × 35 (Samples).The data showed in Section 1.1 was the EEM fluorescence spectra of pure solutions of Pyr (6.0 × 10−5 mol L−1), 1-OHPyr (7.5 × 10−5 mol L−1), BSA (1.0 × 10−6 mol L−1) and their mixed solution (sample 7: CBSA = 1.0 × 10−6 mol L−1, C1-OHPyr = 7.5 × 10−7 mol L−1, CPyr = 6.0 × 10−7 mol L−1).
Table 3.
The concentration setups of Pyr, 1-OHPyr, and BSA in 32 samples [1].
| Nos. | 106 Pyr (mol L−1) | 107 1-OHPyr (mol L−1) | 106 BSA (mol L−1) | Nos. | 106 Pyr (mol L−1) | 107 1-OHPyr (mol L−1) | 106 BSA (mol L−1) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 7.5 | 1.0 | 17 | 0 | 0 | 1.0 |
| 2 | 0.1 | 7.5 | 1.0 | 18 | 0.1 | 0 | 1.0 |
| 3 | 0.2 | 7.5 | 1.0 | 19 | 0.2 | 0 | 1.0 |
| 4 | 0.3 | 7.5 | 1.0 | 20 | 0.3 | 0 | 1.0 |
| 5 | 0.4 | 7.5 | 1.0 | 21 | 0.4 | 0 | 1.0 |
| 6 | 0.5 | 7.5 | 1.0 | 22 | 0.5 | 0 | 1.0 |
| 7 | 0.6 | 7.5 | 1.0 | 23 | 0.6 | 0 | 1.0 |
| 8 | 0.7 | 7.5 | 1.0 | 24 | 0.7 | 0 | 1.0 |
| 9 | 0.6 | 0 | 1.0 | 25 | 0 | 0 | 1.0 |
| 10 | 0.6 | 2.5 | 1.0 | 26 | 0 | 2.5 | 1.0 |
| 11 | 0.6 | 5.0 | 1.0 | 27 | 0 | 5.0 | 1.0 |
| 12 | 0.6 | 7.5 | 1.0 | 28 | 0 | 7.5 | 1.0 |
| 13 | 0.6 | 10.0 | 1.0 | 29 | 0 | 10.0 | 1.0 |
| 14 | 0.6 | 12.5 | 1.0 | 30 | 0 | 12.5 | 1.0 |
| 15 | 0.6 | 15.0 | 1.0 | 31 | 0 | 15.0 | 1.0 |
| 16 | 0.6 | 20.0 | 1.0 | 32 | 0 | 20.0 | 1.0 |
Samples 1–8: mixed BSA with 1-OHPyr first, then addition of Pyr; Samples: 9–16: mixed BSA with Pyr first, then addition of 1-OHPyr.
2.2.2. PARAFAC analysis
Using MATLAB R2016b software (the MathWorks, Inc., Natick, MA, USA), the EEM dataset (size of 33 × 111 × 35) was decomposed by the PARAFAC method, according to the procedures described by Ref. [5]. First, the EEM spectra of the samples were corrected by subtracting the EEM spectra of the blank samples (Tris–HCl buffer) and removing the Rayleigh scattering and the two order Raman scattering peaks to reduce the scattering light and other background interference. Second, the 2–8 component model is used to decompose the EEM dataset by trilinear decomposition. To reduce the time of Matlab analysis, the relative fluorescence intensity values of the 35 EEM spectra were reduced by 100 times; thus, the relative fluorescence intensity values of the three components reported in this section were 0.01 times their practically measured values. Finally, the relative fluorescence intensity values of BSA obtained were used for the fluorescence quenching analysis.
Acknowledgments
We gratefully acknowledge the financial support of the National Major Scientific Instruments Development Project of China (No. 21627814), the National Natural Science Foundation of China (No. 21577110), the Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP, no. 20130121130005), The Natural Science Foundation of Fujian Province (No. 2018J05024) and the Education and Scientific Research Projects of Young and Middle-Aged Teachers in Fujian Province (No. JAT170841).
Footnotes
Transparency data associated with this article can be found in the online version at 10.1016/j.dib.2018.08.140.
Transparency document. Supplementary material
Supplementary material
References
- 1.Zhang J., Chen L.F., Zhu Y.X., Zhang Y. Interactions of pyrene and/or 1-hydroxypyrene with bovine serum albumin based on EEM-PARAFAC combined with molecular docking. Talanta. 2018;186(11):497–505. doi: 10.1016/j.talanta.2018.04.066. [DOI] [PubMed] [Google Scholar]
- 2.Lakowicz J.R. Principles of Fluorescence Spectroscopy. third ed. Springer Science & Business Media; New York: 2006. [Google Scholar]
- 3.Anbazhagan V., Renganathan R. Study on the binding of 2,3-diazabicyclo[2.2.2]oct-2-ene with bovine serum albumin by fluorescence spectroscopy. J. Lumin. 2008;128(9):1454–1458. [Google Scholar]
- 4.Camargo C.R., Caruso Í.P., Gutierrez S.J.C., Fossey M.A., Filho J.M.B., Cornélio M.L. Spectral and computational features of the binding between riparins and human serum albumin. Spectrochim. Acta A. 2018;190(Suppl. C):S81–S88. doi: 10.1016/j.saa.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 5.Stedmon C.A., Bro R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial. Limnol. Oceanogr. Methods. 2008;6(11):572–579. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material