Abstract
We report 2 cases of isolated hepatic hemangiomatosis: a 76-year-old woman who is, to our knowledge, the oldest person with this diagnosis, and a 74-year-old woman. Magnetic resonance imaging of the abdomen showed T2 hyper intense lesions throughout the liver, peripheral nodular arterial enhancement, and filling of contrast on the portal venous and delayed phases. Computed tomography showed liver lesions with peripheral nodular enhancement in the early phase and a centripetal pattern or “filling in” during the late phase; the lesions opacified after a delay of 3 or more minutes and remained isodense or hyperdense on delayed scans. Both images were consistent with hepatic hemangiomatosis. These cases help increase awareness about benign and unusual liver lesions with radiologic characteristics similar to those of malignant liver tumors. The authors also present a review of 15 other cases of isolated hepatic hemangiomatosis reported in English literature from 1970 to present.
Keywords: Hepatic hemangiomatosis
Introduction
Hepatic hemangioma is the most prevalent type of benign liver tumor with an incidence reaching 20% in the overall population [1]. Conversely, hepatic hemangiomatosis, a term used to describe the presence of innumerable hemangiomas, is a rare presentation of liver hemangiomas and is more frequently seen in neonates [2], [3]. Isolated hepatic hemangiomatosis, without extrahepatic involvement, is uncommon, particularly in the adult population [4]. We report 2 cases: 76-year-old and 74-year-old women, both diagnosed with isolated hepatic hemangiomatosis, with a review of 15 other cases reported in English literature from 1970 to 2018.
Case reports
Patient 1
A 76-year-old woman with a past medical history of hypertension, hyperlipidemia, postherpetic neuralgia, and gastroesophageal reflux disease was seen for abdominal pain. She had a history of cigarette smoking more than 35 years ago and denied any alcohol or illicit drug use. Her medications included Atenolol, Lisinopril, Gabapentin, Omeprazole, ASA, and Ativan. The patient saw her primary care provider for right upper quadrant abdominal pain, described as nonradiating, moderate in severity, that she was unable to characterize. Her physical exam was normal and her laboratory tests, including complete blood count, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin, albumin, and prothrombin time were normal. Alpha-fetoprotein was 1.0 ng/mL. Magnetic resonance imaging (MRI) of the abdomen with and without contrast showed multiple T2 hyperintense lesions throughout the liver, and a liver biopsy was performed to exclude a neoplastic process. With a diagnosis of hepatic hemangiomatosis, she was referred to our center in 2018 for specialized care. Upon her visit to our institute, she reported that she was previously diagnosed with liver lesions and had a biopsy performed at a hospital in Iowa in 2009; at that time, she was told she had benign liver tumors.
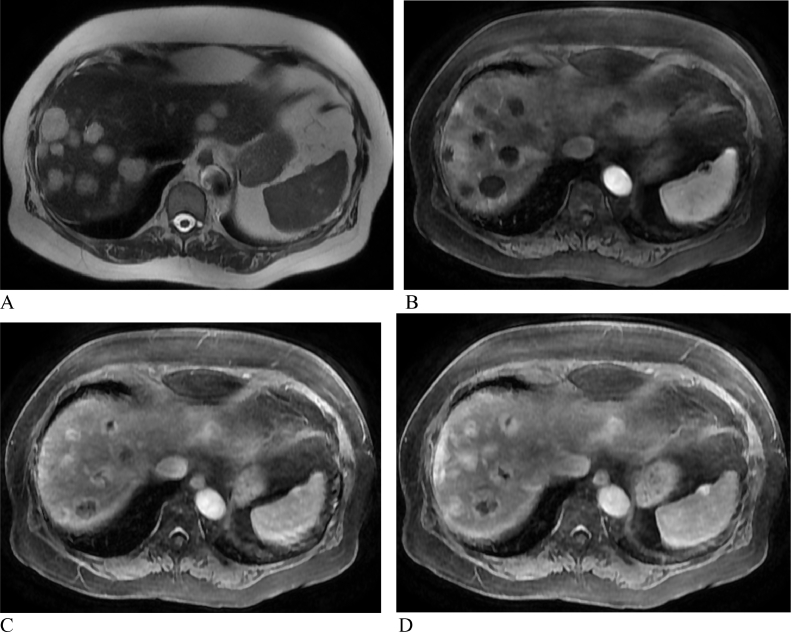
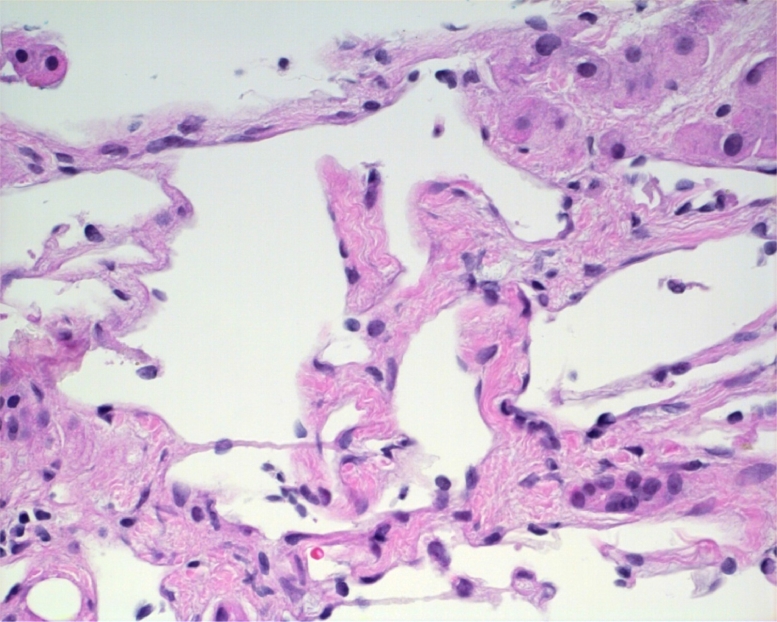
Her most recent MRI showed multiple hyperintense T2 liver lesions. On the postcontrast images, the hepatic lesions demonstrated peripheral nodular arterial enhancement and slowly fill with contrast on the portal venous and delayed phases. This pattern is consistent with hemangiomatosis (Fig. 1). The liver biopsy showed multiple dilated vascular spaces scattered within the otherwise benign liver tissue. The portal areas had a mild chronic inflammatory infiltrate consisting of lymphocytes and plasma cells. The dilated thin-walled vascular channels were lined by bland endothelium. The biopsy confirmed the diagnosis of hepatic hemangiomatosis (Fig. 2).
Fig. 1.
MRI of the abdomen with a pattern consistent with hemangiomatosis. A: The multiple hepatic lesions demonstrate hyperintense T2 signal. B: On the postcontrast images, the lesions demonstrate peripheral nodular arterial enhancement. C: The lesions slowly fill in with contrast on the portal venous phase. D: The lesions slowly fill in with contrast on the delayed phase.
Fig. 2.
Liver biopsy showed multiple-dilated vascular spaces scattered within the otherwise benign liver tissue. The portal areas contained mild chronic inflammatory infiltrate consisting of lymphocytes and plasma cells. The dilated thin-walled vascular channels were lined by bland endothelium.
Patient 2
A 74-year-old woman with a past medical history of primary biliary cholangitis, hyperlipidemia, and hypothyroidism presented to our facility for primary biliary cholangitis follow-up. Her medications include Ursodiol, Obeticholic acid, Pravastatin, ASA, Levothyroxine, magnesium, vitamin D, fish oil, and multivitamin. Her physical exam was normal, including no hepatomegaly, and her laboratory tests including ALT, AST, ALP, bilirubin, albumin, and prothrombin time were normal. Alpha-fetoprotein was 5.2 ng/mL. A computed tomography (CT) of the abdomen with and without contrast in June 2018 showed multiple liver lesions, the 2 largest ones measuring 2.7 cm and 4.1 cm, both of which were unchanged from prior CT in 2017. The lesions showed a peripheral nodular enhancement in the early phase, and a centripetal pattern or “filling in” during the late phase. The lesions opacified after a delay of 3 or more minutes and remained isodense or hyperdense on delayed scans. These characteristics were consistent with the diagnosis of liver hemangiomatosis and biopsy was not performed.
Discussion
Hepatic hemangiomas are also known as cavernous hemangiomas given the cavernous vascular space seen on liver biopsy [5]. Liver hemangiomas are typically isolated lesions, but can be seen in both liver lobes in 40% of cases. The diameter of the hemangioma can vary from only a few millimeters to greater than 20 cm, and the larger lesions are referred to as giant hemangiomas [6]. Hemangiomatosis, or the occurrence of innumerable hemangiomas, is a less common presentation of hepatic hemangiomas and is more commonly seen in neonates diagnosed with hereditary hemorrhagic telangiectasia or systemic hemangiomatosis [2], [7], [8]. Hepatic hemangiomatosis has also been described in association with giant cavernous hemangiomas. In 2011, Jhaveri et al. described hepatic hemangiomatosis in 18 out of 41 patients who were diagnosed with giant cavernous hemangiomas [7]. Additional reports of hemangiomatosis-associated giant hemangioma have been described [4], [15], [18], [21], [24], [30]. Isolated liver hemangiomatosis, without any extrahepatic involvement or association with giant hemangioma, is uncommon in children and even more so in the adult population.
Both of our patients were reassured about the benign nature of the liver lesions and plans were made for consistent follow-up appointments. They were advised against the use of hormonal replacement therapy, and it was recommended that they carry medical alert bracelets with the diagnosis. This would avoid unnecessary liver biopsies and a potential delay in the diagnosis of ruptured hemangiomas.
Demographics and clinical presentations
Table 1 depicts the clinical characteristics of our patients and the other 15 cases of isolated hepatic hemangiomatosis reported in English literature from 1970 to present. The age of patients at the time of publication varied from 21 to 76 with a mean age of 46.4. Of the 17 total cases reported, 13 were women, which is consistent with the previously described predominance of hepatic hemangiomatosis in women [9], [10], [11]. Our patients were 74 and 76 years old and are the oldest patients in this review.
Table 1.
Clinical characteristics of our patients and 15 cases of isolated hepatic hemangiomatosis reported in English literature from 1970 to present.
| Author | Age | Sex | Hormone use | Symptoms | Hepatomegaly |
|---|---|---|---|---|---|
| This manuscript | 76 | F | N | Abdominal pain | N |
| This manuscript | 74 | F | N | None | N |
| Gruttadauria et al. 2015 | 37 | F | N | Abdominal pain, fatigue, anemia | Unk |
| Batista et al. 2014 | 68 | M | N | None | Y |
| Ramos and Coelho, 2014 | 66 | M | N | None | N |
| Supakul et al. 2013 | 53 | M | N | Fatigue, dyspnea on exertion | Y |
| Kim et al. 2008 | 33 | F | N | Abdominal distention, edema | Y |
| Kim et al. 2008 | 33 | F | N | Abdominal distention, shortness of breath | Y |
| Bakhshi et al. 2008 | 65 | M | N | Mass in right hypochondrium and epigastrium | Y |
| Ozakyol and Kebapci, 2006 | 47 | F | Y | Abdominal pain | N |
| Ozakyol and Kebapci, 2006 | 42 | F | Y | Abdominal pain | N |
| Tanaka et al. 2002 | 32 | F | N | Abdominal pain, lower back pain, dyspnea on exertion | N |
| Jayanthi et al. 2000 | 21 | F | N | Abdominal pain, dyspnea | Y |
| Moon et al. 2000 | 50 | F | N | Postprandial abdominal pain and indigestion | N |
| Lehmann et al. 1999 | 35 | F | N | Abdominal pain, weight loss, night sweats, fever | Y |
| Feurle, 1990 | 22 | F | Y | Abdominal pain radiating to right shoulder | N |
| Kositchek and Cullen, 1970 | 36 | F | Y | Abdominal fullness and pain, distention, postprandial fullness, constipation | Y |
Unk: unknown.
While the etiology of hepatic hemangiomatosis remains unknown, one study suggests a role of the vascular endothelial growth factor in its development [12]. In addition, there is a case report that found an association of hemangiomatosis with the use of metoclopramide, as well as regression of the hepatic lesions with discontinuation of the drug [13]. In patients with hepatic hemangiomas, enlargement of the lesions during pregnancy and with estrogen or progesterone therapy, with subsequent regression after the medication is discontinued, suggests hormonal influence over tumor growth. This has not been described in patients with hepatic hemangiomatosis. However, 4 of the 13 women with hepatic hemangiomatosis reported to date had previously taken some form of estrogen [13], [14], [16]. Our patients were not on hormonal replacement; however, our first patient had 3 full-term pregnancies and our second patient had 2 full-term pregnancies.
Out of the 17 cases reported, 13 patients had symptoms of upper abdominal pain and/or sensation of fullness and/or abdominal mass, in either the right upper quadrant and/or epigastrium. Other common symptoms included dyspnea and systemic symptoms of fevers and night sweats. Fatigue was also a reported symptom [17]. The most common finding on physical exam was hepatomegaly [19], [20], [21] reported in 8 of the 17 patients.
Laboratory values and imaging studies
Few of the reported cases included laboratory tests; of those who did, the liver chemistry tests, that is, ALT, AST, ALP, GGT, and bilirubin were normal in the majority of patients. Alpha fetoprotein was reported in 6 cases and was normal in all.
Abdominal ultrasound was performed in 8 out of the 17 cases. On ultrasound exam, the characteristic finding was hyper- or hypoechoic nodules diffusely distributed throughout the liver. Other imaging modalities included CT, MRI, and CT angiography. On CT, performed in 9 out of 17 cases, the lesions of hepatic hemangiomatosis were described as heterogeneous nodular enhancement and hypodense hepatic lesions. MRI, performed in 7 out of 17 cases, showed low signal intensity on T1-weighted images, high signal intensity on T2-weighted sequences, and delayed enhancement in the contrast images.
Differential diagnosis
While the characteristics of the cross-sectional images described above are consistent with the diagnosis of liver hemangiomatosis, there are no definitive radiologic features that will diagnose liver hemangiomatosis with certainty. Therefore, a detailed clinical history and physical exam are paramount. Other liver lesions that may present with similar characteristics are liver angiosarcomas, hepatic epithelioid hemangioendothelioma, and multicentric hepatocellular carcinoma. Table 2 describes the different clinical characteristics of the tumors that should be considered in the differential diagnosis of hepatic hemangiomatosis.
Table 2.
Clinical characteristics of tumors that should be considered in the differential diagnosis of hepatic hemangiomatosis.
| Diagnosis | Age | Sex | History of chronic liver disease | Symptoms | References |
|---|---|---|---|---|---|
| Hepatic hemangiomatosis | 30-50 | F | N | Abdominal pain and fullness | [11], [31], [32] |
| Angiosarcoma | >60 | M | Unk | Abdominal pain, jaundice, ascites, weight loss | [33], [34], [35], [36] |
| Epithelioid hemangioendothelioma | 50′s | F | N | Abdominal pain, mass, weight loss | [37], [38], [39], [40] |
| Hepatocellular carcinoma | 50-60 | M | Y | Upper abdominal pain, weight loss, early satiety, mass in upper abdomen, signs of decompensation (ascites, jaundice, variceal bleeding, encephalopathy) | [41], [42] |
Unk: unknown.
Histology
In 12 out of the 17 reported cases, a liver biopsy was performed to confirm the diagnosis of hepatic hemangiomatosis. As seen in our 76-year-old patient (Fig. 2), the histologic findings of those patients for whom a liver biopsy was performed showed multiple-dilated vascular spaces scattered within the otherwise benign liver tissue. The portal areas contained mild chronic inflammatory infiltrate consisting of lymphocytes and plasma cells. The dilated thin-walled vascular channels were lined by bland endothelium. However, for patients presenting with the characteristic findings on image tests, particularly cross-sectional image, liver biopsy should be avoided, given the vascular nature of these lesions with an increased risk of bleeding.
Follow-up and outcomes
Given the few number of cases reported to date, it is not possible to make statements on the natural history or outcome of hepatic hemangiomatosis. Our patients have done very well and have remained asymptomatic since the diagnoses, 9 years prior to this publication. Other reports described similarly favorable outcomes, although most patients were followed for less than 5 years [13], [14], [16], [22], [23], [25], [26]. Nonetheless, not all reported cases had the same uneventful course. There has been a report of a patient who developed liver failure and eventually expired 10 days after hospitalization [27]. Three other fatalities were reported, but no clear relationship between the hemangiomatosis and the cause of death. One patient was diagnosed with deep venous thrombosis during admission and expired after 1 week [28]. Additionally, there was a patient who was admitted with lactic acidosis and expired after cardiopulmonary arrest [29]. Another patient expired after hemorrhagic shock due to a duodenal ulcer that was present for a few years prior to hospital admission [19].
The scarce number of cases reported and the diversity of the outcome of this unique presentation of isolated liver hemangiomatosis explain the lack of recommendations for treatment, or if any treatment should be considered. Based on the reports of some hemangiomas increasing in size when the patients were exposed to estrogen, recommendations against the use of oral contraceptives and hormonal replacement therapy should be considered. Given the report of an association between metoclopramide and hepatic hemangiomatosis [13], discontinuing this medication may be considered. Otherwise, the treatment approach should be individualized for each patient, based on the symptoms and radiologic findings at presentation. There are reports of patients treated with arterial embolization and hepatectomy [19], [30], and a case of a patient who developed progressively growing hemangiomatosis on the remaining liver parenchyma after left hepatectomy [26]. The above anecdotal reports illustrate the need to individualize the care based on each patient's presentation.
Conclusion
We present 2 cases of isolated hepatic hemangiomatosis: a 76-year-old woman, who is, to our knowledge, the oldest person reported to date with this diagnosis, as well as a 74-year-old woman. These case reports help increase awareness about a benign liver lesion that has radiologic characteristics similar to those of malignant liver tumors. The literature review confirms the rarity of this entity, which is seen more frequently in women and are often asymptomatic. The finding of hepatomegaly on physical exam is likely to prompt imaging of the liver. The importance of a detailed clinical history for the evaluation of these patients cannot be over emphasized. The absence of chronic liver disease, normal laboratory tests, and careful evaluation of the cross-sectional imaging of the liver may obviate the need to perform a liver biopsy. Given the vascular nature of these lesions, liver biopsy is likely to carry an increased risk of bleeding, similar to that seen in biopsies of liver hemangiomas.
Author contributions
Both authors reviewed the information of the patients in the report, reviewed the published literature, and wrote the manuscript.
Informed consent statement
The patients involved in this study gave written informed consent authorizing the use and disclosure of their protected health information.
Acknowledgments
The authors acknowledge the help of Drs. Rishi Gosalia and Wendi Zhou who gave us the images of the patient's MRI and liver biopsy, respectively. We thank our patients who graciously agreed to have the histories of their liver disease reported.
Footnotes
Financial disclosures: None.
Conflict of interest statement: The authors declare there are no conflicts of interest related to this study.
References
- 1.Chiche L, Adam J. Diagnosis and management of benign liver tumors. Semin Liver Dis. 2013;33:236–247. doi: 10.1055/s-0033-1351779. PMid:23943104. [DOI] [PubMed] [Google Scholar]
- 2.Isaacs H., Jr Fetal and neonatal hepatic tumors. J Pediatr Surg. 2007;42:1797–1803. doi: 10.1016/j.jpedsurg.2007.07.047. PMid:18022426. [DOI] [PubMed] [Google Scholar]
- 3.Lopriore E, Markhorst DG. Diffuse neonatal haemangiomatosis: new views on diagnostic criteria and prognosis. Acta Paediatr. 1999;88:93–97. doi: 10.1111/j.1651-2227.1999.tb01276.x. PMid:10090556. [DOI] [PubMed] [Google Scholar]
- 4.Guerra A, Infante A, Rinninella E, Spinelli I, Mazziotti MA, De Gaetano AM. A peculiar case of diffuse hemangiomatosis of the left hepatic lobe in an asymptomatic adult patient: case report and literature review. Eur Rev Med Pharmacol Sci. 2017;21:1593–1597. PMid:28429345. [PubMed] [Google Scholar]
- 5.Evans J, Sabih DE. Hemangioma, Cavernous Liver. [Updated 2017 Dec 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470283/.
- 6.Ribeiro MA, Papaiordanou F, Goncalves JM, Chaib E. Spontaneous rupture of hepatic hemangiomas: a review of the literature. World J Hepatol. 2010;2:428–433. doi: 10.4254/wjh.v2.i12.428. PMid:21191518 PMCid:PMC3010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jhaveri KS, Vlachou PA, Guindi M, Fischer S, Khalili K, Cleary SP. Association of hepatic hemangiomatosis with giant cavernous hemangioma in the adult population: prevalence, imaging appearance, and relevance. Am J Roentgenol. 2011;196:809–815. doi: 10.2214/AJR.09.4143. PMid:21427329. [DOI] [PubMed] [Google Scholar]
- 8.Buscarini E, Buscarini L, Civardi G, Arruzzoli S, Bossalini G, Piantanida M. Hepatic vascular malformations in hereditary hemorrhagic telangiectasia: imaging findings. Am J Roentgenol. 1994;163:1105–1110. doi: 10.2214/ajr.163.5.7976883. PMid:7976883. [DOI] [PubMed] [Google Scholar]
- 9.Bajenaru N, Balaban V, Savulescu F, Campeanu I, Patrascu T. Hepatic hemangioma: review. J Med Life. 2015;8:4–11. PMid:26361504 PMCid:PMC4564031. [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy KR, Kligerman S, Levi J, Livingstone A, Molina E, Franceschi D. Benign and solid tumors of the liver: relationship to sex, age, size of tumors, and outcome. Am Surg. 2001;67:173–178. PMid:11243545. [PubMed] [Google Scholar]
- 11.Gandolfi L, Leo P, Solmi L, Vitelli E, Verros G, Colecchia A. Natural history of hepatic haemangiomas: clinical and ultrasound study. Gut. 1991;32:677–680. doi: 10.1136/gut.32.6.677. PMid:2060877 PMCid:PMC1378888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitajima S, Liu E, Morimoto M, Koike T, Yu Y, Watanabe T. Transgenic rabbits with increased VEGF expression develop hemangiomas in the liver: a new model for Kasabach-Merritt syndrome. Lab Invest. 2005;85:1517–1527. doi: 10.1038/labinvest.3700346. PMid:16200077. [DOI] [PubMed] [Google Scholar]
- 13.Feurle GE. Arteriovenous shunting and cholestasis in hepatic hemangiomatosis associated with metoclopramide. Gastroenterology. 1990;99:258–262. doi: 10.1016/0016-5085(90)91256-6. [DOI] [PubMed] [Google Scholar]
- 14.Ozakyol A, Kebapci M. Enhanced growth of hepatic hemangiomatosis in two adults after postmenopausal estrogen replacement therapy. Tohoku J Exp Med. 2006;210:257–261. doi: 10.1620/tjem.210.257. PMid:17077603. [DOI] [PubMed] [Google Scholar]
- 15.Freedman AN. Liver lesions and oral contraceptives. CMA J. 1982;126:1149–1150. PMid: 7074431. [PMC free article] [PubMed] [Google Scholar]
- 16.Kositchek RJ, Cullen RA. Hemangiomatosis of the liver with thrombosis following use of an oral contraceptive. West J Med. 1970;113:70–74. PMid: 5457518. [PMC free article] [PubMed] [Google Scholar]
- 17.Gruttadauria S, Pagano D, Burgio G, Arcadipane A, Panarello G, Petridis I. Liver transplantation for hemoperitoneum secondary to huge multiple hemangiomatosis: a case report of a tele-intensive care unit in deceased-donor management. Telemed e-Health. 2015;21:499–502. doi: 10.1089/tmj.2014.0145. PMid:25714805. [DOI] [PubMed] [Google Scholar]
- 18.Adam YG, Huvos AG, Fortner JG. Giant hemangiomas of the liver. Ann Surg. 1970;172:239–245. doi: 10.1097/00000658-197008000-00010. PMid:5433290 PMCid:PMC1397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakhshi GD, Shaikh A, Borisa AD, Alagappan C, Patnaik A, Subnis BM. Diffused liver haemangiomatosis in an adult. Bombay Hosp J. 2008;50:669–671. [Google Scholar]
- 20.Kim JD, Chang UI, Yang JM. Clinical challenges and images in GI. Diffuse hepatic hemangiomatosis involving the entire liver. Gastroenterology. 2008;134:1830. doi: 10.1053/j.gastro.2008.05.006. 2197. [DOI] [PubMed] [Google Scholar]
- 21.Ohkura Y, Hashimoto M, Lee S, Sasaki K, Matsuda M, Watanabe G. Right hepatectomy for giant cavernous hemangioma with diffuse hemangiomatosis around Glisson's capsule. World J Gastroenterol. 2014;20:8312–8316. doi: 10.3748/wjg.v20.i25.8312. PMid:25009410 PMCid:PMC4081710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batista A, Matos AP, Oliveira e Neta J, Ramalho M. Diffuse hepatic hemangiomatosis in the adult without extra-hepatic involvement: an extremely rare occurrence. J Clin Imaging Sci. 2014;4:1–4. doi: 10.4103/2156-7514.139733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos LR, Coelho ML. Hepatic haemangiomatosis: multinodular liver in an asymptomatic elderly man. BMJ Case Rep. 2014 doi: 10.1136/bcr-2013-202505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka A, Morimoto T, Yamamori T, Moriyasu F, Yamaoka Y. Atypical liver hemangioma with shunt: long-term follow-up. J Hepatobiliary Pancreat Surg. 2002;9:750–754. doi: 10.1007/s005340200104. PMid:12658411. [DOI] [PubMed] [Google Scholar]
- 25.Moon WS, Yu HC, Lee JM, Kang MJ. Diffuse hepatic hemangiomatosis in an adult. J Korea Med Sci. 2000;15:471–474. doi: 10.3346/jkms.2000.15.4.471. PMid:10983701 PMCid:PMC3054652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehmann FS, Beglinger C, Schnabel K, Terracciano L. Progressive development of diffuse liver hemangiomatosis. J Hepatol. 1999;30:951–954. doi: 10.1016/S0168-8278(99)80152-4. [DOI] [PubMed] [Google Scholar]
- 27.Kim EH, Park SY, Ihn YK, Hwang SS. Diffuse hepatic hemangiomatosis without extrahepatic involvement in an adult patient. Korean J Radiol. 2008;9:559–562. doi: 10.3348/kjr.2008.9.6.559. PMid:19039274 PMCid:PMC2627242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayanthi V, Ravi Shankar T, Ravindran C, Sudalaimuthu S, Chandrasekar TS. Diffuse hepatic hemangiomatosis: case report. Trop Gastroenterol. 2000;21:188–189. PMid:11194583. [PubMed] [Google Scholar]
- 29.Supakul R, Vakili ST, Liangpunsakul S. Adult diffuse hepatic hemangiomatosis: a rare cause of dilated cardiomyopathy and sudden cardiac arrest. J Gen Intern Med. 2013;29:244–245. doi: 10.1007/s11606-013-2531-0. PMid:23868097 PMCid:PMC3889961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH, Yoon CJ, Kim YH, Han H, Cho JY, Kim H. Living-donor liver transplantation for giant hepatic hemangioma with diffuse hemangiomatosis in an adult: a case report. Clin Mol Hepatol. 2017 doi: 10.3350/cmh.2017.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farges O, Daradkeh S, Bismuth H. Cavernous hemangiomas of the liver: are there any indications for resection? 1995. PMid:7740805. [DOI] [PubMed]
- 32.Grieco MB, Miscall BG. Giant hemangiomas of the liver. Surg Gynecol Obstet. 1978;147:783. PMid:362577. [PubMed] [Google Scholar]
- 33.Huerta-Orozco LD, Leonher-Ruezga KL, Ramirez-Gonzalez LR, Hermosillo-Sandoval JM, Sandoval-Alvarado J, Moran-Galaviz RE. Hepatic angiosarcoma and liver transplantation: case report and literature review. Cirugia y Cirujanos (English Edition) 2015;83:510–515. doi: 10.1016/j.circen.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Sherman M. Vinyl chloride and the liver. J Hepatol. 2009;51:1074. doi: 10.1016/j.jhep.2009.09.012. PMid:19836850. [DOI] [PubMed] [Google Scholar]
- 35.Bioulac-Sage P, Laumonier H, Laurent C, Blanc JF, Balabaud C. Benign and malignant vascular tumors of the liver in adults. Semin Liver Dis. 2008;28:302. doi: 10.1055/s-0028-1085098. PMid:18814083. [DOI] [PubMed] [Google Scholar]
- 36.Locker GY, Doroshow JH, Zwelling LA, Chabner BA. The clinical features of hepatic angiosarcoma: a report of four cases and a review of the English literature. Medicine. 1979;58:48–64. doi: 10.1097/00005792-197901000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Baron PW, Amakonah T, Cubas RF, Kore AH, Elihu A, de Vera ME. Diffuse hepatic epithelioid hemangioendothelioma developed in a patient with hepatitis C cirrhosis. Case Rep Transplant. 2014;2014:1–4. doi: 10.1155/2014/694903. PMid:25276467 PMCid:PMC4172934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer. 1999;85:562. doi: 10.1002/(SICI)1097-0142(19990201)85:3%3C562::AID-CNCR7%3E3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 39.Walsh MM, Hytiroglou P, Thung SN, Fiel MI, Siegel D, Emre S. Epithelioid hemangioendothelioma of the liver mimicking Budd-Chiari syndrome. Arch Pathol Lab Med. 1998;122:846. PMid:9740148. [PubMed] [Google Scholar]
- 40.Weiss SW, Ishak KG, Dail DH, Sweet DE, Enzinger FM. Epithelioid hemangioendothelioma and related lesions. Semin Diagn Pathol. 1986;3:259. PMid:3303234. [PubMed] [Google Scholar]
- 41.Sugano S, Miyoshi K, Suzuki T, Kawafune T, Kubota M. Intrahepatic arteriovenous shunting due to hepatocellular carcinoma and cirrhosis, and its change by transcatheter arterial embolization. Am J Gastroenterol. 1994;89:184. PMid:8304300. [PubMed] [Google Scholar]
- 42.Kew MC, Dos Santos HA, Sherock S. Diagnosis of primary cancer of the liver. Br Med J. 1971;4:408. doi: 10.1136/bmj.4.5784.408. PMid:5124443 PMCid:PMC1799483. [DOI] [PMC free article] [PubMed] [Google Scholar]