Abstract
Objective:
We used transcriptomic profiling and immunohistochemistry (IHC) to search for a functional imaging strategy to resolve common problems with morphological imaging of cystic neoplasms and benign cystic lesions of the pancreas.
Methods:
Resected pancreatic cancer (n = 21) and normal pancreas were laser-capture micro-dissected, and transcripts were quantified by RNAseq. Functional imaging targets were validated at the protein level by IHC on a pancreatic adenocarcinoma tissue microarray and a newly created tissue microarray of resected intraductal papillary mucinous neoplasms (IPMNs) and IPMN-associated adenocarcinomas.
Results:
Genes encoding proteins responsible for cellular import of pyruvate, export of lactate, and conversion of pyruvate to lactate were highly upregulated in pancreatic adenocarcinoma compared to normal pancreas. Strong expression of MCT4 and LDHA was observed by IHC in >90% of adenocarcinoma specimens. In IPMNs, the pyruvate-to-lactate signature was significantly elevated in high grade dysplasia (HGD) and IPMN-associated adenocarcinoma. Additionally, cores containing HGD and/or adenocarcinoma exhibited a higher number of peri-lesional stromal cells and a significant increase in peri-lesional stromal cell staining of LDHA and MCT4. Interestingly, the pyruvate-to-lactate signature was significantly upregulated in cores containing only low grade dysplasia (LGD) from patients with histologically confirmed IPMN-associated adenocarcinoma versus LGD cores from patients with non-invasive IPMNs.
Conclusion:
Our results suggest prospective studies with hyperpolarized [1-13C]-pyruvate magnetic resonance spectroscopic imaging are warranted. If these IHC results translate to functional imaging findings, a positive pyruvate-to-lactate imaging signature might be a risk factor for invasion that would warrant resection of IPMNs in the absence of other worrisome features.
Keywords: IPMN, pancreatic adenocarcinoma, pyruvate, hyperpolarized, magnetic resonance spectroscopy
Introduction
For many tumor types, early detection and screening are the main focus of imaging research. Pancreatic cancer, however, is too rare (age-adjusted incidence rate of <13/100,000)1 to warrant screening of the general population.2 In the 20% of patients who present with resectable local disease, the median tumor diameter is 3.2 cm, a size which is seldom missed with conventional morphological imaging techniques (computed tomography [CT], ultrasound [US], endoscopic ultrasound (EUS) or magnetic resonance imaging [MRI]).3
The majority of pancreatic ductal adenocarcinoma (PDAC) arises from pancreatic intraepithelial neoplasm (PanIN) lesions which are typically sub-mm in dimension and generally undetectable with current clinical imaging techniques. However, up to 20% of PDAC arises in the context of radiologically detectable cystic neoplasms which are thought to progress along the adenoma-to-adenocarcinoma axis similar to colon polyps.4, 5 The most common of these cystic precursor lesions is the branch-duct (BD)-intraductal papillary mucinous neoplasm (IPMN).6 There is a wealth of literature on the diagnostic dilemma of BD-IPMNs and the need to resect high-risk lesions while sparing resection for the majority of patients whose lesions are unlikely to progress6–9. While great efforts have been made, including international consensus guidelines and follow-up of these patients with MRI and EUS to capture the small fraction of BD-IPMNs that harbor an invasive cancer, the majority of resections (~75%) for radiologically worrisome BD-IPMNs to date have revealed only low to moderate-grade dysplasia.10–14 The management of these patients remains a significant challenge, and the fact that these lesions are radiologically detectable suggests that a new functional imaging strategy may be the key to progress.
One of the greatest success stories in functional imaging is sodium iodide symporter- (NIS-) mediated imaging for thyroid cancer. NIS (SLC5A5) is a membrane transporter from the solute carrier (SLC) family and is responsible for the uptake of iodine in thyroid follicular cells as the first step in the synthesis of thyroid hormone. The combined action of NIS and a second trapping (or organification) step allow thyroid cancer cells to accumulate radiolabeled iodine >1000-fold above blood levels at 48 hours after administration.15 This efficient 2-compartment system permits highly sensitive detection of primary and metastatic thyroid cancer deposits with gamma-camera, single-photon emission computed tomography (SPECT), and positron emission tomography (PET) imaging.
Our previous work with NIS16–18 and the success of SLC transporter-mediated functional imaging (for example FDG-PET) in other tumors led us to investigate the potential of SLC-mediated functional imaging for pancreatic lesions. In an initial microarray screen of 13 pancreatic tumor samples, we discovered SLC16A3 was upregulated in all 13 pancreatic adenocarcinoma samples versus control pancreas samples.19 SLC16A3 encodes monocarboxylate transporter 4 (MCT4), which is thought to be responsible for cellular export of lactate produced in the glycolytic pathway. While MCT4 activity is not responsible for a direct uptake or trapping process that could be imaged with high resolution SPECT or PET, we reasoned that if this endpoint glycolytic pathway is consistently upregulated versus other competing pathways of pyruvate metabolism in high-risk pancreatic cystic lesions, then it might be worth further exploration as an a potential imaging target for MR spectroscopy following IV injection of hyperpolarized 13C-pyruvate.
In this paper we build on our discovery of MCT4 upregulation in human pancreatic cancer and extend it to the study of endpoint glycolysis in radiologically-detectable but diagnostically-challenging IPMNs, a current and growing clinical problem.
Materials and Methods
Transcriptomic Studies
Patient tissues were obtained from the Mayo SPORE Pancreatic Cancer Tissue Registry following institutional IRB approval. RNA-Sequencing (methods described previously) was performed on total RNA extracted from a cohort of 21 patient pancreatic adenocarcinoma isolated by laser capture micro-dissection (LCM).19 For same-patient control tissue, we laser-captured bulk acinar tissue from 5 patients and normal pancreatic ducts from 16 patients.
Tissue Microarray Creation and Antibody Development
A pancreatic adenocarcinoma tissue microarray (TMA) containing samples from 140 patients (3 cores per patient) without prior chemotherapy 19 was screened. A new TMA from IPMN patients was constructed from 140 surgical specimens (3 cores per patient, 8 TMA blocks total) with various grades of IPMN or IPMN-associated invasive adenocarcinoma at surgical pathology. TMAs were stained with H&E or colorimetrically developed with antibodies against LDHA (sc-137243; Santa Cruz Biotechnology, Dallas, TX) or MCT4 (sc-50329; Santa Cruz) at the Pathology Research Core of Mayo Clinic, Rochester, MN. TMA slides were placed in the BOND III (Leica Biosystems, Chicago, IL) stainer for online processing. Slides were treated with Epitope Retrieval 2 solution for 20 min, stained with LDHA antibody at 1:600 dilution or MCT4 antibody at 1:300 dilution (in Bkg Reducing Diluent, Dako, Carpenteria, CA, S3022) for 30 min. Detection was achieved using the Polymer Refine Detection kit as per the manufacturer’s instructions (Leica Biosystems). Counter staining was performed for 5 min with hematoxylin. Slides were dehydrated through increasing concentrations of alcohol, cleared in xylene, and coverslipped in xylene-based mounting media.
TMA scoring
Tissue microarrays (TMAs) were scanned at 40X using an Aperio (Leica Biosystems) ScanScope AT Turbo Scanner. Scanned images were reviewed for quality by a research technologist who specializes in digital imaging. Images were de-arrayed and scored using PathXL TMA software (PathXL Ltd.). The pancreatic adenocarcinoma TMA was evaluated for antigen expression by a trained pancreatic pathologist and cores were scored as strong, moderate, weak, or absent; and the percent of cells stained at the highest intensity was recorded. Subcellular localization of the staining was noted for each core. IPMN TMA core morphology was assessed on an H&E stained section by an anatomic pathologist, who recorded presence or absence of adenocarcinoma, presence or absence of IPMN and, if present, IPMN subtype (gastric, intestinal, pancreatobiliary) and grade of dysplasia (low grade, LGD; moderate grade, MGD; high grade, HGD). Adjacent TMA sections stained with antibodies against MCT4 and LDHA were manually assessed by a cytotechnologist under the direction of the same anatomic pathologist. Percentage of negative (0), weak (1), moderate (2), and strong (3) staining was assessed, rounding to the nearest 10%. A histoscore was reported as sum of % of cells stained multiplied by each intensity score (histoscore range of 0–300). Staining on the plasma membrane was assessed for MCT4. Cytoplasmic staining was assessed for LDHA. Peri-neoplastic stromal staining was also assessed, categorized as present or absent and, when present, no staining, 1–50% staining, and 51–100% staining, with each antibody.
Statistical analysis
Two-sided paired t-tests were used for transcriptomic data. Tumor RPKM values were compared to same-patient normal pancreas tissue. Core level neoplastic staining (LDHA and MCT4) was compared across the four IPMN groups (LGD, MGD, HGD, or adenocarcinoma). An ANOVA F test was used to assess differences in mean stain histoscore across groups along with a Fisher’s Least Significant Difference (LSD) to determine which group(s) contributed to the overall difference detected by the ANOVA. For each stain, the distribution of percent stromal (0%, 1–50%, 51–100%) was compared between groups (IPMN with LGD/MGD vs. IPMN with HGD/adenocarcinoma) using a Chi-square test. Additional analyses investigated staining in LGD cores that were classified as LGD-only vs LGD in the context IPMN adenocarcinoma. A two sample t-test was used to compare mean stain histoscores and a Chi-square test was used for the distribution of percent stromal staining.
Results
Genes encoding transporters and enzymes responsible for cellular import of pyruvate and export of lactate (including SLC16A1/MCT1 and SLC16A3/MCT4) and conversion of pyruvate to lactate (LDHA) were upregulated in pancreatic adenocarcinoma samples compared to normal pancreatic tissue (Upper Table 1). The competing pathways of mitochondrial pyruvate import/catabolism and pyruvate to alanine conversion were unchanged or downregulated (Lower Table I). Typically when comparing pancreatic adenocarcinoma to patient matched normal tissue, the cell of origin (normal ducts) is used as control tissue, as it provides the most information on changes that are mechanistically important to cancer progression. However, in the unique case of an imaging biomarker for pancreatic lesions, it is important also to rule out putative cancer biomarkers that may be present at high levels in normal acinar cells, which is the predominant cell type in the pancreas. Thus in the group of 21 pancreatic adenocarcinoma patients, we compared 16 adenocarcinomas to matched normal ducts and 5 adenocarcinomas to matched normal acinar tissue. Of note, the magnitude of upregulation was actually higher when comparing pancreatic adenocarcinoma to acinar control tissue, suggesting that the identified upregulation of the pyruvate-to-lactate pathway in the cancer cells might be well-resolved from background normal pancreatic acinar tissue.
Table 1.
RNASseq RPKM values from laser captured microdissected human pancreatic adenocarcinoma cancers and patient-matched normal pancreatic acinar tissue or normal pancreatic ducts.
| Gene | Function | Tumor n = 16 |
Normal duct n = 16 |
Tumor / Normal duct |
Tumor n = 5 |
Normal acinar n = 5 |
Tumor / Normal acinar |
p-value (paired t-test) |
|---|---|---|---|---|---|---|---|---|
| Genes for pyruvate uptake and metabolism to lactate | ||||||||
| SLC2A1 (GLUT1) |
PM glucose transporter | 97.09 | 12.75 | 7.61 | 76.10 | 0.41 | 184.05 | < 0.0001 |
| HK2 | Hexokinase II | 32.67 | 8.11 | 4.03 | 68.15 | 0.77 | 89.01 | < 0.0001 |
| SLC16A1 (MCT1) |
PM pyruvate/lactate transporter | 12.38 | 4.46 | 2.78 | 24.83 | 1.29 | 19.26 | < 0.0001 |
| SLC16A3 (MCT4) |
PM pyruvate/lactate transporter | 21.27 | 7.85 | 2.71 | 5.53 | 0.05 | 113.47 | < 0.0001 |
| LDHA | Lactate dehydrogenase A | 351.6 | 142.31 | 2.47 | 305.27 | 20.67 | 14.77 | < 0.0001 |
| FOXM1 | LDHA-activating transcription factor | 6.17 | 1.45 | 4.27 | 2.21 | 0.12 | 18.40 | 0.0004 |
|
Other competing pyruvate metabolic pathway genes | ||||||||
| BRP44L | Inner mito. Membrane pyruvate carrier | 26.05 | 29.48 | 0.88 | 14.37 | 10.63 | 1.35 | 0.34 |
| GPT2 | Glutamate-pyruvate transaminase | 7.79 | 12.57 | 0.62 | 8.21 | 32.91 | 0.25 | 0.0015 |
| PDHA1 | Mitochondrial pyruvate dehydrogenase | 17.86 | 16.61 | 1.08 | 19.29 | 16.58 | 1.16 | 0.1585 |
| PDHP | Mitochondrial pyruvate dehydrogenase | 35.88 | 36.23 | 0.99 | 28.75 | 16.60 | 1.73 | 0.4035 |
Aerobic glycolysis genes are highly upregulated in pancreatic cancer. Other competing pyruvate metabolic pathway genes are downregulated or largely unchanged. RPKM: average reads per kilobase per million reads. PM: plasma membrane.
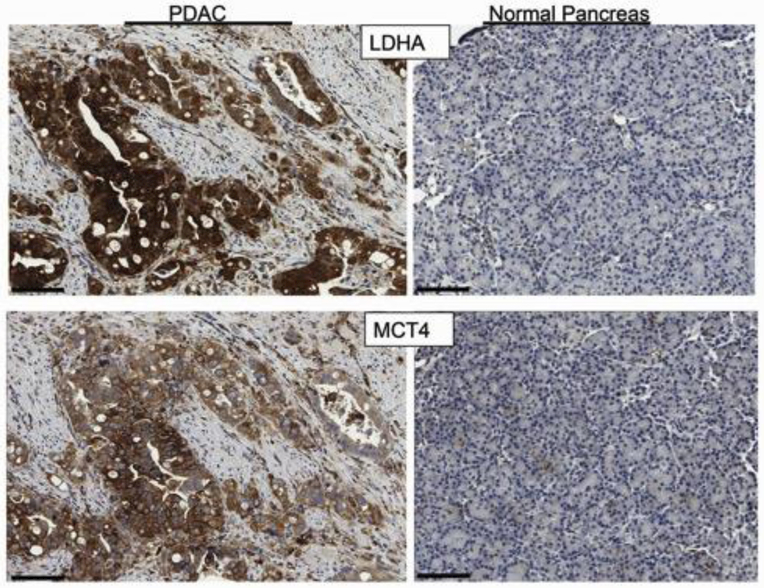
We first performed IHC on a TMA of pancreatic adenocarcinoma (140 pancreatic cancer patient surgical specimens, 3 TMA cores per patient—independent of the patients examined with RNAseq) cores using antibodies against LDHA and MCT4. IHC results were consistent with our transcriptomic profiling studies and showed that the pyruvate-to-lactate metabolic signature was present with both moderate-strong plasma membrane staining of MCT4 and moderate-strong cytoplasmic staining of LHDA in > 90% of patient samples. For LDHA, 152 cores contained sufficient adenocarcinoma tissue for evaluation. Ten cores were scored as weak, 14 as moderate, and 127 as strong. Of the 127 strongly stained cores, all but one exhibited staining in greater than 75% of the tumor cells on the core. For MCT4, 154 cores contained sufficient adenocarcinoma tissue for evaluation. Four cores were scored as weak, 18 as moderate, and 132 as strong. Of the strongly stained cores, all exhibited staining in greater than 75% of the tumor cells on the core.
Since staining was nearly universal in conventional pancreatic adenocarcinoma TMA (primarily PanIN derived) we next explored this IHC signature in a newly created TMA from 140 patients (3 TMA cores per patient) with IPMNs of various histological grades or IPMN-associated adenocarcinoma. A histoscore was employed to quantify the percentage of neoplastic cells (0–100) stained multiplied with the intensity of staining (0,1,2,3). Stromal staining was scored on the presence or absence of peri-lesional stromal cells and the percentage of stained stromal cells. We found a highly significant increase (p<0.0001) in cytoplasmic LDHA staining in HGD and IPMN-associated adenocarcinoma versus cores containing only LGD (Table 2a). MCT4 staining was not significantly elevated in HDG or IPMN-associated adenocarcinoma. However, a combined score of LDHA and MCT4 staining, which might better predict the results of imaging lactate production after injection of HP pyruvate, revealed a highly significant upregulation (p < 0.0007) in HGD and IPMN-adenocarcinoma. In a number of cores, regions of normal pancreas were present, and these regions were largely unstained with any of the antibodies. Representative staining of pancreatic adenocarcinoma and normal pancreas is shown in Fig 1. Figure 2 shows representative cores from a resected specimen with HGD and an adjacent IPMN-adenocarcinoma. In addition to the upregulation in neoplastic cells, the stromal staining index (results are the average of 3 cores per patient) revealed a significant upregulation of MCT4 and LDHA in peri-lesional cells in HGD/ IPMN-adenocarcinoma versus LGD/MGD (Table 2b). As can be seen from the representative figures, the stromal cell staining was quite variable with some LGD exhibiting uniform strong staining (Fig 2, MCT4) and some adenocarcinoma cores exhibiting scant stromal staining (Fig.1). Of note, the 2 additional cores from the IPMN-adenocarcinoma patient in Fig. 1 exhibited strong staining of MCT4 in the majority of stromal cells.
Table 2A.
LDHA and MCT4 histoscores and percent of stained stromal cells in IPMN cores from patients with different grades of dysplasia or adenocarcinoma
| IPMN with LGD n = 216 |
IPMN with MGD n = 0 |
IPMN with HGD n = 20 |
IPMN with Adeno n = 19 |
p-value | |
|---|---|---|---|---|---|
| LDHA | 138.5 (74.9) | 159.0 (87.4) | 206.0 (69.6) | 237.8 (75.1) | <0.0001 |
| Missing | 3 | 1 | 0 | 1 | |
| LSD Different Groups | * | * | ** | ** | |
| MCT4 | 153.4 (79.5) | 162.1 (72.1) | 171.5 (72.0) | 146.7 (92.4) | 0.7103 |
| Missing | 4 | 2 | 0 | 1 | |
| LDHA & MCT4 Average | 145.5 (57.3) | 158.6 (69.5) | 184.3 (65.0) | 192.2 (71.5) | 0.0007 |
| Missing | 6 | 2 | 0 | 1 | |
| LSD Different Groups | * | ** | ** |
p-values for percent stromal staining variables are from a Chi-Square test.
p-values from Mean Expression variables are from ANOVA F test, pairwise comparisons use Fisher’s Least Significant Difference (LSD)
LDHA –
LGD and MGD are not different,
HGD and Adeno are not different, HGD and Adeno are different from LGD and MGD.
MCT4 & LDHA – Adeno (**) is different from LGD (*), HGD (**) is different from LGD (*).
Figure 1.
IHC of IPMN-associated pancreatic adenocarcinoma and a region of normal pancreas. Upper panel) Strong cytoplasmic staining of LDHA was observed in >75% of tumor cells, while normal acinar cells were unstained. Normal ducts exhibited patchy weak staining. Lower panel) Moderate to strong plasma membrane staining of MCT4 was observed in >75% of tumor cells. A few scattered acinar cells and normal ductal cells were weakly or moderately stained. In this particular example, stromal cells adjacent to the adenocarcinoma were predominantly unstained. The normal pancreas core was obtained from a pylorus preserving Whipple procedure. The 70-year-old female patient presented with a mixed MD/BD IPMN and main duct ectasia with associated abdominal pain. There was no evidence of invasion on surgical pathology and the patient is alive 13 years post-resection. Scale bar = 100 microns.
Figure 2.
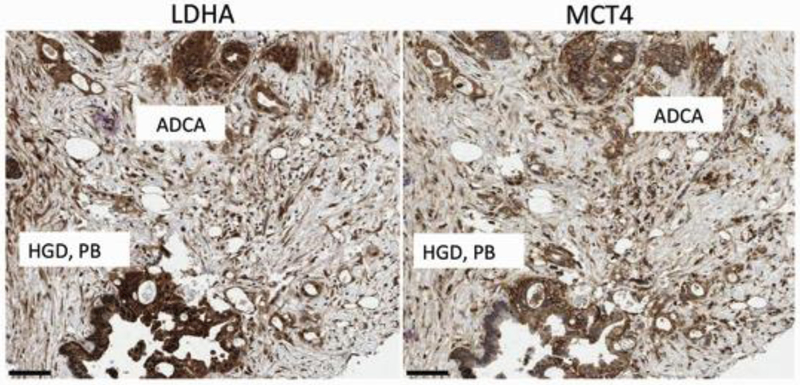
Representative tissue core of a region of invasive adenocarcinoma (grade 3) and nearby region of pancreatobiliary HGD IPMN. The patient was an 81-year-old female who presented with jaundice and a 4.2 cm mass in the pancreas. Surgical pathology revealed a grade 3 adenocarcinoma in association with a mixed-type IPMN with HGD. Left Panel) LDHA IHC revealed strong cytoplasmic staining of all adenocarcinoma cells and neoplastic cells within the adjacent HGD IPMN. The majority of peri-lesional stromal cells were moderately stained. Right panel) MCT4 IHC revealed strong plasma membrane staining in the majority of adenocarcinoma cells and neoplastic cells within the adjacent HGD IPMN. The majority of peri-lesional stromal cells were moderately stained. Scale bar = 100 microns.
Table 2B.
Core Level Stromal Staining Index Results
| IPMN with LGD/MGD n = 247 |
IPMN with HGD/Adeno n =39 |
p-value | |
|---|---|---|---|
| LDHA | 0.0006 | ||
| No Stroma | 21 | 1 | |
| 0 % | 62 (27.4%) | 1 (2.6%) | |
| 1-50 % | 65 (28.8%) | 9 (23.7%) | |
| 51-100 % | 99 (43.8%) | 28 (73.7%) | |
| MCT4 | 0.0085 | ||
| No Stroma | 17 | 2 | |
| 0 % | 15 (6.5%) | 0 (0.0%) | |
| 1-50 % | 95 (41.3%) | 8 (21.6%) | |
| 51-100 % | 120 (52.2%) | 29 (78.4%) |
p-values for percent stromal staining variables are from a Chi-Square test
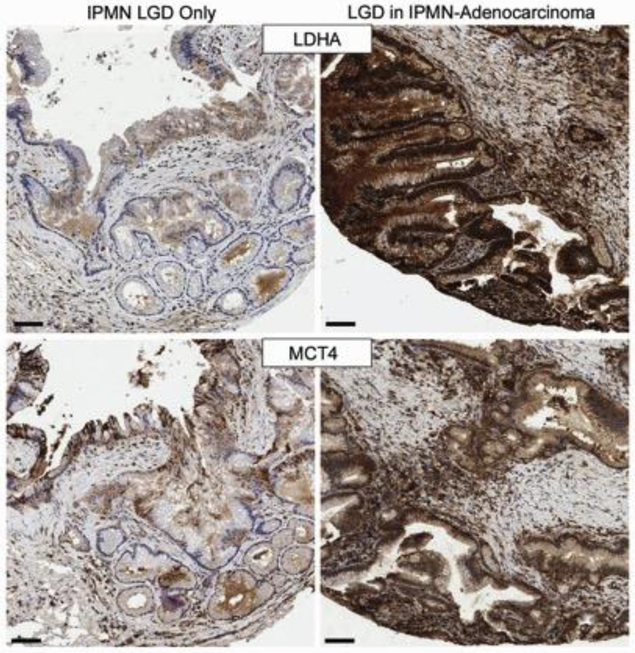
To explore a potential role of the pyruvate-lactate metabolic pathway for predicting progression of IPMN lesions to invasion, we performed a statistical analysis of cores scored as LGD in patients with and without HGD and/or IPMN-associated adenocarcinoma (Table 3). The IPMN-adenocarcinoma group contained 62 cores from 33 unique subjects. 25/33 patients were diagnosed with adenocarcinoma on surgical pathology of the primary resection, while 8/33 were later diagnosed with adenocarcinoma during post-resection follow-up (time to diagnosis was < 2 mos (3 patients), 11 mos, 15 mos, 32 mos, 34 mos, and 74 mos). 9 cores from 4 unique patients with HGD were pooled with the IPMN-adenocarcinoma group. In this analysis, we observed significantly increased (p = 0.0001) cytoplasmic LDHA staining in LDG cores from patients diagnosed with HGD IPMN or IPMN-adenocarcinoma (in the surgical specimens sampled for IPMN cores and patients who later progressed to adenocarcinoma) compared with LGD cores from patients with only benign IPMN (Table 3). In addition, LDHA and MCT4 staining were increased in peri-lesional stromal cells in LGD cores from patients with HDG or IPMN-adenocarcinoma versus patients with LGD only (p = 0.0059 and 0.0083, respectively). Figure 3 shows examples of staining of LDHA and MCT4 in a core from a patient with LGD-only and a core containing IPMN LGD from a patient with histologically-confirmed IPMN-associated adenocarcinoma. Note the higher intensity of MCT4 staining relative to LDHA in the LGD-only core and the much more dramatic increase in LDHA staining between LGD-only and LGD associated with IPMN-adenocarcinoma. Fig 3 also shows the typical increased number of stromal cells and the increase in staining intensity observed in LDG cores from patients with IPMN-adenocarcinoma.
Table 3.
Low Grade Dysplasia Cores Staining Results - by Patient Diagnosis
| IPMN (LGD/MGD) (N=120) |
IPMN (HGD) + IPMN & Adeno (N=71) |
p value | |
|---|---|---|---|
| LDHA | 116.5 (69.0) | 158.7 (74.8) | 0.0001 |
| Missing | 0 | 2 | |
| MCT4 | 150.4 (78.9) | 145.2 (76.4) | 0.6587 |
| Missing | 1 | 1 | |
| LDHA & MCT4 Average | 142.8 (57.2) | 154.1 (57.3) | 0.1913 |
| Missing | 2 | 2 | |
| LDHA stroma | 0.0059 | ||
| No Stroma | 8 | 8 | |
| 0% | 39 (34.8%) | 8 (12.7%) | |
| 1-50% | 28 (25.0%) | 19 (30.2%) | |
| 51-100% | 45 (40.2%) | 36 (57.1%) | |
| MCT4 stroma | 0.0083 | ||
| No Stroma | 7 | 4 | |
| 0% | 10 (8.8%) | 1 (1.5%) | |
| 1-50% | 50 (44.2%) | 20 (29.9%) | |
| 51-100% | 53 (46.9%) | 46 (68.7%) |
p-values for percent stromal staining variables are from a Chi-Square test.
p-values from mean expression variables are from two sample t-test.
Figure 3.
LDHA and MCT4 staining in LGD-only and LGD associated with adenocarcinoma.
Left panels) adjacent sections of a core from a 74-year-old male with an incidentally discovered BD-IPMN. The BD-IPMN increased in size by approximately 1 cm (to 2.9 cm) on follow-up which prompted the resection. Surgical pathology revealed only benign IPMN. Right panels) adjacent sections of a core from a 73-year-old male who presented with a mass in the tail of the pancreas. Surgical pathology revealed a 5.8 cm invasive adenocarcinoma and an adjacent 3.9 cm IPMN with a highest histological grade of HGD involving main and branch ducts. The core shown in the figure contains a region of the IPMN with LGD. Top panel) no or weak staining was observed with LDHA in the LGD-only core, while uniform strong cytoplasmic staining was observed in the LGD-IPMN with associated adenocarcinoma. There is also an increase in both the number of peri-lesional stromal cells and the staining intensity from LDHA. Bottom panel) MCT4 IHC revealed moderate to strong staining in approximately 50% of the neoplastic cells in the LGD-only core, while uniform strong plasma membrane staining was observed in the LGD-IPMN with associated adenocarcinoma. As with LDHA, increased staining intensity of stromal cells with MCT4 was observed. Scale bar = 100 microns
A statiscal analysis of the sensitivity and specificity calculated for a range of LDHA histoscores is shown in Table 4. In this analysis the mean histoscore of the 3 cores per patient (from 123 patients with surgical pathology hisological diagnosis of IPMN or IPMN-associated adenocarcinoma) was used. Subjects were pooled into LDG/MGD or HGD/adenocarcinoma groups based on the highest grade lesion noted on surgical pathology. A sensitivity of 51.9 was observed with a histoscore cutoff of 170 at a specificity of 79.0. As the threshold histoscore was raised to 230, specificity for HGD/Adenocarcinoma reached 96.8, albeit at low sensitivity (25.0). The stromal staining index was not included in these analyses, but the pyruvate-to-lactate signal coming from the stroma would be expected to contribute to improved accuracy.
Table 4.
Area under the ROC Curve (AUC) Sensitivity, Specificity
| LDHA Cutoff for Normal, Elevated (LGD/MGD, Adeno/HGD) |
AUC | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| Continuous | 71.8 | |||
| ≤ 170, > 170 | 65.5 | 51.9 | 79.0 | 66.7 |
| ≤ 180, > 180 | 66.3 | 51.9 | 80.6 | 67.5 |
| ≤ 190, > 190 | 65.8 | 46.2 | 85.5 | 67.5 |
| ≤ 200, > 200 | 65.5 | 42.3 | 88.7 | 67.5 |
| ≤ 210, > 210 | 65.0 | 36.5 | 93.5 | 67.5 |
| ≤ 220, > 220 | 62.0 | 28.9 | 95.1 | 64.9 |
| ≤ 230, > 230 | 60.9 | 25.0 | 96.8 | 64.0 |
Discussion
Our observations with transcriptomic profiling indicate that the metabolic pathway of pyruvate to lactate conversion is highly activated in pancreatic adenocarcinoma. Two of the key proteins, LDHA (to generate lactate) and MCT4 (to import injected pyruvate and export the cytoplasmic lactate produced by LDHA), were examined by IHC and found to be upregulated. Most of the upregulation in HGD and IPMN adenocarcinoma was seen with LDHA; however, a combined LDHA/MCT4 staining index was also significantly upregulated in IPMN HGD and IPMN-adenocarcinoma. This combined staining index is probably most relevant to what we might expect to observe with HP 13C pyruvate metabolic imaging. Additionally, we observed upregulation of LDHA in large regions of LGD in patients that harbored an invasive adenocarcinoma. MCT4 and LDHA were also upregulated in peri-neoplastic stromal cells around lesions with HGD, IPMN-adenocarcinoma, and around LGD in the context of IPMN-adenocarcinoma. Taken together, our results demonstrate a marked increase in monocarboxylate transporters and LDHA in patients with IPMN lesions whose resected pancreas specimens were histologically identified as malignant versus those that were benign on histology. The results presented here provide a window of hope that this metabolic pathway may occur in regions of IPMN lesions large enough to detect with MRSI prior to frank invasion. If this is true, detection of this signature may provide an additional risk factor that could potentially warrant resection. Ultimately, however, it will require a prospective study to determine the clinical predictive value of pyruvate-to-lactate metabolic imaging.
Originally, the Warburg effect20 in cancer (cytoplasmic metabolism of pyruvate to lactate in the presence of enough oxygen to support mitochondrial oxidative phosphorylation) was an enigma, with cancer cells seemingly wasting precious glucose for a paltry 2 molecules of ATP. However, a new line of research has considered the Warburg effect as an anabolic state where production of biological molecules such as lipids and nucleotides from glucose is much more important for rapid cell division of cancer cells than efficient catabolism of glucose to ATP.21 A direct cancer promoting effect of secreted lactate has also been proposed, 22, 23 and a role of cell-cell sharing of lactate between tumor cells or between stromal cells and tumor cells has also been considered.24, 25 It has also been postulated that the Warburg adaptation of tumor cells occurs in clones within pre-invasive lesions prior to invasion, and there is likely a genetic component to fix the aerobic glycolytic pathway and a reversible tumor environmental component in areas of hypoxia.26 It is possible that an environmental component is responsible for the early upregulation of MCT4 in patchy regions of LGD IPMNs, while later genetic hits that that drive histological progression are responsible for the upregulation of LDHA. Ultimately, it will require thorough genomic and epigenetic analyses to answer these questions.
Hyperpolarized MRSI is a rapidly growing field. Recently published data on the first-in-human use of 13C-pyruvate as an imaging agent for patients with prostate cancer has confirmed the safety of this probe, as well as its ability to noninvasively document elevated 13C-pyruvate to 13C-lactate conversion in regions of biopsy-proven prostate cancer.27, 28 While HP-MRSI has not yet been explored clinically in the pancreas, hyperpolarized [1-13C]-pyruvate has been used previously in two pancreatic cancer mechanistic drug studies in mice and one study of pancreatic cancer diagnosis in mice.29–31 The results of the animal studies are consistent with a marked upregulation of the pyruvate-to-lactate pathway that we observed at the level of transcriptomics and immunohistochemistry of human pancreatic cancer specimens.
MRI is the non-invasive imaging technique of choice for evaluation and follow-up of pancreatic cystic lesions. MRSI sequences could be added on to routine pancreatic MRI protocols, as an entire chemical shift imaging (CSI) acquisition or time course study can be obtained in a single breath hold. This technique could document an individual’s baseline IPMN metabolic signature (pyruvate metabolism) at initial detection and then monitor this signature over time. EUS is currently the highest resolution technique for detection and anatomical characterization of pancreatic lesions and more importantly can provide access to FNA and cyst fluid samples. We envision HP-MRSI as non-invasive tool that might lead to invasive procedures such EUS, FNA, and cyst fluid next generation sequencing analysis for confirmation. Our hope is that a change in the pyruvate-lactate signature precedes invasion or “predicts” occult microinvasion within IPMNs.
In summary, our data confirm upregulation of the pyruvate-lactate metabolic signature in IPMN-associated adenocarcinoma, IPMN lesions with HGD, and in regions of LGD from patients with histologically confirmed IPMN-associated adenocarcinoma versus LGD cores from patients without cancer. Based on these findings, it seems reasonable to explore HP-MRSI as a tool to add to the diagnostic imaging of patients with IPMN.
Acknowledgements
SKC acknowledges the support of a generous benefactor gift from Ted L. Shabert, an award from the National Cancer Institute (P50CA102701 [Mayo Clinic SPORE in Pancreatic Cancer]), the Minnesota Partnership for Biotechnology and Medical Genomics (MNP #16.37), the Imaging Biomarker Program within the Center for Individualized Medicine at Mayo Clinic, the Mayo Clinic Center for Clinical and Translational Science (CCaTS) (UL1TR000135), and an award from the Fifth District Fraternal Order of Eagles Cancer Research Fund. We would like to thank Anthony J. Blahnik, LouAnn A. Gross, Sarah N. Tangen, Eileen L. Holicky, Lorna K. Lubinski, , Nathan G. Faiman, Peggy M. Gosse Rahnenfuehrer, and Stephen N. Hart for their technical expertise and Gloria M. Petersen for helpful discussion. We would also like to thank the Mayo Clinic Advanced Genomic Technology Center Gene Expression Core. We acknowledge the assistance of Sonia Watson, PhD, in editing and submission of the manuscript. DKD, EK, and MM acknowledge support of the NIH grant P41 EB015894 and the W.M. Keck Foundation. The authors have no other conflicts of interest to disclose.
Abbreviations
- HGD
high grade dysplasia
- HP
hyperpolarized
- IHC
immunohistochemistry
- IPMN
intraductal papillary mucinous neoplasm
- LDHA
lactate dehydrogenase A
- LCM
laser-capture microdissection
- LGD
low grade dysplasia
- MCT
monocarboxylate transporter
- MGD
moderate grade dysplasia
- MRSI
magnetic resonance spectroscopic imaging
- TMA
tissue microarray
Footnotes
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This was a retrospective study of tissues from the pancreatic cancer tissue registry; for this type of study formal consent is not required. Patient consent was obtained for inclusion of tissues in the registry. For animal studies, all applicable institutional and/or national guidelines for the care and use of animals were followed.
References
- 1.Yeo TP: Demographics, epidemiology, and inheritance of pancreatic ductal adenocarcinoma. Semin Oncol 2015; 42: 8–18. [DOI] [PubMed] [Google Scholar]
- 2.Pannala R, Basu A, Petersen GM, Chari ST: New-onset diabetes: A potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 2009; 10: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA et al. : Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg 2000; 4: 567–579. [DOI] [PubMed] [Google Scholar]
- 4.Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV et al. : A revised classification system and recommendations from the baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol 2015; 39: 1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Distler M, Aust D, Weitz J, Pilarsky C, Grutzmann R: Precursor lesions for sporadic pancreatic cancer: Panin, ipmn, and mcn. Biomed Res Int 2014; 2014: 474905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heckler M, Michalski CW, Schaefle S, Kaiser J, Buchler MW, Hackert T: The sendai and fukuoka consensus criteria for the management of branch duct ipmn - a meta-analysis on their accuracy. Pancreatology 2017; 17: 255–262. [DOI] [PubMed] [Google Scholar]
- 7.Crippa S, Piccioli A, Salandini MC, Cova C, Aleotti F, Falconi M: Treatment of branch-duct intraductal papillary mucinous neoplasms of the pancreas: State of the art. Updates Surg 2016; 68: 265–271. [DOI] [PubMed] [Google Scholar]
- 8.Kim YI, Shin SH, Song KB, Hwang DW, Lee JH, Park KM et al. : Branch duct intraductal papillary mucinous neoplasm of the pancreas: Single-center experience with 324 patients who underwent surgical resection. Korean J Hepatobiliary Pancreat Surg 2015; 19: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahora K, Mino-Kenudson M, Brugge W, Thayer SP, Ferrone CR, Sahani D et al. : Branch duct intraductal papillary mucinous neoplasms: Does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg 2013; 258: 466–475. [DOI] [PubMed] [Google Scholar]
- 10.Adsay V, Mino-Kenudson M, Furukawa T, Basturk O, Zamboni G, Marchegiani G et al. : Pathologic evaluation and reporting of intraductal papillary mucinous neoplasms of the pancreas and other tumoral intraepithelial neoplasms of pancreatobiliary tract: Recommendations of verona consensus meeting. Ann Surg 2016; 263: 162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goh BK, Tan DM, Ho MM, Lim TK, Chung AY, Ooi LL: Utility of the sendai consensus guidelines for branch-duct intraductal papillary mucinous neoplasms: A systematic review. J Gastrointest Surg 2014; 18: 1350–1357. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M: Thirty years of experience with intraductal papillary mucinous neoplasm of the pancreas: From discovery to international consensus. Digestion 2014; 90: 265–272. [DOI] [PubMed] [Google Scholar]
- 13.Khaled YS, Mohsin M, Fatania K, Yee A, Adair R, Sheridan M et al. : Outcome of long interval radiological surveillance of side branch pancreatic duct-involved intraductal papillary mucinous neoplasm in selected patients. HPB (Oxford) 2016; 18: 879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka M: Current best practice and controversies in the follow up of patients with asymptomatic branch duct ipmn of the pancreas. HPB (Oxford) 2016; 18: 709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sisson JC: Practical dosimetry of 131i in patients with thyroid carcinoma. Cancer Biother Radiopharm 2002; 17: 101–105. [DOI] [PubMed] [Google Scholar]
- 16.Penheiter AR, Dingli D, Bender CE, Russell SJ, Carlson SK: Monitoring the initial delivery of an oncolytic measles virus encoding the human sodium iodide symporter to solid tumors using contrast-enhanced computed tomography. J Gene Med 2012; 14: 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penheiter AR, Griesmann GE, Federspiel MJ, Dingli D, Russell SJ, Carlson SK: Pinhole micro-spect/ct for noninvasive monitoring and quantitation of oncolytic virus dispersion and percent infection in solid tumors. Gene Ther 2012; 19: 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penheiter AR, Russell SJ, Carlson SK: The sodium iodide symporter (nis) as an imaging reporter for gene, viral, and cell-based therapies. Curr Gene Ther 2012; 12: 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penheiter AR, Erdogan S, Murphy SJ, Hart SN, Felipe Lima J, Rakhshan Rohakhtar F et al. : Transcriptomic and immunohistochemical profiling of slc6a14 in pancreatic ductal adenocarcinoma. Biomed Res Int 2015; 2015: 593572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warburg O: On the origin of cancer cells. Science 1956; 123: 309–314. [DOI] [PubMed] [Google Scholar]
- 21.Vander Heiden MG, Cantley LC, Thompson CB: Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi SY, Collins CC, Gout PW, Wang Y: Cancer-generated lactic acid: A regulatory, immunosuppressive metabolite? J Pathol 2013; 230: 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doherty JR, Cleveland JL: Targeting lactate metabolism for cancer therapeutics. J Clin Invest 2013; 123: 3685–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Outschoorn UE, Lin Z, Trimmer C, Flomenberg N, Wang C, Pavlides S et al. : Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the warburg effect: Implications for pet imaging of human tumors. Cell Cycle 2011; 10: 2504–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK et al. : Evidence for a stromal-epithelial “lactate shuttle” in human tumors: Mct4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle 2011; 10: 1772–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gatenby RA, Smallbone K, Maini PK, Rose F, Averill J, Nagle RB et al. : Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer 2007; 97: 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M et al. : Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)c]pyruvate. Sci Transl Med 2013; 5: 198ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson DM, Kurhanewicz J: Hyperpolarized 13c mr for molecular imaging of prostate cancer. J Nucl Med 2014; 55: 1567–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajeshkumar NV, Dutta P, Yabuuchi S, de Wilde RF, Martinez GV, Le A et al. : Therapeutic targeting of the warburg effect in pancreatic cancer relies on an absence of p53 function. Cancer Res 2015; 75: 3355–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serrao EM, Kettunen MI, Rodrigues TB, Dzien P, Wright AJ, Gopinathan A et al. : Mri with hyperpolarised [1–13c]pyruvate detects advanced pancreatic preneoplasia prior to invasive disease in a mouse model. Gut 2016; 65: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wojtkowiak JW, Cornnell HC, Matsumoto S, Saito K, Takakusagi Y, Dutta P et al. : Pyruvate sensitizes pancreatic tumors to hypoxia-activated prodrug th-302. Cancer Metab 2015; 3: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]