Abstract
Coccidioidomycosis is a systemic fungal infection for which a vaccine has been sought for over fifty years. The avirulent Coccidioides posadasii strain, Δcps1, which is missing a 6 kb gene, showed significant protection in mice. These studies explore conditions of protection in mice and elucidate the immune response. Mice were vaccinated with different doses and viability states of Δcps1 spores, challenged with virulent C. posadasii, and sacrificed at various endpoints, dependent on experimental objectives. Tissues from vaccinated mice were harvested for in vitro elucidation of immune response. Vaccination with viable Δcps1 spores was required for protection from lethal challenge. Viable spore vaccination produced durable immunity, lasting at least 6 months, and prolonged survival (≥6 months). The C. posadasii vaccine strain also protected mice against C. immitis (survival ≥6 months). Cytokines from infected lungs of vaccinated mice in the first four days after Cp challenge showed significant increases of IFN-γ, as did stimulated CD4+ spleen cells from vaccinated mice. Transfer of CD4+ cells, but not CD8+ or B cells, reduced fungal burdens following challenge. IFN-γ from CD4+ cells in vaccinated mice indicates a Th1 response, which is critical for host control of coccidioidomycosis.
Keywords: Coccidioides, vaccine, mice, IFN-γ, Δcps1
Introduction
Coccidioidomycosis (Valley Fever) is caused by the two fungal species, Coccidioides immitis and C. posadasii. Coccidioides spp. were thought to be restricted to the southwestern United States and northern Mexico in North America, however, the disease has been reported and the fungus recovered from soil in northeastern Utah and southwestern Washington [1–3]. These cases suggest that the historical boundaries of the fungus are expanding, putting even more humans and animals at risk of endemic exposure. Coccidioidomycosis causes significant morbidity. Approximately 40% of infections result in clinical illness. Hospital-related costs of the disease in California alone between 2000 and 2011 totaled $2.2 billion, and attributable deaths averaged 160-170 per year nationally [4, 5]. With a rate and range of disease in dogs similar to humans, Arizonans alone are spending more than $60 million per year caring for dogs with coccidioidomycosis [6, 7].
A vaccine to prevent coccidioidomycosis has the potential to save healthcare dollars and prevent morbidity and mortality in both humans and dogs. We have developed an attenuated, live vaccine candidate by deleting the CPS1 gene in C. posadasii to create Δcps1 strain [8]. We showed that Δcps1 was avirulent in both wild-type and profoundly immunodeficient mice. Vaccination protected against death and high fungal burdens in two different mouse strains and by three different routes of immunization [8]. In this report, we expand on the initial studies by examining the mechanism of protection and the ability of this vaccine to protect against challenge by both C. immitis and C. posadasii. We show that the live vaccine produces protracted survival after lethal challenge, and a long duration of immunity. We tested various doses of Δcps1 to determine the minimal efficacious dose to optimize its use as a vaccine. We examined the Δcps1-induced cytokine responses in the lung and showed that the vaccine produces a Th1 skewed response with little detectable IL-17. These experiments allow us to define the correlates of protection that will be critical in extending these results clinically to dogs and ultimately humans.
Materials and Methods
Mice
Six- to eight-week old female BALB/cAnNHsd (BALB/c) and C57BL/6NHsd (B6) mice were purchased from Envigo (Indianapolis, IN). Mice were housed and used according to NIH guidelines under an approved Institutional Animal Care and Use protocol. All procedures utilizing wild-type Coccidioides strains were performed at animal biosafety level (ABSL) 3. All other experiments were performed under ABSL2 containment.
Fungal strains
Coccidioides posadasii, Silveira strain (Cp), and Coccidioides immitis, RS strain (Ci), were grown to maturity on 2X glucose-yeast extract (GYE) agar, and arthroconidia (spores) were harvested as previously described [9]. Δcps1, an avirulent strain derived from Cp with the 6kb CPS1 gene replaced with the hphB cassette (hygromycin resistance marker)[10], was grown and harvested as above with the addition of hygromycin (50 μg/ml) to medium. To verify the mutant strain, colonies were recovered on 2X GYE containing hygromycin, which suppresses growth of wild-type strains, and the mutation was confirmed by PCR. All growth and use of wild-type Coccidioides strains was performed at BSL3. Δcps1 experiments were performed using BSL2 as authorized by the University of Arizona Institutional Biosafety Committee.
Vaccine preparation and vaccination
Spore suspensions were serially diluted and plated to determine viable numbers and then adjusted to the required concentration in 0.9% USP endotoxin-free saline (saline). Doses of spores used for vaccination ranged from 500-500,000 given once or twice intranasally (IN) or subcutaneously (SC). Doses and route are described in the individual studies. For the irradiated Δcps1 preparation, spores were exposed to 900 grey radiation using a GammaCell 40, (Best Theratronics, Ottawa, ON, Canada), resulting in a >99.9% reduction in viability, and administered IN or SC. For the ethanol-killed preparation, Δcps1 spores were incubated in 70% EtOH for 30 minutes, then washed twice and resuspended in 0.9% saline. Sterility was verified by culture. Spores were administered IN or SC. Saline injection served as a vaccine control.
Challenge with wild-type Coccidioides
Infections with wild-type strains of Cp or Ci (~100 spores/mouse) were administered IN by insufflation of 30 μl of spore suspension in 0.9% saline under ketamine-xylazine anesthesia as previously described [11].
Analysis of lung cytokines
B6 mice were vaccinated IN with Δcps1 twice and infected four weeks after the booster. The right lungs from these mice were collected on days 1, 2, 4, and 6 post-infection (p.i.). Lungs were processed individually for single cell suspensions as previously described [12] Briefly, lungs were minced and fragments digested with 0.5 mg/ml collagenase I (Worthington Biochemica, Lakewood, NJ) and 0.02 mg/ml DNase I (Sigma Aldrich, St. Louis, MO) in RPMI-1640. 5 × 105 cells/well were placed in a 24-well tissue culture plate with 500 μl of complete RPMI medium with 10% fetal calf serum (RPMI/c) and were incubated for 24 hrs at 37°C, 5% CO2, to allow secretion of cytokines. Supernatants were centrifuged at 300 × g for 5 minutes, passed through a 0.2 μm filter for sterilization, and frozen at −80°C until analysis by Luminex using a mouse 31-plex Panel (EMD Millipore, Billerica, MA).
Splenocyte stimulation and flow cytometry
Spleens were collected from mice vaccinated IN or SC with Δcps1, or vaccinated then challenged with Cp spores, and processed into single cell suspensions as previously described [12]. Cells were resuspended in RPMI/c medium, stained with trypan blue, and viability and concentration were determined. Cells (5 × 105) were dispensed into 96-well culture plates and incubated with 10 μg/ml of sterile Coccidioides spherule lysate at 37°C, 5% CO2, for 16 hrs. Protein Transport Inhibitor Cocktail (eBiosciences, San Diego CA) was added for the final four hours of incubation, per the manufacturer’s instructions, to allow accumulation of cytokines for intracellular staining. Cells were then processed for flow cytometric analysis as previously described. Data was collected using a BD-LSR II flow cytometer (BD Bioscience, Mountain View, CA) and analyzed using FlowJo (FlowJo, Ashton, OR) [12].
Statistical Analysis
Lung fungal burden data were log-transformed and analyzed by ANOVA with Tukey’s correction for multiple comparisons. Cytokine secretion was analyzed by False Discovery Rate (FDR) where the FDR was set to 5%. Normally distributed data were analyzed by t-test to detect differences in group means. All calculations were performed using Prism (GraphPad Software, La Jolla, CA).
Results
Viable Δcps1 spores are required to induce protection in mice
To determine if viable Δcps1 spores are required to produce protection, B6 mice (N= 6/group) were vaccinated either IN or SC twice, two weeks apart, with viable spores (100,000), irradiated spores (100,000), or spores killed with EtOH (500,000). Saline injection was used as a control. Four weeks after booster, mice were infected IN with 100 spores of Cp. Mice were sacrificed on day 11 p.i. due to moribund animals in the saline control, irradiated spore, and EtOH-killed spore groups. All mice vaccinated with viable spores were clinically well at sacrifice. Fungal burdens from the saline control group, both irradiated spore groups, and both killed spore groups were significantly higher than from mice vaccinated with the live spores (P<0.01 all comparisons) (Figure 1). In this study, mice vaccinated IN with live spores also had significantly lower lung fungal burdens than those vaccinated SC (P=0.02), but this result is inconsistent with other studies where we have not shown a significant difference in lung fungal burden between IN and SC administration [8]. Spleen cultures from mice given live Δcps1 spores were negative for fungal growth. There was growth in 5/6 spleens from the mice given irradiated spores, and from all mice given EtOH-killed spores or saline. Thus, viable Δcps1 spores are required to induce a protective immune response and none of the inactivated vaccines were effective.
Figure 1.
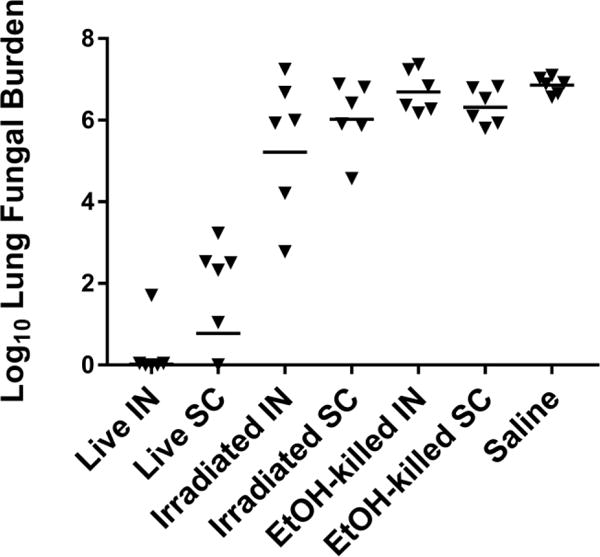
B6 mice (n=6/group) were vaccinated twice with live, irradiated (>99.9% killed), or EtOH-killed spores of Δcps1and challenged intranasally 4 weeks later with lethal Cp. Culture at 11 days p.i. shows that only the live spores significantly reduced lung fungal burden compared to killed preparations or saline control (p<0.001, all comparisons). The route of immunization IN (intranasal) vs. SC (subcutaneous) was not significant (p=0.542). Statistical analysis was by ANOVA with Tukey’s correction for multiple comparisons on log-transformed data. Bar = geometric mean for each group.
Δcps1 induces a response that protects from both C. immitis and C. posadasii
The two species C. immitis and C. posadasii differ in their geographic distribution and at the molecular level, with introgression in overlap regions in southernmost California [13, 14]. Ideally, a single vaccine would induce protection against challenge by either species. We tested this by vaccinating mice IN twice, two weeks apart, with Δcps1 and challenging with a lethal dose of either Ci or Cp four weeks later. As shown in Figure 2, all vaccinated mice survived in good health until scheduled sacrifice 180 days after challenge regardless of the infecting species. All control mice given either Ci or Cp succumbed by day 21.
Figure 2.
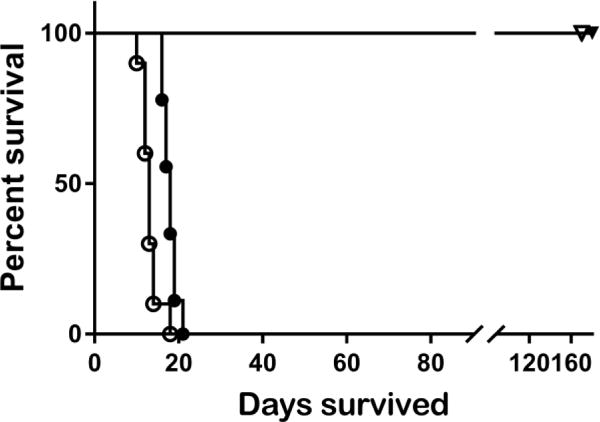
B6 mice were vaccinated twice IN with 10,000 spores of Δcps1or saline and infected 4 weeks later with a lethal dose of Cp or Ci (n=10/group). All control mice died by day 23 p.i, and all vaccinated mice survived until study termination, regardless of which species of wild-type Coccidioides was given. Open symbols = Cp challenge; closed symbols = Ci challenge; circles = control mice; triangles = vaccinated mice.
Δcps1 vaccination produces durable immunity
Because the duration of immunity is an important consideration in development of a vaccine, we vaccinated a cohort of B6 mice either once or twice two weeks apart with 10,000 spores SC and evaluated their resistance to lethal challenge 2, 4 and 6 months later. Mice were challenged with 100 spores of Cp and lung fungal burden was assessed 2 weeks p.i. As seen in Figure 3, all animals had a highly significantly decreased fungal burden (more than 106 fold) compared to unvaccinated animals when challenged at 2, 4 and 6 months post-vaccination. Importantly, there was no difference in the fungal burdens from 2 months to 6 months post-vaccination and no statistical difference between mice vaccinated once or twice. Thus, vaccination with Δcps1 is effective at producing durable, long-lived immunity.
Figure 3.
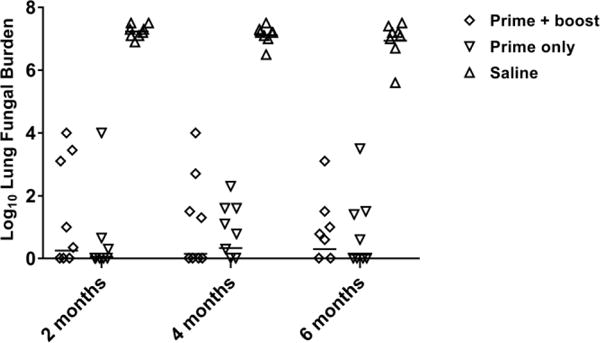
B6 mice (n=8/grp) were vaccinated SC twice two weeks apart or only once and challenged 2, 4, or 6 months after vaccination with ~100 spores of Cp IN. Mean lung fungal burdens 14 days p.i. were <Log 2 in vaccinated groups at all time points, while saline control mice had mean lung fungal burdens >Log 6 (p<0.001, all comparisons). There were no significant differences between prime only or prime + boost groups. Statistical analysis was by ANOVA with Tukey’s correction for multiple comparisons on log-transformed data. Bar = geometric mean.
Reduction of fungal burden depends on vaccine dose
To determine vaccine doses to test in a canine vaccine model, a range of doses was tested in two different strains of mice to determine minimum and maximum doses needed to produce protection. Groups of B6 or BALB/c mice were vaccinated SC twice with Δcps1 spores. Vaccine doses for each group were 500, 10,000, 100,000, or 500,000 spores. One group of each strain received a single vaccination with 500,000 spores, and control mice were given saline. Mice were challenged four weeks after vaccination with a lethal dose of Cp IN, sacrificed two weeks later, and cultured to determine lung fungal burden; livers were cultured whole to assess dissemination. Figure 4 shows a pattern of decreasing fungal burden with increased Δcps1 dose that reaches a clear plateau in B6 mice between 100,000 and 500,000 spores. For B6 mice, doses of at least 10,000 spores resulted in significant reduction compared to saline controls (p<0.001), and there was no difference between 100,000 and 500,000 spores given twice (p= 0.49) (Figure 4A). In BALB/c mice (Figure 4B), which are more difficult to protect [11], 10,000 spores also induced significant reduction in fungal burden compared to saline (p=0.006), but increasing doses to 500,000 continued to reduce fungal burden, possibly because they still had room for improvement in this infection model compared to the B6 mice. In both strains, 500,000 spores given twice suppressed lung fungal burden significantly better than only once (B6, p=0.016 and BALB/c, p=0.021). With at least 100,000 spore vaccine doses, dissemination to the spleen was prevented in all B6 and all but one of the BALB/c mice (data not shown). Based on these studies, maximal protection occurs between 100,000 and 500,000 viable Δcps1 spores and mice need to receive at least 10,000 spores. These data provide information for starting doses in dogs or humans.
Figure 4.
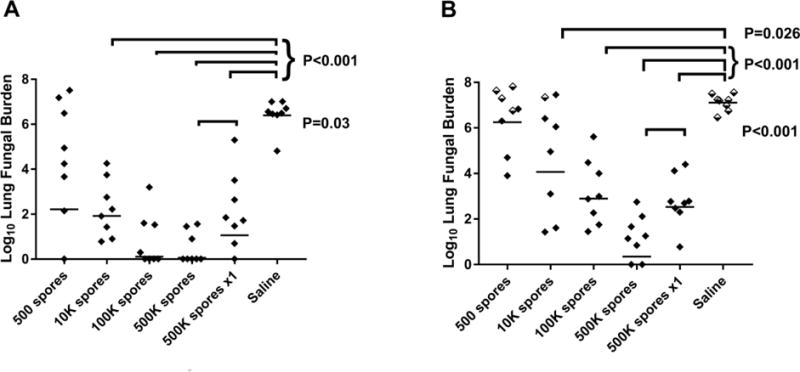
B6 (A) and BALB/c (B) mice (n=8/group) were vaccinated SC with a range of viable spores of Δcps1 twice or 500,000 spores once and challenged IN with 100 spores of Cp. Doses of at least 10,000 spores resulted in significant reduction in lung fungal burdens compared to saline as indicated on graphs. In both strains of mice, 500,000 spores given twice was significantly better than 500,000 spores once. Statistical analysis was by ANOVA with Tukey’s correction for multiple comparisons on log-transformed data. Half-filled diamonds in B indicate BALB/c mice that died before 14 days. Bar = geometric mean.
Proinflammatory cytokines are produced early in vaccinated mice following Cp challenge
To better understand the correlates of a protective immune response to Coccidioides infection, we examined the cytokine response in the lung following IN vaccination with Δcps1 and challenge with wild-type Cp, with the IN vaccination serving as a surrogate for a primary infection. In prior studies, Δcps1 had been cleared from lungs by four weeks following intranasal administration [8]. As a surrogate for prior infection, B6 mice were given 10,000 spores of Δcps1 intranasally twice two weeks apart and allowed to recover for four weeks. Mice given saline IN served as negative controls. Mice were infected with 500 spores of Cp and sacrificed on days 1, 2, 4, and 6 p.i. Mononuclear cells from lungs were cultured overnight without further stimulation and the supernatant tested for cytokines using a Luminex platform for 31 cytokines/chemokines. The complete results of the Luminex assay from all four days is shown in supplementary material. Six of the 31 cytokines tested demonstrated a significant difference between saline- and Δcps1-vaccinated mice following Cp challenge on days 2, 4, and 6, and Table 1 summarizes this data. Two cytokines, IL-4 and LIX, differed significantly in saline and vaccinated mice on day 2, with IL-4 upregulated in the vaccinated mice and LIX in the saline group. Though only day 2 is significant, the IL-4 rose rapidly in the vaccinated mice and appears to be trending back down by day 6, at which time it has just begun to increase in the saline mice. On day 4, which is coincident with spherule rupture [15, 16], five proinflammatory cytokines - MIG, IP-10, MCP-1, IFN-γ, and IL-1β - were significantly elevated in vaccinated compared to control mice, indicating a strong Th1 response as MCP-1, IP-10 and MIG are induced by IFN-γ (Table 1). The cytokine trends, even where not significant, show that the vaccinated mice are quickly able to mobilize the correct inflammatory response for host control by the time the first round of fungal replication takes place, while the saline mice lag until after the spherules rupture. By day 6, significant increases in M-CSF and MIP-1β (macrophage and neutrophil chemoattractants) along with the trends of increase in several other cytokines show that the saline mice are finally mobilizing a response.
Table-1.
| Cytokine | Day 1 | Day 2 | Day 4 | Day 6 | ||||
|---|---|---|---|---|---|---|---|---|
| Saline Vax pg/mla | Δcps1 Vax pg/ml | Saline Vax pg/ml | Δcps1 Vax pg/ml | Saline Vax pg/ml | Δcps1 Vax pg/ml | Saline Vax pg/ml | Δcps1 Vax pg/ml | |
| IL-4 | 1.61 | 5.07 | 1.61 | 17.30 | 1.61 | 37.36 | 19.57 | 10.81 |
| 0.0 | 5.99 | 0.0 | 7.27 | 0.0 | 33.90 | 26.65 | 8.33 | |
|
| ||||||||
|
LIX (CXCL5) |
4478.72 | 3449.55 | 4982.25 | 3580.90 | 4787.18 | 3770.22 | 4083.91 | 4437.54 |
| 649.86 | 362.38 | 224.96 | 275.55 | 1775.24 | 1040.43 | 596.12 | 1383.74 | |
|
| ||||||||
| IL-1β | 23.17 | 57.70 | 12.56 | 20.17 | 14.69 | 41.67 | 149.15 | 55.25 |
| 7.33 | 48.00 | 1.53 | 5.31 | 1.19 | 14.26 | 73.61 | 16.57 | |
|
| ||||||||
|
IP-10 (CXCL10) |
1421.89 | 774.83 | 419.79 | 1919.68 | 398.12 | 978.44 | 852.10 | 784.92 |
| 1213.43 | 230.00 | 37.42 | 1471.52 | 24.90 | 78.11 | 250.37 | 229.03 | |
|
| ||||||||
|
MCP-1 (CCL2) |
1810.84 | 4279.65 | 1423.43 | 2021.21 | 1434.14 | 2247.77 | 3083.74 | 1794.14 |
| 34.75 | 3014.26 | 623.87 | 1233.98 | 103.79 | 40.71 | 2081.47 | 835.70 | |
|
| ||||||||
|
MIG (CXCL9) |
305.22 | 1073.62 | 326.85 | 5053.57 | 138.98 | 2976.02 | 3132.20 | 2936.92 |
| 183.41 | 480.30 | 256.29 | 4242.19 | 12.81 | 608.77 | 2103.75 | 1484.39 | |
|
| ||||||||
| IFN-γ | 10.67 | 20.89 | 6.00 | 608.99 | 5.31 | 210.09 | 28.10 | 42.03 |
| 4.23 | 3.48 | 0.82 | 570.15 | 1.27 | 191.32 | 21.36 | 20.55 | |
|
| ||||||||
| M-CSF | 333.93 | 90.14 | 34.50 | 35.20 | 32.97 | 38.41 | 152.46 | 41.06 |
| 481.74 | 43.96 | 9.51 | 6.71 | 9.56 | 4.45 | 34.64 | 8.22 | |
|
| ||||||||
|
MIP-1β (CCL4) |
40.26 | 59.28 | 34.02 | 34.44 | 27.15 | 27.69 | 270.86 | 29.57 |
| 5.47 | 17.44 | 12.12 | 8.08 | 1.33 | 1.07 | 93.13 | 6.51 | |
Mean ± SD
Spleen CD4+ T-cells prime a Th1 response following Δcps1 vaccination
To determine antigen-specific T-cell responses of vaccinated mice, we performed analysis of the lymphoid populations in B6 mouse spleens A) following vaccination only and B) following Cp challenge four weeks after vaccination with Δcps1. Controls for each study received saline in place of vaccine. Two weeks after SC Δcps1-vaccination only or after the Cp challenge in vaccinated mice, spleens were harvested and processed into single cell suspensions. Splenocytes from pools of four mice were stimulated in vitro for 18 hrs with either Coccidioides spherule lysate or PBS and analyzed for production of IFN-γ and IL-17 by T-cells. In the first experiment, CD4+ T cells from mice vaccinated with Δcps1 made a strong IFN-γ response to spherule lysate (Figure 5), indicating the vaccine induced a Th1 response. This was true in vaccinated mice after wild-type Cp challenge as well, but not in unvaccinated challenged mice. The increase in vaccinated animals’ CD4+ T cells secreting IFN-γ is evident in both the percentage and total number of cytokine producing cells. Interestingly, no IL-17 was detectable after stimulation of the vaccinated mice. Also, CD8+ T cells failed to make either cytokine in response to in vitro stimulation. The upregulation of CD4+ T cells secreting IFN-γ following vaccination and in vitro restimulation supports the cytokine analysis that showed IFN-γ production/Th1 responses in whole lung cultures of vaccinated mice in the first four days following infection. Thus both cytokine secretion detected in the lung and in the spleen confirm that Δcps1 induces the Th1 response that is required for host control of Coccidioides infection [17, 18].
Figure 5.
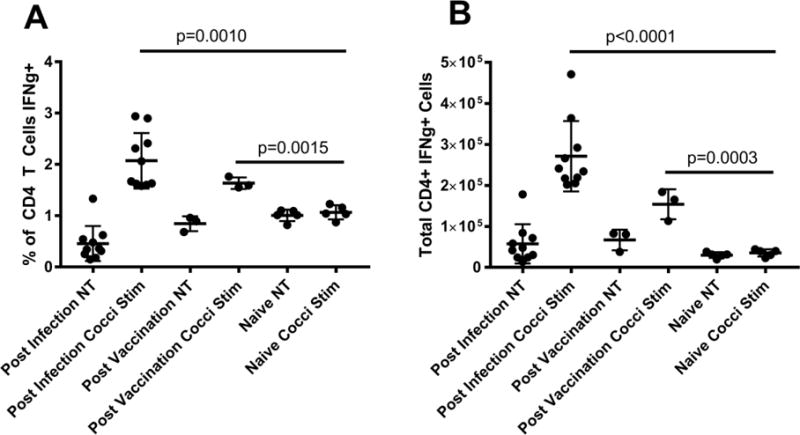
B6 mice (n=12) were vaccinated IN with 10,000 viable spores of Δcps1 or saline (n=8). Two weeks after vaccination splenocytes were harvested and stimulated with 10 ug/ml of Coccidioides spherule lysate (cocci stim) or PBS (NT) for 18 hours, then stained for T-cell cytokine production. There was a significant increase in CD4+ T cells producing IFN-γ in vaccinated mice stimulated with Coccidioides spherule lysate compared to unvaccinated mice (p=0.0008). A second set of vaccinated B6 mice (n=10) was infected with 100 spores of Cp 4 weeks post-vaccination. Two weeks post-infection, spleens were harvested and stimulated as above. As with vaccinated only mice, vaccinated and infected mice also had a significant increase in CD4+ cells making IFN-γ (p<0.0001). Calculation of the total number of CD4+ cells making IFN-γ (B) showed similar results for both vaccinated (p=0.0003) and vaccinated and infected mice (p<0.0001) compared to naïve animals. Dots for vaccinated only mice represent pools of 4 spleens; all other dots represent individual animals. Significance was determined using a Student’s t-test, and error bars indicate SD.
Discussion
Four important points emerged from this research. First, vaccination with Δcps1 provides durable immunity and extended survival following challenge, both for at least six months. Second, vaccination produces a Th1 response that is seen in both the spleen cells of vaccinated mice, and in the lungs of vaccinated mice challenged with Cp. Third, the Δcps1 vaccine must be viable to induce protection after vaccination. Finally, the vaccine provides protection not only against the same species as the vaccine strain, C. posadasii, but also against C. immitis infection.
Coccidioidomycosis vaccine studies seldom monitor vaccinated mice beyond 90 days [19–21], and there are none with a similarly lethal infection (100% deaths in the control mice within three weeks) that have been monitored for six months. Though in past studies with a recombinant protein we have observed a few late deaths near the 2-month termination date [9], the Δcps1-vaccinated mice experienced no late losses up to six months, with healthy-looking survivors that primarily had low or no residual lung fungal burdens. The protection from this vaccine also proved durable, yielding no difference in the high level of protection it provided at 2, 4 or 6 months after vaccination. This supports the utility of the vaccine in a clinical setting for dogs or humans, where it might be found to have even more durable effects.
Previous work investigating the requirements for survival following wild-type Cp or Ci infection has shown T cells and IFN-γ are required [22–24]. Mice deficient in T cells or the ability to produce IFN-γ are more susceptible to lethal Coccidioides infection [25]. These results showed that lungs of mice vaccinated with Δcps1 rapidly produced an IFN-γ response, plus other proinflammatory, IFN-γ-driven cytokines, 2-4 days post-infection. This suggests that the quick anamnestic spike in Th1 immunity provokes the strong host control repeatedly demonstrated in challenge studies herein. In contrast, significant increases in cytokine expression by naïve mice only became apparent on day 6 with the rise of cytokines that attract macrophages and neutrophils (MIP-1 β, M-CSF) and upward trends of the other pro-inflammatory cytokines. These late cytokines in infected, naïve mice are most likely related to increasing fungal burdens compared to vaccinated animals and correlate with spherule rupture after 96 hours [16].
In further support of the Th1 requirement, we showed that resident CD4+T cells in the spleens of vaccinated animals produced IFN-γ following overnight culture with Cp extracts. Although others have detected Th17 cells and IL-17 and consider these a key feature of a vaccine response to Coccidioides and other fungi [17], we observed no evidence of IL-17 in either whole lung cultures or in vitro cytokine responses by isolated cells of B6 mice vaccinated with Δcps1. This result is reminiscent of a previous report that showed that while IL-17 was important in primary responses to Francisella, it was not required in secondary responses [26]. While previous Coccidioides vaccination studies have reported a protective role for CD8+ T cells [27] our study showed no protective capacity of immune CD8+ T cells alone when transferred into an immunocompetent mouse and then challenged. The previous study used differing routes of vaccination and challenge as well as a different vaccinating and challenge strain. The role that CD8+ T cells may play in the establishment of protective immunity in our vaccination and challenge model remains to be examined in greater depth.
Killed, whole-spherule vaccines against coccidioidomycosis have been proven highly efficacious in mice, but both intolerable and not efficacious in people [12, 28]. They are characterized by repeated administration of large quantities (0.8-1 mg) of killed spherules. By enumerating in vitro-grown spherules, we estimate that this is equivalent on a dry weight basis to approximately 1 × 107 spherules per dose. These studies clearly demonstrated that a dose of 10,000 live spores given once or twice induces a high level of protection, while 10-fold or higher doses of irradiated and EtOH-killed spores provided no protection at all. Therefore, the viability of the vaccine is necessary for efficacy. Presumably, the degrading walls and escaping contents of Δcps1 spores as they initiate spherule development in the first hours or days following vaccination [8] provide a wide array of antigenic determinants, similar to what might be encountered following a natural infection. We hypothesize that it is these early proliferative events of the viable Δcps1 prior to its clearance or arrest that result in the durable response with a much lower immunizing dose than is necessary with killed spherule vaccines. The value of booster immunization is equivocal from this data. One study clearly shows that equivalent protection is afforded over six months with either one or two vaccines, and the other demonstrates a greater reduction of fungal burden in two strains of mice given a booster compared to a single vaccine. Studies in a target species may be required to determine the importance of a booster vaccine.
The ability to protect against a heterologous challenge is a major advantage for any vaccine, and critically important for a Coccidioides vaccine due to the inherent small market. Although C. posadasii covers a wider geographic range than C. immitis, approximately 40% of the vulnerable population of humans lives in regions endemic for C. immitis. Our data demonstrate no difference in the ability of Δcps1 to protect mice against C. immitis or C. posadasii. This supports a Δcps1vaccine having utility to prevent disease regardless of the geographic origin of infection.
Supplementary Material
Acknowledgments
Funding
This work was supported by NIAID grants R21 AI117885-01, U01 AI122275-01, and R01 AI132140-01 and by donations to the University of Arizona Foundation Valley Fever Vaccine Project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Footnotes
BSL – biosafety level
GYE – glucose yeast extract
Cp – Coccidioides posadasii
Ci – Coccidioides immitis
IN – intranasal
SC – subcutaneous
IP – intraperitoneal
Conflict of Interest
Marc J. Orbach and Lisa F. Shubitz have a potential conflict of interest as co-discoverers of the Δcps1 vaccine strain on the patent application.
References
- 1.Johnson SM, Carlson EL, Fisher FS, Pappagianis D. Demonstration of Coccidioides immitis and Coccidioides posadasii DNA in soil samples collected from Dinosaur National Monument, Utah. Med Mycol. 2014;52:610–7. doi: 10.1093/mmy/myu004. [DOI] [PubMed] [Google Scholar]
- 2.Litvintseva AP, Marsden-Haug N, Hurst S, Hill H, Gade L, Driebe EM, et al. Valley Fever: Finding New Places for an Old Disease: Coccidioides immitis Found in Washington State Soil Associated With Recent Human Infection. Clinical Infectious Diseases. 2015;60:e1–e3. doi: 10.1093/cid/ciu681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsden-Haug N, Goldoft M, Ralston C, Limaye AP, Chua J, Hill H, et al. Coccidioidomycosis Acquired in Washington State. Clinical Infectious Diseases. 2013;56:847–50. doi: 10.1093/cid/cis1028. [DOI] [PubMed] [Google Scholar]
- 4.Huang JY, Bristow B, Shafir S, Sorvillo F. Coccidioidomycosis-associated Deaths, United States, 1990-2008. Emerg Infect Dis. 2012;18:1723–8. doi: 10.3201/eid1811.120752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sondermeyer G, Lee L, Gilliss D, Tabnak F, Vugia D. Coccidioidomycosis-associated Hospitalizations, California, USA, 2000-2011. Emerg Infect Dis. 2013;19 doi: 10.3201/eid1910.130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shubitz LF. Comparative aspects of coccidioidomycosis in animals and humans. Ann N Y Acad Sci. 2007;1111:395–403. doi: 10.1196/annals.1406.007. [DOI] [PubMed] [Google Scholar]
- 7.Shubitz LF, Butkiewicz CD, Dial SM, Lindan CP. Incidence of Coccidioides infection among dogs residing in a region in which the organism is endemic. Journal of American Veterinary Medical Association. 2005;226:1846–50. doi: 10.2460/javma.2005.226.1846. [DOI] [PubMed] [Google Scholar]
- 8.Narra HP, Shubitz LF, Mandel MA, Trinh HT, Griffin K, Buntzman AS, et al. A Coccidioides posadasii CPS1 Deletion Mutant Is Avirulent and Protects Mice from Lethal Infection. Infect Immun. 2016;84:3007–16. doi: 10.1128/IAI.00633-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng T, Shubitz LF, Simons J, Perrill R, Orsborn KI, Galgiani JN. Localization within a proline-rich antigen (Ag2/PRA) of protecctive antigenicity against infection with Coccidioides immitis in mice. Infection and Immunity. 2002;70:3330–5. doi: 10.1128/IAI.70.7.3330-3335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abuodeh RO, Orbach MJ, Mandel MA, Das A, Galgiani JN. Genetic Transformation of Coccidioides immitis Facilitated by Agvobactevium tumefaciens. Journal of Infectious Diseases. 2000;181:2106–10. doi: 10.1086/315525. [DOI] [PubMed] [Google Scholar]
- 11.Shubitz LF, Peng T, Perrill R, Simons J, Orsborn KI, Galgiani JN. Protection of mice against Coccidioides immitis intranasal infection by vaccination with recombinant antigen 2/PRA. Infection and Immunity. 2002;70:3287–9. doi: 10.1128/IAI.70.6.3287-3289.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrigan LM, Tuladhar S, Brunton JC, Woolard MD, Chen CJ, Saini D, et al. Infection with Francisella tularensis LVS clpB Leads to an Altered yet Protective Immune Response. Infection and Immunity. 2013;81:2028–42. doi: 10.1128/IAI.00207-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neafsey DE, Barker BM, Sharpton TJ, Stajich JE, Park DJ, Whiston E, et al. Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Research. 2010;20:938–46. doi: 10.1101/gr.103911.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher MC, Koenig GL, White TJ, Taylor JW. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia. 2002;94:73–84. [PubMed] [Google Scholar]
- 15.Sun SH, Huppert M. A cytological study of morphogensis in Coccidioides immitis. Sabouraudia. 1976;14:185–98. [PubMed] [Google Scholar]
- 16.Shubitz LF, Perrill R, Lewis ML, Dial SM, Galgiani JN. Early Post-Infection Detection of Coccidioides in Intranasally Infected Mice. Mayo Clinic in Arizona. 2011:40. [Google Scholar]
- 17.Hung CY, Gonzalez A, Wuthrich M, Klein BS, Cole GT. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17) Infect Immun. 2011;79:4511–22. doi: 10.1128/IAI.05726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magee DM, Cox RA. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infection and Immunity. 1995;63:3514–9. doi: 10.1128/iai.63.9.3514-3519.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong YM, Levine HB, Madin SH, Smith CE. Fungal multiplication and histopathological changes in vaccinated mice infected with Coccidioides immitis. Journal of Immunology. 1964;92:779–90. [PubMed] [Google Scholar]
- 20.Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. A Genetically Engineered Live Attenuated Vaccine of Coccidioides posadasii Protects BALB/c Mice against Coccidioidomycosis. Infection and Immunity. 2009;77:3196–208. doi: 10.1128/IAI.00459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson SM, Kerekes KM, Lunetta JM, Pappagianis D. In: Characteristics of the protective subcellular coccidioidal T27K vaccine. Clemons KV, Laniado-Laborin R, Stevens DA, editors. Annals of the New York Academy of Science; 2007. pp. 275–89. [DOI] [PubMed] [Google Scholar]
- 22.Hung CY, Wozniak KL, Cole GT. Flow Cytometric Analysis of Protective T-Cell Response Against Pulmonary Coccidioides Infection. Methods in molecular biology (Clifton, NJ) 2016;1403:551–66. doi: 10.1007/978-1-4939-3387-7_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaman L, Pappagianis D, Benjamini E. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infection and Immunity. 1977;17:580–5. doi: 10.1128/iai.17.3.580-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaman L. Fungicidal activation of murine macrophages by recombinant gamma interferon. Infection and Immunity. 1987;55:2951–5. doi: 10.1128/iai.55.12.2951-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clemons KV, Leathers CR, Lee KW. Systemic Coccidioides immitis infection in nude and beige mice. Infection and Immunity. 1985;47:814–21. doi: 10.1128/iai.47.3.814-821.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts LM, Davies JS, Sempowski GD, Frelinger JA. IFN-gamma, but not IL-17A, is required for survival during secondary pulmonary Francisella tularensis Live Vaccine Stain infection. Vaccine. 2014;32:3595–603. doi: 10.1016/j.vaccine.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fierer J, Waters C, Walls L. Both CD4+ and CD8+ T cells can mediate vaccine-induced protection against Coccidioides immitis infection in mice. The Journal of Infectious Diseases. 2006;193:1323–31. doi: 10.1086/502972. [DOI] [PubMed] [Google Scholar]
- 28.Pappagianis D. Evaluation of the protection efficacy of the killed Coccidioides immitis spherule vaccine in humans. American Review of Respiratory Disease. 1993;148:656–60. doi: 10.1164/ajrccm/148.3.656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


