Abstract
Purpose
Adoptive cell therapy (ACT) using tumor-infiltrating lymphocytes (TIL) has consistently demonstrated clinical efficacy in metastatic melanoma. Recent widespread use of checkpoint blockade has shifted the treatment landscape, raising questions regarding impact of these therapies on response to TIL and appropriate immunotherapy sequence.
Experimental design
Seventy-four metastatic melanoma patients were treated with autologous TIL and evaluated for clinical response according to irRC, overall survival and progression free survival. Immunologic factors associated with response were also evaluated.
Results
Best overall response for the entire cohort was 42%; 47% in 43 checkpoint naïve patients, 38% when patients were exposed to anti-CTLA4 alone (21 patients) and 33% if also exposed to anti-PD1 (9 patients) prior to TIL ACT. Median overall survival was 17.3 months; 24.6 months in CTLA4 naïve patients and 8.6 months in patients with prior CTLA4 blockade. The latter patients were infused with fewer TIL and experienced a shorter duration of response. Infusion of higher numbers of TIL with CD8 predominance and expression of BTLA correlated with improved response in anti-CTLA-4 naive patients, but not in anti-CTLA-4 refractory patients. Baseline serum levels of IL-9 predicted response to TIL ACT, while TIL persistence, tumor recognition and mutation burden did not correlate with outcome.
Conclusion
This study demonstrates the deleterious effects of prior exposure to anti-CTLA4 on TIL ACT response and shows that baseline IL-9 levels can potentially serve as a predictive tool to appropriately select sequence for immunotherapies.
Keywords: TIL ACT, CTLA4, metastatic melanoma
INTRODUCTION
Adoptive cell therapy (ACT) of tumor-infiltrating lymphocytes (TIL) involves infusion of ex-vivo expanded autologous T cells after non myeloablative lymphodepletion (with cyclophosphamide and fludarabine), followed by administration of high dose IL-2 to support TIL post-infusion. Multiple centers have reported overall response rates (ORR) of 40–50% for TIL therapy for metastatic melanoma patients (1–5). In our initial report of 31 treated patients at MD Anderson Cancer Center, we observed an ORR of 48.4%, with a complete response (CR) rate of 6.5% (2). Factors predictive of favorable treatment response included higher number of infused TIL, higher numbers of CD8+ T cells within the infusion product, effector phenotype of the infused CD8+ T cells, and higher frequency of CD8+ T cells co-expressing B-and-T-lymphocyte attenuator (BTLA). These factors, however, are determined based on the features of the generated infusion product. It is critically important to identify biomarker(s) predictive of favorable response to better determine which patients would respond favorably to TIL therapy.
In parallel, other immunotherapy treatment strategies involving checkpoint blockade have achieved FDA approval for metastatic melanoma, thus vastly changing the treatment landscape (6–9). The cytotoxic T lymphocyte associated antigen-4 (CTLA4) blocker ipilimumab was FDA approved in 2011 and consistently shows response rates of 10–15% with potential for durable benefit over 3 years in 25% of treated patients. Single agent anti-PD1 blockade received FDA approval in 2014 and the combination of anti-CTLA4 with PD1 blockade was approved in 2015. As each subsequent newly approved immunotherapy regimen has shown improved response rate, these agents have replaced chemotherapy and high dose interleukin-2 as the standard of care for metastatic melanoma. Thus, our TIL treated patients are now almost universally refractory to either CTLA4 or PD1 blockade which may lead to altered response rates and duration of response if the treatment is consistently administered to patients who are either primarily or secondarily resistant to checkpoint inhibition. While there are preliminary reports citing lower responses rates to TIL ACT in melanoma patients with prior checkpoint exposure, this has not been thoroughly studied in a large patient cohort (3,10).
The primary aims for this study is to assess the response of TIL ACT in the modern age of melanoma therapy, specifically in a cohort of patients who have been previously treated with anti-CTLA4 and/or anti-PD1 therapy. We also sought to assess if biomarkers predicting response to TIL could be identified from pre-therapy samples to identify patients most likely to derive benefit. Reliable predictive biomarkers to guide clinicians towards appropriate first line immunotherapy have yet to be discovered and has become increasingly important as there are now more standard treatment options available for patient care. Since every immunotherapy approach comes with its share of toxicities, it would be highly desirable to orient the patient rapidly to the regimen that he or she is more likely to benefit from using non-invasive methods. The analysis of circulating biomarkers in this cohort of 74 patients resulted in the identification of IL-9 as a factor potentially predictive of favorable response for TIL ACT. This marker represents to our knowledge the first reported potential predictive biomarker for TIL ACT.
MATERIALS AND METHODS
Study Approval and design
The study was approved by the United States Food and Drug Administration and the Institutional Review Board at MD Anderson Cancer Center. Study monitoring was performed by the MD Anderson Investigational New Drugs Office. This study was written and conducted in accordance with the principles from the Declaration of Helsinki. All patient granted a written informed consent prior to treatment initiation.
Patient selection
All metastatic melanoma TIL lines were derived from tumor tissue obtained from patients enrolled on the TIL ACT clinical trial (Institutional review board (IRB)-approved protocol# 2004-0069, NCT00338377) at The University of Texas MD Anderson Cancer Center (MD Anderson). Seventy-four patients were treated between August 2007 and May 2015. This includes the original cohort of 31 patients previously reported (2). Male and female patients over the age of 12 with locally-advanced stage III or stage IV cutaneous, mucosal or uveal melanoma were eligible for enrollment. Prior treatment with any melanoma therapy was allowed, including surgery, radiation, chemotherapy, targeted therapy and/or immunotherapy. Patients with brain metastases 1cm or less in size that were asymptomatic were eligible, as were patients with prior treated and stable brain metastases. All patients had Eastern Cooperative Group Performance Status of 0–2 at time of enrollment and had to have normal bone marrow, hepatic and renal function. All patients were required to have adequate cardiac and pulmonary function as demonstrated by dobutamine stress echocardiogram and pulmonary function by spirometry, respectively. Patients were required to have measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Specific exclusion criteria included any primary or secondary immunodeficiency or use of systemic steroids within 4 weeks of lymphodepletion. Refer to clinical trial NCT00338377 in the NCI website for additional criteria.
Study Design
This is a non-randomized open-label phase 2 study. All patient received a course of non-myeloablative lymphodepletion chemotherapy starting on day −7 (where Day 0 = day of TIL infusion) prior to TIL infusion (60mg/kg of cyclophosphamide on day −7 and −6 and 25mg/m2 of fludarabine from day −5 to day −1) (2). Freshly harvested and washed autologous TIL were infused intravenously on day 0 and a first round of infusion of high dose IL-2 (720,000 IU/kg) was started on day 1 every 8 hours to tolerance for a maximum of 15 doses. Patients were discharged from the hospital once treatment-related toxicities were well managed and then hospitalized once more for a second course of high dose IL-2 approximately day 21 after TIL infusion.
Study Assessments
Blood samples were collected from patients at time of surgery for TIL harvest and/or prior to lymphodepletion (baseline), as well as serially post TIL infusion (days 7, 21, 28, 42 and at the time of imaging). PBMC and serum were preserved when possible for each time points by the MD Anderson Immunomonitoring Core Lab and Melcore Lab. Patient response to therapy was obtained by serial imaging (either PET/CT or contrast enhanced CT of chest/abdomen/pelvis as well as imaging of CNS either with magnetic resonance imaging or CT) which was performed at baseline, 6 weeks, 12 weeks and then every 2–3 months for follow up at the investigator’s discretion. Response evaluation was performed per the Immune Related Response Criteria (irRC) (11). Per protocol, no confirmation scan is needed to define a PR but is required for CR confirmation and is optional for PD. Adverse event monitoring was conducted from time of lymphodepletion initiation, throughout the duration of the hospital stays and follow up on protocol and was assessed per the NIH Common Terminology Criteria for Adverse Events CTCAE version 3.0 (NCI 2006).
TIL expansion and reactivity assessment
TIL were expanded from 1–5mm3 melanoma tumor fragments in TIL complete media (TIL-CM) supplement with 6000IU/mL of IL-2 for a period averaging between 3 to 5 weeks as previously described (2). This constituted the first phase of expansion. In the second phase of expansion, TIL were propagated by a rapid expansion protocol (REP) with anti-CD3 (OKT3, Orthoclone) using pooled allogeneic irradiated PBMC feeder cells (ratio of 1 TIL to 200 feeders). The REP was either initiated in T175 flasks (Nunc) for the first 7 days and then transferred into 3L cell culture bags (OriGen) for the 7 remaining days or done entirely using G-Rex 100M flask (Wilson Wolf Manufacturing) (2,12). All cultures were maintained under the current Good Tissue practice (cGTP) and current Good Manufacturing Practice (cGMP).
Successfully expanded pre-REP TIL were tested for anti-tumor reactivity (autologous setting) by measuring IFNγ production in the supernatant of a co-culture of TIL and autologous tumor cells as previously described (2). IFNγ ELISA kits were obtained from Pierce.
Flow cytometry analysis
TIL were stained in FACS Wash Buffer (1× DPBS with 1% Bovine Serum Albumin) for 30 min on ice using fluorochrome-conjugated monoclonal antibodies from BD Biosciences against CD3, CD4, CD8 and BTLA (clone J168) as described previously (2). Cells were fixed in 1% paraformaldehyde solution for 20min at room temperature following surface staining. Samples were acquired using the BD FACSCanto II and analyzed using FlowJo Software v 7.6.5 (Tree Star). Dead cells were stained using AQUA live/dead dye (Invitrogen) and excluded from the analysis.
TCR Vβ sequencing and tracking
Cell pellets from infusion products of TIL treated patients were flash frozen and stored at −80C. PBMC were collected prior to- and post-TIL infusion. Total DNA was isolated using the Qiagen AllPrep Kit according to the manufacturer’s instructions. TCRβ CDR3 sequencing was performed using the Adaptive Biotechnology (Seattle, WA) pipeline. Survey level sequencing was performed on DNA extracted from TIL and deep level sequencing was performed on DNA extracted from PBMC. Clonality was determined using Immunoseq as previously described (13). Tracking data was extracted from Immunoseq and analyzed at MD Anderson.
Whole Exome Sequencing and Mutation Calls
Library construction and hybrid capture
Whole exome sequencing was performed on DNA extracted from 67 archival formalin-fixed, paraffin-embedded (FFPE) tumor tissues remaining from the tumor harvests used to expand the autologous TIL product. Matched blood samples were used as germ-line controls. Library construction was performed as described in Fisher et al., with the following modifications: initial genomic DNA input into shearing was reduced from 3µg to 10–100ng in 50µL of solution (14). Agilent SureSelect or Illumina Nextera Rapid Capture Exome Kit was used for the exome capture as described in the Supplementary Material.
Preparation of libraries for cluster amplification and sequencing
After post-capture enrichment, library pools were quantified using qPCR (automated assay on the Agilent Bravo), using a kit purchased from KAPA Biosystems with probes specific to the ends of the adapters. Based on qPCR quantification, libraries were normalized to 2nM, then denatured using 0.1 N NaOH on the Hamilton Starlet. After denaturation, libraries were diluted to 20pM using hybridization buffer purchased from Illumina.
Cluster amplification and sequencing
Cluster amplification of denatured templates was performed according to the manufacturer’s protocol (Illumina) using HiSeq 4000 cluster chemistry and HiSeq 4000 flowcells. Flowcells were sequenced on v1 Sequencing-by-Synthesis chemistry for HiSeq 4000 flowcells. The flowcells were then analyzed using RTA v.1.18.64 or later. Each pool of whole exome libraries was run on paired 76bp runs, reading the dual-indexed sequences to identify molecular indices and sequenced across the number of lanes needed to meet coverage for all libraries in the pool.
Somatic mutation detection
Single-nucleotide variants (SNVs) were called by Mutect and filtered to remove FFPE-specific artifacts based on strand-bias (15,16). Short insertions and deletions (indels) were not included in the analysis due to the prevalence of artefactual calls. The SNVs were further filtered to remove artifacts, based on allelic fractions observed in panels of normal samples. Mutations were annotated with Oncotator (17). The number of nonsynonymous mutations in each sample was tallied using MutSigCV (18).
Serum analysis
When available, serum from TIL treated patients collected at baseline (time of surgery for TIL expansion), day 7, pre-IL2 (~week 3) and 3 months post-TIL ACT was assessed using Invitrogen ProcartaPlex multiplex immunoassays (Thermo Fisher Scientific, Vienna, Austria). When multiple baseline samples were available for a patient, the last collection prior to TIL therapy was assessed. All time points were tested for the analytes listed in Supplementary Material.
Statistical Analysis
The Kaplan-Meier product-limit method was used to estimate the OS, PFS, and response duration distributions. OS and PFS were computed from the start of TIL ACT treatment, while response duration was computed from the date of first response. There were 51 deaths and 62 PFS events in 74 patients after a median follow-up of 74 months. Univariable and multivariable Cox proportional hazards regression models were used to estimate hazard ratios along with associated confidence intervals and p-values. Numeric variables were compared between groups using the Mann-Whitney U test, Kruskal-Wallis test, grouped dot plots, box plots, and violin plots, and ROC curve analysis. Analyses were performed using TIBCO S+ 8.2 for Windows. Comparisons of the number of nonsynonymous mutations between patient groups were performed in R [https://www.R-project.org/]. The stratified Mann-Whitney test, used to control for mean target coverage, is from the “coin” package.
RESULTS
Patient characteristics and overall response to TIL therapy
The stage IIIC and IV metastatic melanoma patients (n=74) treated under this protocol received their TIL infusion between August 2007 and May 2015. Patient’s characteristics are listed in Supplementary Table S1. MDACC TIL program employs a banking model as opposed to an “intent to treat” model and thus, we end up treating (proceeding with second phase of expansion, the REP) approximately 1/10 patients that receive a tumor harvest for TIL growth (19). Untreated patients’ pre-REP TIL cultures remain cryopreserved for potential use. Toxicities from therapy were predominantly associated with lymphodepletion and high dose IL-2 (Supplementary Table S2). Grade 3 toxicities included neutropenia, anemia, thrombocytopenia and febrile neutropenia as a consequence of lymphodepletion. The most common grade 3 toxicities from high dose IL-2 include capillary leak syndrome and hyperbilirubinemia. All patients were treated on a dedicated hospital unit and these toxicities are in keeping with, and did not deviate from, those known to occur with TIL ACT and high dose IL-2.
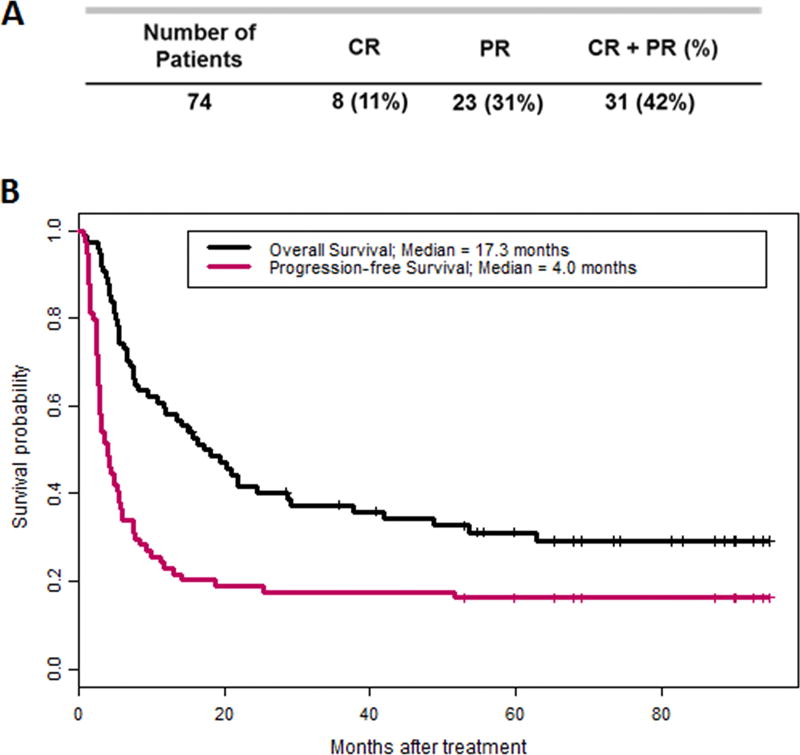
The best overall response (BOR) for the cohort was 42%. Eight patients (11%) achieved a CR and 23 achieved a PR (31%; Fig. 1A). With a median follow-up of 74 months, the median overall survival (OS) was 17.3 months [95% confidence interval (CI), 11.6 – 37.8) and the median progression-free survival (PFS) was 4.0 months (95% CI; 2.9 – 5.8) (Fig. 1B). The 1-year PFS and OS rates were 23% (95% CI; 15%, 35%) and 58% (95% CI; 48%, 70%), respectively. In addition, patient stratification based upon response (CR and PR) demonstrated the durability of response to therapy as the 2 year OS and PFS for CR patients (n=8) was 88% and 75%, respectively (Supplementary Fig. S1). Among the 17 patients that were progression-free for at least one year, the 5 year PFS was 71% (95% CI; 52%, 96%) (Fig. 1B).
Figure 1. Best overall response, OS and PFS of 74 metastatic melanoma patients following TIL ACT.
(A) Clinical response is stratified by complete response (CR) and partial response (PR). (B) Overall survival (black line) and progression-free survival (pink line) post-TIL ACT of the same cohort.
Impact of checkpoint blockade pre-treatment on response to TIL
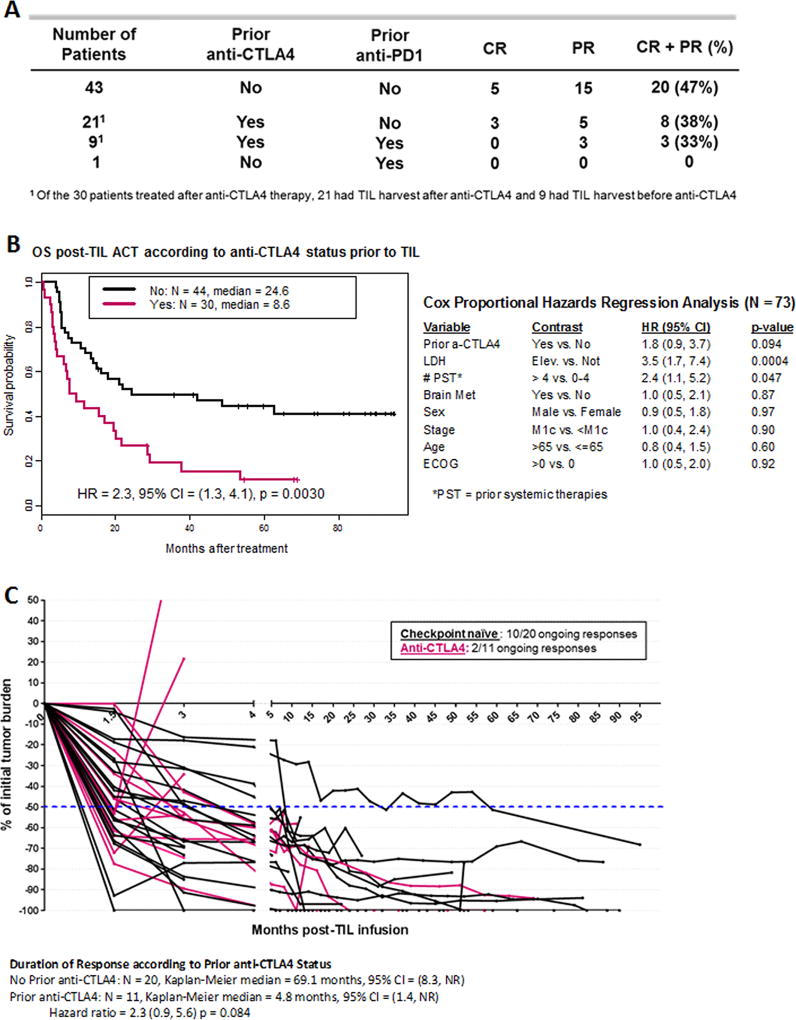
With the recent incorporation of antibodies targeting CTLA4 and PD1 for metastatic melanoma treatment, we sought to evaluate the impact of these therapies on response to TIL ACT. When patients were stratified according to prior checkpoint blockade therapy exposure, the checkpoint naïve patient subset (n=43) had a higher BOR, 47% (Fig. 2A). Surprisingly, when patients were exposed to anti-CTLA4, with or without anti-PD1 (n=9 and 21, respectively) the BOR was 33 and 38% respectively (Fig. 2A). This data clearly suggest a difference between the group however, the number of patients was too low to assess significance. The impact of anti-PD1 alone was not evaluated given the low number of patients (n=1). Consistent with the observed differences in BOR, the median OS was 24.6 months for CTLA4-naïve and 8.6 months for CTLA4 exposed patients (HR 2.3, 95% CI 1.3 – 4.1, p = 0.0030) (Fig. 2B). A similar observation was made for the PFS (Supplementary Fig. S2).
Figure 2. Impact of checkpoint blockade exposure prior to TIL ACT on response and survival.
(A) Best overall response stratified by checkpoint blockade therapy (anti-CTLA4 and/or anti-PD1) prior to TIL ACT (n=74). (B) On the left side, overall survival of checkpoint naïve patients prior to TIL ACT (black line) and anti-CTLA4 exposed patients prior to TIL (pink line). On the right side, multivariable analysis including 8 parameters. (C) Spider plot illustrating tumor regression through time after TIL infusion for checkpoint naïve patients prior to TIL ACT (black line) and anti-CTLA4 exposed patients prior to TIL (pink line). Only responders to TIL ACT are represented.
A multivariate analysis including anti-CTLA4 treatment status and conventional prognostic factors such as lactate dehydrogenase (LDH) at time of TIL infusion, number of prior systemic therapies (PST), presence of brain metastasis, sex, stage, age and ECOG performance status was performed (Fig. 2B, right table). The end point for this analysis was OS. A potential negative impact of progression on anti-CTLA4 prior to TIL ACT (HR 1.8 (0.9, 3.7)) was found but ruled inconclusive rather than no impact (p = 0.094) because of the strong negative effect of high levels of LDH on OS (HR 3.5 (1.7, 7.4) and p = 0.0004) and the limited number of patients in this cohort. A PST value greater than 4 was also a variable linked to poor OS, but contrary to anti-CTLA4, neither high dose of IL-2 or biochemotherapy prior to TIL ACT had an impact on OS post-TIL ACT (Supplementary Fig. S3). Because of the low number of patients treated with BRAF inhibitor prior to TIL infusion, it was not possible to determine if this therapy significantly contributed to changing OS post TIL infusion in an unbiased fashion (Supplementary Fig. S3).
Traditionally, response to TIL ACT has been shown to be durable (10). In our cohort of patients, we observed CR and PR ongoing for more than 7 years (Fig. 1B and 2C). When we stratify the responders by their checkpoint status (naïve or anti-CTLA4) prior to TIL infusion, not only do we observe fewer responders, but the duration of response is also decreased in TIL treated patients who are refractory to anti-CTLA4 (Fig. 2C). The median duration of response was found to be 69.1 months for the checkpoint naïve responders (n = 20) compared to 4.8 months for responders exposed to anti-CTLA4 prior to TIL ACT (n = 11) (HR = 2.3 (0.9, 5.6) p = 0.084). Interestingly, 2 out of 3 patients exposed anti-CTLA4 prior to TIL who achieved a CR after TIL ACT, recurred and died while only 1 out of 5 of the CR patients recurred from the checkpoint naïve group and remains NED post-surgery with no additional therapies. Overall, while the contribution of high LDH and the number of prior systemic therapies cannot be ignored, the data clearly supports the negative impact of anti-CTLA4 over other systemic therapies in this TIL ACT treated patient cohort.
Impact of anti-CTLA4 therapy on TIL persistence post-infusion
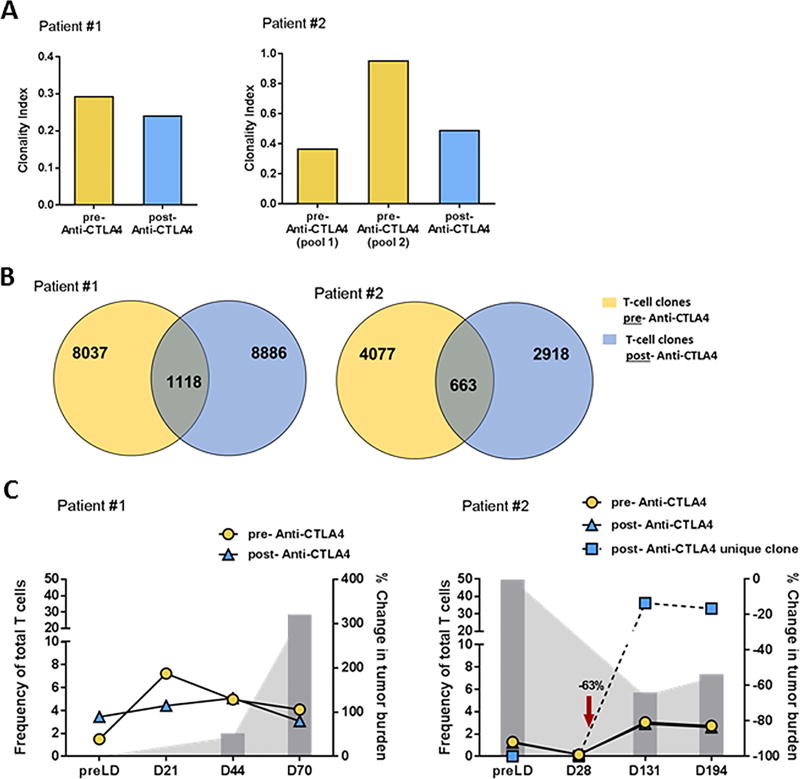
Within this cohort, 2 patients were infused with a mixture of TIL products derived from lesions obtained pre- and post- treatment with anti-CTLA4. These products were rapidly expanded separately and combined at the time of infusion. Prior to product combination, each was sampled for DNA extraction to assess the T-cell repertoire and persistence by CDR3 sequencing. As shown in Figure 3A, both patients maintained a similar level of clonal diversity regardless of their exposure to anti-CTLA4 with the exception of pool 2 for patient 2 (right graph), which was found to be more clonal. In both patients, unique clones were identified in each product, allowing for persistence of each product to be monitored over time (Fig. 3B). A similar percentage of clones unique to each pre- and post-anti-CTLA4 TIL products were detected throughout the assessed time points with the exception of one clone derived from the post-anti-CTLA4 TIL product found in Patient #2 (Fig. 3C, right graph). This clone started to expand in the blood a month post-infusion, coinciding with tumor regression leading to a temporary clinical response in this patient as depicted with the gray bars in Figure 3C right graph. Unfortunately, even though Patient #1’s TIL were able to persist over 2 months, no clinical benefit was observed and the patient progressed rapidly (Fig. 3C, left graph). Overall, we observed persistence of TIL independent of prior anti-CTLA4 exposure for both patients.
Figure 3. Exposure to anti-CTLA does not impact TIL persistence.
(A) Assessment of TIL diversity in the infusion product at the clonal level from tumor harvested pre- (yellow) and post-(blue) anti-CTLA4 therapy for 2 patients. The clonal diversity is expressed by the clonal index (1 = a clonal population, a clonal index closer to 0 = a polyclonal population). (B) Venn diagrams depicting the number of clones found only in one of the 2 conditions [pre (yellow) or post (blue)] as well as the number of REP expanded clones found in both pre and post-anti-CTLA4 TIL products combined in the infusion product (2 patients). (C) Separate tracking TIL post-infusion of both pre- (yellow) and post- (blue) anti-CTLA4 therapy product in both patients over time. For each patients, the percentage change in the tumor burden, baseline being “0” and a negative percentage demonstrating a response.
Correlation of infusion product content (TIL number, CD8+ TIL frequency and BTLA expression) with clinical response
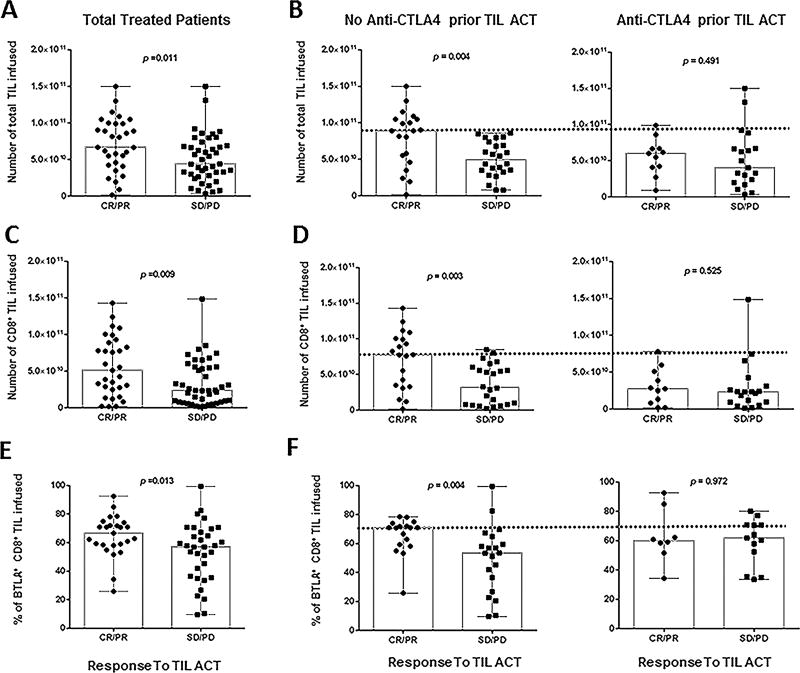
Our first report established that higher numbers of TIL within the infusion product correlated with clinical response (n=31) (2). When we look at this parameter in our entire cohort (n=74), we still observed this correlation (Fig. 4A, p = 0.011). All relevant numbers (median, mean, StD and p-value) related to Figure 4 are compiled in Supplementary Table S3. Interestingly, however, when we stratified the cohort by checkpoint naïve vs prior anti-CTLA4 exposure, the number of TIL infused no longer correlated with response in the anti-CTLA4 prior to TIL group (p = 0.491) whereas this parameter still strongly correlated with response to TIL in the checkpoint naïve group (p = 0.004) (Fig. 4B). The same stratification demonstrated that a high number of infused CD8+ TIL correlated with response except in the anti-CTLA4 prior to TIL group (Total, p = 0.009, CTLA4 p = 0.525 and naive p = 0.003, respectively) (Fig. 4C and D). Overall, patients that were exposed to anti-CTLA4 prior to treatment were infused with both less TIL overall and less CD8+ TIL as underscored by the dashed line in both Figure 4B and D.
Figure 4. Infusion product parameters correlating with clinical response.
(A) Total amount of TIL infused per patient (n=74) stratified by responders (CR, PR) and non-responders (SD, PD). (B) Results reported in A) separated according to their status prior to TIL ACT: exposed to anti-CTLA4 (right) or checkpoint naïve (left). (C) Total amount of CD8+ TIL infused per patient stratified by clinical response. (D) Results reported in (C) separated according to their status prior to TIL ACT: exposed to anti-CTLA4 (right) or checkpoint naïve (left). (E) Percentage of CD8+BTLA+ TIL infused per patient stratified by clinical response. This percentage was evaluated by flow cytometry. (F) Results reported in (E) separated according to their status prior to TIL ACT: exposed to anti-CTLA4 (right) or checkpoint naïve (left). All P-values were generated with the Mann-Whitney test.
The expression of BTLA on CD8+ TIL was previously shown to correlate with response (2). This association was observed among the 43 additional patients in this cohort (p = 0.013) (Fig. 4E). Specifically, BTLA expression on CD8+ TIL was again strongly associated with clinical response in checkpoint naïve patients, but not in patients who received anti-CTLA4 prior to TIL ACT (p = 0.004 and p = 0.972) (Fig. 4F). Overall, the total number of TIL infused, CD8+ TIL frequency and BTLA expression, which our group previously associated with clinical response, are no longer valid markers of response if the patient was treated with anti-CTLA4 prior to TIL ACT.
Tumor recognition and mutation load status according to anti-CTLA4 exposure
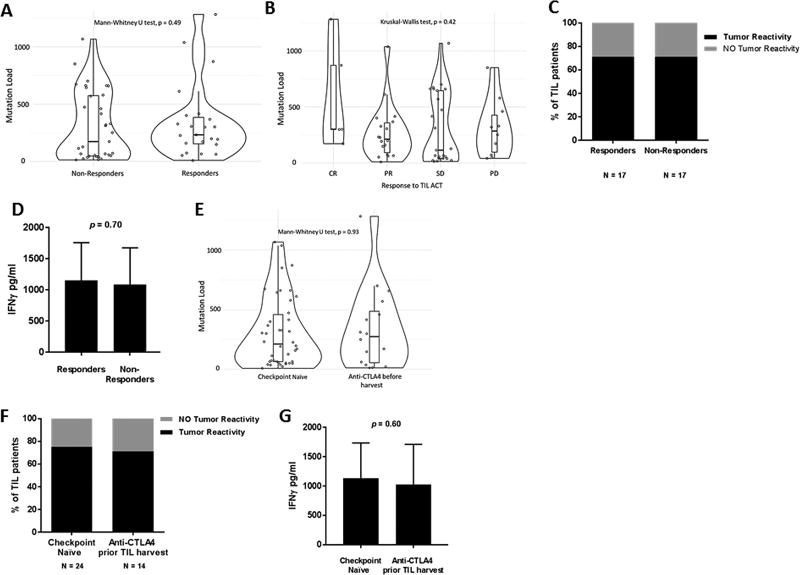
Our findings that patients exposed to anti-CTLA4 prior to TIL ACT had a lower OS, a shorter duration of response, and a TIL infusion with fewer cells prompted us to assess potential differences within the tumor. In view of previous reports that TIL are capable of recognizing epitopes derived from mutated genes that could in turn be linked to tumor regression, we interrogated tumor mutation load of our TIL-treated patients prior to TIL ACT (20,21). As shown in Figure 5A, we found no significant differences in the mutation load of pre-TIL ACT tumor among responders (CR+PR) versus non-responders (SD+PD) (p = 0.49), nor when response was more fully stratified (CR, PR, SD or PD) (Fig. 5B, p = 0.42). Interestingly, the capacity of autologous tumor recognition based on IFN-γ secretion following co-culture with autologous tumor cell line was comparable in responders versus non-responders (Fig. 5C and D, p =0.70). These data suggests that mutation burden or tumor recognition by TIL are not factors contributing to the lack of response to TIL.
Figure 5. Mutation load and tumor recognition are not affected by exposure to anti-CTLA4.
(A–B) Non-synonymous mutation load obtained from whole genome sequencing (WES) from tumor utilized for TIL propagation of treated patient. The graphs compare the mutation load from responders and non-responders (A) and from stratifying these 2 categories: CR and PR for responders and SD and PD for non-responders (B). (C) Pre-REP TIL from treated patients were co-cultured with autologous tumor targets and assessed for IFNg production by ELISA. The percentage of patients displaying tumor recognition (>200 pg/mL) is shown in black. (D) The average of IFNg secreted by the TIL lines in both group of patients capable of autologous tumor recognition shown in (C). (E) Non-synonymous mutation load comparing checkpoint naive tumors vs anti-CTLA4 exposed tumors. (F) Pre-REP TIL (regardless of patient receiving TIL therapy) were co-cultured and assessed for IFNg production as mentioned in (C). (G) The average of IFNg secreted by the TIL lines in both group of patients capable of autologous tumor shown in (F).
We next proceeded to stratify the tumor mutation load according to the anti-CTLA4 status prior to the tumor harvest used to expand the pre-REP TIL. This analysis found no difference in mutation load between the groups (p = 0.93. Fig. 5E). In fact, a similar proportion of pre-REP TIL from patients from each group displayed autologous tumor recognition based on IFN-γ secretion (Fig. 5F and G, p =0.60). Overall, mutation load and tumor recognition did not appear to be the driving factors causing the differential responses between anti CTLA4 treatment naïve or previously treated patients.
In-depth assessment of soluble factors in the serum of TIL treated patients as a comparative analysis and predictive tool
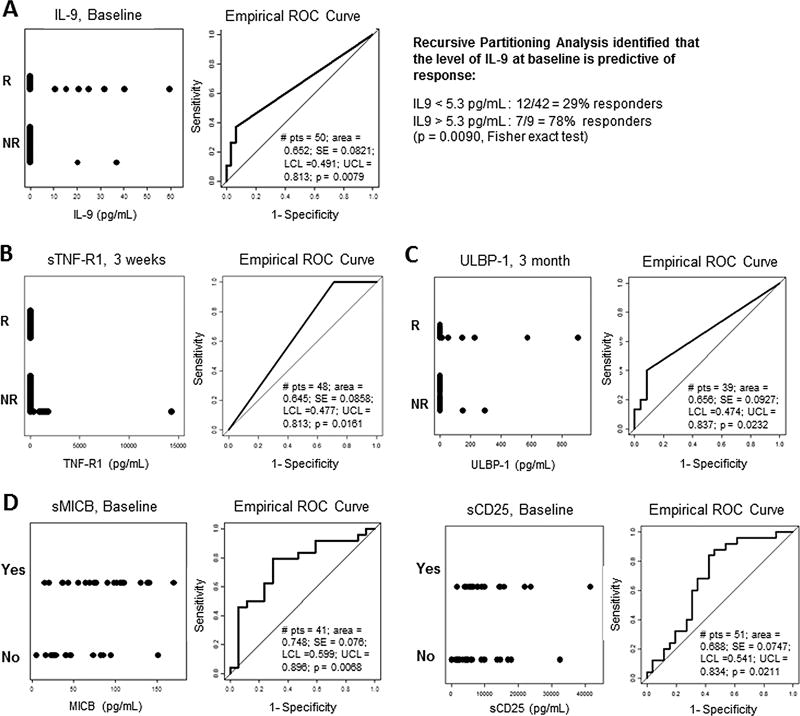
Tumor cells as well as activated immune cells secrete factors that perturb their environment. We reasoned that the level of circulating factors in the serum before therapy may impact response to therapy. Current technologies have given us the opportunity to interrogate patient’s serum for over 100 different soluble factors that can be related to the status of the immune response and the tumor. As a first aim, we explored whether soluble factors could differentiate between responders vs non-responders. Baseline serum IL-9 levels correlated with response to TIL (Fig. 6A, ROC curve, p = 0.0079). Recursive partitioning analysis identified that a serum IL-9 concentration greater than 5.3 pg/mL predicted response in 7 out of 9 patients (Fig. 6A, Fisher exact test, p = 0.009), suggesting that it may be a potential predictive factor in determining response to TIL ACT.
Figure 6. Correlation of serum biomarkers with response to TIL ACT and anti-CTLA4 exposure.
(A–D) Grouped dot plot (left graph) and ROC curve (right graph) of the different time points in the serum of TIL treated patients. (A) Baseline serum level of circulating IL-9 comparing responders (R) from non-responders (NR) (n=50). Using recursive partitioning analysis, baseline IL-9 levels are predictive of response to TIL ACT. (B) Pre-IL-2 serum level of sTNF-R1 comparing responders (R) from non-responders (NR) (second round of IL-2, ~ 3 weeks post-TIL infusion) (n=48). (C) 3 month post-infusion level of sULBP-1 comparing responders (R) from non-responders (NR) (second round of IL-2, ~3 weeks post-TIL infusion) (n=39). (D) Baseline serum level of sMICB (n=41) and sCD25 (n=51) of patients exposed to anti-CTLA4 (YES) or not (NO) prior to TIL ACT. Number of patient in each groups varies depending on availably for either time point or marker panel.
Conversely, levels of soluble TNF-receptor 1 (TNF-R1) in the serum approximately 3 weeks post-TIL infusion (pre-second round of IL-2) were significantly higher in non-responders (Fig. 6B, ROC curve, p = 0.0161). Baseline levels also trended towards correlating with a lack of response (Supplementary Fig. S4). A similar observation was also made in the later time point of 3 month post-infusion but not at day 7 post-TIL infusion (Supplementary Fig. S4). Interestingly, a soluble form of the UL16 binding protein 1 (ULBP-1), an activating ligand expressed on tumor cells that leads to their targeting by cytotoxic CD8+ T cells and NK cells, and an NKG2D ligand, was also found at a higher serum level among responders 3 month post-TIL ACT (Fig. 6C, ROC curve, p = 0.0232)(22).
The second aim of this analysis was to discern differences between patients exposed to anti-CTLA4 prior to TIL therapy vs checkpoint naïve patients. As shown in Figure 6D, the major difference found was at baseline with a marked presence of soluble MICB (p = 0.0068) and soluble CD25 (p = 0.0211) in patients that had previously been treated with anti-CTLA4. Interestingly, both MICB and CD25 have been reported to be associated with lack of response to checkpoint blockade (23–25). Given that this cohort is refractory to checkpoint inhibition, it is not surprising to find those markers and is confirmatory of previous observations. Nevertheless, the major new finding of this circulating factor analysis resides in the discovery of IL-9 as a potential predictive biomarker for response to TIL ACT irrespective of exposure to anti-CTLA4.
DISCUSSION
This constitutes the second report on our TIL ACT trial at MD Anderson. We now report on 74 patients with a median follow-up of 74 months, a BOR of 42% with a median OS of 17.3 months. As reported for our first cohort, high number of TIL infused as well as high number of CD8+ T cells and percentage of BTLA+CD8+ TIL still correlates with clinical response. With this extended cohort, we were able to look for other biomarkers associated with or predictive of response. These are of even more importance now given the strong presence of checkpoint blockade in metastatic melanoma treatment.
Indeed, checkpoint blockade has become a standard of care for metastatic melanoma. However, 60–70% of the patients either do not respond or become refractory to single agent therapy and dual checkpoint inhibition with anti-CTLA4 and anti-PD1 may be associated with prohibitive toxicity (8,9,26,27). There is thus an unmet need to develop other therapies for this patient population. TIL therapy has proven effective for metastatic melanoma but its efficacy in the context of the checkpoint-refractory patient population is unclear. Two groups addressed this question, mainly regarding exposure to anti-CTLA4 and suggested there was no impact on response to TIL ACT (3,10). The major difference between these studies and ours resides in the size of the sample interrogated. This study included 30 patients refractory to anti-CTLA4, of whom 11 responded to therapy, as opposed to the treatment of a total of 5 and 11 anti-CTLA4-refractory patients in prior TIL studies (3,10). In our study, although high LDH levels and the number of systemic therapies were clearly associated with a worse outcome, the only systemic pretreatment observed to correlate with reduced OS was anti-CTLA4 (median of 8.6 months when exposed to CTLA4 vs 24.6 in CTLA4 naïve, HR of 1.8 (0.9–3.7)). This is not surprising given the multiple reports demonstrating a negative impact of high LDH on response to systemic therapies and survival in metastatic melanoma patients (28,29). In addition, while a subset of patients with prior anti-CTLA4 exposure did respond to TIL ACT, the median duration of response was severely impacted (69.1 vs 4.8 months, HR 2.3 (0.9–5.6) p=0.084). We were not able to evaluate the sole impact of anti-PD1 as the majority of our patients exposed to anti-PD1 were also exposed to anti-CTLA4. Nevertheless, we still observed a BOR of >30% if patients received anti-CTLA4 prior to TIL ACT demonstrating that there is a possibility for clinical benefit in this refractory population. In addition, the toxicities observed following TIL ACT can be severe but overall are predictable, manageable and transient. One way of reducing IL-2 associated toxicities could be lowering the dosage of IL-2 post-infusion, which has already shown efficacy in other sites and is a strategy that we are currently exploring in an ongoing trial (30).
Persistence of the TIL, which is generally accepted as an essential element for treatment response, is not materially different between the CTLA-4 exposed or naïve groups, yet the duration of the response to TIL was shorter in the former group. In fact, comparison of persistence of TIL generated from a checkpoint naïve tumor and TIL generated from an anti-CTLA4 exposed tumor in two patients both showed detectable products in the blood up to 7 months after infusion. Moreover, expansion of both products was observed in the blood while patient #2 experienced clinical response, including a massive expansion of a single post-CTLA4 TIL clone, which would argue for clinical activity of TIL grown after anti-CTLA4 therapy. However, as we observed with the non-responding patient (patient #1), expansion of TIL in the blood does not necessarily correlate with response (10). This suggests that anti-CTLA4 exposure does not impair TIL persistence post-infusion and persistence might not be linked to clinical response.
A recent study examined the potential benefit of exposure to ipilimumab on pre-REP TIL (31). Similarly to our study, they did not see any difference in autologous tumor reactivity between the 2 groups at the pre-REP level (31). Interestingly, they reported a higher percentage of CD8+ and CD4+ TIL displaying higher expression of total CTLA4 in the ipilimumab treated group, mainly because of intracellular expression of the molecule (31). This observation could explain in part why patients exposed to anti-CTLA4 prior to TIL were infused with fewer cells, hence fewer CD8+ TIL, after going through the REP process. Indeed, if the TIL exposed to anti-CTLA4 possess a higher intracellular content of the molecule, it is possible that the molecule rapidly gets upregulated following TCR activation and that the feeder cells expressing its ligand could inhibit the TIL growth in the initial step of the REP, when their TCR is activated by the OKT3 loaded on the surface of the feeder cells. One could propose that addition of anti-CTLA4 during the initial REP could help protect the TIL against suppression or even cell death.
Tumors exposed to immunotherapy such as ipilimumab may undergo immunoediting and become less immunogenic. In our study, anti-CTLA4 treatment prior to TIL therapy did not affect autologous tumor recognition by TIL or the amount of mutations found in tumors. This observation could be attributable to the fact that patients in our cohort progressed after anti-CTLA4 therapy. Primary resistance may mean that their tumors may not have been shaped by immune pressure and hence, the tumor mutation landscape was presumably not altered. Given previous reports demonstrating the capacity of TIL to recognize mutated tumor-associated antigens and generate clinical response, these results still advocate for the use of TIL therapy in anti-CTLA4 refractory patient population. This is also supported by the preserved ability of tumor recognition within the anti-CTLA4 group although we demonstrated in our cohort that tumor recognition based on IFNγ secretion does not correlate with clinical response.
Recent reports have demonstrated that predictive biomarkers for outcomes to immunotherapies can be identified in the blood. It was the case for high levels of soluble CD25 (sCD25) in patients who progressed on anti-CTLA4 and sPDL1 in response to anti-PD1 (25,32). To our knowledge, no circulating biomarker, other than normal LDH, has been associated with positive outcome. Comprehensive assessment of soluble biomarkers has never been reported in relation to response to TIL ACT. We sought to bring important knowledge to the field that could be integrated in the design of new clinical trials for TIL ACT and/or patient selection. When first comparing responders and non-responders on treatment, sTNFa-receptor 1 was found in higher levels in non-responder patients three weeks post-infusion. There was also a trend for increased sTNFa-receptor 1 at baseline and 3 months post-treatment. Because of its widespread expression within both innate and adaptive immune cells, it is difficult to speculate on the mechanism underlying the negative impact of such receptor shedding. An accumulation of the soluble form of the TNFa receptor will bind soluble TNF and neutralize its activity, potentially impacting the immune response at multiple levels. At a later time point, responders showed higher levels of sULBP1 in the serum. sULBP1 has been demonstrated to be shed by tumor cells in an effort to avoid detection by NK cells and CD8+ TIL expressing NKG2D (33). Interestingly, detectable levels of sULBP1 pre-therapy have previously been correlated with worse OS in metastatic melanoma patients treated with checkpoint blockade yet, in our study, high sULBP1 at 3 months following TIL infusion positively correlated with response (23). Given the increased level of sULBP1 found in the serum of patients responding to TIL ACT, it could be postulated that the TIL are mounting a selective pressure on the tumor resulting in this attempt to avoid further targeting. When stratifying patients by exposure to anti-CTLA4 prior TIL ACT, our most interesting findings resided in the baseline serum time point. We first reproduced the observation previously reported by Hannani et al. regarding elevated level of sCD25 molecule in anti-CTLA4 progressing patients which solidified our findings (25). We also observed higher level of sMICB in the serum of patients exposed to anti-CTLA4 prior to TIL ACT. Like sULBP1, sMICB is an NKG2D ligand and similar to sULBP1, high levels of sMICB correlates with worse OS in patients treated with checkpoint blockade (23). Further studies of these correlates could help improve the response to TIL or lessen the impact of CTLA4 exposure on TIL but cannot be used as predictive tools to identify patients that will most benefit from TIL therapy.
By studying the baseline serum, we were able to identify IL-9 as a potential predictive biomarker of response to TIL ACT, where 78% of patients having over 5.3pg/ml responded to therapy. IL-9 has been shown to promote anti-tumor immunity in melanoma. The transfer of Th9 cells has been shown to be effective against melanoma in a preclinical tumor model; however, the treatment of tumor-bearing mice with recombinant IL-9 was able to stunt tumor growth as well. Interestingly, the treatment of mice with rIL-9 was effective even in the absence of T and B cells, but not when mast cells were absent (34). It is unclear how pre-therapy IL-9 levels can impact response to TIL ACT since the treatment regimen involves lymphodepletion before TIL infusion. IL-9 is produced by immune cells such as mast cells, NKT cells, Th2, Th17, Treg and innate lymphoid cells (ILC2) (35). Although the lymphodepleting chemotherapy brings the white blood cell count down to zero on the day of TIL infusion, we don’t have a clear idea of the degree of lymphodepletion in the tissues. It is possible that IL-9 signals the presence of certain types of immune cells, including mast cells and that some of these cell types are located in tissues shielding them from the chemotherapy, and therefore can continue to play a role post TIL infusion. Increased frequency of Th9 in the circulation has been previously reported to be positively correlated with response to anti-PD1 in metastatic melanoma (36). In contrast to our observation, this was found to be an on-treatment association of response as opposed to a baseline predictor of response. If our observation can be replicated in other TIL cohorts, IL-9 may truly represent the first biomarker predictive of response to TIL therapy and may help to identify the patients most likely to respond to treatment.
In conclusion, we report reduced overall responses, duration of response and overall survival among patients with metastatic melanoma who received TIL ACT after prior exposure to anti-CTLA4 therapy compared to checkpoint naïve patients. Although multiple parameters were assessed, it is not yet clear what the exact mechanism of resistance may be, and it is possible that a multitude of resistance mechanisms are at play. As there is no lack of tumor recognition or antigen availability, one mechanism of resistance might reside in the immunosuppressive tumor microenvironment which is the focus of our ongoing TIL trial where we block the TGFβ signal in TIL (NCT01955460). Another key difference is the decreased expansion of TIL after anti-CTLA4 which may indicate functional differences and needs to be further investigated. Elucidation of these mechanisms could greatly help improve responses in a checkpoint refractory patient population. The identification of IL-9 as the first potential pre-therapy predictor of response to TIL ACT, independent of exposure to anti-CTLA4, may help with determining which patients will achieve the most benefit with TIL ACT in melanoma.
Supplementary Material
TRANSLATIONAL RELEVANCE.
The treatment of patients with metastatic melanoma using tumor-infiltrating lymphocyte (TIL) adoptive cell therapy (ACT) has shown consistent clinical efficacy. This work evaluates the response to ACT using autologous TIL in the era of checkpoint blockade (primarily anti-CTLA4) and defines IL-9 as a circulating factor predictive of favorable response. Patients previously exposed to anti-CTLA4 were found to have both a reduced overall response rate and reduced median overall survival. Immunologic factors such as TIL persistence, tumor mutation load, and autologous tumor recognition were not found to stratify patient outcomes. However, fewer TIL infused, a shorter duration of response to therapy, and prior exposure to anti-CTLA-4 were found to correlate with one another. Overall, this study describes a negative impact of prior exposure to anti-CTLA4 on response to TIL ACT and identifies a novel predictive factor, IL-9, which could be used to guide future TIL ACT clinical trials designs.
Acknowledgments
The authors would like to thank the Melanoma Medical Oncology nurses and staff members as well as E.J. Shpall and the entire team from the GMP Stem Cell facility and Regulatory Compliance Unit. We would also like to thank the CCIR Regulatory Compliance Unit led by Sapna Parshottam for their constant support and the Melcore Lab for blood processing as well as the MD Anderson blood bank facility. Finally, we would like to acknowledge Prometheus for generously providing IL-2 for TIL manufacturing.
Funding: The study was supported by generous philanthropic contributions to The University of Texas MD Anderson Moon Shots Program, the Miriam and Jim Mulva Melanoma Research Foundation and the Dr. Miriam and Sheldon G Adelson Medical Research Foundation (AMRF). Additional support was provided by National Institutes of Health (1R21CA178580-01 to CB, R01-CA111999 to PH), The University of Texas MD Anderson Cancer Center Support Grant (P30-CA16672) to the flow cytometry core and the MD Anderson Institutional Tissue Bank as well as Institutional provost funds.
Footnotes
Conflict of interest disclosure: The authors have no competing interest to disclose.
References
- 1.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18(24):6758–70. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besser MJ, Shapira-Frommer R, Itzhaki O, Treves AJ, Zippel DB, Levy D, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19(17):4792–800. doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- 4.Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ, et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL-2 regimen. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1879. [DOI] [PubMed] [Google Scholar]
- 5.Goff SL, Dudley ME, Citrin DE, Somerville RP, Wunderlich JR, Danforth DN, et al. Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. J Clin Oncol. 2016;34(20):2389–97. doi: 10.1200/JCO.2016.66.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 12.Forget MA, Haymaker C, Dennison JB, Toth C, Maiti S, Fulbright OJ, et al. The beneficial effects of a gas-permeable flask for expansion of Tumor-Infiltrating lymphocytes as reflected in their mitochondrial function and respiration capacity. Oncoimmunology. 2016;5(2):e1057386. doi: 10.1080/2162402X.2015.1057386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114(19):4099–107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher S, Barry A, Abreu J, Minie B, Nolan J, Delorey TM, et al. A scalable, fully automated process for construction of sequence-ready human exome targeted capture libraries. Genome biology. 2011;12(1):R1. doi: 10.1186/gb-2011-12-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nature biotechnology. 2013;31(3):213–9. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Allen EM, Wagle N, Stojanov P, Perrin DL, Cibulskis K, Marlow S, et al. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nat Med. 2014;20(6):682–8. doi: 10.1038/nm.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos AH, Lichtenstein L, Gupta M, Lawrence MS, Pugh TJ, Saksena G, et al. Oncotator: cancer variant annotation tool. Human mutation. 2015;36(4):E2423–9. doi: 10.1002/humu.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavera RJ, Forget MA, Kim YU, Sakellariou-Thompson D, Creasy CA, Bhatta A, et al. Utilizing T-cell activation signals 1, 2 and 3 for tumor-infiltrating lymphocytes (TIL) expansion: the advantage over the sole use of interleukin-2 in cutaneous and uveal melanoma. Journal of Immunotherapy. 2018 doi: 10.1097/CJI.0000000000000230. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19(6):747–52. doi: 10.1038/nm.3161nm.3161. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verdegaal EM, de Miranda NF, Visser M, Harryvan T, van Buuren MM, Andersen RS, et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature. 2016;536(7614):91–5. doi: 10.1038/nature18945. [DOI] [PubMed] [Google Scholar]
- 22.Paschen A, Baingo J, Schadendorf D. Expression of stress ligands of the immunoreceptor NKG2D in melanoma: regulation and clinical significance. Eur J Cell Biol. 2014;93(1–2):49–54. doi: 10.1016/j.ejcb.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Maccalli C, Giannarelli D, Chiarucci C, Cutaia O, Giacobini G, Hendrickx W, et al. Soluble NKG2D ligands are biomarkers associated with the clinical outcome to immune checkpoint blockade therapy of metastatic melanoma patients. Oncoimmunology. 2017;6(7):e1323618. doi: 10.1080/2162402X.2017.1323618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 25.Hannani D, Vetizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 2015;25(2):208–24. doi: 10.1038/cr.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377(14):1345–56. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damuzzo V, Solito S, Pinton L, Carrozzo E, Valpione S, Pigozzo J, et al. Clinical implication of tumor-associated and immunological parameters in melanoma patients treated with ipilimumab. Oncoimmunology. 2016;5(12):e1249559. doi: 10.1080/2162402X.2016.1249559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin Cancer Res. 2016;22(22):5487–96. doi: 10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellebaek E, Iversen TZ, Junker N, Donia M, Engell-Noerregaard L, Met O, et al. Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J Transl Med. 2012;10:169. doi: 10.1186/1479-5876-10-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjoern J, Lyngaa R, Andersen R, Rosenkrantz LH, Hadrup SR, Donia M, et al. Influence of ipilimumab on expanded tumour derived T cells from patients with metastatic melanoma. Oncotarget. 2017;8(16):27062–74. doi: 10.18632/oncotarget.16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, et al. Soluble PD-L1 as a Biomarker in Malignant Melanoma Treated with Checkpoint Blockade. Cancer Immunol Res. 2017;5(6):480–92. doi: 10.1158/2326-6066.CIR-16-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baragano Raneros A, Suarez-Alvarez B, Lopez-Larrea C. Secretory pathways generating immunosuppressive NKG2D ligands: New targets for therapeutic intervention. Oncoimmunology. 2014;3:e28497. doi: 10.4161/onci.28497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18(8):1248–53. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015;15(5):295–307. doi: 10.1038/nri3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nonomura Y, Otsuka A, Nakashima C, Seidel JA, Kitoh A, Dainichi T, et al. Peripheral blood Th9 cells are a possible pharmacodynamic biomarker of nivolumab treatment efficacy in metastatic melanoma patients. Oncoimmunology. 2016;5(12):e1248327. doi: 10.1080/2162402X.2016.1248327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.