Abstract
Traumatic brain injury (TBI) has historically been viewed as a primarily male problem, since men are more likely to experience a TBI because of more frequent participation in activities that increase risk of head injuries. This male bias is also reflected in preclinical research where mostly male animals have been used in basic and translational science. However, with an aging population in which TBI incidence is increasingly sex-independent due to falls, and increasing female participation in high-risk activities, the attention to potential sex differences in TBI responses and outcomes will become more important. These considerations are especially relevant in designing preclinical animal models of TBI that are more predictive of human responses and outcomes. This review characterizes sex differences following TBI with a special emphasis on the contribution of the female sex hormones, progesterone and estrogen, to these differences. This information is potentially important in developing and customizing TBI treatments.
Keywords: Traumatic brain injury, sex difference, progesterone, estrogen, inflammation, neurodegeneration, vasculature, male, female
1. Introduction
Traumatic brain injury (TBI) is an umbrella term for any blunt or penetrant force to the head, which causes an alteration in brain function. This is in contrast to other types of acquired brain injury where the cause of functional brain damage is a non-traumatic event, such as stroke or infection. It is also distinct from the outdated clinical term, head injury, in that TBI must include functional brain changes. These can include an altered level of consciousness, seizure, behavioral or neuropsychological changes, cognitive deficits, and sensory or motor deficits (Bruns and Hauser, 2003). Injuries classified as a TBI are varied and can range from the mild concussions prevalent in contact sports, to the famous accident that sent a tamping iron through the skull of Phineas Gage (Steegmann, 1962). Indeed, TBI is a major contributor to mortality and morbidity in the United States (US), with over 2.5 million cases per year, accounting for over $7.6 billion in medical and other costs (Center for Disease Control and Prevention, 2015). TBI is also a major cause of lifelong disability (Tagliaferri et al., 2006), and can contribute to the risk of other major health conditions such as Alzheimer’s disease (Lye and Shores, 2000; Tolppanen et al., 2017).
Although the outcome of a TBI is heavily dependent upon the specifics of the injury, certain pre- and post-injury factors can also influence the prognosis (Bazarian et al., 2010; Renner et al., 2012; Stulemeijer et al., 2008). Most obviously, the post-injury factors include the acute and chronic treatments administered after TBI. However, some pre-injury factors, including demographic variables such as age and sex, are increasingly appreciated for their influence on outcomes. Age, for example, is independently associated with both increased incidence (Harvey and Close, 2012) and worsened outcomes of TBI in older adults (Hukkelhoven et al., 2003). Although sex has largely been ignored in preclinical TBI research to date, convergent lines of evidence indicate that this variable may have important implications for TBI outcome and potentially therapeutic development. This review will therefore focus on sex differences in TBI, beginning with epidemiological studies in patients, followed by a detailed analysis of findings from preclinical research. Since the dimorphism that exists between males and females is due in large part to the major sex hormones (estrogen, progesterone and, testosterone), this review will focus on their influence on TBI pathology and their potential use as therapeutics (see (Reddy and Estes, 2016) for further information on the influence of neurosteroids on CNS disorders).
2. Sex differences in TBI: epidemiology
It has long been understood that males are at elevated risk of TBI versus females, particularly in young adulthood, where the male to female incidence ratio approaches or exceeds 3–4:1 (Annegers et al., 1980; Cooper et al., 1983; Durkin et al., 1998; Nell and Brown, 1991). This is generally attributed to the increased interpersonal violence, sports-related injury, and motor vehicle accidents occurring in younger male populations. For example, males represent the majority of emergency department visits and hospitalizations among the pediatric population, particularly in the pubescent age range (Center for Disease Control and Prevention, 2015; Cuthbert et al., 2015). Similarly, the overall death rate from TBI is approximately 3× higher for males than females, and this difference peaks at almost 4× between ages 20 and 24 (Coronado et al., 2011). Consistent with these findings, the difference in incidence between males and females is no longer apparent at age 65 (Cuthbert et al., 2015; Mushkudiani et al., 2007). Although we are using the term sex (biological and physiological characteristics that distinguish females from males) throughout the review, it should be noted that gender (socially constructed roles, behaviors, relative power, relationships, and other traits that societies ascribe to men and women) can play an important role in human TBI epidemiology (for review, see Mollayeva and Colantonio, 2017).
These observations have served to justify the historical focus on male response to TBI, particularly in preclinical research (Figure 1). However, as cultural shifts continue to result in more female involvement in higher-risk activities such as contact sports and military service (National Center for Veterans Analysis and Statistics, 2016; Women’s Sports Foundation, 2007) these differences in incidence may diminish. Furthermore, females are currently at increased risk of certain types of TBI, such as TBI resulting from intimate partner violence (St Ivany and Schminkey, 2016) and sports-related concussions (Covassin et al., 2003; Dick et al., 2007; Gessel et al., 2007; Lincoln et al., 2011; Marar et al., 2012; Powell and Barber-Foss, 1999). Although the latter may be partially attributable to differences in the sports participated in (Schulz et al., 2004) or an under-reporting bias in males (Kerr et al., 2016), there is also evidence that underlying sex differences play a role. For example, anatomical and biomechanical differences (e.g. head mass, neck muscle strength) may contribute to the two-fold increase in concussive injuries suffered by female compared to male soccer players (Mansell et al., 2005; Marar et al., 2012; Tierney et al., 2008).
Figure 1. Distribution of animal sex reported in preclinical TBI studies in 2011 and 2016.
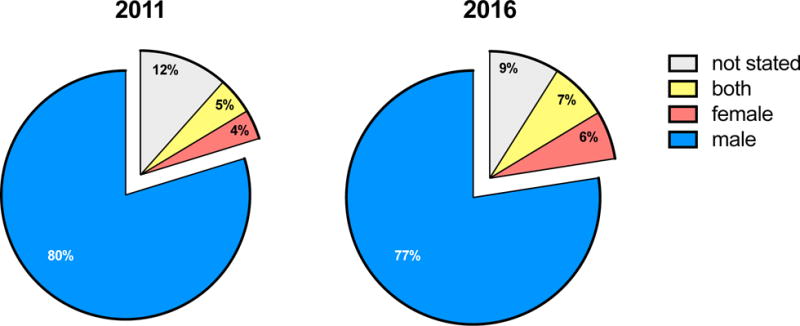
The majority of TBI studies in 2011 (80%) and 2016 (77%) used only male animals. Only a small proportion of studies in 2011 used both males and females (5%), which slightly increased to 7% in 2016. The search strategy included animal studies with the term traumatic brain injury or TBI for articles published in Pubmed between January 1st, 2011 and December 31st, 2011 (2011 graph); or January 1st, 2016 and December 31st, 2016 (2016 graph). Reviews, comments and retracted articles were excluded, as well as studies that used TBI as an abbreviation for total body irradiation. For 2011, 311 studies of TBI in an animal model are included, and 324 studies are included for 2016.
3. Sex differences in TBI: patient outcomes
The literature regarding sex differences in TBI outcomes is controversial, likely reflecting both the diversity of injuries surveyed as well as outcome criteria measured. It has been reported, for instance, that women have significantly fewer complications after moderate to severe TBI (Berry et al., 2009), and that women tend to have better prognoses and outcomes in general (Groswasser et al., 1998; Niemeier et al., 2007; Saban et al., 2011; Sarkaki et al., 2013; Schmidt et al., 2012). Additionally, it has been found that younger women have less secondary injury and better outcomes than older, attributed to neuroprotective effects of sex hormones in pre-menopausal women (Bayir et al., 2004; Kirkness et al., 2004; Ley et al., 2013; Wagner et al., 2004a). Consistent with this, it has been found that the neuroprotective effects of estrogen and progesterone in women decline with age (Niemeier et al., 2013; Roof and Hall, 2000a; Stein, 2008). However, it has also been reported that outcomes are actually better in post-menopausal women compared to men, and pre-menopausal women have the same outcomes as men (Davis et al., 2006).
In apparent disagreement, there is a wealth of information indicating that the reverse is true. Several studies have found that TBI outcome is generally poorer in women versus men (Bayir et al., 2004; Farace and Alves, 2000; Gan et al., 2004; Kirkness et al., 2004; Wagner et al., 2000), including both severe and moderate TBI injuries (Kraus et al., 2000). Female athletes in particular seem to demonstrate a specific susceptibility to concussive injury, in that they report more symptoms initially after concussion and the symptoms persist for longer (Baker et al., 2016; Covassin et al., 2006), they have greater post-concussion deficits (Broshek et al., 2005; Dougan et al., 2014; Preiss-Farzanegan et al., 2009), and they seem to recover worse than males (Berz et al., 2013). Even this is controversial, however, as other studies have shown no sex differences in either acute complications or outcome after TBI (Mushkudiani et al., 2007; Renner et al., 2012; Slewa-Younan et al., 2008), including after sports-related concussion (Covassin et al., 2007).
A recent meta-analysis (Mathias and Wheaton, 2015) found no difference in combined outcome measures, although some differences existed in single outcome measures, and they were split between males and females. For instance, women report different symptoms after TBI compared to males (Covassin et al., 2007; Frommer et al., 2011), and suffer more from: headaches and dizziness (Colantonio et al., 2010), anxiety and depression (Hart et al., 2011; Schopp et al., 2001; Scott et al., 2015), fatigue (Englander et al., 2010), and an increased risk of post-traumatic seizures (Cancelliere et al., 2016). Males, on the other hand, appear more susceptible to paroxysmal sympathetic hyperactivity (Fernandez-Ortega et al., 2012), substance abuse disorders (Scott et al., 2015), and increased aggressive tendencies (McGlade et al., 2015). Further complicating interpretation, however, is the fact that some differences are present prior to injury (e.g. susceptibility to anxiety, migraine) and are not necessarily a result of dimorphic injury responses per se. Nonetheless, prognostic models do appear to benefit from inclusion of sex as a variable (Yuan, Fang et al., 2012), and the evidence from preclinical studies, as outlined below, further supports the need to account for sex differences in a clinical setting.
4. Sex differences in animal models of TBI
To model traumatic brain injuries for preclinical studies, a variety of animal species are currently being used, ranging from classical rodent laboratory animals such as mice and rats but also including pigs, flies, and zebra finches. Since the most commonly used animals are rodents, the studies reviewed used either rats or mice unless otherwise stated. The search strategy for the following data is included in the Supplementary Material (Supplementary Table 1 – Search Strategy). Briefly, (TBI OR traumatic brain injury) AND (sex OR gender) was searched in Pubmed together with the following terms (latest update January 26th 2018): difference, cognition, behavior, maze, rotarod, cognitive, contusion, neuron damage, edema, neuron, estrogen, estrous, progesterone, allopregnanolone, androgen, testosterone, blood brain barrier OR BBB, astroglia, astrocytes, astrogliosis, endothel*, inflammation, microglia, microgliosis, inflammosome, myeloid, monocyte, macrophage, immune, cytokine, chemokine, and biomarker. Reviews, comments, retracted articles, articles in another language than English and articles that used TBI as an abbreviation for total body irradiation were excluded. This led to a final of 132 studies that were analyzed. A table of all the studies including their TBI model (species, strain, impact method, n number, sex used, hormonal status at time of injury, potential hormonal drug, and treatment time point) can be found in the Supplementary Material (Supplementary Table 2 – Literature Overview). The outcomes reported in this review were specifically chosen for the following reasons: 1) importance in the field, 2) scientific interest and 3) widely reported across studies. A summary of all the described data can be found in Table 1. The older clinical term “head injury” was excluded from this search strategy because it includes injuries that are not TBI.
Table 1.
Summary of sex-dependent outcomes in different rodent single TBI models
| Endpoint | weight-drop | CHI | CCI | FPI | ACI | Species | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Behavior | Locomotion deficits | 4 (7–13) | 28 (17–19) | mice | ||||||
| 6 (18) | 8 (10), 9 (12) | 5 (6) | 1 (16–17), 2 (17–18), 3 (12–17), 7 (12–15), 27 (5–13) | 10 (6) | rats | |||||
| Cognitive deficits | 4 (7–13), 28 (17–19) | mice | ||||||||
| 6 (18) | 5 (6) | 11 (19–29) | 1 (16–17), 3 (12–17), 27 (5–13) | rats | ||||||
| Anxiety | 29 (5–10) | mice | ||||||||
| 6 (18) | rats | |||||||||
| Sociability | 29 (5–10) | mice | ||||||||
| 8 (10) | rats | |||||||||
| Inhibitory control | mice | |||||||||
| 9 (12), 12 (6) | rats | |||||||||
| Neurodegeneration | Lesion size | 16 (8) | 4 (7–13), 21 (6–8), 22 (5), 28 (17–19), 29 (5–10) | 15 (3–24) | mice | |||||
| 11 (19–29) | 27 (5–13) | 13 (2–19), 14 (8) | rats | |||||||
| Neuronal count | 16 (8) | mice | ||||||||
| 14 (8) | rats | |||||||||
| Edema | mice | |||||||||
| 23 (5) | 17 (4–6), 18 (5–12), 19 (4–10) | rats | ||||||||
| Cell death | mice | |||||||||
| 20 (5) | rats | |||||||||
| Necrotic neurons | 24 (3–16) | 22 (5) | mice | |||||||
| 13 (2–19) | rats | |||||||||
| Dendritic complexity | 29 (5–10) | mice | ||||||||
| 12 (6) | rats | |||||||||
| Vasculature | CBF | mice | ||||||||
| 25 (8–18) | rats | |||||||||
| Evan's blue extravasation | mice | |||||||||
| 23 (5) | 19 (4–10) | rats | ||||||||
| Inflammation | Microgliosis | 16 (8) | mice | |||||||
| 11 (19–29) | rats | |||||||||
| Astrogliosis | mice | |||||||||
| 6 (18) | 20 (5) | rats | ||||||||
| IL-6 | 26 (28–74) | 21 (6–8) | mice | |||||||
| rats | ||||||||||
| CCL2 | 26 (28–74) | 21 (6–8) | mice | |||||||
| rats | ||||||||||
| TNFalpha | 26 (28–74) | 21 (6–8) | mice | |||||||
| rats | ||||||||||
| IL-1beta | 26 (28–74) | mice | ||||||||
| rats | ||||||||||
| CXCL1 | 26 (28–74) | mice | ||||||||
| rats | ||||||||||
| IL-10 | 26 (28–74) | mice | ||||||||
| rats | ||||||||||
| COX-2 | mice | |||||||||
| 20 (5) | rats | |||||||||
| iNOS | mice | |||||||||
| 20 (5) | rats | |||||||||
| data not found | females better than males | males better than females | no sex difference | variable-dependent | no TBI effect | |||||
The endpoint is presented on the left side and the species (mice or rats) is presented on the right side. The different columns represent different TBI models: CHI (closed head injury), CCI (controlled cortical impact), FPI (fluid percussion injury) and ACI (aseptic cryogenic injury).
Red cells represent a better outcome in females, blue a better outcome in males, yellow no sex difference after TBI, and black represents an outcome that was different in males and females depending on an additional variable such as time point or assay. Grey cells represent end points where no data was available.
The numbers within each cell represent the references below, and the number following in the brackets is the “n” number per group in the corresponding study: 1 Wagner et al. 2002, 2 Wagner et al. 2004, 3 Wagner et al. 2007, 4 Xiong et al. 2007, 5 O’Connor et al. 2003, 6 Mychasiuk et al. 2016, 7 Russell et al. 2011, 8 Mychasiuk et al. 2014, 9 Mychasiuk et al. 2015, 10 Grossman et al. 2000, 11 Hanlon et al. 2017, 12 Hehar et al. 2015, 13 Bramlett et al. 2001, 14 Suzuki et al. 2003, 15 Jones et al. 2005, 16 Igarashi et al. 2001, 17 Roof et al. 1993, 18 Roof et al. 1996, 19 Duvdevani et al. 1995, 20 Günther et al. 2015, 21 Bruce-Keller et al. 2007, 22 Hall et al. 2005, 23 O’Connor et al. 2006, 24 Kupina et al. 2003, 25 Roof et al. 2000, 26 Späni et al. 2018 (this review), 27 Free et al. 2017, 28 Tucker et al. 2016, 29 Semple et al. 2017
4.1 Does sex really matter in traumatic brain injury?
Sex differences in brain injury models have been reported since at least the 1970’s, in several classical brain lesion studies. These early papers indicated that injury response is sexually dimorphic in a regional fashion: damage to the hippocampus favors recovery in females (Loy and Milner, 1980) while damage to the septal nucleus (Flaherty et al., 1979) and the frontal cortex causes less impairment in males (Stein, 1974). Despite these early reports and the aforementioned clinical studies, preclinical traumatic brain injury research has been and still is mostly performed in male animals, belying an implicit assumption that sexually dimorphic responses to TBI are relatively unimportant. The topic of the following section is therefore whether clinically-relevant sex differences are also observed in preclinical TBI models. For the present discussion, we have focused on the following TBI models: closed head injury (CHI), controlled cortical impact (CCI), fluid percussion injury (FPI), aseptic cryogenic injury (ACI), weight-drop injury, or modified versions of these. Unfortunately, there is still only a small body of literature available from which to draw upon in an attempt to address this issue.
4.1.1 Behavior
The most important outcome after a TBI for patients is functional recovery. In animal models, there are several different tests used to measure functional recovery in terms of several common motoric, cognitive, and psychiatric impairments observed following a TBI.
4.1.1.1 Locomotion deficits
Locomotion is a major factor in the wellbeing of human TBI survivors, and also one of the most accessible outcome measures in animal experimentation, and so several studies have compared locomotion-related endpoints between male and female animals following a TBI. Some studies report significantly worse locomotor measures after TBI in male rodents compared to females (Mychasiuk et al., 2016; O’Connor et al., 2003; Russell et al., 2011; Wagner et al., 2007, 2004b, 2002; Xiong et al., 2007; Free et al., 2017), while others report no sex difference (Grossman and Stein, 2000; Mychasiuk et al., 2015, 2014; Tucker et al., 2016; Yamakawa et al., 2017; Hehar et al., 2016). The conflicting findings among the studies could have multiple causes ranging from the use of different species, the variety of TBI models used, to differences in the age of the animals investigated. For example, when the balance beam locomotor test is used to measure recovery of balance and coordination after a controlled cortical impact (CCI) injury, better outcomes are consistently reported in females (Wagner et al., 2007, 2004b, 2002; Free et al., 2017). This finding only holds true for adult rats, however, as the one study which did not find sex differences in the balance beam after CCI used juvenile rats at 17 days (d) old (Russell et al., 2011). Furthermore, when the injury model consists of a weight-drop with rotational free-fall or a lateral injury, balance beam differences between males and females disappear (Mychasiuk et al., 2014, 2015, 2016; Yamakawa et al., 2017; Hehar et al., 2016). Taken together, these data suggest that a milder injury, or injury in younger animals, does not result in appreciable locomotor sex differences. However, in older animals receiving a harsher injury, females have less severe balance beam deficits versus their male counterparts. The rotarod test is another assay for locomotion outcomes including balance, coordination, physical condition, and motor-planning, where the ability of a rodent to stay on a horizontal rotating cylinder is measured. After a closed head injury (CHI), modeling a mild concussion, female rats show a better performance compared to males, with a faster improvement rate after TBI. However, female pre-injury performance was significantly better than in males, and if the post-injury measures are normalized to baseline, females demonstrate a similar recovery rate compared to males (O’Connor et al., 2003). Another study investigated rotarod performance after mild or severe CCI in mice and did not find a difference in performance between males and females (Tucker et al., 2016).
4.1.1.2 Cognitive deficits
In addition to motoric impairments, TBI patients can also suffer from cognitive deficits. Animal models of TBI similarly demonstrate cognitive deficits when assessed in various tests for memory and learning including Morris Water Maze (MWM), Barnes Maze, and Novel Context Mismatch. No sex differences have been observed after single TBI in the MWM with either CHI or CCI (Free et al., 2017; Hanlon et al., 2017; Tucker et al., 2016; Wagner et al., 2002, 2007; Xiong et al., 2007). However, in a multi-hit CHI mouse model of repetitive concussion, female TBI mice showed superior performance during reversal training and during the standard probe trial, but equivalent performance during standard training. This suggests that male mice have more problems to extinguish their initial learning and overwrite it with new information (Velosky et al., 2017). Interestingly, O’Connor et al. (2003) showed that in the Barnes Maze females show fewer deficits than males after TBI, but only if isoflurane is used as anesthetic; anesthesia with halothane abolished the sex difference (O’Connor et al., 2003). Isoflurane previously has been reported to be protective relative to halothane in respect to cognitive outcomes following brain trauma, possibly via reducing excitotoxicity and/or augmenting cerebral blood flow (Statler et al., 2000). Why this beneficial effect is bigger after TBI in females than in males is still unanswered, but it highlights the potential for treatments that take sex into consideration. Unfortunately, it also further complicates interpretation of the literature, in that a variable unrelated to TBI per se (anesthetic choice) can influence outcomes following TBI in a sex-specific manner.
4.1.1.3 Psychiatric disturbances
Aside from locomotor and cognitive deficits, a substantial portion of TBI patients suffer from classic psychiatric problems ranging from depression, to anxiety, to aggressive behavior. As discussed below, these behavioral alterations after TBI can also be successfully modeled in animals, where some sex differences consistent with the clinical literature emerge.
4.1.1.3.1 Anxiety
Mychasiuk et al. (2016) show that only female rats display significantly more anxiety in the Elevated Plus Maze (EPM) after a weight-drop injury. The same can be observed in a lateral injury model with rotation in 30d old juvenile rats (Hehar 2016). Intriguingly, the lateral injury and CCI TBI do not induce a TBI-related increase in anxiety in adult animals (Mychasiuk et al., 2016; Semple et al., 2017; Yamakawa et al., 2017). This provides insight into the heterogeneity of pathophysiological responses, dependent upon the characteristics of the specific biomechanical forces acting on the brain during a TBI, which cause variable symptomatic spectra.
4.1.1.3.2 Sociability
In an earlier study, Mychasiuk et al. (2014) tested whether a brain injury could influence social play behavior in juvenile rats. Rats that underwent a mild TBI (mTBI), introduced via weight-drop with rotational free-fall, showed reduced number of playful initiations than sham animals, and this was particularly pronounced in the female rats. Interestingly, not only did the mTBI animals initiate play less than sham animals, for the female rats the mTBI animals were also significantly less likely to receive playful initiations from either sham or other mTBI rats. Additionally, exposure to normal animals after TBI seems to be an important factor in play behavior influenced by sex: exposure to non-injured animals has a normalizing effect on play behavior in male but not female rats. Indeed, female exposure to normal female animals seemed to further hinder juvenile play behavior after TBI. These observations could be particularly important in the development of an effective treatment for pediatric TBI, since children who suffer a TBI are at risk for psychosocial and behavioral difficulties, which can have life-long consequences (Mychasiuk et al., 2014). Indeed, juvenile CCI leads to same-sex sociability deficits in adulthood in both male and female mice compared to sham controls. However, only male TBI mice showed a deficit in social recognition, whereas only female TBI mice showed a significant increase in sociosexual avoidance behavior (Semple et al., 2017).
4.1.1.3.3 Inhibitory control
The 5-choice serial reaction time task (5-CSRTT) can be used to measure visual attentional processes and impulse control in rats. In short, the animal is required to correctly identify one out of five illuminated apertures via a nose poke to receive a food reward. In between trials, there is a short period wherein the animal must withhold responses, corresponding to inhibitory control behavior. Two different studies using the mild weight-drop with rotational free-fall TBI model show a TBI-associated reduction in accuracy without any observed sex effects in this parameter. However, there was a reduction in inhibitory impulse control in the male rats versus females, as measured by an increased number of impulsive nose-pokes (Hehar et al., 2015; Mychasiuk et al., 2015). Interestingly, these differences corresponded to altered spatial gene expression patterns of impulsivity associated genes (e.g. Comt, Drd2–4, Maoa, Sert, Tph1 & 2) in male versus female rat brains after TBI (Hehar et al., 2015).
4.1.2 Neuropathology
The available behavioral data, particularly for motoric function, provide evidence for a moderate protective effect of female sex, dependent upon the specifics of the injury as well as the age of the animals. The question remains, however, as to whether these differences are simply attributable to the fact that female animals, including their brains, are smaller than males (Mychasiuk et al., 2014), thereby leading to increased brain damage from the same impact force. Interestingly, analysis of ex vivo pig brains showed no difference in mechanical properties between male and female brains (Pervin and Chen, 2011), indicating that at least some of the variability comes from different injury responses.
4.1.2.1 Neurodegeneration
Following a TBI, female sex has been reported as neuroprotective by several different groups using various TBI models and outcome measures, including lesion size (Bramlett and Dietrich, 2001; Free et al., 2017; Igarashi et al., 2001; Jones et al., 2005; Suzuki et al., 2003), neuronal count (Igarashi et al., 2001; Suzuki et al., 2003), edema (Duvdevani et al., 1995; Roof et al., 1993, 1996), and cell death (Günther et al., 2015). In contrast, several different studies report no sex differences in lesion volume after TBI (Bruce-Keller et al., 2007; Hall et al., 2005; Hanlon et al., 2017; Semple et al., 2017; Tucker et al., 2016; Xiong et al., 2007). The study by O’Connor et al. (2006) offers a solution to these apparent contradictory results: while the other studies investigated just a single time point, O’Connor et al. (2006) provides a time line of edema formation and resolution after a TBI. In this study, females show less edema than males at 5 hours (h), 3d and 5d after a weight-drop injury, but more edema at 24h after injury (O’Connor et al., 2006). Given that many studies tend to focus on one or just a couple time points, there may be an underestimation of sexually dimorphic TBI responses more generally. This may also have important implications for the appropriate therapeutic window in males versus females.
On the microscopic level, a study in 1–5d old pigs reported more necrotic neurons are found in males compared to females in the Cornus Ammonis area 1 (CA1) and area 3 (CA3) regions of the hippocampus in the fluid percussion injury (FPI) TBI model (Armstead et al., 2016). In a similar FPI in adult rats, no such difference was found in the CA3 and the hilar regions of the hippocampus (Bramlett and Dietrich, 2001). Whether this discrepancy is due to species or age differences is not known. An intensive characterization study by Kupina et al. (2003) used classical silver staining to show different spatiotemporal patterns of neurodegeneration in females compared to males in response to TBI. Following a moderate weight-drop injury in mice, degenerating neurons could be found as early as 1h after insult in the corpus callosum and near the ventricles of both sexes, with females exhibiting additional silver staining in small, variable regions in the cortex, the medial forebrain bundle and the olfactory tubercle. Three days after injury, degeneration spread in both sexes to the olfactory tract, the cingulum, the external capsule and small selected regions of the cortex, the anterior and posterior commissures, and the thalamus. Only male animals showed silver staining in the caudate and putamen and the optic tract, and they had more extensive staining in the thalamus and the cortex. The peak silver staining for males was earlier than females, at 3 verus 14 days, respectively. Additionally, females had significantly more silver staining 24h after TBI, but reduced staining 3d and 7d after TBI compared to males (Kupina et al., 2003). Use of the more severe CCI model showed a different neurodegeneration profile, with a maximal staining 2d after TBI in both males and females, which started to resolve after 3d. Neurodegeneration could still be detected 4 weeks after TBI, and was significantly higher in females compared to males, suggesting a slower resolution of post-traumatic neurodegenerative debris in females compared to males. Taken together, these findings provide evidence that in the more “diffuse” TBI models, such as weight-drop, female sex shows a neuroprotective effect, whilst this is not the case in the more “focal” CCI injury, and females may actually suffer more neuronal cell death over time. Although the overall volume of neurodegeneration-related staining is similar in the mouse brains subjected to either “diffuse” or “focal” injury (ca. 20 and 18%, respectively), producing a roughly equivalent primary injury severity, the rate of evolution of secondary injury damage is substantially greater in the “focal” CCI versus the “diffuse” weight-drop injury. Hence, the absence of sex difference in the CCI model could be due to the possibility that the neuroprotective effects of female sex hormones are overwhelmed by the rapid evolution of secondary injury (Hall et al., 2005).
In addition to differences in the numbers and spatial distribution of degenerating neurons, there also appear to be morphological differences in those neurons that survive. After a mild weight-drop with rotational free-fall TBI, for example, surviving medium spiny neurons in the nucleus accumbens in male rats have reduced dendritic length whereas neurons from female animals show an increase in dendritic length. The spine density of these neurons is higher at baseline in females, and the reduction after TBI is significantly greater in males compared to female animals (Hehar et al., 2015). Also, pediatric CCI in mice leads to reduced dendritic complexity of pyramidal neurons in the ipsilateral prefrontal cortex and granule cells of the dentate gyrus. These changes persist into adulthood, however the changes are more apparent in males versus females (Semple et al., 2017).
4.1.2.2 Vasculature
Not only are the neurons affected by a physical assault to the brain, but also the cells of the vasculature that supply the neural tissue with nutrients and oxygen. A TBI significantly lowers cerebral blood flow (CBF) in different species including pigs (lateral FPI) and rats (weight-drop injury), and this reduction is consistently more severe in male TBI animals compared to females (Armstead et al., 2013, 2016; Roof and Hall, 2000b). Following a FPI, pial artery diameter in response to hypotension can be measured as an indicator of the protective autoregulatory processes of the vasculature. Normally, the pial arteries will dilate in response to rapid blood withdrawal, to help maintain blood flow. In pigs with a TBI, the pial artery dilates less in response to this hypotension, and the deficit is more severe in male pigs compared to females (Armstead et al., 2012, 2013). Additionally, the same researchers showed that the increase in intracranial pressure (ICP) is more severe in male pigs compared to females (Armstead et al., 2013). This is important because ICP can lead to further physical damage, resulting from a shift in brain structures, hydrocephalus, brain herniation, or restriction of blood supply to the brain. ICP is also an important factor determining outcomes in TBI patients. Evidence from rats indicate that blood brain barrier (BBB) leakage may also be more severe in males: rats subjected to a closed skull weight-drop injury showed higher Evan’s blue extravasation 5h after injury compared to females (O’Connor et al., 2006). However, another study found that no difference in this parameter 24h after CCI (Duvdevani et al., 1995). It is not clear if the observed difference is due to dissimilarity in the observed time point or the TBI method. Unfortunately, there are no data available looking at endothelial markers directly.
4.1.2.3 Inflammation
In TBI, the physical damage to the brain is referred to as the primary injury, and this results in the coordinated activation of immune cells. Although required for healing, if this inflammation is too severe or persists too long, it can lead to additional damage to neuronal tissue, referred to as the secondary injury. Since female animals generally show smaller lesion sites after a TBI, the question arises if this could be associated with differences in inflammatory cascades or levels of inflammatory factors between males and females.
The major resident immune cells of the brain are microglial cells. Very little has been reported regarding sex differences in microglial reactions after TBI, however two studies compared sex-dependent microgliosis after injury. One study analyzed ionized calcium-binding adaptor molecule 1 (Iba1) staining as a measure of general myeloid response (including both microglia and infiltrating macrophages from the periphery) in neonatal rats and did not find sex differences after a single severe closed head injury with skull fractures (Hanlon et al., 2017). However, repetitive mild lateral CHI in rats increased the number of Iba1+ cells in the ventromedial hypothalamus in male but not female animals (Yamakawa et al., 2017). Another study analyzed cluster of differentiation molecule 11B (CD11b) staining in adult mice, a marker expressed by microglia but also neutrophils and infiltrating macrophages; this study also failed to find a difference between males and females after CCI (Igarashi et al., 2001). However, it is still too early to conclude if there are relevant sex-differences in microgliosis after TBI due to several reasons: 1) the developing brain could be different from the adult brain; 2) the study in adult mice had groups of only 8 mice which may be too small to reveal small sex differences; and 3) these are very generic measures and more targeted approaches of looking at microglial responses are available that have yet to be used in this context. As with the morphological differences reported within neurons after TBI, microglial-specific sex differences are likely to be subtle if present.
In addition to microglia, astrocytes also contribute to neuroinflammatory responses. Microgliosis after injury is paralleled by astrogliosis, the response of astrocytes to central nervous system (CNS) damage. Astrocytes perform a wide range of essential functions in the CNS including but not limited to the maintenance of extracellular ion balance, biochemical support of endothelial cells that form the BBB, and provision of nutrients to neurons. Günther et al. (2015) observed increased astrogliosis 24h after a modified CCI injury (acceleration of a lead pellet with air pressure directly on the brain tissue) without any sex differences (Günther et al., 2015). However, astrogliosis after repetitive CHI was significantly higher in certain white matter tracts in male mice compared to female (Velosky et al., 2017). Also, mRNA levels for the astrocyte activation marker glial fibrillary acidic protein (GFAP) in the rat seem to be dependent on sex, brain region and injury type: GFAP mRNA was reduced in the prefrontal cortex in a weight-drop model in males, and increased in a lateral impact with rotation TBI model in females. In the hippocampus, only the lateral injury showed a sex difference where males had an increase and females a decrease in GFAP mRNA (Mychasiuk et al., 2016). Repetitive injury in rats reduces GFAP mRNA expression in the prefrontal cortex and does not change levels in the hippocampus, and neither region shows any sex difference (Yamakawa et al., 2017).
Another measure of inflammatory processes in the brain is the release of soluble cytokines and chemokines that act as proinflammatory factors. Particularly potent factors include the cytokines interleukin 1 beta (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNFα), and the chemokines C-C motif chemokine ligand 2 (CCL2) and C-X-C motif chemokine ligand 1 (CXCL1). There are also anti-inflammatory messengers, such as the cytokine interleukin 10 (IL-10). After a brain injury, proinflammatory factors are usually acutely increased. Bruce-Keller et al. (2007) report an increase of IL-6 and CCL2 but not TNFα and IL-4 3d after a CCI in adult mice, without observable sex differences; however this may be due to limited group sizes of 6–8 per group (Bruce-Keller et al., 2007).
We performed a meta-analysis of brain cytokine and chemokine levels of several CHI studies conducted in our lab over the past several years in adult mice aged 3 to 7 months (for methods see (Bachstetter et al., 2015)). At 9h after injury, there is a clear increase of pro-inflammatory factors IL-1β, IL-6, TNFα, IL-10, CXCL1, and CCL2 when the data from both sexes are combined. When the data are separated based on sex (Figure 2), we found that IL-6, TNFα, and CCL2 are significantly more elevated in female compared to male mice. Interestingly, the anti-inflammatory cytokine IL-10 was elevated in males but not in females. Since female mice are smaller compared to their male littermates, we were concerned that the data merely reflect a more severe injury in females due to body size. However, normalization by weight did not change the observed sex differences, nor were the results an artifact of age. We therefore conclude that at least in the CHI mouse model of TBI, females show a more severe early inflammatory profile compared to males. These data are supported by the fact that additional pro-inflammatory factors, cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS), also respond differently in males and females. Both are elevated 24h after a modified CCI in rats, but COX-2 is significantly higher in males whereas iNOS is significantly elevated in females compared to males (Günther et al., 2015). Sex differences in the opposite direction have also been reported for IL-6 in the cerebrospinal fluid of pigs, where males show a higher IL-6 elevation after FPI than females (Armstead et al., 2016). Whether this is primarily an effect of species, compartment, age, injury, or time-point has yet to be determined.
Figure 2. Female mice show a different cytokine profile than males 9h after CHI.
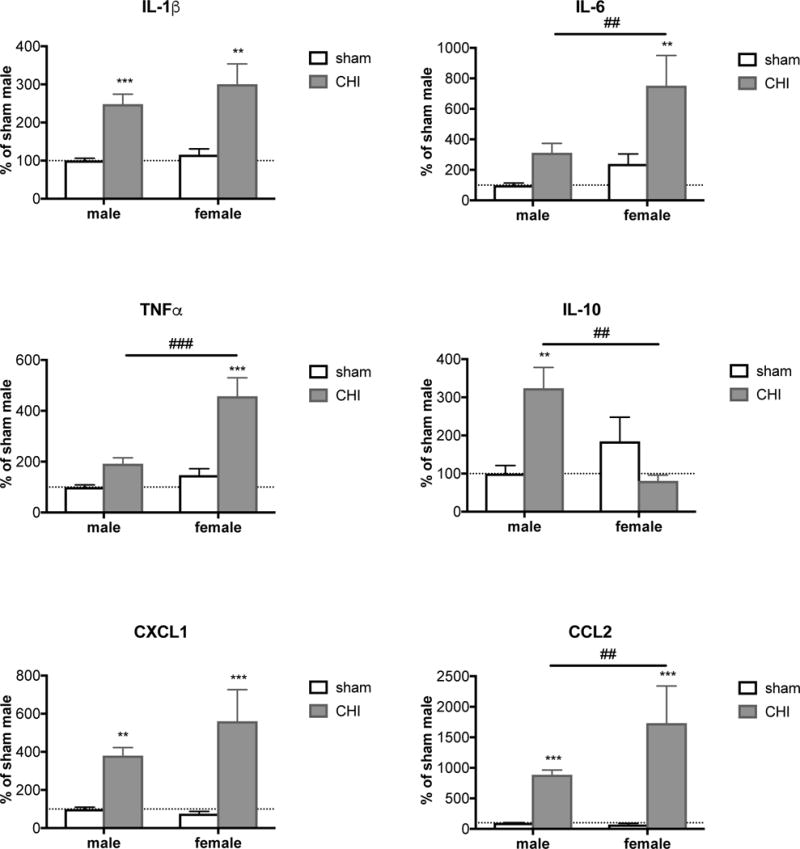
9h after CHI, brain levels of different proinflammatory cytokines and chemokines, including IL-1β, IL-6, TNFα, CCL2 and CXCL1, are increased. IL-6, TNFα and CCL2 are significantly more elevated in females compared to male mice. Analysis of the anti-inflammatory cytokine IL-10 revealed a significant increase 9h after CHI only in male mice, whereas in female mice a non-significant reduction of IL-10 can be observed. Sham and CHI animals from 9 different studies (9h after CHI in C57/BL6 mice age 5 ± 2 months) were included in the meta-analysis. Values are measured in cortical brain homogenates with Meso Scale Discovery enzyme-linked immunosorbent assay and normalized to the corresponding male sham animals. n=28–74. Error bars are mean ± SEM. * sham versus CHI within same sex, # male CHI versus female CHI. ** or ## p<0.01, *** or ### p<0.001.
4.2 How important is the status of sex hormones at time of injury?
An obvious major difference between males and females are the sex hormones. Progesterone and estrogen are the main female sex hormones and testosterone is the male analog. Female hormonal status can be experimentally manipulated in various ways, primarily via ovariectomy to decrease hormonal levels, or induction of pseudopregnancy to increase them. Side effects of an ovariectomy other than a reduction in female sex hormones include an increased life span and a decreased incidence of tumors (Murray, 1936). Male hormones are generally manipulated by the administration of anabolic-androgenic steroids (AAS), including natural androgens like testosterone as well as synthetic androgens structurally related to and exerting similar effects as testosterone. There are no data available about the influence of reduced testosterone levels via male castration on TBI outcomes. The following section focuses on the influence of sex hormone levels on various outcome measures including behavior, inflammation, and neuronal and vascular injury.
4.2.1 Behavior
The influence of hormonal status at time of brain injury has been known to affect behavioral outcomes since at least the late 1980s, when it was shown that pseudopregnant female rats were less impaired in a delayed-spatial alternation task after aspiration lesion compared to normal cycling females (Attella et al., 1987). Female sex hormone levels did not influence post-CCI cognition measured via MWM (Wagner et al., 2002, 2004b), however, though they may influence post-TBI motoric outcomes. Wagner et al. (2004b) showed a small but statistically significant relationship between higher estrogen levels and longer beam walk latencies after CCI in rats, with no such relationship for progesterone levels (Wagner et al., 2004b). Interestingly, the administration of estrogen 4h before FPI in males significantly increased motor scores, especially in the forelimb flexion test. A composite neuroscore trended better for estrogen-treated males but did not reach significance (n=6) (Emerson et al., 1993). After CCI in pseudopregnant female rats, however, the increased estrogen and progesterone did not influence motoric performance as evaluated with the Footfault test, measuring the number of slipped or missed forelimb steps and forelimb usage (Grossman and Stein, 2000). Similarly, male sex hormone elevation in male rodents at time of injury, administered as a triple cocktail of AAS, did not have an influence on any motoric measures in the rotarod after TBI using the CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration) TBI-model (Namjoshi et al., 2016).
4.2.2 Neuropathology
4.2.2.1 Neurodegeneration
Several studies show that reduction of progesterone and estrogen via ovariectomy before FPI abolishes the reduction in lesion volume otherwise seen in females compared to males (Bramlett and Dietrich, 2001; Suzuki et al., 2003, 2004). There are also conflicting data, however, indicating no difference between males, intact females and ovariectomized females following a CCI (Bruce-Keller et al., 2007). Other lines of evidence do support a protective role of estrogen and progesterone in TBI-associated neurodegeneration in the FPI model: fewer surviving cortical neurons close to the injury site in ovariectomized females and males compared to intact females (Suzuki et al., 2003, 2004), as well as increased edema in ovariectomized versus intact females in a weight-drop TBI model (Maghool et al., 2013). This latter neuroprotective effect is dose-dependent in that pseudopregnant females, with the highest levels of sex hormones, show less edema even than intact females in different TBI models (Attella et al., 1987; Duvdevani et al., 1995; Roof et al., 1993). Supplementation of sex hormones in ovariectomized females had a profound effect on edema formation in a concentration-dependent manner following a weight-drop injury (Maghool et al., 2013). The authors showed that even the relatively small hormonal changes during normal female estrous cycle had an influence on edema formation: females in their proestrous stage at time of injury had less edema formation compared to females in their nonproestrous stage. Neuronal degeneration measured by Fluorojade staining did not change as a function of sex hormone levels in females after a CCI (Bruce-Keller et al., 2007), although the number of amyloid precursor protein positive cells (APP, a marker of axonal injury) is increased in ovariectomized compared to intact females that underwent a FPI (Suzuki et al., 2004).
Progesterone supplementation in ovariectomized rats before CCI did not show an effect on lesion volume 7d post-injury, although the loss of neuronal cells in the CA3 and CA1 regions of the hippocampus was significantly reduced with progesterone implants (Robertson et al., 2006). Estrogen administration before the induction of weight-drop injury with craniotomy in females that underwent an ovariectomy significantly reduced markers of programmed cell death, including terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and cleaved caspase 3 (Li et al., 2011).
AAS administration in males before injury had no influence on axonal damage as measured by APP staining following a weight-drop injury (Mills et al., 2012), however AAS-treatment increased neurodegeneration in response to induction of CHIMERA injury when measured with silver staining (Namjoshi et al., 2016). Interestingly, these data imply that it may not simply be the case that female sex hormones are protective, but in certain contexts male sex hormones may be detrimental.
4.2.2.2 Vasculature
Since the constituents of the vasculature also express receptors for the major sex hormones, including testosterone, progesterone and estrogen (McNeill et al., 2002), it is likely that differences in hormones could underlie the differences highlighted in the previous section. And indeed, the drop in CBF and the increase in ICP after a TBI are significantly more pronounced in ovariectomized females in a weight-drop as well as a CCI model of TBI (Maghool et al., 2013; Roof and Hall, 2000b). Estrogen treatment in ovariectomized females and in males can significantly increase CBF following CCI (Roof and Hall, 2000b), and ICP can be positively influenced by supplementing ovariectomized females with estrogen or progesterone before the traumatic weight-drop event (Maghool et al., 2013). Unfortunately, no data are available about the influence of AAS-treatment on vascular TBI outcomes.
4.2.2.3 Inflammation
Sex hormones exert effects on TBI response not only in neuronal and vascular outcome measures, but also inflammatory factors. Measurement of brain cytokine levels in ovariectomized females provides evidence that the female sex hormones may at least partially explain the increased inflammatory responses observed in females, as reported above: reduced brain concentrations of IL-6 and CCL2 were found in ovariectomized compared to intact females, although there was also an increase in TNFα. Estrogen replacement before CCI did not affect levels of IL-6 and CCL2 but reduced TNFα to the level of intact females (Bruce-Keller et al., 2007).
Unfortunately, there are no data available about the influence of female hormonal status at time of injury on microglial or astroglial cells. But AAS administration in male animals before TBI (CHIMERA) did not have any influence on Iba1+ microgliosis (Namjoshi et al., 2016).
4.3 So, can we use female sex hormones as TBI therapeutics?
Estrogen receptors (ER) and progesterone receptors (PR) exist throughout the brain, and their expression may vary depending on sex, hormonal status, age, cell type and brain region (Auger and De Vries, 2002; Camacho-Arroyo et al., 1998; Guerra-Araiza et al., 2000, 2001, 2002, 2003, Hagihara et al., 1992, 1992, Kato et al., 1984, 1993; Kato and Onouchi, 1983; Wagner et al., 2001) (for more information see (Pfaff and Joels, 2016). However, the precise mechanisms by which progesterone and estrogen provide their neuroprotective effects on neurons, astrocytes, and the BBB are still unclear. Several mechanisms have been shown to potentially contribute (for review see: Brotfain et al., 2016): 1) regulation of protective gene transcription changes within neurons, 2) binding and activation of gamma-Aminobutyric acid-A (GABA-A) receptors to increase cellular survival, 3) scavenging of oxygen free radicals, 4) enhancement of astroglial glutamate metabolism and cycling (estrogen only), 5) reduction of proinflammatory mediators from microglial cells (estrogen only), 6) modulation of Aquaporin-4 channel expression resulting in less brain edema (progesterone only), 7) increased CBF or promotion of angiogenesis (estrogen only), and 8) scavenging and increased efflux of excitotoxic glutamate.
4.3.1 Estrogen
The effects of estrogen treatment on TBI-induced behavioral deficits including locomotor, cognitive and classical psychiatric problems, are currently not well defined and will require further studies. However, as discussed in the following section, there are some reports describing estrogen effects on neurodegeneration, vascular outcomes, and inflammation in preclinical TBI models.
4.3.1.1 Estrogen effects on neuropathology
4.3.1.1.1 Estrogen effects on neurodegeneration
The effect of estrogen on brain edema after weight-drop TBI is consistent, with all eight studies investigating this demonstrating a beneficial effect of estrogen treatment (Asl et al., 2013; Dehghan et al., 2015; Khaksari et al., 2011, 2015a, 2015b; O’Connor et al., 2005; Shahrokhi et al., 2010, 2012). Similar beneficial effects of estrogen are reported in terms of neuronal loss, with significantly more neuronal cells and fewer FluoroJade+ cells following a FPI (Day et al., 2013) and reduced TUNEL staining in different TBI models (Bao et al., 2011; Soustiel et al., 2005).
4.3.1.1.2 Estrogen effects on the vasculature
Estrogen treatment has been found to help reduce the increased ICP after weight-drop TBI (Dehghan et al., 2015; Shahrokhi et al., 2010), as well as BBB dysfunction as measured by Evans blue leakage following a weight-drop injury (Asl et al., 2013; Dehghan et al., 2015; Khaksari et al., 2015b; O’Connor et al., 2005; Shahrokhi et al., 2012). No data are available whether estrogen treatment after TBI has an influence on CBF.
4.3.1.1.3 Estrogen effects on inflammation
Not much is known to date about estrogen’s effects on inflammation, although it has been reported that its administration following lateral FPI can ameliorate astrogliosis (Day et al., 2013). It has also been reported that estrogen can reduce the elevations of IL-1β, TNFα, and IL-6 following a weight-drop TBI (Khaksari et al., 2011; Sarkaki et al., 2013; Shahrokhi et al., 2010), and it can increase brain levels of the anti-inflammatory cytokines transforming growth factor beta (TGFβ) and IL-10 after a weight-drop injury (Khaksari et al., 2011, 2015a; Sarkaki et al., 2013).
4.3.1.2 Estrogen Dosing
An important question during drug development is proper dosing; it should be high enough to exert beneficial effects but also not too high to cause adverse reactions. For edema, even physiological doses of estrogen (33.3 μg/kg) show a benefit, although a much bigger decrease in brain water content can be achieved with a pharmacological dose of estrogen following a weight-drop injury (1 mg/kg) (Asl et al., 2013; Khaksari et al., 2011, 2015b; Shahrokhi et al., 2012). For positive effects on BBB integrity after a weight-drop TBI, a high pharmacological dose is necessary (1 mg/kg) (Asl et al., 2013; Khaksari et al., 2015b). Conversely, the early increase in TGFβ 6h after a weight-drop TBI seems only to be exerted by the physiological low but not the high dose. Twenty-four hours after TBI, however, both doses effectively increase TGFβ levels (Khaksari et al., 2011; Sarkaki et al., 2013). Similarly, the reduction in IL-6 after injury only occurs with the low dose of estrogen. In contrast, IL-1β was further elevated 6h after a weight-drop TBI with the high therapeutic dose, without an effect of the low dose. At 24h after injury, both doses are capable of reducing IL-1β brain levels (Khaksari et al., 2011; Sarkaki et al., 2013).
4.3.2 Progesterone
For a summary of all progesterone effects in rodent models see Table 2.
Table 2.
Summary of progesterone effects in different rodent TBI models
| Endpoint | weight-drop | CHI | CCI | FPI | ACI | Species | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Behavior | Locomotion deficits | 3 (6–10) | mice | |||||||
| 17 (15), 21 (7), 23 (5–8) | 1 (8), 5 (7–8), 6 (3–5), 7 (8), 11 (12), 13 (8–9), 16 (3–8), 18 (6–10), 19 (5), 20 (6), 22 (6–9) | 24 (5–6), 25 (6) | 15 (5–12) | rats | ||||||
| Cognitive deficits | 3 (6–10) | 4 (3–24) | mice | |||||||
| 10 (7), 12 (6), 14 (6), 17 (15) | 2 (10–12), 5 (7–8), 6 (3–5), 7 (8), 9 (7–9), 11 (12), 13 (8–9), 16 (3–8) | 1 (8) | 8 (5–12), 15 (5–12) | rats | ||||||
| Anxiety | mice | |||||||||
| 21 (7) | 18 (6–10), 26 (8) | rats | ||||||||
| Sociability | mice | |||||||||
| rats | ||||||||||
| Inhibitory control | mice | |||||||||
| rats | ||||||||||
| Neurodegeneration | Lesion size | 3 (6–10) | 4 (3–24) | mice | ||||||
| 5 (7–8), 13 (8–9), 16 (3–8), 18 (6–10), 22 (6–9), 25 (6) | 1 (8), 2 (10–12), 6 (3–5), 7 (8), 9 (7–9), 11 (12), 27 (5–6) | 28 (4–7) | rats | |||||||
| Neuronal count | mice | |||||||||
| 10 (7), 14 (6), 21 (7), 33 (2–5) | 2 (10–12), 5 (7–8). 6 (3–5) | rats | ||||||||
| Edema | 4 (3–24) | mice | ||||||||
| 33 (2–5), 34 (5), 35 (10) | 16 (3–8), 29 (5–12), 30 (5–6), 31 (5), 32 (6–12) | 27 (5–6) | rats | |||||||
| Cell death | mice | |||||||||
| rats | ||||||||||
| Necrotic neurons | mice | |||||||||
| 36 (6) | 22 (6–9), 38 (6) | 9 (7–9), 11 (12), 37 (3–13) | rats | |||||||
| Dendritic complexity | mice | |||||||||
| rats | ||||||||||
| Vasculature | CBF | mice | ||||||||
| rats | ||||||||||
| Evan's blue extravasation | 39 (10) | mice | ||||||||
| 34 (5), 35 (10), 40 (7) | rats | |||||||||
| Inflammation | Microgliosis | mice | ||||||||
| 16 (3–8) | rats | |||||||||
| Astrogliosis | mice | |||||||||
| 41 (5) | 16 (3–8), 18 (6–10), 22 (6–9) | 11 (12) | 15 (5–12) | rats | ||||||
| IL-6 | 45 (7–9) | mice | ||||||||
| 36 (6), 44 (7) | 43 (4) | 19 (5) | rats | |||||||
| CCL2 | 45 (7–9) | mice | ||||||||
| rats | ||||||||||
| TNFalpha | 45 (7–9) | mice | ||||||||
| 33 (2–5), 36 (6) | 26 (8), 32 (6–12), 42 (5), 43 (4) | 19 (5) | rats | |||||||
| IL-1beta | 45 (7–9) | 4 (3–24) | mice | |||||||
| 36 (6) | 40 (7), 44 (7) | 19 (5), 32 (6–12), 42 (5) | rats | |||||||
| CXCL1 | 45 (7–9) | mice | ||||||||
| rats | ||||||||||
| IL-10 | mice | |||||||||
| rats | ||||||||||
| COX-2 | mice | |||||||||
| 12 (6) | 43 (4) | rats | ||||||||
| iNOS | mice | |||||||||
| 14 (6) | rats | |||||||||
| data not found | beneficial effect | detrimental effect | no effect | variable-dependent | no TBI effect | |||||
The endpoints are presented on the left side and the species (mice or rats) on the right side. The different columns represent different TBI models: CHI (closed head injury), CCI (controlled cortical impact), FPI (fluid percussion injury) and ACI (aseptic cryogenic injury).
Red-colored cells represent beneficial progesterone effects, blue represent detrimental progesterone effects, yellow represent no progesterone effect, black indicate a variable-dependent progesterone effect (sex, time point or assay), and green indicate no deficit in the TBI model that could be influenced with progesterone. Grey cells represent end points where no data was available.
The numbers within each cell represent the references below, and the number following in the brackets is the “n” number per group in the corresponding study. The sex(es) used in the study is indicated in parentheses after the reference citation:
1 Nudi et al. 2015 (males), 2 Roof et al. 1994 (males), 3 Mannix et al. 2014 (both), 4 Jones et al. 2005 (both), 5 Shear et al. 2002 (males), 6 Djebaili et al 2004 (males), 7 Wali et al. 2001 (males), 8 Li et al. 2012 (males), 9 Hua et al. 2012 (males), 10 Uysal et al. 2013 (both), 11 Tang et al. 2013 (males), 12 Si et al. 2013 (males), 13 Geddes et al. 2014 (males), 14 Si et al. 2014 (males), 15 Webster et al. 2015 (males), 16 Wali et al. 2016 (males), 17 O’Connor et al. 2007 (both), 18 Cutler et al. 2006 (males), 19 Cekic et al. 2011 (males), 20 Cekic et al. 2012 (males), 21 Baykara et al. 2013 (both), 22 Peterson et al. 2015 (males), 23 Allitt et al. 2016 (males), 24 Grossman et al. 2000 (both), 25 Grossman et al. 2011 (males), 26 Cutler et al. 2005 (males), 27 Gilmer et al. 2008 (males), 28 Robertson et al. 2015 (both), 29 Roof et al. 1996 (both), 30 Wright et al. 2001 (males), 31 Guo et al. 2006 (males), 32 VanLandingham et al. 2006 (males), 33 Pan et al. 2007 (males), 34 O’Connor et al. 2005 (both), 35 Shahrokhi et al. 2012 (females). 36 Chen et al. 2008 (males), 37 Djebaili et al. 2005 (males), 38 Barha et al. 2011 (males), 39 Pascual et al. 2013 (males), 40 Khaksari et al. 2011 (females), 41 Liu et al. 2014 (males), 42 He et al. 2004 (males), 43 Cutler et al. 2007 (males), 44 Sarkaki et al. 2013 (females), 45 Hua et al. 2011 (males)
4.3.2.1 Progesterone effects on behavioral measures
The potential of progesterone as a therapeutic drug for TBI treatment has been studied extensively in preclinical models. Sixteen studies alone have investigated the effect of progesterone treatment following TBI in males and females. In terms of cognitive outcomes, all but one CCI study (Nudi et al., 2015) report beneficial effects of progesterone treatment on hippocampal-dependent spatial learning in the MWM in a variety of different TBI models (Djebaili et al., 2004; Geddes et al., 2014; Hua et al., 2012; Jones et al., 2005; Li et al., 2012; Mannix et al., 2014; Roof et al., 1994; Shear et al., 2002; Si et al., 2013, 2014; Tang et al., 2013; Uysal et al., 2013; Wali et al., 2011, 2016; Webster et al., 2015). Two additional studies measuring hippocampal-dependent spatial learning with the Barnes Maze also reported beneficial effects of progesterone treatment after TBI induced with different methods (Nudi et al., 2015; O’Connor et al., 2007).
Additionally, motoric alterations after TBI using a wide range of different models show improvement with progesterone treatment in both male and female animals (Allitt et al., 2016; Baykara et al., 2013; Cekic et al., 2011, 2012; Cutler et al., 2006a; Djebaili et al., 2004; Geddes et al., 2014; Nudi et al., 2015; O’Connor et al., 2007; Peterson et al., 2015; Shear et al., 2002; Tang et al., 2013; Wali et al., 2011, 2016; Webster et al., 2015) with the exception of some studies (Grossman et al., 2011; Grossman and Stein, 2000). Interestingly, in a study using the CCI method in 4 week old mice, progesterone treatment only improved wire grip scores in male mice, but worsened performance in females (Mannix et al., 2014), suggesting that the hormonal status prior to injury could influence whether progesterone treatment is beneficial or not. General neurological function, measured with the modified Neurological Severity Score (mNSS), can likewise be improved with progesterone treatment following a TBI in rats (Li et al., 2012; Pan et al., 2007), or mice (Kumasaka et al., 2014) independent of the injury model used. Classical psychiatric problems, like anxiety, also seem to be affected by progesterone treatment, since progesterone reduces anxiety levels after a weight-drop TBI in male and female rats in the EPM (Baykara et al., 2013). Two other studies could not replicate these findings since their CCI TBI models did not induce injury-dependent anxiety, although progesterone treatment in these studies reduced baseline anxiety levels (Cutler et al., 2005, 2006b).
4.3.2.2 Progesterone effects on neuropathology
4.3.2.2.1 Progesterone effects on neurodegeneration
Although the behavioral analyses show overwhelming positive effects of progesterone treatment after TBI, this is not as obvious in analysis of the lesion volume using different TBI animal models. Only about half of the studies show a beneficial progesterone effect, with smaller lesion areas in both sexes compared to their vehicle treated controls (Cutler et al., 2006a; Geddes et al., 2014; Grossman et al., 2011; Jones et al., 2005; Peterson et al., 2015; Shear et al., 2002; Wali et al., 2016), while the others report no difference (Djebaili et al., 2004; Gilmer et al., 2008; Hua et al., 2012; Mannix et al., 2014; Nudi et al., 2015; Roof et al., 1994; Tang et al., 2013; Wali et al., 2011). Interestingly, Robertson et al. (2015) show less percentage tissue loss in progesterone-treated CCI animals only in females, without sex differences in lesion volume (Robertson and Saraswati, 2015).
Conflicting reports regarding the effects of progesterone on edema formation in different animal TBI models have also been reported, with some showing beneficial effects (Guo et al., 2006; O’Connor et al., 2005; Pan et al., 2007; Roof et al., 1996; Shahrokhi et al., 2012; VanLandingham et al., 2006, 2007; Wali et al., 2016; Wright et al., 2001) and others reporting no difference (Gilmer et al., 2008; Jones et al., 2005). Wright et al. (2001) show an inverse correlation between the degree of cerebral edema and serum progesterone levels, with less progesterone correlating with more edema in a CCI rat TBI model (Wright et al., 2001). Additionally, various studies have reported that neuronal loss after TBI in a variety of different models is reduced in progesterone-treated animals (Baykara et al., 2013; Djebaili et al., 2004; Pan et al., 2007; Roof et al., 1994; Shear et al., 2002; Si et al., 2014; Uysal et al., 2013). Also, axonal injury as assessed by APP measurement was profoundly diminished with progesterone treatment following weight-drop TBI (O’Connor et al., 2007). In line with the evidence that more neuronal cells survive, fewer apoptotic cells have been reported in progesterone treated weight-drop TBI males compared to controls (Chen et al., 2008; Uysal et al., 2013), but at least one study has reported no difference in this measure using the CCI TBI method (Djebaili et al., 2005). Reduction in FluoroJade labeled cells after progesterone treatment in TBI males was also only reported in some (Barha et al., 2011; Peterson et al., 2015) but not all studies (Hua et al., 2012; Tang et al., 2013), all using the CCI method.
4.3.2.2.2 Progesterone effects on the vasculature
Progesterone effects are not limited to amelioration of behavioral and neuronal changes, but extend even to the vasculature. Progesterone treatment after TBI in male and female animals reduces BBB damage, measured as vascular leakage of albumin following a CCI (Pascual et al., 2013) or Evan’s Blue in a weight-drop TBI model (Khaksari et al., 2011; O’Connor et al., 2005; Shahrokhi et al., 2012). Also the increased ICP in ovariectomized females is significantly reduced with progesterone treatment in weight-drop injured animals (Shahrokhi et al., 2010).
4.3.2.2.3 Progesterone effects on inflammation
Regarding astroglial responses, the data at hand are similarly conflicted, with the majority of studies using different TBI models showing that progesterone treatment reduces astrogliosis, as measured by GFAP staining (Cutler et al., 2006b; Liu et al., 2014; Peterson et al., 2015; Wali et al., 2016; Webster et al., 2015). One other study could not find a beneficial progesterone effect on astrogliosis after CCI, potentially due to the advanced age (13 months) of the rats used (Tang et al., 2013). Nothing is known about astrocyte responses to progesterone in females, since all of the studies used only male animals.
Progesterone treatment also seems to have anti-inflammatory effects on microglial cells after CCI, since it is able to reduce microgliosis, measured as IBA1 upregulation, in female and male rats (Wali et al., 2016). Notably, the ability of progesterone treatment to reduce proinflammatory cytokine levels after TBI seems to be dependent on the type of injury. For instance, the increase of IL-1β protein or mRNA levels is only reduced with progesterone treatment in the more severe TBI models that include a craniotomy (Cekic et al., 2011; Chen et al., 2008; He et al., 2004; Hua et al., 2011; VanLandingham et al., 2006). Milder injury models that left the skull intact showed no IL-1β reduction with progesterone treatment (Jones et al., 2005; Khaksari et al., 2011; Sarkaki et al., 2013). One other possible explanation for the different findings could be the sex of the animals treated, since all experiments using only male animals showed a significant IL-1β reduction with progesterone treatment while studies using either both or only females did not report a beneficial progesterone effect on TBI-induced IL-1β enhancement. For TNFα the picture looks similar, although not as clear as with IL-1β: despite similar injuries, some studies report a reduction with progesterone treatment (Chen et al., 2008; Cutler et al., 2005, 2007; He et al., 2004; Pan et al., 2007; VanLandingham et al., 2006), while some studies could not find a progesterone effect (Cekic et al., 2011; Hua et al., 2011). The story grows even more complicated when other cytokines are considered: whereas most studies report a reduction of upregulated IL-6 after TBI, independent of the injury model and sex (Chen et al., 2008; Cutler et al., 2007; Hua et al., 2011; Sarkaki et al., 2013), one experiment performed by Cekic et al. (2011) reports that progesterone treatment enhanced IL-6 protein even further 24h after CCI in male animals (Cekic et al., 2011). For CCL2 and CXCL1 only one report in male mice is available showing a reduction of the increased protein and mRNA levels with progesterone administration after CCI (Hua et al., 2011). Similarly, the COX-2 increase in male rats has been reported responsive to progesterone treatment in different TBI models, while no data are available for females (Cutler et al., 2007; Si et al., 2013). The same holds true for iNOS following a weight-drop injury (Si et al., 2014).
4.3.2.3 Progesterone Dosing and Withdrawal
As for estrogen, dosing is an important question in the potential use of progesterone as a TBI therapeutic. Cardiovascular disease and breast cancer are possible severe negative side effects of progesterone (Writing Group for the Women’s Health Initiative Investigators, 2002). Several studies explored the effects of different doses of progesterone treatment after TBI. The study by Geddes et al. (2014) shows a dose response of progesterone on CCI-induced lesion size with 8 and 16 mg/kg being most effective in limiting tissue damage, and motoric and cognitive deficits (Geddes et al., 2014). Cutler et al. (2007) also report a beneficial effect of a 16mg/kg dose on edema size in male rats following a bilateral CCI injury, but no such benefit at 8 mg/kg (Cutler et al., 2007). An even higher 32 mg/kg dose of progesterone leads to fewer useful cellular and behavioral effects than lower doses following a CCI (Cutler et al., 2007; Goss et al., 2003).
Interestingly, the consistent dose response curve reported for neuronal injury and behavior does not apply equally to all the inflammatory changes. For example, in female rats that underwent a weight-drop injury, brain levels of the proinflammatory cytokine IL-1β are unchanged with an 8 mg/kg dose of progesterone, whereas they are increased with a 1,7 mg/kg dose. For IL-6 there is no effect at 1,7 mg/kg but a significant reduction at the 8 and 16 mg/kg dosages (Cutler et al., 2007; Sarkaki et al., 2013). Progesterone can also reduce TNFα at either the 8 or 16 mg/kg dosage (Cutler et al., 2007). More sensitive to the concentration of progesterone administered is the BBB, since reduced leakage of Evans Blue can be achieved with the administration of a low physiological dose (1,7 mg/kg) whereas a higher pharmacological dose (8 mg/kg) increases BBB leakage in the weight-drop model of TBI (Khaksari et al., 2011; Shahrokhi et al., 2012). Taken together, these findings indicate that progesterone may be too blunt of an instrument to be useful as a treatment, and more targeted combination therapies may be necessary.
One final factor to consider for the usage of progesterone as a potential TBI therapeutic is the mode of withdrawal. Several studies report that a tapered withdrawal (reducing the last doses to half or partial doses) shows much better outcomes than an acute progesterone withdrawal including lesion size, astrogliosis, motoric measurements (Cutler et al., 2006b), and brain TNFα mRNA levels following CCI (Cutler et al., 2005).
5. Female sex hormone treatments in human clinical trials
Given the protective effects of female sex hormones in certain animal models of TBI, and their relative safety in humans, several clinical trials have been performed to ascertain whether such hormonal treatments confer protection to TBI patients. Unfortunately, the results have been disappointing (Ma et al., 2016). Although one study (Wright et al., 2007) reported a reduced 30d mortality rate and increased likelihood of better outcomes after progesterone treatment, other studies have reported no significant effect, including the two large Phase III ProTECT and SYNAPSE trials (Skolnick et al., 2014; Wright et al., 2014). It is worth highlighting some potential limitations of these studies, however. A major factor that could potentially influence the results of the ProTECT trial is the large heterogeneity of enrolled patients including differences in sex (75% male), age (median of 35 years but including subjects up to 94 years), race and cultural background, and severity (29% moderate, 54% moderate-to-severe and 18% severe) (Stein, 2015; Wright et al., 2014). Similarly, the SYNAPSE III trial included patients across many different countries, ages (16–70 years) and sex, however they only included severe TBI cases (Skolnick et al., 2014). Both trials used the Glasgow Outcome Scale-extended (GOS-E) 6m after injury as their primary outcome measure (the SYNAPSE III additionally used the GOS), thereby lacking any mechanistic early outcome end-points and biomarkers, that potentially could yield a positive result (Skolnick et al., 2014; Wright et al., 2014). Some other factors that could have played a role are: no follow-up past 6 months to assess possible late recovery, errors of execution of approved protocols, and effects of other therapies and rehabilitation on outcome measures. Both of those Phase III trials also did not incorporate any drug optimization studies into their design, which could have been essential since we know from pre-clinical studies that variables such as treatment paradigm (treatment window, dosing, duration, mode of withdrawal and route of administration), age and sex influence drug treatment outcome (Stein, 2015). Further, the endogenous levels of these hormones in the patients at time of injury and recovery are highly variable and also likely to affect the efficacy of hormone treatment. In line with this idea, the ProTECT trial indicated potentially worse outcomes in females versus males after progesterone treatment: while the male progesterone group did not differ from placebo, there was a trend toward better recovery in the female placebo group (Wright et al., 2014). While none of the other clinical trials addressed this directly, one small study of only male patients did indicate a protective effect of progesterone (Shakeri et al., 2013). To address these issues, future TBI trial designs should have more strict inclusion criteria to reduce the impact of varying clinical characteristics and comorbid conditions. In addition, grouping patients by the following factors should be considered: sex, age, biomarkers, comorbidities, genotypes, and severity. Optimal dosage, pharmacokinetics and time suitability of different drugs should also be considered to better assess drug efficacy (Lu et al., 2016).
Taken together, it is not yet clear if progesterone will provide detectable benefits across TBI patients as a whole. Maybe progesterone treatment is useful in some subpopulation of TBI patients, dependent on a combination of 1) sex, 2) type of TBI, 3) severity of TBI, 4) age, 5) hormonal status at time of injury, and 6) other medications. Nonetheless, progesterone currently provides a valid research tool to uncover specific beneficial pathways that 1) are more or less pertinent to different types of injury and 2) may be better targeted in ways other than with a broadly-acting hormone. A similar point of view might also hold true for estrogen, however this has yet to be established: a single Phase II study investigating the effects of intravenous estrogen after TBI was completed in 2012 (NCT00973674), but the results have yet to be reported as of January 2018.
6. Conclusion
Despite the limited number of studies available, the differences in species, TBI methods, and ages, the small animal numbers used in many of the studies, and the general lack of studies including female animals, there is reason to be optimistic. Recent National Institutes of Health (NIH) policy changes require researchers to account for sex as a biological variable when they apply for funding. Along with the adoption of more rigorous practices including randomization and blindedness, adequate powering, common data elements, and the addition of more translational endpoints, such enhancements to preclinical research should lead to a better understanding of potentially important sex differences, as well as increase our understanding of TBI-related processes more generally. Better comprehension of relevant preclinical variables in TBI will allow us to design better clinical trials and ultimately bring us closer to developing truly effective treatments.
Supplementary Material
Highlights.
Sex is an understudied variable in TBI research
Sex and hormonal status at time of injury influence different outcome measures
Female mice show a stronger brain inflammation response acutely post-TBI than males
Female sex hormone treatment can improve certain TBI aspects in preclinical models
More research into sex differences will enable better treatment strategies for TBI
Acknowledgments
The authors would like to acknowledge Dr. Adam D. Bachstetter for contributing data for the meta analysis, Dr. Erin Abner for statistical assistance, and Dr. Josh J. Morganti for scientific input. Further, we would like to thank Danielle S. Goulding, Edgardo Dimayuga and all the former Van Eldik lab members for their contributions to the different studies combined in the cytokine and chemokine meta-analysis.
Funding
This work was supported in part by the National Institutes of Health [R01 NS093920].
Abbreviations
- AAS
anabolic-androgenic steroids
- APP
beta-amyloid precursor protein
- BBB
blood brain barrier
- 5-CSRTT
5-choice serial reaction time task
- CA1
Cornu Ammonis area 1
- CA3
Cornu Ammonis area 3
- CBF
cerebral blood flow
- CCI
controlled cortical impact
- CCL2
chemokine (C-C motif) ligand 2
- CD11b
cluster of differentiation molecule 11B
- CHI
closed head injury
- CHIMERA
closed-head impact model of engineered rotational acceleration)
- CNS
central nervous system
- COX-2
cyclooxygenase 2
- CPP
cerebral perfusion pressure
- CXCL1
chemokine (C-X-C motif) ligand 1
- d
day(s)
- EPM
Elevated Plus Maze
- ER
estrogen receptor
- FPI
fluid percussion injury
- GFAP
glial fibrillary acidic protein
- h
hour(s)
- IBA1
ionized calcium-binding adaptor molecule 1
- ICP
intracranial pressure
- IL-1β
interleukin 1 beta
- IL-6
interleukin 6
- IL-10
interleukin 10
- iNOS
inducible nitric oxide synthase
- mNSS
modified Neurological Severity Score
- mRNA
messenger ribonucleic acid
- mTBI
mild traumatic brain injury
- PR
progesterone receptor
- MWM
Morris Water Maze
- NIH
National Institutes of Health
- SEM
standard error of mean
- TBI
traumatic brain injury
- TGFβ
transforming growth factor beta
- TNFα
tumor necrosis factor alpha
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors have no competing interests to declare.
References
- Allitt BJ, Johnstone VPA, Richards K, Yan EB, Rajan R. Progesterone Exacerbates Short-Term Effects of Traumatic Brain Injury on Supragranular Responses in Sensory Cortex and Over-Excites Infragranular Responses in the Long Term. J Neurotrauma. 2016;33:375–389. doi: 10.1089/neu.2015.3946. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Grabow JD, Kurland LT, Laws ER. The incidence, causes, and secular trends of head trauma in Olmsted County, Minnesota, 1935–1974. Neurology. 1980;30:912–919. doi: 10.1212/wnl.30.9.912. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Riley J, Vavilala MS. Preferential Protection of Cerebral Autoregulation and Reduction of Hippocampal Necrosis With Norepinephrine After Traumatic Brain Injury in Female Piglets. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2016;17:e130–137. doi: 10.1097/PCC.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Armstead WM, Riley J, Vavilala MS. Dopamine prevents impairment of autoregulation after traumatic brain injury in the newborn pig through inhibition of Up-regulation of endothelin-1 and extracellular signal-regulated kinase mitogen-activated protein kinase. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2013;14:e103–111. doi: 10.1097/PCC.0b013e3182712b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead WM, Riley J, Vavilala MS. TBI sex dependently upregulates ET-1 to impair autoregulation, which is aggravated by phenylephrine in males but is abrogated in females. J Neurotrauma. 2012;29:1483–1490. doi: 10.1089/neu.2011.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asl SZ, Khaksari M, Khachki AS, Shahrokhi N, Nourizade S. Contribution of estrogen receptors alpha and beta in the brain response to traumatic brain injury. J Neurosurg. 2013;119:353–361. doi: 10.3171/2013.4.JNS121636. [DOI] [PubMed] [Google Scholar]
- Attella MJ, Nattinville A, Stein DG. Hormonal state affects recovery from frontal cortex lesions in adult female rats. Behav Neural Biol. 1987;48:352–367. doi: 10.1016/s0163-1047(87)90918-6. [DOI] [PubMed] [Google Scholar]
- Auger CJ, De Vries GJ. Progestin receptor immunoreactivity within steroid-responsive vasopressin-immunoreactive cells in the male and female rat brain. J Neuroendocrinol. 2002;14:561–567. doi: 10.1046/j.1365-2826.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Webster SJ, Goulding DS, Morton JE, Watterson DM, Van Eldik LJ. Attenuation of traumatic brain injury-induced cognitive impairment in mice by targeting increased cytokine levels with a small molecule experimental therapeutic. J Neuroinflammation. 2015;12:69. doi: 10.1186/s12974-015-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG, Leddy JJ, Darling SR, Shucard J, Makdissi M, Willer BS. Gender Differences in Recovery From Sports-Related Concussion in Adolescents. Clin Pediatr (Phila ) 2016;55:771–775. doi: 10.1177/0009922815606417. [DOI] [PubMed] [Google Scholar]
- Bao YJ, Li LZ, Li XG, Wang YJ. 17Beta-estradiol differentially protects cortical pericontusional zone from programmed cell death after traumatic cerebral contusion at distinct stages via non-genomic and genomic pathways. Mol Cell Neurosci. 2011;48:185–194. doi: 10.1016/j.mcn.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Barha CK, Ishrat T, Epp JR, Galea LAM, Stein DG. Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Exp Neurol. 2011;231:72–81. doi: 10.1016/j.expneurol.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayir H, Marion DW, Puccio AM, Wisniewski SR, Janesko KL, Clark RSB, Kochanek PM. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma. 2004;21:1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- Baykara B, Aksu I, Buyuk E, Kiray M, Sisman AR, Baykara B, Dayi A, Tas A, Ozdemir D, Arda MN, Uysal N. Progesterone treatment decreases traumatic brain injury induced anxiety and is correlated with increased serum IGF-1 levels; prefrontal cortex, amygdala, hippocampus neuron density; and reduced serum corticosterone levels in immature rats. Biotech Histochem Off Publ Biol Stain Comm. 2013;88:250–257. doi: 10.3109/10520295.2013.769630. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP. Sex differences in outcome after mild traumatic brain injury. J Neurotrauma. 2010;27:527–539. doi: 10.1089/neu.2009.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C, Ley EJ, Tillou A, Cryer G, Margulies DR, Salim A. The effect of gender on patients with moderate to severe head injuries. J Trauma. 2009;67:950–953. doi: 10.1097/TA.0b013e3181ba3354. [DOI] [PubMed] [Google Scholar]
- Berz K, Divine J, Foss KB, Heyl R, Ford KR, Myer GD. Sex-specific differences in the severity of symptoms and recovery rate following sports-related concussion in young athletes. Phys Sportsmed. 2013;41:58–63. doi: 10.3810/psm.2013.05.2015. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001;18:891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- Broshek DK, Kaushik T, Freeman JR, Erlanger D, Webbe F, Barth JT. Sex differences in outcome following sports-related concussion. J Neurosurg. 2005;102:856–863. doi: 10.3171/jns.2005.102.5.0856. [DOI] [PubMed] [Google Scholar]
- Brotfain E, Gruenbaum SE, Boyko M, Kutz R, Zlotnik A, Klein M. Neuroprotection by Estrogen and Progesterone in Traumatic Brain Injury and Spinal Cord Injury. Curr Neuropharmacol. 2016;14:641–653. doi: 10.2174/1570159X14666160309123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Dimayuga FO, Reed JL, Wang C, Angers R, Wilson ME, Dimayuga VM, Scheff SW. Gender and estrogen manipulation do not affect traumatic brain injury in mice. J Neurotrauma. 2007;24:203–215. doi: 10.1089/neu.2006.0163. [DOI] [PubMed] [Google Scholar]
- Bruns J, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- Camacho-Arroyo I, Guerra-Araiza C, Cerbón MA. Progesterone receptor isoforms are differentially regulated by sex steroids in the rat forebrain. Neuroreport. 1998;9:3993–3996. doi: 10.1097/00001756-199812210-00001. [DOI] [PubMed] [Google Scholar]
- Cancelliere C, Donovan J, Cassidy JD. Is Sex an Indicator of Prognosis After Mild Traumatic Brain Injury: A Systematic Analysis of the Findings of the World Health Organization Collaborating Centre Task Force on Mild Traumatic Brain Injury and the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2016;97:S5–18. doi: 10.1016/j.apmr.2014.11.028. [DOI] [PubMed] [Google Scholar]
- Cekic M, Cutler SM, VanLandingham JW, Stein DG. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32:864–874. doi: 10.1016/j.neurobiolaging.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic M, Johnson SJ, Bhatt VH, Stein DG. Progesterone treatment alters neurotrophin/proneurotrophin balance and receptor expression in rats with traumatic brain injury. Restor Neurol Neurosci. 2012;30:115–126. doi: 10.3233/RNN-2011-0628. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Report to Congress on Traumtic Brain Injury in the United States: Epidemiology and Rehabilitation [WWW Document] 2015 URL https://www.cdc.gov/traumaticbraininjury/pdf/tbi_report_to_congress_epi_and_rehab-a.pdf (accessed 5.7.17)
- Chen G, Shi J, Jin W, Wang L, Xie W, Sun J, Hang C. Progesterone administration modulates TLRs/NF-kappaB signaling pathway in rat brain after cortical contusion. Ann Clin Lab Sci. 2008;38:65–74. [PubMed] [Google Scholar]
- Colantonio A, Harris JE, Ratcliff G, Chase S, Ellis K. Gender differences in self reported long term outcomes following moderate to severe traumatic brain injury. BMC Neurol. 2010;10:102. doi: 10.1186/1471-2377-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KD, Tabaddor K, Hauser WA, S K, Feiner C, Factor PR. The Epidemiology of Head Injury in the Bronx; pp. 79–88. Neuroepidemiology. 1983;2:79–88. doi: 10.1159/000110513. [DOI] [Google Scholar]
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD, Centers for Disease Control Prevention (CDC) Surveillance for traumatic brain injury-related deaths–United States, 1997-2007. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002. 2011;60:1–32. [PubMed] [Google Scholar]
- Covassin T, Schatz P, Swanik CB. Sex differences in neuropsychological function and post-concussion symptoms of concussed collegiate athletes. Neurosurgery. 2007;61:345–350. doi: 10.1227/01.NEU.0000279972.95060.CB. discussion 350-351. [DOI] [PubMed] [Google Scholar]
- Covassin T, Swanik CB, Sachs M, Kendrick Z, Schatz P, Zillmer E, Kaminaris C. Sex differences in baseline neuropsychological function and concussion symptoms of collegiate athletes. Br J Sports Med. 2006;40:923–927. doi: 10.1136/bjsm.2006.029496. discussion 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin T, Swanik CB, Sachs ML. Sex Differences and the Incidence of Concussions Among Collegiate Athletes. J Athl Train. 2003;38:238–244. [PMC free article] [PubMed] [Google Scholar]
- Cuthbert JP, Harrison-Felix C, Corrigan JD, Kreider S, Bell JM, Coronado VG, Whiteneck GG. Epidemiology of adults receiving acute inpatient rehabilitation for a primary diagnosis of traumatic brain injury in the United States. J Head Trauma Rehabil. 2015;30:122–135. doi: 10.1097/HTR.0000000000000012. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Cekic M, Miller DM, Wali B, VanLandingham JW, Stein DG. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J Neurotrauma. 2007;24:1475–1486. doi: 10.1089/neu.2007.0294. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Pettus EH, Hoffman SW, Stein DG. Tapered progesterone withdrawal enhances behavioral and molecular recovery after traumatic brain injury. Exp Neurol. 2005;195:423–429. doi: 10.1016/j.expneurol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Cutler SM, VanLandingham JW, Murphy AZ, Stein DG. Slow-release and injected progesterone treatments enhance acute recovery after traumatic brain injury. Pharmacol Biochem Behav. 2006a;84:420–428. doi: 10.1016/j.pbb.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Vanlandingham JW, Stein DG. Tapered progesterone withdrawal promotes long-term recovery following brain trauma. Exp Neurol. 2006b;200:378–385. doi: 10.1016/j.expneurol.2006.02.137. [DOI] [PubMed] [Google Scholar]
- Davis DP, Douglas DJ, Smith W, Sise MJ, Vilke GM, Holbrook TL, Kennedy F, Eastman AB, Velky T, Hoyt DB. Traumatic brain injury outcomes in pre- and post-menopausal females versus age-matched males. J Neurotrauma. 2006;23:140–148. doi: 10.1089/neu.2006.23.140. [DOI] [PubMed] [Google Scholar]
- Day NL, Floyd CL, D’Alessandro TL, Hubbard WJ, Chaudry IH. 17β-estradiol confers protection after traumatic brain injury in the rat and involves activation of G protein-coupled estrogen receptor 1. J Neurotrauma. 2013;30:1531–1541. doi: 10.1089/neu.2013.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan F, Khaksari M, Abbasloo E, Shahrokhi N. The Effects of Estrogen Receptors’ Antagonist on Brain Edema, Intracranial Pressure and Neurological Outcomes after Traumatic Brain Injury in Rat. Iran Biomed J. 2015;19:165–171. doi: 10.7508/ibj.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick R, Hootman JM, Agel J, Vela L, Marshall SW, Messina R. Descriptive epidemiology of collegiate women’s field hockey injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42:211–220. [PMC free article] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Dougan BK, Horswill MS, Geffen GM. Athletes’ age, sex, and years of education moderate the acute neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc JINS. 2014;20:64–80. doi: 10.1017/S1355617712001464. [DOI] [PubMed] [Google Scholar]
- Durkin MS, Olsen S, Barlow B, Virella A, Connolly ES. The epidemiology of urban pediatric neurological trauma: evaluation of, and implications for, injury prevention programs. Neurosurgery. 1998;42:300–310. doi: 10.1097/00006123-199802000-00052. [DOI] [PubMed] [Google Scholar]
- Duvdevani R, Roof RL, Fülöp Z, Hoffman SW, Stein DG. Blood-brain barrier breakdown and edema formation following frontal cortical contusion: does hormonal status play a role? J Neurotrauma. 1995;12:65–75. doi: 10.1089/neu.1995.12.65. [DOI] [PubMed] [Google Scholar]
- Emerson CS, Headrick JP, Vink R. Estrogen improves biochemical and neurologic outcome following traumatic brain injury in male rats, but not in females. Brain Res. 1993;608:95–100. doi: 10.1016/0006-8993(93)90778-l. [DOI] [PubMed] [Google Scholar]
- Englander J, Bushnik T, Oggins J, Katznelson L. Fatigue after traumatic brain injury: Association with neuroendocrine, sleep, depression and other factors. Brain Inj. 2010;24:1379–1388. doi: 10.3109/02699052.2010.523041. [DOI] [PubMed] [Google Scholar]
- Farace E, Alves WM. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg. 2000;93:539–545. doi: 10.3171/jns.2000.93.4.0539. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ortega JF, Prieto-Palomino MA, Garcia-Caballero M, Galeas-Lopez JL, Quesada-Garcia G, Baguley IJ. Paroxysmal sympathetic hyperactivity after traumatic brain injury: clinical and prognostic implications. J Neurotrauma. 2012;29:1364–1370. doi: 10.1089/neu.2011.2033. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Powell G, Hamilton LW. Septal lesion, sex, and incentive shift effects on open field behavior of rats. Physiol Behav. 1979;22:903–909. doi: 10.1016/0031-9384(79)90335-4. [DOI] [PubMed] [Google Scholar]
- Free KE, Greene AM, Bondi CO, Lajud N, de la Tremblaye PB, Kline AE. Comparable impediment of cognitive function in female and male rats subsequent to daily administration of haloperidol after traumatic brain injury. Exp Neurol. 2017;296:62–68. doi: 10.1016/j.expneurol.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer LJ, Gurka KK, Cross KM, Ingersoll CD, Comstock RD, Saliba SA. Sex differences in concussion symptoms of high school athletes. J Athl Train. 2011;46:76–84. doi: 10.4085/1062-6050-46.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan BK, Lim JHG, Ng IHB. Outcome of moderate and severe traumatic brain injury amongst the elderly in Singapore. Ann Acad Med Singapore. 2004;33:63–67. [PubMed] [Google Scholar]
- Geddes RI, Sribnick EA, Sayeed I, Stein DG. Progesterone treatment shows benefit in a pediatric model of moderate to severe bilateral brain injury. PloS One. 2014;9:e87252. doi: 10.1371/journal.pone.0087252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessel LM, Fields SK, Collins CL, Dick RW, Comstock RD. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42:495–503. [PMC free article] [PubMed] [Google Scholar]
- Gilmer LK, Roberts KN, Scheff SW. Efficacy of progesterone following a moderate unilateral cortical contusion injury. J Neurotrauma. 2008;25:593–602. doi: 10.1089/neu.2007.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: a progesterone dose-response study. Pharmacol Biochem Behav. 2003;76:231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Grossman KJ, Goss CW, Stein DG. Sickness behaviors following medial frontal cortical contusions in male rats. Behav Brain Res. 2011;217:202–208. doi: 10.1016/j.bbr.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman KJ, Stein DG. Does endogenous progesterone promote recovery of chronic sensorimotor deficits following contusion to the forelimb representation of the sensorimotor cortex? Behav Brain Res. 2000;116:141–148. doi: 10.1016/s0166-4328(00)00275-8. [DOI] [PubMed] [Google Scholar]
- Groswasser Z, Cohen M, Keren O. Female TBI patients recover better than males. Brain Inj. 1998;12:805–808. doi: 10.1080/026990598122197. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Cerbón MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression pattern in the rat brain during the estrous cycle. Life Sci. 2000;66:1743–1752. doi: 10.1016/s0024-3205(00)00497-5. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I. Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull. 2002;59:105–109. doi: 10.1016/s0361-9230(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Reyna-Neyra A, Salazar AM, Cerbón MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression in the prepuberal and adult male rat brain. Brain Res Bull. 2001;54:13–17. doi: 10.1016/s0361-9230(00)00410-x. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Villamar-Cruz O, González-Arenas A, Chavira R, Camacho-Arroyo I. Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol. 2003;15:984–990. doi: 10.1046/j.1365-2826.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- Günther M, Plantman S, Davidsson J, Angéria M, Mathiesen T, Risling M. COX-2 regulation and TUNEL-positive cell death differ between genders in the secondary inflammatory response following experimental penetrating focal brain injury in rats. Acta Neurochir (Wien) 2015;157:649–659. doi: 10.1007/s00701-014-2331-2. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sayeed I, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp Neurol. 2006;198:469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Hagihara K, Hirata S, Osada T, Hirai M, Kato J. Distribution of cells containing progesterone receptor mRNA in the female rat di- and telencephalon: an in situ hybridization study. Brain Res Mol Brain Res. 1992;14:239–249. doi: 10.1016/0169-328x(92)90179-f. [DOI] [PubMed] [Google Scholar]
- Hall ED, Gibson TR, Pavel KM. Lack of a gender difference in post-traumatic neurodegeneration in the mouse controlled cortical impact injury model. J Neurotrauma. 2005;22:669–679. doi: 10.1089/neu.2005.22.669. [DOI] [PubMed] [Google Scholar]
- Hanlon LA, Raghupathi R, Huh JW. Differential effects of minocycline on microglial activation and neurodegeneration following closed head injury in the neonate rat. Exp Neurol. 2017;290:1–14. doi: 10.1016/j.expneurol.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart T, Brenner L, Clark AN, Bogner JA, Novack TA, Chervoneva I, Nakase-Richardson R, Arango-Lasprilla JC. Major and minor depression after traumatic brain injury. Arch Phys Med Rehabil. 2011;92:1211–1219. doi: 10.1016/j.apmr.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Harvey LA, Close JCT. Traumatic brain injury in older adults: characteristics, causes and consequences. Injury. 2012;43:1821–1826. doi: 10.1016/j.injury.2012.07.188. [DOI] [PubMed] [Google Scholar]
- He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Hehar H, Yeates K, Kolb B, Esser MJ, Mychasiuk R. Impulsivity and Concussion in Juvenile Rats: Examining Molecular and Structural Aspects of the Frontostriatal Pathway. PloS One. 2015;10:e0139842. doi: 10.1371/journal.pone.0139842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehar H, Yu K, Ma I, Mychasiuk R. Paternal age and diet: The contributions of a father’s experience to susceptibility for post-concussion symptomology. Neuroscience. 2016;332:61–75. doi: 10.1016/j.neuroscience.2016.06.039. [DOI] [PubMed] [Google Scholar]
- Hua F, Reiss JI, Tang H, Wang J, Fowler X, Sayeed I, Stein DG. Progesterone and low-dose vitamin D hormone treatment enhances sparing of memory following traumatic brain injury. Horm Behav. 2012;61:642–651. doi: 10.1016/j.yhbeh.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Wang J, Ishrat T, Wei W, Atif F, Sayeed I, Stein DG. Genomic profile of Toll-like receptor pathways in traumatically brain-injured mice: effect of exogenous progesterone. J Neuroinflammation. 2011;8:42. doi: 10.1186/1742-2094-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkelhoven CWPM, Steyerberg EW, Rampen AJJ, Farace E, Habbema JDF, Marshall LF, Murray GD, Maas AIR. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg. 2003;99:666–673. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Huang TT, Noble LJ. Regional vulnerability after traumatic brain injury: gender differences in mice that overexpress human copper, zinc superoxide dismutase. Exp Neurol. 2001;172:332–341. doi: 10.1006/exnr.2001.7820. [DOI] [PubMed] [Google Scholar]
- Jones NC, Constantin D, Prior MJW, Morris PG, Marsden CA, Murphy S. The neuroprotective effect of progesterone after traumatic brain injury in male mice is independent of both the inflammatory response and growth factor expression. Eur J Neurosci. 2005;21:1547–1554. doi: 10.1111/j.1460-9568.2005.03995.x. [DOI] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Mouri N. The ontogeny of gene expression of progestin receptors in the female rat brain. J Steroid Biochem Mol Biol. 1993;47:173–182. doi: 10.1016/0960-0760(93)90072-5. [DOI] [PubMed] [Google Scholar]
- Kato J, Onouchi T. Progestin receptors in female rat brain and hypophysis in the development from fetal to postnatal stages. Endocrinology. 1983;113:29–36. doi: 10.1210/endo-113-1-29. [DOI] [PubMed] [Google Scholar]
- Kato J, Onouchi T, Takamatsu M. Decreased progestin receptors in the cerebral cortex of hypothyroid postnatal rats. J Steroid Biochem. 1984;20:817–819. [PubMed] [Google Scholar]
- Kerr ZY, Register-Mihalik JK, Kroshus E, Baugh CM, Marshall SW. Motivations Associated With Nondisclosure of Self-Reported Concussions in Former Collegiate Athletes. Am J Sports Med. 2016;44:220–225. doi: 10.1177/0363546515612082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaksari M, Abbasloo E, Dehghan F, Soltani Z, Asadikaram G. The brain cytokine levels are modulated by estrogen following traumatic brain injury: Which estrogen receptor serves as modulator? Int Immunopharmacol. 2015a;28:279–287. doi: 10.1016/j.intimp.2015.05.046. [DOI] [PubMed] [Google Scholar]
- Khaksari M, Hajializadeh Z, Shahrokhi N, Esmaeili-Mahani S. Changes in the gene expression of estrogen receptors involved in the protective effect of estrogen in rat’s trumatic brain injury. Brain Res. 2015b;1618:1–8. doi: 10.1016/j.brainres.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Khaksari M, Soltani Z, Shahrokhi N, Moshtaghi G, Asadikaram G. The role of estrogen and progesterone, administered alone and in combination, in modulating cytokine concentration following traumatic brain injury. Can J Physiol Pharmacol. 2011;89:31–40. doi: 10.1139/y10-103. [DOI] [PubMed] [Google Scholar]
- Kirkness CJ, Burr RL, Mitchell PH, Newell DW. Is there a sex difference in the course following traumatic brain injury? Biol Res Nurs. 2004;5:299–310. doi: 10.1177/1099800404263050. [DOI] [PubMed] [Google Scholar]
- Kraus JF, Peek-Asa C, McArthur D. The independent effect of gender on outcomes following traumatic brain injury: a preliminary investigation. Neurosurg Focus. 2000;8:e5. doi: 10.3171/foc.2000.8.1.156. [DOI] [PubMed] [Google Scholar]
- Kumasaka K, Marks JA, Eisenstadt R, Murcy MA, Samadi D, Li S, Johnson V, Browne KD, Smith DH, Schwab CW, Pascual JL. In vivo leukocyte-mediated brain microcirculatory inflammation: a comparison of osmotherapies and progesterone in severe traumatic brain injury. Am J Surg. 2014;208:961–968. doi: 10.1016/j.amjsurg.2014.08.004. discussion 967-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupina NC, Detloff MR, Bobrowski WF, Snyder BJ, Hall ED. Cytoskeletal protein degradation and neurodegeneration evolves differently in males and females following experimental head injury. Exp Neurol. 2003;180:55–73. doi: 10.1016/s0014-4886(02)00048-1. [DOI] [PubMed] [Google Scholar]
- Ley EJ, Short SS, Liou DZ, Singer MB, Mirocha J, Melo N, Bukur M, Salim A. Gender impacts mortality after traumatic brain injury in teenagers. J Trauma Acute Care Surg. 2013;75:682–686. doi: 10.1097/TA.0b013e31829d024f. [DOI] [PubMed] [Google Scholar]
- Li LZ, Bao YJ, Zhao M. 17beta-estradiol attenuates programmed cell death in cortical pericontusional zone following traumatic brain injury via upregulation of ERalpha and inhibition of caspase-3 activation. Neurochem Int. 2011;58:126–133. doi: 10.1016/j.neuint.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang B, Kan Z, Zhang B, Yang Z, Chen J, Wang D, Wei H, Zhang J, Jiang R. Progesterone increases circulating endothelial progenitor cells and induces neural regeneration after traumatic brain injury in aged rats. J Neurotrauma. 2012;29:343–353. doi: 10.1089/neu.2011.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln AE, Caswell SV, Almquist JL, Dunn RE, Norris JB, Hinton RY. Trends in concussion incidence in high school sports: a prospective 11-year study. Am J Sports Med. 2011;39:958–963. doi: 10.1177/0363546510392326. [DOI] [PubMed] [Google Scholar]
- Liu F, Liao F, Li W, Han Y, Liao D. Progesterone alters Nogo-A, GFAP and GAP-43 expression in a rat model of traumatic brain injury. Mol Med Rep. 2014;9:1225–1231. doi: 10.3892/mmr.2014.1967. [DOI] [PubMed] [Google Scholar]
- Loy R, Milner TA. Sexual dimorphism in extent of axonal sprouting in rat hippocampus. Science. 1980;208:1282–1284. doi: 10.1126/science.7375941. [DOI] [PubMed] [Google Scholar]
- Lu XY, Sun H, Li QY, Lu PS. Progesterone for Traumatic Brain Injury: A Meta-Analysis Review of Randomized Controlled Trials. World Neurosurg. 2016;90:199–210. doi: 10.1016/j.wneu.2016.02.110. [DOI] [PubMed] [Google Scholar]
- Lye TC, Shores EA. Traumatic brain injury as a risk factor for Alzheimer’s disease: a review. Neuropsychol Rev. 2000;10:115–129. doi: 10.1023/a:1009068804787. [DOI] [PubMed] [Google Scholar]
- Ma J, Huang S, Qin S, You C, Zeng Y. Progesterone for acute traumatic brain injury. Cochrane Database Syst Rev. 2016;12:CD008409. doi: 10.1002/14651858.CD008409.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghool F, Khaksari M, Siahposht Khachki A. Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: involvement of female sex steroid hormones. Brain Res. 2013;1497:61–72. doi: 10.1016/j.brainres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Mannix R, Berglass J, Berkner J, Moleus P, Qiu J, Jantzie LL, Meehan WP, Stanley RM, Robinson S. Sex differences in the effect of progesterone after controlled cortical impact in adolescent mice: a preliminary study. J Neurosurg. 2014;121:1337–1341. doi: 10.3171/2014.8.JNS14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell J, Tierney RT, Sitler MR, Swanik KA, Stearne D. Resistance training and head-neck segment dynamic stabilization in male and female collegiate soccer players. J Athl Train. 2005;40:310–319. [PMC free article] [PubMed] [Google Scholar]
- Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747–755. doi: 10.1177/0363546511435626. [DOI] [PubMed] [Google Scholar]
- Mathias JL, Wheaton P. Contribution of brain or biological reserve and cognitive or neural reserve to outcome after TBI: A meta-analysis (prior to 2015) Neurosci Biobehav Rev. 2015;55:573–593. doi: 10.1016/j.neubiorev.2015.06.001. [DOI] [PubMed] [Google Scholar]
- McGlade E, Rogowska J, Yurgelun-Todd D. Sex differences in orbitofrontal connectivity in male and female veterans with TBI. Brain Imaging Behav. 2015;9:535–549. doi: 10.1007/s11682-015-9379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke. 2002;33:1685–1691. doi: 10.1161/01.str.0000016325.54374.93. [DOI] [PubMed] [Google Scholar]
- Mills JD, Bailes JE, Turner RC, Dodson SC, Sakai J, Maroon JC. Anabolic steroids and head injury. Neurosurgery. 2012;70:205–209. doi: 10.1227/NEU.0b013e3182250918. discussion 209-210. [DOI] [PubMed] [Google Scholar]
- Mollayeva T, Colantonio A. Gender, Sex and Traumatic Brain Injury: Transformative Science to Optimize Patient Outcomes. Healthc Q Tor Ont. 2017;20:6–9. doi: 10.12927/hcq.2017.25144. [DOI] [PubMed] [Google Scholar]
- Murray WS. Some effects of ovariectomy during the period of declining reproductive powers in mice. J Exp Med. 1936;63:893–900. doi: 10.1084/jem.63.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushkudiani NA, Engel DC, Steyerberg EW, Butcher I, Lu J, Marmarou A, Slieker F, McHugh GS, Murray GD, Maas AIR. Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:259–269. doi: 10.1089/neu.2006.0028. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Candy S, Ma I, Esser MJ. The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J Neurosci Methods. 2016;257:168–178. doi: 10.1016/j.jneumeth.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Esser MJ. A mild traumatic brain injury (mTBI) induces secondary attention-deficit hyperactivity disorder-like symptomology in young rats. Behav Brain Res. 2015;286:285–292. doi: 10.1016/j.bbr.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Farran A, Esser MJ. Mean girls: sex differences in the effects of mild traumatic brain injury on the social dynamics of juvenile rat play behaviour. Behav Brain Res. 2014;259:284–291. doi: 10.1016/j.bbr.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Namjoshi DR, Cheng WH, Carr M, Martens KM, Zareyan S, Wilkinson A, McInnes KA, Cripton PA, Wellington CL. Chronic Exposure to Androgenic-Anabolic Steroids Exacerbates Axonal Injury and Microgliosis in the CHIMERA Mouse Model of Repetitive Concussion. PloS One. 2016;11:e0146540. doi: 10.1371/journal.pone.0146540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Veterans Analysis and Statistics. Profile of Veterans: 2014 [WWW Document] 2016 URL https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2014.pdf (accessed 1.10.18)
- Nell V, Brown DS. Epidemiology of traumatic brain injury in Johannesburg–II. Morbidity, mortality and etiology. Soc Sci Med 1982. 1991;33:289–296. doi: 10.1016/0277-9536(91)90363-h. [DOI] [PubMed] [Google Scholar]
- Niemeier JP, Marwitz JH, Lesher K, Walker WC, Bushnik T. Gender differences in executive functions following traumatic brain injury. Neuropsychol Rehabil. 2007;17:293–313. doi: 10.1080/09602010600814729. [DOI] [PubMed] [Google Scholar]
- Niemeier JP, Marwitz JH, Walker WC, Davis LC, Bushnik T, Ripley DL, Ketchum JM. Are there cognitive and neurobehavioural correlates of hormonal neuroprotection for women after TBI? Neuropsychol Rehabil. 2013;23:363–382. doi: 10.1080/09602011.2012.761944. [DOI] [PubMed] [Google Scholar]
- Nudi ET, Jacqmain J, Dubbs K, Geeck K, Salois G, Searles MA, Smith JS. Combining Enriched Environment, Progesterone, and Embryonic Neural Stem Cell Therapy Improves Recovery after Brain Injury. J Neurotrauma. 2015;32:1117–1129. doi: 10.1089/neu.2014.3618. [DOI] [PubMed] [Google Scholar]
- O’Connor CA, Cernak I, Johnson F, Vink R. Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp Neurol. 2007;205:145–153. doi: 10.1016/j.expneurol.2007.01.034. [DOI] [PubMed] [Google Scholar]
- O’Connor CA, Cernak I, Vink R. The temporal profile of edema formation differs between male and female rats following diffuse traumatic brain injury. Acta Neurochir Suppl. 2006;96:121–124. doi: 10.1007/3-211-30714-1_27. [DOI] [PubMed] [Google Scholar]
- O’Connor CA, Cernak I, Vink R. Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 2005;1062:171–174. doi: 10.1016/j.brainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- O’Connor CA, Cernak I, Vink R. Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats. J Neurotrauma. 2003;20:533–541. doi: 10.1089/089771503767168465. [DOI] [PubMed] [Google Scholar]
- Pan DS, Liu WG, Yang XF, Cao F. Inhibitory effect of progesterone on inflammatory factors after experimental traumatic brain injury. Biomed Environ Sci BES. 2007;20:432–438. [PubMed] [Google Scholar]
- Pascual JL, Murcy MA, Li S, Gong W, Eisenstadt R, Kumasaka K, Sims C, Smith DH, Browne K, Allen S, Baren J. Neuroprotective effects of progesterone in traumatic brain injury: blunted in vivo neutrophil activation at the blood-brain barrier. Am J Surg. 2013;206:840–845. doi: 10.1016/j.amjsurg.2013.07.016. discussion 845-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervin F, Chen WW. Effect of inter-species, gender, and breeding on the mechanical behavior of brain tissue. NeuroImage. 2011;54(Suppl 1):S98–102. doi: 10.1016/j.neuroimage.2010.03.077. [DOI] [PubMed] [Google Scholar]
- Peterson TC, Hoane MR, McConomy KS, Farin FM, Bammler TK, MacDonald JW, Kantor ED, Anderson GD. A Combination Therapy of Nicotinamide and Progesterone Improves Functional Recovery following Traumatic Brain Injury. J Neurotrauma. 2015;32:765–779. doi: 10.1089/neu.2014.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Joels M. Hormones, Brain and behavior - 3rd Edition. (3rd) 2016 [Google Scholar]
- Powell JW, Barber-Foss KD. Traumatic brain injury in high school athletes. JAMA. 1999;282:958–963. doi: 10.1001/jama.282.10.958. [DOI] [PubMed] [Google Scholar]
- Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R. 2009;1:245–253. doi: 10.1016/j.pmrj.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Estes WA. Clinical Potential of Neurosteroids for CNS Disorders. Trends Pharmacol Sci. 2016;37:543–561. doi: 10.1016/j.tips.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner C, Hummelsheim H, Kopczak A, Steube D, Schneider HJ, Schneider M, Kreitschmann-Andermahr I, Jordan M, Uhl E, Stalla GK. The influence of gender on the injury severity, course and outcome of traumatic brain injury. Brain Inj. 2012;26:1360–1371. doi: 10.3109/02699052.2012.667592. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol. 2006;197:235–243. doi: 10.1016/j.expneurol.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Saraswati M. Progesterone protects mitochondrial function in a rat model of pediatric traumatic brain injury. J Bioenerg Biomembr. 2015;47:43–51. doi: 10.1007/s10863-014-9585-5. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129:64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Heyburn JW, Stein DG. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp Neurol. 1996;138:246–251. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993;607:333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000a;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J Neurotrauma. 2000b;17:1155–1169. doi: 10.1089/neu.2000.17.1155. [DOI] [PubMed] [Google Scholar]
- Russell KL, Kutchko KM, Fowler SC, Berman NEJ, Levant B. Sensorimotor behavioral tests for use in a juvenile rat model of traumatic brain injury: assessment of sex differences. J Neurosci Methods. 2011;199:214–222. doi: 10.1016/j.jneumeth.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban KL, Smith BM, Collins EG, Pape TLB. Sex differences in perceived life satisfaction and functional status one year after severe traumatic brain injury. J Womens Health 2002. 2011;20:179–186. doi: 10.1089/jwh.2010.2334. [DOI] [PubMed] [Google Scholar]
- Sarkaki AR, Khaksari Haddad M, Soltani Z, Shahrokhi N, Mahmoodi M. Time- and dose-dependent neuroprotective effects of sex steroid hormones on inflammatory cytokines after a traumatic brain injury. J Neurotrauma. 2013;30:47–54. doi: 10.1089/neu.2010.1686. [DOI] [PubMed] [Google Scholar]
- Schmidt AT, Hanten GR, Li X, Vasquez AC, Wilde EA, Chapman SB, Levin HS. Decision making after pediatric traumatic brain injury: trajectory of recovery and relationship to age and gender. Int J Dev Neurosci Off J Int Soc Dev Neurosci. 2012;30:225–230. doi: 10.1016/j.ijdevneu.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopp LH, Shigaki CL, Johnstone B, Kirkpatrick HA. Gender Differences in Cognitive and Emotional Adjustment to Traumatic Brain Injury. J Clin Psychol Med Settings. 2001;8:181–188. doi: 10.1023/A:1011369620254. [DOI] [Google Scholar]
- Schulz MR, Marshall SW, Mueller FO, Yang J, Weaver NL, Kalsbeek WD, Bowling JM. Incidence and risk factors for concussion in high school athletes, North Carolina, 1996-1999. Am J Epidemiol. 2004;160:937–944. doi: 10.1093/aje/kwh304. [DOI] [PubMed] [Google Scholar]
- Scott C, McKinlay A, McLellan T, Britt E, Grace R, MacFarlane M. A comparison of adult outcomes for males compared to females following pediatric traumatic brain injury. Neuropsychology. 2015;29:501–508. doi: 10.1037/neu0000074. [DOI] [PubMed] [Google Scholar]
- Semple BD, Dixit S, Shultz SR, Boon WC, O’Brien TJ. Sex-dependent changes in neuronal morphology and psychosocial behaviors after pediatric brain injury. Behav Brain Res. 2017;319:48–62. doi: 10.1016/j.bbr.2016.10.045. [DOI] [PubMed] [Google Scholar]
- Shahrokhi N, Haddad MK, Joukar S, Shabani M, Keshavarzi Z, Shahozehi B. Neuroprotective antioxidant effect of sex steroid hormones in traumatic brain injury. Pak J Pharm Sci. 2012;25:219–225. [PubMed] [Google Scholar]
- Shahrokhi N, Khaksari M, Soltani Z, Mahmoodi M, Nakhaee N. Effect of sex steroid hormones on brain edema, intracranial pressure, and neurologic outcomes after traumatic brain injury. Can J Physiol Pharmacol. 2010;88:414–421. doi: 10.1139/y09-126. [DOI] [PubMed] [Google Scholar]
- Shakeri M, Boustani MR, Pak N, Panahi F, Salehpour F, Lotfinia I, Meshkini A, Daghighi S, vahedi P, Khani M, Taghiloo D. Effect of progesterone administration on prognosis of patients with diffuse axonal injury due to severe head trauma. Clin Neurol Neurosurg. 2013;115:2019–2022. doi: 10.1016/j.clineuro.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- Si D, Wang H, Wang Q, Zhang C, Sun J, Wang Z, Zhang Z, Zhang Y. Progesterone treatment improves cognitive outcome following experimental traumatic brain injury in rats. Neurosci Lett. 2013;553:18–23. doi: 10.1016/j.neulet.2013.07.052. [DOI] [PubMed] [Google Scholar]
- Si D, Yang P, Jiang R, Zhou H, Wang H, Zhang Y. Improved cognitive outcome after progesterone administration is associated with protecting hippocampal neurons from secondary damage studied in vitro and in vivo. Behav Brain Res. 2014;264:135–142. doi: 10.1016/j.bbr.2014.01.049. [DOI] [PubMed] [Google Scholar]
- Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, Nelson NR, Stocchetti N, SYNAPSE Trial Investigators A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 2014;371:2467–2476. doi: 10.1056/NEJMoa1411090. [DOI] [PubMed] [Google Scholar]
- Slewa-Younan S, van den Berg S, Baguley IJ, Nott M, Cameron ID. Towards an understanding of sex differences in functional outcome following moderate to severe traumatic brain injury: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:1197–1201. doi: 10.1136/jnnp.2008.147983. [DOI] [PubMed] [Google Scholar]
- Soustiel JF, Palzur E, Nevo O, Thaler I, Vlodavsky E. Neuroprotective anti-apoptosis effect of estrogens in traumatic brain injury. J Neurotrauma. 2005;22:345–352. doi: 10.1089/neu.2005.22.345. [DOI] [PubMed] [Google Scholar]
- St Ivany A, Schminkey D. Intimate Partner Violence and Traumatic Brain Injury: State of the Science and Next Steps. Fam Community Health. 2016;39:129–137. doi: 10.1097/FCH.0000000000000094. [DOI] [PubMed] [Google Scholar]
- Statler KD, Kochanek PM, Dixon CE, Alexander HL, Warner DS, Clark RS, Wisniewski SR, Graham SH, Jenkins LW, Marion DW, Safar PJ. Isoflurane improves long-term neurologic outcome versus fentanyl after traumatic brain injury in rats. J Neurotrauma. 2000;17:1179–1189. doi: 10.1089/neu.2000.17.1179. [DOI] [PubMed] [Google Scholar]
- Steegmann AT. Dr. Harlow’s famous case: the “impossible” accident of Phineas. P Gage Surgery. 1962;52:952–958. [PubMed] [Google Scholar]
- Stein DG. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015;29:1259–1272. doi: 10.3109/02699052.2015.1065344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2008;57:386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG. Functional recovery after lesions of the nervous system. V. Neural plasticity and behavioral recovery in the central nervous system. Sequential versus single lesions and some other variables contributing to the recovery of function in the rat. Neurosci Res Program Bull. 1974;12:260–268. [PubMed] [Google Scholar]
- Stulemeijer M, van der Werf S, Borm GF, Vos PE. Early prediction of favourable recovery 6 months after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:936–942. doi: 10.1136/jnnp.2007.131250. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bramlett HM, Dietrich WD. The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184:1017–1026. doi: 10.1016/S0014-4886(03)00389-3. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bramlett HM, Ruenes G, Dietrich WD. The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J Neurotrauma. 2004;21:842–853. doi: 10.1089/0897715041526186. [DOI] [PubMed] [Google Scholar]
- Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 2006;148:255–268. doi: 10.1007/s00701-005-0651-y. discussion 268. [DOI] [PubMed] [Google Scholar]
- Tang H, Hua F, Wang J, Sayeed I, Wang X, Chen Z, Yousuf S, Atif F, Stein DG. Progesterone and vitamin D: Improvement after traumatic brain injury in middle-aged rats. Horm Behav. 2013;64:527–538. doi: 10.1016/j.yhbeh.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney RT, Higgins M, Caswell SV, Brady J, McHardy K, Driban JB, Darvish K. Sex differences in head acceleration during heading while wearing soccer headgear. J Athl Train. 2008;43:578–584. doi: 10.4085/1062-6050-43.6.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolppanen AM, Taipale H, Hartikainen S. Head or brain injuries and Alzheimer’s disease: A nested case-control register study. Alzheimers Dement J Alzheimers Assoc. 2017;13:1371–1379. doi: 10.1016/j.jalz.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Tucker LB, Fu AH, McCabe JT. Performance of Male and Female C57BL/6J Mice on Motor and Cognitive Tasks Commonly Used in Pre-Clinical Traumatic Brain Injury Research. J Neurotrauma. 2016;33:880–894. doi: 10.1089/neu.2015.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal N, Baykara B, Kiray M, Cetin F, Aksu I, Dayi A, Gurpinar T, Ozdemir D, Arda MN. Combined treatment with progesterone and magnesium sulfate positively affects traumatic brain injury in immature rats. Turk Neurosurg. 2013;23:129–137. doi: 10.5137/1019-5149.JTN.5582-11.1. [DOI] [PubMed] [Google Scholar]
- VanLandingham JW, Cekic M, Cutler S, Hoffman SW, Stein DG. Neurosteroids reduce inflammation after TBI through CD55 induction. Neurosci Lett. 2007;425:94–98. doi: 10.1016/j.neulet.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanLandingham JW, Cutler SM, Virmani S, Hoffman SW, Covey DF, Krishnan K, Hammes SR, Jamnongjit M, Stein DG. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology. 2006;51:1078–1085. doi: 10.1016/j.neuropharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Velosky AG, Tucker LB, Fu AH, Liu J, McCabe JT. Cognitive performance of male and female C57BL/6J mice after repetitive concussive brain injuries. Behav Brain Res. 2017;324:115–124. doi: 10.1016/j.bbr.2017.02.017. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Bayir H, Ren D, Puccio A, Zafonte RD, Kochanek PM. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J Neurotrauma. 2004a;21:125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Ren D, Willard LA, Wenger MK, Zafonte RD, Dixon CE. Gender associations with chronic methylphenidate treatment and behavioral performance following experimental traumatic brain injury. Behav Brain Res. 2007;181:200–209. doi: 10.1016/j.bbr.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Sokoloski J, Zafonte RD, Capulong E, Dixon CE. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci Lett. 2002;334:165–168. doi: 10.1016/s0304-3940(02)01103-5. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Sasser HC, Hammond FM, Wiercisiewski D, Alexander J. Intentional traumatic brain injury: epidemiology, risk factors, and associations with injury severity and mortality. J Trauma. 2000;49:404–410. doi: 10.1097/00005373-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Willard LA, Kline AE, Wenger MK, Bolinger BD, Ren D, Zafonte RD, Dixon CE. Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 2004b;998:113–121. doi: 10.1016/j.brainres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Pfau JL, De Vries GJ, Merchenthaler IJ. Sex differences in progesterone receptor immunoreactivity in neonatal mouse brain depend on estrogen receptor alpha expression. J Neurobiol. 2001;47:176–182. doi: 10.1002/neu.1025. [DOI] [PubMed] [Google Scholar]
- Wali B, Sayeed I, Guthrie DB, Natchus MG, Turan N, Liotta DC, Stein DG. Evaluating the neurotherapeutic potential of a water-soluble progesterone analog after traumatic brain injury in rats. Neuropharmacology. 2016;109:148–158. doi: 10.1016/j.neuropharm.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Wali B, Sayeed I, Stein DG. Improved behavioral outcomes after progesterone administration in aged male rats with traumatic brain injury. Restor Neurol Neurosci. 2011;29:61–71. doi: 10.3233/RNN-2011-0579. [DOI] [PubMed] [Google Scholar]
- Webster SJ, Van Eldik LJ, Watterson DM, Bachstetter AD. Closed head injury in an age-related Alzheimer mouse model leads to an altered neuroinflammatory response and persistent cognitive impairment. J Neurosci Off J Soc Neurosci. 2015;35:6554–6569. doi: 10.1523/JNEUROSCI.0291-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Women’s Sports Foundation. The Women’s Sports Foundation Report: Addressing the Health and Physical Activity Needs of Girls in the Boston Metropolitan Area [WWW Document] 2007 URL https://www.womenssportsfoundation.org/wp-content/uploads/2016/08/boston_metro_full.pdf (accessed 1.10.18)
- Wright DW, Bauer ME, Hoffman SW, Stein DG. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J Neurotrauma. 2001;18:901–909. doi: 10.1089/089771501750451820. [DOI] [PubMed] [Google Scholar]
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. 402.e1–2. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M, Goldstein FC, Caveney AF, Howlett-Smith H, Bengelink EM, Manley GT, Merck LH, Janis LS, Barsan WG, NETT Investigators Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014;371:2457–2466. doi: 10.1056/NEJMoa1404304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Group for the Women’s Health Initiative Investigators. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Lu D, Qu C, Goussev A, Schallert T, Chopp M. Role of gender in outcome after traumatic brain injury and therapeutic effect of erythropoietin in mice. Brain Res. 2007;1185:301–312. doi: 10.1016/j.brainres.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa GR, Lengkeek C, Salberg S, Spanswick SC, Mychasiuk R. Behavioral and pathophysiological outcomes associated with caffeine consumption and repetitive mild traumatic brain injury (RmTBI) in adolescent rats. PloS One. 2017;12:e0187218. doi: 10.1371/journal.pone.0187218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Fang, Ding Jun, Chen Hao, Guo Yan, Wang Gan, Gao Wen-Wei, Chen Shi-Wen, Tain Heng-Li. Predicting outcomes after traumatic brain injury: The development and validation of prognostic models based on admission characteristics. J Trauma Acute Care Surg. 2012;73:137–145. doi: 10.1097/TA.0b013e31824b00ac. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


