Abstract
Gamma oscillations (~25-100 Hz) are believed to play a role in cognition. Accordingly, aberrant gamma oscillations have been observed in several cognitive disorders, including Alzheimer’s disease and Fragile X syndrome. Here, we review how recent results showing abnormal gamma rhythms in Alzheimer’s disease and Fragile X syndrome help reveal links between cellular disturbances and cognitive impairments. We also discuss how gamma results from rodent models of Alzheimer’s disease and Fragile X syndrome may provide insights about unique functions of distinct slow (~25-50 Hz) and fast gamma (~55-100 Hz) subtypes. Finally, we consider studies employing brain stimulation paradigms in Alzheimer’s disease and discuss how such studies may reveal causal relationships between gamma impairments and memory disturbances.
Introduction
Gamma oscillations are rhythmic fluctuations in local field potentials (LFPs) that span a broad range of frequencies (~25-100 Hz). Gamma oscillations are prominent across multiple brain regions including the hippocampus, where they are believed to play a role in attentional selection and memory operations [1]. Accumulating evidence suggests that the broad range of frequencies of oscillations that are described as gamma rhythms may actually be two functionally distinct rhythms, slow (~25-50 Hz) and fast (~55-100 Hz) gamma [2,3]. These different frequencies of gamma rhythms are thought to be locally generated by circuits involving some distinct and some overlapping classes of GABAergic interneurons [3]. Although slow and fast gamma are thought to be locally generated, gamma oscillators exhibiting similar frequencies in different brain regions can become coupled by anatomical connections between the regions.
Fast gamma rhythms in the hippocampus are coupled with fast gamma inputs from the medial entorhinal cortex [2], an area that processes current sensory information. Thus, it has been proposed that fast gamma promotes the transmission of current sensory information to the hippocampus during new memory encoding [3]. In agreement with this, hippocampal fast gamma dominates during exploration of novel object-place pairings [4], and hippocampal place cells represent recent locations and current trajectories during periods of fast gamma [5,6]. Moreover, fast gamma has been shown to be dominant, relative to slow gamma, when mice attend to external landmarks to navigate to a goal location [7].
Conversely, slow gamma rhythms in hippocampal subfield CA1 are coupled with inputs from CA3 [2], a neighboring hippocampal subfield from which stored memories are thought to be retrieved [8–10]. In line with this proposed memory retrieval role of CA3, slow gamma has been hypothesized to promote memory retrieval by facilitating CA3 inputs to CA1 [3]. This hypothesis remains controversial, in part due to reports of enhanced slow gamma measures during exploration of novel objects [11] and places [12]. Still, other evidence supports a memory retrieval role for slow gamma. In familiar environments, hippocampal place cells were found to predict upcoming locations and trajectories when slow gamma was present in CA1 [5,6]. Another study observed increases in the magnitude of slow gamma during correct performance of an associative memory task at a time when cue-evoked memory retrieval would be expected to occur [13]. Also, slow gamma power and coherence between CA3 and CA1 increases during sharp wave-ripples (SWRs) [5,14], high frequency (~150-250 Hz) events that arise in the hippocampus during inactive behaviors (e.g., waking rest, slow wave sleep, eating, grooming [15]). These results may support a memory retrieval function for slow gamma, given that sharp wave-ripples are thought to play a key role in memory retrieval [14–16].
Given the evidence suggesting that gamma rhythms are important for hippocampal memory processing, it is perhaps not surprising that several brain disorders that involve memory impairments are associated with disturbances in gamma rhythms. However, the question of whether gamma abnormalities are responsible for cognitive impairments, or instead are just a by-product of cellular and molecular disturbances that produce cognitive symptoms, remains unanswered. In this review, we will discuss recent evidence showing impaired gamma oscillations in two major disorders that affect memory: 1) Alzheimer’s disease (AD); and 2) fragile X syndrome (FXS). We discuss how reported impairments in gamma rhythms may relate to both cellular disturbances and memory impairments associated with these disorders. We also discuss novel therapeutic strategies that attempt to alleviate cognitive deficits in these disorders by restoring normal gamma activity. Experiments incorporating such strategies are expected to provide answers to the question of whether cognitive disturbances in brain disorders are explained by aberrant gamma rhythms.
AD
AD is a progressive neurodegenerative disease that exhibits characteristic cellular and molecular pathologies in the brain, including the accumulation of amyloid-β (Aβ) -containing amyloid deposits in the extracellular space and formation of tau neurofibrillary tangles inside neurons [17]. The entorhinal cortex and hippocampus, key brain regions for spatial and episodic memory, are particularly vulnerable to cellular pathologies that characterize AD [18]. Accordingly, early cognitive symptoms of AD involve episodic and spatial memory impairments [19]. However, it remains unclear how cellular and molecular disturbances in AD affect coordinated activity across the distributed networks of neurons that subserve memory operations.
Recently, a novel hypothesis has been proposed to explain memory impairments in AD, namely that patients with AD are able to encode memories but are unable to later retrieve these memories [20]. In accordance with this hypothesis, and the purported role of slow gamma in memory retrieval described above, disruptions in slow gamma rhythms have been observed in several rodent models of AD. We recently reported reductions in slow gamma power in CA1 of 3×Tg mice navigating a familiar circular track [21]. Also, CA1 place cell representations of space were unstable, and slow gamma coordination of CA1 place cell firing was decreased [21]. It is possible that slow gamma impairments in these mice caused incomplete retrieval of stored spatial information from CA3 to CA1. Deficits in hippocampal slow gamma power and concomitant spatial memory impairments have also been observed in a mouse model of tau pathology [22]. However, in this study, reduced power was observed at fast gamma frequencies also, making it difficult to identify memory impairments selectively associated with slow gamma rhythms.
Decreased SWR-associated slow gamma has also been observed in multiple AD mouse models. A series of studies using mice that had undergone targeted replacement of endogenous mouse ApoE with the AD-linked human ApoE4 gene (ApoE4 KI mice) showed that manipulations that alleviated slow gamma impairments in ApoE4 KI mice rescued learning and memory deficits [23,24]. Specifically, elimination of ApoE4 in GABAergic interneurons rescued SWR-associated slow gamma and abolished memory impairments in ApoE4 KI mice [23,24], highlighting a role for interneuron abnormalities in slow gamma disturbances and linking disturbed slow gamma to memory impairments in AD. Also, Iaccarino et al. [25] observed reduced SWR-associated slow gamma in the 5×FAD mouse model of AD [25], a line with high levels of Aβ accumulation from an early age [26]. Furthermore, they demonstrated that rescuing slow gamma rhythms alleviated AD pathology. Specifically, optogenetic excitation of hippocampal fast-spiking parvalbumin-positive interneurons at slow gamma frequency (i.e., 40 Hz) attenuated Aβ production and promoted microglial engulfment of Aβ [25].
The results of these studies suggest that lessening slow gamma disturbances may be a promising new strategy for treatment of memory impairments in AD. The studies discussed above demonstrate converging mechanisms in different models of AD, one modeling late-onset AD (ApoE4 KI mice) and others modeling familial AD (3×Tg; 5×AD mice). Both ApoE4 and Aβ are known to disrupt the excitatory/inhibitory (E/I) balance of neuronal networks by interfering with GABAergic transmission [27–29]. Moreover, memory impairments in both ApoE4 and Aβ AD mouse models can be attenuated by restoring interneuron function [24,30,31]. Thus, it is conceivable that treatments designed to alleviate disturbances in inhibitory transmission will reinstate healthy patterns of slow gamma rhythms, and lessen memory impairments, in AD.
FXS
FXS is a prevalent inherited intellectual disability [32]. FXS is caused by mutation of the FMR1 gene, resulting in reduced production of fragile X mental retardation protein, which is linked to alterations in synaptic development and function, and disrupted E/I balance across multiple brain regions [33,34]. Individuals with FXS commonly exhibit attention deficits and hypersensitivity to sensory input [35]. indicating that FXS involves disturbances in selective attention. Selective attention, the process of filtering salient stimuli from irrelevant stimuli, is thought to involve gamma rhythmic coordination of cells responding to salient stimuli [36]. Therefore, a plausible hypothesis is that disturbances in gamma activity underlie some of the behavioral disturbances observed in FXS. Indeed, recent electroencephalography recordings from FXS patients suggest that gamma activity in auditory cortex is less entrained by auditory stimuli, compared to controls [37]. Furthermore, these electrophysiological aberrations accompanied parental reports of hypersensitivity and behavioral deficits. In accord with these findings in humans, a study employing a rat model of FXS (i.e., FMR1 knockout rats) found that FXS rats’ visual cortex failed to show the switch from an elevated gamma state to a reduced gamma state that normally occurs during transitions from active sensing to rest [38]. Such results may indicate an inability to “ignore” unattended stimuli.
Attentional selection is important for proper memory formation. Thus, one possibility is that attentional disturbances caused by gamma abnormalities in sensory cortices explain memory problems in FXS. However, recent studies suggest that hippocampal gamma rhythm abnormalities may also relate to memory disturbances in FXS. In one recent study, FMR1 KO mice showed impaired performance on a shock-zone avoidance discrimination task when the location of the shock-zone was altered, a phase of the task requiring mice to suppress previous memories [39]. A subsequent study by the same group investigated slow and fast gamma activity in the same shock-zone avoidance task and found that the ratio of slow to fast gamma in CA1 of control mice was increased prior to successful avoidance of the shock-zone [40]. Furthermore, the slow to fast gamma ratio in control mice was diminished during trials in which the shock-zone location was altered; however, a similar attenuation in the slow to fast gamma ratio was not observed in FMR1 KO mice [40]. If slow gamma promotes the retrieval of stored memories, as has been proposed [2,14]. a weakening of slow gamma would be expected to occur when retrieval of memories of previously learned locations is suppressed. In FMR1 KO mice, abnormal domination of hippocampal network activity by slow gamma rhythms may also interfere with the transmission of current sensory information from the superficial layers of the medial entorhinal cortex to CA1 by fast gamma. This could perhaps result in persistent recall of the old shock zone, rather than encoding of a new shock zone.
What mechanisms underlie abnormally dominant slow gamma in FXS? Hippocampal slice studies may provide clues. FMR1 KO mice exhibit excessive learning-dependent Schaffer collateral long-term potentiation and synaptic transmission [41]. as well as a lower threshold for induction of long-term potentiation (LTP) at Schaffer collateral synapses [42]. In contrast, LTP of temporoammonic inputs to CA1 pyramidal neurons is reportedly impaired in FXS mice (G. Ordemann and D.H. Brager, abstract in Soc Neurosci Abstracts 2017, 118.18). These different LTP effects in FMR1 KO animals may be explained by elevated h-currents in FXS [43]. considering that h-currents are enhanced in the distal apical dendrites [44] where temporoammonic synapses are found. These differential effects on LTP for CA3 and entorhinal inputs in FXS may provide CA3 inputs to CA1 with an advantage and thereby promote slow gamma activity in CA1. However, in one report, CA1 networks in FMR1 KO mice were less synchronized by slow gamma than in control mice [41]. These results are seemingly at odds with those discussed above, which found abnormally dominant slow gamma in CA1 of FMR1 KO mice [40]. A possible explanation for this seeming discrepancy is that slow gamma in FMR1 KO mice was only shown to abnormally dominate during periods of high cognitive demand when the shock-zone location was moved. In the study by Talbot and colleagues [41]. the shock-zone location remained constant. In any event, the nature of slow gamma abnormalities in FMR1 KO animals, and their potential contributions to behavioral disturbances, deserve further attention.
Applying brain stimulation methods to unravel the contribution of gamma rhythms to disease
Deep brain stimulation (DBS) can be used to stimulate specific brain regions at particular frequencies. Thus, DBS techniques may provide an opportunity to probe the role of gamma rhythm disturbances in memory disorders and to develop targeted therapeutic strategies. With regard to DBS as a therapy for AD, promising results have been obtained using high frequency (130 Hz) stimulation of the entorhinal cortex in AD mouse models [45,46]. DBS has also been tested in human AD patients with mixed results [47,48]. However, most DBS studies in AD patients thus far have used open loop, high frequency (e.g., 130 Hz) stimulation of pathways in the entorhinal-hippocampal network [48]. Such stimulation may not facilitate rhythmic coordination of cells but may instead improve cognition through other means, such as by reducing inflammation or increasing synaptic proteins [48]. More targeted approaches may be needed to affect gamma rhythmic coordination.
The above-discussed slow and fast gamma findings suggest that stimulation of different entorhinal-hippocampal pathways at different frequencies may be necessary to selectively affect memory encoding or memory retrieval at different times. Both the pathway that is stimulated and the frequency at which stimulation is delivered are likely important. Perhaps theta-modulated bursts of fast gamma stimulation, delivered to the perforant path at times when new information is presented, can enhance memory encoding. In line with this idea, theta burst microstimulation of the right entorhinal cortex during memory encoding improved memory in a group of epilepsy patients [49]. Conversely, slow gamma stimulation of intrahippocampal pathways may be better suited for memory retrieval. In agreement with this hypothesis, slow gamma (50 Hz) stimulation of the human hippocampus or entorhinal cortex during memory encoding has been shown to impair, not improve, memory [50]. With regard to the efficacy of different frequencies of stimulation, the relatively long period of a slow gamma cycle (i.e., ~25-40 ms) may be optimal for memory retrieval because it allows sufficient time for cued retrieval of previously learned sequences of information on a compressed time scale [6]. On the other hand, the short period of a fast gamma cycle (~10 ms) may preclude retrieval of previously learned sequences. Rapidly recurring inhibitory events during fast gamma may instead allow the hippocampal network to continuously update its responses based on current sensory inputs and thereby encode ongoing experiences in real-time [6].
Still, it remains unclear whether stimulation patterns designed to restore healthy gamma rhythms in the entorhinal-hippocampal network will be sufficient to alleviate cognitive impairments in AD and other brain disorders. Slow and fast gamma rhythms may reflect a general brain mechanism for routing different streams of information, considering that analogous low and high frequency rhythms have been reported in other brain networks during top-down and bottom-up processing [51,52]. Thus, it is possible that slow and fast gamma modes in the entorhinal-hippocampal network, and other brain networks, are regulated by ascending inputs from lower brain nuclei. If so, it will be necessary to understand how targeted stimulation of entorhinal-hippocampal pathways interacts with ongoing modulation by inputs from the lower brain.
Much work remains to be done to test the above-discussed hypotheses using targeted stimulation protocols in animal models of AD and other brain disorders. DBS studies in human disorders will then provide the ultimate test of whether gamma rhythmic disturbances cause cognitive impairments or are a by-product of cellular disturbances. It is exciting to imagine that such studies will lead to the development of novel therapies, and also identify reliable gamma rhythm biomarkers, for memory disorders.
Figure 1.
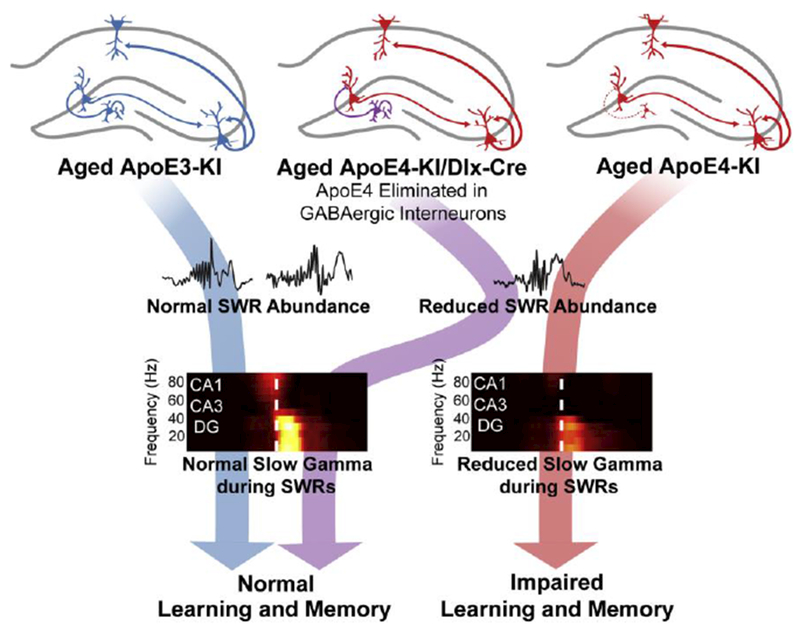
Aged mice expressing the non-AD related ApoE3 allele show normal SWR abundance and SWR-associated slow gamma, and perform well in memory tasks. Conversely, aged mice expressing the AD-related ApoE4 allele have reduced SWR abundance, decreased SWR-associated slow gamma, and impaired learning and memory. Importantly, when the ApoE4 mutation is eliminated from GABAergic interneurons, SWR-associated slow gamma deficits are alleviated, and learning and memory impairments do not develop. This indicates that SWR-associated slow gamma is crucial for normal learning and memory in this aged AD model. Reproduced with permission from [23].
Acknowledgements
Funding was provided by grant 1R01MH102450-01A1 from NIMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colgin LL, Moser El: Gamma oscillations in the hippocampus. Physiology (Bethesda) 2010, 25:319–329. [DOI] [PubMed] [Google Scholar]
- 2.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser El: Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 2009, 462:353–357. [DOI] [PubMed] [Google Scholar]
- 3.Colgin LL: Rhythms of the hippocampal network. Nat Rev Neurosci 2016, 17:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng C, Bieri KW, Hwaun E, Colgin LL: Fast Gamma Rhythms in the Hippocampus Promote Encoding of Novel Object-Place Pairings. eNeuro 2016, 3:ENEURO.0001–0016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieri KW, Bobbitt KN, Colgin LL: Slow and fast gamma rhythms coordinate different spatial coding modes in hippocampal place cells. Neuron 2014, 82:670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng C, Bieri KW, Hsiao YT, Colgin LL: Spatial Sequence Coding Differs during Slow and Fast Gamma Rhythms in the Hippocampus. Neuron 2016, 89:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study investigated sequences of place cells firing during distinct periods of slow and fast gamma rhythms nested within theta cycles. Place cell sequences tended to represent upcoming locations ahead of the animal in a temporally compressed manner during slow gamma rhythms. Fast gamma-associated place cell sequences tended to track an animal’s actual location in real time. These observations provide support for distinct roles of slow and fast gamma in hippocampal function, with slow gamma rhythms possibly promoting retrieval of previously stored representations of upcoming locations and fast gamma potentially promoting the encoding of ongoing experiences.
- 7.Cabral HO, Vinck M, Fouquet C, Pennartz CM, Rondi-Reig L, Battaglia FP: Oscillatory dynamics and place field maps reflect hippocampal ensemble processing of sequence and place memory under NMDA receptor control. Neuron 2014, 81:402–415. [DOI] [PubMed] [Google Scholar]
- 8.Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, et al.: Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 2002, 297:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steffenach HA, Sloviter RS, Moser El, Moser MB: Impaired retention of spatial memory after transection of longitudinally oriented axons of hippocampal CA3 pyramidal cells. Proceedings of the National Academy of Sciences of the United States of America 2002, 99:3194–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treves A, Rolls ET: Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 1992, 2:189–199. [DOI] [PubMed] [Google Scholar]
- 11.Trimper JB, Galloway CR, Jones AC, Mandi K, Manns JR: Gamma Oscillations in Rat Hippocampal Subregions Dentate Gyrus, CA3, CA1, and Subiculum Underlie Associative Memory Encoding. Cell Rep 2017, 21:2419–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitanishi T, Ujita S, Fallahnezhad M, Kitanishi N, Ikegaya Y, Tashiro A: Novelty-Induced Phase-Locked Firing to Slow Gamma Oscillations in the Hippocampus: Requirement of Synaptic Plasticity. Neuron 2015, 86:1265–1276. [DOI] [PubMed] [Google Scholar]
- 13.Rangel LM, Rueckemann JW, Riviere PD, Keefe KR, Porter BS, Heimbuch IS, Budlong CH, Eichenbaum H: Rhythmic coordination of hippocampal neurons during associative memory processing. Elife 2016, 5:e09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr MF, Karlsson MP, Frank LM: Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 2012, 75:700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzsaki G: Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 2015, 25:1073–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr MF, Jadhav SP, Frank LM: Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nature neuroscience 2011, 14:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soto C: Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 2003, 4:49–60. [DOI] [PubMed] [Google Scholar]
- 18.Hyman BT, Trojanowski JQ: Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 1997, 56:1095–1097. [DOI] [PubMed] [Google Scholar]
- 19.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al.: The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011, 7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, Tonegawa S: Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 2016, 531:508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study used pre-plaque AD mouse models to investigate engram cells, neuronal assemblies that undergo biochemical changes during the formation of a memory and thus can be reactivated to recall the memory, and their role in memory impairment. Neurons in the dentate gyrus (DG) that were activated during fear conditioning were tagged with channelrhodopsin such that light could subsequently be used to reactivate them. AD mice showed reduced freezing during recall, but light-induced reactivation of these engram cells elicited a freezing response in a context distinct from the training context in both AD and control mice. DG engram cells were also observed to have decreased dendritic spine density, and an engram cell-specific optical long-term potentiation protocol was able to restore spine density and rescue memory retrieval in AD mice. These results imply that deficits in memory retrieval, rather than encoding or consolidation, underlie cognitive impairments in early AD.
- 21.Mably AJ, Gereke BJ, Jones DT, Colgin LL: Impairments in spatial representations and rhythmic coordination of place cells in the 3×Tg mouse model of Alzheimer’s disease. Hippocampus 2017, 27:378–392. [DOI] [PubMed] [Google Scholar]; * This study investigated CA1 place cell representations of space and rhythmic modulation of place cell spiking in the 3×Tg mouse model of AD. Spatial representations were found to be less stable in 3×Tg mice than in control mice. Furthermore, modulation of CA1 place cell firing by slow gamma rhythms was attenuated in 3×Tg mice compared to control mice. These findings suggest that slow gamma rhythmic coordination of place cells may be important for stable representations of space and further suggest that this mechanism is disrupted in AD mice.
- 22.Booth CA, Witton J, Nowacki J, Tsaneva-Atanasova K, Jones MW, Randall AD, Brown JT: Altered Intrinsic Pyramidal Neuron Properties and Pathway-Specific Synaptic Dysfunction Underlie Aberrant Hippocampal Network Function in a Mouse Model of Tauopathy. The Journal of neuroscience : the official journal of the Society for Neuroscience 2016, 36:350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillespie AK, Jones EA, Lin YH, Karlsson MP, Kay K, Yoon SY, Tong LM, Nova P, Carr JS, Frank LM, et al.: Apolipoprotein E4 Causes Age-Dependent Disruption of Slow Gamma Oscillations during Hippocampal Sharp-Wave Ripples. Neuron 2016, 90:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study investigated network abnormalities in an aged ApoE4 KI mouse model of AD. Mutant mice were found to have a decreased number of SWRs and reduced coincident slow gamma power. Using a line of mice with ApoE4 specifically removed from interneurons, the authors were able to show that interneuronal ApoE4 was responsible for reduced SWR-associated slow gamma power. In an earlier paper from the same group, removal of ApoE4 from interneurons was also shown to rescue interneuron loss, as well as learning and memory deficits. SWR reduction was not ameliorated by ApoE4 removal from interneurons, suggesting that slow gamma impairments underlie learning and memory deficts in this mouse model.
- 24.Knoferle J, Yoon SY, Walker D, Leung L, Gillespie AK, Tong LM, Bien-Ly N, Huang Y: Apolipoprotein E4 produced in GABAergic interneurons causes learning and memory deficits in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 2014, 34:14069–14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, et al.: Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study investigated hippocampal rhythms in the 5XFAD mouse model of AD. The authors found diminshed slow gamma activity during sharp wave ripples in 5XFAD mice. Channelrhodopsin was expressed in fast-spiking parvalbumin-positive interneurons in these mice, and slow gamma rhythms were induced by optically stimulating these interneurons at 40 Hz. Rescuing slow gamma rhythms in this manner resulted in a reduction in Aβ levels. Furthermore, genome-wide RNA sequencing of CA1 tissue revealed an upregulation of genes that promote microglial engulfment of Aβ following 40 Hz stimulation. Entrainment of slow gamma in the visual cortex by non-invasive light-flicker methods was also able to decrease Aβ deposition, and promote microglial engulfment of Aβ, in this region. These findings indicate a broad role of slow gamma rhythms in AD pathogenesis, including effects on both neuronal and non-neuronal cell types.
- 26.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, et al.: Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 2006, 26:10129–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Jackson RJ, Hong W, Taylor WM, Corbett GT, Moreno A, Liu W, Li S, Frosch MP, Slutsky I, et al.: Human Brain-Derived Abeta Oligomers Bind to Synapses and Disrupt Synaptic Activity in a Manner That Requires APP. J Neurosci 2017, 37:11947–11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurudenkandy FR, Zilberter M, Biverstal H, Presto J, Honcharenko D, Stromberg R, Johansson J, Winblad B, Fisahn A: Amyloid-beta-induced action potential desynchronization and degradation of hippocampal gamma oscillations is prevented by interference with peptide conformation change and aggregation. J Neurosci 2014, 34:11416–11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung L, Andrews-Zwilling Y, Yoon SY, Jain S, Ring K, Dai J, Wang MM, Tong L, Walker D, Huang Y: Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice. PLoS One 2012, 7:e53569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong LM, Djukic B, Arnold C, Gillespie AK, Yoon SY, Wang MM, Zhang O, Knoferle J, Rubenstein JL, Alvarez-Buylla A, et al.: Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Abeta accumulation. J Neurosci 2014, 34:9506–9515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Losa M, Tracy TE, Ma K, Verret L, Clemente-Perez A, Khan AS, Cobos I, Ho K, Gan L, Mucke L, et al.: Nav1.1-Overexpressing Interneuron Transplants Restore Brain Rhythms and Cognition in a Mouse Model of Alzheimer’s Disease. Neuron 2018, https://doi.Org/10.1016/j.neuron.2018.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study showed that the voltage-gated sodium channel subunit Nav1.1 is essential for interneuron function, gamma oscillations, and associated learning and memory operations. When transplanted into wildtype mice, Nav1.1-deficient interneurons impaired behavioral performance on mnemonic tasks. Furthermore, Nav1.1-overexpressing interneuron transplants alleviated behavior-dependent gamma (30-80 Hz) deficits and improved behavioral performance in a mouse model of Alzheimer’s disease.
- 32.Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, Warren ST: Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. American journal of human genetics 2009, 85:503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassell GJ, Warren ST: Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 2008, 60:201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis JK, Broadie K: Multifarious Functions of the Fragile X Mental Retardation Protein. Trends Genet 2017, 33:703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross C, Hoffmann A, Bassell GJ, Berry-Kravis EM: Therapeutic Strategies in Fragile X Syndrome: From Bench to Bedside and Back. Neurotherapeutics 2015, 12:584–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fries P: Rhythms for Cognition: Communication through Coherence. Neuron 2015, 88:220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ethridge LE, White SP, Mosconi MW, Wang J, Byerly MJ, Sweeney JA: Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in Fragile X Syndrome. Transl Psychiatry 2016, 6:e787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berzhanskaya J, Phillips MA, Gorin A, Lai C, Shen J, Colonnese MT: Disrupted Cortical State Regulation in a Rat Model of Fragile X Syndrome. Cereb Cortex 2017, 27:1386–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radwan B, Dvorak D, Fenton AA: Impaired cognitive discrimination and discoordination of coupled theta-gamma oscillations in Fmr1 knockout mice. Neurobiol Dis 2016, 88:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dvorak D, Radwan B, Sparks FT, Talbot ZN, Fenton AA: Control of recollection by slow gamma dominating mid-frequency gamma in hippocampus CA1. PLoS Biol 2018, 16:e2003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talbot ZN, Sparks FT, Dvorak D, Curran BM, Alarcon JM, Fenton AA: Normal CA1 Place Fields but Discoordinated Network Discharge in a Fmr1-Null Mouse Model of Fragile X Syndrome. Neuron 2018, 97:684–697. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** In this study, LFP and single neuronal responses were assessed in FMR1 KO and control mice. Experience-dependent synaptic changes were enhanced in CA1 of FMR1 KO mice compared to control mice. However, spatial properties of place cells were normal in FMR1 KO mice, and place cells exhibited normal remapping in response to altered contexts. Importantly, the organization of CA1 putative pyramidal cell and interneuron firing by theta and slow gamma rhythms was disrupted in FMR1 KO mice, and this was suggested to underlie the cognitive flexibilty deficits seen in these mice.
- 42.Routh BN, Johnston D, Brager DH: Loss of functional A-type potassium channels in the dendrites of CA1 pyramidal neurons from a mouse model of fragile X syndrome. J Neurosci 2013, 33:19442–19450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brager DH, Akhavan AR, Johnston D: Impaired dendritic expression and plasticity of h-channels in the fmr1(−/y) mouse model of fragile X syndrome. Cell Rep 2012, 1(3):225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magee JC: Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 1998, 18(19):7613–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia F, Yiu A, Stone SSD, Oh S, Lozano AM, Josselyn SA, Frankland PW: Entorhinal Cortical Deep Brain Stimulation Rescues Memory Deficits in Both Young and Old Mice Genetically Engineered to Model Alzheimer’s Disease. Neuropsychopharmacology 2017, 42:2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mann A, Gondard E, Tampellini D, Milsted JAT, Marillac D, Hamani C, Kalia SK, Lozano AM: Chronic deep brain stimulation in an Alzheimer’s disease mouse model enhances memory and reduces pathological hallmarks. Brain Stimul 2018, 11:435–444. [DOI] [PubMed] [Google Scholar]
- 47.Lozano AM, Fosdick L, Chakravarty MM, Leoutsakos JM, Munro C, Oh E, Drake KE, Lyman CH, Rosenberg PB, Anderson WS, et al.: A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer’s Disease. J Alzheimers Dis 2016, 54:777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senova S, Chaillet A, Lozano AM: Fornical Closed-Loop Stimulation for Alzheimer’s Disease. Trends Neurosci 2018. [DOI] [PubMed] [Google Scholar]
- 49.Titiz AS, Hill MRH, Mankin EA, Z MA, Eliashiv D, Tchemodanov N, Maoz U, Stern J, Tran ME, Schuette P, Behnke E et al.: Theta-burst microstimulation in the human entorhinal area improves memory specificity. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobs J, Miller J, Lee SA, Coffey T, Watrous AJ, Sperling MR, Sharan A, Worrell G, Berry B, Lega B, Jobst BC et al.: Direct Electrical Stimulation of the Human Entorhinal Region and Hippocampus Impairs Memory. Neuron 2016, 92(5):983–990. [DOI] [PubMed] [Google Scholar]
- 51.Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, Fries P: Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 2015, 85(2):390–401. [DOI] [PubMed] [Google Scholar]
- 52.Zheng C, Colgin LL: Beta and gamma rhythms go with the flow. Neuron 2015, 85(2):236–237. [DOI] [PMC free article] [PubMed] [Google Scholar]


