Abstract
The relationship between fat, bone and systemic metabolism is a growing area of scientific interest. Marrow adipose tissue is a well-recognized component of the bone marrow milieu and is metabolically distinct from current established subtypes of adipose tissue. Despite recent advances, the functional significance of marrow adipose tissue is still not clearly delineated. Bone and fat cells share a common mesenchymal stem cell (MSC) within the bone marrow, and hormones and transcription factors such as growth hormone, leptin, and peroxisomal proliferator-activated receptor γ influence MSC differentiation into osteoblasts or adipocytes. MSC osteogenic potential is more vulnerable than adipogenic potential to radiation and chemotherapy, and this confers a risk for an abnormal fat-bone axis in survivors following cancer therapy and bone marrow transplantation. This review provides a summary of data from animal and human studies describing the relationship between marrow adipose tissue and hematopoiesis, bone mineral density, bone strength, and metabolic function. The significance of marrow adiposity in other metabolic disorders such as osteoporosis, diabetes mellitus, and estrogen and growth hormone deficiency are also discussed. We conclude that marrow adipose tissue is an active endocrine organ with important metabolic functions contributing to bone energy maintenance, osteogenesis, bone remodeling, and hematopoiesis. Future studies on the metabolic role of marrow adipose tissue may provide the critical insight necessary for selecting targeted therapeutic interventions to improve altered hematopoiesis and augment skeletal remodeling in cancer survivors.
Keywords: Bone marrow milieu, Bone marrow adipose tissue, White adipose tissue, Hematopoeisis, Mesenchymal stem cells, Hematopoeitic stem cells, Adipocytes, Adipokines, Bone mineral density
Introduction
Marrow adipose tissue is a well-recognized component of the bone marrow microenvironment and is metabolically distinct from other subtypes of adipose tissue. The functional significance of marrow adipose tissue remains unknown. However, growing evidence suggests an inverse association between marrow adipocytes and measures of hematopoiesis, as well as bone mineral density.[1] Recent advances in imaging modalities have provided improved tools to measure marrow adiposity; to investigate the underlying physiology; and to study the function of this intriguing fat depot. This review summarizes our current understanding of the following: (1) the role of stem cell interaction in the bone marrow niche in regulating hematopoiesis, marrow adiposity and bone formation; (2) current delineated subtypes of adipose tissue and their physiologic function; and (3) marrow adipose tissue as a distinct endocrine organ with future therapeutic implications.
Stem Cells and the bone marrow niche
The bone marrow microenvironment provides a critical regulatory milieu for the differentiation of hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs). HSCs are the developmental origin of the hematopoietic system and comprise 0.001% of total bone marrow cells.[2] They arise from dorsal aortic section of the aorta-gonad-mesonephros region to populate the fetal liver and subsequently migrate to the spleen and eventually to the bone marrow.[3] Differentiated hematopoietic cells include erythrocytes, platelets, and white blood cells, that give rise to innate and adaptive immune function.[4] MSCs, on the other hand, are the origin of connective tissue cells such as osteoblasts, adipocytes, chondrocytes and myocytes. In addition to the remodeling and repair of various organ systems, MSCs play a critical role in maintaining the HSCs population within the bone marrow microenvironment.[5]
The endosteal bone surface is the principal component of the hematopoietic niche, and plays an influential role in HSC differentiation and interaction with osteoblasts, osteoclasts, and MSCs.[6] While the primary function of osteoblasts is to secrete osteoid for bone mineralization, these cells also play a major role in HSC regulation.[7] Osteoblasts prevent HSC mobilization from the bone marrow niche and promote HSC quiescence through the secretion of soluble stromal-cell derived factor 1 (also known as CXCL12) and angiopoietin-1.[8, 9] Osteoblasts and MSCs are closely coupled to HSC proliferation, and increases in osteoblast population lead to concomitant increases in HSC numbers.[7, 10] This expansion is mediated by osteoblastic Notch signaling[7] and other factors such as osteopontin,[11] Wnt, N-cadherin, thrombopoietin,[12] and angiopoietin.[13] The delicate interaction between these cell populations is further highlighted in conditions such as inflammation, obesity, aging,[14] type 1 diabetes mellitus [15], or cancer therapy that change the number and activity of osteoblasts and MSCs, and invariably demonstrate an effect on HSCs.[16] Additionally, knockout of MSC severely impairs the maintenance of HSC progenitors and their ability to home to the bone marrow, further highlighting the critical role that MSCs play in HSC maintenance.[17] Therefore, the complex cellular interactions in conjunction with the properties of the bone marrow microenvironment form the marrow regulatory niche that influences the actions and activities of these marrow progenitor cells.
On the other hand, osteoclasts are multinucleated cells that arise from hematopoietic cells and are predominantly responsible for bone resorption. In addition to bone remodeling, osteoclasts are also involved in HSC mobilization within the bone marrow milieu through enzymatically cleaving CXCL12.[18] Thus, a competitive balance between osteoblasts and osteoclasts is necessary for the regulation of HSC in the marrow microenvironment (Figure 1). Osteoclast-mediated bone resorption increases calcium levels and this further enables HSCs (via calcium receptors) to navigate and lodge within the bone marrow endosteal surface.[19] While the size of the HSC population is closely associated with osteoclast numbers, bisphosphonate therapy, which drastically slows osteoclast activity, results in curtailed osteoblast-mediated increases in HSC numbers.[20] Hence, bisphosphonate treatment increases the risk of impaired hematopoietic engraftment, as functional osteoclasts are required for the regulation of hematopoiesis both independently and through co-operation with other marrow cells.
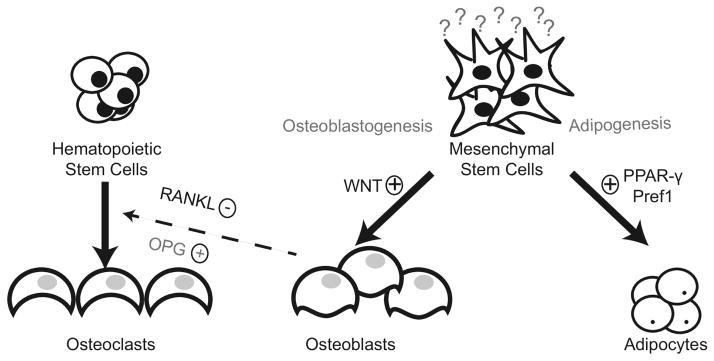
Figure 1.
The endosteal bone surface is the principal component of the hematopoietic niche, and plays an influential role in hematopoietic stem cell (HSC) differentiation and interaction with osteoblasts, osteoclasts, and mesenchymal stem cells (MSCs). Osteoblasts and MSCs are closely coupled to HSC proliferation. Knockout of MSC severely impairs the maintenance of HSC progenitors and their ability to home to the bone marrow, further highlighting the critical role that MSCs play in HSC differentiation. Activation of the canonical Wnt pathway, targeted by most cancer treatment regimens, play a critical role in MSC differentiation. Hormones and transcription factors such as Pref 1, growth hormone, leptin, and peroxisomal proliferator-activated receptor γ (PPAR- γ) can influence MSC differentiation into either osteoblasts or adipocytes. Osteoclasts are multinucleated giant cells that arise from hematopoietic cells and are predominantly responsible for bone resorption. In addition to bone remodeling, osteoclasts are also involved in HSC mobilization within the bone marrow milieu through enzymatically cleaving soluable stromal-cell derived factor 1 or CXCL12. The receptor activator of NF-κB ligand (RANKL) plays a critical role in osteoclast formation, and the biological activity of RANKL is moderated by osteoprogerin (OPG), a physiological decoy receptor. Thus, a competitive balance between osteoblasts and osteoclasts is necessary for the regulation of HSC in the bone marrow milieu.
Accumulating evidence indicates that multiple niches are required for each hematopoietic process.[1] The physical and functional interaction of the different niches and cells residing within the bone marrow (i.e. changes in the bone marrow composition with enhanced adiposity) can affect HSC and hematopoiesis. For example, osteoblast lineage Gsα-dependent signaling allows for normal B-cell development, thus emphasizing the importance of bone-cell interaction on B-cell fate.[21] Similar to HSCs, B-cells require exposure to CXCL12 during development. CXCL12 is critical for the maintenance of multipotent progenitors in differentiation to the B-cell lineage. By intercalating within the hematopoietic milieu and disrupting the cellular composition of the bone marrow niche, adipocytes displace and interfere with the connection between HSCs and other niche cells to exert a negative influence on hematopoiesis. Thus, even small changes in the microenvironment, such as enhanced bone marrow adiposity, can affect a particular niche or disrupt cellular trafficking.[22]
Adipocytes share the bone marrow milieu with osteoblasts, MSCs, osteoclasts, and vascular cells. The role of adipocytes on hematopoiesis in this niche is complex, though predominantly characterized as inhibitory.[1, 23] The increased bone marrow adiposity seen after chemotherapy and radiation treatment is antagonistic to hematopoietic recovery.[1] The peroxisome proliferator-activated receptor-c (PPAR-c) inhibitor bisphenol A diglycidyl ether (BADGE) prevents bone marrow adipocyte formation in vitro and in vivo in mice models of streptozocin-induced diabetes.[1, 24] Administration of BADGE to lethally irradiated mice two weeks after bone-marrow transplantation results in the inhibition of bone marrow adipocyte formation, with robust cellular engraftment and higher peripheral white blood cell counts.[1] This suggests that PPAR-c inhibitors, or other adipocyte inhibitors, might serve as adjuvants to hematopoietic recovery following hematopoietic stem cell transplantation (HSCT).
β-catenin signaling and activation of the canonical Wnt pathway, targeted by most cancer treatment regimens, play a critical role in MSC differentiation and are required for hematopoietic regeneration following injury.[25] Total body irradiation, used as part of the treatment regimens in allogeneic HSCT, is associated with enhanced marrow adiposity, suggesting that MSC interaction with HSCs within the bone marrow niche is required for successful engraftment.[26, 27] We previously demonstrated markedly increased marrow adiposity, abnormal bone microarchitecture, and abnormal fat distribution in long-term childhood HSCT recipients after total body irradiation.[26] Importantly, these patients also had occult vertebral compression fractures as well as widespread vertebral deformities, highlighting the fracture risk associated with increased marrow adiposity.
Bone marrow adipocytes may directly modify HSC differentiation through paracrine effects,[28–30] and adipocyte-derived factors including adiponectin, leptin, prostaglandins, and sex steroids can regulate hematopoiesis.[31, 32] Bone and fat cells share a common MSC within the bone marrow. Human cell culture studies suggest that MSC osteogenic potential is more vulnerable to radiation and chemotherapy than adipogenic potential. Consequently, hormones and transcription factors such as growth hormone, leptin, and peroxisomal proliferator-activated receptor γ (PPAR- γ) can influence MSC differentiation into either osteoblasts or adipocytes. Secreted by adipocytes, leptin regulates appetite and energy metabolism. Leptin also plays a critical role in skeletal metabolism through sympathetic neuronal signaling within the hypothalamus.[33] Recent data indicate that human bone marrow adipocytes produce leptin in a regulated manner that becomes suppressed during caloric restriction and systemic inflammation.[34] While the systemic function of marrow-adipose tissue derived leptin has yet to be determined, increasing evidence suggests that leptin produced by bone marrow adipocytes acts predominantly as an autocrine and paracrine factor within the bone marrow milieu to influence hematopoiesis and osteoblastogenesis.[30, 35]
Adipose tissue an intriguing endocrine organ
Adipose tissue is a metabolically active tissue comprised of mature adipocytes, endothelial cells, immune cells, pre-adipocytes, and adipose progenitor cells. Mammalian adipose tissue is traditionally classified into two distinct subtypes: white adipose tissue (WAT); and brown adipose tissue (BAT), and further divided into regional depots based on structural organization, cellular composition, biochemical profile, and biological function.[36] The traditional role of WAT is long-term energy storage. Excess energy stimulates lipogenic enzymes that synthesize triglycerides for storage,[37] while reduced caloric intake stimulates enzymatic lipid hydrolysis and release of free fatty acids from fat stores into the blood stream for metabolism by other organs.[38] WAT is dispersed throughout the body. The largest WAT depots are located within the visceral and subcutaneous regions and exhibit notable region-specific metabolic differences. In general, the expansion of visceral adipose tissue is associated with an increased risk of type 2 diabetes mellitus (T2DM), cardiometabolic disease and the metabolic syndrome.[39] In mice, transplanting subcutaneous fat into the visceral cavity improves glucose metabolism, further highlighting the intrinsic difference of these two fat depots.[40] Visceral adipocytes are also more responsive to lipolytic signals which upregulate the transport of free fatty acids, while subcutaneous adipocytes serve as stable energy reserves.[41] During periods of caloric excess, WAT mass expands through adipocyte hypertrophy and hyperplasia by terminal differentiation of committed pre-adipocytes into mature adipocytes, a process dependent on PPAR-γ.[41] As WAT deposits expand in states of obesity, the fat tissue undergoes remodeling to facilitate tissue expansion. Dead adipocytes are removed by adipose tissue macrophages that infiltrate the fat tissue in response to adipocyte death,[42] and these macrophages contribute to an increased inflammatory profile in WAT depots present in states of obesity and often associated with the development of insulin resistance.[43]
In contrast to WAT, BAT appears as discrete adipose tissue located along the neck, supraclavicular, paravertebral, and peri-renal regions. Brown adipocytes originate from MYF-5 positive dermomyotomes and become active upon cold exposure.[44, 45] BAT is rich in mitochondria and functions in basal and inducible energy expenditure by uncoupling protein 1 (UCP1),[46] which stimulates proton leak from the mitochondrial membrane to uncouple respiration from ATP synthesis and produce heat. BAT’s thermogenic activity is typically controlled by catecholamines, including the β-adrenergic signaling, as well as thyroid hormone.[47] BAT inversely correlates with body mass index[48] and, along with its role in adaptive thermogenesis, also functions in protecting against obesity, insulin resistance and T2DM.[49] In the past decade, a “third” fat tissue (the so-called “beige” adipose tissue) has been described and sparked much research interest. Beige adipose tissue is an inducible thermogenic adipose tissue that forms in WAT after exposure to different environmental triggers, including chronic cold exposure.[50] Beige fat resembles BAT in terms of displaying thermogenic activity, and originates from mesenchymal stem cells that express Pdgfrα, MYF5-negative mesoderm precursors, with a subset (approximately 15%) originating from MYH11-positive smooth muscle-like precursors.[44, 51] Unlike the firmly established metabolic and endocrine role of WAT in various physiologic states and disorders, markers and pathways associated with brown and beige adipose tissue are currently under active investigation. There is growing scientific interest in activating these specific fat tissues as potential therapeutic options to reduce metabolic disorders. Additional studies are needed to better elucidate metabolic properties and systemic regulating factors for “browning” of WAT as future therapeutic targets for treating obesity, T2DM and other metabolic disorders in cancer survivors.[45]
Marrow adipose tissue, a distinct fat tissue
Situated within the bone marrow cavity, marrow adipose tissue (MAT) accounts for approximately 10% of the total fat mass in healthy adult humans.[52] The origin of bone marrow adipocytes remains unclear and it is thought that these adipocytes differentiate from MSCs located within the bone marrow cavity, where they subsequently differentiate into white and beige adipose tissue. During early childhood, bone marrow is predominantly composed of hematopoietic tissue.[53] However, in both males and females, exponential accumulation of MAT begins at birth, starting with the distal bones,[53] with males demonstrating greater amounts of MAT compared to females.[54] By age 25 years, approximately 70% of the human bone marrow consists of MAT, with continued gradual accumulation of MAT throughout life.[52] While MAT was originally recognized as a distinct adipose depot by the mid to late twentieth century,[55] recent advances in medical research along with newer imaging modalities such as magnetic resonance spectroscopy, positron emission tomography-computed tomography (PET-CT), and osmium tetroxide staining coupled with micro-CT, have provided the necessary tools to study the function and physiology of this unique fat depot.[56]
MAT’s origin is distinct to both WAT and BAT and is derived from progenitors that express osterix (Sp7), a transcription factor essential for osteoblastogenesis and bone formation.[57] Recent gene profiling comparing adult bone marrow-derived adipocytes to epididymal adipocytes also reveal different gene patterns, further highlighting MAT differences from WAT and BAT.[58] For example, bone marrow adipocytes demonstrate low expression of adipocyte-specific genes such as PPARγ, but high expression of genes associated with early adipocyte differentiation (C/EBPβ, RGS2), as well as genes that regulate bone cell function (SFRP4, TNFα, TFG).[59]
The distinct developmental origin and lipid composition of marrow adipocytes has generated new-found scientific interest into the role and metabolic function of MAT.[60] Similar to WAT, the lipid content of MAT is entirely composed of triglycerides,[23] but, unlike WAT, the MAT fatty acid component consists of saturated, monounsaturated, and polyunsaturated fat.[52] Fatty acid metabolism is critical for HSC and MSC proliferation and function. During times of metabolic need, adipose tissue lipases break down triglycerides to release free fatty acids for use as an energy source to regulate osteoblasts, osteoclasts, and hematopoietic cell populations.[61] In humans, the fatty acid composition of MAT is significantly higher in saturated fat content, which is distinct from fatty acid composition of subcutaneous WAT.[62] The difference in fatty acid content of adipocytes located within hematopoietic dominant regions of the bone marrow compared to non-hematopoietic regions suggest that bone marrow adiposity influences hematopoiesis by providing local source of fatty acids [23, 60]. Thus bone marrow adiposity can also influence hematopoiesis by providing a local source of fatty acids.
Theories regarding the functional role of MAT have varied over the past few decades, particularly as MAT accumulation is associated with aging, osteoporosis, type 1 diabetes mellitus (T1DM), T2DM, anorexia nervosa, estrogen and growth hormone deficiency.[52] During states of nutritional deprivation, MAT and WAT show marked differences, with varying responses to nutritional cues. In human models of caloric restriction, such as anorexia nervosa, MAT stores are increased compared with healthy weight controls, while WAT stores are low.[63–65] The mechanism of how caloric restriction triggers the development of MAT is unclear and a signal, such as the hormone ghrelin released systemically or locally, may trigger other hormonal responses to induce marrow adipogenesis.[66] The increased MAT stores seen in anorexia nervosa and caloric restriction have been discussed extensively in prior reviews.[57, 65]
MAT exists in two distinct subtypes designated as constitutive and regulated MAT, each with different characteristics and function.[60] Regulated MAT (rMAT) is predominantly located in proximal skeletal sites and is interspersed within regions of active hematopoiesis. In contrast, constitutive MAT (cMAT) forms in the distal skeletal regions in early postnatal life and remains largely unchanged in the face of systemic or environmental challenges. The distinct metabolic role and function of these MAT subtypes are further highlighted by variations in lipid composition and gene expression.[60] However, future studies are required to better delineate the role of these distinct MAT subpopulations, where cMAT may serve an important function in early vertebrate development, in contrast to rMAT’s role in hematopoiesis and skeletal remodeling.[56, 60]
Is marrow adipose tissue an endocrine organ?
Increases in MAT with aging and other clinical conditions such as anorexia nervosa, T1DM, T2DM, glucocorticoid treatment and cancer therapy raises the fundamental question regarding the function of this unique adipose tissue. MAT expresses and secretes adiponectin and this exerts systemic metabolic effects, prompting investigators to classify it as a functional endocrine organ.[57] In humans, low circulating levels of adiponectin are present in states of obesity, and enhanced WAT is a well-established biomarker of insulin resistance and cardiovascular disease.[67] Conversely, serum adiponectin concentrations increase in lean states, such as caloric restriction in humans with anorexia nervosa.[68] Reduced circulating levels of adiponectin in obesity likely derives from reduced adiponectin expression and secretion due to mitochondrial dysfunction from increased inflammation, hypoxia, as well as endoplasmic reticulum stress.[67] Findings in animal models collectively suggest that MAT expansion is required for increased adiponectin production during periods of caloric restriction, supporting the conclusion that MAT contributes to the increases in circulating adiponectin measured in this context. In addition, the increased adiponectin concentrations seen during caloric restriction may also play a role in skeletal muscle adaptation.[57] However, the consequences of adiponectin production from MAT have yet to be fully delineated.
Fat and bone: the role of adipose tissue and the skeleton
MAT is found across all skeletal sites in humans, and compromises up to 15% of total fat stores in adults.[57] Skeletal homeostasis is actively mediated through MAT interaction with osteoblasts.[69–71] While endosteal adipocytes are rare in neonates, these cells steadily accumulate throughout the lifespan and occupy a greater proportion of the bone marrow cavity in the axial skeleton with aging.[72] MAT is increased in metabolic disorders with low bone mass (e.g. T1DM or anorexia nervosa).[63, 73] As noted, osteoblasts and adipocytes derive from a common pool of mesenchymal progenitors, superficially suggesting a simple tradeoff between bone and fat mass. Pref-1, a member of the epidermal growth factor-like family of proteins, is a known regulator of adipocyte and osteoblast differentiation.[65] Wren et al. were the first to report an inverse association between femoral cortical bone area and MAT in both young and older subjects.[74] Furthermore, an inverse relationship between bone mineral density (BMD) and MAT was also demonstrated in groups of healthy Caucasian women[75] and middle-age Caucasian and African American men and women.[76] Yet, the negative association of high marrow adiposity and low bone mass is variable and far from a simple inverse relationship. In healthy individuals, marrow adipocytes increase rapidly in long, axial bones around peak skeletal mass acquisition during puberty. As noted previously, males have greater amounts of MAT when compared with females, despite higher BMD.[54, 72] Several animal models have also demonstrated high bone mass despite increased marrow adipose tissue.[52, 77] These findings suggest that simultaneous accumulation of bone mass and MAT can occur, and that the MAT in healthy individuals somehow differs from the marrow fat accumulation seen in various disease processes, including cancer survivors following radiation and chemotherapy. Similarly, the relationship between WAT and bone is equally complex and highly dependent on the location of the fat depot. High body mass confers greater mechanical loading and enhanced bone mass, yet greater visceral WAT has deleterious effects on bone and contributes to osteoporosis by disrupting bone remodeling through the release of inflammatory cytokines, such as IL6 and TNFα.[78] In our study of long-term HSCT survivors following total body irradiation, MAT volume was two-fold greater when compared with age- and sex-matched controls. The enhanced MAT was also associated with greater visceral adiposity and fat infiltration of muscle, reduced bone volume fraction, and abnormal bone microarchitecture.[26] Similarly, adult patients receiving pelvic radiation therapy in combination with chemotherapy experience significant bone marrow cell depletion, bone loss with increased fracture risks, and enhanced MAT.[79]
Increased MAT is present in osteoporosis, and MAT is an important indicator of bone integrity.[80] Iliac bone biopsies in osteoporotic individuals demonstrate increased MAT volume and decreased trabecular bone volume compared with age-matched controls, suggesting increased fracture risk in individuals with increased MAT.[81, 82] Similarly, Wehrli et al. demonstrated that enhanced vertebral adiposity is an independent predictor of fracture risk.[83] Lower proportion of unsaturated lipid content is noted in MAT of individuals with osteoporosis and osteopenia based on proton spectroscopy imaging.[84] However, it is not known whether marrow fat saturation or unsaturation contributes to increased fracture risks.
Mechanical loading also serves as an important player in the bone-fat interaction for skeletal homeostasis. PPAR-γ is required for adipocyte differentiation, and treadmill running in rats prevents ovariectomy-induced bone loss by limiting PPAR-γ expression.[85] Unloading in humans and animal models is associated with increased MAT and low bone mass.[86] In rat models exposed to hind limb unloading, impaired bone acquisition and greater marrow adiposity is seen, and these abnormalities normalize upon skeletal reloading.[87, 88] At the cellular level, MSCs subjected to subtle mechanical signals in vivo demonstrate an increased propensity towards osteoblastogenesis, even if situated in highly adipogenic environments.[89, 90] Similarly, in vitro stretching of MSCs reduces PPAR-γ signaling and adipogenesis, even during PPAR-γ activation.[91] Interestingly, β-catenin signaling is also increased during mechanical stretching and serves as an important mechanosensitive regulatory mechanism in the stem cell niche and a further explanation of how exercise can influence the bone marrow microenvironment.[92] Recent intervention studies in healthy children demonstrate increases in BMD along with significant decreases in femoral MAT with activity.[93, 94]
Lastly, in addition to exercise, growth hormone serves as another key factor in the bone-fat interaction, particularly as growth hormone is secreted in response to exercise.[95] During aging, the bone marrow cavity gradually becomes filled with adipocytes and bone is lost. Concomitantly, levels of growth hormone also decline. In mice and humans with growth hormone deficiency, adipocytes accumulate within the bone marrow cavity and these levels normalize with growth hormone replacement.[96] In these individuals, growth hormone replacement is also accompanied by parallel increases in osteoblast activity and increased BMD.[96]
Marrow adipose tissue and metabolic disorders
T1DM is a significant risk factor for impaired cortical geometry, low bone mass, and fractures.[73, 97] Increased MAT is present in patients with T1DM regardless of disease severity,[98] yet it is still unclear if marrow adipocyte infiltration in these patients plays a central role in bone loss. In animal models of streptozocin-induced T1DM, expression of proadipocyte genes such as PPARγ was increased in long bones along with reduced expression of osteocalcin.[99, 100] Interestingly, subsequent treatment with PPARγ antagonist, BADGE, in these animal models prevented the accumulation of marrow adiposity, without improvement in the accompanied skeletal loss.[100] These investigations suggest that the PPARγ-mediated interaction between bone formation and enhanced marrow adiposity is probably not the sole mechanism responsible for bone loss in T1DM. On the other hand, treatment with thiazolidinediones (TZD), agonists of the PPARγ receptors and strong inducers of MAT, are linked with bone loss in the appendicular skeleton of rodents.[47, 101] Yet, conflicting results are noted in humans with respect to TZD treatment and marrow adipose tissue expansion, suggesting need for more detailed investigation.[102]
Skeletal fragility is also a well-recognized feature of T2DM even in the presence of normal BMD.[103] Despite elevated fracture risks, increased MAT is not consistently present in patients with T2DM. To date, studies using magnetic resonance spectroscopy suggest an increased saturated to unsaturated lipid ratio within the marrow cavity of women with T2DM who have fractures.[104] While marrow adiposity is not a feature of insulin resistance, in women with T2DM who have hemoglobin A1C levels >7%, higher levels of MAT were noted compared with those who have levels ≤ 7%, alluding that perhaps MAT is affected by glycemic control.[105]
The decline in estradiol and dihydrotestosterone levels, as seen with aging or as a consequence of cancer therapy, increases expression of PPARγ and differentiation of MSCs into adipocytes. In animal models following ovariectomy, adipocyte infiltration and marked increase in bone marrow adiposity are seen.[106] In addition, postmenopausal women undergoing estrogen treatment demonstrate a decline in bone marrow adipocyte number and size as well as in MAT, suggesting a regulatory action of estrogen and androgens on bone marrow cells.[107] Finally, mice deficient in 11β-hydroxysteroid dehydrogenase 1, an isoenzyme that interconverts active glucocorticoids to its inert 11-keto forms, lack marrow adipocytes, suggesting a role for active glucocorticoids in MAT expansion.[108] Hence, the increased MAT seen in anorexia nervosa may also reflect an impact of elevated circulating cortisol levels.[109, 110]
Future directions and therapeutic implications of marrow adiposity
Over the past few decades, the majority of studies have focused on discerning the basic function and endocrine role of MAT, an intriguing source of adiposity in mammals. Animal studies indicate that MAT is an endocrine organ capable of undergoing pathologic changes and evolving in the presence of various disease states. The bone marrow niche regulates hematopoiesis and osteoblastogenesis. Factors influencing this process occur through delicate cellular, physical and chemical interactions within the bone marrow microenvironment. Thus, even small changes to the niche composition (e.g. cancer therapy) can have profound impacts on hematopoiesis, adiposity and skeletal health. While data from animal and human studies support the hypothesis that MAT is associated with skeletal remodeling, many questions still remain unanswered regarding the source, origin, and function of MAT and the local role of MAT in the skeletal microenvironment. Although animal studies have informed our basic understanding of MAT, future comprehensive clinical studies are needed to determine its relevance in treating metabolic disorders, improving skeletal health, and enhancing hematopoiesis. These efforts will provide the foundation for future targeted therapeutic interventions with the aim to address altered hematopoiesis and maximize skeletal remodeling in different patient groups including survivors of cancer and bone marrow transplantation.
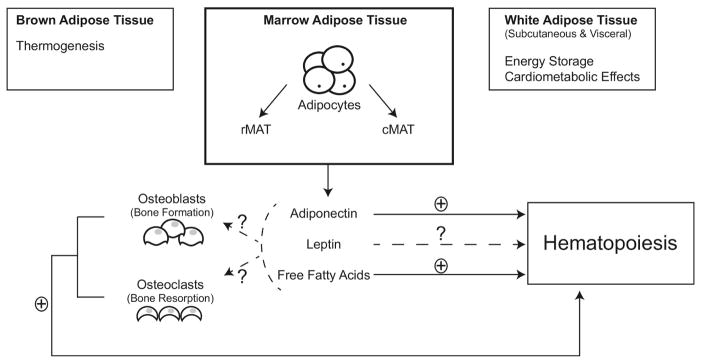
Figure 2.
Mammalian adipose tissue is currently classified into distinct subtypes of white adipose tissue (WAT), brown adipose tissue (BAT), and marrow adipose tissue (MAT). Not shown in this figure, is another subtype referred to as “beige” adipose tissue further described in the review text. These adipose tissues are further divided into regional depots based on structural organization, cellular composition, biochemical profile, and biological function. MAT has endocrine and paracrine functions. Recent gene profiling of marrow-derived adipocytes reveal different gene patterns, further highlighting its difference from WAT and BAT. MAT is further divided into two distinct subtypes: regulated MAT (rMAT) and constitutive MAT (cMAT). rMAT is predominantly located in the proximal skeletal sites and interspersed within regions of active hematopoiesis, while cMAT is found predominantly in the distal skeletal regions with no corresponding interspersed areas of active hematopoiesis. MAT expresses and secretes adiponectin to exert systemic metabolic effects. However, many systemic effects of adiponectin and other MAT-derived endocrine factors have yet to be delineated. Local production of leptin or adiponectin might influence osteoblast and osteoclast function. The positive and negative effects of these factors are indicated by “+” or “−”, while inconclusive effects by a “?”. Future clinical studies are needed to better delineate the paracrine and endocrine functions of MAT as potential targeted interventions for treatment of various hematopoietic, metabolic and skeletal disorders.
Acknowledgments
Funding Sources: This review was supported by the NIH grant K07 CA166177 (SMM).
Abbreviations
- BADGE
Bisphenol A diglycidyl ether
- BAT
Brown adipose tissue
- BMD
Bone mineral density
- cMAT
Constitutive marrow adipose tissue
- HSC
Hematopoietic stem cell
- HSCT
hematopoietic stem cell transplantation
- MAT
Marrow adipose tissue
- MSC
Mesencymal stem cell
- PPAR-c
Peroxisome proliferator-activated receptor-c
- PPAR- γ
Peroxisomal proliferator-activated receptor γ
- rMAT
Regulated marrow adipose tissue
- Sp7
Ostirix
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TZD
Thiazolidinediones
- UCP1
Uncoupling protein 1
- WAT
White adipose tissue
Footnotes
Disclosure Summary: The authors have no financial relationships to disclose relevant to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challen GA, Boles N, Lin KK, Goodell MA. Mouse hematopoietic stem cell identification and analysis. Cytometry A. 2009;75(1):14–24. doi: 10.1002/cyto.a.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9(2):129–36. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357(9270):1777–89. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 5.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–30. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8(4):290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 7.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 8.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Tzeng YS, Li H, Kang YL, Chen WC, Cheng WC, Lai DM. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117(2):429–39. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 11.Chiba H, Ataka K, Iba K, Nagaishi K, Yamashita T, Fujimiya M. Diabetes impairs the interactions between long-term hematopoietic stem cells and osteopontin-positive cells in the endosteal niche of mouse bone marrow. Am J Physiol Cell Physiol. 2013;305(7):C693–703. doi: 10.1152/ajpcell.00400.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox N, Priestley G, Papayannopoulou T, Kaushansky K. Thrombopoietin expands hematopoietic stem cells after transplantation. J Clin Invest. 2002;110(3):389–94. doi: 10.1172/JCI15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang LD, Wagers AJ. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat Rev Mol Cell Biol. 2011;12(10):643–55. doi: 10.1038/nrm3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almeida M. Aging mechanisms in bone. Bonekey Rep. 2012;1 doi: 10.1038/bonekey.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2(1):35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 16.Adler BJ, Kaushansky K, Rubin CT. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol. 2014;10(12):737–48. doi: 10.1038/nrendo.2014.169. [DOI] [PubMed] [Google Scholar]
- 17.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 19.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175(2):266–76. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 20.Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2011;117(5):1540–9. doi: 10.1182/blood-2010-05-282855. [DOI] [PubMed] [Google Scholar]
- 21.Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, Scadden DT, Kronenberg HM. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105(44):16976–81. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009;24(5):759–64. doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavassoli M, Houchin DN, Jacobs P. Fatty acid composition of adipose cells in red and yellow marrow: A possible determinant of haematopoietic potential. Scand J Haematol. 1977;18(1):47–53. doi: 10.1111/j.1600-0609.1977.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 24.Wright HM, Clish CB, Mikami T, Hauser S, Yanagi K, Hiramatsu R, Serhan CN, Spiegelman BM. A synthetic antagonist for the peroxisome proliferator-activated receptor gamma inhibits adipocyte differentiation. The Journal of biological chemistry. 2000;275(3):1873–7. doi: 10.1074/jbc.275.3.1873. [DOI] [PubMed] [Google Scholar]
- 25.Lento W, Ito T, Zhao C, Harris JR, Huang W, Jiang C, Owzar K, Piryani S, Racioppi L, Chao N, Reya T. Loss of beta-catenin triggers oxidative stress and impairs hematopoietic regeneration. Genes Dev. 2014;28(9):995–1004. doi: 10.1101/gad.231944.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mostoufi-Moab S, Magland J, Isaacoff EJ, Sun W, Rajapakse CS, Zemel B, Wehrli F, Shekdar K, Baker J, Long J, Leonard MB. Adverse Fat Depots and Marrow Adiposity Are Associated With Skeletal Deficits and Insulin Resistance in Long-Term Survivors of Pediatric Hematopoietic Stem Cell Transplantation. J Bone Miner Res. 2015;30(9):1657–66. doi: 10.1002/jbmr.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muruganandan S, Sinal CJ. The impact of bone marrow adipocytes on osteoblast and osteoclast differentiation. IUBMB Life. 2014 doi: 10.1002/iub.1254. [DOI] [PubMed] [Google Scholar]
- 29.Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015;3(2):141–7. doi: 10.1016/S2213-8587(14)70007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulston RJ, Cawthorn WP. Bone marrow adipose tissue as an endocrine organ: close to the bone? Horm Mol Biol Clin Investig. 2016;28(1):21–38. doi: 10.1515/hmbci-2016-0012. [DOI] [PubMed] [Google Scholar]
- 31.Umemoto Y, Tsuji K, Yang FC, Ebihara Y, Kaneko A, Furukawa S, Nakahata T. Leptin stimulates the proliferation of murine myelocytic and primitive hematopoietic progenitor cells. Blood. 1997;90(9):3438–43. [PubMed] [Google Scholar]
- 32.Yokota T, Meka CS, Kouro T, Medina KL, Igarashi H, Takahashi M, Oritani K, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW. Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. J Immunol. 2003;171(10):5091–9. doi: 10.4049/jimmunol.171.10.5091. [DOI] [PubMed] [Google Scholar]
- 33.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 34.Laharrague P, Truel N, Fontanilles AM, Corberand JX, Penicaud L, Casteilla L. Regulation by cytokines of leptin expression in human bone marrow adipocytes. Horm Metab Res. 2000;32(10):381–5. doi: 10.1055/s-2007-978658. [DOI] [PubMed] [Google Scholar]
- 35.Hamrick MW. Leptin, bone mass, and the thrifty phenotype. J Bone Miner Res. 2004;19(10):1607–11. doi: 10.1359/JBMR.040712. [DOI] [PubMed] [Google Scholar]
- 36.Fruhbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol Biol. 2008;456:1–22. doi: 10.1007/978-1-59745-245-8_1. [DOI] [PubMed] [Google Scholar]
- 37.Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715–36. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- 38.Ahmadian M, Wang Y, Sul HS. Lipolysis in adipocytes. Int J Biochem Cell Biol. 2010;42(5):555–9. doi: 10.1016/j.biocel.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–8. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 40.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7(5):410–20. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 42.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–8. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 43.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185(3):1836–45. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125(2):478–86. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 46.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Lecka-Czernik B, Ackert-Bicknell C, Adamo ML, Marmolejos V, Churchill GA, Shockley KR, Reid IR, Grey A, Rosen CJ. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology. 2007;148(2):903–11. doi: 10.1210/en.2006-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 49.Kozak LP, Koza RA, Anunciado-Koza R. Brown fat thermogenesis and body weight regulation in mice: relevance to humans. Int J Obes (Lond) 2010;34(Suppl 1):S23–7. doi: 10.1038/ijo.2010.179. [DOI] [PubMed] [Google Scholar]
- 50.Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5(5):1196–203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 51.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ, Jr, Rosen ED, Spiegelman BM. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19(5):810–20. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A. Marrow fat and bone--new perspectives. J Clin Endocrinol Metab. 2013;98(3):935–45. doi: 10.1210/jc.2012-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14(1):10–9. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]
- 54.Schellinger D, Lin CS, Fertikh D, Lee JS, Lauerman WC, Henderson F, Davis B. Normal lumbar vertebrae: anatomic, age, and sex variance in subjects at proton MR spectroscopy--initial experience. Radiology. 2000;215(3):910–6. doi: 10.1148/radiology.215.3.r00jn42910. [DOI] [PubMed] [Google Scholar]
- 55.Zakaria E, Shafrir E. Yellow bone marrow as adipose tissue. Proc Soc Exp Biol Med. 1967;124(4):1265–8. doi: 10.3181/00379727-124-31983. [DOI] [PubMed] [Google Scholar]
- 56.Suchacki KJ, Cawthorn WP, Rosen CJ. Bone marrow adipose tissue: formation, function and regulation. Curr Opin Pharmacol. 2016;28:50–6. doi: 10.1016/j.coph.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, Soliman SS, DelProposto JL, Lumeng CN, Mitra A, Pandit SV, Gallagher KA, Miller JD, Krishnan V, Hui SK, Bredella MA, Fazeli PK, Klibanski A, Horowitz MC, Rosen CJ, MacDougald OA. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20(2):368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu LF, Shen WJ, Ueno M, Patel S, Kraemer FB. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011;12:212. doi: 10.1186/1471-2164-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW, Maye P. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8(8):e71318. doi: 10.1371/journal.pone.0071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, Wu B, Ding SY, Bredella MA, Fazeli PK, Khoury B, Jepsen KJ, Pilch PF, Klibanski A, Rosen CJ, MacDougald OA. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:7808. doi: 10.1038/ncomms8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cornish J, MacGibbon A, Lin JM, Watson M, Callon KE, Tong PC, Dunford JE, van der Does Y, Williams GA, Grey AB, Naot D, Reid IR. Modulation of osteoclastogenesis by fatty acids. Endocrinology. 2008;149(11):5688–95. doi: 10.1210/en.2008-0111. [DOI] [PubMed] [Google Scholar]
- 62.Griffith JF, Yeung DK, Ahuja AT, Choy CW, Mei WY, Lam SS, Lam TP, Chen ZY, Leung PC. A study of bone marrow and subcutaneous fatty acid composition in subjects of varying bone mineral density. Bone. 2009;44(6):1092–6. doi: 10.1016/j.bone.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 63.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK, Rosen CJ, Gordon CM. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25(2):298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, Breggia A, Miller KK, Klibanski A. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab. 2010;95(1):407–13. doi: 10.1210/jc.2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC, Wells T. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145(1):234–42. doi: 10.1210/en.2003-0899. [DOI] [PubMed] [Google Scholar]
- 67.Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2(3):133–41. doi: 10.1016/j.molmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dolezalova R, Lacinova Z, Dolinkova M, Kleiblova P, Haluzikova D, Housa D, Papezova H, Haluzik M. Changes of endocrine function of adipose tissue in anorexia nervosa: comparison of circulating levels versus subcutaneous mRNA expression. Clin Endocrinol (Oxf) 2007;67(5):674–8. doi: 10.1111/j.1365-2265.2007.02944.x. [DOI] [PubMed] [Google Scholar]
- 69.Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology. 2013;154(8):2687–701. doi: 10.1210/en.2012-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen W, Chen J, Gantz M, Punyanitya M, Heymsfield SB, Gallagher D, Albu J, Engelson E, Kotler D, Pi-Sunyer X, Shapses S. Ethnic and sex differences in bone marrow adipose tissue and bone mineral density relationship. Osteoporos Int. 2012;23(9):2293–301. doi: 10.1007/s00198-011-1873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheu Y, Cauley JA. The role of bone marrow and visceral fat on bone metabolism. Current osteoporosis reports. 2011;9(2):67–75. doi: 10.1007/s11914-011-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology. 1990;175(1):219–23. doi: 10.1148/radiology.175.1.2315484. [DOI] [PubMed] [Google Scholar]
- 73.McCabe LR. Understanding the pathology and mechanisms of type I diabetic bone loss. J Cell Biochem. 2007;102(6):1343–57. doi: 10.1002/jcb.21573. [DOI] [PubMed] [Google Scholar]
- 74.Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab. 2011;96(3):782–6. doi: 10.1210/jc.2010-1922. [DOI] [PubMed] [Google Scholar]
- 75.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18(5):641–7. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen W, Scherzer R, Gantz M, Chen J, Punyanitya M, Lewis CE, Grunfeld C. Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and Caucasian participants: the CARDIA study. J Clin Endocrinol Metab. 2012;97(4):1337–46. doi: 10.1210/jc.2011-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ackert-Bicknell CL, Shockley KR, Horton LG, Lecka-Czernik B, Churchill GA, Rosen CJ. Strain-specific effects of rosiglitazone on bone mass, body composition, and serum insulin-like growth factor-I. Endocrinology. 2009;150(3):1330–40. doi: 10.1210/en.2008-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carmona R, Pritz J, Bydder M, Gulaya S, Zhu H, Williamson CW, Welch CS, Vaida F, Bydder G, Mell LK. Fat composition changes in bone marrow during chemotherapy and radiation therapy. Int J Radiat Oncol Biol Phys. 2014;90(1):155–63. doi: 10.1016/j.ijrobp.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 81.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–71. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 82.Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ, Vittinghoff E, Siggeirsdottir K, Sigurdsson G, Oskarsdottir D, Shet K, Palermo L, Gudnason V, Li X. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98(6):2294–300. doi: 10.1210/jc.2012-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG. Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology. 2000;217(2):527–38. doi: 10.1148/radiology.217.2.r00nv20527. [DOI] [PubMed] [Google Scholar]
- 84.Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22(2):279–85. doi: 10.1002/jmri.20367. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y, Wang S, Bu S, Wang Y, Duan Y, Yang S. Treadmill training prevents bone loss by inhibition of PPARgamma expression but not promoting of Runx2 expression in ovariectomized rats. Eur J Appl Physiol. 2011;111(8):1759–67. doi: 10.1007/s00421-010-1820-0. [DOI] [PubMed] [Google Scholar]
- 86.Qin W, Bauman WA, Cardozo C. Bone and muscle loss after spinal cord injury: organ interactions. Ann N Y Acad Sci. 2010;1211:66–84. doi: 10.1111/j.1749-6632.2010.05806.x. [DOI] [PubMed] [Google Scholar]
- 87.Jee WS, Wronski TJ, Morey ER, Kimmel DB. Effects of spaceflight on trabecular bone in rats. Am J Physiol. 1983;244(3):R310–4. doi: 10.1152/ajpregu.1983.244.3.R310. [DOI] [PubMed] [Google Scholar]
- 88.Wronski TJ, Morey ER. Recovery of the rat skeleton from the adverse effects of simulated weightlessness. Metab Bone Dis Relat Res. 1983;4(6):347–52. [PubMed] [Google Scholar]
- 89.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007;104(45):17879–84. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24(1):50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Case N, Thomas J, Xie Z, Sen B, Styner M, Rowe D, Rubin J. Mechanical input restrains PPARgamma2 expression and action to preserve mesenchymal stem cell multipotentiality. Bone. 2013;52(1):454–64. doi: 10.1016/j.bone.2012.08.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149(12):6065–75. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meyer U, Romann M, Zahner L, Schindler C, Puder JJ, Kraenzlin M, Rizzoli R, Kriemler S. Effect of a general school-based physical activity intervention on bone mineral content and density: a cluster-randomized controlled trial. Bone. 2011;48(4):792–7. doi: 10.1016/j.bone.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 94.Casazza K, Hanks LJ, Hidalgo B, Hu HH, Affuso O. Short-term physical activity intervention decreases femoral bone marrow adipose tissue in young children: a pilot study. Bone. 2012;50(1):23–7. doi: 10.1016/j.bone.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smilios I, Tsoukos P, Zafeiridis A, Spassis A, Tokmakidis SP. Hormonal responses after resistance exercise performed with maximum and submaximum movement velocities. Appl Physiol Nutr Metab. 2014;39(3):351–7. doi: 10.1139/apnm-2013-0147. [DOI] [PubMed] [Google Scholar]
- 96.Menagh PJ, Turner RT, Jump DB, Wong CP, Lowry MB, Yakar S, Rosen CJ, Iwaniec UT. Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res. 2010;25(4):757–68. doi: 10.1359/jbmr.091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18(4):427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 98.Slade JM, Coe LM, Meyer RA, McCabe LR. Human bone marrow adiposity is linked with serum lipid levels not T1-diabetes. J Diabetes Complications. 2012;26(1):1–9. doi: 10.1016/j.jdiacomp.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146(8):3622–31. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148(1):198–205. doi: 10.1210/en.2006-1006. [DOI] [PubMed] [Google Scholar]
- 101.Grey A, Beckley V, Doyle A, Fenwick S, Horne A, Gamble G, Bolland M. Pioglitazone increases bone marrow fat in type 2 diabetes: results from a randomized controlled trial. Eur J Endocrinol. 2012;166(6):1087–91. doi: 10.1530/EJE-11-1075. [DOI] [PubMed] [Google Scholar]
- 102.Harslof T, Wamberg L, Moller L, Stodkilde-Jorgensen H, Ringgaard S, Pedersen SB, Langdahl BL. Rosiglitazone decreases bone mass and bone marrow fat. J Clin Endocrinol Metab. 2011;96(5):1541–8. doi: 10.1210/jc.2010-2077. [DOI] [PubMed] [Google Scholar]
- 103.Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, Donaldson MG, Cauley JA, Harris TB, Koster A, Womack CR, Palermo L, Black DM G. Study of Osteoporotic Fractures Research, G. Osteoporotic Fractures in Men Research, A. Health, G. Body Composition Research, Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. Jama. 2011;305(21):2184–92. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, Link TM. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013;28(8):1721–8. doi: 10.1002/jbmr.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C, Nevitt MC, Lynch J, McCulloch CE, Link TM. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the osteoarthritis initiative. J Magn Reson Imaging. 2012;35(2):370–8. doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Riggs BL, Khosla S, Melton LJ. 3rd, Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 107.Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008;19(9):1323–30. doi: 10.1007/s00198-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Justesen J, Mosekilde L, Holmes M, Stenderup K, Gasser J, Mullins JJ, Seckl JR, Kassem M. Mice deficient in 11beta-hydroxysteroid dehydrogenase type 1 lack bone marrow adipocytes, but maintain normal bone formation. Endocrinology. 2004;145(4):1916–25. doi: 10.1210/en.2003-1427. [DOI] [PubMed] [Google Scholar]
- 109.Lawson EA, Misra M, Meenaghan E, Rosenblum L, Donoho DA, Herzog D, Klibanski A, Miller KK. Adrenal glucocorticoid and androgen precursor dissociation in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(4):1367–71. doi: 10.1210/jc.2008-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, Wexler T, Herzog DB, Klibanski A. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94(12):4710–6. doi: 10.1210/jc.2009-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]