Abstract
The amygdala is a key structure mediating emotional processing. Few studies have used direct electrical stimulation of the amygdala in humans to examine stimulation-elicited physiological and emotional responses, and the nature of such effects remains unclear. Determining the effects of electrical stimulation of the amygdala has important theoretical implications for current discrete and dimensional neurobiological theories of emotion, which differ substantially in their predictions about the emotional effects of such stimulation. To examine the effects of amygdala stimulation on physiological and subjective emotional responses we examined epilepsy patients undergoing intracranial EEG monitoring in which depth electrodes were implanted unilaterally or bilaterally in the amygdala. Nine subjects underwent both sham and acute monopolar electrical stimulation at various parameters in electrode contacts located in amygdala and within lateral temporal cortex control locations. Stimulation was applied at either 50 Hz or 130 Hz, while amplitudes were increased stepwise from 1-12 V, with subjects blinded to stimulation condition. Electrodermal activity (EDA), heart rate (HR), and respiratory rate (RR) were simultaneously recorded and subjective emotional response was probed after each stimulation period. Amygdala stimulation (but not lateral control or sham stimulation) elicited immediate and substantial dose-dependent increases in EDA and decelerations of HR, generally without affecting RR. Stimulation elicited subjective emotional responses only rarely, and did not elicit clinical seizures in any subject. These physiological results parallel stimulation findings with animals and are consistent with orienting/defensive responses observed with aversive visual stimuli in humans. In summary, these findings suggest that acute amygdala stimulation in humans can be safe and can reliably elicit changes in emotion physiology without significantly affecting subjective emotional experience, providing a useful approach for investigation of amygdala-mediated modulatory effects on cognition.
Keywords: Amygdala, Brain Stimulation, Autonomic Reactivity, Psychophysiology, Electrodermal Activity, Heart Rate, Emotional Experience
The amygdala is an almond-shaped brain structure in the medial temporal lobe that is implicated in a wide range of emotional functions. The amygdala is comprised of multiple subnuclei that differ in their function and projections, yielding extensive modulatory connections to widespread brain regions (Adolphs et al., 1995; Pessoa, 2008; Pessoa and Adolphs, 2010; Ledoux, 2000; Burgdorf and Panksepp, 2006). The role of the amygdala in mediating emotional responses has attracted particular attention with respect to subjective responses (e.g., fear) and physiological affective changes in autonomic nervous system (ANS) activity (Mangina and Beuzeron-Mangina, 1996; Asahina et al., 2003; Guillory and Bujarski, 2014). Considerable evidence from nonhuman animal, human neuroimaging, and lesion studies have shown that the amygdala modulates ANS activity in the context of the processing of affectively salient stimuli (Adolphs et al., 1994; Ledoux, 2000; Bradley et al., 2001; Critchley, 2002; Critchley, 2005; Feinstein, Adolphs, Damasio, & Tranel, 2011). Amygdala lesions have been observed to blunt normal physiological and subjective emotional responses to some types of emotionally salient stimuli, particularly fear-related responses to aversive stimuli (Adolphs et al., 1997; Ledoux, 2017; Davis and Whalen, 2001), though the amygdala’s role in emotion likely extends beyond aversive stimuli and fear to encompass positive emotion, reward, and aspects of social processing (Adolphs, 2010; Anderson & Adolphs, 2014; Hamann et al., 1999).
An important current debate in the cognitive neuroscience of emotion concerns the neural representation of emotion in the human brain. Key in this debate is whether individual brain regions have specific emotion functions, or alternately, whether emotions are an emergent property of processing that is distributed across multiple brain regions and networks. Reflecting this debate, current neurobiological theories of emotion propose markedly different functions of the amygdala in mediating emotional responses (Anderson & Adolphs, 2014; Vytal and Hamann, 2010; Barrett and Satpute, 2013; Lindquist et al., 2012; Ekman, 1992; Bradley et al., 2001; Barrett et al., 2007). One theoretical issue concerns whether the amygdala specifically mediates the basic emotion of fear, or alternately, whether the amygdala’s role is more general and intrinsic to brain networks mediating multiple emotions (Hamann, 2012; Lindquist et al., 2012; Pessoa, 2010). A closely related issue is the extent to which the amygdala may mediate the emotional dimensions of intensity (arousal) or valence (degree of pleasantness or unpleasantness) that have been proposed by influential psychological dimensional theories of emotion (Barrett and Satpute, 2013; Hamann, 2012; Bradley et al., 2001; Pessoa, 2010).
Although human neuroimaging and neuropsychological lesion studies have provided important evidence regarding the neural representation of emotion in the human brain and the role of the amygdala, both approaches have important limitations (Adolphs, 2016; Vytal and Hamann, 2010; Barrett and Satpute, 2013). Neuroimaging can provide correlational evidence suggesting the amygdala’s role in emotion, but such evidence cannot be used to infer a causal or necessary role of the amygdala. Neuropsychological studies of patients with brain lesions incorporating the amygdala have reported variable impairments in the subjective experience of fear in some patients with bilateral amygdala lesions (Feinstein et al., 2011; Adolphs et al., 1999). However, although studies of patients with bilateral lesions have contributed greatly to understanding of amygdala function, such patients are rare and thus these studies have limitations related to small sample sizes, individual differences in post-lesion neural reorganization, developmental differences, and other factors. In addition, human lesions caused by degenerative, ischemic, or post-surgical processes are typically not restricted to the amygdala, although a few patients with bilateral lesions restricted to the amygdala have been extensively studied (Adolphs et al., 1999; Adolphs, 2016).
By contrast, acute electrical brain stimulation of the amygdala and other brain regions may provide more direct causal evidence for specific emotional functions (Burgdorf et al., 2000; Panksepp, 1982), complementing the correlational evidence obtained using methods such as functional neuroimaging and electrophysiological recording (Rutishauser et al., 2015). Discrete focal brain stimulation can produce reliable, temporally precise changes in electrophysiological brain states, measures of peripheral physiology, and subjective mental state in awake human subjects, lending causal evidence to evaluate the predictions of current neurobiological theories of emotion (Mayberg et al., 2005; Selimbeyoglu and Parvizi, 2010; Guillory and Bujarski, 2014). In the current study, we examined both subjective and psychophysiological responses to direct electrical stimulation of the amygdala. While direct amygdala stimulation has been reported in a few studies to elicit subjective emotional responses and changes in emotional physiology, the relationships among stimulation dosage and subjective and physiological measures of emotion have not been determined (Lanteaume et al., 2007; Meletti et al., 2006; Bijanki et al., 2014; Smith et al., 2006).
Several studies have examined the subjective emotional responses associated with stimulation of several different brain regions (Guillory and Bujarski, 2014). In contrast, the current study focused specifically on the amygdala, a region which has been investigated less frequently and systematically (Halgren et al., 1978; Halgren et al., 1992; Mangina and Beuzeron-Mangina, 1996; Meletti et al., 2006; Lanteaume et al., 2007). Four studies have used stimulation mapping of the amygdala and other brain structures in epilepsy patients undergoing intracranial electroencephalographic (iEEG) monitoring to examine the effects of electrical stimulation on subjective emotional experience (Lanteaume et al., 2007; Meletti et al., 2006; Bijanki et al., 2014; Smith et al., 2006). Electrical stimulation of the amygdala typically elicits subjective responses relatively rarely (Meletti et al., 2006). For example, Meletti and colleagues reported evoked subjective emotional responses in only 12% of amygdala stimulation trials. With respect to the nature of elicited subjective responses, stimulation to the amygdala can induce both positive and negative emotional experiences (e.g., elation or fear), although negative emotional responses are more frequently observed (Bijanki et al., 2014; Lanteaume et al., 2007; Meletti et al., 2006; Smith et al., 2006). Across stimulation of various regions in the left or right hemisphere, Smith et al. (2006) found that right-hemisphere stimulation produced more dysphoric responses than left-hemisphere stimulation. Similarly, Lanteaume et al. reported that right amygdala stimulation elicited only negative responses (e.g., fear and sadness), whereas left amygdala stimulation elicited both negative and positive responses. In addition, Lanteaume et al. found that amygdala stimulation elicited a larger electrodermal response (EDA) when a subjective emotional experience was also elicited, relative to stimulation conditions in which no emotional experience was elicited. In contrast, Bijanki et al. (2014) reported a significant positive response with relatively high-amplitude, intermittent stimulation (15 V, 5 s On, 5 s Off) to the right amygdala in a patient with severe comorbid depression. Taken together, these studies establish that direct amygdala stimulation can elicit changes in emotion physiology (EDA only) and subjective emotional experience. However, the extent to which these autonomic effects (EDA, heart rate, or respiration) are dependent upon the dose of stimulation (i.e., stimulation amplitude) and the presence or absence of a subjective emotional response, has yet to be established.
Importantly, emotional responses involve changes in three primary domains: neural, behavioral/subjective, and physiological (Bradley and Lang, 2000a; Bradley and Lang, 2000b; Lang, 1995; Lang et al., 1997; Hamann, 2001). For example, encountering a poisonous snake elicits brain activation related to processing threat, behavioral responses such as recoiling or freezing, and physiological changes in autonomic nervous system activity, such as changes in heart rate and skin conductance. The extent to which changes in each affective domain are mediated independently by the amygdala remains to be fully characterized. Accordingly, in the current study we examined how direct electrical stimulation of the amygdala influenced concurrent physiological and behavioral/subjective responses, relative to sham stimulation and stimulation to a positive control region (i.e., the lateral temporal cortex). In addition, the present study examined changes in autonomic reactivity to varying parameters of direct electrical stimulation to the human amygdala (stimulation amplitude, hemisphere, and frequency). Specifically, we measured changes in electrodermal activity (EDA), heart rate (HR), and respiration rate (RR) while delivering long (30 s), intermittent electrical stimulation trains to the human amygdala. We also examined whether there was a relationship between these autonomic changes and the subjective experience of emotion. We hypothesized that even below subjective thresholds, stimulation would produce an amplitude- and location-dependent increase in autonomic arousal when stimulating the amygdala, shown by increased SCR, decreased heart rate, and respiration consistent with orienting and defensive responses observed with aversive visual stimuli in humans (Bradley et al., 2001; Lang & Bradley, 2010) and previous studies strongly implicating the amygdala in emotional arousal processes (Adolphs, 2010; Anderson, 2003; Lang & Bradley, 2010).
Methods
2.1 Subjects
Nine patients undergoing intracranial EEG monitoring for drug-resistant epilepsy were recruited to participate in the study (4 female; all right-handed; M(SD)age=36(10); see Table 1 for detailed patient demographics and epilepsy information). All patients gave written informed consent to a study protocol approved by the Emory University Institutional Review Board. In all 6 of 9 subjects in which functional MRI was obtained, left-hemisphere language dominance was confirmed. Standard stereotactic EEG depth electrode arrays (Ad-Tech; 0.86 mm diameter, 2 mm length platinum-coated contacts, spaced along 5 mm intervals) were implanted into the brain parenchyma by either of two neurosurgeons (J.T.W., R.E.G.) for the sole purposes of clinical seizure investigation. One patient was excluded from all further autonomic analysis due to poor psychophysiology recordings (S9). All 9 analyzed patients had stimulated electrode contacts localized within the amygdala (Figure 1). On average, patients were monitored in the Emory University Hospital Epilepsy Monitoring Unit for 2 weeks. Study inclusion required subjects to be English speaking adults (>18 years, regardless of gender, race or ethnicity), implanted with intracranial depth electrodes, including those localized to the left and/or right amygdala. All patients had low average to average full scale intelligence quotients and were able to converse and respond to questions about their subjective feelings. Subjects also had to be able to understand an informed consent (comprehend potential risks and benefits), and give written and verbal informed consent to all experiments.
Table 1.
Demographics and Epilepsy Table. L, Left; R, Right; Sz, Seizure; M, Male; F, Female. ICM, Intracranial Monitoring; SLAH, Stereotactic Laser Amygdalohippocampectomy; *
| Subject | Sex | Age | Stimulation Hemisphere | Prior Resection | Post-ICM Surgery | Outcome |
|---|---|---|---|---|---|---|
| 1 | F | 36 | L & R | None | Further ICM | Multifocal Sz |
| 2 | F | 45 | L | None | R SLAH | Sz Free |
| 3 | M | 30 | L | R Frontal Resection | R Frontal Laser Ablation | Sz Free |
| 4 | F | 36 | L | R Temporal Lobectomy | R Inferio-Temporal Resection | Sz Free |
| 5 | M | 47 | R | None | L Inferior Temporo-Parietal Resection | Not Sz Free |
| 6 | F | 23 | R | None | None | Multifocal Sz |
| 7 | M | 44 | R | None | None | Sz Free |
| 8 | M | 26 | L & R | R Lateral Temporal Astrocytoma | R Lateral Temporal Laser Ablation | Sz Free |
| 9 | M | 46 | R | None | R Anterior Temporal Lobectomy | Not Sz Free |
Figure 1. Precise localization of stimulation sites overlaid on illustrated coronal slices through the human amygdala.

Black circles indicate estimated centroids of stimulation in or near BLA in all 9 patients (white border on circles denotes right-sided stimulation). Distance in mm from the AC (0,0,0) point in the anterior to posterior direction (y-axis). Adapted with permission from Mai atlas. BLA = basolateral complex of the amygdala; La = lateral amygdala nucleus; BL = basolateral amygdala nucleus; BM = basomedial nucleus; BLVM = basolateral amygdala, ventromedial Part; Me = medial amygdala nucleus; Ce = central amygdala nucleus; ACo = anterior cortical amygdala nucleus; PCo = posterior cortical amygdala nucleus; AC = anterior commissure; TH-LV = temporal horn of the lateral ventricle; Cl = claustrum; Peri = perirhinal cortex; EC = entorhinal cortex; PHG = parahippocampal gyrus; CA1 = CA1 field of the hippocampus; GP = globus pallidus; Put = putamen; PuV = ventral putamen; st = stria terminalis; Sub = subiculum; PrS = pre-subiculum; AHi = amygdalahippocampal area; BLPL = basolateral nucleus, paralaminar part.
2.2 Stimulation Electrode Localization
High-resolution pre-surgical and post-implantation anatomical scans were gathered on each patient (T1 MPRAGE, Siemens 1.5T, TR = 1900, TE = 3.5). We applied the following method to localize the precise position of the stimulating electrode contacts relative to the BLA using custom MATLAB scripts (Fig. 1). Preoperative and postoperative T1 images were aligned with an automated linear co-registration using the FLIRT module of FSL (Jenkinson and Smith, 2001), which was then corrected for postsurgical tissue displacement and deformation using a manually-guided nonlinear thin-plate spline warping (Bookstein, 1989; Rohr et al., 2001). Control points for the nonlinear warping were selected at locations where the anatomical correspondence between pre- and post-operative images could be unambiguously identified in a side-by-side visual comparison, with emphasis on features bounding the electrode location as closely as imaging artifacts allowed. Amygdala nuclei were then projected into the space of the preoperative image through nonlinear warping of deformable meshes representing the structures of interest. The meshes were obtained by digitizing a stereotactic atlas of the human brain (Mai et al., 2008; Oya et al., 2009) and were projected into the pre-operative image space by first aligning the outer boundary of the atlas-derived amygdala with an amygdala boundary surface obtained through automated subcortical segmentation (FSL FIRST; Patenaude et al., 2011), with manual adjustment of the latter to improve accuracy, when necessary. These respective surfaces then provided control points for a nonlinear thin-plate spline warping, which allowed the atlas-derived meshes to be projected into the space of the subject’s preoperative image. Locations of stimulating and recording electrodes in the amygdala were further verified retrospectively in each patient, by automated co-registration of each postoperative structural brain T1 MRI and head CT images with each preoperative brain MRI using a stereotactic neurosurgical planning computer workstation (ROSA Surgical Planning Software, MedTech Surgical, Inc., New York, NY, USA). A neurosurgeon (J.T.W.) directly compared contact locations to standard MRI and tissue sections atlases of the human brain (Duvernoy, 2005; Mai et al., 2008). Prior prospective BLA localizations were consistent with this direct retrospective method.
2.3 Procedure
2.3.1. Stimulation Experiment
Amygdala stimulation was alternated with stimulation at lateral temporal lobe (i.e. middle temporal gyrus) control locations and a sham, no-stimulation condition presented unpredictably interspersed between trials of escalating stimulation amplitude, with the patient blinded to stimulation condition. The patient was asked to sit quietly and minimize movement throughout testing. Respiration, heart rate, and intracranial EEG were continuously monitored by a neurologist or neurosurgeon. No after-discharges or seizure-like activity were detected during any stimulation periods included in the analysis.
Stimulation amplitude (V, Volts) and frequency (Hz, Hertz) were manipulated within a block of either amygdala or lateral temporal cortex control stimulation. A block was constituted by stimulation within one region, for one set of amplitudes and frequency, with randomly interspersed sham control trials. Stimulation was delivered using a voltage-controlled clinical neurostimulator typically used to stimulate percutaneous leads for treatment of movement and pain disorders (Model 3628 Dual Screen, Medtronic, Inc., Minneapolis, MN, USA) that utilized a bidirectional pulse pair designed to avoid thermal and electrolytic damage. Each trial consisted of stimulation for 30 seconds on and at least 30 seconds off. These stimulation and inter-trial interval durations were selected to maximize the number of parameters that could be tested in a given session (~90 min), while allowing adequate time between each stimulation trial for autonomic measures (described below) to return to baseline, as determined by real time data interpretation by a researcher (C.S.I), before beginning the next trial. After each stimulation, patients were asked to report any emotional or physiological sensation and the 30 second intertrial interval began at least 15 seconds after the patient had stopped talking to allow for physiological measures to return to a steady baseline.
Frequency was set to either 50 or 130 Hz with a pulse width 300 or 90 μsec, respectively. Pulse width was adjusted with frequency to maintain approximate equivalence in the charge density delivered between the two frequencies. Initially in early subjects, 50 and 130 Hz were tested, but after finding these frequencies to be indistinguishable, we performed stimulation only at 50 Hz thereafter. Stimulation amplitude varied in a stepwise fashion in either 1 or 2 V increments from 1 to 12 V, with subjects blinded to stimulation condition. It was considered desirable to escalate voltages in a stepwise manner (interspersed with sham stimulation) in the design of the study to a) determine the lowest dosage of any individual subjective responses that might confound subsequent trials by carry-over effects and to b) minimize the risk that eliciting afterdischarges or seizures (although neither were detected in this study) at higher dosages would preclude data collection.
The clinician delivering the stimulation stated audibly “On” and “Off” at the onset and offset of each stimulation trial, regardless of active stimulation, positive control (lateral temporal cortex), or sham condition (see Supplementary Tables 1 and 2 for average trial counts per condition). To summarize, participants received blocked stimulation including either a) active amygdala stimulation interleaved with sham or b) active positive control stimulation targeting non-limbic control site (lateral temporal lobe neocortex) interleaved with sham. Stimulation was delivered on a voltage-controlled basis in stepwise ascending order. Sham trials were interspersed unpredictably with actual stimulation trials to blinded subjects (Figure 2). The research procedure session was completely independent of any clinical mapping procedures (e.g. cortical function and/or seizure network mapping), and the only communication in the room was the announcement of the beginning and end of trials referred to aloud as an ascending condition number (e.g. “Condition 1 on”, Condition 1 off”, “Condition 2 on”, “Condition 2 off”, etc.), with the associated stimulation target (i.e. amygdala or lateral temporal lobe) and parameters (actual versus sham stimulation) being predetermined by the investigators and unknown to the subject. The patient was asked to comment on any subjective experience after each and every trial, including sham conditions.
Figure 2.

Illustration of one block of the stimulation procedure. Participants received blocked stimulation including either a) active amygdala stimulation interleaved with sham or b) active positive control stimulation targeting non-limbic control site interleaved with sham. Stimulation was delivered on a voltage-controlled basis (V, Volts) in stepwise ascending order. Sham trials were interspersed unpredictably with actual stimulation trials to blinded subjects. Each trial consisted of stimulation for 30 seconds on and at least 30 seconds off. Electrodermal Activity (EDA), Heart Rate (HR), and Respiration Rate (Resp) were measured throughout all stimulation blocks.
While we did not directly measure impedance for every stimulation electrode, we did determine the impedance range of depth electrode contacts to be between 800 and 1000 Ohms in a patient by measuring multiple contacts (localized to gray matter by post-implant MRI) along different electrode arrays using the impedance measurement function of a clinical radiofrequency lesion generator (G4, Cosman Medical, Inc. Burlington, MA) connected by alligator clips to externalized electrode tails. These measurements are consistent with the reported impedance range of Ad-Tech reduced diameter depth electrode contacts that provide valid measurements of iEEG activity. Using this impedance (resistance) range, the study voltage range of 1-12 V corresponds to expected currents of 1-1.25 mA for 1 V and 12-15 mA for 12 V (overall range ~1-15 mA), according to Ohm’s Law.
2.3.2. Measures of autonomic response
Electrodermal activity (EDA), electrocardiogram (ECG), and respiration (RSP) were recorded while behavior was monitored and videotaped. Data were recorded and analyzed using Acqknowledge 4.2 with a MP150 amplifier and wireless Bionomadix transceivers (Biopac, Inc., Goleta, CA, USA). Heart rate (HR) and respiration rate (RR) were calculated from the ECG and RSP data, respectively. EDA leads were placed on the left and right palms for each patient to minimize movement and dropout artifacts that are often found when recording from the fingers. For each subject, whichever hand recording was of the highest quality (the least occurrence of signal dropout artifacts) was selected for analysis throughout the entire session, with the lower quality recording being completely omitted from further analysis. EDA amplitude (natural log (ln) transformed to correct for known skew in EDA distributions; Boucsein et al., 2012) was calculated as the magnitude of the trough-to-peak change in EDA from stimulation onset to offset and in two 15-second intervals during stimulation. All changes in EDA amplitude were z-scored within each patient to adjust for overall levels of EDA reactivity across patients. The ECG leads were placed just under the patient’s right collar bone (cathode), just below the patient’s lowest left rib (anode), and on the patients left collar bone (ground) in a lead III configuration. All dropout artifacts were corrected with linear interpolation from the last physiological signal to the next physiological signal. Heart rate was calculated using a sliding window assessment of the ECG R-waves (R-to-R interval). The respiratory data were collected using a chest band with force transducer. All artifacts were manually smoothed using the Acqknowledge software by linearly interpolating across the nonphysiological recording. For respiration rate analyses, after apparent movement artifacts were corrected with linear interpolation, the respiration signal was first resampled from 1000 samples/s to 62.5 samples/s, then digitally band pass filtered between 0.5 Hz and 1 Hz with a Finite Impulse Response function (FIR) to remove slow and fast variations in the signal that were likely non-respiratory. The respiration rate calculation, used the positive peaks of the respiration signal and calculated the time between these peaks to generate an ongoing change in respiration rate over time in accordance with the hardware/software manufacturer’s instructions (Biopac, Inc; https://www.biopac.com/knowledge-base/respiration-recording/). Average changes in HR and RR were calculated after subtracting a 15 second pre-stimulus baseline for the 30-second stimulation periods and in 15-second windows across the stimulation periods.
2.4 Statistical analyses
Mixed linear models with random and fixed effects were used to estimate the effects of stimulation voltage, location (amygdala, lateral temporal control), and hemisphere (left, right) on three outcome measures of autonomic arousal. Outcome variables included 1) natural log-transformed (ln) maximum of EDA amplitude across 30 seconds of stimulation, 2) change in average HR across 30 seconds of stimulation compared to pre-stimulus baseline, and 3) change in average RR across 30 seconds of stimulation compared to pre-stimulus baseline. EDA amplitude was very strongly skewed, and therefore was ln-transformed to better meet the model assumptions of normality and to allow for greater interpretability of the parameter estimates than other normalization methods (z-scoring or square root transformation). The overall model design was as follows:
Yi is a vector of the outcome responses for the ith subject and i = 1 to 7, where β0 represents the model intercept, β1 represents stimulation volts (continuous variable, fixed effect), β2 represents location of stimulation (indicator variable, fixed effect), β3 represents hemisphere (indicator variables, fixed effects), and γ01 indicates a random intercept modeled for each participant. Modeling was performed with SAS 9.4 using the proc mixed procedure. The two patients that had subjective responses to higher voltages of amygdala stimulation (S8 and S9) were excluded from the models because they had notably different subjective responses to the stimulation.
Results
Linear mixed models were fit to a data set collected from 7 participants who completed an average of 45 trials each (range 22-98) across a stimulation range of 0 to 12 volt amplitudes. 84% of trials were stimulated below 9 V; there were 6 trials at 11 volts and 13 trials at 12 volts. Testing also included 72 single-blinded sham trials interleaved with active stimulation conditions. 150 active trials took place in the amygdala, and 91 trials at positive control locations (lateral temporal cortex), for a total of 241 total active stimulations. Of those, 101 were to the left hemisphere, 127 to the right hemisphere, and 13 were bilateral stimulations (including both amygdala trials and positive control trials). 201 trials were completed at 50 Hz, 40 trials were completed at 130 Hz. Differences in frequency (50 Hz vs. 130 Hz) did not contribute significantly to differences in autonomic responses, and thus frequency was not included as a parameter in the final models. Typical raw traces from several stimulation and sham condition trials an exemplar patient are presented in Figure 3 (S1). From visual inspection of the raw data of this exemplar and all other patients, nearly immediate (within 3 seconds) changes in EDA and HR are apparent.
Figure 3.

Example dose response from one patient that did not have a concurrent emotional experience for A) electrodermal activity (EDA), B) Heart Rate, and C) Respiration in arbitrary units from a maximal expansion to contraction of the chest band with force transducer. Note that electrodermal activity markedly increased and heart rate markedly decelerated with higher stimulation amplitudes, while respiration rate is similar across conditions. V, Volts; BPM, Beats Per Minute; μS, microsiemens.
3.1 Electrodermal Activity (EDA)
The mixed linear model to predict EDA amplitude measures included the following predictors: stimulation voltage, location (amygdala, positive control), and hemispheric laterality (left, right). Bilateral stimulation was excluded from the model due to an inadequate number of trials relative to other conditions. Voltage was a significant linear predictor of the change in EDA amplitude (p = 0.002; see Table 2 for full model statistics); every 1 V increase of amygdala stimulation is predicted to evoke a 1.06 microsiemen (μS) change in skin conductance, controlling for effects of other predictors. Given this observation, this model would predict that 12 volts of stimulation would lead to a 12.77 μS increase in EDA amplitude. The application of stimulation to the amygdala as opposed to sham also significantly predicted increased EDA amplitude (p = .01), while positive control location stimulation was not a significant predictor of change in EDA amplitude in this model relative to sham trials (p = .12). Using post-hoc contrasts to compare left versus right amygdala stimulation the model would predict that left amygdala stimulation would produce a 2.01 μS greater increase in EDA than right amygdala stimulation. See Figure 4 for plots of the amplitude-dependent effects of amygdala stimulation on change in EDA per patient.
Table 2.
Mixed linear effects model for each autonomic arousal outcome variable.
| Outcome Variable | Predictors | Coef. β | SE(β) | df | t | p |
|---|---|---|---|---|---|---|
| Electrodermal Response | ||||||
| Intercept | −1.12 | 0.43 | 6 | −2.59 | 0.04 | |
| Volt Level | 0.06 | 0.02 | 301 | 3.59 | 0.0004 | |
| Amygdala | 0.74 | 0.29 | 301 | 2.52 | 0.01 | |
| Positive Control | 0.47 | 0.3 | 301 | 1.58 | 0.12 | |
| Left Hemisphere | −0.7 | 0.29 | 301 | −2.47 | 0.01 | |
| Right Hemisphere | −0.55 | 0.28 | 301 | −1.96 | 0.051 | |
| Change in Heart Rate | ||||||
| Intercept | −1.48 | 0.54 | 6 | −2.74 | 0.03 | |
| Volt Level | −0.24 | 0.07 | 301 | −3.15 | 0.002 | |
| Amygdala | −1.86 | 0.51 | 301 | −3.67 | 0.0003 | |
| Left Hemisphere | 1.47 | 0.77 | 301 | 1.91 | 0.06 | |
| Right Hemisphere | 1.79 | 0.74 | 301 | 2.4 | 0.017 | |
| Change in Respiration Rate | ||||||
| Intercept | −0.84 | 0.76 | 4 | −1.11 | 0.33 | |
| Volt Level | −0.21 | 0.07 | 244 | −2.89 | 0.004 | |
| Amygdala | −0.89 | 0.52 | 244 | −1.73 | 0.09 | |
| Left Hemisphere | 1.11 | 0.84 | 244 | 1.32 | 0.19 | |
| Right Hemisphere | 0.24 | 0.71 | 244 | 0.33 | 0.74 |
Figure 4.
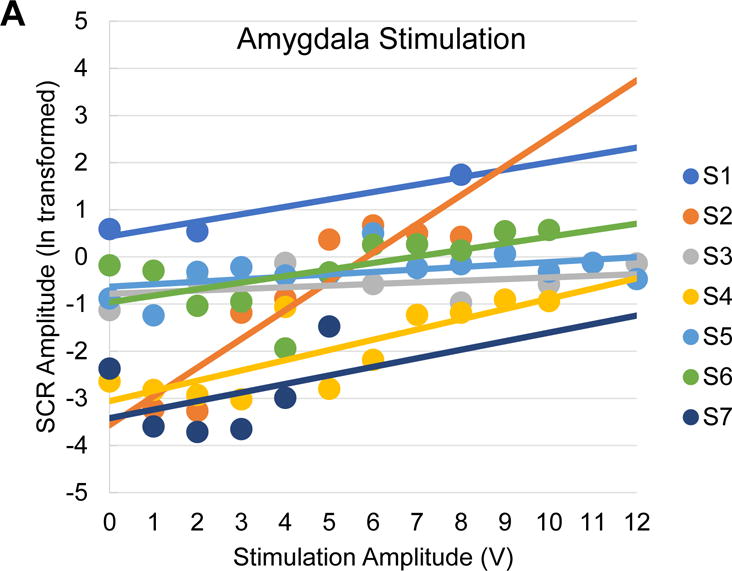

Amplitude-dependent effects of amygdala stimulation on electrodermal responses. A, Scatter plot of mean skin conductance response (SCR) amplitudes for each patient as a function of amygdala stimulation voltage (dots) with a patient-wise linear trend line. Stimulation-related changes in EDA is indicated by SCR amplitude. Note the consistency of increases in SCR amplitude with increases in stimulation voltage. B, Scatter plot of mean SCR amplitudes for each patient as a function of positive control (lateral temporal cortex) stimulation voltage (dots) with a patient-wise linear trend line. Note the inconsistency of changes in SCR amplitude with increases in stimulation voltage. SCR, Skin Conductance Response; μS, microseimens.
3.2 Heart Rate (HR)
The mixed linear model to predict a change in HR included the following predictors: stimulation voltage, location (amygdala only), hemisphere (left, right). Bilateral stimulation was excluded from the model due to an inadequate number of trials relative to other conditions. The impact of positive control stimulation upon heart rate did not significantly differ from sham stimulation, so the model includes sham and positive control as a pooled comparison group. Voltage was a significant predictor of the change in HR (p = 0.002; see Table 2 for full model statistics), with every 1 V increase of amygdala stimulation leading to a −0.24 beat per minute (BPM) decrease in HR, holding all other predictors constant. Given this observation, this model would predict that 12 volts of stimulation would lead to a −2.8 BPM decrease in HR. Stimulation to the amygdala as opposed to the positive control and sham, significantly predicted a decrease in HR (p = .0003). Using post-hoc contrasts to compare left versus right amygdala stimulation, we found that left amygdala stimulation would be predicted to elicit a −2.18 BPM greater deceleration in heart rate than right amygdala stimulation. See Figure 5 for plots of the amplitude-dependent effects of amygdala stimulation on change in HR per patient.
Figure 5.


Amplitude-dependent effects of amygdala stimulation on change in heart rate. A, Scatter plot of mean change in heart rate for each patient as a function of amygdala stimulation voltage (dots) with a patient-wise linear trend line. Note the consistency of decreases in heart rate with increases in stimulation voltage. B, Scatter plot of mean change in heart rate for each patient as a function of positive control (lateral temporal cortex) stimulation voltage (dots) with a patient-wise linear trend line. Note the inconsistency of changes in heart rate with increases in stimulation voltage. BPM, Beats Per Minute; V, Volts.
3.3 Respiration Rate (RR)
The mixed linear model to predict changes in RR included the following predictors: stimulation voltage, location (amygdala only), and hemisphere (left, right). Bilateral stimulation was excluded from the model due to an inadequate number of trials relative to other conditions. The impact of positive control stimulation upon respiration rate did not significantly differ from sham stimulation, so the model includes sham and positive control as a pooled comparison group. Voltage was a significant predictor of the change in RR (p = 0.004; see Table 2 for full model statistics), with every 1 V increase of amygdala stimulation leading to a −0.21 breaths per minute (BrPM) decrease in RR, holding all other predictors constant. Given this observation, this model would predict that 12 volts of stimulation would lead to a −2.5 (BrPM) decrease in RR. Amygdala stimulation was a non-significant predictor of a change in RR relative to sham and positive control trials (p = .08). For contrasts based on the hemisphere of stimulation, left and right hemisphere stimulation were non-significant predictors of change in RR relative to sham trials (p = 0.19 and p= 0.73, respectively). When directly contrasting left versus right amygdala stimulation, our model predicts a −0.021 BrPM difference between left and right amygdala stimulation where left stimulation produces a stronger suppression in respiratory rate. See Figure 6 for plots of the amplitude-dependent effects of amygdala stimulation on change in respiration per patient.
Figure 6.
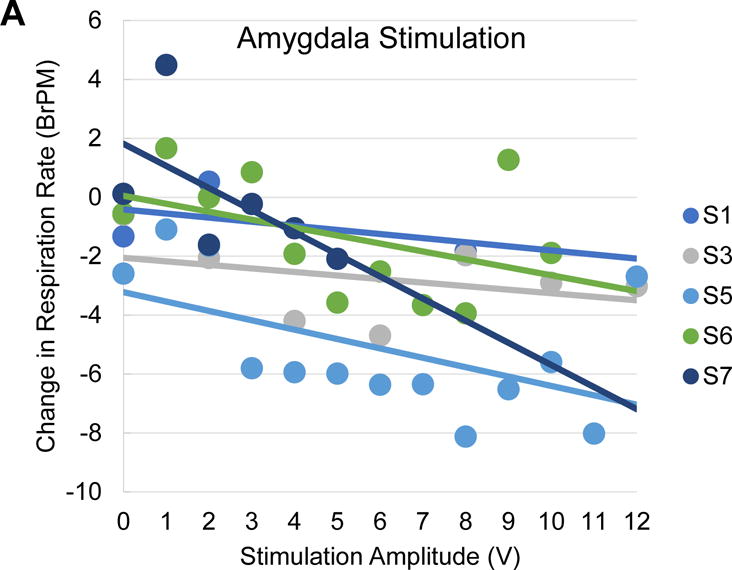

Amplitude-dependent effects of amygdala stimulation on change in respiration rate. A, Scatter plot of mean change in respiration rate for each patient as a function of amygdala stimulation voltage (dots) with a patient-wise linear trend line. Note the consistency of decreases in respiration rate with increases in stimulation voltage. B, Scatter plot of mean change in respiration rate for each patient as a function of positive control (lateral temporal cortex) stimulation voltage (dots) with a patient-wise linear trend line. Note the inconsistency of changes in respiration rate with increases in stimulation voltage. BrPM, Breaths Per Minute.
3.4 Emotional and autonomic changes in patients with subjective emotional responses
Only two of the nine patients (subjects 8 and 9) had amplitude-dependent subjective emotional responses during stimulation. Both had stimulated electrodes that were within the basolateral amygdala complex and resembled those of several other subjects that did not report subjective experiences. Subject 8 was excluded from the mixed linear models because of apparent differences in his autonomic response to stimulation above their subjective emotional response threshold, while subject 9 was excluded from any psychophysiological modeling analyses due to insufficient autonomic recordings. Similar to most subjects reported in this study, subject 9 reported no subjective affective responses with unilateral stimulation to the left or right amygdala. With bilateral stimulation, subject 9 experienced an olfactory sensation in which he smelled an unfamiliar “burnt peppermint” odor between 4-8 V and with bilateral stimulation at 10 V, two instances of a spontaneous brief laughter and when asked to describe this initially he used descriptors like ‘joy’ and ‘weird’ (130 Hz, 300 μS pulse width) and one instance of an anxious sensation in which he described a mild unpleasant sense that everyone that had been in the room with him now felt unfamiliar (50 Hz, 300 μS pulse width). The patient denied that these experiences were anything like his typical auras, and no after-discharges or other seizure-like activity were observed.
Subject 8 had both a subjective response to right and left amygdala stimulation and sufficient autonomic recordings. He described a much more vivid and reliable subjective experience of fear that increased with stimulation amplitudes between 5 and 8 V (see video 1 in supplementary materials). This patient did not report feeling any anxiety or fear during any sham trials or during stimulation between 1-4 V and had no knowledge of the brain area or hemisphere that was being stimulated. Specifically, after receiving 5 V stimulation to his right amygdala for 30 seconds, S8 spontaneously reported:
“It just felt like, um, half of my body was scared. You know when you get scared and you get that attack. It pretty much just goes down the back of your neck, throughout the rest of your body. It’s just only on my left side.”
At 6 V he reported:
“It was, um, it was terrifying, it was just…it was like I was about to get attacked by a dog. Like the moment, like someone unleashes a dog on you, and it’s just like it’s so close, and you feel like you’re going to **** your pants. It’s terrifying. It’s not pain as much as fear.”
He reported feeling similar at 7 V and afterwards also spontaneously reported “this is fun.” He further explained that he could distinguish feelings in his body that would normally be associated with fear recognized the absence of an actual threat, making the experience “fun”. He reported no sensations during sham trials that were randomly interspersed with stimulation amplitudes that elicited an emotional response. At 8 V he asked to stop 15 seconds in to the stimulation and reported:
“That was so scary it was nauseating. It’s like, um, I went zip-lining a few weeks ago…and this was worse…it’s just, that last one, it just felt like I was leaving my body. It was so intense”
After describing the initial feeling, he was able to laugh and make jokes to the researchers and his wife. He even went as far as saying “I could do it again”. A similar fear effect was replicated as we stepped down from 8 V back to 1 V of stimulation in 30 sec on/30 sec off blocks (the patient did not know the stimulation amplitude was stepping down because he was blinded to the condition). A similar subjective fear effect was replicated several times without autonomic recordings one week later with the same stimulation parameters. None of these stimulation amplitudes provoked any signs of after-discharges or electrographic seizure activity. Stimulation was also delivered to this patient’s left amygdala immediately after the initial right amygdala stimulation, with sham conditions randomly interspersed. Left amygdala stimulation produced a much weaker fear response at 5 V of stimulation in which he reported that he felt a negative feeling of “shame” similar to being “sent to the corner.” Left amygdala stimulation was discontinued after 5 V due to emergence of after-discharges above this amplitude.
Subject 8’s emotion physiology responses were also voltage-dependent, but showed a different pattern of responsivity across the EDA, HR, and RR that was concurrently recorded during amygdala stimulation (Fig. 7 and Fig. 8). In particular, he showed a voltage-dependent increase in EDA amplitude, with stimulation amplitudes greater than 4 V producing large changes in EDA amplitude immediately after the onset of stimulation. In contrast to the findings from patients with no subjective response, this patient showed a notable increase in HR with greater stimulation amplitudes. RR did not appear to change significantly during stimulation trials. As in all of the other patients, sham trials and control location stimulation did not produce any significant change in EDA, HR, or RR.
Figure 7.

Example dose response from one patient that had a concurrent negative fear response at higher stimulation amplitudes. Note that electrodermal activity and heart rate markedly increases with higher stimulation amplitudes to the right amygdala, but for higher levels of left amygdala stimulation the data show an initial decrease in heart rate. V, Volts; BPM, Beats Per Minute; μS, microsiemens.
Figure 8.

Amplitude-dependent effects of amygdala stimulation on change in electrodermal activity and heart rate in a patient with an emotional fear response to high stimulation amplitudes. A, Scatter plot of mean SCR amplitudes for patient S8 as a function of amygdala stimulation (red dots) and positive control stimulation (purple dots) voltage with separate linear trend lines. Note the consistency of increases in heart rate with increases in amygdala stimulation voltage and the lack of changes in heart rate with increases in positive control stimulation voltage. B, Scatter plot of mean change in heart rate for patient S8 as a function of amygdala stimulation (red dots) and positive control stimulation (purple dots) voltage with separate linear trend lines. Note the consistency of decreases in heart rate with increases in amygdala stimulation voltage and the lack of changes in heart rate with increases in positive control stimulation voltage.
Discussion
The current study’s examination of the effects of human amygdala stimulation on physiological and subjective emotional responses yielded three primary findings. First, we found that amygdala stimulation strongly modulated autonomic activity without eliciting concurrent subjective emotional responses in most patients. Second, we found that these effects were specific to the amygdala relative to lateral temporal lobe stimulation, indicating that the effects were not a general effect of brain or temporal lobe stimulation. Further, we found that the effects of amygdala stimulation were immediate and dose-dependent: stepwise increases in voltage elicited linear, dose-dependent increases in EDA amplitude and decreases in HR within seconds of the stimulation onset. Third, we found that only in a minority of patients, amygdala stimulation provoked subjective emotional responses. In one patient, these subjective emotional responses were strongly negative in valence whereas the other patient’s responses were primarily negative with a few positively-valenced responses. In our most extensively documented patient with subjective emotional responses, fear responses were accompanied by dose-dependent increases in heart rate rather than decreases, which was a markedly different pattern of physiological response than what was observed in the patients who did not show subjective emotional responses. The fact that amygdala stimulation elicited physiological changes in the absence of after-discharges or seizure-like activity suggests that acute amygdala stimulation may be used carefully in humans to study autonomic and behavioral-physiological responses without confounding effects of seizures.
A key novel finding of the current study was that amygdala stimulation can elicit substantial changes in autonomic physiology without necessarily eliciting concurrent subjective emotional responses. Indeed, although the majority of patients (7 of 9) reported no subjective emotional responses when queried, relatively large stimulation-induced changes in SCR and HR were still observed in each of these patients. These findings strongly suggest that stimulation-induced physiological changes are not a consequence of concurrent subjective emotional responses (Lanteaume et al., 2007), and that they are dissociable from subjective responses. These results also highlight the importance of measuring both physiological and subjective emotional responses to brain stimulation to fully assess responses in different domains of emotional response. These findings also raise the question of why these patients did not report any subjective emotional responses despite having substantial physiological responses. One possibility is that the patients were either unaware of their physiological responses, perhaps because these responses were below the threshold of interoceptive awareness, or because attention was not focused on interoceptive stimuli. It seems unlikely that the patients were aware of these physiological responses, but interpreted or appraised them as non-emotional (e.g., somatic) as no somatic changes were spontaneously reported.
Notably, we did not observe significant changes in respiratory rate and observed no episodes of outright apnea or respiratory arrest under our experimental conditions. It has long been observed that direct electrical stimulation in the region of the temporal pole, uncus, amygdala, orbitofrontal region, and anterior insula can cause respiratory depression in animals (Spencer, 1894; Kaada, 1951) and also an awake patient using 30-60 Hz sine wave pulses at 1-4 V (Kaada and Jasper, 1952). In the latter study, respiratory arrest could still be elicited by stimulating the insula and temporal lobe white matter, even after complete resection of the ipsilateral medial temporal lobe including amygdala. A more recent study of direct amygdala stimulation using depth electrodes and 50 Hz stimulation at high amplitudes (15-20 V) likewise reported reproducible respiratory arrests in 3 human subjects (Dlouhy et al., 2015). Our study, by contrast, examined autonomic changes induced by amygdala stimulation in a larger sample size at lower, possibly more physiological, stimulation amplitudes. We observed dose-response effects on HR and SCR, but not RR, at low stimulation doses. Further studies are required, but it seems unlikely that the amygdala represents an “apnea center”. High amplitude stimulation of the amygdala and nearby structures (possibly spreading to hypothalamus and brainstem, which are rarely directly stimulated in humans) may engage a wider integrated autonomic/visceral emotional motor outflow network.
In previous studies of amygdala stimulation effects, this relationship between physiological and subjective emotional responses was either not assessed or was assessed in a way that precluded valid statistical analysis. In the one study that did examine this relationship, statistical evidence bearing directly on this issue was not provided (Lanteaume et al., 2007). Specifically, although the frequency with which amygdala stimulation elicited a significant SCR with vs. without subjective responses was assessed (i.e., 100% of subjective response trials and 43% or 12/28 trials with no subjective response), this comparison was not tested statistically, possibly because the small number of total stimulations that did not elicit a subjective emotional response (28 stimulations) precluded valid statistical comparisons. Moreover, the mean SCR magnitude reported for these 28 stimulation trials in was close to zero, with error bars indicating the standard error of the mean overlapped with zero (Fig. 4 in Lanteaume et al., 2007), consistent with the interpretation that the mean SCRs for stimulation trials that did not elicit subjective responses did not differ significantly from zero (the relevant statistical test was not reported). Thus, this prior study did not provide evidence that significant SCR responses to amygdala stimulation can occur in the absence of subjective emotional responses (Lanteaume et al., 2007).
Another important finding of the current study was that the stimulation-induced physiological changes were dose-dependent and were associated specifically with amygdala stimulation and not observed with either sham stimulation or stimulation of the positive control site in the lateral temporal cortex. Including a control stimulation site that was consistent across patients in the current study allowed us to more conclusively establish the amygdala as the proximal location of the stimulation effects, in contrast to previous studies which did not include a control stimulation site (e.g., Lanteaume et al., 2007), in which general effects of temporal lobe stimulation could not be ruled out. Although there was inter-subject variability in response patterns, testing responses across multiple stimulation voltage levels also allowed the shape of the dose-dependent response profile to be assessed for the first time, confirming that the relationship between stimulation voltage and ANS changes was approximately linear.
In line with most prior studies of amygdala stimulation in humans, we found that subjective emotional responses were relatively infrequent, observed in only 2 of the 9 patients studied at relatively high-doses of stimulation (> 5 V; Halgren et al., 1978; Halgren et al., 1992; Mangina and Beuzeron-Mangina, 1996; Meletti et al., 2006). Though infrequent, these subjective emotional responses could be highly intense, particularly in the case of subject 8, who reported intense feelings of fear and anxiety. In the two patients who reported subjective emotional responses, the valence of their subjective response was most often affectively negative and most commonly was reported as being experienced as fear or anxiety, though a few more ambiguous positive and negative emotions were also reported. These findings parallel most previous amygdala stimulation studies in that reported subjective emotional responses have been typically predominantly aversive, with fear and anxiety the most commonly reported emotions (Meletti et al., 2006; Smith et al., 2006; Lanteume et al., 2007). Notably, we elicited negatively-valenced emotional responses from both left (“guilt”) and right (“fear”) amygdala stimulations, with the left possibly being the less intense subjectively negative experience for the patient. A few studies have reported positive subjective emotional responses following left-hemisphere amygdala stimulation (Meletti et al., 2006; Lanteume et al., 2007) and one case study (Bijanki et al., 2014) reported a significant positive response with high-amplitude, intermittent stimulation (15 V; 50 Hz; 5 s On, 5 s Off) to the right amygdala in a patient with severe comorbid depression. Bilateral stimulation at 10 V in subject 9 was the only instance in which amygdala stimulation in the present study elicited a positive ‘joyful’, but ‘weird’ response, suggesting that perhaps activation of a broader emotion-related network connected to the amygdala using higher-amplitude stimulation is needed to elicit subjectively experienced positive emotions.
Future studies should use more sensitive measures of emotional experience, such as affective bias tasks (Bijanki et al., 2014), to supplement the subjective report of the patients. Finally, all subjective emotional responses were transient, subsiding after the offset of stimulation and subjects often reflected on the experience as ‘interesting’, ‘weird’, or ‘fun’, even in the case of stimulation-induced fearful or anxious emotions. In addition, subjects reported that their emotional responses did not seem to be triggered by their immediate environment, such as stimuli in the room. Future studies should examine the extent to which amygdala stimulation, both below and above thresholds for subjective awareness, modulates emotional responses to external stimuli and contexts.
The subjective emotional responses in patient 8 were accompanied by dose-dependent increases rather than decreases in heart rate, a markedly different pattern of physiological response than what was observed in the patients who did not experience subjective emotional responses during amygdala stimulation. It is difficult to know which specific factors may account for the differences in subject 8’s response to amygdala stimulation. Speculatively, the location of his right amygdala stimulation appears closer to the central nucleus than all but one other patient (subject 6, in whom a subjective response was absent), potentially resulting in stronger stimulation of the central nucleus, which has direct efferent connections to subcortical structures controlling the ANS, such as the hypothalamus (Critchley & Harrison, 2013). However, the factors determining whether subjective emotional responses are observed or not will require further study to provide a more definite answer to this question.
What implications do these findings have for current debates about the neural representation of emotion in the human brain? With regard to dimensional and discrete basic emotion theories, the current results indicate that amygdala stimulation elicits responses that have both dimensional and discrete aspects. The robust increases in SCR, a well-established physiological measure of sympathetic ANS activation, are characteristic of a sympathetic arousal response and fit with a large body of research implicating the amygdala in emotional arousal processes (Adolphs, 2010; Anderson, 2003; Lang & Bradley, 2010; Critchley, 2002; Critchley, 2005). The pattern of SCR increases and heart rate decreases we observed is consistent with a defensive cascade response characteristic of responses to negatively-valenced stimuli (Bradley and Lang, 2000a; Lang, 1995; Lang et al., 1997). However, this response pattern is also generally consistent with the known functional connectivity of the amygdala to regions mediating physiological responses and therefore does not unambiguously reflect a negatively-valenced emotional response. In contrast, the observed subjective emotional responses were primarily negative in valence, consistent with prior literature that suggests that the amygdala has a preferential (but not exclusive) role in mediating negatively-valenced emotion (Anderson & Adolphs, 2014; Hamann, 2012; Zald, 2003).
Paralleling prior reports, the observed subjective emotional responses were predominantly self-reported as being fear or anxiety, rather than other basic emotions such as disgust, anger, sadness, or happiness. Subject 9’s responses were not clearly related to fear or anxiety, but rather an unpleasant feeling of unfamiliarity and occasionally a ‘weird joyful’ feeling. These subjective responses suggest that amygdala stimulation elicits a response profile that is predominantly biased towards eliciting a specific basic emotion state (fear and anxiety) but is not exclusively specialized for fear or anxiety. This response profile is consistent with other evidence from neuroimaging and human lesion studies that suggest that processing of basic emotions (and emotional dimensions such as arousal and valence) is preferentially associated with some brain regions (such as the amygdala) more than other regions. Individual brain regions typically participate in processing multiple emotions rather than being dedicated to processing individual basic emotions (Anderson & Adolphs, 2014; Hamann, 2012; Pessoa, 2010). That is, the subjective experience of emotion appears to be an emergent property of complex brain networks rather than a product of activity within a single brain structure.
This study has additional important implications for the role of the amygdala in the modulation of both emotion physiology and emotional experience. By measuring several psychophysiological measures of ANS activity concurrently with amygdala stimulation, this study provides novel evidence that amygdala stimulation produces physiological responses predicted by theoretical views that propose that aversive stimuli and threats trigger a defensive motivation response and an ensuing cascade of physiological responses that change with the imminence and severity of threat (Bradley et al., 2001). Defensive motivation responses are characterized as a series of autonomic and somatic reflexive behaviors that prepare an organism for defensive behavior and facilitate processing of the threat context (Bradley et al., 2001). These defensive motivation responses are mediated by projections from the basolateral nuclei of the amygdala (BLA), the most frequent sub-nuclear amygdala location in the present study’s stimulation electrodes (Fig. 1; Critchley & Harrison, 2013). Responses initiated by activation of projections from the BLA are both behavioral and physiological. Behavioral responses to BLA activation include both freezing and active flight (Fanselow, 1994). Previously reported physiological responses to BLA activation include acute increases in electrodermal activity, bradycardia or slowing of the heart rate (Kapp, Frysinger, Gallagher, & Haselton, 1979; Kapp et al., 1982), increased blood pressure (LeDoux, 1990), and potentiation of the startle response (Davis, 2000).
In the current study we found that, within the range of parameters tested, increasing amplitudes of amygdala stimulation reliably increased electrodermal activity, significantly slowed heart rate, and was associated with marginal and non-significant decreases in respiration rate. The defensive cascade model (Bradley and Lang, 2000a; Lang, 1995; Lang et al., 1997) suggests that this pattern of increased skin conductance and heart rate deceleration reflects an orienting response, in which perceptual processing is facilitated in the early stages of defense upon exposure to a threat, but prior to overt action. This pattern of coactivation of sympathetic and parasympathetic systems (Cacioppo & Berntson, 1994; Cacioppo, Gardner, & Berntson, 1999) can be provoked by the perception of unpleasant pictures in humans (Lang et al., 1997). These findings suggest that direct electrical stimulation to the amygdala may provoke a state of defensive readiness (i.e. orienting response) in the human autonomic nervous system even in the absence of subjective emotional responses.
In infrequent cases, higher amplitudes of amygdala stimulation (>4 volts) produced subjective emotional responses consistent with negative defensive behavioral responses typically elicited by exposure to threat, such as fear and anxiety. Physiological responses during amygdala stimulation trials that evoked an emotional response were more indicative of later stages of the defensive motivation cascade during which electrodermal activity increases even further and heart rate transitions from deceleration to acceleration. Notably, these overt negative fear responses and their concurrent physiological responses were consistently provoked immediately after the onset of amygdala stimulation greater than 5 V. Specifically, the patient’s skin conductance amplitude and heart rate acutely increased with stimulation amplitudes that elicited an emotional response. In addition, initially after receiving lower amplitudes of stimulation in a blinded fashion to the right amygdala, 5 V of stimulation prompted the patient to spontaneously report that the left side of his body “felt scared”. This finding suggests that there may have been perceptible contralateral autonomic effects of right hemisphere amygdala stimulation on the left side of the patient’s body. During subsequent testing a week later, these effects were replicated, but no evidence of specific lateralized physiological changes was detected (i.e., piloerection only on the left extremities could not be observed). Consistent with previous studies (Smith et al., 2006; Lanteaume et al., 2007), this patient also had a much more prominent fear response with right amygdala stimulation than left amygdala stimulation. In addition, higher amplitudes of right amygdala stimulation produced an increase in heart rate, while similar amplitudes of left amygdala stimulation produced a deceleration in heart rate, consistent with the responses seen in patients that had no subjective emotional experience. Taken together, these findings suggest that direct electrical amygdala stimulation can provoke a subjective fear or anxiety response at higher amplitudes of stimulation and drive the ANS to more closely resemble an animal in an overt defensive state.
The current study has a number of limitations. First, as in any study examining the effects of direct electrical stimulation in patients undergoing intracranial monitoring, our study necessarily consisted of patients with intractable epilepsy, rather than neurotypical patients. Second, given the vast number of possible combinations of stimulation amplitude, duration, frequency, location, and hemisphere, some parameter combinations were not adequately sampled here (e.g., bilateral stimulation, longer durations, and a wider range of frequencies). Further, some patients only received a single trial for higher voltage stimulation conditions due to time constraints. By automating stimulation delivery and adjusting stimulation durations, future studies may be able to more efficiently sample a broader range of stimulation parameter combinations across multiple instances of each stimulation condition. In addition, stimulation of the amygdala for longer than the 30 second stimulation blocks used in the current study may reveal different patterns of change in measures of autonomic physiology and subjective emotional responses at longer time scales. Future studies should stimulate for longer durations to determine whether prolonged amygdala stimulation at higher amplitudes produces more frequent subjective emotional responses and examine the modulatory effect of amygdala stimulation on the subjective and physiological response to emotionally evocative stimuli. Further, the stimulator in the present study delivered constant voltage rather than constant current. However, we measured impedances across multiple contacts in electrode arrays in a patient to be relatively consistent, and thus calculated that voltages of 1-12 V correspond to a current range of ~1-15 mA. Importantly, although impedance (and therefore current) could vary modestly at different contacts, the impedance at a given contact is expected to remain constant across the experimental session, allowing dose-response assessments of stimulation effects at that contact in a subject to be validly recorded. Future extensions of the present work should examine the psychophysiological changes associated with current-controlled amygdala stimulation to ensure full control over stimulation amplitude, especially among patients. Finally, our method of assessing subjective emotional responses may not have been sufficiently sensitive to exhaustively probe whether patients were experiencing subtle changes in emotional experience. For instance, although we asked patients to report any change in emotional experience, they may have elected to not report subtle or transient emotional responses. Future studies will also be aided by the use of a double-blind design, above and beyond the present study’s patient-blinded design, when examining the effects of stimulation on physiology and emotional experience.
The present study suggests several fruitful future directions to pursue in expanding understanding of the amygdala’s causal role in modulating emotional experience, autonomic physiology, and activity in other functionally connected brain regions. First, future studies should simultaneously measure other informative indices of ANS activity, such as blood pressure, heart rate variability, pupillometry, and cardiac output. Additional measures of ANS activity can potentially provide a more complete picture of the sympathetic and parasympathetic influences of direct electrical stimulation to the human amygdala. Second, because prior studies suggest that the emotional responses elicited by stimulation to the amygdala region should depend on the location and volume of tissue activated (including both grey and white matter structures), future studies should further investigate the physiological and subjective effects of precisely stimulating each of the major amygdala nuclei. More precise electrical field modeling of the volume of tissue activated in nucleated structures will also be needed to fully examine this issue (Butson et al., 2007). In addition, future studies should use diffusion tensor imaging on patients to examine the spatial distribution of electrophysiological effects of stimulation in regions connected to the amygdala (Riva-Posse et al., 2014). Mapping the precise location of each stimulation electrode and their volume of tissue activated based on stimulation dose relative to subnuclei of the amygdala and specific white matter tracts may help to explain inter-subject variability in dose responses in future studies. Further, future studies may be able to test the physiological and neural effects of acute amygdala stimulation using concurrent measures of local field potentials or functional MRI Finally, although lower stimulation amplitudes did not elicit physiological responses in the present study, there is evidence that at even lower stimulation amplitudes (<2 V), amygdala stimulation can have beneficial effects on cognitive processes such as memory (Inman et al., 2017). Future studies should use more sensitive measures of neural and autonomic processing to examine the potential impact of lower stimulation amplitudes on central and peripheral nervous system activity.
In conclusion, the current study found that amygdala stimulation in humans can have a strong, dose-dependent impact upon ANS activity in the absence of concurrent subjective emotional responses. When subjective emotional responses elicited by amygdala stimulation were reported, they were primarily negative in valence, and were most commonly described as fear or anxiety, consistent with a response profile for the amygdala that preferentially but not exclusively involves fear and anxiety. These results parallel previous stimulation findings with animals and humans and are consistent with responses predicted by theoretical views positing a defensive cascade of physiological responses to aversive situations. In general, stimulation-induced amygdala responses further validate the proposal that the amygdala is involved in multiple emotions but has a preferential involvement in processing emotional arousal as well as fear and anxiety. Further investigation of the effects of direct electrical stimulation to the human amygdala should consider the factors determining whether subjective emotional responses are elicited, and the factors that account for the variable nature of such responses. The results of the current study provide novel evidence that the human amygdala plays a causal role in the modulation of both emotion physiology and emotional experience.
Supplementary Material
Highlights.
Increasing amplitudes of amygdala stimulation elicited dose-dependent increases in electrodermal activity and decreases in heart rate.
In one patient, amygdala stimulation elicited subjective experiences of fear and anxiety, accompanied by increased heart rate.
Amygdala stimulation reliably elicits changes in autonomic activity in a dose-dependent and safe manner, and elicits subjective emotional experiences only infrequently.
Acknowledgments
We would like to thank the patients, physicians, and staff of the Emory University Hospital Epilepsy Monitoring Unit for their contributions to this project. We also thank Nigel Pedersen for helpful conversation and providing useful historical brain stimulation context. KRB was supported in part by career development awards from the American Foundation for Suicide Prevention and the NIH (KL2TR000455). JTW was supported in part by career development awards from the Sleep Research Society Foundation and the Neurosurgery Research Education Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
C.S.I., K.R.B., D.I.B, S.H., and J.T.W. contributed to the study design. C.S.I., K.R.B., D.I.B., R.F., and J.T.W. contributed to the data collection. J.T.W. and R.E.G. conducted the surgeries. C.S.I., K.R.B., and J.T.W. contributed to the electrode localization. C.S.I. and K.R.B. performed the behavioral, psychophysiological, and statistical analyses. K.R.B. designed the mixed linear models. C.S.I, K.R.B., S.H., and J.T.W. contributed to the interpretation. C.S.I., K.R.B., S.H., and J.T.W. wrote the manuscript. C.S.I., J.T.W., K.R.B., D.I.B., R.E.G., and S.H. edited the manuscript. All authors discussed and commented on the manuscript.
References
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. Journal of Neuroscience. 1995;15(9):5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, et al. Recognition of facial emotion in nine individuals with amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. Human Lesion Studies in the 21st Century. Neuron. 2016;90(6):1151–1153. doi: 10.1016/j.neuron.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Adolphs R. A Framework for Studying Emotions across Species. Cell. 2014;157(1):187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina M, Suzuki A, Mori M, Kanesaka T, Hattori T. Emotional sweating response in a patient with bilateral amygdala damage. International Journal of Psychophysiology. 2003;47(1):87–93. doi: 10.1016/S0167-8760(02)00123-X. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Current Opinion in Neurobiology. 2013;23(3):361–372. doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The Experience of Emotion. Annual Review of Psychology. 2007;55(1):373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijanki KR, Kovach CK, McCormick LM, Kawasaki H, Dlouhy BJ, Feinstein J, Howard MA., III Case Report: Stimulation of the Right Amygdala Induces Transient Changes in Affective Bias. Brain Stimulation. 2014;7(5):690–693. doi: 10.1016/j.brs.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein FL. Principal warps: Thin-plate splines and the decomposition of deformations. Pattern Analysis and Machine Intelligence, IEEE Transactions on. 1989;11:567–585. [Google Scholar]
- Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT, Dawson ME, Filion DL. Publication recommendations for electrodermal measurements. Psychophysiology. 2012;49:1017–1034. doi: 10.1111/j.1469-8986.2012.01384.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Emotion and motivation. Handbook of psychophysiology. 2000a;2:602–642. [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: Behavior, feeling, and physiology. Cognitive neuroscience of emotion. 2000b;25:49–59. [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276–298. doi: 10.1037/A528-3542.L3.276. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behavioral Neuroscience. 2000;114(2):320–327. doi: 10.1037/0735-7044.114.2.320. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neuroscience & Biobehavioral Reviews. 2006;30(2):173–187. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34(2):661–670. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: A critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115(3):401–423. doi: 10.1037/0033-2909.115.3.401. [DOI] [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. The affect system has parallel and integrative processing components: Form follows function. Journal of Personality and Social Psychology. 1999;76:839–855. [Google Scholar]
- Critchley HD. Electrodermal Responses: What Happens in the Brain. The Neuroscientist. 2002;5(2):132–142. doi: 10.1177/107385840200800209. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77(4):624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The amygdala. Vol. 2. Oxford, England: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry; New York. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, Richerson GB. Breathing Inhibited When Seizures Spread to the Amygdala and upon Amygdala Stimulation. Journal of Neuroscience. 2015;35(28):10281–10289. doi: 10.1523/JNEUROSCI.0888-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. Springer Science & Business Media; 2005. [Google Scholar]
- Ekman P. An argument for basic emotions. Cognition and Emotion. 1992;6(3-4):169–200. doi: 10.1080/02699939208411068. [DOI] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio AR, Tranel D. The human amygdala and the induction and experience of fear. Current Biology. 2011;21(1):34–38. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillory SA, Bujarski KA. Exploring emotions using invasive methods: review of 60 years of human intracranial electrophysiology. Social Cognitive and Affective Neuroscience. 2014;9(12):1880. doi: 10.1093/scan/nsu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. TICS. 2001;5(9):394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hamann S. Mapping discrete and dimensional emotions onto the brain: controversies and consensus. TICS. 2012;16(9):458–466. doi: 10.1016/j.tics.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Halgren E, Walter RD, Cherlow DG, Crandall PH. Mental phenomena evoked by electrical stimulation of the human hippocampal formation and amygdala. Brain. 1978;101:83–117. doi: 10.1093/brain/101.1.83. [DOI] [PubMed] [Google Scholar]
- Halgren E. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York, NY, US: Wiley-Liss; 1992. Emotional neurophysiology of the amygdala within the context of human cognition; pp. 191–228. [Google Scholar]
- Inman CS, Manns JR, Bijanki KR, Bass DI, Hamann S, Drane DL, Fasano R, Kovach CK, Gross RE, Willie JT. Direct electrical stimulation of the amygdala enhances declarative memory in humans. Proceedings of the National Academy of Sciences USA. 2017 doi: 10.1073/pnas.1714058114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimization method for robust affine registration of brain images. Medical image analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kaada BR. Stomato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat, and dog; a study of responses from the limbic, subcallosal, orbito-insular, piriform and temporal cortex, hippocampus-fornix and amygdala. Acta Physiologica Scandinavica Supplementum. 1951;24(83):1–262. [PubMed] [Google Scholar]
- Kaada BR, Jasper H. Respiratory responses to stimulation of temporal pole, insula, and hippocampal and limbic gyri in man. A M A Archives of Neurology and Psychiatry. 1952;68(5):609–619. doi: 10.1001/archneurpsyc.1952.02320230035004. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50(5):372–385. doi: 10.1037/0003-066X.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Hillsdale, NJ: Erlbaum; 1997. pp. 97–135. [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biological Psychology. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteaume L, et al. Emotion induction after direct intracerebral stimulations of human amygdala. Cerebral Cortex. 2007;17(6):1307–1313. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Information flow from sensation to emotion plasticity in the neural computation of stimulus value. In: Gabriel M, Moore J, editors. Learning and computational neuroscience: Foundations of adaptive networks. Cambridge, MA: Bradford Books/MIT Press; 1990. pp. 3–52. [Google Scholar]
- LeDoux JE. Emotion Circuits in the Brain. Annual Review of Neuroscience. 2000;23(1):155–184. doi: 10.1146/annurev.neuro.23.L155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Semantics, Surplus Meaning, and the Science of Fear. Trends in Cognitive Sciences. 2017;21(5):303–306. doi: 10.1016/j.tics.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Barrett LF. A functional architecture of the human brain: emerging insights from the science of emotion. Trends in Cognitive Sciences. 2012:1–8. doi: 10.1016/j.tics.2012.09.005. [DOI] [PMC free article] [PubMed]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. 3rd. Academic Press; 2008. [Google Scholar]
- Mangina CA, Beuzeron-Mangina JH. Direct electrical stimulation of specific human brain structures and bilateral electrodermal activity. International Journal of Psychophysiology. 1996;22(1-2):1–8. doi: 10.1016/0167-8760(96)00022-0. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Kennedy SH. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Meletti S, et al. Emotions induced by intracerebral electrical stimulation of the temporal lobe. Epilepsia. 2006;47.s5:47–51. doi: 10.1111/j.1528-1167.2006.00877.x. [DOI] [PubMed] [Google Scholar]
- Oya H, Kawasaki H, Dahdaleh NS, Wemmie JA, Howard MA., III Stereotactic atlas-based depth electrode localization in the human amygdala. Stereotactic and functional neurosurgery. 2009;87:219–228. doi: 10.1159/000225975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Toward a general psychobiological theory of emotions. Behavioral and Brain Sciences. 1982;5(3):407–422. doi: 10.1017/S0140525X00012759. [DOI] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nature Reviews Neuroscience. 2010;11(11):773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. Emotion and cognition and the amygdala: from “what is it?” to “what’s to be done? Neuropsychologia. 2010;48(12):3416–3429. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, Mayberg HS. Defining Critical White Matter Pathways Mediating Successful Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression. Biological Psychiatry. 2014;76(12):963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr K, et al. Landmark-based elastic registration using approximating thin-plate splines. IEEE Transactions on medical imaging. 2001;20(6):526–534. doi: 10.1109/42.929618. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Adolphs R. The primate amygdala in social perception – insights from electrophysiological recordings and stimulation. Trends in Neurosciences. 2015;38(5):295–306. doi: 10.1016/j.tins.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimbeyoglu A, Parvizi J. Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Frontiers in Human Neuroscience. 2010;4 doi: 10.3389/fnhum.2010.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Lee GP, Fountas K, King DW, Jenkins PD. Intracranial stimulation study of lateralization of affect. Epilepsy & Behavior. 2006;8(3):534–541. doi: 10.1016/j.yebeh.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Spencer WG. The Effect Produced upon Respiration by Faradic Excitation of the Cerebrum in the Monkey, Dog, Cat, and Rabbit. Philosophical Transactions of the Royal Society of London B. 1894;185:609–657. [Google Scholar]
- Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. Journal of Cognitive Neuroscience. 2010;22(12):2864–2885. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


