Abstract
Neurological conditions associated with HIV remain major contributors to morbidity and mortality and are increasingly recognized in the aging population on long-standing combination antiretroviral therapy (cART). Importantly, growing evidence shows that the CNS may serve as a reservoir for viral replication, which has major implications for HIV eradication strategies. Though there has been major progress in the last decade in our understanding of the pathogenesis, burden, and impact of neurological conditions associated with HIV infection, significant scientific gaps remain. In many resource-limited settings, ARVs considered second or third line in the U.S., which carry substantial neurotoxicity, remain mainstays of treatment, and patients continue to present with severe immunosuppression and central nervous system (CNS) opportunistic infections. Despite this, increased global access to cART has coincided with an aging HIV+ population with cognitive sequelae, cerebrovascular disease, and peripheral neuropathy. Further neurological research in low- and middle-income countries (LMICs) is needed to address the burden of neurological complications in HIV+ patients, particularly regarding CNS viral reservoirs and their effects on eradication.
Keywords: Neurology, HIV, aging, cerebrovascular disease, antiretroviral therapy, CNS escape, viral reservoir, global health
Introduction
The landscape of global HIV is changing with increased access to combination antiretroviral therapy (cART). In 2015, the number of people living with HIV on antiretroviral (ARV) therapy reached 17 million individuals, increasing by approximately one third from the previous year.[1] In sub-Saharan Africa, the world’s most affected region, the number of people on treatment has more than doubled in the last 5 years. Since 2003, annual AIDS-related deaths have decreased by approximately 43% globally.[1] Despite these achievements, significant challenges remain as there were 1.8 million new infections in 2016 (a 16% decrease since 2010) and approximately 36.7 million people worldwide continue to live with HIV.[1 2]
HIV-associated neurological syndromes cause significant morbidity and mortality globally and may be due to primary HIV infection, secondary to opportunistic infection (OI) or immune reconstitution, or associated with ARV treatment. HIV can primarily or secondarily affect all parts of the nervous system, including the brain, meninges, spinal cord, nerve roots, peripheral nerves and muscles. Neurological disease is the first manifestation of symptomatic HIV infection in approximately 10-20% of individuals, and about 60% of patients with advanced HIV have clinical evidence of neurologic dysfunction during the course of their illness.[3–5] Given increased global access to cART, neurological conditions are becoming more frequent in an aging HIV+ population with longstanding HIV including cognitive sequelae, cerebrovascular disease, and peripheral neuropathy. There is also an ongoing impact of neurotoxicity due to continued use of older generation ARVs in resource-limited regions. Here we review the most common neurological complications of HIV with an emphasis on their impact in low- and middle-income countries (LMICs), as well as the current knowledge gaps in the field including the impact of the central nervous system (CNS) as a reservoir for HIV and its effects on eradication efforts. [6]
Methods
A review of available literature was performed to identify data on the prevalence, etiology, outcomes, and treatment of neurologic disorders in patients living with HIV in resource-limited settings. Online databases (Ovid Medline and PubMed) were searched to identify relevant articles published in English using combinations of the terms “neurology,” “nervous system diseases,” “developmental disabilities,” “neurological impairment,” and “HIV” with the specifiers “developing countries,” “global health,” and “low-and middle-income countries.” Abstracts from any potentially relevant study were reviewed for inclusion, and the most relevant publications were included in the final review. Web sites from the World Health Organization were scanned for further references. The abstracts of any articles identified in this search strategy were screened for relevance and electronic or paper copies of the full text were obtained for all relevant articles. References from each relevant article were scanned for further relevant articles and a snowball search was performed. Commercial search engines, including Google, were then used to check for any missing publications. Identified publications were then prioritized for relevance to the topic and coded for themes and key emerging themes were summarized in each section below.
Neurological Disorders Associated with Primary HIV Infection
HIV does not productively infect neurons, instead damaging neurons and neuronal support cells through inflammatory mediators and excitotoxicity.[7 8] HIV invades the central nervous system early in infection, crossing the blood brain barrier through infected monocytes and lymphocytes in what is often described as a “Trojan Horse” mechanism.[8] Infected monocytes then differentiate into resident macrophages, and establish low level viral replication, typically infecting neighboring microglia. Astrocytes may also be susceptible to infection, but do not develop productive infection. Viral proteins (e.g. Tat) released from infected monocyte-derived cells may directly damage neurons.[9 10] Simultaneously an increase in translocation of bacteria across a leaky gut membrane may increase lipopolysaccharide (LPS) derived factors, further increasing systemic inflammation and monocyte activation. [11 12] Activated monocytes and macrophages produce a series of cytokines and chemokines which increase transmigration of inflammatory cells as well producing increased concentrations of excitatory neurotransmitters.[7 8] The increase in excitatory amino acids and excessive activation of NMDA receptors may increase intraneuronal calcium concentrations to toxic levels, with this process compounded by increased oxidative stress and dysregulation of normal autophagic processes. [13–15]
HIV has been cultured from brain, nerve, and muscle tissue in individuals at all stages of infection. HIV enters the nervous system in the beginning stages of infection, with evidence of the virus in cerebrospinal fluid (CSF) as early as one week after initial infection and changes in brain structure visualized within three months of primary infection. [16–18]The most favored theory on viral entry and migration into the CNS suggests trafficking of HIV-infected CD4+ cells into the CNS as part of routine immune inspection. HIV-1 virions may also cross the blood brain barrier (BBB) or blood-CSF barrier in the setting of high blood viral load.[19] This is supported by a recent study which found evidence of BBB disruption early in the course of primary HIV infection that was associated with CSF markers of neuronal injury and persisted essentially unchanged over the first year of cART treatment.[20] HIV’s initial seeding of the nervous system is thought to be asymptomatic, though mounting evidence suggests this may not be true. A study performed in Thailand showed a significant number of patients have mild neurological manifestations at the time of acute infection, including mild cognitive impairment (MCI), motor findings, and neuropathy. [6] Another Thai study identified neurocognitive impairments in one-quarter of participants with acute HIV infection which did not improve in the first six months of cART treatment. [21] However, these neurological signs and symptoms are only captured clinically with thorough neurological and cognitive evaluations, which remains a challenge since identifying the more subtle neurologic signs and symptoms of HIV is often challenging in resource-limited settings.[16] The first study identified higher plasma HIV RNA levels at diagnosis in those with one or more neurologic findings, and the latter study found higher CSF HIV RNA levels correlated with the presence of neurocognitive impairment. This suggests that both systemic disease severity and early CNS invasion play a role in the development of neurologic symptoms. More severe neurological manifestations may occur during seroconversion, including acute meningoencephalitis and acute inflammatory demyelinating polyneuropathy (AIDP). In patients presenting with aseptic meningitis, a strong correlation with CSF viral load has been identified.[22] The true prevalence of acute CNS manifestations due to HIV in resource-poor settings at the time of seroconversion is not known, as individuals may not present reliably for care, and if they do, most resource-poor settings do not have access to p24-sensitive tests and there lacks structured practices of follow-up antibody testing post-discharge. [23 24]
Peripheral Manifestations of HIV
HIV infection can also cause deleterious effects on the peripheral nervous system (PNS). Distal symmetric polyneuropathy (DSP) is the most common HIV-associated peripheral nerve disorder, affecting up to half of HIV+ patients.[25] A recent cohort study of 400 HIV+ and 400 HIV- Ugandans found high rates of symptomatic neuropathy in both HIV+ and HIV- participants, and 20% of total participants had objective signs but no symptoms of neuropathy [26]. Older age was also associated with increased neuropathy risk in HIV+ individuals. HIV- individuals were notably healthy with low rates of diabetes. This suggests that additive effects, which may be environmental or diet-related, may increase neuropathy risk in both HIV+ and HIV- individuals in this region. A rural Zambian study of HIV+ adults prior to the initiation of cART found that food insecurity and lower body mass index but not HIV stage were risk factors for peripheral neuropathy symptoms further supporting the potential importance of diet in HIV-associated neuropathies in sub-Saharan Africa.[27]
Other PNS conditions include polyradiculopathy, mononeuropathies, mononeuropathy multiplex, and autonomic neuropathy. Moreover, there are multiple reports of motor neuron diseases in HIV+ patients, including primary lateral sclerosis, brachial amyotrophic diplegia, pseudobulbar syndrome, and classic amyotrophic lateral sclerosis (ALS).[28] Table 1 highlights the most common peripheral manifestations of HIV.
Table 1.
Peripheral Nervous System Manifestations of HIV.
| Peripheral Manifestation | Clinical Presentation | Mechanism |
|---|---|---|
| Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) [231–235] |
|
|
| Acute Inflammatory Demyelinating Polyneuropathy (AIDP) [231–235] |
|
|
| Distal Symmetric Polyneuropathy (DSP) [108 236–239] |
|
|
| Diffuse Infiltrative Lymphocytosis Syndrome (DILS) [240 241] |
|
|
| HIV-Associated Myopathy [104 242–245] |
|
|
| Bell’s Palsy [246] |
|
|
| Vasculitic Neuropathy/Mononeuritis Multiplex [247] |
|
|
| Brachial Plexopathy (Parsonage Turner Syndrome) [96 248] |
|
|
| Motor Neuron Disease [249] |
|
|
HIV-Associated Cerebrovascular Disorders
There is evidence of an increased stroke risk in adults and children in HIV infected patients when controlling for traditional cerebrovascular risk factors.[29–33] Data adjusted for demographics and ischemic stroke risk factors show incidence ratios of 1.05 and 1.76 for HIV-infected men and women, respectively, compared to HIV- individuals, suggesting a more pronounced risk in females. [34] HIV also increases the incidence ratio of intracerebral hemorrhage (ICH) by 1.85. [35] In high HIV-prevalence pediatric studies, HIV may be one of the main contributors to stroke. In fact, the effect of HIV on ICH poses a lesser risk with increasing age. [29 36]
Several mechanisms have been proposed for HIV-associated stroke, including premature atherosclerosis, OIs, cardioembolism, coagulopathy, and HIV vasculopathy (Table 2).[29 31 37–39] Immunosuppression due to HIV resulting in low CD4 counts (≤ 200) and high viral loads has been identified as another significant risk factor for strokes.[34] A recent study in the U.S. of 60 HIV-infected and 60 HIV-uninfected stroke cases found that the among those with HIV, the most recent plasma HIV viral load prior to the stroke predicted stroke subtype. Among HIV-infected patients with virologic suppression, a trend toward a greater proportion of strokes attributable to large artery atherosclerosis was observed.[40] Furthermore, with the widespread global use of cART, increasing rates of HIV+ patients face previously rare complications of treatment. [41] One 2008 study found a 26% increase in vascular disease incidence for each year of exposure to cART, and particular cART drugs, including lopinavir and ritonavir, may lower cerebral vasoreactivity by weakening endothelial function, independent of vascular risk factors, cART duration, and time since HIV diagnosis. [39] Interestingly, a 2017 study found association in illegal drug use, low CD4 count, and high viral load with ischemic cerebral events and no association with cART use or treatment compliance. [42] A thinner media layer might be a preclinical stage in HIV vasculopathy development.[43] A study in China found that cerebrovascular endothelial dysfunction associated with HIV seropositivity may be important in the pathogenesis of cerebrovascular dysfunction.[44]
Table 2.
Summary of key HIV-associated cerebrovascular disorders in adults and children with HIV.
| Stroke Mechanism | Evidence | Key Studies | |
|---|---|---|---|
| Opportunistic Infection or Neoplasia |
|
Benjamin et al. 2016; Narayan et al. 2002 | |
| Tuberculosis (TB) |
|
Lammie et al. 2009; Berenguer et al. 1992 | |
| Varicella Zoster Virus (VZV) |
|
Gutierrez et al. 2011; Gilden et al. 2009; Nagel et al. 2008 | |
| Syphilis |
|
Zetola et al. 2007; Timmermans et al. 2004 | |
| Neoplasia |
|
Tipping et al. 2007; Chetty et al. 2000 | |
| Cardioembolism |
|
Goyal et al. 2014 | |
| Cardioembolism | Non-atherosclerotic vasculopathy |
|
Gutierrez et al. 2015 |
| Bacterial and Marantic Endocarditis |
|
Berger et al. 1990 | |
| Thrombotic thrombocytopenic purpura (TPP) |
|
Brecher et al. 2008 | |
| Ischemic Heart Disease and HIV-Associated Cardiac Dysfunction |
|
Barbaro 2001 | |
| HIV-Associated Hyperviscosity |
|
Garderet et al. 2004; Hague et al. 1990 | |
| Coagulopathy E.g.: protein S and protein C deficiency |
|
Zimba et al. 2017; Mochan et al. 2005 | |
| Coagulopathy E.g. antiphospholipid antibodies |
|
Ortiz et al. 2007; Abuaf 1997 | |
| Cerebral Venous Thrombosis (CVT) |
|
Netravathi et al. 2017 | |
| CNS Vasculopathy |
|
Benjamin et al. 2016, Tipping et al. 2007 | |
| Premature Atherosclerosis |
|
Chow et al. 2017; Narayan et al. 2002 | |
| Low CD4 Count and HIV-Associated Vasculopathy |
|
Benjamin et al. 2012 | |
| Pediatric Cerebrovascular Disease |
|
Blockhuis et al. 2017; Potchen et al. 2016; Park et al. 1990 | |
CD8+ encephalitis, an emerging clinical entity pathologically associated with marked perivascular infiltrates with polyclonal CD8+ lymphocytes, may be a newly recognized HIV vasculopathy though further studies are needed to fully delineate the pathophysiological processes underlying this condition. A 2013 case-series of 14 cases of CD8+ encephalitis all exhibited radiographic features of diffuse hyperintensity of the white matter and multiple punctate or linear lesions in patients with, on average, a decade of treated HIV infection. [45–47] Most reported cases of CD8+ encephalitis have occurred in patients with systemic viral suppression. Multinucleated giant cells, typically seen in HIV encephalitis, are not present in CD8+ encephalitis. Since this CD8+ encephalitis responds well to glucocorticoids, it is an important condition to include in the differential diagnosis of individuals with well-controlled, long standing HIV who present with acute or subacute CNS dysfunction.
The incidence rate of HIV-associated cerebrovascular diseases in LMIC remains underestimated due to limited access to neuroimaging, the subtlety of clinical presentations, and misdiagnosis of HIV-associated cerebrovascular conditions as HIV encephalopathy. [48] In such regions, HIV is becoming a more significant contributor to the growing global burden of cerebrovascular disease.[49]
CNS Opportunistic Infections
Many CNS OIs are AIDS-defining conditions with high mortality risk, including Progressive Multifocal Encephalopathy (PML), CNS cytomegalovirus (CMV), CNS tuberculosis (TB), cryptococcal meningitis, and cerebral toxoplasmosis.[50 51] CNS OIs most commonly occur when the CD4 cell count is ≤200 cells/μl, and in up to 15% of HIV infected patients, multiple CNS OIs exist concurrently.[52 53] Unfortunately, clinical manifestations of CNS OIs are often non-specific (headaches, fevers, delirium, seizures, focal neurologic deficits) and the rather broad differential may be only marginally narrowed with imaging and CSF analyses. Therefore, diagnosis is especially challenging in resource-limited settings with limited laboratory testing and neuroimaging and can be further complicated by underutilization of lumbar puncture in these regions.[54 55] The differential diagnosis can be further guided by the degree of immunosuppression in the host, as CNS mass lesions are most common in acutely immunosuppressed patients with CD4 cell counts ≤200 cells/μl.[56] But in reality, a patient with advanced AIDS who presents with fever and headache could suffer from toxoplasmosis, TB, lymphoma, syphilis, or PML, or a combination of more than one of these OIs simultaneously.
cART is the most important strategy to prevent CNS OIs: the restoration of cellular immunity brought about by cART decreases the risk of CNS OIs among HIV+ patients who discontinue antimicrobial therapy following adequate immune recovery.[57–59] Strategies for patients who do not receive cART or are not adherent remain exposure avoidance and antimicrobial therapy. Many infections, such as Streptococcus pneumoniae and hepatitis B virus, can be prevented by vaccination; however, vaccine efficacy may be compromised in advanced HIV. [60] Of note, timely initiation of prophylaxis can substantially reduce incidence of OIs. However, of the CNS OIs, only toxoplasmosis and varicella zoster virus (VZV) have effective prophylactic regimens available. For CNS toxoplasmosis, prophylaxis consists of one double-strength trimethoprim-sulfamethoxazole tablet daily, while prevention of VZV infection is achieved with vaccination in patients with CD4 counts ≥200 cells/μL who have no known prior exposure to VZV or are known to be VZV seronegative.[61] For a comprehensive review, refer to the 2016 review by Albarillo & O’Keefe.[62] See Table 3 for an overview of the epidemiology, clinical presentation, prophylaxis, and treatment of the most common CNS OIs.
Table 3.
Epidemiology, Clinical Characteristics, Prophylaxis and Treatment of the Most Common CNS OIs
| CNS Opportunistic Infection | Epidemiology | Clinical Characteristics | Prophylaxis/Treatment |
|---|---|---|---|
| CNS Toxoplasmosis [250–253] | The most commonly reported CNS OI since cART was introduced. Rates vary according to the seroprevalence of Toxoplasma gondii in the population. | Presents with headache, fever, and subacute neurologic deficits. Seizures are common. Diagnosis is often established by clinical and radiographic improvements after empirical treatment and by a positive test for IgG antibodies to T. gondii in serum. Radiographically defined by multiple ring enhancing lesions frequently involving basal ganglia structures. | Combined pyrimethamine, folinic acid, and sulfadiazine has traditionally been used. Trimethoprim-sulfamethoxazole was equally effective in a small randomized trial, and is the more economical in resource-poor regions. Corticosteroids are indicated when substantial mass effect is seen. Induction treatment should be continued for at least 6 weeks (until significant clinical improvement). Maintenance therapy should be continued in all patients until immune reconstitution is achieved. Brain biopsy is indicated if no significant improvement within two weeks of therapy initiation to evaluate for primary CNS lymphoma, which has a similar clinical and radiographic presentation. |
| Primary CNS Lymphoma [254–256] | Pre-HAART 5.33 per 1,000 person years Post-HAART 0.32 per 1,000 person years | Usually presents with headaches, mental status changes, seizures, and focal neurological deficits in the absence of fever. Imaging usually reveals a solitary ring-enhancing lesion but can be multiple. CSF Epstein Barr Virus (EBV) PCR is suggestive but not specific for the diagnosis. The primary differential diagnosis is toxoplasmosis. Brain biopsy is often needed for definitive diagnosis but is usually not obtained until empiric therapy for toxoplasmosis is unsuccessful. | Immediate ART initiation is essential to any treatment regimen. High-dose methotrexate and ART are associated with good long-term survival and low relapse rates. Whole brain radiation and chemotherapy can also be considered. |
| Progressive multifocal leukoencephalopathy (PML) [96 257–261] | PML incidence has declined in the cART era and prognosis has improved with initiation of cART upon PML diagnosis. | Characterized by demyelinating infection of oligodendrocytes by the JC polyomavirus (JCV), PML presents with slowly progressive multifocal neurological deficits. Visual symptoms are most common, and seizures often occur. Definitive diagnosis requires clinical (compatible history and examination), radiographic (multifocal lesions in the subcortical and periventricular white matter), and virological evidence (positive CSF JCV PCR or typical histopathological findings on brain biopsy). | Primary treatment is immune reconstitution with immediate cART initiation as no JCV-specific treatment exists. Anecdotal reports of improved outcomes with mirtazapine and interleukin-7 have not been confirmed in clinical trials. Topotecan showed promise in a phase 2 trial, but no phase 3 trial has been performed. |
| Cryptococcal Meningitis (CM) [262–274] | In sub-Saharan Africa, cryptococcal infection is a leading cause of meningitis in adult HIV+ pts. Accounted for 63% of cases of adult meningitis in study in South Africa. CM is responsible for 1/5 of global AIDS-related mortality. | Initially presents with non-specific headache followed by focal neurological deficits. Definitive diagnosis is made with a positive CSF cryptococcal antigen (CrAg) or CSF fungal culture. If LP is unavailable, serum CrAg titers ≥ 1:160 are highly suggestive of CM and virtually all individuals with serum CrAg titer >1:640 have CM. Cryptococcal antigen lateral flow assay (CrAg LFA), a dipstick immunochromatographic assay, was recently developed; it requires little to no lab infrastructure and has applicability in resource-limited settings. | Aggressive management of raised ICP is crucial to lower the risk of early death. Repeated high-volume lumbar puncture leads to immediate symptomatic relief and can reverse neurological morbidity; therapeutic LPs were associated with a 69% relative improvement in survival. Intravenous amphotericin B and oral flucytosine ×2 weeks followed by oral fluconazole ×8 weeks is the recommended antifungal treatment. Monotherapy with high dose fluconazole is often used in low resource settings. |
| Cytomegalovirus (CMV) [275] | With increased cART availability, CMV incidence has decreased as this is a late stage manifestation of HIV infection usually occurring at CD4 count < 50 cells/μL. | Clinical syndromes include ventriculoencephalitis, micronodular encephalitis, retinitis, and polyradiculitis. CSF CMV PCR is sensitive and specific for the diagnosis. | No specific treatment. |
| Latent Tuberculous Meningitis (TBM) [102 122 276–285] | Occurs in ~1% of TB cases. Exact incidence of HIV-associated TBM is unknown due to limited epidemiological data. | Characterized by progressive granulomatous inflammation in the basal meninges (may result in hydrocephalus, vasculitis-associated strokes, and death if left untreated). Diffuse brain involvement often exists in HIV+ pts. Rapid detection is crucial in HIV+ pts. Cohort studies have evaluated the use of GeneXpert, a PCR-based diagnostic tool, on CSF in possible TBM cases. According to the WHO, Xpert should be used as the initial CSF diagnostic test for potential TBM pts (strong recommendation given the urgency of rapid diagnosis, very low-quality evidence). | Early treatment with anti-TB chemotherapy and adjunctive treatment with glucocorticoids reduce the rate of death and disability from TBM. Current WHO guidelines recommend treatment with 4 anti-TB drugs for at least the first 2 months of therapy, followed by treatment with 2 drugs (rifampin and isoniazid) for an additional 7-10 months. Current standard dosing regimens may put pts. at risk of treatment failure from suboptimal rifampicin exposure and may potentially increase the risk of adverse CNS events independently correlated with pyrazinamide CSF exposure. Optimum timing of cART initiation with anti-TB therapy remains controversial. |
| Varicella Zoster Virus (VZV) Vasculitis [286] | Can present with encephalitis, cranial neuropathies, strokes, seizures and myelitis. Often occurs in the weeks or months after a typical shingles rash. Diagnosis is confirmed with positive CSF VZV PCR or IgM. | Intravenous acyclovir for at least 14 days. |
CNS-Immune Reconstitution Syndrome
Although the use of cART markedly improves immune function and prognosis in HIV-infected patients, immune reconstitution inflammatory syndrome (IRIS) is a significant complication of ARV initiation.[63 64] IRIS describes a constellation of symptoms and clinical features that may occur in previously immunosuppressed patients during rapid restoration of immune function in the presence of a pathogen or foreign antigen. Low CD4 cell count and high viral load at treatment initiation, as well as recent diagnosis of an opportunistic infection, are the most significant risk factors.[65 66] Those in LMIC regions may be at particularly high risk given lower CD4 counts and higher prevalence of opportunistic infections at treatment initiation. Prevalence of CNS-IRIS in resource-limited settings is unknown, but at least 25% of individuals in these settings present to medical attention with CD4 cell counts <100 cells/μl.[67] Comparatively, a meta-regression reported mean CD4 count at presentation in developed countries was 336 cells/μl in 2011.[68]
IRIS affects a quarter of patients starting ARV, and though CNS-IRIS is a rare phenomenon compared to other organ system involvement (including the lymphatic and pulmonary system), it has the highest associated morbidity and mortality.[69 70] A recent epidemiological study in Southern India found one-third of patients experienced at least one IRIS event at a median of 27 days post-cART initiation.[71] Two forms of IRIS have been described: paradoxical IRIS manifests with recurrence of symptoms of a previously recognized and treated OI, and unmasking IRIS manifests with the inflammatory presentation of a newly diagnosed OI.[72 73] IRIS can be challenging to distinguish from progression of an underlying opportunistic infection.
Tuberculosis, Cryptococcus, and PML are the most frequent pathogens associated with CNS-IRIS, though a number of other causes of CNS-IRIS have been identified.[70 74] Prospective studies have reported an incidence of paradoxical cryptococcal meningitis-IRIS (CM-IRIS) in 13%–30% of persons with CM surviving to receive ARV.[75–80] High serum cryptococcal antigen titer and level of immunosuppression at ARV initiation are the main risk factors for paradoxical CM-IRIS. Among those who are diagnosed, mortality is unacceptably high (>50%).[76 81] In patients who develop unmasking Cryptococcosis, pre-ARV cryptococcal antigen screening with preemptive fluconazole therapy for those positive is recommended for patients with CD4 counts <100 cells/μl.[82] Ongoing studies in resource-limited settings are studying the utility of screening and treatment for cryptococcal infection pre-ARV initiation as a public health strategy to prevent unmasking CM-IRIS.[70] The high risk of CM-IRIS in those who are ARV-naïve makes the decision of ARV initiation timing challenging; clinicians must weigh the risks of CM-IRIS and high mortality associated with deferring ARV in advanced HIV patients.[83]
Tuberculosis-associated-IRIS (TB-IRIS) is the most common form of IRIS in high HIV/TB co-infection settings, with neurological involvement in up to 30% of cases.[84–86] A study in South Africa found paradoxical neurological TB-IRIS to be the most common cause (21% of cases) for CNS deterioration in patients within one year of starting ARV.[87] TB-IRIS can be challenging to differentiate from progression of TB due to drug resistance, but should be suspected in patients who appear to initially respond to anti-TB therapy followed by deterioration after initiation of cART. Neurologic TB-IRIS tends to present later than other forms of IRIS, often occurring 5-10 months after cART initiation. Paradoxical neurologic TB-IRIS is a potentially life- threatening condition, often presenting with signs and symptoms of meningitis and increased intracranial pressure. Mortality is high, ranging from 12-25%. Risk factors for TB-IRIS include low CD4 count and recent diagnosis of TB. However, the optimal time to start ARV in patients with HIV-associated neuro-TB remains uncertain. Patients with HIV and comorbid TB treated earlier with cART have higher rates of IRIS, but lower overall mortality.[88–93]. Oral and intravenous steroids are typically utilized in cases of neuro-TB-IRIS, and a single randomized controlled trial demonstrated improved outcomes in patients with TB IRIS treated with a 4-week course of oral prednisone. [66]
PML, a demyelinating disease of the brain caused by JC-polyomavirus, occurs in 3%–5% of persons with advanced HIV.[94 95] This is likely an underestimation, since diagnosis requires ancillary data that are often not available in resource-limited settings.[96] Additionally, in a recent study, PCR for JCV in CSF was positive in only 84% of cases of clinical PML, yet brain biopsy confirmed PML diagnosis in 90% of cases and demonstrated histological signs of IRIS in 95% of cases. [76] Diagnosis of PML is reliant on histology, lab evidence, and neuroimaging findings, which are not often available in developing countries. [97] Fortunately, incidence of PML in HIV+ individuals has declined substantially in the post-cART era.[94] An observational study in Northeastern India reported HIV+ PML patients as having very low CD4 counts (107±87.5 cells/μl).[98] Up to 16% of HIV+ patients develop PML-IRIS upon commencing ARVs, with a median ARV start-to-IRIS time of 4 weeks for paradoxical IRIS and 7-8 weeks for unmasking IRIS.[99 100] PML-IRIS can typically be distinguished from PML without IRIS through the presence of contrast enhancement on Magnetic Resonance Imaging (MRI), often with significant mass effect. [76] In the absence of typical MRI findings, brain biopsy may be necessary. There are no specific strategies known to prevent or treat PML-IRIS. The role of steroids in PML-IRIS is controversial. Patients with increased intracranial pressure in the setting of PML-IRIS may benefit from steroids, but there are no randomized controlled trials and observational studies have yielded conflicting results[76,81].
Neurotoxic Effects of HIV Treatment
ARVs may lead to a wide spectrum of adverse effects along the neuro-axis, sometimes leading to adherence problems, regimen changes, or withdrawal from therapy.[101–110] The lack of access to newer, less neurotoxic ARVs is particularly concerning in resource-limited settings. Table 4 highlights some of the major adverse neurological effects associated with ARVs; ARV side effects tend to vary by drug class.
Table 4.
Major adverse neurological effects associated with ARVs.
| Family/Drug | Mechanism of Injury | Neurological Adverse Effects |
|---|---|---|
|
Nucleoside Analogs (“D Drugs”) ddI, d4T, ddC [102 103 109 287] |
|
|
|
Nucleoside Reverse Transcriptase Inhibitor (NRTI) Zidovudine Abacav [104] |
|
|
|
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) Efavirenz Nevirapine Rilpivirine [44 106 114] |
|
Efavirenz
Nevirapine-few neurologic side effects Rilpivirine-side effects similar to Efavirenz but lower incidence of these |
|
Protease Inhibitors Ritonavir Saquinavir Darunavir Lopina [107 110] |
|
|
|
Integrase Inhibitors Raltegravir Dolutegravir Elutegravir |
|
|
The nucleoside analogues (NAs) didanosine (ddI), stavudine (d4T), and zalcitabine (ddC) are most frequently associated with peripheral neuropathy, and as a result have largely been phased out in high-resource settings.[103] However, in many resource-limited settings these drugs remain in use. Zidovudine (AZT) and Abacavir both remain cornerstones of therapy in many resource-limited settings, and have minimal association with peripheral neuropathy, though both have been reported to cause myopathy and psychiatric symptoms in some patients. [103 104]
The NNRTI Efavirenz has the highest rate of CNS side effects, affecting more than 50% of patients in some studies.[101] Side effects include insomnia, confusion, nightmares, and psychiatric symptoms including anxiety and depression. In contrast, nevirapine appears to have few CNS side effects. The newer NNRTI Etravirine has also been associated with peripheral neuropathy, though at lower rates than the older NAs. Rilpivirine appears to have relatively few CNS side effects, though depression, headache, and insomnia have been reported.
Protease inhibitors have largely been thought to have minimal CNS side effects, although in vitro data suggests that there is at least a theoretical risk of neurotoxicity. [105] In addition, protease inhibitor induced hyperlipidemia presents theoretically could induce incidence of stroke, though this has yet to demonstrated in studies.
The integrase inhibitors (Raltegravir, Dolutegravir, Elutegravir) have only recently become available in many resource-limited settings, and are primarily used in those settings as third-line or salvage therapy. Although the profile of CNS side effects is similar to those reported for Efavirenz (sleep disturbance, confusion, and psychiatric changes), the incidence is much lower and in practice discontinuation due to side effects is rare with these drugs. [111–113]
Given growing evidence that the CNS is a viral reservoir, another important aspect of ARV therapy is its ability to cross the blood brain barrier and penetrate CNS brain parenchyma. The CNS is an immune-privileged compartment and few ARVs are efficient with effective penetration. Newer therapeutic agents with good CNS penetration and minimal neurotoxicity are appealing but are often unavailable in resource-limited settings where older-generation ARVs remain the mainstay of treatment due to cost.[88 114]
Comorbid Conditions
Co-Infection with Malaria and Tuberculosis (TB)
Globally, a significant number of HIV-infected individuals reside in sub-tropical climates where infections or exposures present comorbid health risks. Malaria remains among the most common devastating illness in Africa. HIV+ people living in malaria-endemic regions experience frequent malaria infections; some have suggested that malaria co-infections can speed the progress of HIV, but studies in both adults and children have found acute mortality rates comparable in HIV-infected and uninfected individuals.[115] A significantly higher prevalence of co-infection has also been observed among non-cART patients compared to cART patients.[116] However, cotrimoxazole prophylaxis has demonstrated a 69%–80% reduction in the risk of malaria in HIV+ adults.[116–118] HIV-infected pregnant women did not demonstrate reduced risk of placental or maternal malaria nor improved birth conditions with daily treatment of trimethoprim-sulfamethoxazole (TMP-SMX) compared to control.[119] Newborns of mothers co-infected with HIV and malaria are at high risk of congenital malaria when maternal CD4 count is <200 cells/μl.[120] Among children with cerebral malaria, HIV-coinfection is associated with marked blunting of the inflammatory response but does not affect parasite density or outcome.[121]
Tuberculosis (TB) is a leading cause of death among HIV+ individuals worldwide. HIV significantly increases the risk of TB co-infection, including central nervous system (CNS) TB, which often manifests as meningitis, tuberculomas, and radiculomyelitis. Early diagnosis and treatment of CNS TB is essential to minimizing morbidity and mortality.[122] However, CNS TB recognition in HIV+ populations is especially challenging due to the increased risk of CNS OIs and malignancies that may present similar to or along with CNS TB.[123] HIV-TB coinfection management is complicated by drug-drug interactions, uncertainty of optimal ARV start time, and IRIS.[88–93 124 125]
Mental and Substance Use Disorders
Mental and substance use disorders are common among HIV+ individuals.[126] When HIV+ individuals suffer from multiple stigma-laden medical conditions, the effect is more than additive.[127] Adherence to cART is negatively associated with depressive symptoms and drug use.[128 129]
There is some evidence that depression may be associated with the same underlying HIV-mediated inflammatory process that causes HAND.[27 130] Elevated plasma pro-inflammatory cytokine levels contribute to the development of depression and depressive-like behaviors in HIV+ subjects.[131] A study of HIV+ patients in an Irish clinic reported 51.1% (N=604) screened positively for cognitive impairment; of those positive, 9.1% positively screened for depression and 24.5% positively screened for anxiety.[132] Moreover, depression prevalence among HIV+ individuals has been found to increase with age.[133]
Seizures and Chronic Seizure Disorders
Seizures are thought to occur in at least 11% of people with HIV.[134] OIs and associated CNS structural lesions are among the most common cause of HIV-associated seizures. In resource-limited settings, key questions about seizures and seizure disorders in caring for people with HIV include: (1) what is the underlying cause of the seizure(s), (2) who warrants long-term treatment with antiepileptic drugs (AEDs) (i.e. who is going to have further seizures), and (3) given the limited AED options available, when and what AEDs should be utilized?
A cohort study of HIV-Associated Seizures and Epilepsy (CHASE) Study in HIV+ Zambian adults with new onset seizure has identified significant functional impairment in 44% of patients, with 25% of patients experiencing recurrent seizures.[135 136] The only identifiable risk factor for seizure recurrence was survival with 37% of patients dying within less than 1 year of the index seizure.[137 138] High early mortality in the setting of advanced immune suppression highlights the urgent need for immune reconstitution and necessary avoidance of non-urgent initiation of any enzyme-inducing medications (including AEDs) that might decrease the ARV efficacy. Seizure etiologies most commonly include co-infection with CNS OIs (Cryptococcus, JC-virus, TB, CMV, and VZV) and very low CD4 counts (median: 112 cells/μl). Failure to identify a seizure etiology in the CHASE study population, which underwent imaging, EEG, and extensive CSF PCR evaluation for OIs, was associated with a higher mortality, suggesting that primary HIV effects on the CNS might be responsible for the seizure and be a marker for more fatal disease.[138]
More than 80% of people with epilepsy live in LMICs, and most live in tropical areas.[139] Evidence-based guidelines for the co-treatment of epilepsy and HIV are largely informed by small pharmacokinetic studies and case reports that conclude levetiracetam, lacosamide, gabapentin and pregabalin are the most ideal agents for HIV/epilepsy co-treatment.[140] All of these AEDs are costly, and none are routinely available in most LMIC settings. Enzyme-inducing AEDs (phenobarbital, phenytoin and carbamazepine) are the most widely available treatment options in LMIC, but their co-use with ARVs is not recommended since single pill cART therapies do not allow for dose adjustment and subtherapeutic levels of PIs or NRTS due to AED-associated enzyme induction could result in the development of ARV-resistant HIV strains (Table 5).[141 142] Among low-cost, older generation AEDs, valproic acid is probably the safest medication to combine with ARV medication though adverse effects to the liver and/or mitochondrial dysfunction remain a concern. Adverse AED-ARV interactions can also occur due to HIV-associated hypoalbuminemia, which can lead to elevated free levels of highly protein-bound AEDs, increased AED hypersensitivity responses in people with HIV, and increased risk of bone loss from combining AEDs and ARVs than can independently lead to osteoporosis.[143]
Table 5.
Specific Reported AED-ARV Interactions.
| Interaction | Effect |
|---|---|
| Ritonavir + valproic acid | Decreased VPA levels |
| Efavirenz + valproic acid | Decreased VPA levels |
| Phenytoin + lopinavir/ritonavir | Decreased lopinavir by 33% and ritonavir by 28% |
| Valproic acid + lopinavir | Increased lopinavir by 38% |
| Atazanavir/ritonavir + lamotrigine | Decreased lamotrigine by 32% |
| Lopinavir/ritonavir + lamotrigine | Decreased lamotrigine by 50% |
| Lopinavir/ritonavir + phenytoin | Decreased phenytoin by 31% |
| Carbamazepine + Efavirenz | Decrease Efavirenz by 36% |
| Carbamazepine + nevirapine | Decrease nevirapine half-life by ~20% |
| Phenytoin + nevirapine | Decrease nevirapine half-life |
| Valproic acid + zidovudine | Doubled zidovudine level |
| Efavirenz + carbamazepine | Decreased carbamazepine by 27% |
Aging in the HIV+ Population
The widespread availability of cART in resource-rich settings has led to the transition of HIV from an acute, deadly illness to a chronic disease. In LMIC regions, HIV remains a cause of significant morbidity and mortality in younger populations due to ongoing sociocultural challenges, stigma, and minimal access to newer, less toxic ARVs; however, global trends demonstrate an aging population of HIV+ individuals who receive adequate ARVs in resource-limited regions.[144] For example, HIV+ Ugandans in their 40s who are receiving ARVs can expect to live into their 60s.[145] Approximately 1/8 HIV+ adults and 1/10 HIV+ patients receiving ARVs in sub-Saharan Africa are >50 years old.[146] Despite global decreases in HIV-associated mortality, infection remains a leading cause of disability-adjusted life years (DALYs).[147] Neurological disease remains common among treated HIV+ patients due to early viral entry into the CNS and ongoing inflammation and immune activation that persist in chronic infection. Progressive brain atrophy despite persistent viral suppression in HIV+ adults over age 60 is also of concern.[148] Co-existing conditions and risk factors, including hypertension, hyperlipidemia, substance abuse, and ARV treatment effects, contribute to the effects of primary HIV infection on the nervous system. In addition, neurological conditions often associated with older age, including stroke and dementia, are occurring at younger ages in people with chronic HIV infection, a phenomenon referred to as “accelerated aging.” This may be due to chronic systemic inflammation, which occurs despite virological suppression, long-term ARV toxicity, lifestyle factors, or a combination thereof. [149 150] Therefore, it is important for providers to be aware of this and screen for neurologic diseases of older age in younger adults with chronic HIV infection.
Systemically, HIV is associated with frailty, a geriatric syndrome characterized by unintentional weight loss, diminished gait speed and grip strength, exhaustion, and low energy expenditure.[151 152] In Western HIV+ cohorts, frailty has been associated with increased mortality and morbidity, including hospitalizations, and occurs at younger ages than in HIV-uninfected populations. [153–159] Furthermore, frailty at the time of cART initiation was predictive of lower AIDS-free survival, increased mortality rates, and was inversely related to CD4 counts. Frailty is likely a major contributor to neurological deterioration in aging HIV+ patients. Studies have shown an increased risk of cognitive impairment among HIV- older adults with frailty, and HIV+ adults with cognitive impairment have significantly increased odds of frailty. [160] However, little is known about the burden of frailty in LMIC, particularly in sub-Saharan Africa, and gaps in knowledge regarding the health of older people in this region is increasingly being recognized as a public health concern.[161 162]
HAND refers to a spectrum of neurocognitive impairment that includes asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD). HAND is diagnosed using neuropsychological testing and functional status assessments, and HAND stages are differentiated based on the severity of deficits on these tests. [163] Although HAND stabilizes and sometimes improves with initiation of cART, it rarely resolves completely, even in the setting of optimal systemic virological suppression, and incident HAND can occur in patients on cART. Thus, while less severe HAND stages predominate today, the overall prevalence of HAND remains relatively unchanged compared to the pre-cART era, affecting up to 50% of HIV-infected persons and remaining one of the most common neurological complications of HIV. [164–166] Recognition of HAND is important as individuals with HAND have higher rates of cART non-adherence and steeper declines in adherence over time compared to individuals without HAND.[167–169]
The reported prevalence of HAND in LMIC varies widely, ranging from 6%–64% in children and adults,[148 170–176] likely reflecting vast differences in methodology (e.g. use of screening tests versus full neuropsychological test batteries for diagnosis) and patient populations (e.g. cART-naïve versus cART-treated patients). In a study of adult HIV+ Malawians on cART, 15% reported symptomatic neurocognitive impairment (12% MND, 3% HAD) while 55% met criteria for ANI.[177] In a meta-analysis on the epidemiology of neurodegenerative disease in sub-Saharan Africa, 47/144 (33%) studies reported HAND as a major contributor to neurodegenerative disease.[178] Accurate epidemiologic data about HAND in sub-Saharan Africa is important, however, as rates may differ from Western population due to differences in risk factors including differing genetics and rates of co-morbidities known to increase HAND risk such as hypertension and diabetes. The biology of HIV itself also differs in this region with different circulating HIV subtypes compared to Western populations, but results conflict regarding the effect of HIV subtypes on HAND (Supplemental Table 8). Some studies suggest subtype D is most neurovirulent, followed by subtypes B, C, and A, respectively, but other studies suggest HAND prevalence is not affected by subtype. [179] Methodological limitations may account for at least some of these differences, but further investigation is needed to definitively answer these questions.
The impact of HAND will likely continue to grow as the global HIV+ population ages as mounting evidence suggests that HIV exacerbates age-associated cognitive decline[180–183] and older age is associated with increased risk of HAND.[168, 169, 184–187] Older HIV+ individuals also demonstrate greater than expected brain atrophy on neuroimaging studies which was associated with impaired performance in multiple cognitive domains compared to older HIV- individuals.[188] Longitudinal studies demonstrate synergistic effects of HIV and aging on cognitive function.[189–193] Cognitive decline is likely multifactorial due to direct damage from the virus and indirect damage through secondary risk factors, including vascular disease, chronic drug use, and toxic long-term effects of ARVs. Importantly, premature age-associated neurocognitive decline correlated with structural and functional brain changes on neuroimaging and histopathology, including signs of Alzheimer’s disease pathology, is observed in some HIV+ patients at younger ages than would otherwise be expected and may be related to accelerated aging as discussed above.[194–196]
Currently, no HAND-specific therapies exist, but small trials of paroxetine and maraviroc showed some benefit in improving neurocognitive function in HIV+ cART-treated adults while trials of intranasal insulin are ongoing after in vitro evidence suggested insulin may have neuroprotective effects in HIV infection. [197–201] Development of validated biomarkers and improved clinical neurocognitive tests that can holistically and accurately assess the risk of developing HAND are also needed to facilitate future trials of novel HAND therapies. Table 6 includes a review of key biomarkers associated with HIV-associated cognitive impairment.
Table 6.
Review of key biomarkers associated with HIV-associated cognitive impairment.
| Biomarker | Pathway | Outcome | Key Studies |
|---|---|---|---|
| C-Reactive Protein (CRP) | General marker of systemic inflammation; acute phase reactant. Associated with complement system activation. | Associated with cognitive impairment and mortality in adults; associated with cognitive impairment in children when associated with other biomarkers. | Kuller et al 2008 Boulware et al 2011 Kapetanovic et al 2014 De Luca et al 2013 |
| Interleukin-6 (IL-6) | Marker of T-cell and macrophage activation; Stimulates immune response in response to infection. | Associated with cognitive impairment and mortality in adults; associated with cognitive impairment in children when associated with other biomarkers. | Kuller et al 2008 Boulware et al 2011 Tenorio et al 2014 Ancuta et al 2008 Kapetanovic 2014 |
| Fibrinogen | Involved in blood clotting; Marker of systemic inflammation and vascular injury. | Associated with cognitive impairment and mortality in adults; associated with cognitive impairment in children when associated with other biomarkers. | Kapetanovic et al 2014 |
| Soluble TNF receptors 1 and 2 (sTNFR1 and sTNFR2) | Produced in response to increased levels of TNF; may modulate effects of TNF. | Associated with cognitive impairment and non-AIDS events in adults. Stronger association noted for sTNFR2 than for sTNFR1. | Tenorio et al 2014 |
| Soluble CD163 (sCD163) | Marker of monocyte/macrophage activation; induced by endotoxin and toll-like receptor activation | Associated with coronary events and cognitive impairment in adults | Burdo et al 2013 |
| Soluble CD14 (sCD14) | Produced by monocytes in response to lipopolysaccharide (LPS); marker of monocyte response to LPS. | Associated with cognitive impairment and mortality in adults. | Ancuta et al 2008 |
| Soluble CD40 ligand (sCD40L) | Produced by activated platelets; pro-inflammatory; promotes monocyte adhesion to endothelium and increases blood-brain barrier permeability | Associated with atherosclerosis and dementia in adults. | Sui et al 2007Davidson 2012 |
| Heme-oxygenase 1 (HO-1) | Marker of oxidative stress | Associated with dementia in adults; associated with cognitive decline in children. | Gill et al 2014Bearden et al 2017 |
Aspects of Pediatric HIV Neurology
More than 3 million children worldwide are HIV+, with >90% residing in low-resource settings.[202] HIV may cause neurologic disorders in children through primary viral effects or immune suppression resulting in OIs.[203] In addition, perinatally-infected HIV+ children are at risk of complications through in utero exposure to maternal HIV.[204] Pediatric HIV infection may affect any part of the nervous system and is more likely to cause brain disorders than affect the spinal cord or peripheral nerves in children compared with adults.[134 203] The primary effects of HIV in the pediatric nervous system may manifest differently than in adults, particularly with HIV-associated cognitive impairment and HIV-associated cerebrovascular disease.[205 206] The spectrum of OIs in children is similar to that in adults, though certain OIs, such as JC-virus-associated progressive multifocal leukoencephalopathy (PML), are rare in children.[203] Cerebral toxoplasmosis is uncommon in younger children but may be seen in older children and adolescents.[207] For a summary of the most common neurologic manifestations of HIV in children, see Table 7.
Table 7.
Summary of key primary neurologic complications in children with HIV.
| Complication | Prevalence | Key Studies |
|---|---|---|
| Progressive encephalopathy | Untreated children: 30–50% With early cART: <1% |
Donald et al. 2015; Patel et al. 2009 |
| Other cognitive impairment | Untreated children: >90% With early cART: 10–50% |
Abubakar 2008; Nachman et al. 2009 |
| Stroke | Uncommon (exact prevalence unknown) | Izbudak 2013; Shah et al. 1996 |
| Seizures/Epilepsy | 3–14% | Samia et al. 2013; Bearden et al. 2015 |
| Myelopathy | Rare (exact prevalence unknown) | Sharer et al. 1990 |
| Peripheral neuropathy | 5–50% | Floeter et al. 1997; Peters et al. 2014 |
HIV-Associated Neurocognitive Effects in Children
Since HIV was first described in the 1980s, neurocognitive sequelae have been a common occurrence in HIV-infected children.[140, 203] The spectrum of HIV-associated neurocognitive impairment is broad, ranging from a severe and often fatal encephalopathy in untreated children to milder forms of cognitive impairment largely characterized by deficits in attention and executive function (Figure 1). However, determining the extent of the contribution of HIV to cognitive impairment in children can be challenging due to confounding effects of multiple overlapping factors, including parental illness, low socioeconomic status (SES), stigma, malnutrition, and reduced educational opportunities.[208]
Figure 1.
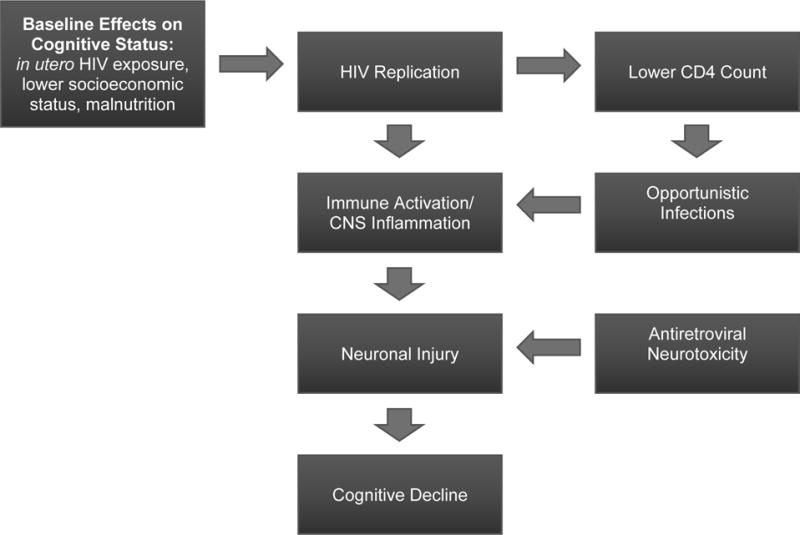
Conceptual model of HIV-associated neurocognitive impairment in children.
HIV-associated progressive encephalopathy (HPE) is the most severe form of HIV-associated neurocognitive impairment, and is characterized by impaired brain growth associated with developmental regression. In the pre-cART era HPE was thought to affect up to 50% of children with perinatally-acquired HIV; in the current era, it is almost exclusively seen in children who do not receive early treatment with cART. HPE is rarely encountered in resource-rich settings due to ubiquitous early treatment with cART but remains a major cause of morbidity and mortality in resource-poor settings; thus, early initiation of cART is an important preventive measure.[203] If HPE is detected early, cognitive deterioration may be arrested and partially reversed by cART initiation, yet static encephalopathy often remains in treated patients.[209]
Studies suggest that 10%–50% of cART-treated children will develop significant cognitive deficits.[143, 203, 205, 210] This may be due to poor ARV-CNS penetration, sustained immune activation leading to neuronal excitotoxicity, ARV neurotoxicity, or comorbidities such as depression impacting cognitive functioning.[211] One South African study found 45% of HIV+ youths met criteria for HIV-associated neurocognitive disorders.[212]
Effects of In Utero Exposure to Maternal HIV and ARVs
Multiple studies have found an increased risk of certain neurologic disorders and an effect on development in children who are born to HIV-infected mothers even in the absence of active HIV infection in the child.[213–216] The precise mechanisms are unclear but may be due to systemic maternal illness, teratogenic effects of ARVs, or in utero exposure to high levels of inflammatory cytokines. HIV-uninfected children born to infected mothers have higher rates of premature birth, and maternal HIV infection is a risk factor for pediatric cerebral palsy. [207] Whether this is associated with in utero antiretroviral drug toxicity or secondary to direct effects of HIV is unclear. [217] There may be selective toxicity related to specific ARV regimens used to treat pregnant women with HIV.[218]
Compartmentalization of HIV in the CNS
The CNS is rich in macrophages and microglia, which house the virus similarly to CD4+ T cells.[19] It is also a unique anatomic compartment that is “immune privileged” due to the semipermeable nature of the BBB which leads to differing mechanisms of immune surveillance in the CNS compared to the periphery. As a result, the CNS may serve as a reservoir for viral replication outside the reach of systemic immune surveillance, giving rise to two important phenomena – CSF viral escape and CNS compartmentalization - that have major implications for HIV eradication strategies.[219]
CNS infection is generally well-controlled by systemic suppressive cART, yet there are some instances when HIV RNA can be detected in the CSF despite plasma virus suppression below measureable clinical limits. This is known as CSF viral escape. It occurs in approximately 10% of cART-treated patients with plasma viral suppression and most likely originates from local viral reservoirs. [220] It is clinically relevant as it may be an important mechanism in the development of HIV-associated neurocognitive disorders (HAND) in cART-treated patients with plasma viral suppression. [221–223] In some cases, disproportionate viral replication in the CSF or CSF viral escape may occur in addition to attack on HIV-infected CD4+ lymphocytes and autoreactive CD8+ cells. A better understanding of CSF viral escape could lead to important insights about CNS HIV infection including the CNS cell types producing HIV during infection and whether CNS infection could re-seed systemic infection over time.[224] However, studies of CSF viral escape are limited in all settings by its relatively low prevalence and the need for a lumbar puncture for identification, and diagnosis of viral escape is currently impossible in most low-resource settings.[224–225]
CNS compartmentalization occurs when virus that enters the CNS undergoes divergent evolution from systemic virus resulting in genetically distinct viral strains in the CNS.[226] This process usually begins during primary or uncontrolled chronic HIV infection and has also been associated with the development of neurocognitive impairment. [227] Even in virologically suppressed cART-treated patients without CSF viral escape, HIV may persist in CNS reservoirs in a dormant state of infection that is capable of reactivation and replication of new viral particles.[19 228] This viral population will likely pose a major barrier to HIV cure strategies. Further research is necessary to understand the reservoir role of the CNS and to develop CNS-active latency-reversing agents (LRAs) and biomarkers to enable monitoring and treatment evaluation.[229–230]
Conclusion
Neurological conditions associated with HIV infection continue to cause major disability and mortality, particularly in resource-limited settings, where a large portion of patients present severely immunosuppressed. Barriers continue to limit quality of life in HIV+ patients suffering from major neurological manifestations. There is mounting evidence that the CNS plays an essential role in HIV persistence and is likely a major barrier to disease eradication. Lastly, acute and chronic effects of HIV infection on the nervous system are increasing in aging populations around the world with synergistic effects on the risk of cognitive dysfunction and cerebrovascular disease.
Supplementary Material
Footnotes
Funding Disclosures: None
References
- 1.UNAIDS. Global AIDS Update. Geneva: Joint United Nations Programme on HIV/AIDS; 2016. Global AIDS Update 2016. [Google Scholar]
- 2.UNAIDS. UNAIDS, ed Global AIDS Update. Geneva: Joint United Nations Programme on HIV/AIDS; 2017. UNAIDS Data 2017. [Google Scholar]
- 3.Levy RM, Bredesen DE, Rosenblum ML. Neurological manifestations of the acquired immunodeficiency syndrome (AIDS): experience at UCSF and review of the literature. J Neurosurg. 1985;62(4):475–95. doi: 10.3171/jns.1985.62.4.0475. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 4.Koppel BS, Wormser GP, Tuchman AJ, Maayan S, Hewlett D, Jr, Daras M. Central nervous system involvement in patients with acquired immune deficiency syndrome (AIDS) Acta Neurol Scand. 1985;71(5):337–53. doi: 10.1111/j.1600-0404.1985.tb03211.x. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 5.Snider WD, Simpson DM, Nielsen S, Gold JW, Metroka CE, Posner JB. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983;14(4):403–18. doi: 10.1002/ana.410140404. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 6.Birbeck GL, Meyer AC, Ogunniyi A. Nervous system disorders across the life course in resource-limited settings. Nature. 2015;527(7578):S167–71. doi: 10.1038/nature16031. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zayyad Z, Spudich S. Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND) Curr HIV/AIDS Rep. 2015;12(1):16–24. doi: 10.1007/s11904-014-0255-3. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol. 2010;5(3):294–309. doi: 10.1007/s11481-010-9205-z. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll A, Brew B. HIV-associated neurocognitive disorders: recent advances in pathogenesis, biomarkers, and treatment. F1000Res. 2017;6:312. doi: 10.12688/f1000research.10651.1. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones BM, Chiu SS, Wong WH, Lim WW, Lau YL. Cytokine profiles in human immunodeficiency virus-infected children treated with highly active antiretroviral therapy. J Int AIDS Soc. 2005;7(2):71. doi: 10.1186/1758-2652-7-2-71. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilakka-Kanthikeel S, Huang S, Fenton T, Borkowsky W, Cunningham CK, Pahwa S. Increased gut microbial translocation in HIV-infected children persists in virologic responders and virologic failures after antiretroviral therapy. Pediatr Infect Dis J. 2012;31(6):583–91. doi: 10.1097/INF.0b013e31824da0f5. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallet MA, Rodriguez CA, Yin L, et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS. 2010;24(9):1281–90. doi: 10.1097/QAD.0b013e328339e228. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dever SM, Rodriguez M, Lapierre J, Costin BN, El-Hage N. Differing roles of autophagy in HIV-associated neurocognitive impairment and encephalitis with implications for morphine co-exposure. Front Microbiol. 2015;6:653. doi: 10.3389/fmicb.2015.00653. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields J, Dumaop W, Eleuteri S, et al. HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J Neurosci. 2015;35(5):1921–38. doi: 10.1523/JNEUROSCI.3207-14.2015. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez AB, Kaul M. Neuronal Stress and Injury Caused by HIV-1, cART and Drug Abuse: Converging Contributions to HAND. Brain Sci. 2017;7(3) doi: 10.3390/brainsci7030025. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellmuth J, Fletcher JL, Valcour V, et al. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology. 2016;87(2):148–54. doi: 10.1212/WNL.0000000000002837. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–82. doi: 10.1093/infdis/jis326. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ragin AB, Wu Y, Gao Y, et al. Brain alterations within the first 100 days of HIV infection. Ann Clin Transl Neurol. 2015;2(1):12–21. doi: 10.1002/acn3.136. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tompkins L, Dukhovlinova E, Swanstrom R. HIV Reservoirs in the Central Nervous System. In: al. TJHe, editor. Encyclopedia of AIDS. New York: 2016. [Google Scholar]
- 20.Rahimy E, Li FY, Hagberg L, et al. Blood-Brain Barrier Disruption Is Initiated During Primary HIV Infection and Not Rapidly Altered by Antiretroviral Therapy. J Infect Dis. 2017;215(7):1132–40. doi: 10.1093/infdis/jix013. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kore I, Ananworanich J, Valcour V, et al. Neuropsychological Impairment in Acute HIV and the Effect of Immediate Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2015;70(4):393–9. doi: 10.1097/QAI.0000000000000746. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newton PJ, Newsholme W, Brink NS, Manji H, Williams IG, Miller RF. Acute meningoencephalitis and meningitis due to primary HIV infection. BMJ. 2002;325(7374):1225–7. doi: 10.1136/bmj.325.7374.1225. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kranick SM, Nath A. Neurologic complications of HIV-1 infection and its treatment in the era of antiretroviral therapy. Continuum (Minneap Minn) 2012;18(6 Infectious Disease):1319–37. doi: 10.1212/01.CON.0000423849.24900.ec. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma SK, Soneja M. HIV & immune reconstitution inflammatory syndrome (IRIS) Indian J Med Res. 2011;134(6):866–77. doi: 10.4103/0971-5916.92632. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aziz-Donnelly A, Harrison TB. Update of HIV-Associated Sensory Neuropathies. Curr Treat Options Neurol. 2017;19(10):36. doi: 10.1007/s11940-017-0472-3. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 26.Saylor D, Nakigozi G, Nakasujja N, et al. Peripheral neuropathy in HIV-infected and uninfected patients in Rakai, Uganda. Neurology. 2017 doi: 10.1212/WNL.0000000000004136. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birbeck GL, Kvalsund MP, Byers PA, et al. Neuropsychiatric and socioeconomic status impact antiretroviral adherence and mortality in rural Zambia. Am J Trop Med Hyg. 2011;85(4):782–9. doi: 10.4269/ajtmh.2011.11-0187. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alfahad T, Nath A. Retroviruses and amyotrophic lateral sclerosis. Antiviral Res. 2013;99(2):180–7. doi: 10.1016/j.antiviral.2013.05.006. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamin LA, Corbett EL, Connor MD, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: A case-control study. Neurology. 2016;86(4):324–33. doi: 10.1212/WNL.0000000000002278. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izbudak I, Chalian M, Hutton N, et al. Perinatally HIV-infected youth presenting with acute stroke: progression/evolution of ischemic disease on neuroimaging. J Neuroradiol. 2013;40(3):172–80. doi: 10.1016/j.neurad.2012.08.001. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007;68(16):1257–61. doi: 10.1212/01.wnl.0000259515.45579.1e. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 32.Legido A, Lischner HW, de Chadarevian JP, Katsetos CD. Stroke in pediatric HIV infection. Pediatr Neurol. 1999;21(2):588. doi: 10.1016/S0887-8994(99)00057-0. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 33.Moriarty DM, Haller JO, Loh JP, Fikrig S. Cerebral infarction in pediatric acquired immunodeficiency syndrome. Pediatr Radiol. 1994;24(8):611–2. doi: 10.1007/bf02012751. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 34.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60(4):351–8. doi: 10.1097/QAI.0b013e31825c7f24. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chow FC, He W, Bacchetti P, et al. Elevated rates of intracerebral hemorrhage in individuals from a US clinical care HIV cohort. Neurology. 2014;83(19):1705–11. doi: 10.1212/WNL.0000000000000958. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khurana E, Bearden D. Stroke in Children with Human Immunodeficiency Virus in Botswana: A Report of Six Cases. Neurology. 2014;82(10):4.303. [Google Scholar]
- 37.Su T, Mutsaerts HJ, Caan MW, et al. Cerebral blood flow and cognitive function in HIV-infected men with sustained suppressed viremia on combination antiretroviral therapy. AIDS. 2017;31(6):847–56. doi: 10.1097/QAD.0000000000001414. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 38.Watson C, Busovaca E, Foley JM, et al. White matter hyperintensities correlate to cognition and fiber tract integrity in older adults with HIV. J Neurovirol. 2017;23(3):422–29. doi: 10.1007/s13365-016-0509-5. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabin CA, Ryom L, De Wit S, et al. Associations between immune depression and cardiovascular events in HIV infection. AIDS. 2013;27(17):2735–48. doi: 10.1097/01.aids.0000432457.91228.f3. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 40.Chow FC, Price RW, Hsue PY, Kim AS. Greater Risk of Stroke of Undetermined Etiology in a Contemporary HIV-Infected Cohort Compared with Uninfected Individuals. J Stroke Cerebrovasc Dis. 2017;26(5):1154–60. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.010. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruse B, Cysique LA, Markus R, Brew BJ. Cerebrovascular disease in HIV-infected individuals in the era of highly active antiretroviral therapy. J Neurovirol. 2012;18(4):264–76. doi: 10.1007/s13365-012-0092-3. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 42.Silva-Pinto A, Costa A, Serrao R, Sarmento A, Abreu P. Ischaemic stroke in HIV-infected patients: a case-control study. HIV Med. 2017;18(3):214–19. doi: 10.1111/hiv.12415. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 43.Gutierrez J, Menshawy K, Goldman J, et al. Metalloproteinases and Brain Arterial Remodeling Among Individuals With and Those Without HIV Infection. J Infect Dis. 2016;214(9):1329–35. doi: 10.1093/infdis/jiw385. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chow FC, Li Y, Hu Y, et al. Relationship Between HIV Infection, Antiretroviral Therapy, Inflammatory Markers, and Cerebrovascular Endothelial Function Among Adults in Urban China. J Acquir Immune Defic Syndr. 2017;74(3):339–46. doi: 10.1097/QAI.0000000000001254. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morioka H, Yanagisawa N, Sasaki S, et al. CD8 Encephalitis Caused by Persistently Detectable Drug-resistant HIV. Intern Med. 2016;55(10):1383–6. doi: 10.2169/internalmedicine.55.5783. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 46.Gray F, Lescure FX, Adle-Biassette H, et al. Encephalitis with infiltration by CD8+ lymphocytes in HIV patients receiving combination antiretroviral treatment. Brain Pathol. 2013;23(5):525–33. doi: 10.1111/bpa.12038. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kugathasan R, Collier DA, Haddow LJ, et al. Diffuse White Matter Signal Abnormalities on Magnetic Resonance Imaging Are Associated With Human Immunodeficiency Virus Type 1 Viral Escape in the Central Nervous System Among Patients With Neurological Symptoms. Clin Infect Dis. 2017;64(8):1059–65. doi: 10.1093/cid/cix035. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammond CK, Eley B, Wieselthaler N, Ndondo A, Wilmshurst JM. Cerebrovascular disease in children with HIV-1 infection. Dev Med Child Neurol. 2016;58(5):452–60. doi: 10.1111/dmcn.13080. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 49.Afzal A, Benjamin M, Gummelt KL, Afzal S, Shamim S, Tribble M. Ascending paralysis associated with HIV infection. Proc (Bayl Univ Med Cent) 2015;28(1):25–8. doi: 10.1080/08998280.2015.11929176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang R, Zhang H, Xiong Y, et al. Molecular diagnosis of central nervous system opportunistic infections and mortality in HIV-infected adults in Central China. AIDS Res Ther. 2017;14:24. doi: 10.1186/s12981-017-0150-2. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mocroft AJ, Lundgren JD, d’Armino Monforte A, et al. Survival of AIDS patients according to type of AIDS-defining event. The AIDS in Europe Study Group. Int J Epidemiol. 1997;26(2):400–7. doi: 10.1093/ije/26.2.400. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 52.Tan IL, Smith BR, von Geldern G, Mateen FJ, McArthur JC. HIV-associated opportunistic infections of the CNS. Lancet Neurol. 2012;11(7):605–17. doi: 10.1016/S1474-4422(12)70098-4. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 53.Siddiqi OK, Ghebremichael M, Dang X, et al. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected Zambian adults. Clin Infect Dis. 2014;58(12):1771–7. doi: 10.1093/cid/ciu191. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sikazwe I, Elafros MA, Bositis CM, et al. HIV and new onset seizures: slipping through the cracks in HIV care and treatment. HIV Med. 2016;17(2):118–23. doi: 10.1111/hiv.12283. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thakur KT, Mateyo K, Hachaambwa L, et al. Lumbar puncture refusal in sub-Saharan Africa: A call for further understanding and intervention. Neurology. 2015;84(19):1988–90. doi: 10.1212/WNL.0000000000001561. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smego RA, Jr, Orlovic D, Wadula J. An algorithmic approach to intracranial mass lesions in HIV/AIDS. Int J STD AIDS. 2006;17(4):271–6. doi: 10.1258/095646206776253390. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 57.Ledergerber B, Egger M, Erard V, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA. 1999;282(23):2220–6. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 58.Brooks JT, Kaplan JE, Holmes KK, Benson C, Pau A, Masur H. HIV-associated opportunistic infections--going, going, but not gone: the continued need for prevention and treatment guidelines. Clin Infect Dis. 2009;48(5):609–11. doi: 10.1086/596756. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 59.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 60.Prevention; CfDCa, Health; NIo, America HMAotIDSo. Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. 2017 [Google Scholar]
- 61.Health NIo. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. AIDSInfo. 2017 [Google Scholar]
- 62.Albarillo F, O’Keefe P. Opportunistic Neurologic Infections in Patients with Acquired Immunodeficiency Syndrome (AIDS) Curr Neurol Neurosci Rep. 2016;16(1):10. doi: 10.1007/s11910-015-0603-8. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 63.Meintjes G, Scriven J, Marais S. Management of the immune reconstitution inflammatory syndrome. Curr HIV/AIDS Rep. 2012;9(3):238–50. doi: 10.1007/s11904-012-0129-5. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 64.French MA. Disorders of immune reconstitution in patients with HIV infection responding to antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4(1):16–21. doi: 10.1007/s11904-007-0003-z. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 65.Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10(11):791–802. doi: 10.1016/S1473-3099(10)70170-5. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.French MA, Mallal SA, Dawkins RL. Zidovudine-induced restoration of cell-mediated immunity to mycobacteria in immunodeficient HIV-infected patients. AIDS. 1992;6(11):1293–7. doi: 10.1097/00002030-199211000-00009. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 67.WHO, UNAIDS, UNICEF. Global Update on HIV Treatment 2013: Results, Impact and Opportunities HIV Treatment. Geneva: World Health Organization; 2013. [Google Scholar]
- 68.Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ. A systematic review and meta-regression of temporal trends in adult CD4(+) cell count at presentation to HIV care, 1992–2011. Clin Infect Dis. 2013;57(7):1027–37. doi: 10.1093/cid/cit421. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 69.Janssen S, Osbak K, Holman R, et al. Low incidence of the immune reconstitution inflammatory syndrome among HIV-infected patients starting antiretroviral therapy in Gabon: a prospective cohort study. Infection. 2017 doi: 10.1007/s15010-017-1000-9. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bahr N, Boulware DR, Marais S, Scriven J, Wilkinson RJ, Meintjes G. Central nervous system immune reconstitution inflammatory syndrome. Curr Infect Dis Rep. 2013;15(6):583–93. doi: 10.1007/s11908-013-0378-5. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thambuchetty N, Mehta K, Arumugam K, Shekarappa UG, Idiculla J, Shet A. The Epidemiology of IRIS in Southern India: An Observational Cohort Study. J Int Assoc Provid AIDS Care. 2017 doi: 10.1177/2325957417702485. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miravalle A, Jensen R, Kinkel RP. Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol. 2011;68(2):186–91. doi: 10.1001/archneurol.2010.257. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 73.Narayanan S, Banerjee C, Holt PA. Cryptococcal immune reconstitution syndrome during steroid withdrawal treated with hydroxychloroquine. Int J Infect Dis. 2011;15(1):e70–3. doi: 10.1016/j.ijid.2010.09.006. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 74.van Bilsen WPH, van den Berg C, Rijnders BJA, et al. Immune reconstitution inflammatory syndrome associated with toxoplasmic encephalitis in HIV-infected patients. AIDS. 2017;31(10):1415–24. doi: 10.1097/QAD.0000000000001492. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 75.Chang CC, Dorasamy AA, Gosnell BI, et al. Clinical and mycological predictors of cryptococcosis-associated immune reconstitution inflammatory syndrome. AIDS. 2013;27(13):2089–99. doi: 10.1097/QAD.0b013e3283614a8d. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 76.Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7(12):e1000384. doi: 10.1371/journal.pmed.1000384. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.da Cunha Colombo ER, Mora DJ, Silva-Vergara ML. Immune reconstitution inflammatory syndrome (IRIS) associated with Cryptococcus neoformans infection in AIDS patients. Mycoses. 2011;54(4):e178–82. doi: 10.1111/j.1439-0507.2010.01870.x. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 78.Bicanic T, Meintjes G, Rebe K, et al. Immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis: a prospective study. J Acquir Immune Defic Syndr. 2009;51(2):130–4. doi: 10.1097/QAI.0b013e3181a56f2e. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 79.Haddow LJ, Easterbrook PJ, Mosam A, et al. Defining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis. 2009;49(9):1424–32. doi: 10.1086/630208. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 80.Sungkanuparph S, Filler SG, Chetchotisakd P, et al. Cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: a prospective multicenter study. Clin Infect Dis. 2009;49(6):931–4. doi: 10.1086/605497. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 81.Rhein J, Boulware DR. Prognosis and management of cryptococcal meningitis in patients with human immunodeficiency virus infection. Neurobehavioral HIV Medicine. 2012;2012(4):45–61. doi: 10.2147/NBHIV.S24748. published Online First: Epub Date. [DOI] [Google Scholar]
- 82.Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51(4):448–55. doi: 10.1086/655143. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370(26):2487–98. doi: 10.1056/NEJMoa1312884. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–23. doi: 10.1016/S1473-3099(08)70184-1. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pepper DJ, Marais S, Maartens G, et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009;48(11):e96–107. doi: 10.1086/598988. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 86.Agarwal U, Kumar A, Behera D, French MA, Price P. Tuberculosis associated immune reconstitution inflammatory syndrome in patients infected with HIV: meningitis a potentially life threatening manifestation. AIDS Res Ther. 2012;9(1):17. doi: 10.1186/1742-6405-9-17. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Asselman V, Thienemann F, Pepper DJ, et al. Central nervous system disorders after starting antiretroviral therapy in South Africa. AIDS. 2010;24(18):2871–6. doi: 10.1097/QAD.0b013e328340fe76. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Health SANDo. National Consolidated Guidelines for the Prevention of Mother-To-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults Pretoria. Department of Health, Republic of South Africa: 2015. [Google Scholar]
- 89.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365(16):1492–501. doi: 10.1056/NEJMoa1014181. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471–81. doi: 10.1056/NEJMoa1013911. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365(16):1482–91. doi: 10.1056/NEJMoa1013607. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Torok ME, Yen NT, Chau TT, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)--associated tuberculous meningitis. Clin Infect Dis. 2011;52(11):1374–83. doi: 10.1093/cid/cir230. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Torok ME, Farrar JJ. When to start antiretroviral therapy in HIV-associated tuberculosis. N Engl J Med. 2011;365(16):1538–40. doi: 10.1056/NEJMe1109546. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 94.Fournier A, Martin-Blondel G, Lechapt-Zalcman E, et al. Immune Reconstitution Inflammatory Syndrome Unmasking or Worsening AIDS-Related Progressive Multifocal Leukoencephalopathy: A Literature Review. Front Immunol. 2017;8:577. doi: 10.3389/fimmu.2017.00577. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. doi: 10.1146/annurev.med.080708.082655. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 96.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430–8. doi: 10.1212/WNL.0b013e31828c2fa1. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cinque P, Koralnik IJ, Gerevini S, Miro JM, Price RW. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect Dis. 2009;9(10):625–36. doi: 10.1016/S1473-3099(09)70226-9. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sharma SR, Hussain M, Habung H. Neurological manifestations of HIV-AIDS at a tertiary care institute in North Eastern India. Neurol India. 2017;65(1):64–68. doi: 10.4103/0028-3886.198203. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 99.Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology. 2009;72(17):1458–64. doi: 10.1212/01.wnl.0000343510.08643.74. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cinque P, Pierotti C, Vigano MG, et al. The good and evil of HAART in HIV-related progressive multifocal leukoencephalopathy. J Neurovirol. 2001;7(4):358–63. doi: 10.1080/13550280152537247. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 101.Decloedt EH, Maartens G. Neuronal toxicity of efavirenz: a systematic review. Expert Opin Drug Saf. 2013;12(6):841–6. doi: 10.1517/14740338.2013.823396. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 102.WHO. Guidelines for Treatment of Tuberculosis. 4. Geneva: World Health Organization; 2010. [Google Scholar]
- 103.Kallianpur AR, Hulgan T. Pharmacogenetics of nucleoside reverse-transcriptase inhibitor-associated peripheral neuropathy. Pharmacogenomics. 2009;10(4):623–37. doi: 10.2217/pgs.09.14. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robinson-Papp J, Simpson DM. Neuromuscular diseases associated with HIV-1 infection. Muscle Nerve. 2009;40(6):1043–53. doi: 10.1002/mus.21465. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ellis RJ, Marquie-Beck J, Delaney P, et al. Human immunodeficiency virus protease inhibitors and risk for peripheral neuropathy. Ann Neurol. 2008;64(5):566–72. doi: 10.1002/ana.21484. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boly L, Cafaro V, Dyner T. Depressive symptoms predict increased incidence of neuropsychiatric side effects in patients treated with efavirenz. J Acquir Immune Defic Syndr. 2006;42(4):514–5. doi: 10.1097/01.qai.0000221691.61972.34. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 107.Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology. 2011;76(5):444–50. doi: 10.1212/WNL.0b013e31820a0cfc. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morgello S, Estanislao L, Simpson D, et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol. 2004;61(4):546–51. doi: 10.1001/archneur.61.4.546. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 109.Dalakas MC, Semino-Mora C, Leon-Monzon M. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2′3′-dideoxycytidine (ddC) Lab Invest. 2001;81(11):1537–44. doi: 10.1038/labinvest.3780367. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 110.Markowitz M, Saag M, Powderly WG, et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333(23):1534–9. doi: 10.1056/NEJM199512073332204. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 111.Padilla M, G-C A, Rojas J, Blanco JL, Blanch J, Lonca M, Torres B, Martinez M, Laguno M, Tricas A, Rodriguez A, Mallolas J, de Lazzari E, Peñafiel J, Martinez E. Tolerability of integrade inhibitors in a real-life setting. Antivir Ther. 2016;21((1):Suppl 1):A28. [Google Scholar]
- 112.Lepik KJNA, Yip B, Toy KJ, Akagi L, Montaner JSG, Guillemi S, Hogg R, Barrios R. Adverse drug reactions associated with integrase strand transfer inhibitors (INSTI) in clinical practice: post-marketing experience with raltegravir, elvitegravir-cobicistat and dolutegravir. Toronto: International AIDS Society; 2015. [Google Scholar]
- 113.de Boer M, van den Berk G, van Holten N, et al. Intolerance of dolutegravir containing cART regimens in real life clinical practice. AIDS. 2016 doi: 10.1097/QAD.0000000000001279. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 114.Bertrand L, Dygert L, Toborek M. Antiretroviral Treatment with Efavirenz Disrupts the Blood-Brain Barrier Integrity and Increases Stroke Severity. Sci Rep. 2016;6:39738. doi: 10.1038/srep39738. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hochman S, Kim K. The Impact of HIV Coinfection on Cerebral Malaria Pathogenesis. J Neuroparasitology. 2012;3:1–18. doi: 10.4303/jnp/235547. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jegede FE, Oyeyi TI, Abdulrahman SA, et al. Effect of HIV and malaria parasites co-infection on immune-hematological profiles among patients attending anti-retroviral treatment (ART) clinic in Infectious Disease Hospital Kano, Nigeria. PLoS One. 2017;12(3):e0174233. doi: 10.1371/journal.pone.0174233. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eholie SP, Ello FN, Coffie PA, Hema A, Minta DK, Sawadogo A. Effect of cotrimoxazole prophylaxis on malaria occurrence among HIV-infected adults in West Africa: The MALHIV Study. Trop Med Int Health. 2017 doi: 10.1111/tmi.12919. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 118.Ottichilo RK, Polyak CS, Guyah B, et al. Malaria Parasitemia and Parasite Density in Antiretroviral-Treated HIV-Infected Adults Following Discontinuation of Cotrimoxazole Prophylaxis. J Infect Dis. 2017;215(1):88–94. doi: 10.1093/infdis/jiw495. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Natureeba P, Kakuru A, Muhindo M, et al. Intermittent Preventive Treatment with Dihydroartemisinin-piperaquine for the Prevention of Malaria among HIV-infected Pregnant Women. J Infect Dis. 2017 doi: 10.1093/infdis/jix110. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eki-Udoko FE, Sadoh AE, Ibadin MO, Omoigberale AI. Prevalence of congenital malaria in newborns of mothers co-infected with HIV and malaria in Benin city. Infect Dis (Lond) 2017;49(8):609–16. doi: 10.1080/23744235.2017.1312667. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 121.Mbale EW, Moxon CA, Mukaka M, et al. HIV coinfection influences the inflammatory response but not the outcome of cerebral malaria in Malawian children. J Infect. 2016;73(3):189–99. doi: 10.1016/j.jinf.2016.05.012. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741–51. doi: 10.1056/NEJMoa040573. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 123.Thwaites GE, Duc Bang N, Huy Dung N, et al. The influence of HIV infection on clinical presentation, response to treatment, and outcome in adults with Tuberculous meningitis. J Infect Dis. 2005;192(12):2134–41. doi: 10.1086/498220. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 124.Grinsztejn B, De Castro N, Arnold V, et al. Raltegravir for the treatment of patients co-infected with HIV and tuberculosis (ANRS 12 180 Reflate TB): a multicentre, phase 2, non-comparative, open-label, randomised trial. Lancet Infect Dis. 2014;14(6):459–67. doi: 10.1016/S1473-3099(14)70711-X. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 125.Dooley KE, Sayre P, Borland J, et al. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin or once daily with rifabutin: results of a phase 1 study among healthy subjects. J Acquir Immune Defic Syndr. 2013;62(1):21–7. doi: 10.1097/QAI.0b013e318276cda9. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 126.Chibanda D, Cowan FM, Healy JL, Abas M, Lund C. Psychological interventions for Common Mental Disorders for People Living With HIV in Low- and Middle-Income Countries: systematic review. Trop Med Int Health. 2015;20(7):830–9. doi: 10.1111/tmi.12500. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 127.Atadzhanov M, Elafros MA, Gardiner J, et al. The Impact of Comorbid HIV Infection on Epilepsy-Associated Stigma among Zambian Adults. Neurology. 2016;86(16):4.203. [Google Scholar]
- 128.Betancur MN, Lins L, Oliveira IR, Brites C. Quality of life, anxiety and depression in patients with HIV/AIDS who present poor adherence to antiretroviral therapy: a cross-sectional study in Salvador, Brazil. Braz J Infect Dis. 2017 doi: 10.1016/j.bjid.2017.04.004. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li L, Luo S, Lan CW, et al. Alcohol Use, HIV Treatment Adherence, and Sexual Risk Among People with a History of Injecting Drug Use in Vietnam. AIDS Behav. 2017 doi: 10.1007/s10461-017-1860-0. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–86. doi: 10.1016/S1473-3099(13)70269-X. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rivera-Rivera Y, Vazquez-Santiago FJ, Albino E, Sanchez MD, Rivera-Amill V. Impact of Depression and Inflammation on the Progression of HIV Disease. J Clin Cell Immunol. 2016;7(3) doi: 10.4172/2155-9899.1000423. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McNamara PH, Coen R, Redmond J, Doherty CP, Bergin C. A High Prevalence Rate of a Positive Screen for Cognitive Impairment in Patients With Human Immunodeficiency Virus Attending an Irish Clinic. Open Forum Infect Dis. 2017;4(1):ofw242. doi: 10.1093/ofid/ofw242. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Langebeek N, Kooij KW, Wit FW, et al. Impact of comorbidity and ageing on health-related quality of life in HIV-positive and HIV-negative individuals. AIDS. 2017;31(10):1471–81. doi: 10.1097/QAD.0000000000001511. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 134.Govender R, Eley B, Walker K, Petersen R, Wilmshurst JM. Neurologic and neurobehavioral sequelae in children with human immunodeficiency virus (HIV-1) infection. J Child Neurol. 2011;26(11):1355–64. doi: 10.1177/0883073811405203. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 135.Kalungwana L, Elafros MA, Siddiqi OK, et al. Cognitive impairment and psychiatric morbidity in HIV+ Zambians with new-onset seizure. Am J Trop Med Hyg. 2014;91(6):1254–8. doi: 10.4269/ajtmh.13-0758. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Siddiqi OK, Elafros MA, Sikazwe I, et al. Acute EEG findings in HIV-infected Zambian adults with new-onset seizure. Neurology. 2015;84(13):1317–22. doi: 10.1212/WNL.0000000000001411. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Siddiqi AE, Hu X, Hall HI, Centers for Disease C, Prevention . MMWR Morb Mortal Wkly Rep. CDC; 2015. Mortality among blacks or African Americans with HIV infection--United States, 2008–2012; pp. 81–6. [PMC free article] [PubMed] [Google Scholar]
- 138.Siddiqi OK, Elafros MA, Bositis CM, et al. New-onset seizure in HIV-infected adult Zambians: A search for causes and consequences. Neurology. 2017;88(5):477–82. doi: 10.1212/WNL.0000000000003538. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moog C, Mayr L, Tolstrup M. New insights on the phenotype of HIV reservoirs. AIDS. 2016;30(10):1675–6. doi: 10.1097/QAD.0000000000001069. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 140.Birbeck GL, French JA, Perucca E, et al. Evidence-based guideline: Antiepileptic drug selection for people with HIV/AIDS: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Ad Hoc Task Force of the Commission on Therapeutic Strategies of the International League Against Epilepsy. Neurology. 2012;78(2):139–45. doi: 10.1212/WNL.0b013e31823efcf8. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Okulicz JF, Grandits GA, French JA, et al. Virologic outcomes of HAART with concurrent use of cytochrome P450 enzyme-inducing antiepileptics: a retrospective case control study. AIDS Res Ther. 2011;8:18. doi: 10.1186/1742-6405-8-18. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Okulicz JF, Grandits GA, French JA, et al. The impact of enzyme-inducing antiepileptic drugs on antiretroviral drug levels: a case-control study. Epilepsy Res. 2013;103(2–3):245–53. doi: 10.1016/j.eplepsyres.2012.07.009. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wilmshurst JM, Donald KA, Eley B. Update on the key developments of the neurologic complications in children infected with HIV. Curr Opin HIV AIDS. 2014;9(6):533–8. doi: 10.1097/COH.0000000000000101. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 144.Mills EJ, Barnighausen T, Negin J. HIV and aging--preparing for the challenges ahead. N Engl J Med. 2012;366(14):1270–3. doi: 10.1056/NEJMp1113643. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 145.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155(4):209–16. doi: 10.7326/0003-4819-155-4-201108160-00358. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 146.Negin J, Cumming RG. HIV infection in older adults in sub-Saharan Africa: extrapolating prevalence from existing data. Bull World Health Organ. 2010;88(11):847–53. doi: 10.2471/BLT.10.076349. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ortblad KF, Lozano R, Murray CJ. The burden of HIV: insights from the Global Burden of Disease Study 2010. AIDS. 2013;27(13):2003–17. doi: 10.1097/QAD.0b013e328362ba67. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Clifford KM, Samboju V, Cobigo Y, et al. Progressive Brain Atrophy Despite Persistent Viral Suppression in HIV Over Age 60. J Acquir Immune Defic Syndr. 2017 doi: 10.1097/QAI.0000000000001489. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69(7):833–42. doi: 10.1093/gerona/glt168. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Raghavan A, Rimmelin DE, Fitch KV, Zanni MV. Sex Differences in Select Non-communicable HIV-Associated Comorbidities: Exploring the Role of Systemic Immune Activation/Inflammation. Curr HIV/AIDS Rep. 2017;14(6):220–28. doi: 10.1007/s11904-017-0366-8. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 153.Althoff KN, Jacobson LP, Cranston RD, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci. 2014;69(2):189–98. doi: 10.1093/gerona/glt148. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pathai S, Gilbert C, Weiss HA, et al. Frailty in HIV-infected adults in South Africa. J Acquir Immune Defic Syndr. 2013;62(1):43–51. doi: 10.1097/QAI.0b013e318273b631. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Piggott DA, Muzaale AD, Mehta SH, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One. 2013;8(1):e54910. doi: 10.1371/journal.pone.0054910. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Terzian AS, Holman S, Nathwani N, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health (Larchmt) 2009;18(12):1965–74. doi: 10.1089/jwh.2008.1090. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62(11):1279–86. doi: 10.1093/gerona/62.11.1279. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 158.Akgun KM, Tate JP, Crothers K, et al. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr. 2014;67(4):397–404. doi: 10.1097/QAI.0000000000000341. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Desquilbet L, Jacobson LP, Fried LP, et al. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci. 2011;66(9):1030–8. doi: 10.1093/gerona/glr097. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Smith B, SR, Mateen F, Becker JT, Martin E, Miller E, Margolick JB, Selnes O, Sacktor N. Longitudinal association of HIV-associated neurocognitive disorders with frailty in HIV-1+ men. Conference on Retroviruses and Opportunistic Infections; Atlanta, Georgia. 2013. [Google Scholar]
- 161.Bernard C, Dabis F, de Rekeneire N. Physical function, grip strength and frailty in people living with HIV in sub-Saharan Africa: systematic review. Trop Med Int Health. 2017;22(5):516–25. doi: 10.1111/tmi.12852. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Teguo MT, Kuate-Tegueu C, Dartigues JF, Cesari M. Frailty in sub-Saharan Africa. Lancet. 2015;385(9983):2151. doi: 10.1016/S0140-6736(15)61021-2. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 163.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Coban H, Robertson K, Smurzynski M, et al. Impact of aging on neurocognitive performance in previously antiretroviral-naive HIV-infected individuals on their first suppressive regimen. AIDS. 2017;31(11):1565–71. doi: 10.1097/QAD.0000000000001523. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Foca E, Magro P, Motta D, et al. Screening for Neurocognitive Impairment in HIV-Infected Individuals at First Contact after HIV Diagnosis: The Experience of a Large Clinical Center in Northern Italy. Int J Mol Sci. 2016;17(4):434. doi: 10.3390/ijms17040434. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wagner GA, Chaillon A, Liu S, et al. HIV-associated neurocognitive disorder is associated with HIV-1 dual infection. AIDS. 2016;30(17):2591–97. doi: 10.1097/QAD.0000000000001237. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kamal S, Locatelli I, Wandeler G, et al. The Presence of Human Immunodeficiency Virus-Associated Neurocognitive Disorders Is Associated With a Lower Adherence to Combined Antiretroviral Treatment. Open Forum Infect Dis. 2017;4(2):ofx070. doi: 10.1093/ofid/ofx070. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tsai YT, Chen YC, Hsieh CY, Ko WC, Ko NY. Incidence of Neurological Disorders Among HIV-Infected Individuals With Universal Health Care in Taiwan From 2000 to 2010. J Acquir Immune Defic Syndr. 2017;75(5):509–16. doi: 10.1097/QAI.0000000000001448. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 169.Tsegaw M, Andargie G, Alem G, Tareke M. Screening HIV-associated neurocognitive disorders (HAND) among HIV positive patients attending antiretroviral therapy in South Wollo, Ethiopia. J Psychiatr Res. 2017;85:37–41. doi: 10.1016/j.jpsychires.2016.10.016. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 170.Patel MR, Nana M, Yotebieng M, Tabala M, Behets F, Van Rie A. Delayed antiretroviral therapy despite integrated treatment for tuberculosis and HIV infection. Int J Tuberc Lung Dis. 2014;18(6):694–9. doi: 10.5588/ijtld.13.0807. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nakku J, Kinyanda E, Hoskins S. Prevalence and factors associated with probable HIV dementia in an African population: a cross-sectional study of an HIV/AIDS clinic population. BMC Psychiatry. 2013;13:126. doi: 10.1186/1471-244X-13-126. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ruel TD, Boivin MJ, Boal HE, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis. 2012;54(7):1001–9. doi: 10.1093/cid/cir1037. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Joska JA, Westgarth-Taylor J, Myer L, et al. Characterization of HIV-Associated Neurocognitive Disorders among individuals starting antiretroviral therapy in South Africa. AIDS Behav. 2011;15(6):1197–203. doi: 10.1007/s10461-010-9744-6. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 174.Lawler K, Jeremiah K, Mosepele M, et al. Neurobehavioral effects in HIV-positive individuals receiving highly active antiretroviral therapy (HAART) in Gaborone, Botswana. PLoS One. 2011;6(2):e17233. doi: 10.1371/journal.pone.0017233. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Robertson K, Kumwenda J, Supparatpinyo K, et al. A multinational study of neurological performance in antiretroviral therapy-naive HIV-1-infected persons in diverse resource-constrained settings. J Neurovirol. 2011;17(5):438–47. doi: 10.1007/s13365-011-0044-3. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Holguin A, Faudon JL, Labernardiere JL, Soriano V. Susceptibility of HIV-1 non-B subtypes and recombinant variants to Enfuvirtide. J Clin Virol. 2007;38(2):176–80. doi: 10.1016/j.jcv.2006.09.002. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 177.Kelly CM, van Oosterhout JJ, Ngwalo C, et al. HIV associated neurocognitive disorders (HAND) in Malawian adults and effect on adherence to combination anti-retroviral therapy: a cross sectional study. PLoS One. 2014;9(6):e98962. doi: 10.1371/journal.pone.0098962. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lekoubou A, Echouffo-Tcheugui JB, Kengne AP. Epidemiology of neurodegenerative diseases in sub-Saharan Africa: a systematic review. BMC Public Health. 2014;14:653. doi: 10.1186/1471-2458-14-653. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tyor W, Fritz-French C, Nath A. Effect of HIV clade differences on the onset and severity of HIV-associated neurocognitive disorders. J Neurovirol. 2013;19(6):515–22. doi: 10.1007/s13365-013-0206-6. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ding Y, Duan S, Ye R, et al. More improvement than progression of liver fibrosis following antiretroviral therapy in a longitudinal cohort of HIV-infected patients with or without HBV and HCV co-infections. J Viral Hepat. 2017;24(5):412–20. doi: 10.1111/jvh.12658. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 181.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26(3):307–19. doi: 10.1080/13803390490510031. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 182.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8(2):136–42. doi: 10.1080/13550280290049615. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 183.Heaton RK, Grant I, Butters N, et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1(3):231–51. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 184.Wendelken LA, Valcour V. Impact of HIV and aging on neuropsychological function. J Neurovirol. 2012;18(4):256–63. doi: 10.1007/s13365-012-0094-1. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18(Suppl 1):S11–8. doi: 10.1097/00002030-200418001-00003. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 186.Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS. 2004;18(Suppl 1):S79–86. doi: 10.1097/00002030-200418001-00012. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wilkie FL, Goodkin K, Khamis I, et al. Cognitive functioning in younger and older HIV-1-infected adults. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S93–S105. doi: 10.1097/00126334-200306012-00006. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 188.Cole JH, Underwood J, Caan MW, et al. Increased brain-predicted aging in treated HIV disease. Neurology. 2017;88(14):1349–57. doi: 10.1212/WNL.0000000000003790. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hellmuth J, Milanini B, Valcour V. Interactions between ageing and NeuroAIDS. Curr Opin HIV AIDS. 2014;9(6):527–32. doi: 10.1097/COH.0000000000000104. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Seider TR, Luo X, Gongvatana A, et al. Verbal memory declines more rapidly with age in HIV infected versus uninfected adults. J Clin Exp Neuropsychol. 2014;36(4):356–67. doi: 10.1080/13803395.2014.892061. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59(5):469–77. doi: 10.1097/QAI.0b013e318249db17. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Becker JT, Cuesta P, Fabrizio M, et al. Brain structural and functional recovery following initiation of combination antiretroviral therapy. J Neurovirol. 2012;18(5):423–7. doi: 10.1007/s13365-012-0115-0. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Morgan EE, Woods SP, Smith C, et al. Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND) AIDS Behav. 2012;16(8):2279–85. doi: 10.1007/s10461-012-0229-7. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol. 2009;4(2):163–74. doi: 10.1007/s11481-008-9143-1. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 195.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111(6):529–38. doi: 10.1007/s00401-006-0037-0. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 196.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19(4):407–11. doi: 10.1097/01.aids.0000161770.06158.5c. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 197.Sacktor N, Skolasky RL, Moxley R, et al. Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: results from a double-blind, placebo-controlled trial. J Neurovirol. 2017 doi: 10.1007/s13365-017-0587-z. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ndhlovu LC, Umaki T, Chew GM, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND) J Neurovirol. 2014;20(6):571–82. doi: 10.1007/s13365-014-0279-x. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gates TM, Cysique LA, Siefried KJ, Chaganti J, Moffat KJ, Brew BJ. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS. 2016;30(4):591–600. doi: 10.1097/QAD.0000000000000951. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 200.Mamik MK, Asahchop EL, Chan WF, et al. Insulin Treatment Prevents Neuroinflammation and Neuronal Injury with Restored Neurobehavioral Function in Models of HIV/AIDS Neurodegeneration. J Neurosci. 2016;36(41):10683–95. doi: 10.1523/JNEUROSCI.1287-16.2016. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gerena Y, Menendez-Delmestre R, Skolasky RL, et al. Soluble insulin receptor as a source of insulin resistance and cognitive impairment in HIV-seropositive women. J Neurovirol. 2015;21(2):113–9. doi: 10.1007/s13365-014-0310-2. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang H, Wolock TM, Carter A, et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3(8):e361–87. doi: 10.1016/S2352-3018(16)30087-X. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Donald KA, Hoare J, Eley B, Wilmshurst JM. Neurologic complications of pediatric human immunodeficiency virus: implications for clinical practice and management challenges in the African setting. Semin Pediatr Neurol. 2014;21(1):3–11. doi: 10.1016/j.spen.2014.01.004. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 204.Gomez C, Archila ME, Rugeles C, Carrizosa J, Rugeles MT, Cornejo JW. A prospective study of neurodevelopment of uninfected children born to human immunodeficiency virus type 1 positive mothers. Rev Neurol. 2009;48(6):287–91. [PubMed] [Google Scholar]
- 205.Nachman SA, Chernoff M, Gona P, et al. Incidence of noninfectious conditions in perinatally HIV-infected children and adolescents in the HAART era. Arch Pediatr Adolesc Med. 2009;163(2):164–71. doi: 10.1001/archpedi.163.2.164. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shah SS, Zimmerman RA, Rorke LB, Vezina LG. Cerebrovascular complications of HIV in children. AJNR Am J Neuroradiol. 1996;17(10):1913–7. [PMC free article] [PubMed] [Google Scholar]
- 207.Bearden D, Steenhoff AP, Dlugos DJ, et al. Early antiretroviral therapy is protective against epilepsy in children with human immunodeficiency virus infection in botswana. J Acquir Immune Defic Syndr. 2015;69(2):193–9. doi: 10.1097/QAI.0000000000000563. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hochhauser CJ, Gaur S, Marone R, Lewis M. The impact of environmental risk factors on HIV-associated cognitive decline in children. AIDS Care. 2008;20(6):692–9. doi: 10.1080/09540120701693982. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 209.Prato M, Venturini E, Chiappini E, de Martino M, Galli L. Starting treatment in pediatric HIV infection: try to clarify a gray area. Pediatr Infect Dis J. 2015;34(5 Suppl 1):S31–5. doi: 10.1097/INF.0000000000000662. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 210.Abubakar A, Van Baar A, Van de Vijver FJ, Holding P, Newton CR. Paediatric HIV and neurodevelopment in sub-Saharan Africa: a systematic review. Trop Med Int Health. 2008;13(7):880–7. doi: 10.1111/j.1365-3156.2008.02079.x. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 211.Brew BJ. HIV, the brain, children, HAART and ‘neuro-HAART’: a complex mix. AIDS. 2009;23(14):1909–10. doi: 10.1097/QAD.0b013e32832ec4c6. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 212.Hoare J, Phillips N, Joska JA, et al. Applying the HIV-associated neurocognitive disorder diagnostic criteria to HIV-infected youth. Neurology. 2016;87(1):86–93. doi: 10.1212/WNL.0000000000002669. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gonzalez R, Ruperez M, Sevene E, et al. Effects of HIV infection on maternal and neonatal health in southern Mozambique: A prospective cohort study after a decade of antiretroviral drugs roll out. PLoS One. 2017;12(6):e0178134. doi: 10.1371/journal.pone.0178134. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis. 2016;16(6):e92–e107. doi: 10.1016/S1473-3099(16)00055-4. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 215.Filteau S, Rowland-Jones S. Cytomegalovirus Infection May Contribute to the Reduced Immune Function, Growth, Development, and Health of HIV-Exposed, Uninfected African Children. Front Immunol. 2016;7:257. doi: 10.3389/fimmu.2016.00257. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis. 2012;12(4):330–40. doi: 10.1016/S1473-3099(11)70341-3. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 217.Nicholson L, Chisenga M, Siame J, Kasonka L, Filteau S. Growth and health outcomes at school age in HIV-exposed, uninfected Zambian children: follow-up of two cohorts studied in infancy. BMC Pediatr. 2015;15:66. doi: 10.1186/s12887-015-0386-8. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Landreau-Mascaro A, Barret B, Mayaux MJ, Tardieu M, Blanche S, French Perinatal Cohort Study G Risk of early febrile seizure with perinatal exposure to nucleoside analogues. Lancet. 2002;359(9306):583–4. doi: 10.1016/S0140-6736(02)07717-6. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 219.Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog. 2015;11(3):e1004720. doi: 10.1371/journal.ppat.1004720. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Eden A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202(12):1819–25. doi: 10.1086/657342. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26(14):1765–74. doi: 10.1097/QAD.0b013e328355e6b2. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Khoury MN, Tan CS, Peaslee M, Koralnik IJ. CSF viral escape in a patient with HIV-associated neurocognitive disorder. J Neurovirol. 2013;19(4):402–5. doi: 10.1007/s13365-013-0175-9. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Arenas-Pinto A, Stohr W, Clarke A, et al. Evaluation of cerebrospinal fluid virological escape in patients on long-term protease inhibitor monotherapy. Antivir Ther. 2017;22(6):535–38. doi: 10.3851/IMP3146. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Joseph J, Cinque P, Colosi D, et al. Highlights of the Global HIV-1 CSF Escape Consortium Meeting, 9 June 2016, Bethesda, MD, USA. J Virus Erad. 2016;2(4):243–50. [PMC free article] [PubMed] [Google Scholar]
- 225.Ferretti F, Gisslen M, Cinque P, Price RW. Cerebrospinal Fluid HIV Escape from Antiretroviral Therapy. Curr HIV/AIDS Rep. 2015;12(2):280–8. doi: 10.1007/s11904-015-0267-7. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 226.Choi JY, Hightower GK, Wong JK, et al. Genetic features of cerebrospinal fluid-derived subtype B HIV-1 tat. J Neurovirol. 2012;18(2):81–90. doi: 10.1007/s13365-011-0059-9. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Harrington PR, Schnell G, Letendre SL, et al. Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS. 2009;23(8):907–15. doi: 10.1097/QAD.0b013e3283299129. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol. 2016;14(1):55–60. doi: 10.1038/nrmicro.2015.5. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 229.Gray LR, Brew BJ, Churchill MJ. Strategies to target HIV-1 in the central nervous system. Curr Opin HIV AIDS. 2016;11(4):371–5. doi: 10.1097/COH.0000000000000278. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 230.Spudich SS. Immune activation in the central nervous system throughout the course of HIV infection. Curr Opin HIV AIDS. 2016;11(2):226–33. doi: 10.1097/COH.0000000000000243. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs. 2000;59(6):1251–60. doi: 10.2165/00003495-200059060-00005. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 232.Moyle GJ, Sadler M. Peripheral neuropathy with nucleoside antiretrovirals: risk factors, incidence and management. Drug Saf. 1998;19(6):481–94. doi: 10.2165/00002018-199819060-00005. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 233.Parry O, Mielke J, Latif AS, Ray S, Levy LF, Siziya S. Peripheral neuropathy in individuals with HIV infection in Zimbabwe. Acta Neurol Scand. 1997;96(4):218–22. doi: 10.1111/j.1600-0404.1997.tb00272.x. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 234.Barohn RJ, Gronseth GS, LeForce BR, et al. Peripheral nervous system involvement in a large cohort of human immunodeficiency virus-infected individuals. Arch Neurol. 1993;50(2):167–71. doi: 10.1001/archneur.1993.00540020045016. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 235.Cornblath DR. Treatment of the neuromuscular complications of human immunodeficiency virus infection. Ann Neurol. 1988;23(Suppl):S88–91. doi: 10.1002/ana.410230723. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 236.Lee AJ, Bosch RJ, Evans SR, et al. Patterns of peripheral neuropathy in ART-naive patients initiating modern ART regimen. J Neurovirol. 2015;21(2):210–8. doi: 10.1007/s13365-015-0327-1. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Schifitto G, McDermott MP, McArthur JC, et al. Incidence of and risk factors for HIV-associated distal sensory polyneuropathy. Neurology. 2002;58(12):1764–8. doi: 10.1212/wnl.58.12.1764. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 238.Simpson DM, Haidich AB, Schifitto G, et al. Severity of HIV-associated neuropathy is associated with plasma HIV-1 RNA levels. AIDS. 2002;16(3):407–12. doi: 10.1097/00002030-200202150-00012. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 239.Wang SX, Ho EL, Grill M, et al. Peripheral neuropathy in primary HIV infection associates with systemic and central nervous system immune activation. J Acquir Immune Defic Syndr. 2014;66(3):303–10. doi: 10.1097/QAI.0000000000000167. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Basu D, Williams FM, Ahn CW, Reveille JD. Changing spectrum of the diffuse infiltrative lymphocytosis syndrome. Arthritis Rheum. 2006;55(3):466–72. doi: 10.1002/art.21980. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 241.Gherardi RK, Chretien F, Delfau-Larue MH, et al. Neuropathy in diffuse infiltrative lymphocytosis syndrome: an HIV neuropathy, not a lymphoma. Neurology. 1998;50(4):1041–4. doi: 10.1212/wnl.50.4.1041. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 242.Illa I, Nath A, Dalakas M. Immunocytochemical and virological characteristics of HIV-associated inflammatory myopathies: similarities with seronegative polymyositis. Ann Neurol. 1991;29(5):474–81. doi: 10.1002/ana.410290505. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 243.Simpson DM, Bender AN. Human immunodeficiency virus-associated myopathy: analysis of 11 patients. Ann Neurol. 1988;24(1):79–84. doi: 10.1002/ana.410240114. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 244.Petito CK, Navia BA, Cho ES, Jordan BD, George DC, Price RW. Vacuolar myelopathy pathologically resembling subacute combined degeneration in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985;312(14):874–9. doi: 10.1056/NEJM198504043121402. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 245.Lloyd TE, Pinal-Fernandez I, Michelle EH, et al. Overlapping features of polymyositis and inclusion body myositis in HIV-infected patients. Neurology. 2017;88(15):1454–60. doi: 10.1212/WNL.0000000000003821. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Riancho J, Delgado-Alvarado M, Valero C, Echevarria S, Farinas MC. Clinical spectrum of peripheral facial paralysis in HIV-infected patients according to HIV status. Int J STD AIDS. 2013;24(1):39–41. doi: 10.1177/0956462412472308. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 247.Kaku M, Simpson DM. HIV neuropathy. Curr Opin HIV AIDS. 2014;9(6):521–6. doi: 10.1097/COH.0000000000000103. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 248.Finney KA, David L. Brachial plexus neuritis in the context of acute HIV seroconversion illness: a case report. Int J STD AIDS. 2012;23(2):143–4. doi: 10.1258/ijsa.2011.011176. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 249.Bowen LN, Tyagi R, Li W, et al. HIV-associated motor neuron disease: HERV-K activation and response to antiretroviral therapy. Neurology. 2016;87(17):1756–62. doi: 10.1212/WNL.0000000000003258. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Martin-Iguacel R, Ahlstrom MG, Touma M, et al. Incidence, presentation and outcome of toxoplasmosis in HIV infected in the combination antiretroviral therapy era. J Infect. 2017 doi: 10.1016/j.jinf.2017.05.018. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 251.Ene L, Marcotte TD, Umlauf A, et al. Latent toxoplasmosis is associated with neurocognitive impairment in young adults with and without chronic HIV infection. J Neuroimmunol. 2016;299:1–7. doi: 10.1016/j.jneuroim.2016.08.003. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.USAID. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. USAID; 2010. [Google Scholar]
- 253.Dedicoat M, Livesley N. Management of toxoplasmic encephalitis in HIV-infected adults (with an emphasis on resource-poor settings) Cochrane Database Syst Rev. 2006;(3):CD005420. doi: 10.1002/14651858.CD005420.pub2. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 254.Moulignier A, Lamirel C, Picard H, et al. Long-term AIDS-related PCNSL outcomes with HD-MTX and combined antiretroviral therapy. Neurology. 2017;89(8):796–804. doi: 10.1212/WNL.0000000000004265. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 255.Rubenstein J, Ferreri AJ, Pittaluga S. Primary lymphoma of the central nervous system: epidemiology, pathology and current approaches to diagnosis, prognosis and treatment. Leuk Lymphoma. 2008;49(Suppl 1):43–51. doi: 10.1080/10428190802311441. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rios A. HIV-related hematological malignancies: a concise review. Clin Lymphoma Myeloma Leuk. 2014;14(Suppl):S96–103. doi: 10.1016/j.clml.2014.06.020. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 257.Stroke NIoNDa. Progressive Multifocal Leukoencephalopathy Information Page. What research is being done? 2017 [Google Scholar]
- 258.Brew BJ, Davies NW, Cinque P, Clifford DB, Nath A. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol. 2010;6(12):667–79. doi: 10.1038/nrneurol.2010.164. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 259.Cettomai D, McArthur JC. Mirtazapine use in human immunodeficiency virus-infected patients with progressive multifocal leukoencephalopathy. Arch Neurol. 2009;66(2):255–8. doi: 10.1001/archneurol.2008.557. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 260.Miskin DP, Chalkias SG, Dang X, Bord E, Batson S, Koralnik IJ. Interleukin-7 treatment of PML in a patient with idiopathic lymphocytopenia. Neurol Neuroimmunol Neuroinflamm. 2016;3(2):e213. doi: 10.1212/NXI.0000000000000213. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Royal W, 3rd, Dupont B, McGuire D, et al. Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. J Neurovirol. 2003;9(3):411–9. doi: 10.1080/13550280390201740. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 262.Das S, Datt S, Roy P, Saha R, Xess I. Sporadic occurrence of cryptococcal meningitis in HIV-seronegative patients: Uncommon etiology? Indian J Pathol Microbiol. 2017;60(2):236–38. doi: 10.4103/IJPM.IJPM_599_16. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 263.Hu Z, Yang Y, Cheng J, Cheng C, Chi Y, Wei H. The use of mannitol in HIV-infected patients with symptomatic cryptococcal meningitis. Drug Discov Ther. 2017;10(6):329–33. doi: 10.5582/ddt.2016.01054. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 264.Montgomery MP, Nakasujja N, Morawski BM, et al. Neurocognitive function in HIV-infected persons with asymptomatic cryptococcal antigenemia: a comparison of three prospective cohorts. BMC Neurol. 2017;17(1):110. doi: 10.1186/s12883-017-0878-2. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tenforde MW, Mokomane M, Leeme T, et al. Advanced HIV disease in Botswana following successful antiretroviral therapy rollout: Incidence of and temporal trends in cryptococcal meningitis. Clin Infect Dis. 2017 doi: 10.1093/cid/cix430. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Vidal JE, Boulware DR. Lateral Flow Assay for Cryptococcal Antigen: An Important Advance to Improve the Continuum of Hiv Care and Reduce Cryptococcal Meningitis-Related Mortality. Rev Inst Med Trop Sao Paulo. 2015;57(Suppl 19):38–45. doi: 10.1590/S0036-46652015000700008. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rolfes MA, Hullsiek KH, Rhein J, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis. 2014;59(11):1607–14. doi: 10.1093/cid/ciu596. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jarvis JN, Meintjes G, Harrison TS. Outcomes of cryptococcal meningitis in antiretroviral naive and experienced patients in South Africa. J Infect. 2010;60(6):496–8. doi: 10.1016/j.jinf.2010.03.007. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010;10(67) doi: 10.1186/1471-2334-10-67. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hamill RJ, Sobel JD, El-Sadr W, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safety. Clin Infect Dis. 2010;51(2):225–32. doi: 10.1086/653606. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 272.Sloan D, Dlamini S, Paul N, Dedicoat M. Treatment of acute cryptococcal meningitis in HIV infected adults, with an emphasis on resource-limited settings. Cochrane Database Syst Rev. 2008;(4):CD005647. doi: 10.1002/14651858.CD005647.pub2. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 273.Beyene T, Zewde AG, Balcha A, et al. Inadequacy of High-Dose Fluconazole Monotherapy Among Cerebrospinal Fluid Cryptococcal Antigen (CrAg)-Positive Human Immunodeficiency Virus-Infected Persons in an Ethiopian CrAg Screening Program. Clin Infect Dis. 2017;65(12):2126–29. doi: 10.1093/cid/cix613. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wake RM, Britz E, Sriruttan C, et al. High Cryptococcal Antigen Titers in Blood are Predictive of Subclinical Cryptococcal Meningitis Among HIV-Infected Patients. Clin Infect Dis. 2017 doi: 10.1093/cid/cix872. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Savva GM, Pachnio A, Kaul B, et al. Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell. 2013;12(3):381–7. doi: 10.1111/acel.12059. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 276.Merkler AE, Reynolds AS, Gialdini G, et al. Neurological complications after tuberculous meningitis in a multi-state cohort in the United States. J Neurol Sci. 2017;375:460–63. doi: 10.1016/j.jns.2017.02.051. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Torok ME, Aljayyoussi G, Waterhouse D, et al. Suboptimal Exposure to Anti-TB Drugs in a TBM/HIV+ Population is not Related to Anti-retroviral Therapy. Clin Pharmacol Ther. 2017 doi: 10.1002/cpt.646. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 278.Nhu NT, Heemskerk D, Thu do DA, et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol. 2014;52(1):226–33. doi: 10.1128/JCM.01834-13. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Patel VB, Connolly C, Singh R, et al. Comparison of amplicor and GeneXpert MTB/RIF tests for diagnosis of tuberculous meningitis. J Clin Microbiol. 2014;52(10):3777–80. doi: 10.1128/JCM.01235-14. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.WHO. Technical and operational ‘how-to’: practical considerations Xpert MTB/RIF Implementation Manual. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 281.Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12(10):999–1010. doi: 10.1016/S1474-4422(13)70168-6. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 282.Brancusi F, Farrar J, Heemskerk D. Tuberculous meningitis in adults: a review of a decade of developments focusing on prognostic factors for outcome. Future Microbiol. 2012;7(9):1101–16. doi: 10.2217/fmb.12.86. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 283.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24(2):351–76. doi: 10.1128/CMR.00042-10. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–12. doi: 10.1016/S1473-3099(10)70138-9. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 285.Prasad K, Singh MB. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2008;(1):CD002244. doi: 10.1002/14651858.CD002244.pub3. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 286.Iten A, Chatelard P, Vuadens P, et al. Impact of cerebrospinal fluid PCR on the management of HIV-infected patients with varicella-zoster virus infection of the central nervous system. J Neurovirol. 1999;5(2):172–80. doi: 10.3109/13550289909021999. [DOI] [PubMed] [Google Scholar]
- 287.Estanislao L, Thomas D, Simpson D. HIV neuromuscular disease and mitochondrial function. Mitochondrion. 2004;4(2–3):131–9. doi: 10.1016/j.mito.2004.06.007. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 288.Rumbaugh JA, Tyor W. HIV-associated neurocognitive disorders: Five new things. Neurol Clin Pract. 2015;5(3):224–31. doi: 10.1212/CPJ.0000000000000117. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Caruana G, Vidili G, Serra PA, et al. The burden of HIV-associated neurocognitive disorder (HAND) in post-HAART era: a multidisciplinary review of the literature. Eur Rev Med Pharmacol Sci. 2017;21(9):2290–301. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


