Abstract
Obstructive sleep apnea syndrome (OSAS) is a sleep disorder characterized with upper airway obstructions. Some studies showed cognitive and electrophysiological changes in patients with OSAS; however, contradictory results were also reported. The purpose of the present study was twofold: (1) to investigate cognitive changes in severe OSAS patients by using neuropsychological tests and electrophysiological methods together, (2) to investigate influence of hypoxemia levels on cognition. Fifty-four severe OSAS patients and 34 age-, gender- and education matched healthy subjects were participated. OSAS patients were further divided into two subgroups according to minimum oxygen saturation levels. All participants underwent a detailed neuropsychological test battery. A classical visual oddball task was used to elicit ERP P300 and mean P300 amplitudes were measured from Fz, Cz and Pz electrode sites. OSAS patients showed reduced mean P300 amplitudes up to 43–51% on all electrode sites compared to healthy controls. Subgroup analysis revealed significant differences in neuropsychological test scores between healthy controls and high hypoxemia OSAS group, as well as between low and high hypoxemia groups. Moreover, both low and high hypoxemia OSAS groups had lower P300 amplitudes compared with healthy controls. P300 amplitudes showed a gradual decline in parallel with increasing hypoxemia severity; however, the difference between high and low hypoxemia OSAS groups did not reach significance. Moderate correlations were found between sleep parameters, neuropsychological test scores and P300 amplitudes. These results suggest that electrophysiological measures could be better indicators of cognitive changes than neuropsychological tests in OSAS, particularly in mildly affected patients.
Keywords: Obstructive sleep apnea syndrome, OSAS, Event-related potentials, P300, Neuropsychological tests, Hypoxemia
Introduction
Obstructive sleep apnea syndrome (OSAS) is a sleep disorder characterized with complete or partial upper airway obstructions. OSAS can be seen in all age groups, affecting up to 2% of women and 4% of men and factors such as aging, obesity and use of sedatives are known to increase the risk of OSAS (American Academy of Sleep Medicine 2005).
Excessive daytime sleepiness, loud snoring and witnessed apneas are the main signs of OSAS which are helpful for the diagnostic processes (American Academy of Sleep Medicine 2005). However, OSAS is reported to be underdiagnosed (Kapur et al. 2002), because these breathing events occur during sleep and patients usually do not become aware of these signs and/or ignore the symptoms. The risk of cardiovascular diseases, hypertension (Carlson et al. 1994; Drager et al. 2010), coronary heart disease (Peker et al. 2002; Pepperell 2011), pulmonary hypertension, stroke, psychiatric disorders, metabolic diseases and even sudden death increase over time when patients with OSAS remain untreated. Moreover, adverse effects of OSAS on cognitive functions were reported.
Cognitive dysfunction in OSAS is researched over 40 years and neuropsychological testing has been the most commonly used evaluation method. A recent meta-analysis reported that executive functions are substantially affected in patients with OSAS (for a review see: Stranks and Crowe 2016). Nevertheless, some studies comparing OSAS patients with healthy controls have not found any differences between groups in executive functions or reported confounding results (Salorio et al. 2002; Verstraeten et al. 2004). For instance, Salorio et al. (2002) found that OSAS patients had poorer letter fluency scores but similar Wisconsin Card Sorting Test (WCST) performances in comparison with healthy controls. Other cognitive functions such as memory, attention, processing speed and visual construction showed inconsistent results among previous studies. Recent studies were focused on the effects of sleep fragmentation and intermittent hypoxemia on cognition, which are considered as the underlying causes of cognitive dysfunction in OSAS (for a review, see Gagnon et al. 2014). Some authors suggested that progressive and irreversible decline in executive functions is related to long-term exposure to intermittent hypoxemia (Beebe and Gozal 2002). Findley et al. (1986) compared hypoxemic and non-hypoxemic patients with OSAS and reported that patients with hypoxemia had more severe cognitive impairment than those without, particularly in measures of attention, verbal and spatial short-term memory and complex problem-solving. Moreover, some studies reported persistent deficits in executive functions even after continuous positive airway pressure (CPAP) treatment (Bédard et al. 1993; Naegele et al. Naëgelé et al. 1995–1998; Feuerstein et al. 1997; Ferini-Strambi et al. 2003). Beebe and Gozal (2002) proposed a model which included both hypoxemia and sleep fragmentation as key mechanisms behind prefrontal cortical dysfunction in OSAS. This model emphasized that preserved executive functioning is essential to perform other cognitive abilities. Conversely, some authors suggested that the sleep fragmentation alone could be sufficient to induce cognitive impairment in OSAS patients (Verstraeten et al. 2004; Verstraeten and Cluydts 2004). Executive functions require attention and previous studies showed that attentional abilities are affected by sleep fragmentation (for a review, see Gagnon et al. 2014). Based on this idea, some authors controlled attentional capacity and the results revealed similar performances between OSAS patients and healthy controls on executive functioning tasks (Verstraeten et al. 2004). The majority of recent reports consider both intermittent hypoxemia and sleep fragmentation as contributing factors to cognitive deficits in OSAS.
Many studies examined cognitive dysfunction in OSAS through electrophysiological techniques. Event-related potentials (ERPs) are beneficial methods for investigating cognitive processes and can be obtained by averaging electrophysiological brain responses to stimulus which are presented during an electroencephalogram (EEG) recording. ERP component P300 is known to reflect higher cortical functions that involve classifying/updating memory representations of stimuli (Polich 2007). P300 can be elicited by an oddball paradigm, where an individual is asked to distinguish rare stimuli from the standard stimuli (Picton 1992). In OSAS studies, ERPs were used for distinguishing OSAS from other sleep disorders (Sangal and Sangal 1995; Sforza and Haba-Rubio 2006) or assessing treatment efficacy (Walsleben et al. 1989; Kotterba et al. 1998; Neau et al. 1996; Vakulin et al. 2012). P300 is the most investigated component and the oddball paradigm is the most common used task in these studies (Raggi and Ferri 2012). Several studies reported prolonged P300 latency in OSAS (Kotterba et al. 1998; Walsleben et al. 1989; Rumbach et al. 1991; Vakulin et al. 2012; Sangal and Sangal 1995, 1997a, b; Inoue et al. 2001; Gosselin et al. 2006; Sforza and Haba-Rubio 2006; Peng et al. 2004; Akçalı et al. 2015). Abnormal P300 latency was thought to be related to altered stimulus classification and cognitive processing speed. Considerable number of studies also reported reduced P300 amplitudes in OSAS patients compared with healthy controls (Rumbach et al. 1991; Wong et al. 2006; Martins et al. 2011; Vakulin et al. 2012; Akçalı et al. 2015). These findings indicate that attentional resources activated during the stimulus evaluation processes could be impaired in patients with OSAS. Despite the majority of significant findings, some studies did not report any differences in P300 amplitude (Sangal and Sangal 1995, 1997a, b; Afifi et al. 2003) or latency (Martins et al. 2011; Afifi et al. 2003).
The purpose of the present study was twofold: (1) to investigate cognitive changes in patients with severe OSAS by using neuropsychological tests and electrophysiological methods together, and (2) to investigate the influence of hypoxemia levels on cognition. As neuropsychological test scores are known to be influenced by age and educational level (Strauss et al. 2006), an age- and education-restricted sample was preferred in the present study. Only participants aged between 21 and 55 years with a high educational level were included to the study. Akçalı et al. 2015 also reported P300 differences only in younger participants and found no differences between older healthy subjects and older OSAS patients. The authors suggested forming study samples with a narrow age range for OSAS in order to control for variability (Akçalı et al. 2015). In the present study, a detailed neuropsychological test battery and ERP P300 were used together in order to provide a broader perspective on the subject. Furthermore, the OSAS patients were divided into two subgroups according to their hypoxemia levels to observe the effects of hypoxemia on cognitive functions. As far as we know, this is the first study examining the effects of hypoxemia on ERP P300 responses in patients with OSAS. According to previous literature, we hypothesized that patients with severe OSAS would have poorer neuropsychological test performances on attention and executive function tasks and decreased P300 amplitudes especially over parietal regions. Furthermore, we assume a gradual decline in neuropsychological test scores and P300 amplitudes in accordance with increasing hypoxemia levels.
Materials and methods
Participants
The sample of the study consisted of patients who were administered to the sleep disorders clinic in the Department of Neurology at the Dokuz Eylul University Hospital and healthy volunteers who were recruited from various community sources.
All participants had an active working life and were mostly young adults. Fifty-four untreated patients with severe OSAS with an age range of 21–51 (M = 38.94, SD = 7.43) years and 34 age-, gender- and education-matched healthy controls with an age range of 22–55 (M = 36.97, SD = 10.83) years participated to the study. All OSAS patients and 16 of healthy controls underwent a full-night polysomnographic (PSG) evaluation. Severe OSAS (Apnea–Hypopnea Index (AHI) > 30) was diagnosed according to the American Academy of Sleep Medicine criteria (Iber et al. 2007). Epworth Sleepiness Scale (ESS) was used to evaluate excessive daytime sleepiness for all participants. The healthy controls without PSG evaluation had ESS scores lower than 10. None of the healthy controls had a history of snoring or witnessed apnea and their bed partners confirmed this information. The study only included participants who had at least 11 years of education in order to prevent the education effect on neuropsychological test scores. The clinical and demographic characteristics of participants were presented in Table 1 and the PSG data of patients with OSAS are shown in Table 2.
Table 1.
Clinical and demographic characteristics of participants
| Healthy controls (n = 34) | OSAS patients (n = 54) | p | |
|---|---|---|---|
| Age | 36.97 ± 10.83 | 38.94 ± 7.43 | .355a |
| Gender (M/F) | 31/3 | 53/1 | .126b |
| Education (year) | 14.59 ± 1.07 | 14.15 ± 1.43 | .105a |
| BMI (kg/m2) | 26.28 ± 2.70 | 31.57 ± 5.63 | < .001*a |
| ESS | 2.66 ± 2.91 | 10.52 ± 4.93 | < .001*a |
| BDI | 7.21 ± 5.25 | 8.85 ± 5.63 | .175a |
Bold values indicate statistically significant differences between groups
Data are presented as mean ± standard deviation (SD)
OSAS obstructive sleep apnea, M male, F female, BMI body-mass index, ESS Epworth Sleepiness Scale, BDI Beck’s depression inventory
*p < .005
aIndependent sample t test
bChi square test
Table 2.
Polysomnographic sleep parameters of patients with OSAS
| Mean ± SD | Median | Range | |
|---|---|---|---|
| AHI (events/h) | 58.20 ± 21.05 | 54.70 | 30.00–104.10 |
| Total sleep time (min) | 437.40 ± 48.77 | 444.50 | 324.90–534.10 |
| Time with SO2 < 90% (%) | 17.04 ± 21.01 | 9.25 | 0.00–74.50 |
| Time with SO2 < 80% (%) | 4.07 ± 8.87 | .20 | 0.00–38.00 |
| Sleep efficiency (%) | 90.59 ± 6.74 | 93.45 | 70.10–99.70 |
Data are presented as mean ± standard deviation (SD)
AHI apnea/hypopnea index, h hour, min minutes, SO2 oxygen saturation
In the present study, the OSAS group was divided into two subgroups (OSAS–low hypoxemia and OSAS–high hypoxemia) to investigate the effect of hypoxemia on cognition. The subgroups were created according to the oxygen saturation levels. OSAS—low hypoxemia group (n = 24) presents the patients with minimum SO2 over 80% and OSAS–high hypoxemia group (n = 30) presents the patients with minimum SO2 below 80% in total sleep time. Clinical and demographic characteristics of participants were presented in Table 3.
Table 3.
Clinical, demographic and polysomnographic characteristics of healthy controls and OSAS subgroups
| Healthy controls (n = 34) | OSAS-low hypoxemia (n = 24) | OSAS-high hypoxemia (n = 30) | p | |
|---|---|---|---|---|
| Age | 36.97 ± 10.83 | 38.04 ± 7.43 | 39.67 ± 7.47 | .484 |
| Gender (M/F) | 31/3 | 23/1 | 30/0 | .238 |
| Education (year) | 14.59 ± 1.07 | 14.08 ± 1.55 | 14.20 ± 1.34 | .300 |
| BMI (kg/m2) | 26.28 ± 2.70 | 29.42 ± 4.86 | 33.30 ± 5.68 | < .001a,b,c |
| ESS | 2.66 ± 2.91 | 8.25 ± 3.75 | 12.33 ± 5.06 | < .001a,b,c |
| BDI | 7.21 ± 5.25 | 8.08 ± 5.80 | 9.47 ± 5.51 | .263 |
| AHI (events/h) | – | 45.93 ± 16.15 | 68.01 ± 19.47 | < .001a |
| Total sleep time (min) | – | 444.40 ± 43.67 | 431.81 ± 52.56 | .351 |
| Time with O2 < 90% (%) | – | 3.03 ± 4.18 | 28.25 ± 22.37 | .001 a |
| Time with O2 < 80% (%) | – | – | 7.34 ± 10.91 | NA |
| Sleep efficiency (%) | – | 90.74 ± 7.11 | 90.47 ± 6.56 | .884 |
Bold values indicate statistically significant differences between groups
Data are presented as mean ± standard deviation (SD). p values were obtained from One-Way ANOVA and Chi Square test for continuous and categorical data respectively
OSAS obstructive sleep apnea, M male, F female, BMI body-mass index, ESS Epworth Sleepiness Scale, BDI Beck’s depression inventory, NA non-applicable
Significant differences between groups were symbolized with: aHealthy controls versus OSAS low hypoxemia, bHealthy controls versus OSAS high hypoxemia, cOSAS low hypoxemia versus OSAS high hypoxemia
Our exclusion criteria included factors that may affect cognitive functions such as other sleep disorders, head trauma, psychiatric disorders (i.e. depression, psychosis and anxiety disorders), dementia, cerebrovascular disease, uncontrolled hypertension, uncontrolled diabetes and alcohol/drug abuse. All participants underwent a brain MRI scan to exclude any structural abnormalities. The ethical committee of Dokuz Eylul University approved the study and all participants signed an informed consent.
Neuropsychological testing
All participants underwent a detailed neuropsychological evaluation before the EEG recordings. General cognitive status, verbal and visual memory processes, attention, executive functions, visuospatial skills and language were assessed. The test battery included the following tests: The Mini Mental State Examination (MMSE—Folstein et al. 1975), Oktem Verbal Memory Processes Test (OVMPT—Öktem 1992), Wechsler Memory Scale-Revised (WMS-R) Visual Reproduction Subtest (Wechsler 1987), WMS-R Digit Span (Wechsler 1987), Wechsler Adult Intelligence Scale-Fourth (WAIS-IV) Similarities Subtest (Wechsler 2008), verbal fluency tests (semantic and lexical), clock drawing test, Wisconsin Card Sorting Test (WCST—Heaton et al. 1993), Stroop Test (Stroop 1935), simple copying test and Boston Naming Test (Kaplan et al. 2001).
Electrophysiological recording, paradigm and analysis
EEG data was collected in an electrically shielded and sound attenuated room from 30 Ag/AgCl electrodes located on an elastic cap, according to the international 10–20 system (EasyCap; Brain Products GmbH; Gilching, Germany). Linked earlobe electrodes (A1 + A2) were used as references and electrooculogram (EOG) were recorded from medial upper and lateral orbital rim of the right eye. All electrode impedances were kept below 10 kΩ. EEG was amplified from 0.03 to 70 Hz and digitalized at 500 Hz sampling rate with a BrainAmp 32-channel DC amplifier (Brain Products GmbH; Gilching, Germany).
A classical oddball paradigm was used to elicit ERPs. A total of 120 stimuli were presented visually. The standard stimulus was the light given in 10 cd/cm2 luminance (probability 0.66) and the target stimulus was the light given in 40 cd/cm2 luminance (probability 0.33). The stimuli had 10 ms r/f time and 1 s duration. The inter-stimulus interval varied randomly from 3 to 7 s. Participants were asked to mentally count the target stimuli and maximum 10% error was allowed. The number of counted stimuli did not differ between groups (p = .560).
Offline data analysis was performed with Brain Vision Analyzer 2.1 (Brain Products GmbH; Gilching, Germany). Raw EEG data were filtered between 0.5 and 30 Hz at 12 dB per octave and segmented in 1000 ms time window epochs, time-locked to the target stimulus onset (800 ms post-stimulus with a 200 ms pre-stimulus baseline). Baseline correction was applied to all data with respect to the 200 ms time interval before target stimulus presentation. Vertical and horizontal ocular artifacts were corrected offline using the Gratton et al. (1983) method. Artifacts were automatically rejected based on the following criteria: (1) maximum amplitude in an epoch was ± 100 μV; (2) maximum allowed voltage step was 50 μV/ms; (3) maximum allowed difference in a 200 ms interval was 50 μV and (4) lowest activity in a 100 ms interval was 0.5 μV. EEG data with at least 20 artifact-free epochs were averaged to obtain ERPs. Fifteen patients with severe OSAS and five healthy controls were excluded at the beginning of the study due to insufficient epoch numbers (number of epochs < 20). The remaining 54 patients with severe OSAS and 34 healthy controls were included in further analysis.
Mean P300 amplitudes in 250–550 ms time window after target onset were automatically measured from F3, Fz, F4, C3, Cz, C4, P3, Pz and P4 electrode sites. P300 amplitudes are generally larger along the midline electrodes, thus, only Fz, Cz and Pz measurements were included in the statistical analysis. Grand average ERP waveforms were computed for groups.
Statistical analysis
Statistical analysis was performed using SPSS v.20.0 (SPSS IBM, Armonk, New York). Neuropsychological test scores of healthy controls and OSAS patients were compared with independent samples t test. Mean P300 amplitude values were analyzed using repeated measures ANOVA. The factor structure in repeated measures ANOVA was 2 × 3, with two levels of group (healthy controls and OSAS patients) and three levels of anterior–posterior electrode distribution (AP—frontal, central and parietal). Corrected Greenhouse–Geisser p values were reported. Post-hoc analysis was performed with independent samples t test. Difference between groups (%) for mean P300 amplitudes were calculated with “(Healthy controls-OSAS patients)/Healthy controls” formula.
Furthermore, the OSAS group was divided into two subgroups (OSAS-low hypoxemia and OSAS-high hypoxemia) according to the oxygen saturation levels to investigate hypoxemia effects. Neuropsychological test scores of OSAS subgroups and healthy controls were compared with one-way ANOVA. Mean P300 amplitudes of the three groups were analyzed with a 3 × 3 repeated measures ANOVA [Three levels of group (Healthy controls, OSAS- low hypoxemia, and OSAS-high hypoxemia) × three levels of anterior–posterior electrode distribution (AP—frontal, central and parietal)]. Corrected Greenhouse–Geisser p values were reported and post hoc analysis was performed with independent samples t test. The relationships between neuropsychological test scores, polysomnographic sleep parameters and mean P300 amplitudes were investigated with Pearson correlation. p < 0.05 was considered as statistically significant in all analysis.
Results
In all neuropsychological tests, group means were within the expected range of age and education norms. Only two of fifty-four patients (4%) with OSAS were classified as cognitively impaired by a neuropsychologist; one patient had impaired short-term verbal memory and the second patient had impaired attention and verbal memory (both short- and long-term). Statistical analysis revealed lower OVMPT total learning scores and lower OVMPT immediate recall scores in patients with OSAS compared to healthy controls (Table 4). Other test scores did not differ between groups.
Table 4.
Neuropsychological test scores of participants
| Tests | Healthy controls (n = 34) | OSAS patients (n = 54) | p |
|---|---|---|---|
| OVMPT total learning scores | 130.15 ± 9.93 | 124.09 ± 11.91 | .016 |
| OVMPT immediate recall scores | 7.53 ± 1.71 | 6.28 ± 1.65 | .001 |
Data are presented as mean ± standard deviation (SD)
OSAS obstructive sleep apnea syndrome, OVMPT Oktem verbal memory processes test
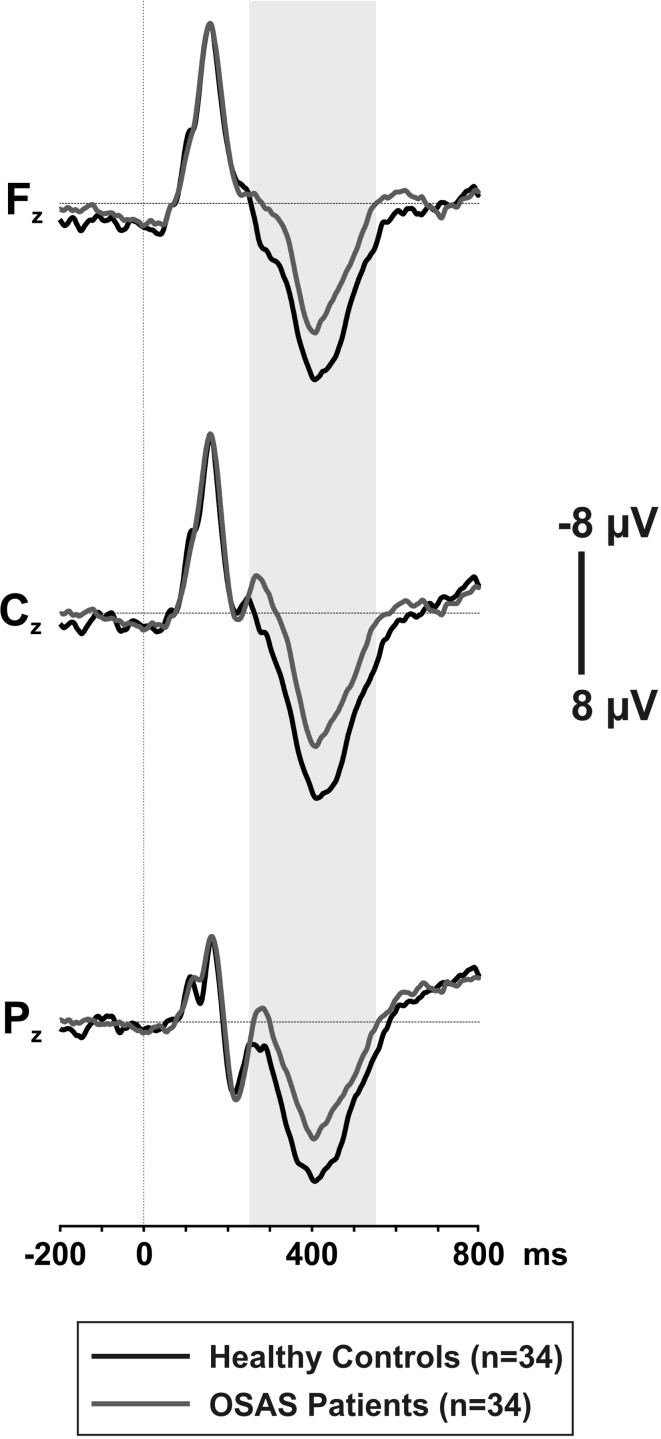
Mean P300 amplitudes showed a significant main effect of GROUP [F(1.86) = 29.315, p < .001], indicating lower mean P300 amplitudes in patients with OSAS compared with healthy controls. Post-hoc analysis revealed that patients with OSAS had decreased mean P300 amplitudes by 43–51% at Fz (p < .001), Cz (p < .001) and Pz (p < .001) electrode sites. Mean P300 amplitudes for both groups are presented in Table 5 and the grand average ERP waveforms are shown in Fig. 1. The grand averages were created with equal number of subjects in both groups, as the number of epochs/subjects is known to have an influence on grand averages.
Table 5.
Mean P300 amplitudes (µV) of healthy controls and patients with OSAS at Fz, Cz and Pz electrode sites
| Electrode | Healthy controls (n = 34) | OSAS patients (n = 54) | p | Difference % |
|---|---|---|---|---|
| Fz | 3.89 ± 1.63 | 2.03 ± 1.68 | < .001 | 48 |
| Cz | 4.03 ± 1.50 | 1.97 ± 1.85 | < .001 | 51 |
| Pz | 3.69 ± 1.48 | 2.09 ± 1.73 | < .001 | 43 |
Data are presented as mean ± standard deviation (SD)
OSAS obstructive sleep apnea syndrome, µV microvolt. Difference % is calculated with “(Healthy controls-OSAS patients)/Healthy controls × 100” formula
Fig. 1.
The grand average ERP waveforms, time-locked to the target stimulus onset at Fz, Cz and Pz electrode sites
Subgroup analysis
The OSAS group was divided into two subgroups (OSAS-low hypoxemia and OSAS-high hypoxemia) in order to investigate the effect of hypoxemia on cognition. OVMPT total learning scores (p = .005) and OVMPT immediate recall scores (p = .001) significantly differed between three groups. Post-hoc analysis revealed that the OSAS-high hypoxemia group had significantly lower OVMPT total learning and OVMPT immediate recall scores than both healthy controls (p = .002; p < .001, respectively) and the OSAS-low hypoxemia group (p = .038; p = .040, respectively). There were no significant differences between healthy controls and the OSAS-low hypoxemia group (p > 0.05).
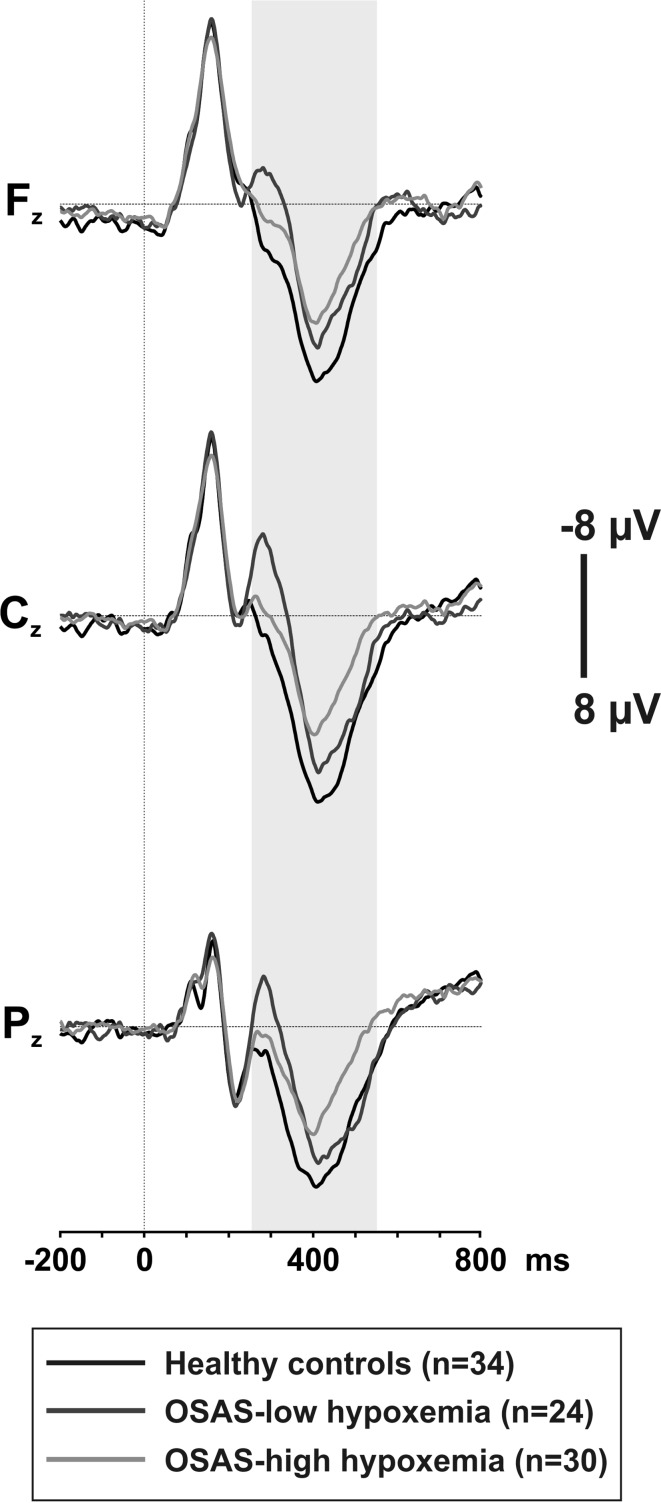
Repeated measures of ANOVA revealed a significant main effect of GROUP for mean P300 amplitudes [F(2.85) = 14.811 p < .001]. Mean P300 amplitudes of healthy controls were significantly higher compared to both OSAS subgroups (Table 6). Mean P300 amplitudes showed a gradual decline as oxygen desaturation increases; however, the observed difference between the OSAS subgroups did not reach significant levels. The grand average ERP waveforms for the three groups are presented in Fig. 2.
Table 6.
Mean P300 amplitudes (µV) of healthy controls, OSAS-low hypoxemia subgroup and OSAS-high hypoxemia subgroup at Fz, Cz and Pz electrode sites
| Electrode | Healthy controls (n = 34) | OSAS-low hypoxemia (n = 24) | OSAS-high hypoxemia (n = 30) | p |
|---|---|---|---|---|
| Fz | 3.89 ± 1.63 | 2.07 ± 1.63 | 2.00 ± 1.75 | <.001a,b |
| Cz | 4.03 ± 1.50 | 2.10 ± 1.82 | 1.86 ± 1.89 | <.001a,b |
| Pz | 3.69 ± 1.48 | 2.41 ± 1.73 | 1.83 ± 1.71 | <.001a,b |
Data are presented as mean ± standard deviation (SD)
OSAS obstructive sleep apnea syndrome, µV microvolt
Significant differences between groups were symbolized with: aHealthy controls versus OSAS low hypoxemia, bHealthy controls versus OSAS high hypoxemia
Fig. 2.
Grand average ERP waveforms for healthy controls, OSAS-low hypoxemia and OSAS-high hypoxemia subgroups at Fz, Cz and Pz electrode sites
Correlation analysis between ERP data, neuropsychological test scores and sleep parameters
Pearson correlation analysis (including both groups) revealed moderate correlations between neuropsychological test scores, sleep parameters and mean P300 amplitudes. OVMPT total learning scores were correlated with sleep efficiency (r = 0.267, p = .030), BMI (r = − 0.276, p = .014), and mean P300 amplitudes at Fz (r = 0.225, p = .035), Cz (r = 0.273, p = .010) and Pz (r = 0.256, p = .016) electrode sites. OVMPT immediate recall scores showed correlations with sleep efficiency (r = 0.256, p = .038), AHI (r = − 0.391, p = .001), and percentage of time with oxygen saturation below 90% (r = − 0.255, p = .039). WMS-R digit span forward scores were correlated with mean P300 amplitudes at Fz (r = 0.298, p = .005), Cz (r = 0.308, p = .003) and Pz (r = 0.293, p = .006) electrode sites. Furthermore, ESS scores were negatively correlated with OVMPT total learning scores (r = − 0.299, p = .006), OVMPT immediate recall scores (r = -0.391, p = .001), and mean P300 amplitudes at Fz (r = − 0.359, p = .001), Cz (r = − 0.399, p < .001) and Pz (r = − 0.319, p = .003) electrode sites. Percentage of time with oxygen saturation below 90% showed correlations with WCST number of trials to achieve the first category (r = 0.344, p = .005).
Pearson correlation analyses were conducted separately for OSAS-low hypoxemia and OSAS-high hypoxemia groups. Mean P300 amplitudes and sleep parameters did not reveal any significant correlations in both subgroups. In OSAS-high hypoxemia group, sleep efficiency showed moderate correlations with OVMPT total learning scores (r = 0.367, p = .046) and OVMPT free recall scores (r = 0.451, p = .012).
Discussion
OSAS is reported to be an underdiagnosed sleep disorder which could lead to cognitive impairment when remained untreated. In the present study, we aimed to investigate cognitive changes in patients with severe OSAS by using both neuropsychological testing and ERP P300. Moreover, we explored the influence of hypoxemia levels on cognition.
In the present study, patients with severe OSAS showed reduced mean P300 amplitudes compared to healthy controls and increased hypoxemia levels appeared to have an adverse effect on mean P300 amplitudes. Only two patients with OSAS were classified as cognitively impaired by a neuropsychologist; however, all other patients had neuropsychological test scores within normal limits. Statistical analyses revealed group differences on verbal memory test scores, indicating lower scores in patients with OSAS compared to healthy controls. The OSAS patients with higher levels of hypoxemia had the lowest verbal memory test scores when compared to healthy controls and the OSAS patients with lower levels of hypoxemia.
The a priori hypothesis of the present study was that the patients with OSAS would show significantly decreased mean P300 amplitudes over parietal regions. Nevertheless, the mean P300 amplitudes of OSAS patients showed a widespread reduction up to 43–51% compared to healthy controls. Previous studies reported reduced P300 amplitudes in patients with OSAS (Rumbach et al. 1991; Wong et al. 2006; Martins et al. 2011; Vakulin et al. 2012; Akçalı et al. 2015) and some studies indicated that the decreased P300 amplitudes remained abnormal even after CPAP treatment (Neau et al. 1996; Kotterba et al. 1998; Vakulin et al. 2012). These irreversible changes were associated with chronic effects of hypoxemia (Kotterba et al. 1998), as the effects of sleep fragmentation on cognition should be returned to normal levels after treatment. The current study extends these earlier findings by further exploring the negative effects of hypoxemia on cognition. The gradual decline in the mean P300 amplitudes in parallel with the increasing levels of hypoxemia suggested that hypoxemia is an important factor linking OSAS to cognitive impairment.
ERP P300 component has been associated with memory and attentional processes (Polich and Herbst 2000). In the present study, the scores of memory and attention tests showed moderate correlations with the mean P300 amplitudes. Moreover, subjective sleep complaints (ESS scores) were negatively correlated with the mean P300 amplitudes in frontal, central and parietal areas. To our knowledge, this is the first study that investigates the impact of hypoxemia on P300 amplitudes in patients with severe OSAS. Only one previous study investigated the effects of hypoxemia on N270-LPC and N270–N430-LPC ERP components by dividing OSAS patients into two subgroups according to their oxygen desaturation in the total sleep time (Zhang et al. 2002). ERPs associated with processing of conflicting information found to be altered in the OSAS high hypoxemia group but not in the low hypoxemia group (Zhang et al. 2002).
Cognitive dysfunction in OSAS has been studied for many years by using neuropsychological tests and numerous studies reported impairment in several cognitive domains including memory, attention, executive functions, working memory, motor performance and psychomotor speed (Greenberg et al. 1987; Salorio et al. 2002; Findley et al. 1986). According to a recent meta-analysis, executive functions are predominantly impaired in OSAS while other cognitive domains such as memory and attention are affected to a lesser extent (Stranks and Crowe 2016). Based on previous studies, we hypothesized that the patients with severe OSAS would show impairment in these domains. However, there were no significant differences between groups on the attention and executive functioning tests in the current study. Patients with OSAS had lower scores only on short-term verbal memory compared to healthy controls; however, this statistical significance does not imply a clinical significance, as 96% of patients with OSAS performed within normal ranges and classified as cognitively normal by a neuropsychologist. One explanation for the lack of significant clinical differences on neuropsychological test performances could be a ceiling effect, as all our participants were highly educated young adults. Moreover, comorbidities were suggested to play a major role in cognitive changes in OSAS (Borges et al. 2013). Borges et al. (2013) found that when all the comorbidities (i.e., neurological and psychiatric diseases, diabetes, obesity and hypertension) were excluded, patients and controls performed equally well on neuropsychological tests. In the present study, all comorbidities except obesity were also excluded and the neuropsychological test results were consistent with the findings of the previous study.
Intermittent hypoxemia is known to affect the hippocampus (Sapin et al. 2015) and frontal lobes (Morrell and Twigg 2006), which might cause deficits in memory and executive functions over time. In the present study, we found that the short-term verbal memory scores were negatively correlated with the measures of hypoxemia and the OSAS patients with high levels of hypoxemia performed significantly poorer than those with low levels of hypoxemia. Findley et al. (1986) found that the OSAS patients with hypoxemia had impairment in several cognitive domains including short-term verbal memory in comparison to the OSAS patients without hypoxemia. Other studies also reported correlations between hypoxemia measures and cognitive performance (Greenberg et al. 1987, Quan et al. 2006). Nevertheless, Hoth et al. 2013 suggested a protective advantage of higher levels of hypoxemia on memory. The authors discussed the beneficial effects of intermittent hypoxemia on the cardiovascular system, brain, and memory with a focus on the evidence from clinical and animal studies (Manukhina et al. 2006; Hoth et al. 2013). In an experimental study on rats, aging was found to be a protective factor against the harmful effects of intermittent hypoxemia (Quintero et al. 2016). These contradictory results between studies may stem from the differences in the age ranges of study participants, as the participants in our study were relatively younger compared to the participants in Hoth et al.’s (2013) study which included both young adult and elderly participants. Another possible explanation might be the exposure time to hypoxemia (disease duration without treatment), which can only be determined by subjective reports and thus cannot be analyzed or reported as an objective variable in studies.
Early diagnosis and intervention in OSAS is important, as patients are at increased risk for life-threatening comorbidities when remain untreated (Carlson et al. 1994; Drager et al. 2010; Peker et al. 2002; Peperell 2011). Moreover, patients with OSAS were found to be more likely to carry APOE ε4 allele, which increases the risk of developing Alzheimer’s disease (Gottlieb et al. 2004). Beside the genetic tendency, exposure to hypoxemia was also reported to be a risk factor for developing mild cognitive impairment and dementia (Yaffe et al. 2011). In the present study, neuropsychological tests failed to reveal significant differences between the OSAS patients with low levels of hypoxemia and healthy controls, yet electrophysiological measures appeared to be a more sensitive method in identifying patients with OSAS, even in mild conditions. These results indicate that neuropsychological tests may be inadequate to demonstrate cognitive deficits in young patients with higher levels of education.
Beebe et al. (2003) suggested that daytime neuropsychological sequelae should also be taken into consideration in treatment planning for patients with OSAS. Determining the effect of CPAP treatment on cognitive functions could be difficult for OSAS patients who were classified as cognitively normal according to the neuropsychological test results. Nevertheless, electrophysiological methods may provide a more useful measurement for monitoring treatment efficiency.
In conclusion, the present study underlines the fact that neuropsychological tests could be inadequate for identifying cognitive changes in some patient groups and electrophysiological methods may provide a more sensitive measurement. Thus, future studies should investigate the use of ERP P300 as a potential cognitive biomarker of OSAS in individual basis. Moreover, combining ERPs and other brain imaging techniques would contribute to our understanding of cognitive dysfunction in OSAS, and provide more information on the relation between cognition and sleep in general.
Acknowledgements
This study was supported by Dokuz Eylul University Scientific Research Projects (Project Number: 2014.KB.SAG.058).
References
- Afifi L, Guilleminault C, Colrain IM. Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir Physiol Neurobiol. 2003;136(2–3):221–234. doi: 10.1016/S1569-9048(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Akcali A, Sahin E, Ergenoglu T, Neyal M. Latency of auditory P300 response is related with cognitive deficits in Obstructive Sleep Apnea Syndrome. Sleep Biol Rhythms. 2015;13:49–56. doi: 10.1111/sbr.12076. [DOI] [Google Scholar]
- American Academy of Sleep Medicine . Diagnostic and coding manual. 2. Westchester: American Academy of Sleep Medicine; International classification of sleep disorders; 2005. [Google Scholar]
- Bédard MA, Montplaisir J, Malo J, Richer F, Rouleau I. Persistent neuropsychological deficits and vigilance impairment in sleep apnea syndrome after treatment with continuous positive airways pressure (CPAP) J Clin Exp Neuropsychol. 1993;15(2):330–341. doi: 10.1080/01688639308402567. [DOI] [PubMed] [Google Scholar]
- Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11(1):1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26(3):298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- Borges JG, Ginani GE, Hachul H, Cintra FD, Tufik S, Pompéia S. Executive functioning in obstructive sleep apnea syndrome patients without comorbidities: focus on the fractionation of executive functions. J Clin Exp Neuropsychol. 2013;35(10):1094–1107. doi: 10.1080/13803395.2013.858661. [DOI] [PubMed] [Google Scholar]
- Carlson JT, Hedner JA, Ejnell H, Peterson LE. High prevalence of hypertension in sleep apnea patients independent of obesity. Am J Respir Crit Care Med. 1994;150:72–77. doi: 10.1164/ajrccm.150.1.8025776. [DOI] [PubMed] [Google Scholar]
- Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, Lorenzi-Filho G. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105:1135–1139. doi: 10.1016/j.amjcard.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, Cappa SF. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003;61(1):87–92. doi: 10.1016/S0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Feuerstein C, Naegelé B, Pépin JL, Lévy P. Frontal lobe-related cognitive functions in patients with sleep apnea syndrome before and after treatment. Acta Neurol Belg. 1997;97(2):96–107. [PubMed] [Google Scholar]
- Findley LJ, Barth JT, Powers DC, Wilhoit SC, Boyd DG, Suratt PM. Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest. 1986;90(5):686–690. doi: 10.1378/chest.90.5.686. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gagnon K, Baril AA, Gagnon JF, Fortin M, Décary A, Lafond C, Desautels A, Montplaisir J, Gosselin N. Cognitive impairment in obstructive sleep apnea. Pathol Biol (Paris) 2014;62(5):233–240. doi: 10.1016/j.patbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Gosselin N, Mathieu A, Mazza S, Petit D, Malo J, Montplaisir J. Attentional deficits in patients with obstructive sleep apnea syndrome: an event-related potential study. Clin Neurophysiol. 2006;117(10):2228–2235. doi: 10.1016/j.clinph.2006.07.130. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, DeStefano AL, Foley DJ, Mignot E, Redline S, Givelber RJ, Young T. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology. 2004;63(4):664–668. doi: 10.1212/01.WNL.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Greenberg GD, Watson RK, Deptula D. Neuropsychological dysfunction in sleep apnea. Sleep. 1987;10(3):254–262. doi: 10.1093/sleep/10.3.254. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin card sorting test manual: revised and expanded. Florida: Psychological Assessment Resources; 1993. [Google Scholar]
- Hoth KF, Zimmerman ME, Meschede KA, Arnedt JT, Aloia MS. Obstructive sleep apnea: impact of hypoxemia on memory. Sleep Breath. 2013;17(2):811–887. doi: 10.1007/s11325-012-0769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF (2007) Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, pp 48–49
- Inoue Y, Nanba K, Kojima K, Mitani H, Arai AH. P300 abnormalities in patients with severe sleep apnea syndrome. Psychiatry Clin Neurosci. 2001;55(3):247–248. doi: 10.1046/j.1440-1819.2001.00844.x. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston naming test. Austin: Pro-ed; 2001. [Google Scholar]
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6(2):49–54. doi: 10.1055/s-2002-32318. [DOI] [PubMed] [Google Scholar]
- Kotterba S, Rasche K, Widdig W, Duscha C, Blombach S, Schultze-Werninghaus G, Malin JP. Neuropsychological investigations and event-related potentials in obstructive sleep apnea syndrome before and during CPAP-therapy. J Neurol Sci. 1998;159(1):45–50. doi: 10.1016/S0022-510X(98)00131-2. [DOI] [PubMed] [Google Scholar]
- Manukhina EB, Downey HF, Mallet RT. Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia. Exp Biol Med (Maywood) 2006;231(4):343–365. doi: 10.1177/153537020623100401. [DOI] [PubMed] [Google Scholar]
- Martins CH, Castro Júnior Nd, Costa Filho OA, Souza Neto OM. Obstructive sleep apnea and P300 evoked auditory potential. Braz J Otorhinolaryngol. 2011;77(6):700–705. doi: 10.1590/S1808-86942011000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell MJ, Twigg G. Neural consequences of sleep disordered breathing: the role of intermittent hypoxia. Adv Exp Med Biol. 2006;588:75–88. doi: 10.1007/978-0-387-34817-9_8. [DOI] [PubMed] [Google Scholar]
- Naëgelé B, Thouvard V, Pépin JL, Lévy P, Bonnet C, Perret JE, Pellat J, Feuerstein C. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18(1):43–52. [PubMed] [Google Scholar]
- Neau JP, Paquereau J, Meurice JC, Chavagnat JJ, Pinon-Vignaud ML, Vandel B, Recard P, Gil R. Auditory event-related potentials before and after treatment with nasal continuous positive airway pressure in sleep apnea syndrome. Eur J Neurol. 1996;3(1):29–35. doi: 10.1111/j.1468-1331.1996.tb00185.x. [DOI] [Google Scholar]
- Öktem Ö. A verbal test of memory processes. Arch Neuropsychiatry. 1992;29:196–206. [Google Scholar]
- Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- Peng B, Li SW, Kang H, Huang XZ. Cognitive and emotional impairment in obstructive sleep apnea syndrome. Chin Med Sci J. 2004;19(4):262–265. [PubMed] [Google Scholar]
- Pepperell JC. Sleep apnoea syndromes and the cardiovascular system. Clin Med. 2011;11:275–278. doi: 10.7861/clinmedicine.11-3-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9(4):456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Herbst KL. P300 as a clinical assay: rationale, evaluation, and findings. Int J Psychophysiol. 2000;38(1):3–19. doi: 10.1016/S0167-8760(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Quan SF, Wright R, Baldwin CM, Kaemingk KL, Goodwin JL, Kuo TF, Kaszniak A, Boland LL, Caccappolo E, Bootzin RR. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med. 2006;7(6):498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Quintero M, Olea E, Conde SV, Obeso A, Gallego-Martin T, Gonzalez C, Monserrat JM, Gómez-Niño A, Yubero S, Agapito T. Age protects from harmful effects produced by chronic intermittent hypoxia. J Physiol. 2016;594(6):1773–1790. doi: 10.1113/JP270878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggi A, Ferri R. Cognitive evoked potentials in obstructive sleep apnea syndrome: a review of the literature. Rev Neurosci. 2012;23(3):311–323. doi: 10.1515/revneuro-2012-0027. [DOI] [PubMed] [Google Scholar]
- Rumbach L, Krieger J, Kurtz D. Auditory event-related potentials in obstructive sleep apnea: effects of treatment with nasal continuous positive airway pressure. Electroencephalogr Clin Neurophysiol. 1991;80(5):454–457. doi: 10.1016/0168-5597(91)90094-E. [DOI] [PubMed] [Google Scholar]
- Salorio CF, White DA, Piccirillo J, Duntley SP, Uhles ML. Learning, memory, and executive control in individuals with obstructive sleep apnea syndrome. J Clin Exp Neuropsychol. 2002;24(1):93–100. doi: 10.1076/jcen.24.1.93.973. [DOI] [PubMed] [Google Scholar]
- Sangal RB, Sangal JM. P300 latency: abnormal in sleep apnea with somnolence and idiopathic hypersomnia, but normal in narcolepsy. Clin Electroencephalogr. 1995;26(3):146–153. doi: 10.1177/155005949502600305. [DOI] [PubMed] [Google Scholar]
- Sangal RB, Sangal JM. Obstructive sleep apnea and abnormal P300 latency topography. Clin Electroencephalogr. 1997;28(1):16–25. doi: 10.1177/155005949702800104. [DOI] [PubMed] [Google Scholar]
- Sangal RB, Sangal JM. Abnormal visual P300 latency in obstructive sleep apnea does not change acutely upon treatment with CPAP. Sleep. 1997;20(9):702–704. doi: 10.1093/sleep/20.9.702. [DOI] [PubMed] [Google Scholar]
- Sapin E, Peyron C, Roche F, Gay N, Carcenac C, Savasta M, Levy P, Dematteis M. Chronic intermittent hypoxia induces chronic low-grade neuroinflammation in the dorsal hippocampus of mice. Sleep. 2015;38(10):1537–1546. doi: 10.5665/sleep.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforza E, Haba-Rubio J. Event-related potentials in patients with insomnia and sleep-related breathing disorders: evening-to-morning changes. Sleep. 2006;29(6):805–813. doi: 10.1093/sleep/29.6.805. [DOI] [PubMed] [Google Scholar]
- Stranks EK, Crowe SF. The cognitive effects of obstructive sleep apnea: an updated meta-analysis. Arch Clin Neuropsychol. 2016;31(2):186–193. doi: 10.1093/arclin/acv087. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. Oxford: Oxford University Press; 2006. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- Vakulin A, Catcheside PG, Baulk SD, Antic NA, van den Heuvel CJ, Banks S, McEvoy RD. Auditory evoked potentials remain abnormal after CPAP treatment in patients with severe obstructive sleep apnoea. Clin Neurophysiol. 2012;123(2):310–317. doi: 10.1016/j.clinph.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Verstraeten E, Cluydts R. Executive control of attention in sleep apnea patients: theoretical concepts and methodological considerations. Sleep Med Rev. 2004;8(4):257–267. doi: 10.1016/j.smrv.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Verstraeten E, Cluydts R, Pevernagie D, Hoffmann G. Executive function in sleep apnea: controlling for attentional capacity in assessing executive attention. Sleep. 2004;27(4):685–693. [PubMed] [Google Scholar]
- Walsleben JA, Squires NK, Rothenberger VL. Auditory event-related potentials and brain dysfunction in sleep apnea. Electroencephalogr Clin Neurophysiol. 1989;74(4):297–311. doi: 10.1016/0168-5597(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale-revised (WMS-R) San Antonio: Psychological Corporation; 1987. [Google Scholar]
- Wechsler D (2008) Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio, TX: NCS Pearson 22, 498
- Wong KK, Grunstein RR, Bartlett DJ, Gordon E. Brain function in obstructive sleep apnea: results from the Brain Resource International Database. J Integr Neurosci. 2006;5(1):111–121. doi: 10.1142/S0219635206001033. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang Y, Li S, Huang X, Cui L. Early detection of cognitive impairment in patients with obstructive sleep apnea syndrome: an event-related potential study. Neurosci Lett. 2002;325(2):99–102. doi: 10.1016/S0304-3940(02)00249-5. [DOI] [PubMed] [Google Scholar]