Abstract
Background
The aim of this study was to assess the effect of combined use of Astragaloside IV(AsIV) and atorvastatin (AV) on the expression of PPAR-γ and inflammation-associated cytokines in atherosclerosis rats.
Material/Methods
High-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) in plasma were detected through automatic biochemical analyzer and the histopathological analysis was performed via HE staining. The levels of oxidized low-density lipoprotein (oxLDL) and tumor necrosis factor-α (TNF-α), and interleukins (IL)-6 and IL-18 in serum were detected by ELISA. The expressions of proliferator-activated receptor-gamma (PPAR-γ), cluster of differentiation 36 (CD36), matrix metalloprotein-9 (MMP-9), intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1(VCAM-1), and p38 and P-p38 levels were detected by Western blot. RT-PCR was used to detect the mRNA expressions of nuclear factor-κB (NF-κB), PPAR-γ, CD36, MMP-9, ICAM-1, and VCAM-1.
Results
Administration of AsIV and AV significantly decreased the lipid content and oxLDL in plasma. The levels of TNF-α, IL-6, and IL-18 were significantly decreased in AsIV, AV, and AsIV + AV groups, especially in the AsIV + AV group. Administration decreased the levels of NF-κB, CD36, MMP-9, ICAM-1, VCAM-1, and P-p38 expression and increased the expression of peroxisome PPAR-γ. Compared with the NC group, the atherosclerotic lesions significantly increased in the HD group, while the combined administration significantly inhibited the development of atherosclerotic disease.
Conclusions
Combined administration of AV and AsIV showed potent effects against atherosclerosis through the NF-κB/PPARγ pathway, which may be a new therapy for treatment of atherosclerosis in the future.
MeSH Keywords: Accessory Atrioventricular Bundle, Atherosclerosis, Inflammation
Background
As a chronic inflammatory disease, atherosclerosis is the main reason for the rising mortality worldwide. In the early stage, endothelial dysfunction is a critical event in atherosclerosis, which recruits macrophages into intima, leading to the further initiation of atherosclerosis [1]. Hence, elucidating the molecular mechanisms in the inflammatory process of early atherosclerosis is important in the development of novel intervention strategies for atherosclerosis.
The oxidized low-density lipoprotein (oxLDL) can cause a series of atherosclerosis-related inflammation. Monocytes stimulated by oxLDL from peripheral blood migrate to the subendothelial space, where they become macrophages. Moreover, tumor necrosis factor-α (TNF-α) and interleukins (IL-18, IL-6, and IL-1β) can induce the inflammatory cascade and accelerate unlimited lipid uptake, foam cell generation, and progression of atherosclerosis [2]. Therefore, when the inflammation is inhibited, the atherosclerosis can be effectively prevented and cured.
In the past decade, it has been demonstrated that nuclear factor-κB (NF-κB) is a multi-faceted transcription factor, which plays an important role in many physiological and pathological processes [3]. Therefore, the NF-κB pathway plays a central role in activating multiple atherogenic mechanisms and further causing atherosclerosis. In addition, mitogen-activated protein kinase (MAPK) signaling downstream of the NF-κB pathway seems to play an important role in pro-angiogenic cells [4]. A previous study has showed that the p38 MAP kinase pathway can accelerate the damage of cultured vasculogenic cells in vitro [5]. Furthermore, it has been demonstrated that peroxisome proliferator-activated receptor-gamma (PPAR-γ) is closely related with the inflammatory cytokine production by macrophages [6,7].
Astragaloside IV (AsIV) has pharmacological effects in anti-inflammation [8], anti-oxidative stress [9,10] and anti-endoplasmic reticulum stress [11]. Statins and reductase inhibitor are widely used to lower blood lipids, especially cholesterol. It has been reported that statin administration can significantly decrease morbidity and mortality in the perioperative period [12,13].
Since inflammation and blood lipids are the major factors accounting for the pathogenesis of atherosclerosis, we hypothesized that the combined use of AsIV and atorvastatin (AV) would have great effects on the high-fat diet (HD)-induced rat model of atherosclerosis.
Material and Methods
Establishment of atherosclerosis (AS) and administration
We purchased 8-week-old male Sprague-Dawley rats (260–290 g) from Beijing Vitonlihua Experimental Animal Technology Co., LTD (Beijing, China). All animals were kept in a 23±2°C 12-h dark/light cycle environment with free access to water and food. Rats were randomly divided into 5 groups (n=10): a normal control group (NC), a model group (HD), an Astragaloside IV group (AsIV), an atorvastatin group (AV), and a combination group (AsIV + AV). The rats in the NC group were fed the basic diet for 13 weeks, and the rats in the NC group were given physiological saline for another 8 weeks. The rats in the 4 other groups were fed with a high-fat diet (2% cholesterol) for 5 weeks [14], then continued to receive the basic diet for 8 weeks. Eight weeks later, the model group was given saline at the same time, and the other 3 groups were administered with AsIV 20 mg/(kg·d), atorvastatin 10 mg/(kg·d) and AsIV 20 mg/(kg·d) +AV 10 mg/(kg·d) by oral gavage for 8 weeks.
All animal experimental procedures were performed strictly according to the protocols approved by the Committee for Animal Care and Use (Ethics code SCXK Beijing −+−+−+2012-0001).
Biochemical analysis
Before the end of the experiment, the rats were fasted for at least 8 h and then anesthetized with 0.3% pentobarbital sodium. Blood was taken from the carotid artery at room temperature and centrifuged (3500 rpm, rt.) for 15 min, then supernatant was collected for biochemical analysis. The carotid artery was stored in liquid nitrogen for Western blot analysis. Other carotid artery tissues were fixed with 4% paraformaldehyde for HE detection. High-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) were detected by automatic biochemical analyzer (Ortho-Clinical Diagnostics Inc, USA). Experiments were performed strictly in accordance with the manufacturer’s instructions.
Detection of ox LDL, TNF-α, IL-18, and IL-6 level in plasma
The expression levels of oxLDL, IL-18, IL-6, and TNF-α in plasma were detected by ELISA according to the ELISA kit instructions (MBS 729489, My BioSource Ltd., USA).
Carotid artery pathological evaluation
The carotid arteries were isolated, fixed with 4% paraformaldehyde (Beijing North of Conde Clinical Reagent Co., Beijing, China), embedded in paraffin, and sliced into 4-μm sections. The sections were rinsed twice with xylene for 15 min, then dehydrated stepwise with different concentrations of ethanol. The nuclei and tissues were stained with hematoxylin and eosin (Beijing North of Conde Clinical Reagent Co., Beijing, China) for 10 min, then washed twice with 100% alcohol and xylene (5 min each time). The pathological features of the carotid arteries were observed with a microscope (Nikon Eclipse TE2000-U, NIKON, Japan).
RT-PCR assay
The expressions of PPAR-γ, cluster of differentiation 36 (CD36), NF-κB, matrix metalloprotein-9 (MMP-9), intercellular cell adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) mRNA in carotid artery were detected by real-time quantitative PCR. Trizol reagent (Invitrogen Corporation, CA, USA) was used to extract total mRNA from the carotid artery. RT-PCR was performed using SYBR Green master mix on a 7900HTFast-Real-Time PCR System (Applied Biosystems). The change in mRNA expression was calculated by the 2−ΔΔCT method. β-actin mRNA was used as the internal reference mRNA. The primers for the relevant genes are shown in Table 1.
Table 1.
The primer sequences used for RT-PCR assay.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| β-actin | GCATTGCTGACAGGATGCAG | CCTGCTTGCTGATCCACATC |
| PPAR-γ | GACCTGAAGCTCCAAGAATACCA | GCTGGGTCTTTTCAGAATAATAAGG |
| NF-κB | TGGGGGCCTTGCTTGGCAAC | GAGGTCCCCAGGGGTGTGGG |
| CD36 | ACATTTGCAGGTCTATCTACG | AATGGTTGTCTGGATTCTGG |
| MMP-9 | CATTCGCGTGGATAAGGAGT | CACTGCAGGAGGTCGTAGG |
| ICAM-1 | CGCAAGTCCAATTCACACTGA | CCAGAGCGGCAGAGCAA |
| VCAM-1 | TGACAAGTCCCCATCGTTGA | ACCTCGCGACGGCATAATT |
Western blotting assay
The protein expression of NF-κB, PPAR-γ, CD36, MMP-9, ICAM-1, VCAM-1, p38, and P-p38 were detected by Western blot. Experimental procedures were strictly in accordance with the BCA kit (Solarbio, Beijing, PC0020) manual. Carotid artery was sonicated with pre-cooled lysate. The supernatant was centrifuged (13 000 rpm, 30 min) at 4°C, and the protein concentration was detected by BCA method. The total proteins were separated by SDS-PAGE and transferred to a PVDF membrane (Merck, Darmstadt, Germany). The PVDF membrane was immersed in 5% milk powder in TBST buffer (20 mM Tris, 137 mM NaCl, pH 7.6, with 0.1% Tween 20) and blocked for 1 h. Then, the PVDF membrane was incubated with the corresponding primary antibody (1: 1000, Abcam) for 2 h at room temperature followed by washing 3 times with TBST buffer for 10 min at room temperature, and the corresponding HRP-conjugated secondary antibody (1: 2000, Abcam) was added and incubated for 1 h at room temperature. The ECL chemiluminescence system (Thermo Fisher Scientific, Waltham, MA, USA) was used to detect the signal on the specimen membrane and the X-ray film recorded the experimental results. The spectral densities of the bands were analyzed using AlphaView SA software (Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
All data are expressed as mean ±SD. Statistical analysis was performed using the GraphPad Prism 5.0 software. Statistical analysis between multiple groups was performed by one-way ANOVA followed by Tukey post-test. Comparison between 2 groups were performed by t test. p<0.05 were considered to be statistically significant.
Results
Effect of combined use of AsIV and AV on blood lipids
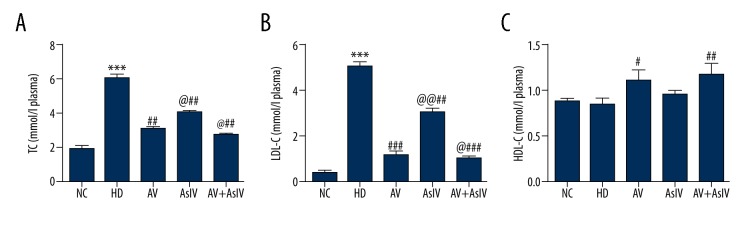
As shown in Figure 1, the level of TC in the HD group was about 3 times higher than that of the NC group. The combined administration led to a significant decrease in the TC content compared with the single administration (p<0.05). The LDL-C content was approximately 5.2 mmol/L in the HD group. The LDL-C content was significantly decreased to 1 mmol/L after combined administration of Astragaloside IV and atorvastatin. Compared with the NC group, the HDL-C had no significant change in the HD group, and HDL-C content increased significantly (p<0.01) after combined administration. Combined administration treatment decreased the levels of blood lipids caused by HD in rats.
Figure 1.
Combination treatment of AsIV and AV attenuated the elevated level of TC, HDL-C, and LDL-C in hypercholesterolemic rats. Data are presented as mean ±SD (n=10). Compared with the NC, *** p<0.001; compared with HD, ## p<0.01, ### p<0.001; compared with AV, @ p<0.05, @@ p<0.01.
Effect of combined use of AsIV and AV on oxLDL in plasma
OxLDL is an oxidized form of LDL, and the accumulation of oxLDL and LDL-C in plasma can induce the production of atherosclerosis. As seen from Figure 2, the levels of oxLDL in the HD group was approximately 3-fold higher than in the NC group. However, the drug combination significantly lowered the level of oxLDL in comparison with the AV group and the AsIV alone group. Combined administration significantly reduced the levels of oxLDL.
Figure 2.
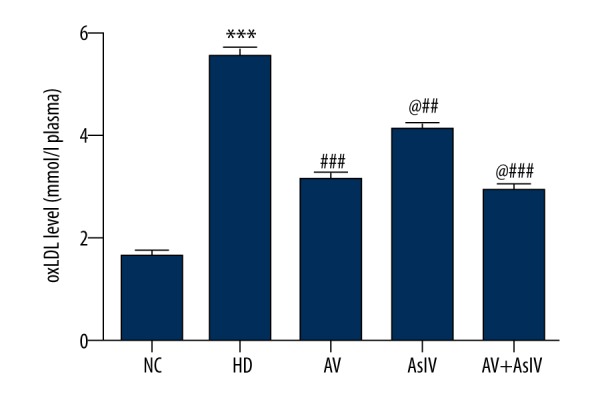
Bar graph showing the attenuating effect of combination administration oxLDL in plasma of hypercholesterolemic rats. Data are presented as mean ± SD (n=10). Compared with NC, *** p<0.001; compared with HD, ### p<0.001; compared with AV, @ p<0.05.
Effect of combined use of AsIV and AV on inflammatory cytokines
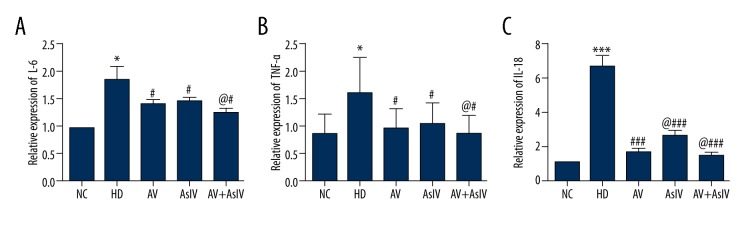
The effects of drug combination on the levels of IL-6, IL-18, and TNF-α in plasma of rats are shown in Figure 3. Compared with the HD group, AsIV, AV, and AsIV + AV significantly decreased the levels of IL-6, IL-18, and TNF-α, with a significant difference (p<0.05 or 0.001), and the AsIV + AV group had the strongest effect, reducing inflammatory cytokines by 23%, 29.1%, and 25.6% compared with the AV group (p<0.05.
Figure 3.
The detection of plasma IL-6 (A), TNF-α (B) and IL-18 (C) inflammatory factor expression levels through ELISA methods. The data are expressed as mean ±SD (n=5). Compared with NC, * p<0.05, *** p<0.001; compared with HD, # p<0.05, ### p<0.001; compared with AV, @ p<0.05.
Effect of combined use of AsIV and AV on histopathology change
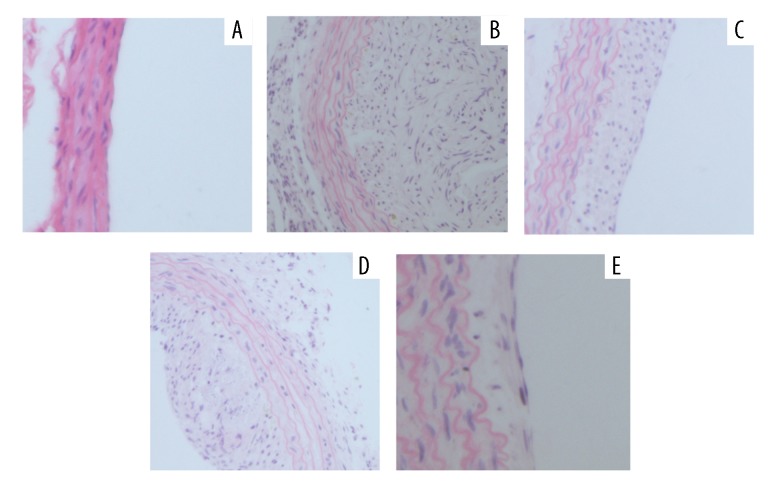
As seen from Figure 4, the histopathology of the carotid artery showed a large amount of endothelial cells lipid deposition, thickening of the wall, carotid artery atherosclerotic lesions, and a serious degree of proliferation in the HD group compared with the NC group. However, AsIV or AV alone and AsIV + AV treated groups showed significantly less microvesicular fatty change, decreased atherosclerosis-induced damage, and the thickness of intimal hyperplasia, especially in the AsIV + AV treated group. Combination therapy of AsIV + AV significantly improved the intimal hyperplasia of vascular disease caused by HD.
Figure 4.
Hematoxylin and eosin staining of histological sections of the carotid artery. (A) NC control group; (B) HD model group; (C) atorvastatin (AV) administration group; (D) astragaloside (AsIV) administration group; (E) combination group (AsIV + AV) (magnification 200×).
Effect of combined use of AsIV and AV on relative mRNA expressions
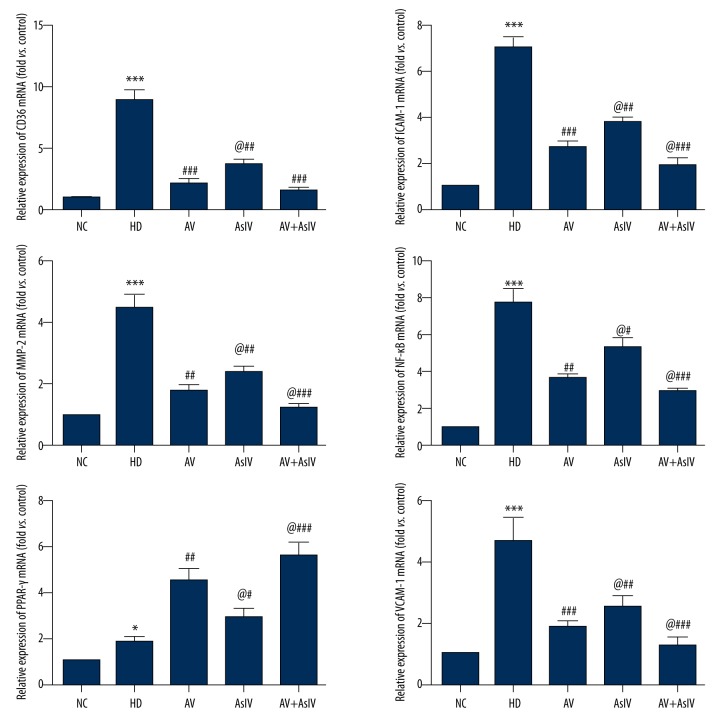
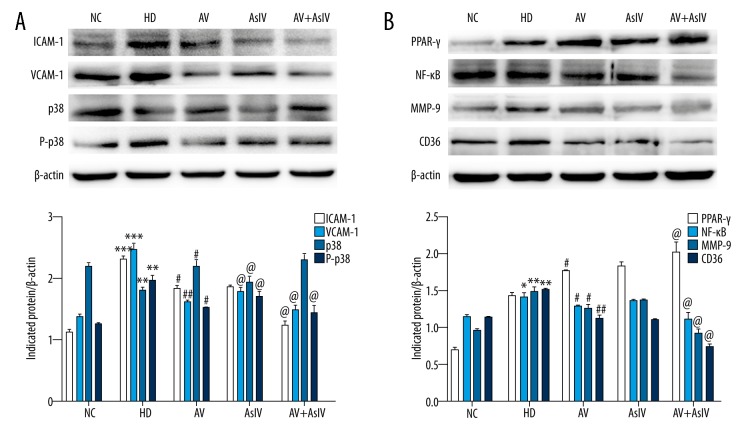
As shown in Figure 5, the mRNA expression level of ICAM-1, VCAM-1, NF-κB, CD36, and MMP-9 was significantly decreased by AsIV or AV alone and AsIV + AV compared with the HD group (p<0.05 or p<0.001), while the PPAR-γ level was increased. In addition, compared with the AV group, AsIV + AV significantly reduced the expression of NF-κB by 23% (p<0.05) to further relieve symptoms of atherosclerosis via the NF-κB/PPAR-γ pathway.
Figure 5.
The expressions of ICAM-1, VCAM-1, NF-κB, PPAR-γ, CD36, and MMP-9 mRNA were analyzed by RT-PCR. β-actin mRNA was used as the internal control. Data are expressed as the mean ±SD. Compared with NC, * p<0.05, *** p<0.001; compared with HD, # p<0.05, ## p<0.01, ### p<0.001; compared with AV, @ p<0.05.
Effect of combined use of AsIV and AV on relative protein expressions
As shown in Figure 6, the protein expressions of ICAM-1, VCAM-1, NF-κB, p-p38, CD36, and MMP-9 were reduced, while the expression of PPARγ was up-regulated in AsIV, AV, and AsIV + AV groups as compared to the HD group (p<0.05 or p<0.001). Compared with the AV group, the AsIV + AV group had the strongest effect (p<0.05), especially for p-p38 and NF-κB.
Figure 6.
The expression levels of ICAM-1, VCAM-1, p38 and P-p38 (A) and NF-κB, PPAR-γ, CD36 and MMP-9 (B) were detected by Western Blot. Graphs were generated using GraphPad Prism 5.0 software. Data are expressed as the mean ±SD. Compared with NC, * p<0.05, *** p<0.001; compared with HD, # p<0.05, ## p<0.01, ### p<0.001; compared with AV, @ p<0.05.
Discussion
Atherosclerosis is a worldwide disease with a causal relationship with complex diseases such as coronary heart disease and stroke [15]. However, the roles of the target genes PPAR-γ and inflammation in AS development have been unclear. In the present research, AV and AsIV combined administration showed potent effects against atherosclerosis through the NF-κB/PPAR-γ pathway. Our results indicate that the expression levels of TC, HDL-C, LDL-C, ox LDL, and classic histopathology were all restored by AV and AsIV, which further demonstrates that AV and AsIV combined administration is a strong candidate for treatment of AS.
In this study, we found that activation of the NF-κB signaling pathway plays a key role in HD-induced atherosclerosis. Activation of NF-κB is a driver of inflammation, which is involved in all stages of typical atherosclerosis [16]. Inflammatory cytokines, such as TNF-α, IL-18, and IL-6, are all produced by inflammatory cells, and their expression levels are regulated by inflammatory response systems [17]. Some studies have shown that NF-κB modulates the function of inflammatory cytokines and adhesion molecules and plays an important role in the regulation of inflammatory responses [18,19]. In addition, TNF-α results in the expression of ICAM-1 and VCAM-1, which accelerate coronary artery disease [20]. We demonstrated that AV and AsIV combined administration ameliorated the levels of TNF-α, IL-18, IL-6, ICAM-1, and VCAM-1 compared with AV or AsIV alone in HD-induced atherosclerosis. These findings suggested that the protective effect of AV and AsIV combined administration against atherosclerosis may be through inhibiting the NF-κB pathway and the related inflammation reaction.
In addition, there is growing evidence that oxLDL is a major risk factor for atherosclerosis, existing in multiple forms characterized by varying degrees of oxidation and mixtures of different bioactive ingredients [21]. Endothelial dysfunction may be induced by oxLDL. In fact, resident macrophages or endothelial cells are able to oxidize low-density lipoproteins that infiltrate through the intima [22]. CD36 is a membrane glycoprotein with a molecular weight of ~88 kD that can bind oxLDL and enters macrophages as scavenger receptors, and it further induces macrophages to differentiate into foam cells that cause the core of atherosclerotic lesions [23]. Tontonoz et al. reported that monocyte CD36 plays a key role in the pathogenesis and progression of atherosclerotic lesions. The ability of CD36 significantly reduces the size of vascular lesions and endocytose oxLDL in ApoE-deficient animals and oxLDL induces CD36 by activating transcription factor PPAR-γ [24]. In addition, PPAR-γ also plays a vital role in development and progression of atherosclerosis and pretreatment of monocytes with PPAR-γ agonists reduced their adhesion to vascular endothelium and their transendothelial migration [25]. Based on these data, it appears that at least some of the antiatherogenic effects of macrophage PPAR-γ may be attributed to monocyte recruitment. In this work, the expressions of PPAR-γ, CD36, and oxLDL were up-regulated in HD-treated groups, and Cand36, oxLDL were reduced by AV or AsIV alone, except for PPAR-γ, and the combined administration had the most significant effect. These results indicate that AV and AsIV exerts anti-atherosclerotic effects by increasing PPAR-γ activity.
Besides the listed inflammatory cytokines and related proteins, the MAP kinase signaling pathway may also be a key pathway in pro-angiogenic cells [26]. A recent study found that when the p38 MAP kinase pathway is activated, vascular cell injury can be induced in vitro [27]. However, the significant anti-atherosclerotic effects of p38 MAPK inhibition in vivo may not only be caused by the promotion of the number and function of angiogenic cells, but also involve additional mechanisms, such as the anti-inflammatory activity of anti-p38 inhibitor [28]. p38 MAP kinase has an atheroprotective effect, so in order to determine the effect of AV and AsIV, we detected the expression levels of p-p38/p38. Our results demonstrate that p-p38/p38 was up-regulated in HD-treated groups, which were all reduced by AV or AsIV and the combined administration, and the combined administration had the most significant effect. These results indicate that AV and AsIV exert anti-atherosclerotic effects by inhibiting the p38 signaling pathway.
Conclusions
The combination of AV and AsIV has an important effect on the NF-κB/PPAR-γ and p38 MAPK signaling pathways in the inhibition of inflammation. The combined treatment of AV and AsIV decreased the proinflammatory cytokines and increased the PPAR-γ levels. Notably, compared with AV or AsIV alone, the combined administration exerted a stronger protective effect on atherosclerosis, providing new therapeutic strategies for the treatment of atherosclerosis.
Footnotes
Source of support: Departmental sources
References
- 1.Dorobantu M, Tautu OF, Darabont R, et al. Objectives and methodology of Romanian SEPHAR II Survey. Project for comparing the prevalence and control of cardiovascular risk factors in two East-European countries: Romania and Poland. Arch Med Sci. 2015;11(4):715–23. doi: 10.5114/aoms.2015.53290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19(9):1166–72. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. J Anesth. 2006;290(4):L622–45. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 4.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat Med. 2011;17(7):883–87. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song D, Fang G, Mao SZ, et al. Chronic intermittent hypoxia induces atherosclerosis by NF-κB-dependent mechanisms. Biochim Biophys Acta. 2012;1822(11):1650–59. doi: 10.1016/j.bbadis.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Brocks D, Assenov Y, Minner S, et al. Intratumor DNA methylation heterogeneity reflects clonal evolution in aggressive prostate cancer. Cell Rep. 2014;8(3):798–806. doi: 10.1016/j.celrep.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 7.Oh J, Riek AE, Darwech I, et al. Deletion of macrophage vitamin d receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell Rep. 2015;10(11):1872–86. doi: 10.1016/j.celrep.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gui D, Huang J, Guo Y, et al. Astragaloside IV ameliorates renal injury in streptozotocin-induced diabetic rats through inhibiting NF-κB-mediated inflammatory genes expression. Cytokine. 2013;61(3):970–77. doi: 10.1016/j.cyto.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Zhou W, Chen Y, Zhang X. Astragaloside IV alleviates lipopolysaccharide-induced acute kidney injury through down-regulating cytokines, CCR5 and p-ERK, and elevating anti-oxidative ability. Med Sci Monit. 2017;23:1413–20. doi: 10.12659/MSM.899618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Chi YF, Yuan ZT, et al. Astragaloside IV inhibits renal tubulointerstitial fibrosis by blocking TGF-β/Smad signaling pathway in vivo and in vitro. Exp Biol Med. 2014;239(10):1310–24. doi: 10.1177/1535370214532597. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Gui D, Chen J, et al. Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside IV is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats. Cell Physiol Bioche. 2014;33(6):1975–87. doi: 10.1159/000362974. [DOI] [PubMed] [Google Scholar]
- 12.Olafsdottir E, Aspelund T, Sigurdsson G, et al. Similar decline in mortality rate of older persons with and without type 2 diabetes between 1993 and 2004 the Icelandic population-based Reykjavik and AGES-Reykjavik cohort studies. BMC Public Health. 2013;13:36. doi: 10.1186/1471-2458-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva S, Lourenço P, Paulo C, et al. Statin-induced low cholesterol is not associated with poor outcome in chronic heart failure. J Cardiovasc Pharmacol Ther. 2012;17(3):284–90. doi: 10.1177/1074248411429967. [DOI] [PubMed] [Google Scholar]
- 14.Bayatmakoo R, Rashtchizadeh N, Yaghmaei P, et al. Atorvastatin inhibits cholesterol-induced caspase-3 cleavage through down-regulation of p38 and up-regulation of Bcl-2 in the rat carotid artery. Cardiovasc J Afr. 2017;28(5):298–303. doi: 10.5830/CVJA-2017-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: From pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li RC, Haribabu B, Mathis SP, et al. Leukotriene B4 eeceptor-1 mediates intermittent hypoxia-induced atherogenesis. J Am Coll Cardiol. 2011;184(1):124–31. doi: 10.1164/rccm.201012-2039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Wang Z, Kwong SQ, et al. Inhibition of PDGF, TGF-β, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011;55(3):612–25. doi: 10.1016/j.jhep.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 18.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43(S1):63–74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 19.Hu M, Xu L, Yin L, et al. Cytotoxicity of dioscin in human gastric carcinoma cells through death receptor and mitochondrial pathways. J Appl Toxicol. 2013;33(8):712–22. doi: 10.1002/jat.2715. [DOI] [PubMed] [Google Scholar]
- 20.Miwa K, Igawa A, Inoue H. Soluble E-selectin, ICAM-1 and VCAM-1 levels in systemic and coronary circulation in patients with variant angina. Cardiovasc Res. 1997;36(1):37–44. doi: 10.1016/s0008-6363(97)00143-0. [DOI] [PubMed] [Google Scholar]
- 21.Reddy MA, Natarajan R. Epigenetic mechanisms in diabetic vascular complications. Cardiovasc Res. 2011;90(3):421–29. doi: 10.1093/cvr/cvr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levitan I, Volkov S, Subbaiah PV. Oxidized LDL: Diversity, patterns of recognition, and pathophysiology. Antioxid Redox Signal. 2010;13(1):39–75. doi: 10.1089/ars.2009.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talle MA, Rao PE, Westberg E, et al. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983;78(1):83–89. doi: 10.1016/0008-8749(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 24.Tontonoz P, Nagy L, Alvarez JG, et al. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93(2):241–52. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 25.Collins AR, Meehan WP, Kintscher U, et al. Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21(3):365–71. doi: 10.1161/01.atv.21.3.365. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich EB, Werner C, Walenta K, et al. Role of extracellular signal-regulated kinase for endothelial progenitor cell dysfunction in coronary artery disease. Basic Res Cardiol. 2009;104(5):613–20. doi: 10.1007/s00395-009-0022-6. [DOI] [PubMed] [Google Scholar]
- 27.Sun C, Liang C, Ren Y, et al. Advanced glycation end products depress function of endothelial progenitor cells via p38 and ERK 1/2 mitogen-activated protein kinase pathways. Basic Res Cardiol. 2009;104(1):42–49. doi: 10.1007/s00395-008-0738-8. [DOI] [PubMed] [Google Scholar]
- 28.Pettus LH, Wurz RP. Small molecule p38 MAP kinase inhibitors for the treatment of inflammatory diseases: Novel structures and developments during 2006–2008. Curr Top Med Chem. 2008;8(16):1452–67. doi: 10.2174/156802608786264245. [DOI] [PubMed] [Google Scholar]