Abstract
Background
Allograft inflammatory factor-1 (AIF-1) is a cytoplasmic protein cloned from activated macrophages in human and rat allografts. AIF-1 has been identified as a modulator of inflammatory response, and recently published studies have shown its increased expression in carcinogenesis. However, there are still limited data on the potential functional role of AIF-1 in hepatocellular carcinoma (HCC).
Material/Methods
We evaluated the expression of AIF-1 in 104 cases of paired HCC and adjacent non-cancerous liver tissues using immunohistochemistry, Western blotting, and qPCR analysis, and sought to determine whether its expression was correlated with clinicopathological features. In vitro assays, including cell proliferation and migration assays, were used to study the effects of AIF-1 knockdown in L02 human hepatocyte, and Huh7 and SMMC7721 liver cancer cell lines.
Results
Expression of AIF-1 was increased in HCC compared to adjacent normal liver tissues and was positively correlated with median tumor size (p=0.046), number of tumor deposits (p=0.009), the Barcelona Clinic Liver Cancer (BCLC) stage (p=0.004), and portal vein tumor thrombus (PVTT) (p<0.001). Huh7 and SMMC7721 human HCC cells demonstrated upregulated AIF-1 expression compared to normal hepatocytes. Small interfering RNA (siRNA)-mediated silencing of AIF-1 expression resulted in a reduction in cell proliferation and migration in human HCC cells.
Conclusions
These findings suggest AIF-1 may have roles as a diagnostic or prognostic biomarker and a promising therapeutic target in HCC.
MeSH Keywords: Carcinoma, Hepatocellular; Cell Migration Assays; Cell Proliferation
Background
Hepatocellular carcinoma (HCC) is the main primary liver cancer and ranks third as the leading cause of cancer-related deaths worldwide [1,2]. Although available treatment options for HCC continue to improve due to developments in hepatic resection, radiofrequency tumor ablation, transarterial chemoembolization, and liver transplantation, most current therapeutic strategies have only limited efficacy and the mortality of patients remains persistently high [3]. Therefore, it is important to continue to investigate new and potentially more reliable diagnostic and prognostic biomarkers, and the molecular mechanisms involved in hepatic carcinogenesis to provide new treatment targets. Molecular links between inflammation and carcinogenesis continue to be discovered [4–8]. Inflammatory cytokines involved in liver carcinogenesis include interleukin 1 (IL-1), interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF-α) [9–12].
Allograft inflammatory factor 1 (AIF-1) is a novel inflammatory mediator that was originally identified by Utans et al. from rat cardiac allografts undergoing chronic rejection [13]. AIF-1 is a 17-kDa, cytoplasmic, interferon-γ-inducible, calcium-binding, and inflammation-responsive scaffold protein produced mainly by circulating monocytes and tissue macrophages [14]. Recent studies have shown that AIF-1 participates in modulating inflammatory responses in rheumatoid arthritis, inflammatory skin disorders, and systemic sclerosis [15–17]. Data from several recent studies have also shown that AIF-1 is involved in promoting proliferation and migration of breast cancer cells in vitro, which indicate a role for AIF-1 in the progression of malignant tumors [18,19]. Moreover, AIF-1 is expressed in activated Kupffer cells lining the walls of liver sinusoids [20]. However, the role of AIF-1 expression in the development and progression of HCC and its significance for patients with HCC remain unknown.
The aims of this study were to evaluate the expression of AIF-1 in 104 cases of paired HCC and adjacent non-cancerous liver tissues and to determine whether AIF-1 expression is correlated with clinicopathological features of patients with HCC.
Material and Methods
Patients
A total of 104 patients with histologically diagnosed HCC were enrolled in the study. All patients underwent partial hepatectomy (PH) at Nanfang Hospital, Guangzhou, Guangdong, China, from July 2016 to July 2017.
A total of 104 paired HCC and adjacent non-cancerous liver tissues were collected. Patients with other coexisting severe systemic diseases or serious infections were excluded from this study.
The Medical Ethics Committee of Nanfang Hospital approved the study protocol. Informed consent for using the tissue samples for research purposes was signed by all patients who participated in the study. Patient clinical information is summarized in Table 1.
Table 1.
Correlation between allograft inflammatory factor-1 (AIF-1) and clinicopathological characteristics in patients with hepatocellular carcinoma (HCC).
| Variable | Relative AIF-1 Protein Levels | |||
|---|---|---|---|---|
| n | Value | t | P | |
| Age (years) | 1.097 | 0.141 | ||
| ≤60 | 60 | 64.064±2.989 | ||
| >60 | 44 | 58.781±3.877 | ||
| Gender | 0.153 | 0.071 | ||
| Female | 92 | 61.961±2.602 | ||
| Male | 12 | 60.812±5.535 | ||
| HBsAg | −1.126 | 0.850 | ||
| Negative | 28 | 66.231±4.566 | ||
| Positive | 76 | 60.207±2.787 | ||
| AFP (ng/ml) | 1.872 | 0.154 | ||
| ≤200 | 76 | 64.502±2.903 | ||
| >200 | 28 | 54.571±3.765 | ||
| Cirrhosis | −1.141 | 0.837 | ||
| No | 44 | 58.694±3.505 | ||
| Yes | 60 | 64.127±3.223 | ||
| Tumor size (cm) | −7.140 | 0.046 | ||
| ≤5 | 52 | 47.872±2.877 | ||
| >5 | 52 | 75.785±2.645 | ||
| Tumor number | −5.130 | 0.009 | ||
| Single | 92 | 57.875±2.343 | ||
| Multiple | 12 | 92.142±4.224 | ||
| BCLC stage | −5.596 | 0.004 | ||
| 0-A | 56 | 50.981±3.074 | ||
| B-C | 48 | 74.485±2.776 | ||
| PVTT | −9.559 | <0.001 | ||
| No | 64 | 48.691±2.436 | ||
| Yes | 40 | 82.849±2.282 | ||
| Encapsulation | 3.557 | 0.110 | ||
| Complete | 97 | 89.647±6.021 | ||
| Incomplete | 8 | 59.510±2.388 | ||
AFP – alpha-fetoprotein; BCLC – Barcelona clinic liver cancer; PVTT – portal vein tumor thrombus.
Immunohistochemistry
Immunohistochemical (IHC) analysis was performed as previously described [21,22]. Briefly, HCC and matched adjacent non-cancerous tissues were fixed in 4% paraformaldehyde, embedded in paraffin wax, and sectioned at 4-μm thickness. Sections were then deparaffinized in xylene and rehydrated with graded concentrations of ethanol. Antigen retrieval was performed, followed by blocking of endogenous peroxidase with 3% hydrogen peroxide and nonspecific antigen binding with normal goat serum. Sections were then incubated with primary antibodies to AIF-1 (Abcam, Cambridge, MA, USA) at a 1: 1000 dilution at 4°C overnight, followed by incubation with anti-rabbit horseradish peroxidase-conjugated secondary antibody (Zhongshanjinqiao, Beijing, China) at 37°C for 1 h. The tissue sections were stained with 3, 3-diaminobenzidine tetrahydrochloride (DAB) and counterstained with hematoxylin.
Images of all histopathological slides were analyzed using the image analysis software IMAGE PRO PLUS version 6.0 (Media Cybernetics, MD, USA) as previously reported [23]. Photomicrographs were taken from 5 random visual fields within the areas of histologically identified HCC, and from the confirmed normal liver tissue, and submitted for software analysis. The mean integral optical density (IOD) was measured and used as the relative expression levels for AIF-1 in HCC and normal liver.
Cell lines and culture
The following cell lines were used for in vitro experiments: normal human hepatocyte cell line L02 and HCC cell lines Huh7 and SMMC7721. The cell lines were purchased from the Cell Bank of the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China).
L02 cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Life Technologies, CA, USA) containing 10% fetal bovine serum (FBS) (Life Technologies, CA, USA), and Huh7 and SMMC7721 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, CA, USA) containing 10% FBS (Life Technologies, CA, USA). Cells were incubated in an atmosphere of 5% CO2 at 37°C.
Western blotting
Western blotting analysis was carried out according to the method previously reported [24]. Briefly, cell lysates from liver tissue and cultured liver cancer cells were collected. Total protein was extracted from cell lysates using ice-cold lysis buffer (RIPA buffer, a cocktail of protease inhibitors, Sigma, USA), and the insoluble material was discarded after 15-min centrifugation at 4°C. The samples were mixed with 5× loading buffer (Thermo Fisher Scientific, MA, USA), and boiled for 5 min at 95°C. Equal amounts (30 μg) of total protein were separated by 10% SDS-PAGE and then transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories). The PVDF transfer membranes were blocked with 5% skimmed milk powder in TBS-Tween solution (0.05% Tween 20 in Tris-buffered saline) and then incubated overnight at 4°C with AIF-1 primary antibody at a 1: 1000 dilution (Abcam, Cambridge, MA, USA). The protein bands were washed 3 times with TBS-Tween solution and incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Beverly, MA, USA) for 2 h at room temperature. The protein bands were washed 3 times again with TBS-tween, detected using chemiluminescence reagents, and quantified by measuring protein band intensity using Gel-Pro Analyzer (Media Cybernetics, Sarasota, FL, USA).
Quantitative real-time PCR
Quantitative real-time PCR (qPCR) analysis was performed as previously described [25]. Briefly, total RNA was extracted from liver tissues using the TRIzol RNA isolation system (Takara, Dalian, China) according to the manufacturer’s instructions. First-strand cDNA was reverse-transcribed using PrimeScript™ RT Master Mix (Takara, Dalian, China) and qPCR was performed using SYBR Green mix (Takara, Dalian, China). The conditions for qPCR were as follows: 1 cycle at 95°C for 30 s, 40 cycles of denaturing at 90°C for 5 s, and annealing at 64°C for 20 s. The LightCycler 480 System (Roche, Basel, Switzerland) was used for determination of mRNA levels. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as internal normalization control.
The specific primers for AIF-1 are as follows:
Forward, 5′-ACGTTCAGCTACCCTGACTTTCT-3′;
Reverse, 5′-CCTGTTGGCTTTTCCTTTTCTCT-3′.
The specific primers for GAPDH are as follows:
Forward, 5′-GCACCGTCAAGGCTGAGAAC-3′;
Reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
RNA interference
Huh7 and SMMC7721 HCC cell lines were seeded in 6-well plates and cultured in complete medium until 60–80% confluent. The cells were then transiently transfected with AIF-1 small (or short) interfering RNA (siRNA) or negative control siRNA (Genepharma, Suzhou, China) in serum-free media using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After incubation for 6–8 h, the transfection medium was replaced with fresh medium containing 10% FBS. Analysis of AIF-1 protein and mRNA expression was performed at 48 h post-transfection. The sequence of siRNA against AIF-1 is: GAAACGAAUGCUGGAGAAATT.
Cell proliferation assay
Cell proliferation was evaluated using the Cell Counting Kit-8 (CCK-8) (Dojindo, Kumamoto, Japan) assay. Huh7 and SMMC7721 cells transfected with AIF-1 siRNA and negative control siRNA were placed in a 96-well plate. Following cultivation for 48 h, 10 μl of the CCK-8 reagent was added to each well for 2 h at 37°C. Immediately after the incubation, cell proliferation was detected by measuring absorbance at 450 nm using an absorbance microplate reader (Bio-Tek, Seattle, WA, USA).
Wound-healing assay
The AIF-1 siRNA transfected Huh7 and SMMC7721, and negative control cells were plated in 6-well plates (Corning, PA, USA) and cultured to 90% confluence. Cultured cells were starved in 0.1% FBS for 24 h and then washed once with Dulbecco’s phosphate-buffered saline (PBS). Following scraping with a sterile 10-μL pipette tip in 3 separate places in each well, L02 cells were washed with PBS twice to remove debris and cultured in serum-free medium. After 18 h, cell migration across the wounded area was quantified by counting 3 different fields using an inverted phase-contrast microscope (Olympus, Tokyo, Japan) at 100× objective magnification.
Transwell migration assay
The Huh7 and SMMC7721 cells transfected with AIF-1 siRNA and negative control cells were seeded at a concentration of 2×105 cells/well in the upper chamber (Corning, NY, USA). The upper Transwell chamber was pre-coated with 8-μm polycarbonate sterile membranes (Corning, NY, USA) and filled with DMEM without FBS. The lower Transwell chamber was filled with complete medium to serve as a chemoattractant. After 48 h of incubation, non-migrating cells in the upper chamber were cleaned by wiping with cotton swabs, and the filters were processed first by fixing in methanol followed by staining with 0.05% crystal violet. The total number of migrated cells was quantified by counting 5 different fields using an inverted phase-contrast microscope (Olympus, Tokyo, Japan) at 40× objective magnifications.
Statistical analysis
The association between levels of AIF-1 protein and mRNA expression with clinicopathological parameters of patients studied was evaluated by the Pearson χ2 test. A two-tailed t test was used to compare means of data between the 2 types of tissue studied: HCC and matched non-tumor control tissue. Multiple comparisons were performed by one-way analysis of variance (ANOVA), and pairwise comparisons were evaluated by the least significant difference (LSD) test or Dunnett’s T3 method, where appropriate. A two-tailed P value of less than 0.05 was considered statistically significant. All experiments were performed in triplicate. Statistical analysis was carried out using SPSS 13.0 software (SPSS Inc., Chicago, USA).
Results
Increased expression of AIF-1 in human HCC tissues
To explore the possible role of AIF-1 in the progression of HCC, we detected AIF-1 expression in 104 paired tumor and adjacent non-cancerous liver tissues from patients with HCC. IHC results showed that AIF-1 was remarkably upregulated in tumor tissues compared to matched non-cancerous liver tissues (Figure 1A). To confirm IHC results, Western blotting analysis was performed to further detect AIF-1 protein expression (Figure 1B). Compared to adjacent non-cancerous liver tissues, AIF expression was significantly increased in HCC tissues (p<0.001) (Figure 1C). Furthermore, we performed qPCR analysis to determine the expression of AIF-1 mRNA. Consistent with the results of IHC and Western blotting analyses, HCC tissues expressed significantly enhanced levels of AIF-1 mRNA compared to adjacent non-tumor control tissues (p<0.001) (Figure 1D). These results indicate AIF-1 expression was upregulated in HCC tissues.
Figure 1.
Allograft inflammatory factor-1 (AIF-1) protein and mRNA expression in hepatocellular carcinoma (HCC) and non-cancerous liver tissues. (A) Representative immunohistochemical analysis of allograft inflammatory factor-1 (AIF-1) expression levels in HCC and corresponding adjacent non-cancerous liver tissues (adjacent). (B) The expression of AIF-1 protein was detected in 104 HCC tissue specimens (C) and corresponding adjacent non-cancerous liver tissues (N) by Western blotting analysis. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as an internal control. Representative Western blots show an enhanced expression of AIF-1 protein in HCC tissue specimens. (C, D) Western blotting and qPCR analysis were conducted to measure the protein and mRNA levels of AIF-1, respectively, in 104 HCC and corresponding adjacent non-cancerous liver tissue samples. * p<0.05 versus HCC tissues.
Correlation between AIF-1 expression and clinicopathological characteristics of patients with HCC
To evaluate the clinical significance of AIF-1 expression in HCC, analysis of correlation between AIF-1 protein expression and clinicopathological patient parameters was performed. The parameters studied are shown in Table 1 (n=104). We found no significant relationship between upregulation of AIF-1 expression and patient clinicopathological parameters, including age, sex, hepatitis B surface antigen (HBsAg), serum alpha fetoprotein (AFP) levels, the presence of liver cirrhosis, or tumor encapsulation (all p>0.05). However, we found significantly increased AIF-1 expression in patients with large primary liver tumor diameters (diameter >5 cm, p=0.046) and multiple tumor nodules (number ≥2, p=0.009).
American Association for the Study of Liver Diseases and European Association for the Study of the Liver guidelines have recommended the Barcelona Clinic Liver Cancer (BCLC) classification staging system for the management of HCC [26]. The clinical staging of cancers was classified according to the BCLC staging systems. In this study, increased expression of AIF-1 was detected in the group of patients with advanced BCLC stage (B-C) compared to early BCLC stage (0-A) (p=0.004). HCC cells could invade the portal venous system and subsequently develop into portal vein tumor thrombosis (PVTT), which is the main route for intrahepatic metastasis of HCC [27]. Our results also showed that elevated AIF-1 expression was significantly associated with the presence of PVTT (p<0.001). These findings suggest that AIF-1 expression may be involved in the progression of human HCC.
Effect of AIF-1 on in vitro proliferation of HCC cell lines
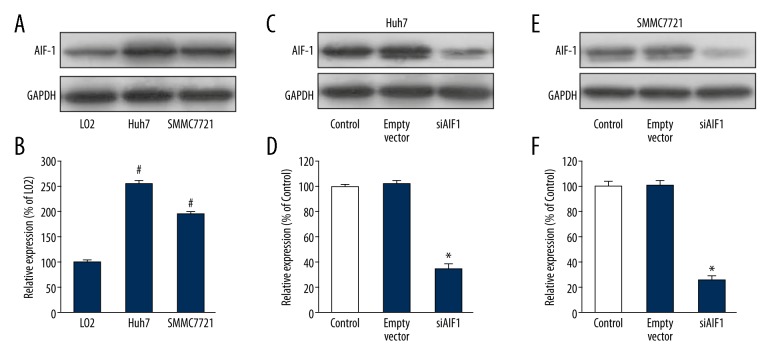
The findings of this study, described in sections 3.1 and 3.2 above, show that AIF-1 expression was significantly increased in HCC compared to normal liver tissues, and this was supported by in vitro cell studies. We also analyzed AIF-1 expression levels in 2 liver cancer cell lines (Huh7 and SMMC7721) and a normal liver cell line (L02) by Western blotting and qPCR. The data showed that, compared to normal liver cells, AIF-1 expression was increased in both liver cancer cell lines (all p<0.05) (Figure 2A, 2B).
Figure 2.
Allograft inflammatory factor-1 (AIF-1) expression and the effect of siAIF-1 transfection in hepatocellular carcinoma (HCC) cells. (A) Western blotting analysis was used to examine the expression levels of AIF-1 in 2 human liver cancer cell lines, Huh7 and SMMC7721, as well as in an L02 normal human liver cell line. (B) Bar graphs show that AIF-1 expression was higher in both liver cancer cell lines than that in L02 normal human liver cell line. Data are shown as mean ±SD. # p<0.05 versus L02 cells. (C) Western blotting analysis confirms the effect of AIF-1 siRNA transfection in Huh7 cells. (D) Bar graphs show that siRNA transfection effectively silenced the AIF-1 expression in Huh7 cells. Data are shown as mean ±SD. * p<0.05 versus control. (E) Western blotting analysis confirms the effect of AIF-1 siRNA transfection in SMMC7721 cells. (F) Bar graphs show that siRNA transfection effectively silenced the AIF-1 expression in SMMC7721 cells. Data are shown as mean ±SD. * p<0.05 versus control.
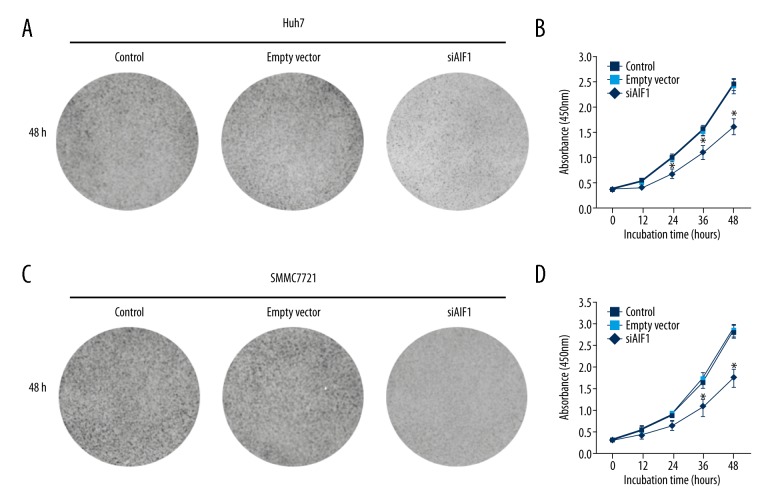
To characterize the functional significance of AIF-1 in cell proliferation, the transfection experiment included a specific siRNA to silence endogenous AIF-1 expression. Figure 2C–2F shows that the efficiency of siRNA transfection against AIF-1 was effectively tested by Western blotting analysis of Huh7 and SMMC7721 cells. We further investigated the proliferation rate of AIF-1-silenced cells using CCK-8 assay. Data indicated that the proliferation rate was inhibited in liver cancer cells infected with AIF-1 siRNA compared to control cells (all p<0.05) (Figure 3A–3D). These findings indicate AIF-1 might have a role in the proliferation of liver cancer cells.
Figure 3.
Effects of allograft inflammatory factor-1 (AIF-1) on proliferation of hepatocellular carcinoma (HCC) cells. (A) Representative photomicrograph of cell viability assay of Huh7 cells following transfection with siAIF-1 or empty vector for 48 h. (B) Cell viability of Huh7 cells was determined by measuring the absorbance at 450 nm and is shown as a growth curve after normalization. Data are mean ±SD. * p<0.05 versus control. (C) Representative photomicrograph of cell viability assay of SMMC7721 cells following transfection with siAIF-1 or empty vector for 48 h. (D) Cell viability of SMMC7721 cell was determined by measuring the absorbance at 450 nm and is shown as a growth curve after normalization. Experiments were performed in triplicate. Data are mean ±SD. * p<0.05 versus control.
Effect of AIF-1 on migration of HCC cell lines
The findings from this study showed that AIF-1 expression was significantly correlated with PVTT (p<0.001), which suggests AIF-1 has a role in HCC cell metastasis. To investigate this hypothesis, we studied the effect of AIF-1 on cell migration using Huh7 and SMMC7721 cells. The in vitro wound-healing assay showed that the both liver cancer cell lines transfected with siRNA stimulated reduced wound closure ability compared to control cells (Figure 4A–4D). Furthermore, the Transwell migration assay also showed that silenced AIF-1 expression in Huh7 and SMMC7721 cells was associated with reduced cell migration properties into the lower Transwell chamber compared to control cells (Figure 5A–5D). Taken together, these data suggest increased expression of AIF-1 may be associated with metastatic potential of human HCC cells.
Figure 4.
Effects of allograft inflammatory factor-1 (AIF-1) on migration of hepatocellular carcinoma (HCC) cells. (A) Representative photomicrographs of wound-healing assay using Huh7 cells following transfection with siAIF-1 or empty vector for 24 h. (B) Distance traveled in μM by Huh7 cells was measured and shown as a histogram, after normalization. Data are shown as mean ±SD. * p<0.05 versus control. (C) Representative photomicrographs of wound-healing assay using SMMC7721 cells following transfection with siAIF-1 or empty vector for 24 h. (D) Distance traveled in μM by SMMC7721 cells was measured and shown as a histogram, after normalization. Experiments were performed in triplicate. Data are shown as mean ±SD. * p<0.05 versus control.
Figure 5.
Effects of allograft inflammatory factor-1 (AIF-1) on migration of hepatocellular carcinoma (HCC) cells in vitro. (A) Representative photomicrographs of Transwell cell migration assay using Huh7 cells following transfection with siAIF-1 or empty vector for 48 h. (B)The number of migrated Huh7 cells per field was quantified and shown as a histogram, after normalization. Data are shown as mean ±SD. * p<0.05 versus control. (C) Representative photomicrographs of Transwell migration assay using SMMC7721 cells following transfection with siAIF-1 or empty vector for 48 h. (D) The number of migrated SMMC7721 cells per field was quantified and shown as a histogram, after normalization. Experiments were performed in triplicate. Data are shown as mean ±SD. * p<0.05 versus control.
Discussion
The findings of this study reveal increased expression of AIF-1 protein and mRNA in human liver cancer compared to adjacent non-cancerous liver tissues. We also investigated the correlation between the increased AIF-1 expression and clinicopathologic parameters in patients with HCC. The main clinical associations with increased expression of AIF-1 were in the group of patients with advanced BCLC stage (stage B and C, p=0.004) and the presence of PVTT (p<0.001). These findings suggest that AIF-1 expression may be involved in the progression of human HCC and may have potential as a prognostic marker.
The findings of increased expression of AIF-1 in human malignancy are supported by some recently published studies. Liu et al. showed increased expression of AIF-1 in breast cancer duct epithelial cells, which was associated with increased proliferation of breast cancer cell line MDA-MB-231 via activation of the NF-kappaB/cyclin D1 pathway [18]. Additionally, Liu et al. provided further evidence that AIF-1 enhanced migration of breast cancer cell lines MDA-MB-231 and MCF-7 through activation of the p38 MAPK signaling pathway [19]. Consistent with these previously published studies, we found increased expression of AIF-1 in HCC cell lines compared to control L02 cells, and further demonstrated that suppression of AIF-1 expression resulted in reduced HCC cell proliferation in vitro. The findings also provide new data in support of reduced HCC cell migration through blockage of AIF-1 in vitro. When combined with the results from previous studies, we have evidence suggesting that AIF-1 has a role in promoting the progression of HCC.
Inflammatory mediators, including inflammatory cytokines, have been shown to be involved in carcinogenesis, tumor progression, and metastasis in several tumor types [28,29]. AIF-1 is a key inflammatory cytokine that has been demonstrated to be involved in malignancy, as shown by the 2012 study by Song et al. showing increased expression of AIF-1 mRNA levels in human cervical cancer compared to adjacent normal cervical tissues [30]. Deininger et al. identified increased expression of AIF-1 in activated microglial cells and a subset of tumor-infiltrating macrophages within tumors in rat and human gliomas [31]. These previous studies support the possibility that AIF-1 acts as an oncogene. However, in contrast to our findings, Ye et al. showed significantly lower AIF-1 protein levels in gastric cancer tissues than in matched adjacent non-cancerous tissues and provided novel evidence of a negative correlation between AIF-1 expression and clinical and histopathological parameters [32]. These data suggest that the mechanisms by which AIF-1 is involved in cancer progression remain unclear. Currently, there have been few studies on the expression of AIF-1 and its clinicopathological significance in HCC.
In HCC, tumor burden has been shown to be an important prognostic marker [33]. Maximal tumor size and number of tumor nodules are related to overall survival of patients with HCC [34]. Therefore, we initially categorized the patients into subgroups according to maximal tumor size and number of tumor nodules. Our results showed that increased AIF-1 expression was associated with maximal tumor size (diameter >5 cm) and presence of multiple tumor nodules. Also, siRNA-mediated AIF-1 silencing inhibited liver cancer cell proliferation and migration. These findings suggest that AIF-1 plays an essential role in the progression of human HCC. This was a preliminary study and is limited because it had a small sample size and was conducted in a single center. Based on the above results, further studies with larger sample sizes are needed to confirm our findings.
Conclusions
This study revealed that AIF-1 was upregulated in HCC. Expression of AIF-1 was closely associated with distinct clinical characteristics in HCC patients. Furthermore, silencing of AIF-1 expression resulted in a reduction in cell proliferation and migration in human HCC cells. Thus, AIF-1 may be a novel prognostic biomarker and therapeutic target for HCC patients.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the National Key Clinical Specialty Discipline Construction Program, the National Natural Science Foundation of China (Grant number 81500398), the Natural Science Foundation of Guangdong Province (Grant number 2015A030310480), and the President’s Foundation of Nanfang Hospital, Southern Medical University (Grant number 2016C013)
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Thomas MB, Jaffe D, Choti MM, et al. Hepatocellular carcinoma: Consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Grinberg-Bleyer Y, Ghosh S. A novel link between inflammation and cancer. Cancer Cell. 2016;30:829–30. doi: 10.1016/j.ccell.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nature Immunol. 2016;17:230–40. doi: 10.1038/ni.3384. [DOI] [PubMed] [Google Scholar]
- 6.Pribluda A, Elyada E, Wiener Z, et al. A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell. 2013;24:242–56. doi: 10.1016/j.ccr.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Li P, Liu C, et al. Sinomenine hydrochloride inhibits breast cancer metastasis by attenuating inflammation-related epithelial-mesenchymal transition and cancer stemness. Oncotarget. 2017;8:13560–74. doi: 10.18632/oncotarget.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Si H, Lu H, Yang X, et al. TNF-alpha modulates genome-wide redistribution of DeltaNp63alpha/TAp73 and NF-kappaB cREL interactive binding on TP53 and AP-1 motifs to promote an oncogenic gene program in squamous cancer. Oncogene. 2016;35:5781–94. doi: 10.1038/onc.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He G, Dhar D, Nakagawa H, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell. 2013;155:384–96. doi: 10.1016/j.cell.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–19. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakamoto K, Hikiba Y, Nakagawa H, et al. Inhibitor of kappaB kinase beta regulates gastric carcinogenesis via interleukin-1alpha expression. Gastroenterol. 2010;139:226–38. doi: 10.1053/j.gastro.2010.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utans U, Arceci RJ, Yamashita Y, Russell ME. Cloning and characterization of allograft inflammatory factor-1: A novel macrophage factor identified in rat cardiac allografts with chronic rejection. J Clin Invest. 1995;95:2954–62. doi: 10.1172/JCI118003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Autieri MV. cDNA cloning of human allograft inflammatory factor-1: Tissue distribution, cytokine induction, and mRNA expression in injured rat carotid arteries. Biochem Biophys Res Comm. 1996;228:29–37. doi: 10.1006/bbrc.1996.1612. [DOI] [PubMed] [Google Scholar]
- 15.Kimura M, Kawahito Y, Obayashi H, et al. A critical role for allograft inflammatory factor-1 in the pathogenesis of rheumatoid arthritis. J Immunol. 2007;178:3316–22. doi: 10.4049/jimmunol.178.5.3316. [DOI] [PubMed] [Google Scholar]
- 16.Orsmark C, Skoog T, Jeskanen L, Kere J, Saarialho-Kere U. Expression of allograft inflammatory factor-1 in inflammatory skin disorders. Acta Derm Venereol. 2007;87:223–27. doi: 10.2340/00015555-0225. [DOI] [PubMed] [Google Scholar]
- 17.Kadoya M, Yamamoto A, Hamaguchi M, et al. Allograft inflammatory factor-1 stimulates chemokine production and induces chemotaxis in human peripheral blood mononuclear cells. Biochem Biophys Res Comm. 2014;448:287–91. doi: 10.1016/j.bbrc.2014.04.106. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Tan WY, Chen QR, et al. Daintain/AIF-1 promotes breast cancer proliferation via activation of the NF-kappaB/cyclin D1 pathway and facilitates tumor growth. Cancer Sci. 2008;99:952–57. doi: 10.1111/j.1349-7006.2008.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T, Feng Z, Jia S, et al. Daintain/AIF-1 promotes breast cancer cell migration by up-regulated TNF-alpha via activate p38 MAPK signaling pathway. Breast Cancer Res Treat. 2012;131:891–98. doi: 10.1007/s10549-011-1519-x. [DOI] [PubMed] [Google Scholar]
- 20.Nagakawa Y, Nomoto S, Kato Y, et al. Over-expression of AIF-1 in liver allografts and peripheral blood correlates with acute rejection after transplantation in rats. Am J Transplant. 2004;4:1949–57. doi: 10.1111/j.1600-6143.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 21.Sun S, Xie F, Zhang Q, et al. Advanced oxidation protein products induce hepatocyte epithelial-mesenchymal transition via a ROS-dependent, TGF-beta/Smad signaling pathway. Cell Biol Int. 2017;41:842–53. doi: 10.1002/cbin.10792. [DOI] [PubMed] [Google Scholar]
- 22.Sun S, Xie F, Xu X, et al. Advanced oxidation protein products induce S-phase arrest of hepatocytes via the ROS-dependent, β-catenin-CDK2-mediated pathway. Redox Biol. 2018;14:338–53. doi: 10.1016/j.redox.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan N, Liu Q, Liu X, et al. Low expression of B-cell-associated protein 31 in human primary hepatocellular carcinoma correlates with poor prognosis. Histopathology. 2016;68:221–29. doi: 10.1111/his.12738. [DOI] [PubMed] [Google Scholar]
- 24.Xie F, Sun S, Xu A, et al. Advanced oxidation protein products induce intestine epithelial cell death through a redox-dependent, c-jun N-terminal kinase and poly (ADP-ribose) polymerase-1-mediated pathway. Cell Death Dis. 2014;5:e1006. doi: 10.1038/cddis.2013.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, Sun S, Xie F, et al. Advanced oxidation protein products induce epithelial-mesenchymal transition of intestinal epithelial cells via a PKC delta-mediated, redox-dependent signaling pathway. Antiox Redox Signal. 2017;27:37–56. doi: 10.1089/ars.2015.6611. [DOI] [PubMed] [Google Scholar]
- 26.Mitsunobu M, Toyosaka A, Oriyama T, et al. Intrahepatic metastases in hepatocellular carcinoma: the role of the portal vein as an efferent vessel. Clin Exp Metastasis. 1996;14:520–29. doi: 10.1007/BF00115112. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Chen L, Gu J, et al. Recurrently deregulated lncRNAs in hepatocellular carcinoma. Nat Commun. 2017;8:14421. doi: 10.1038/ncomms14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Incio J, Liu H, Suboj P, et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016;6:852–69. doi: 10.1158/2159-8290.CD-15-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruber R, Panayiotou R, Nye E, et al. YAP1 and TAZ control pancreatic cancer initiation in mice by direct up-regulation of JAK-STAT3 signaling. Gastroenterol. 2016;151:526–39. doi: 10.1053/j.gastro.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song JY, Bae HS, Koo do H, et al. Candidates for tumor markers of cervical cancer discovered by proteomic analysis. J Korean Med Sci. 2012;27:1479–85. doi: 10.3346/jkms.2012.27.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deininger MH, Seid K, Engel S, et al. Allograft inflammatory factor-1 defines a distinct subset of infiltrating macrophages/microglial cells in rat and human gliomas. Acta Neuropath. 2000;100:673–80. doi: 10.1007/s004010000233. [DOI] [PubMed] [Google Scholar]
- 32.Ye Y, Miao S, Lu R, et al. Allograft inflammatory factor-1 is an independent prognostic indicator that regulates beta-catenin in gastric cancer. Oncol Rep. 2014;31:828–34. doi: 10.3892/or.2013.2915. [DOI] [PubMed] [Google Scholar]
- 33.Markowitz GJ, Yang P, Fu J, et al. Inflammation-dependent IL18 signaling restricts hepatocellular carcinoma growth by enhancing the accumulation and activity of tumor-infiltrating lymphocytes. Cancer Res. 2016;76:2394–405. doi: 10.1158/0008-5472.CAN-15-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191–99. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]