Abstract
Early-life trauma has been linked to increased risks for anxiety, depression, and chronic pain. We used rodent models of acute and inflammatory neonatal pain to explore effects on fear conditioning and somatosensory function. Hindpaw needle pricks or handling on postnatal days (PNDs) 1–7 caused lasting impacts on affective and somatosensory function when assessed at later ages, PNDs 24 (post-weaning), 45 (adolescence) or 66 (adulthood). First, auditory, but not contextual, freezing was mildly disrupted regardless of age. Second, a profound post-fear conditioning tactile hypersensitivity was observed in neonatally stressed, post-weaning rats. In the absence of fear conditioning the mechanical hypersensitivity was not observed, consistent with a two-hit model of psychopathology. Injections of 2% λ-carrageenan did not have the same lasting impact but was slightly protective against observed effects of neonatal vehicle injections. Basal and elicited corticosterone levels post-weaning was not altered by neonatal pain or handling. These data demonstrate that neonatal adversity can have lasting impacts on affective and somatosensory function that differs regardless of age.
Keywords: Neonatal Pain, Stress, Fear Conditioning, Sensory Function
In humans, a growing body of literature suggests that neonatal pain exposure, such as that experienced by infants in the neonatal intensive care unit (NICU), can contribute to negative outcomes later in life, including depression, anxiety, and heightened pain sensitivity (Anand et al., 2006; Grunau, Holsti, & Peters, 2006; Taddio, Katz, Ilersich, & Koren, 1997). Despite some evidence showing that analgesic treatment produces few long-term neurodevelopmental problems in children, many medical professionals are apprehensive to use analgesics when performing painful procedures (Anand, 2016).
Due to the confounding influence of illness or birth complications in humans, preclinical animal studies are often utilized for observing the effects of early-life pain. In terms of sensory function, acute neonatal pain produced by two hindpaw pricks per day for the first 15 days of life produced tactile hypersensitivity in rats as old as 180 days (Carmo, Sanada, Machado, & Fazan, 2016). Thermal hypersensitivity has also been observed in 22 day-old rats following four hindpaw pricks per day for the first seven days of life (Anand, Coskun, Thrivikraman, Nemeroff, & Plotsky, 1999). There also appears to be a sex difference, with males showing greater tactile hypersensitivity than females when tested at maturity (14–23 weeks) following eight days of hindpaw pricks (Page, Hayat, & Kozachik, 2013). Neonatal pain has also been shown to alter fear and anxiety-like behaviors. For example, Schellinck et al., (2003) demonstrated that repeated neonatal hindpaw pricks (but not a single acute tail snip) produced an increase in anxiety-like behaviors in an elevated plus maze, as well as a deficit in spatial memory learning.
Whereas acute pain appears to produce hypersensitivity later in life, chronic inflammatory pain in neonates tends to produce the opposite effect. Wang et al. (2004) showed that rats injected in the hindpaw with carrageenan as neonates displayed thermal hyposensitivity and attenuated visceromotor responses to colorectal distention as adults (60–70 days old). Additionally, neonatal rats that received hindpaw injections of 10% formalin on days 1–7, showed longer hot plate and tail withdrawal latencies indicative of thermal hyposensitivity (Bhutta et al., 2001). As with acute pain, there appears to be a sex difference in neonatal inflammatory pain and somatosensory function. Specifically, females showed greater thermal hyposensitivity than males following neonatal carrageenan hindpaw injections when tested at postnatal day (PND) 60 (LaPrairie & Murphy, 2007). In terms of fear responses following inflammatory pain, rats given the inflammatory agent lipopolysaccharide (LPS) as neonates did not differ from controls in either auditory or contextual fear conditioning development, however, LPS-treated rats did show a delay in fear extinction, suggesting perinatal inflammation can preserve fearful memories in rodents (Doenni, Song, Hill, & Pittman, 2017).
The hypothalamic pituitary adrenal (HPA) axis has been heavily implicated in the consequences of neonatal pain with long-term impacts of neonatal pain/stress reported. Preterm infants who experienced more painful procedures had significantly elevated cortisol levels when they were school-aged (Brummelte et al., 2015). It has also been demonstrated that early-life stress can alter HPA and amydgalar activity in humans (Heim & Nemeroff, 2002; Iorio et al., 2017).
Despite these data, a few key questions remain in understanding the long-term effects of neonatal pain and trauma. First, “two-hit” models have become increasingly popular to explain the interaction between early-life environments and later-life events in predicting cognitive and physiological outcomes. However, these models have been understudied in terms of neonatal trauma and susceptibility to pain throughout the lifespan. Second, although the effects of acute pain and inflammatory pain appear to be different, to the best of our knowledge, they have not been compared within a single paradigm in the same laboratory, leaving open the possibility that procedural differences can account for the differential outcomes. Third, the possibility that the effects of neonatal pain or stress differ over the course of the lifespan remains under-investigated. The purpose of the current project is to directly examine these questions over three experiments.
Methods
Experiment 1A examined the effects of repeated acute hindpaw needle pricks on subsequent fear conditioning and somatosensory function in post-weaning (PND 24), adolescence (PND 45) and adulthood (PND 66). In Experiment 1B rats underwent the same neonatal pain manipulation as in Experiment 1A, but sensory testing occurred in the absence of fear conditioning. Experiment 2 examined the effects of inflammatory pain using two 25-μl injections of λ-carrageenan or vehicle into the left hindpaw on PNDs 1 and 4. Rats underwent the same fear conditioning protocol and sensory function tests as Experiment 1. To investigate whether neonatal pain alone can produce disruptions in somatosensory function or if a second vulnerability is required, Experiment 3, assessed levels of corticosterone to examine HPA-axis function.
Subjects.
Experiments 1 and 3 used timed-pregnant female Sprague-Dawley rats (Charles River) which arrived on gestational day (GD) 12 and gave birth on GD 22, which was considered PND 0. Experiment 2 used a combination of timed-pregnant shipped and timed-pregnant in-house bred rats using a protocol modified from Ogilvie and Rivier (1997). As no differences were found between them, their data were otherwise combined for analysis. All litters were housed in 43 cm x 44 cm x 20 cm closed-environment cages (Innovive, San Diego CA). On PND 1, pups were removed from their mother, placed on a heating pad, sexed, marked (via tail tattoo for Experiment 1A; via crystal violet stain for Experiments 2 and 3), and culled to no more than 10 rats per litter (5 males and 5 females when possible). Pups were weaned on PND 21 and lived with their same-sex littermates (approximately 5 per cage). If the rats were tested beyond PND 45 (Experiments 1 and 2), rats were housed 2–3 per cage with same-sex littermates. All rats were maintained on a 12:12 light/dark cycle with lights on at 07:00. Food and water was available ad libitum, and at the end of experimentation, rats were euthanized via CO2 overdose (or pentobarbital overdose and cardiac puncture in the case of Experiment 4).
Apparatus.
Fear conditioning was performed in four Startfear chambers (Harvard Apparatus/Panlab model #58722) with two separate contextual cues that differed in shape (square vs. circle), color (black vs. white walls), and scent (70% ethanol vs. 1% ammonia). Tactile allodynia was measured by using the up/down technique with von Frey monofilaments (North Coast Medical, Gilroy CA) of varying gauges (equating to 0.4 g – 15 g pressure). Thermal hyperalgesia was assessed using a Hargreaves apparatus (Ugo Basile Plantar Test model #7371, Collegeville PA). Blood corticosterone concentration was measured using an ELISA kit (Enzo Life Sciences, Farmingdale NY).
Drugs and Chemicals.
λ-Carrageenan (Sigma-Aldrich, St. Louis MO) was dissolved in 0.9% physiological saline in a 2% w/v concentration. Carrageenan or vehicle was administered i.pl.at a 25 μl volume.
Neonatal Pain.
For Experiments 1 and 3, on PNDs 1–7, pups were preassigned (in a pseudo-random fashion) to receive either a needle prick in the left hindpaw with a 23-gauge needle tip every 2hr, four times per day (needle prick), or a non-painful handling where the left hindpaw was lightly touched with the experimenter’s index finger (handled) on the same schedule (see Figure 1B). For Experiment 2, the thickness of each rat’s left hindpaw was measured (in mm) via digital calipers daily until PND 7. This was performed as a confirmation that the inflammatory agent was producing edema, a measure of inflammation. On PNDs 1 and 4 rats received a 25 μl injection of either vehicle or carrageenan into the plantar surface of the left hindpaw (see Figure 1B). Rats in this experimental group will henceforth be referred to as “inflammation” or “inflammatory pain”.
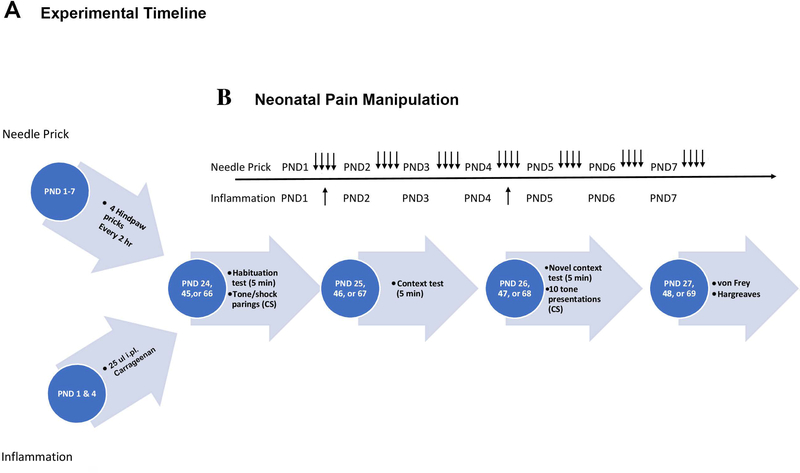
Figure 1.
Timeline representations for the Experimental Timeline (A). Rats were exposed to neonatal pain and stress manipulations during the first week of life. Then underwent a 3-day fear conditioning protocol across subsequent days in different age cohorts starting at PND 24 (post-weaning), 45 (adolescence), or 66 (adulthood). Sensory testing occurred on the fourth day. The timeline for the neonatal manipulation (B) occurred from PND 1–7. Arrows represent experiment-specific insults, a single needle prick(occurring 4 times per day) for the acute pain experiments and an i.pl. injection for the inflammatory pain experiment.
Fear Conditioning (Experiments 1A and 2).
On PNDs 24 (post-weaning), 45 (adolescence), or 66 (adulthood), rats underwent the fear conditioning protocol (see Figure 1A). Experiment 1 initially included PND 17 subjects. However, they were excluded from the current experiment because both control and experimental subjects failed to demonstrate specific fear conditioning (see also Burman et al 2014; Deal et al 2016). On Day 1 rats were placed in their preassigned (counterbalanced) fear conditioning chamber (FCC) and the program was initiated. The percent of time spent freezing during the first 5 mins was recorded (Habituation). Freezing was defined as movement falling below a predefined threshold based on weight (see Burman et al., 2014). Following the habituation period, a 67-dB tone (CS) was presented for 10 sec, immediately followed by a 2-sec 1.0 mA foot shock (UCS). There were 10 tone-shock pairings, and interval trial intervals (ITIs) were pseudorandomized with an average ITI of 2.25 min to prevent temporal learning effects. The following day rats were placed in the same FCC and the percent freezing for 5 mins was recorded (Context). The third day, rats were placed in the FCC with the opposite contextual cues than the FCC where initial fear conditioning took place, and the percent freezing for the first 5 mins was recorded (Novel Context). Subsequently, there were 10 presentations of the original CS (10-sec 67-dB tone) every 30 sec, and the percent freezing for the 10-sec period during each CS presentation was recorded and averaged (AVG Tone). The change in freezing caused by the tone (Tone Difference Score) was calculated by subtracting novel context freezing from the AVG Tone score.
Somatosensory Testing (Experiments 1 and 2).
24h after the Novel Context and Tone tests (Experiments 1A and 2), rats underwent sensory testing. This occurred at the same age (PND 27) but without fear conditioning for Experiment 1B. An additional test was performed on PND 34 in Experiment 1 to test the duration of the observed hypersensitivity. Rats naïve to the experimental testing room were placed in wire mesh-floored chambers and allowed to habituate for 15 mins. Tactile allodynia of the left hindpaw was measured using the up-down method with von Frey monofilaments ranging from 2–15 g as previously described by (Chaplan, Bach, Pogrel, Chung, & Yaksh, 1994; Bonin, Bories, & De Koninck, 2014). The monofilament was placed on the plantar surface of the rat’s left hindpaw and pressure was increased until there was approximately a 30-degree bend in the monofilament. The threshold (in g) to withdraw the hindpaw from the filament was calculated by recording which filament gauge produced a withdrawal response and titrating the gauges to narrow down how much force is necessary to produce a paw withdrawal. Upon completion, rats were transferred to glass-floored cages and allowed to habituate for an additional 15 min. Light intensity on the apparatus was set to 50% which was selected based on a pilot study (data not shown) where adult rats had an average withdrawal latency of 15 sec. The cutoff for the stimulus was set to 30 sec to avoid tissue damage. The light-emitting diode of the Hargreaves apparatus was placed under the left hindpaw, and the latency for the rat to withdraw its hindpaw (in sec) was recorded twice and averaged.
Corticosterone (Experiment 3).
On PND 24, rats were separated into groups that either received a stressor or a non-painful control (basal) condition. For the stressed group, rats were placed in the aforementioned fear conditioning chambers and the Day 1 fear conditioning protocol, in which a tone was paired with a foot shock 10 times, was initiated. Basal rats were left in their home cage. 15 min after the stress program ended, rats were injected with an overdose of pentobarbital, and trunk blood was collected via cardiac puncture and refrigerated for 24 hrs. Blood was spun down at 15,000 rpm for 18 mins and serum was collected and frozen at −20 C. A corticosterone ELISA kit (Enzo Life Sciences, Farmingdale NY) was used to measure the total concentration of corticosterone (ng/ml) for each rat in each condition.
Experimental Design and Analysis.
Data were analyzed with IBM SPSS version 22 with mixed model ANOVAs, with Greenhouse-Geisser corrections when sphericity was violated. A Tukey posthoc analysis was performed when there were more than two variables to compare. Experiment 1A started with 72 male and 68 female rats (from 19 litters). Ten male and 6 female rats were removed for either neonate attrition, or experimenter error leaving a final count of 62 males and 62 females. Experiment 2 started with 76 males and 78 females (from 23 litters). One male and 2 female rats were removed due to experimenter error leaving a final count of 75 males and 76 females respectively. Experiment 1B started with 45 male and 46 female rats (from 17 litters) and no rats were removed from this experiment. Experiment 3 started with 34 males and 29 females (from 17 litters). Two males and 2 females were removed due to abnormally high sample values, resulting in a final count of 32 males and 27 females. No more than one same sex littermate was assigned to any experimental group. In the rare situation where that was violated, the data from the two subjects were averaged. Data are reported as mean ± SEM and a p-value of ≤ 0.05 was considered statistically significant.
Results
Experiment 1A: Acute neonatal pain on fear conditioning and somatosensory function.
This experiment examines the effects of neonatal needle pricks on subsequent fear conditioning and sensory function across the lifespan.
Effects of neonatal pain
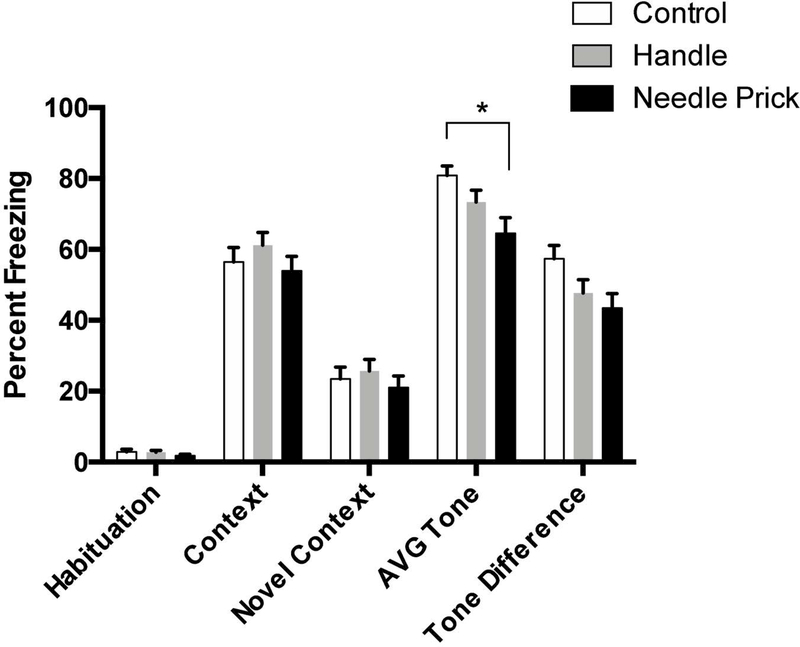
Needle pricking, but not handling, disrupted expression of auditory fear conditioning irrespective of age and sex (see Figures 3 and 4). This was shown by a 2 (Sex: male, female) × 3 (Condition: control, handled, needle prick) × 3 (Age: post-weaning, adolescence, adulthood) ANOVA which revealed a statistically significant main effect of neonatal pain condition on the average level of freezing during the tone presentations following the novel context (F(2,106)=3.83, p<0.05; see Figure 2). There was no interaction with Sex or Age. Post hoc analyses further revealed that only needle pricked rats showed attenuated freezing (M=64.52, SEM=4.44) in comparison to controls (M=80.83, SEM=2.69). This conclusion was further supported by a trend towards a significant effect of neonatal pain condition on the Tone Difference score (F(2,106)=2.87, p=0.06). Post hoc analyses revealed that the needle pricked rats (but not handled) had lower tone difference scores than the control condition (see Figure 2 and 3). Needle pricked and handled rats did not differ from each other. The effects of neonatal pain on the pattern of freezing across the 10 tone presentations during the test session simply reaffirmed the statistically significant main effect (F(2,106)=3.52, p<0.05) of neonatal pain with needle pricked rats and not handled rats (see Figure 4) differing from controls. Although there was a significant effect of tone presentation (F(5.091,954)=17.50, p<0.001, Greenhouse-Geisser corrected), it did not interact with the neonatal pain condition (p > 0.1)
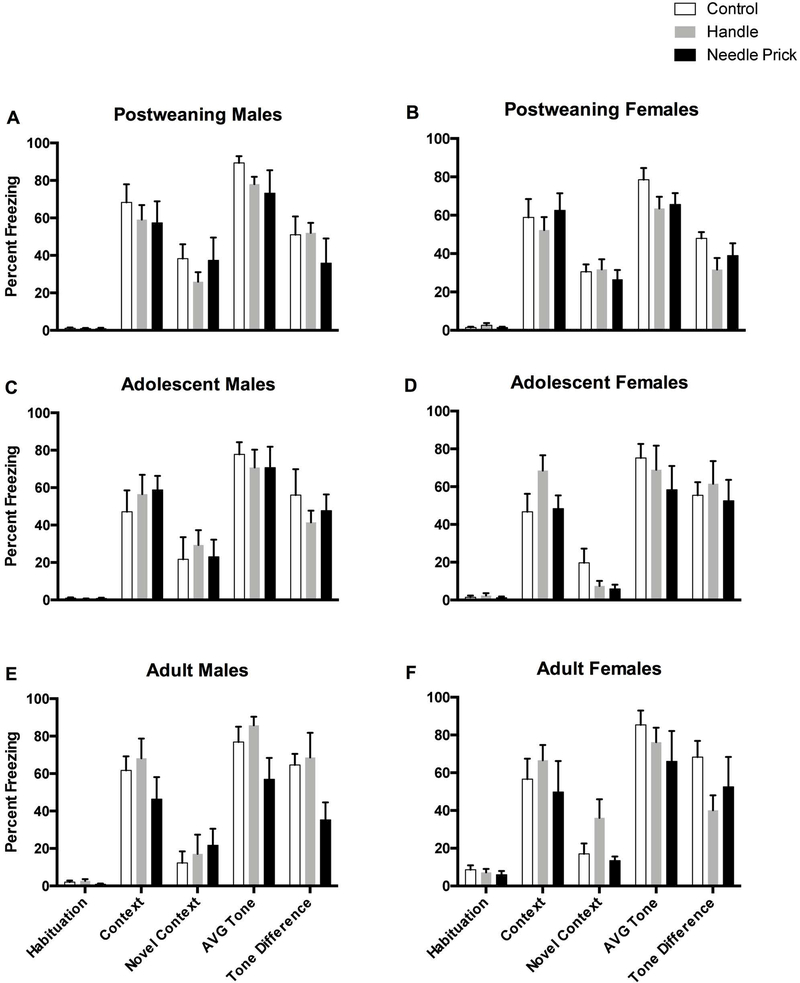
Figure 3.
Percent freezing for rats on the 5 freezing variables (Habituation, Context, Novel Context, Average Tone, and Tone Difference Scores). Top two panels represent post-weaning rats in the control (N=6M, 6F), handle (N=7M, 9F), and needle prick (N=5M, 8F) groups. Adolescent rats are represented in the middle two panels in the control (N=7M, 5F), handle (N=10M, 7F), and needle prick (N=7M, 7F) groups. Adult rats are depicted on the bottom two panels in the control (N=5M 7F), handle (N=7M, 8F), and needle prick (N=8M, 5F) groups. These data demonstrate that the younger rats show greater freezing to the novel context,suggesting greater contextual generalization. There was also a significant effect of needle pricks on auditory freezing which is observed better in Figures 2 and 4.
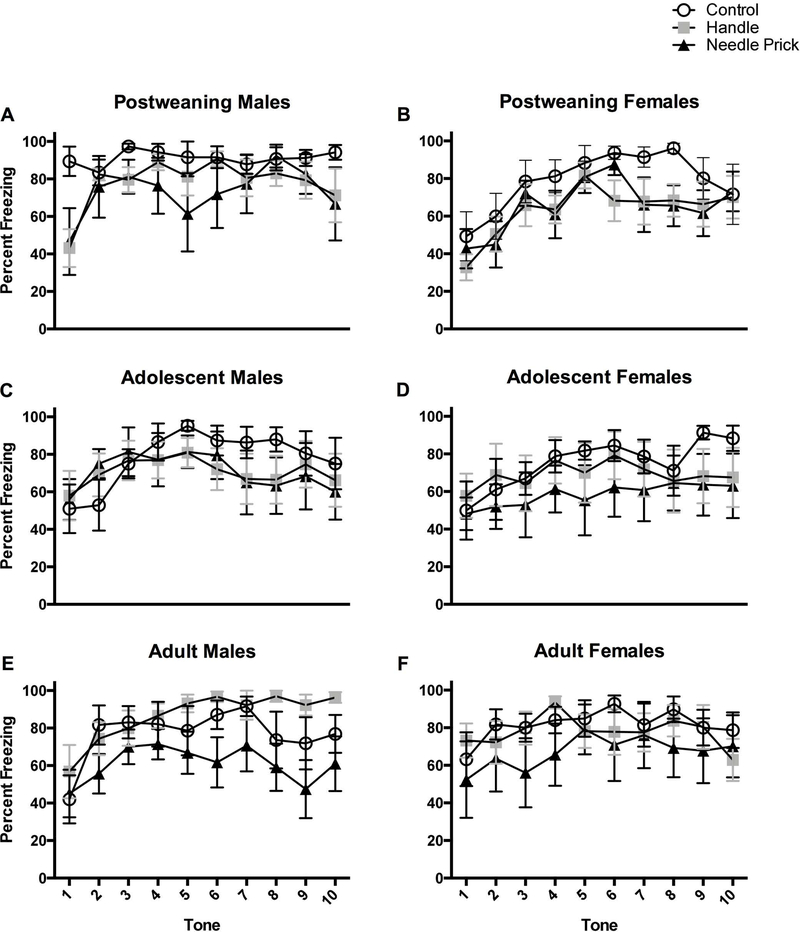
Figure 4.
Percent freezing for rats after each of the 10 CS tone presentations in the novel environment. The top two panels represent post-weaning in the control (N=6M, 6F), handle (N=7M, 9F), and needle prick (N=5M, 8F) groups. Adolescent rats are represented in the middle two panels in the control (N=7M, 5F), handle (N=10M, 7F), and needle prick (N=7M, 7F) groups. Adult rats are depicted on the bottom two panels in the control (N=5M 7F), handle (N=7M, 8F), and needle pricks (N=8M, 5F). This figure highlights the main effect of neonatal pain throughout the 10 tone presentations.
Figure 2.
Percent freezing for acute pain rats on the 5 freezing variables (Habituation, Context, Novel Context, Average Tone, and Tone Difference Scores) collapsed across age and sex to highlight effects between Control (N=36), Handle (N=49), and 4-Prick (N=40) rats. * represents a statistically significant difference between neonatal pain conditions. These data indicate that neonatal pain disrupt auditory fear conditioning when collapsed across age and sex.
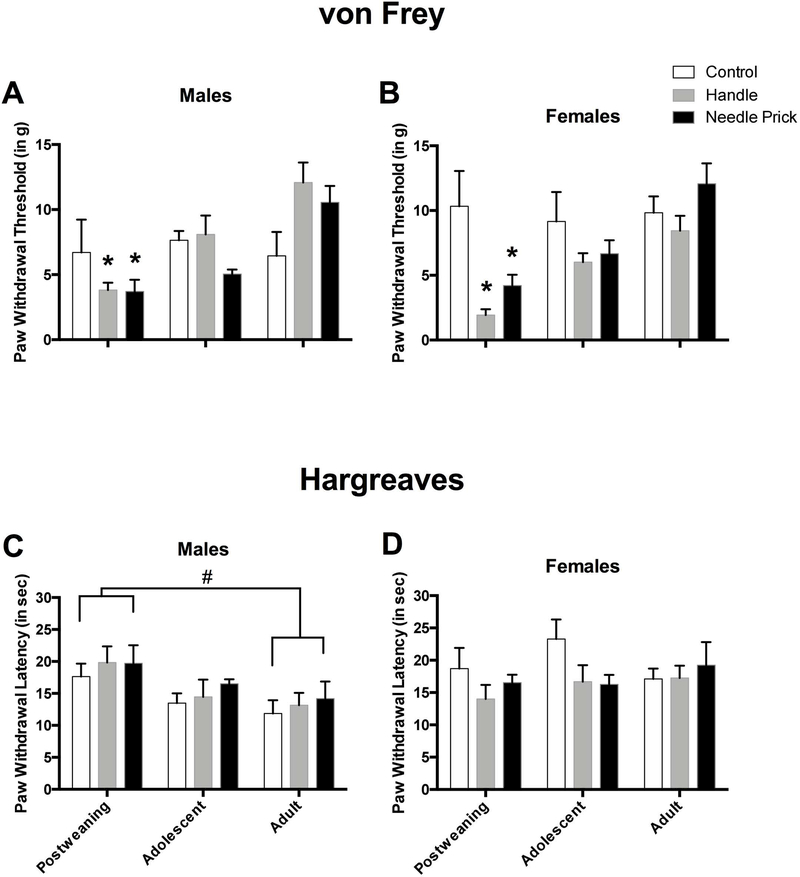
Both needle pricks and non-painful handling also caused subsequent hypersensitivity when assessed by the von Frey mechanical allodynia measure, especially in post-weaning female subjects. This was shown by a statistically significant interaction between neonatal pain condition and age (F(4,71)=4.23, p<0.01). To follow up on this interaction, the effects of neonatal pain condition at each age was analyzed using 1-way ANOVAs. Only at post-weaning period was there was a statistically significant effect of the neonatal pain manipulation (F(2,27)=6.36, p<0.01), and post hoc analyses revealed that rats in the handled (M=2.72, SEM=0.49) and needle prick (M=3.98, SEM=0.64) conditions showed greater hypersensitivity compared to controls (M=8.16, SEM=1.98) (see Figure 5A and B). There was no effect of neonatal pain condition in either adolescent (F(2,18)=1.19, n.s.), or adults rats (F(2,26)=1.93, n.s.). Neonatal pain also interacted with sex on the von Frey measure (F(2,71)=4.05, p<0.05). To determine where the individual effects were found, analyses were performed comparing each sex and neonatal pain condition. Analyses revealed that there was as statistically significant main effect of neonatal pain in females (F(2,35)=5.16, p<0.05), but not males (F(2,36)=0.68, n.s). Post hoc analyses revealed that handled females (M=5.28, SEM=0.94), but not needle pricked females (M=7.15, SEM=1.14), showed greater hypersensitivity respective to their control counterparts (M=9.83, SEM=1.25) (see Figure 5B).
Figure 5.
Paw withdrawal thresholds on the von Frey mechanical allodynia measure for males (A) and females (B) shows post-weaning, adolescent, and adult male rats in the control, handle, and needle prick groups. (all N’s identical to that of Figure 4). Paw withdrawal latencies on the Hargreaves thermal hyperalgesia measure for acute pain male (C) and female (D) rats during post-weaning, adolescence, and adulthood. * represents a statistically significant difference from control. # represents a statistically significant difference between age groups when collapsed across neonatal pain condition. These data indicate that both male and female post-weaning rats that received needle pricks or handling showed a mechanical hypersensitivity the dissipated with age, and that older male rats are hypersensitive to thermal stimuli irrespective of neonatal pain condition.
In summary, neonatal pain condition did not affect freezing during habituation, upon return to the conditioning context (Contextual Freezing), nor in the Novel Context. Moreover, only tactile (von Frey test) and not thermal (Hargreaves) reactivity was affected (see Figure 5C and D).
Effects of age
The amount of time spent freezing during each of the individual test sessions varied depending on the age of the rat, irrespective of sex or neonatal pain condition. This was shown by a 2 (Sex: male, female) × 3 (Condition: control, handled, needle prick) × 3 (Age: post-weaning, adolescence, adulthood) repeated measures ANOVA (with percent freezing during the Habituation, Context, Novel Context tests serving as the repeated variables) revealing a statistically significant interaction between age and test sessions (F(4, 202)=668.41, p<0.001). To characterize this interaction further, a separate 3-way univariate ANOVA was performed for each test session. Although of small size, there was a statistically significant main effect of age during the Habituation test (F(2,117)=13.11, p<0.001) with adult rats showing greater freezing compared to post-weaning, but not adolescent, rats (see Figure 3). There was no statistically significant age difference during the context freezing test (p > 0.1).
The effect of age became more apparent when examining freezing to the novel context. Younger rats appeared to have difficulty differentiating between contexts as there was a statistically significant main effect of age during the Novel Context (F(2,121)=4.32, p<0.05), with post-weaning rats showing a higher freezing percentage than both adolescent and adult rats (see Figure 3).
The age of the rats may have also played a role in mechanical sensitivity, although this was largely driven by the experimental group. There was a main effect of age (F(2,71)=14.11, p<0.001), and post hoc analyses revealed a strong age-dependent effect with post-weaning rats being the most hypersensitive (M=4.79, SEM=0.76). This threshold increased partially but not significantly by adolescence (M=7.06, SEM=0.63), and by adulthood there was a statistically significant increase in paw withdrawal threshold values (M=9.99, SEM=0.68). It is important to note, however that this effect is largely driven by the effects of handling and needle pricks on post-weaning subjects (see above and Figure 5).
Effects of sex
There was only one notable sex difference, which interacted with age, that emerged in the Hargreaves thermal hyperalgesia measure (F(2,71)=3.32, p<0.05). Univariate ANOVAs were performed on each sex and revealed that males, but not females, showed a statistically significant age effect, with post-weaning male rats having longer withdrawal latencies (M=18.97, SEM=1.41) than adult (M=13.28, SEM=1.48), but not adolescent males (M=14.79, SEM=1.54; see Figure 5C).
Experiment 1B: Neonatal pain and later-life somatosensory function.
This experiment examined the effects of neonatal needle pricks on subsequent sensory function during the post-weaning period in the absence of prior fear conditioning.
Mechanical sensitivity.
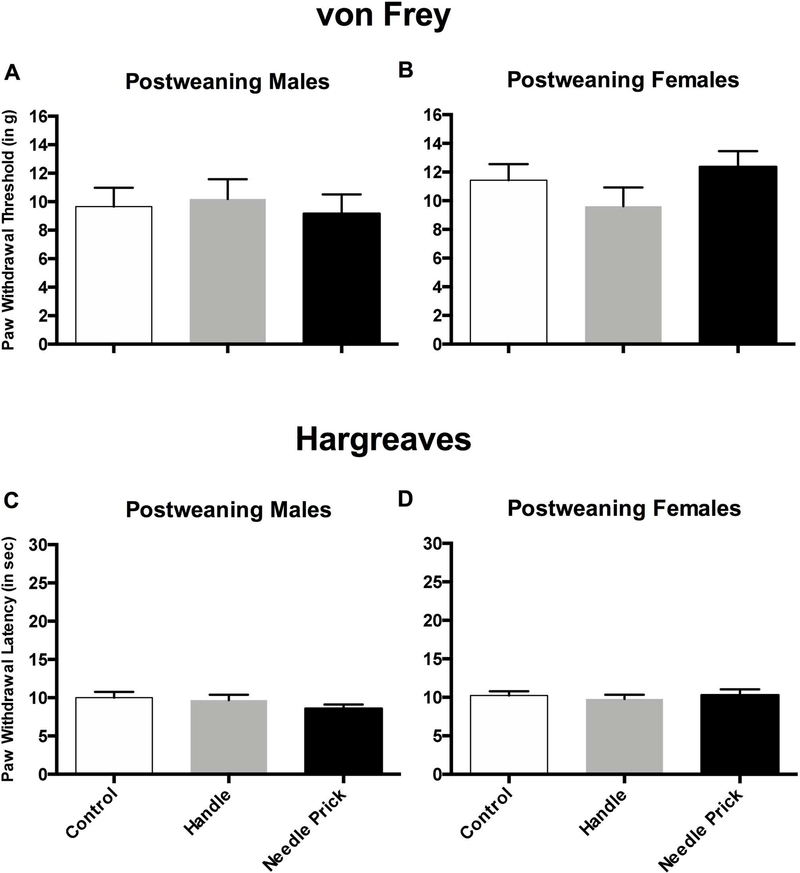
A 2 (Sex: male, female) × 3 (Condition: control, handle, needle prick) ANOVA was performed to observe the effects of neonatal pain alone on post-weaning mechanical allodynia data (see Figure 6A and B). There were no statistically significant main effect or interactions observed with sex or neonatal pain condition (all ps > 0.10).
Figure 6.
Paw withdrawal thresholds for the von Frey mechanical allodynia measure (top two panels) and paw withdrawal latencies for the Hargreaves thermal hyperalgesia measure (bottom two panels) without exposure to fear conditioning. Data for male (left panels) and female (right panels) in the control (N=17M, 14F), handle (N=13M, 17F), and needle prick (N=15M, 15F) conditions are represented. These data demonstrate that a second activating trauma is necessary to produce hypersensitivity seen in Figure 5.
Thermal sensitivity.
A 2 (Sex: male, female) × 3 (Condition: control, handle, needle prick) ANOVA was performed to observe the effects of neonatal pain alone on post-weaning thermal hyperalgesia data (see Figure 6C and D). There were no statistically significant main effect or interactions observed with sex or neonatal pain condition (all ps > 0.10).
Experiment 2: Chronic inflammatory pain on fear conditioning and somatosensory function.
This experiment examined the effects of neonatal inflammatory pain on subsequent fear conditioning and sensory function across the lifespan.
Paw Inflammation
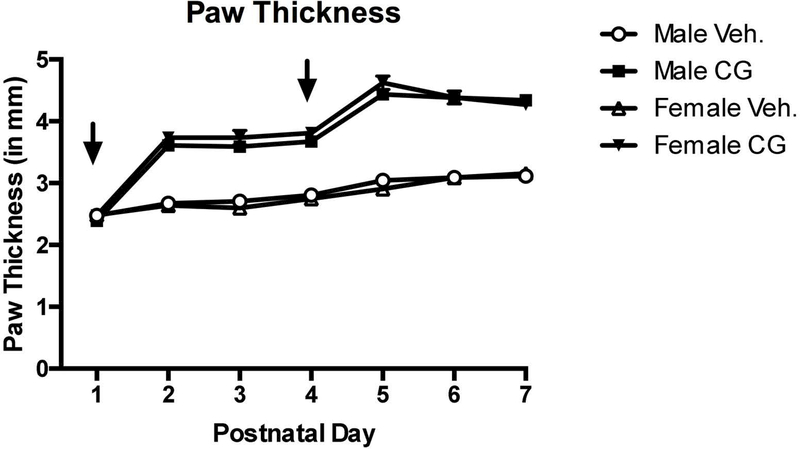
Each rat in the inflammation experiment had paw thickness measured as a confirmation that carrageenan was inducing an inflammatory response. A 2 (Sex: male, female) × 2 (Condition: vehicle, inflammation) repeated measures ANOVA (with each paw thickness measurements taken on the first seven days of life serving as the repeated variable) was performed on each rat that received i.pl. injections. There was a statistically significant effect of test day (F(6,570)=296.28, p<0.001), which is indicative of general growth over the seven days. Additionally, there was a strong main effect of condition (F(1,95)=494.22, p<0.001) with carrageenan treated male and female rats showing greater edema than vehicle-treated rats (see Figure 7)
Figure 7.
Paw thickness measurements of male and female neonatal rat pups following injection of vehicle (open symbols) or carrageenan (closed symbols) from PNDs 1–7. Arrows indicate injection day. These data serve as a confirmation that the inflammatory agent in fact produced inflammation as measured by edema.
Effects of neonatal pain
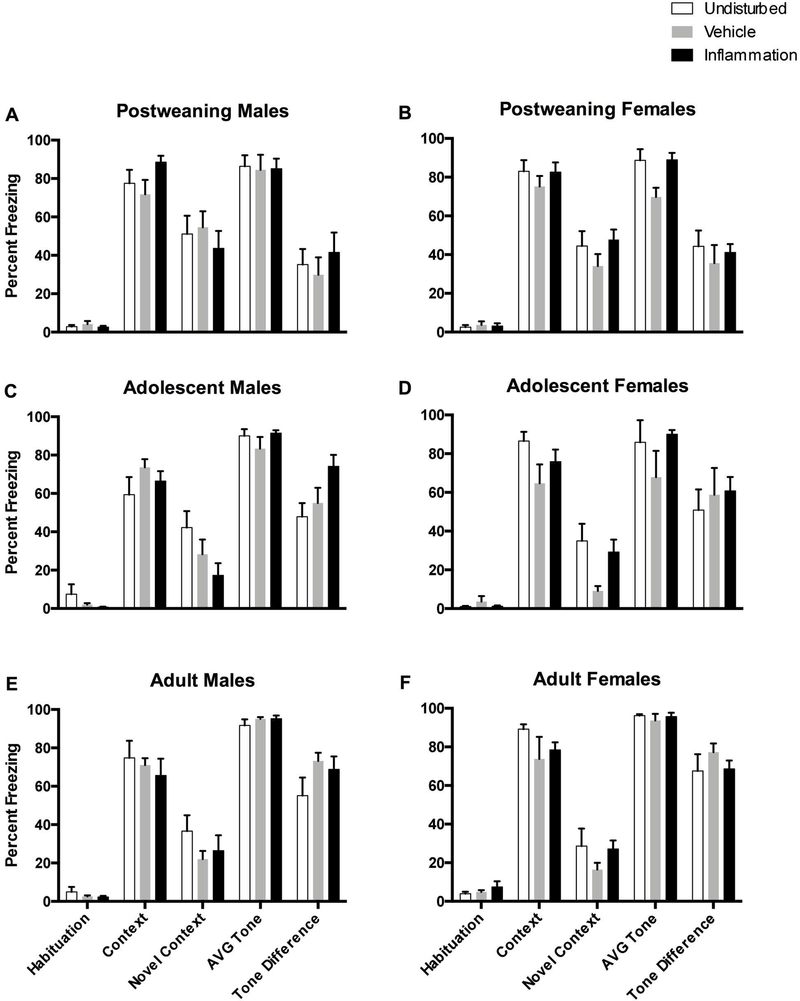
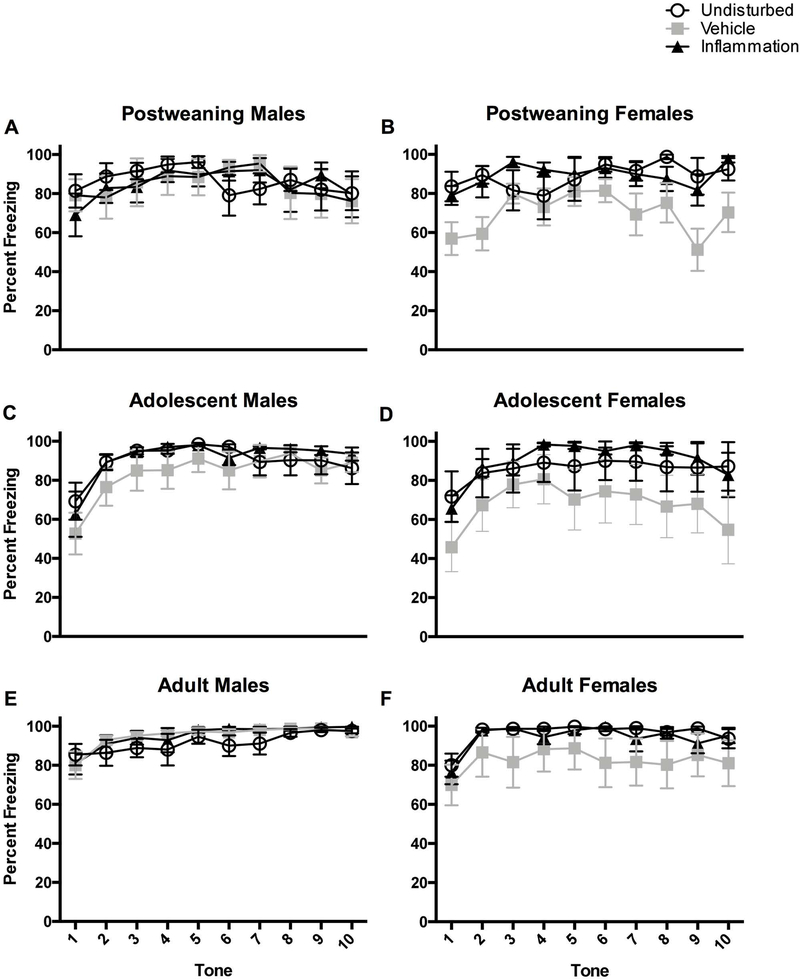
Inflammation itself did not appear to disrupt fear conditioning relative to undisturbed rats, however the vehicle injection did attenuate freezing, perhaps due to the acute pain or stress, irrespective of age, sex, or test session (see Figure 8). This was shown by a 2 (Sex: male, female) × 3 (Condition: undisturbed, vehicle, inflammation) × 3 (Age: post-weaning, adolescence, adulthood) repeated measures ANOVA (with percent freezing during the Habituation, Context, Novel Context tests serving as the repeated variables). This revealed a statistically significant main effect of neonatal pain condition (F(2,133)=3.72, p<0.05), and post hoc analyses revealed that vehicle-treated rats froze less (M=27.47, SEM=3.03) compared to undisturbed (M=39.74, SEM=3.50) but not inflammation (M=31.82, SEM=3.02) rats (see Figure 8). It should be noted that there was a statistically significant Sex × Condition × Test Session interaction (see Effects of sex below).
Figure 8.
Percent freezing inflammatory pain rats on the 5 freezing variables. Top two panels represent post-weaning in the undisturbed (N=9M, 9F), vehicle (N=8M, 9F), and inflammation (N=8M, 8F) groups. Adolescent rats are represented in the middle two panels in the undisturbed (N=10M, 8F), vehicle (N=8M, 8F), and inflammation (N=6M, 8F) groups. Adult rats are depicted on the bottom two panels in the undisturbed (N=10M, 9F), vehicle (N=9M 8F), and inflammation (N=7M, 9F) groups. Any effect of the inflammatory pain is likely obscured by a ceiling effect, particularly with auditory freezing (AVG tone variable).
The effects of neonatal pain condition on the pattern of freezing across the 10 tone presentations during the Novel Context session provided more specific insight into freezing behavior as there was a statistically significant Tone × Sex × Age interaction (F(18, 1206)=1.71, p<0.05). Separate ANOVAs performed on each sex revealed that only vehicle-treated adolescent females showed attenuated freezing during all tone presentations compared to undisturbed rats (F(2, 23)=5.33, p<0.05; see Figure 9D). Inflammation did not produce any significant main effects or interactions on the Tone Difference variable (all ps > 0.1).
Figure 9.
Percent freezing for rats after each of the 10 CS tone presentations in the novel environment. The top two panels represent post-weaning in the undisturbed (N=9M, 9F), vehicle (N=8M, 9F), and inflammation (N=8M, 8F) groups. Adolescent rats are represented in the middle two panels in the undisturbed (N=10M, 8F), vehicle (N=8M, 8F), and inflammation (N=6M, 8F) groups. Adult rats are depicted on the bottom two panels in the undisturbed (N=10M, 9F), vehicle (N=9M 8F), and inflammation (N=7M, 9F) groups. These data do show the attenuation of freezing in adolescent vehicle-treated females as well as a general high freezing percentage across all rats.
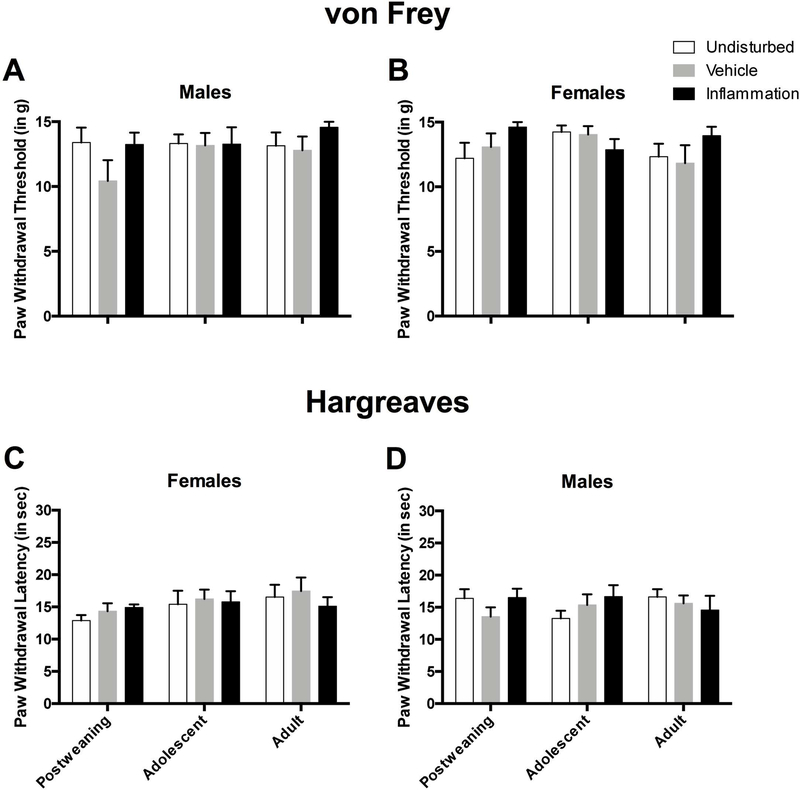
Inflammation did not produce any statistically significant main effects or interactions on the von Frey (see Figure 10A and B) mechanical allodynia measure nor the Hargreaves (see Figure 10C and D) thermal hyperalgesia measure (all ps > 0.1).
Figure 10.
Paw withdrawal thresholds on the von Frey mechanical allodynia measure in male (A) and female (B) rats during post-weaning, adolescence, and adulthood in the undisturbed, vehicle, and inflammation groups (all N’s identical to that of Figure 9). Paw withdrawal latencies on the Hargreaves thermal hyperalgesia measure for male (C) and female (D) rats during post-weaning, adolescence, and adulthood in the undisturbed, vehicle, and inflammation groups. These data highlight that rats in each condition, age, and sex have a high withdrawal threshold (mechanical sensitivity) indicating a ceiling effect in these animals. Likewise, a lack of group differences in rats on the thermal hyperalgesia measure may also suggest a ceiling effect.
Effects of age
The amount of time spent freezing during each of the individual test sessions varied depending on the age of the rat irrespective of sex or neonatal pain condition. This was shown by a 2 (Sex: male, female) × 3 (Condition: undisturbed, vehicle, inflammation) × 3 (Age: post-weaning, adolescence, adulthood) repeated measures ANOVA (with percent freezing during the Habituation, Context, Novel Context tests serving as the repeated variables) revealing a statistically significant interaction between age and test session (F(4, 266)=8.80, p<0.001). To characterize this interaction further, a separate 3-way univariate ANOVA was performed for each test session. Similar to Experiment 1, these subsequent analyses revealed that younger rats have difficulty differentiating between contexts as post-weaning rats spent more time freezing during the Novel Context (M=45.80, SEM=3.17) than both adolescent (M=27.66, SEM=3.24) or adult (M=26.60, SEM=2.75) rats (see Figure 8). Age also played a role in the expression of auditory fear conditioning. A 2 (Sex: male, female) × 3 (Condition: undisturbed, vehicle, inflammation) × 3 (Age: post-weaning, adolescence, adulthood) univariate ANOVA performed on AVG tone freezing revealed a statistically significant main effect of age (F(2, 134)=6.80, p<0.01). Again, as shown earlier, older animals seem to freeze the most, as post hoc analyses revealed that adult rats spent considerably more time freezing (M=94.54, SEM=0.92) than both adolescent (M=84.84, SEM=3.22) and post-weaning (M=83.79, SEM=2.37) rats.
The age of the rat did not influence sensory function as there were no statistically significant main effects or interactions on the von Frey (see Figure 10A and B) mechanical allodynia measure nor the Hargreaves (see Figure 10C and D) thermal hyperalgesia measure (all ps > 0.1).
Effect of sex
As mentioned previously, neonatal inflammatory pain differentially affected females and males, and this sex difference was dependent on the test session. A 2 (Sex: male, female) × 3 (Condition: undisturbed, vehicle, inflammation) × 3 (Age: post-weaning, adolescence, adulthood) repeated measures ANOVA (with percent freezing during the Habituation, Context, Novel Context tests serving as the repeated variables) revealed a statistically significant Sex × Condition × Test Session interaction (F(4, 266)=3.66, p<0.01). Subsequent analyses were performed in each sex individually to determine which test sessions were affected by inflammation. Further univariate ANOVAs performed at each test session revealed that neonatal pain produced differential freezing only with vehicle-treated female freezing less than both inflammation and undisturbed rats during the Context (F(2, 67)=4.07, p<0.05) and Novel Context (F(2, 67)=5.76, p<0.01) sessions (see Figure 8B, D, and F). There were no further sex-related main effects or interactions for the average tone, tone difference score, von Frey, or Hargreaves variables.
Experiment 3: Neonatal pain and blood corticosterone.
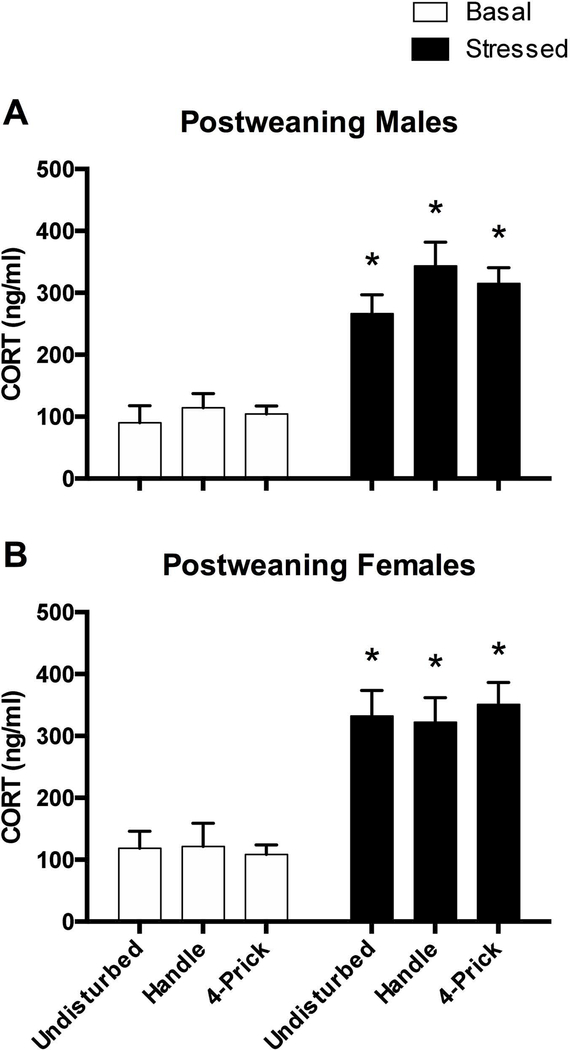
A 2 (Sex: male, female) × 2 (Stress: basal, stressed) × 3 (Condition: control, handle, needle prick) ANOVA was performed to observe the effects of neonatal pain on post-weaning blood corticosterone levels. There was a statistically significant main effect of stress (F(1, 55)=127.32, p<0.001) with rats exposed to the stressor showing elevated blood corticosterone levels (M=336.81, SEM=12.86) compared to basal rats (M=112.42, SEM=8.37). There were no other statistically significant main effects or interactions (all ps > 0.10) (see Figure 11).
Figure 11.
Blood corticosterone concentration for post-weaning males (top panel) and females (bottom panel) in both the basal (white bars) and stressed (black bars) conditions. Basal rats are subdivided into undisturbed (N=4M, 5F), handle (N=6M, 6F), and needle prick (N=5M, 6F) conditions. Stressed rats are likewise subdivided into undisturbed (N=4M, 4F), handle (N=5M, 5F), and needle prick (N=5M, 4F) groups. * represents a statistically significant difference from each respective basal group. These data show that acute stress, but not neonatal pain produce general increases in blood stress hormone levels.
Discussion
The major findings of these studies are that needle pricks, but not inflammation produced a disruption in auditory fear conditioning when collapsed across age and sex, and both needle pricks and non-painful handling produced a subsequent tactile hypersensitivity in post-weaning-aged rats, consistent with a two-hit model of pain-susceptibility. Although other studies that have also found effects of neonatal pain and trauma (Carmo et al., 2016; Chocyk et al., 2014), the current work expands upon them by demonstrating the super-additive effects of neonatal pain and later trauma, as well as directly comparing repeated-acute and chronic (inflammatory) pain models.
Experiment 1 demonstrated that acute neonatal pain induced by repeated hindpaw needle pricks can disrupt fear conditioning with regard to the subsequent freezing produced when the original conditioned stimulus was presented. There are several possible explanations for this effect. The disruption could be a result of dysfunction in the ability of these rats to express freezing (i.e. a motor deficit). However, because rats in each condition demonstrated equivalent freezing to the fearful context, it appears unlikely that there were gross changes in motor function. Rather, this effect is more likely due to trauma-induced specific differences in fear conditioning or fear expression to discrete auditory cues. Similar results have been reported in rats that experienced maternal separation as a stressor rather than pain (Chocyk et al., 2014). However, it should be noted that the diminished freezing was observed to both the context and CS in that paper, whereas in the current experiment only cued freezing to the tone CS was affected. Similar results have also been found in adult rats experiencing acute and chronic pain states, but likewise a reduction of freezing behavior was found in both the cued and context tests (Johnston, Maier, Rudy, & Watkins, 2012).
Although it may seem counterintuitive to see reduced freezing in rats exposed to early-life stress, there are several potential explanations as to how this could occur. One is that early-life pain/stress disrupts learning processes. In fear conditioning, this would manifest as reduced freezing, although other, non-fear-based learning tasks, could also be disrupted. Second, it is possible that neonatal pain reduces the sensitivity to subsequent nociceptive stimuli, such as foot shocks, resulting in reduced fear conditioning. In our data, needle pricked and handled rats were both hypersensitive respective to controls on measures of mechanical sensitivity, so differences in pain sensitivity appear unlikely to account for the observed impairment in fear expression. Additionally, there is evidence to suggest that intermittent maternal separation (15 or fewer mins) rather than prolonged separation can produce resilience to immediate stressors and thus alter later-life behavioral responses (Francis & Meaney, 1999; Plotsky et al., 2005). As, the repeated maternal separations and reunions in the current experiments were fairly similar to resiliency manipulations, we may have inadvertently increased pup resilience during the painful event and subsequently dampened their fear response to a later-life stressor. Finally, it is also possible that neonatal trauma simply immunizes subjects against later trauma (Beggs, 2015; Beggs, Currie, Salter, Fitzgerald, & Walker, 2012; Schwaller & Fitzgerald, 2014).
In contrast to the needle prick groups, there were few fear conditioning effects in rats that experienced inflammation as neonates. Our fear conditioning data indicate that neonatal inflammatory pain does not disrupt fear conditioning as hypothesized. It is notable that the inflammation did not produce changes in the fear conditioning paradigm as was observed to the needle pricked rats, which demonstrated reduced freezing to the tone CS, however vehicle-treated female rats did show attenuated contextual freezing. This may be due to the vehicle injection serving as an acute pain state, which has been shown to disrupt fear responses. Whether the neonatal inflammation has the opposite effect of enhancing fear conditioning is still unclear as we observed a ceiling effect, in that all groups were close to 100% freezing to the tone. Future studies with reduced intensity of the UCS and resultant lower freezing percentages will allow us to examine whether neonatal carrageenan enhances fear conditioning in later age groups.
Neonatal inflammation also failed to alter tactile or thermal sensory thresholds of rats in any age group. Of interest, this differs from other reports that chronic inflammatory pain induced during the neonatal period can produce a hyposensitivity to painful and non-painful stimuli later in life (Bhutta et al., 2001; G. Wang et al., 2004). Our data again indicate that a ceiling effect may be present in assessing mechanical sensitivity. Future studies will need to utilize a tactile measure that is capable of detecting decreases in mechanical sensitivity.
One question that we addressed was whether neonatal trauma alone was sufficient to cause lasting changes in mechanical or thermal sensitivity. We demonstrate that neonatal needle pricks failed to alter mechanical or thermal sensory thresholds in the absence of a fear conditioning experience. Instead, our data support a “two-hit” model, in that exposure to the stress of fear conditioning was required for the induction of mechanical hypersensitivity and that the hypersensitivity resolved within seven days. Two-hit models, in which both neonatal and subsequent trauma add together to create a disordered phenotype, have been proposed for a variety of psychological and physiological disorders, including anxiety (Catuzzi & Beck, 2014). Moreover, a two-hit model has also been shown as necessary for the development of visceral pain hypersensitivity in rodents (Prusator & Greenwood-Van Meerveld, 2016). Although to best of our knowledge, these data are the first to demonstrate a developmental two-hit effect in acute cutaneous pain, it is conceptual similar to the “nociceptor priming” demonstrated in adults (e.g., Kandasamy & Price, 2015).
Finally, we assessed blood corticosterone levels in rats subjected to the needle prick manipulation both with, and without an acute stressor prior to the point of collection. Consistent with previous reports, there was a general increase in blood corticosterone levels following the acute stressor, however there were no group differences due to neonatal pain conditions in either the basal or stressed post-weaning rats, similar to findings in adult rats (Anand et al., 1999). This suggests that neonatal pain alone is not enough to produce lasting changes in HPA function. Interestingly, we also did not observe any sex effect on corticosterone levels. In other studies observing neonatal stress manipulations (3 hr maternal separation), marked sex differences have been reported in adulthood corticosterone levels in both basal and acutely stressed rats, with females showing greater corticosterone concentration than males (Couto-Pereira et al., 2016). The most obvious explanation as to why we did not observe sex differences is the fact we measured corticosterone levels in pre-pubescent rats and thus the sex difference had not yet developed.
Our data also shed light on several other issues. For example, we observed differences in novel-context freezing, consistent with a greater generalization across contexts in the younger animals. A greater generalization of learning in younger animals has been shown previously (Brown & Stanton, 2008). Also, we have previously published (Burman, Erickson, Deal, & Jacobson, 2014; Deal, Erickson, Shiers, & Burman, 2016) that both auditory and contextual freezing emerge gradually prior to PND 24. The current data are consistent with those conclusions and further demonstrate a lack of age-related differences on these variables after PND 24. The current findings regarding contextual generalization/discrimination are attributable to maturation of the hippocampus or other spatial learning structures.
The current data also provide insight into the neurological mechanisms underlying different forms of fear conditioning. It is well-established that cued and contextual fear conditioning differ in that dorsal hippocampus function is only required for contextual freezing (Kim & Fanselow, 1992; Phillips & LeDoux, 1992). Less clear is whether the role of the amygdala also dissociates in these forms of learning. Although a great deal of work suggests that the basolateral amygdala is required for both cued and contextual freezing (Castro-Gomes et al., 2016; Yen et al., 2012) some work suggests that amygdala disruption can preferentially disrupt cued over contextual fear conditioning (Selden, Everitt, Jarrard, & Robbins, 1991). Our data, in which needle pricks disrupt cued, but not contextual, freezing, appears to favor the later interpretation. Additionally, there are several studies which directly support changes in amygdalar structure and function following both early-life stress (Holschneider, Guo, Mayer, & Wang, 2016; Roth & Sullivan, 2005; Thompson, Sullivan, & Wilson, 2008) and early-life pain (Victoria, Inoue, Young, & Murphy, 2013a; 2013b).
To summarize, these data collectively indicate that repeated hindpaw needle pricking and handling, but not inflammation-induced neonatal pain, can increase susceptibility to stress-induced mechanical hypersensitivity at PND 24. In addition, repeated needle pricks also produces a disruption in fear conditioning, particularly when the original CS is presented in a novel environment. Of interest, neonatal pain alone does not seem to alter subsequent sensory thresholds in the absence of stress or alter stress-induced increase in blood corticosterone levels, suggesting that behavioral changes later in life do not result solely from long-term alterations in pain sensory thresholds or responsivity of the HPA. Though more research is necessary, it is possible that neonatal pain in rats causes a dysfunction in the amygdala, resulting in a disruption of the recall of fearful stimuli. Overall, these data have valuable clinical relevance, as many painful procedures in NICUs are acute in nature (i.e., heel lances) and therefore special consideration should be given to prevent later-life dysfunction.
Acknowledgements:
This work was funded by NIGMS P20GM103643 (Meng PI) and NICHD/NIGMS 1R15HD091841 (Burman PI), as well as the generous support of the UNE summer undergraduate research experience (SURE) program, the UNE Office of the Vice-President for Research and the College of Arts and Sciences. The authors report no conflicts of interest. We’d like to thank Cassandra Simmons and all the other members of the Burman and King laboratories for their technical assistance. We’d also like to Dr. Gordon Barr and Dr. Ian Meng for their helpful feedback on earlier versions of this manuscript.
References
- Anand KJS (2016). Revisiting a dilemma: repetitive pain vs. opioid exposures? Acta Paediatrica (Oslo, Norway : 1992), 105(7), 736–737. 10.1111/apa.13442 [DOI] [PubMed] [Google Scholar]
- Anand KJS, Aranda JV, Berde CB, Buckman S, Capparelli EV, Carlo W, et al. (2006). Summary proceedings from the neonatal pain-control group. (Vol. 117, pp. S9–S22). Presented at the Pediatrics, American Academy of Pediatrics. 10.1542/peds.2005-0620C [DOI] [PubMed] [Google Scholar]
- Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, & Plotsky PM (1999). Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiology & Behavior, 66(4), 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S (2015). Long-Term Consequences of Neonatal Injury. Canadian Journal of Psychiatry. Revue Canadienne De Psychiatrie, 60(4), 176–180. 10.1177/070674371506000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Currie G, Salter MW, Fitzgerald M, & Walker SM (2012). Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain, 135(Pt 2), 404–417. 10.1093/brain/awr288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta AT, Rovnaghi C, Simpson PM, Gossett JM, Scalzo FM, & Anand KJ (2001). Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects. Physiology & Behavior, 73(1–2), 51–58. [DOI] [PubMed] [Google Scholar]
- Bonin RP, Bories C, & De Koninck Y (2014). A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filiments. Molecular Pain, 10(26), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KL, & Stanton ME (2008). Cross-modal transfer of the conditioned eyeblink response during interstimulus interval discrimination training in young rats. Developmental Psychobiology, 50(7), 647–664. 10.1002/dev.20335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Chau CMY, Cepeda IL, Degenhardt A, Weinberg J, Synnes AR, & Grunau RE (2015). Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology, 51, 151–163. 10.1016/j.psyneuen.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman MA, Erickson KJ, Deal AL, & Jacobson RE (2014). Contextual and auditory fear conditioning continue to emerge during the periweaning period in rats. PloS One, 9(6), e100807 10.1371/journal.pone.0100807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo E. de C. D., Sanada LS, Machado NLB, & Fazan VPS (2016). Does Pain in the Neonatal Period Influence Motor and Sensory Functions in a Similar Way for Males and Females During Post-Natal Development in Rats? Pain Medicine (Malden, Mass.), 17(8), 1520–1529. 10.1093/pm/pnv117 [DOI] [PubMed] [Google Scholar]
- Castro-Gomes V, Bergstrom HC, McGuire JL, Parker CC, Coyner J, Landeira-Fernandez J, et al. (2016). A dendritic organization of lateral amygdala neurons in fear susceptible and resistant mice. Neurobiology of Learning and Memory, 127, 64–71. 10.1016/j.nlm.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Catuzzi JE, & Beck KD (2014). Anxiety vulnerability in women: a two-hit hypothesis. Experimental Neurology, 259, 75–80. 10.1016/j.expneurol.2014.01.023 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, & Yaksh TL (1994). Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods, 53(1), 55–63. [DOI] [PubMed] [Google Scholar]
- Chocyk A, Przyborowska A, Makuch W, Majcher-Maślanka I, Dudys D, & Wędzony K (2014). The effects of early-life adversity on fear memories in adolescent rats and their persistence into adulthood. Behavioural Brain Research, 264, 161–172. 10.1016/j.bbr.2014.01.040 [DOI] [PubMed] [Google Scholar]
- Couto-Pereira N. de S., Ferreira CF, Lampert C, Arcego DM, Toniazzo AP, Bernardi JR, et al. (2016). Neonatal interventions differently affect maternal care quality and have sexually dimorphic developmental effects on corticosterone secretion. International Journal of Developmental Neuroscience : the Official Journal of the International Society for Developmental Neuroscience, 55, 72–81. 10.1016/j.ijdevneu.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Deal AL, Erickson KJ, Shiers SI, & Burman MA (2016). Limbic system development underlies the emergence of classical fear conditioning during the third and fourth weeks of life in the rat. Behavioral Neuroscience, 130(2), 212–230. 10.1037/bne0000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenni VM, Song CM, Hill MN, & Pittman QJ (2017). Early-life inflammation with LPS delays fear extinction in adult rodents. Brain, Behavior, and Immunity, 63, 176–185. 10.1016/j.bbi.2016.11.022 [DOI] [PubMed] [Google Scholar]
- Francis DD, & Meaney MJ (1999). Maternal care and the development of stress responses. Current Opinion in Neurobiology, 9(1), 128–134. 10.1016/S0959-4388(99)80016-6 [DOI] [PubMed] [Google Scholar]
- Grunau RE, Holsti L, & Peters JWB (2006). Long-term consequences of pain in human neonates. Seminars in Fetal & Neonatal Medicine, 11(4), 268–275. 10.1016/j.siny.2006.02.007 [DOI] [PubMed] [Google Scholar]
- Heim C, & Nemeroff CB (2002). Neurobiology of early life stress: clinical studies. Seminars in Clinical Neuropsychiatry, 7(2), 147–159. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Guo Y, Mayer EA, & Wang Z (2016). Early life stress elicits visceral hyperalgesia and functional reorganization of pain circuits in adult rats. Neurobiology of Stress, 3, 8–22. 10.1016/j.ynstr.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio CRD, Carey CE, Michalski LJ, Corral-Frias NS, Conley ED, Hariri AR, & Bogdan R (2017). Hypothalamic-pituitary-adrenal axis genetic variation and early stress moderates amygdala function. Psychoneuroendocrinology, 80, 170–178. 10.1016/j.psyneuen.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Maier SF, Rudy JW, & Watkins LR (2012). Post-conditioning experience with acute or chronic inflammatory pain reduces contextual fear conditioning in the rat. Behavioural Brain Research, 226(2), 361–368. 10.1016/j.bbr.2011.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, & Price TJ (2015). The pharmacology of nociceptor priming. Handbook of Experimental Pharmacology, 227(Chapter 2), 15–37. 10.1007/978-3-662-46450-2_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, & Fanselow MS (1992). Modality-specific retrograde amnesia of fear. Science (New York, NY), 256(5057), 675–677. [DOI] [PubMed] [Google Scholar]
- LaPrairie JL, & Murphy AZ (2007). Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain, 132 Suppl 1, S124–33. 10.1016/j.pain.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie KM, & Rivier C (1997). Effect of alcohol on the proestrous surge of luteinizing hormone (LH) and the activation of LH-releasing hormone (LHRH) neurons in the female rat. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 17(7), 2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page GG, Hayat MJ, & Kozachik SL (2013). Sex differences in pain responses at maturity following neonatal repeated minor pain exposure in rats. Biological Research for Nursing, 15(1), 96–104. 10.1177/1099800411419493 [DOI] [PubMed] [Google Scholar]
- Phillips RG, & LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106(2), 274–285. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, & Meaney MJ (2005). Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 30(12), 2192–2204. 10.1038/sj.npp.1300769 [DOI] [PubMed] [Google Scholar]
- Prusator DK, & Greenwood-Van Meerveld B (2016). Sex differences in stress-induced visceral hypersensitivity following early life adversity: a two hit model. Neurogastroenterology and Motility : the Official Journal of the European Gastrointestinal Motility Society, 28(12), 1876–1889. 10.1111/nmo.12891 [DOI] [PubMed] [Google Scholar]
- Roth TL, & Sullivan RM (2005). Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry, 57(8), 823–831. 10.1016/j.biopsych.2005.01.032 [DOI] [PubMed] [Google Scholar]
- Schellinck HM, Stanford L, & Darrah M (2003). Repetitive acute pain in infancy increases anxiety but does not alter spatial learning ability in juvenile mice. Behavioural Brain Research, 142(1–2), 157–165. [DOI] [PubMed] [Google Scholar]
- Schwaller F, & Fitzgerald M (2014). The consequences of pain in early life: injury-induced plasticity in developing pain pathways. The European Journal of Neuroscience, 39(3), 344–352. 10.1111/ejn.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, & Robbins TW (1991). Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience, 42(2), 335–350. [DOI] [PubMed] [Google Scholar]
- Taddio A, Katz J, Ilersich AL, & Koren G (1997). Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet (London, England), 349(9052), 599–603. 10.1016/S0140-6736(96)10316-0 [DOI] [PubMed] [Google Scholar]
- Thompson JV, Sullivan RM, & Wilson DA (2008). Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Res, 1200, 58–65. 10.1016/j.brainres.2008.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria NC, Inoue K, Young LJ, & Murphy AZ (2013a). A single neonatal injury induces life-long deficits in response to stress. Developmental Neuroscience, 35(4), 326–337. 10.1159/000351121 [DOI] [PubMed] [Google Scholar]
- Victoria NC, Inoue K, Young LJ, & Murphy AZ (2013b). Long-term dysregulation of brain corticotrophin and glucocorticoid receptors and stress reactivity by single early-life pain experience in male and female rats. Psychoneuroendocrinology, 38(12), 3015–3028. 10.1016/j.psyneuen.2013.08.013 [DOI] [PubMed] [Google Scholar]
- Wang G, Ji Y, Lidow MS, & Traub RJ (2004). Neonatal hind paw injury alters processing of visceral and somatic nociceptive stimuli in the adult rat. The Journal of Pain : Official Journal of the American Pain Society, 5(8), 440–449. 10.1016/j.jpain.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Yen Y-C, Mauch CP, Dahlhoff M, Micale V, Bunck M, Sartori SB, et al. (2012). Increased levels of conditioned fear and avoidance behavior coincide with changes in phosphorylation of the protein kinase B (AKT) within the amygdala in a mouse model of extremes in trait anxiety. Neurobiology of Learning and Memory, 98(1), 56–65. 10.1016/j.nlm.2012.04.009 [DOI] [PubMed] [Google Scholar]