Abstract
Parkinson's disease (PD) is the second most common neurodegenerative disorder characterized by progressive loss of dopaminergic neurons in the substantia nigra. The precise mechanism underlying pathogenesis of PD is not fully understood, but it has been widely accepted that excessive reactive oxygen species (ROS) are the key mediator of PD pathogenesis. The causative factors of PD such as gene mutation, neuroinflammation, and iron accumulation all could induce ROS generation, and the later would mediate the dopaminergic neuron death by causing oxidation protein, lipids, and other macromolecules in the cells. Obviously, it is of mechanistic and therapeutic significance to understand where ROS are derived and how ROS induce dopaminergic neuron damage. In the present review, we try to summarize and discuss the main source of ROS in PD and the key pathways through which ROS mediate DA neuron death.
1. Introduction
Parkinson's disease (PD) is an age-dependent, progressive neurodegenerative disease, characterized by selective loss of dopaminergic (DA) neurons residing in an area of the midbrain known as the substantia nigra [1, 2]. As the second most common neurodegenerative disease, PD remains incurable, which might be underlined by the fact that mechanism for PD pathogenesis is not fully illustrated.
With the intensive studies, it is now widely accepted that genetic background, environment factors, and aging are the key contributors of PD pathogenesis. In recent years, some PD-associated genes have been identified, including α-synuclein (SNCA), PTEN-induced putative kinase 1 (PINK1), parkin, DJ-1 (PARK7), and leucine rich repeat kinase 2 (LRRK2), mutations of which lead to the familial forms of PD (early-onset) [3]. Even in the rest of 90% of the sporadic cases of PD, mutations of those genes could also increase the PD susceptibility [4]. Environmental factors such as heavy metals, drugs, and exposure to neurotoxic compounds can induce PD via interfering dopamine transporter activity, dopamine metabolism, mitochondrial function, and proteasome activity [5–7]. Aging could result in misfolding of proteins as well as mitochondrial dysfunction, which are all closely related to the PD pathogenesis [8]. Although the underlying mechanisms of neuronal degeneration in PD remain to be better understood, it is well established that all of the PD-related factors mentioned above can cause excessive generation of ROS [9].
ROS, as the by-products of cellular metabolism, are defined as a group of reactive molecules derived from molecular oxygen, which include superoxide anion (O2−), hydroxyl radical (·OH), and hydrogen peroxide (H2O2) [10]. ROS are essential for maintaining many physiological processes such as apoptosis, autophagy, and immunological defense [11]. But if the balance between production and elimination of ROS is disturbed, pathogenic consequences such as neurodegeneration would happen [12].
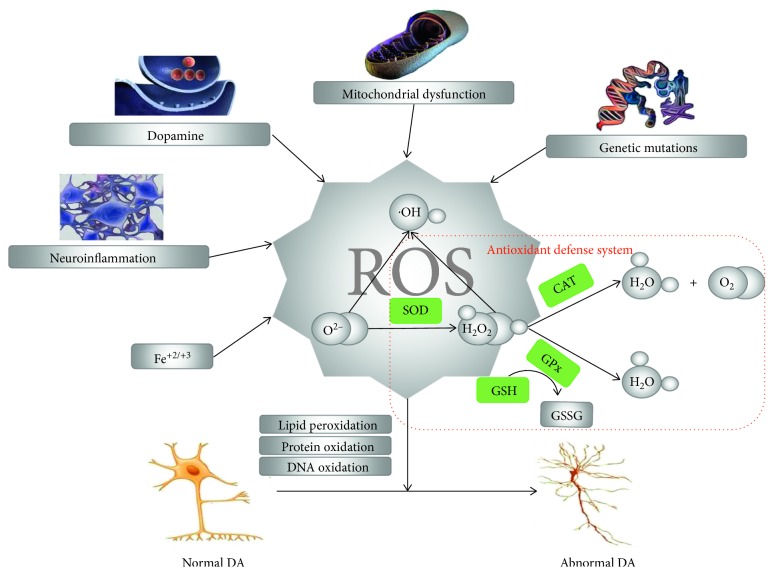
In this review, we will focus on discussing how the PD-associated factors induce ROS generation and how ROS lead to dopaminergic neuron death in PD (Figure 1).
Figure 1.
Schematic pathway of ROS generation and induction of DA neurons death. Mitochondria dysfunction, dopamine, neuroinflammation, iron, and genetic mutations solely or synergistically induce ROS generation, which could induce dopaminergic neurons death via protein, lipid, and DNA oxidation.
2. ROS and PD-Associated Factors
Numerous evidences suggest that PD-associated factors such as genes mutation, mitochondrial dysfunction, dopamine auto-oxidation, neuroinflammation, iron accumulation, and external toxicants accumulation, all could induce ROS generation.
2.1. PD-Related Genetic Mutations and ROS
It has been recognized that the genetic mutations such as α-synuclein, PINK1, parkin, DJ-1, and LRRK2 are causative factors of the familial forms of PD [13, 14]. Mutation or multiplication of the α-synuclein gene facilitates the accumulation of α-synuclein, which is a major component of Lewy bodies, the pathological hallmark of PD [15]. It was indicated that accumulation of α-synuclein caused oxidative stress by two parallel pathways: directly stimulating the generation of excessive ROS or indirectly interfering scavenge of damaged mitochondria from which majority of ROS were derived [16, 17]. PINK1 is the kinase that could phosphorylate and activate parkin in the process of damaged mitochondria clearance by autophagy, which exerts neuroprotection against ROS overproduction [18]. It was reported that loss of PINK1 or parkin induced mitochondrial dysfunction and consequent overproduction of ROS, while overexpression of PINK1 or parkin protected against ROS-induced cell death [19, 20]. Parkin is an E3 ubiquitin ligase, and loss of function leads to autosomal recessive PD [21]. Mutation of parkin impairs its function in the elimination of damaged mitochondria, the latter generated ROS [22]. DJ-1 is a small compact protein that localized on the outer mitochondrial membrane (OMM). The sulfhydryl group of DJ-1 could react with ROS to form the cysteine sulfinic acid, which functions as a ROS quencher [23]. Loss of DJ-1 renders increased ROS levels and ultimately caused dopaminergic neuron death [19]. LRRK2 is a large multidomain protein and its mutation leads to autosomal dominant PD. A proposed mechanism for the increased vulnerability of LRRK2 mutant cells to oxidative stress is via the kinase-dependent interaction between LRRK2 and dynamin-like protein (DLP1), which facilitates DLP1 translocation to mitochondria and subsequent mitochondrial fission [24, 25]. Another mechanism is through the interaction of LRRK2 with peroxiredoxin 3 (PRDX3), which is a mitochondrial member of the antioxidant family of thioredoxin peroxidases. Mutations in the LRRK2 kinase domain increase phosphorylation of PRDX3 leading to decreased peroxidase activity, increased ROS production, and increased cell death [26, 27]. Notably, postmortem analysis of brains from PD patients carrying the G2019S mutation in the kinase domain of LRRK2 has shown marked increase in phosphorylated PRDX3 compared to normal brains [28].
2.2. Mitochondrial Dysfunction and ROS
Mitochondria are known as the “power houses” of cells, the place generating adenosine triphosphate (ATP) through oxidative phosphorylation (OXPHOS) [29]. During ATP production, ROS also generate from the electron transport chain [30]. The ROS from complex I are released to the mitochondrial matrix, while the ROS from complex III are released to both the mitochondrial matrix and the inner membrane space (IMS) [31]. Mitochondrial dysfunction leads to increased ROS generation; in return, ROS are also harmful to the electron transport chain itself, leading to even higher production of ROS [32, 33]. It was suggested that mitochondria-induced overproduction of ROS was a key factor responsible for cell death and the progression of late-onset neurodegenerative diseases, particularly in idiopathic PD [32, 34]. Mitochondrial dysfunction leads to the deficiency of ATP, which is indispensable especially to dopaminergic neurons to propagate electrical signals, maintain ionic gradients and secrete dopamine [35]. The fact that the activity of the mitochondrial electron transport chain in the substantia nigra of PD patients was decreased compared with age-matched controls, further supported the role of mitochondrial dysfunction in PD [36]. In summary, mitochondrial dysfunction can cause PD though the overproduction of ROS, which underlines the dopaminergic neuron death in PD.
2.3. Dopamine and ROS
Dopamine (DA), the neurotransmitter produced from DA neurons, is responsible for the regulation of excitatory and inhibitory synaptic transmission for ensuring smooth coordinated movement [37]. The movement disorder displayed in PD patients is basically underlined by the deficiency of DA. Noteworthy, dopamine is an unstable molecule that may auto-oxidize to form quinones and H2O2 [38, 39]. H2O2 could react with iron or oxygen to form more active ˙OH [40]. DA quinones could react with the sulfhydryl groups of the cysteine in proteins, particularly glutathione (GSH), a ROS scavenger, resulting in lower GSH levels, and higher ROS level [41]. In addition, ROS, especially H2O2, are generated as by product in the process of dopamine oxidative metabolism by monoamine oxidases B [42, 43]. Besides the synthesis and degradation, the transport and storage of dopamine also contribute to elevated ROS production. Dopamine is synthesized in the cytosol and rapidly stored into synaptic vesicles for providing a stable environment for DA before released out [15], which is dependent on vesicular monoamine transporter 2 (VMAT2). Dopamine reuptake, occurred with the help of dopamine active transporter (DAT), is essential for precisely tuning the dopamine level in synaptic cleft [44]. Obviously, any perturbation to the storage and reuptake of dopamine would elevate cytoplasmic dopamine, which enhances the susceptibility to be oxidation. Consist with that, mutant α-synuclein, which linked to inherited forms of PD, is associated with enhanced dopamine reuptake and down regulates VMAT2 [45]. In addition, DAT is involved in dopamine neurotoxicity by reuptake dopamine from extracellular space to cytosol leading to accumulation of dopamine [46]. Conclusively, dopamine is an unstable molecule and prone to auto-oxidize in cytoplasm. Any perturbation elevating cytoplasmic dopamine can increase dopamine auto-oxidization and subsequently ROS and eventually PD pathogenesis.
2.4. Neuroinflammation and ROS
Neuroinflammation is a protective response of nervous system to various kinds of tissue insults and damage. It would induce release of trophic factors and ROS to protect against stimulus so as to facilitate the regeneration and the repair [47]. Once inflammation is overwhelmed, it would cause accumulation of ROS and consequently cell death [48]. A large body of research shows that chronic inflammation involves in chronic neurodegenerative diseases, particularly the pathogenesis of PD.
Microglial cells, resident immune cells in the central nervous system (CNS), are main participants of the inflammatory response. Activated microglia releases various cytokines and chemokines to initiate corresponding processes to recruit additional microglia and leukocytes to the site of injury [49]. Cytokines such as, TNF-α, IL-1β, and IFN-γ, are proinflammatory, which will activate NADPH oxidases (Nox). Nox2, one isoform of Nox, is mainly expressed in the nervous system involved in the production of ROS as a result of the catalyzing the electron transfer from NADPH to oxygen [50]. In addition, TNF-α could cause the depletion of endogenous antioxidants such as GSH of DA neurons, which renders DA neurons more susceptible to ROS [51]. IL-1β causes aberrant mitochondrial membrane potential and the depletion of ATP through facilitating the formation of peroxynitrite, ultimately leading to mitochondria dysfunction and consequent increased ROS [52, 53]. Beside cytokines and chemokines, microglia can also be activated by endogenous proteins such as α-synuclein [54]. α-Synuclein directly promotes activation of Nox2 in microglia leading to a burst of ROS. Conclusively, cytokines and chemokines released by microglia can induce NAPDH oxidase activity, which are capable of markedly enhancing the level of ROS and therefore PD pathogenesis.
2.5. Iron and ROS
Iron accumulation is another important hallmark of PD, which has been supported by multiple of evidences, especially increased iron level observed in the substantia nigra of PD patients compared to age-matched controls [55]. Iron is indispensable for many fundamental biological processes, but excessive iron is cytotoxic. Neurons therefore tightly regulate iron levels via controlling both iron uptake and iron storage. As established, the homeostasis of cellular iron is coordinated mainly by two iron regulatory proteins (IRP1 and IRP2) [56, 57], which could bind to DNA iron-response elements (IREs) and regulate their translations [58]. With aging, the regulation machinery of iron tends to be compromised and abnormal iron accumulation and increased free iron concentration subsequently occurred [59].
Excessive iron ions can cause an exacerbated ROS production via Fenton and Haber–Weiss reactions. Iron also catalyzes the conversion of excess dopamine to neuromelanin, during which ROS are generated [60]. Consistent with that, N-acetyl-l-cysteine (NAC), an antioxidant, which could decrease iron levels, showed neuroprotective effect in PD models [61]. Moreover, desferrioxamine (DFO) and VAR10303 (VAR), two kinds of iron chelator, reduced the ROS and rescued the MPTP induced PD mouse phenotypes [62, 63]. Collectively, iron can also contribute to pathogenesis of PD via aggravating ROS production.
3. Pathological Role of ROS in the PD Pathogenesis
In cells, ROS are strictly regulated by antioxidant defense systems, which mainly consist of superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), ascorbic acid (vitamin C), α-tocopherol (vitamin E), and GSH [64] (Table 1). Once the formation of ROS overwhelms the antioxidant defense system, oxidative stress will be induced. As motioned above, various PD causative factors can lead to excessive ROS generation, which further emphasizes the pivotal role of ROS in the PD pathogenesis. ROS participated in PD pathogenesis involving the peroxidation of lipid, protein, and nucleic acid [65].
Table 1.
Antioxidant defense systems and proposed mechanisms against ROS.
| Classifications | Antioxidants | Functions |
|---|---|---|
| Enzymatic antioxidant defenses | Superoxide dismutase (SOD) | SOD catalyzes two O2− anions to convert into a molecule of H2O2 and oxygen 2 O2− + 2H+ → H2O2 + O2 |
| Glutathione peroxidase (GPx) | GPx, a family of multiple isoenzymes containing selenium, catalyzes the degradation of H2O2 and lipid peroxides. Moreover, GPx can utilize GSH as an electron donor for the reduction of peroxides [64]. | |
| Catalase (GPx) | Catalase, mainly existing in peroxisomes, is responsible for converting H2O2 into water 2 H2O2 → 2 H2O + O2 |
|
|
| ||
| Nonenzymatic antioxidants | Ascorbic acid (vitamin C) | Vitamin C, a water-soluble antioxidant, is capable of removing ROS by electron transfer. In addition, vitamin C can act as a cofactor for antioxidant enzymes [88]; [90] |
| α-Tocopherol (vitamin E) | Vitamin E, a lipid-soluble antioxidant, can attenuate the effects of peroxide. In particular, it can protect against lipid peroxidation in cell membranes [88] | |
| Glutathione (GSH) | GSH, in its reduced form, is known to react with ROS for the removal of ROS. Moreover, GSH is the electron donor for the reduction of peroxides in the GPx reaction [64] | |
3.1. ROS-Induced Lipid Peroxidation
Lipid is the main component of the membrane for cell as well as the organelles, such as mitochondria and nuclear. Lipid, especially polyunsaturated fatty acids, is very vulnerable to the attack of ROS [66]. A hydrogen moiety of unsaturated carbon of polyunsaturated fatty acids could easily be attacked and consequently captured by ROS to form water, leaving an unpaired electron on the polyunsaturated fatty acids, which was converted into a peroxyl radical [67]. Once formed, peroxyl radicals would eventually produce malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and other toxic products [68, 69]. It was suggested that MDA was the major mutagenic and carcinogenic product of lipid peroxidation, whereas 4-HNE was less mutagenic and carcinogenic but the most toxic [70]. 4-HNE could trigger caspase activation and ultimately cause neuronal apoptosis [71]. In addition, 4-HNE could also reduce the GSH levels via interplaying with sulfhydryl groups [72]. Peroxided lipid reacts with polyunsaturated fatty acids leading to further oxidation, ultimately disrupting plasma membranes [73]. Accordingly, ROS-induced lipid peroxidation can cause neuronal damage and contribute to PD progression.
3.2. ROS-Induced Protein Oxidation
It has been demonstrated that ROS initiates protein oxidation by two parallel pathways: directly inducing protein chain and side chain oxidation and indirectly inducing protein oxidation in the process of lipid peroxidation and glycosylation [74, 75]. Protein oxidation includes the cross-linking and fragmentation of protein and carbonyl group formation [76–78]. It is noteworthy that surface-exposed methionine and cysteine residues of proteins are particularly sensitive to oxidation by almost all forms of ROS. ROS-induced protein oxidation potentially effects cell survival via disrupting the active site of enzymes and consequently protein-protein and protein-DNA interactions [79]. It was demonstrated that loss function mutation in DJ-1, one familial PD-related gene, leaded to protein oxidative damage [80]. Supplementation of antioxidant, vitamin C, could decrease the H2O2 and oxidized protein level [81]. Therefore, protein oxidation by ROS involves in PD pathogenesis.
3.3. ROS-Induced DNA Oxidation
It is acknowledged that OH can bind with DNA molecule, leading to oxidation of bases and the deoxyribose backbone [82]. The key product of DNA oxidation is 8-hydroxy-deoxyguanosine (8-OHdG), which results in transcriptional mutagenesis and generation of mutated species of protein that contributed to PD pathogenesis [83, 84]. Notably, mitochondrial DNA (mtDNA) oxidation by ROS would lead to mtDNA abnormality and consequently trigger the expression of aberrant mitochondrial proteins and mitochondrial dysfunction, collectively exacerbating ROS production [85, 86]. It is therefore unsurprising to note that there is a vicious cycle between mtDNA oxidation and increased ROS production, which ultimately leads to neuronal death and PD pathogenesis.
4. Anti-ROS with Compounds for the Therapeutics of PD
In light of the above-mentioned evidence on the crucial role of ROS in the pathogenesis of PD, anti-ROS therapy has been an attractive strategy to counteract the oxidative stress-induced neuronal cell death in PD [87]. Classic antioxidants mainly include vitamin C, vitamin E, Coenzyme Q10 (CoQ10), GSH, NAC, and creatine. Vitamin C and vitamin E are members of antioxidant defense systems. Vitamin E could scavenge hydroxyl and peroxyl radicals, thus protecting against lipid peroxidation [88]. Vitamin C could not only directly remove O2− and ˙OH, but also indirectly facilitate vitamin E to counteract overproduced ROS to show neuroprotection in PD [89, 90]. It was reported that a combination of vitamin C and vitamin E administered to patients with early PD may slow the progression of the disease [91, 92]. CoQ10, a constituent of the mitochondrial electron transport chain (ETC), prevented electrons leaking along the ETC which would generate ROS [93]. It was reported that oral administration of CoQ10 in PD animal models and PD patients attenuated mitochondrial dysfunction and deficit of dopamine [94]. Mechanically, CoQ10 acted as antioxidant to scavenge H2O2 or as a cofactor and activator of mitochondrial uncoupling proteins to decrease the generation of ROS [93, 95]. GSH, the major endogenous antioxidant molecule, was found to reduce in the substantia nigra of PD patients [96]. However, direct administration of GSH did not achieve expected effect of scavenging ROS due to its susceptibility to oxidation by various ROS [97]. NAC, a precursor of GSH, was alternatively utilized to restore GSH levels by providing the rate-limiting substrate for GSH synthesis [98]. Moreover, NAC could also directly act as a scavenger of ROS and ameliorate dopaminergic neuronal loss in PD models [99, 100]. Creatine is a nitrogenous guanidine molecule with antioxidant properties, which could retain mitochondrial dysfunction and protect DA neuron death in PD models [101, 102]. As known, most of the ROS are produced during ATP production though OXPHOS. Resveratrol, a natural polyphenolic compound, is showed to protect against Parkin deficiency-induced mitochondria dysfunction and oxidative stress via activating AMPK/SIRT1/PGC-1α axis [103]. Pinocembrin (PB) could mitigate MPP (+) induced SH-SY5Y cells oxidative stress and apoptosis [104].
Nuclear factor erythroid 2-related factor 2 (Nrf2) controls the antioxidant and detoxifying response in mammalian [105]. Recently, it was reported that carnosic acid (CA) exerts antioxidant effects through activation of Nrf2, the latter upregulating expression of some of endogenous antioxidants such as GPx, glutathione reductase (GR) [106]. Moreover, isothiocyanate sulforaphane (SFN), another Nrf2 activator, also displays neuroprotective effects in PD models [107]. All those studies suggest that Nrf2 is a pivotal mediator of cellular antioxidative stress system.
Noteworthy, antioxidants show the promising effect for antagonizing oxidative stress in animal PD models, and they do not display the equivalent efficacy in clinical trials. More work need to do before antioxidant could be applied for PD treatment in clinic.
5. Conclusions
PD is the second most common neurodegenerative disorder, and the mechanisms of neuronal degeneration in PD are poorly known and remain to be fully illustrated. It is widely accepted that genetic mutations, mitochondrial dysfunction, dopamine auto-oxidation, neuroinflammation, and iron accumulation contribute significantly to the pathogenesis of PD. Interestingly, all of the PD-related factors can cause excessive generation of ROS. Once ROS overwhelm antioxidant defense systems, excess ROS can induce lipid peroxidation, protein oxidation, and DNA oxidation to trigger PD-related cell loss in the SN. In the future, the molecular signal pathway of ROS inducing PD pathogenesis needs be further explored. Antioxidants which could be utilized for PD treatment should be developed.
Acknowledgments
This study was supported by the National Science Foundation of China (Grant nos. 81672508 and 61505076), Jiangsu Provincial Foundation for Distinguished Young Scholars (BK20170041), Key University Science Research Project of Jiangsu Province (no. 16KJA180004), and Jiangsu key Research and Development program (BE2015699).
Contributor Information
Cheng-wu Zhang, Email: iamcwzhang@njtech.edu.cn.
Lin Li, Email: iamlli@njtech.edu.cn.
Conflicts of Interest
There are no any conflicts of interest on this paper.
References
- 1.Jellinger K. Neuropathological substrates of Alzheimers disease and Parkinsons disease. Journal of Neural Transmission. 1987;24:109–129. [PubMed] [Google Scholar]
- 2.Moon H. E., Paek S. H. Mitochondrial dysfunction in Parkinson’s disease. Experimental Neurobiology. 2015;24:103–116. doi: 10.5607/en.2015.24.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitworth A. J., Pallanck L. J. Genetic models of Parkinson’s disease: mechanisms and therapies. SEB Experimental Biology Series. 2008;60:93–113. [PubMed] [Google Scholar]
- 4.Shadrina M. I., Slominsky P. A. Molecular genetics of Parkinson’s disease. Russian Journal of Genetics. 2006;42(8):858–871. doi: 10.1134/s1022795406080035. [DOI] [PubMed] [Google Scholar]
- 5.Chou A. P., Maidment N., Klintenberg R., et al. Ziram causes dopaminergic cell damage by inhibiting e1 ligase of the proteasome. Journal of Biological Chemistry. 2008;283(50):34696–34703. doi: 10.1074/jbc.m802210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doorn J. A., Florang V. R., Schamp J. H., Vanle B. C. Aldehyde dehydrogenase inhibition generates a reactive dopamine metabolite autotoxic to dopamine neurons. Parkinsonism & Related Disorders. 2014;20:S73–S75. doi: 10.1016/s1353-8020(13)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J., Fitsanakis V. A., Gu G. Y., et al. Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: a link through mitochondrial dysfunction. Journal of Neurochemistry. 2003;84(2):336–346. doi: 10.1046/j.1471-4159.2003.01525.x. [DOI] [PubMed] [Google Scholar]
- 8.Ross J. M., Olson L., Coppotelli G. Mitochondrial and ubiquitin proteasome system dysfunction in ageing and disease: two sides of the same coin? International Journal of Molecular Sciences. 2015;16(8):19458–19476. doi: 10.3390/ijms160819458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim K.-L., Zhang C.-W. Molecular events underlying Parkinson’s disease—an interwoven tapestry. Frontiers in Neurology. 2013;4 doi: 10.3389/fneur.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliwell B. Oxidative stress and neurodegeneration: where are we now? Journal of Neurochemistry. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 11.Covarrubias L., Hernandez-Garcia D., Schnabel D., Salas-Vidal E., Castro-Obregon S. Function of reactive oxygen species during animal development: passive or active? Developmental Biology. 2008;320(1):1–11. doi: 10.1016/j.ydbio.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 12.Casetta I., Govoni V., Granieri E. Oxidative stress, antioxidants and neurodegenerative diseases. Current Pharmaceutical Design. 2005;11(16):2033–2052. doi: 10.2174/1381612054065729. [DOI] [PubMed] [Google Scholar]
- 13.Chai C., Lim K.-L. Genetic insights into sporadic Parkinson’s disease pathogenesis. Current Genomics. 2013;14(8):486–501. doi: 10.2174/1389202914666131210195808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youle R. Autophagy functions of genes mutated in ALS and Parkinson’s disease. Free Radical Biology and Medicine. 2017;112:p. 15. doi: 10.1016/j.freeradbiomed.2017.10.368. [DOI] [Google Scholar]
- 15.Basu S., Je G., Kim Y.-S. Transcriptional mutagenesis by 8-oxodG in α-synuclein aggregation and the pathogenesis of Parkinson’s disease. Experimental and Molecular Medicine. 2015;47(8):p. e179. doi: 10.1038/emm.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Maio R., Barrett P. J., Hoffman E. K., et al. Alpha-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Science Translational Medicine. 2016;8(342) doi: 10.1126/scitranslmed.aaf3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Junn E., Mouradian M. M. Human alpha-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine. Neuroscience Letters. 2002;320(3):146–150. doi: 10.1016/s0304-3940(02)00016-2. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Q., Huang C., Guo J., et al. Hsp70 participates in PINK1-mediated mitophagy by regulating the stability of PINK1. Neuroscience Letters. 2018;662:264–270. doi: 10.1016/j.neulet.2017.10.051. [DOI] [PubMed] [Google Scholar]
- 19.Yang H., Zuo J., Liu W. Parkin, PINK1, DJ-1 and mitochondria dysfunction with Parkinson’s disease. Chinese Bulletin of Life Sciences. 2010;22:1009–1012. [Google Scholar]
- 20.Zhang H.-T., Mi L., Wang T., et al. PINK1/parkin-mediated mitophagy play a protective role in manganese induced apoptosis in SH-SY5Y cells. Toxicology In Vitro. 2016;34:212–219. doi: 10.1016/j.tiv.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C. W., Hang L. T., Yao T. P., Lim K. L. Parkin regulation and neurodegenerative disorders. Frontiers in Aging Neuroscience. 2016;7 doi: 10.3389/fnagi.2015.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cartelli D., Amadeo A., Calogero A. M., et al. Parkin absence accelerates microtubule aging in dopaminergic neurons. Neurobiology of Aging. 2017;61:66–74. doi: 10.1016/j.neurobiolaging.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Junn E., Jang W. H., Zhao X., Jeong B. S., Mouradian M. M. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. Journal of Neuroscience Research. 2009;87(1):123–129. doi: 10.1002/jnr.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu J., Yu M., Wang C., Xu Z. Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via dynamin-like protein. Journal of Neurochemistry. 2012;122(3):650–658. doi: 10.1111/j.1471-4159.2012.07809.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Yan M. H., Fujioka H., et al. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Human Molecular Genetics. 2012;21(9):1931–1944. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angeles D. C., Gan B.-H., Onstead L., et al. Mutations in LRRK2 increase phosphorylation of peroxiredoxin 3 exacerbating oxidative stress-induced neuronal death. Human Mutation. 2011;32(12):1390–1397. doi: 10.1002/humu.21582. [DOI] [PubMed] [Google Scholar]
- 27.Johnson W. M., Yao C., Chen S., Wilson-Delfosse A. L., Mieyal J. J. Loss of redoxin proteins exacerbates LRRK2-mediated Parkinson’s disease phenotype in C. elegans. FASEB Journal. 2013;27:20–24. [Google Scholar]
- 28.Angeles D. C., Ho P., Chua L. L., et al. Thiol peroxidases ameliorate LRRK2 mutant-induced mitochondrial and dopaminergic neuronal degeneration in Drosophila. Human Molecular Genetics. 2014;23(12):3157–3165. doi: 10.1093/hmg/ddu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee R., Starkov A. A., Beal M. F., Thomas B. Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2009;1792(7):651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadenas E., Davies K. J. A. Mitochondrial free radical generation, oxidative stress, and aging. Free Radical Biology and Medicine. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 31.Muller F., Liu Y. H., Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. Journal of Biological Chemistry. 2004;279(47):49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 32.Bhat A. H., Dar K. B., Anees S., et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomedicine & Pharmacotherapy. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 33.Hang L., Thundyil J., Lim K.-L., Sci N. Y. A. Mitochondrial dysfunction and Parkinson disease: a Parkin-AMPK alliance in neuroprotection. Mitochondrial Research in Translational Medicine. 2015;1350(1):37–47. doi: 10.1111/nyas.12820. [DOI] [PubMed] [Google Scholar]
- 34.Reddy P. H., Beal M. F. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends in Molecular Medicine. 2008;14(2):45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su Y.-H., Lee Y.-L., Chen S.-F., et al. Essential role of ss-human 8-oxoguanine DNA glycosylase 1 in mitochondrial oxidative DNA repair. Environmental and Molecular Mutagenesis. 2013;54(1):54–64. doi: 10.1002/em.21742. [DOI] [PubMed] [Google Scholar]
- 36.Schapira A. H. V. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. The Lancet Neurology. 2008;7(1):97–109. doi: 10.1016/s1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 37.Hauber W. Involvement of basal ganglia transmitter systems in movement initiation. Progress In Neurobiology. 1998;56:507–540. doi: 10.1016/s0301-0082(98)00041-0. [DOI] [PubMed] [Google Scholar]
- 38.Biosa A., Arduini I., Soriano M. E., Bubacco L., Bisaglia M. Analysis of the functional effects mediated by dopamine oxidation products at the mitochondrial level. Journal of Neurochemistry. 2017;142:p. 106. [Google Scholar]
- 39.Hastings T. G. The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson’s disease. Journal of Bioenergetics and Biomembranes. 2009;41:469–472. doi: 10.1007/s10863-009-9257-z. [DOI] [PubMed] [Google Scholar]
- 40.Dusek P., Schneider S. A., Aaseth J. Iron chelation in the treatment of neurodegenerative diseases. Journal of Trace Elements in Medicine and Biology. 2016;38:81–92. doi: 10.1016/j.jtemb.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Park S. U., Ferrer J. V., Javitch J. A., Kuhn D. M. Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: potential mechanism of neurotoxicity in dopamine neurons. Journal of Neuroscience. 2002;22(11):4399–4405. doi: 10.1523/jneurosci.22-11-04399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saller S., Kunz L., Berg D., et al. Dopamine in human follicular fluid is associated with cellular uptake and metabolism-dependent generation of reactive oxygen species in granulosa cells: implications for physiology and pathology. Human Reproduction. 2014;29(3):555–567. doi: 10.1093/humrep/det422. [DOI] [PubMed] [Google Scholar]
- 43.Youdim M. B. H., Edmondson D., Tipton K. F. The therapeutic potential of monoamine oxidase inhibitors. Nature Reviews Neuroscience. 2006;7(4):295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 44.Gainetdinov R. R., Caron M. G. Monoamine transporters: from genes to behavior. Annual Review of Pharmacology and Toxicology. 2003;43(1):261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 45.Lee F. J. S., Liu F., Pristupa Z. B., Niznik H. B. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB Journal. 2001;15(6):916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- 46.Su P., Liu F. A peptide disrupting the D2R-DAT interaction protects against dopamine neurotoxicity. Experimental Neurology. 2017;295:176–183. doi: 10.1016/j.expneurol.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Farooqui A. A., Horrocks L. A., Farooqui T. Modulation of inflammation in brain: a matter of fat. Journal of Neurochemistry. 2007;101(3):577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh H.-L., Yang C.-M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed Research International. 2013;2013:18. doi: 10.1155/2013/484613.484613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dheen S. T., Kaur C., Ling E.-A. Microglial activation and its implications in the brain diseases. Current Medicinal Chemistry. 2007;14(11):1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 50.Seredenina T., Schiavone S., Maghzal G., et al. Inhibition of NOX NADPH oxidases as a potential treatment for neuroinflammation. Free Radical Biology and Medicine. 2013;65:p. S14. doi: 10.1016/j.freeradbiomed.2013.08.137. [DOI] [Google Scholar]
- 51.Hayter H. L., Pettus B. J., Ito F., Obeid L. M., Hannun Y. A. TNF alpha-induced glutathione depletion lies downstream of cPLA(2) in L929 cells. FEBS Letters. 2001;507(2):151–156. doi: 10.1016/s0014-5793(01)02967-2. [DOI] [PubMed] [Google Scholar]
- 52.Kim E. K., Kwon K. B., Han M. J., et al. Coptidis rhizoma extract protects against cytokine-induced death of pancreatic beta-cells through suppression of NF-kappa B activation. Experimental and Molecular Medicine. 2007;39(2):149–159. doi: 10.1038/emm.2007.17. [DOI] [PubMed] [Google Scholar]
- 53.Ramdial K., Franco M. C., Estevez A. G. Cellular mechanisms of peroxynitrite-induced neuronal death. Brain Research Bulletin. 2017;133(4):4–11. doi: 10.1016/j.brainresbull.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Block M. L., Zecca L., Hong J.-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature Reviews Neuroscience. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 55.Wang R. D. Iron metabolism in Parkinson’s disease patients with olfactory disorder. Journal of the American Geriatrics Society. 2016;64:p. S374. [Google Scholar]
- 56.Lee D. W., Andersen J. K. Iron elevations in the aging parkinsonian brain: a consequence of impaired iron homeostasis? Journal of Neurochemistry. 2010;112(2):332–339. doi: 10.1111/j.1471-4159.2009.06470.x. [DOI] [PubMed] [Google Scholar]
- 57.Theil E. C., Eisenstein R. S. Combinatorial mRNA regulation: Iron regulatory proteins and iso-iron-responsive elements (Iso-IREs) Journal of Biological Chemistry. 2000;275(52):40659–40662. doi: 10.1074/jbc.r000019200. [DOI] [PubMed] [Google Scholar]
- 58.Kaur D., Andersen J. Does cellular iron dysregulation play a causative role in Parkinson’s disease? Ageing Research Reviews. 2004;3(3):327–343. doi: 10.1016/j.arr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Sian-Huelsmann J., Mandel S., Youdim M. B. H., Riederer P. The relevance of iron in the pathogenesis of Parkinson’s disease. Journal of Neurochemistry. 2011;118(6):939–957. doi: 10.1111/j.1471-4159.2010.07132.x. [DOI] [PubMed] [Google Scholar]
- 60.Zucca F. A., Segura-Aguilar J., Ferrari E., et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Progress in Neurobiology. 2017;155:96–119. doi: 10.1016/j.pneurobio.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nunez-Millacura C., Tapia V., Munoz P., Maccioni R. B., Nunez M. T. An oxidative stress-mediated positive-feedback iron uptake loop in neuronal cells. Journal of Neurochemistry. 2002;82(2):240–248. doi: 10.1046/j.1471-4159.2002.00971.x. [DOI] [PubMed] [Google Scholar]
- 62.Bar-Am O., Amit T., Kupershmidt L., et al. Neuroprotective and neurorestorative activities of a novel iron chelator-brain selective monoamine oxidase-A/monoamine oxidase-B inhibitor in animal models of Parkinson’s disease and aging. Neurobiology of Aging. 2015;36(3):1529–1542. doi: 10.1016/j.neurobiolaging.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 63.Gotsbacher M. P., Telfer T. J., Witting P. K., Double K. L., Finkelstein D. I., Codd R. Analogues of desferrioxamine B designed to attenuate iron-mediated neurodegeneration: synthesis, characterisation and activity in the MPTP-mouse model of Parkinson’s disease. Metallomics. 2017;9(7):852–864. doi: 10.1039/c7mt00039a. [DOI] [PubMed] [Google Scholar]
- 64.Dringen R. Metabolism and functions of glutathione in brain. Progress in Neurobiology. 2000;62(6):649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 65.Valko M., Leibfritz D., Moncol J., Cronin M. T. D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry & Cell Biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Angelova P. R., Horrocks M. H., Klenerman D., Gandhi S., Abramov A. Y., Shchepinov M. S. Lipid peroxidation is essential for alpha-synuclein-induced cell death. Journal of Neurochemistry. 2015;133(4):582–589. doi: 10.1111/jnc.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patten D. A., Germain M., Kelly M. A., Slack R. S. Reactive oxygen species: stuck in the middle of neurodegeneration. Journal of Alzheimers Disease. 2010;20(s2):S357–S367. doi: 10.3233/jad-2010-100498. [DOI] [PubMed] [Google Scholar]
- 68.Miletic J., Ilic T., Stefanovic A., Miljkovic M., Stojanov M. 4-Hydroxynonenal in Parkinson’s disease. Amino Acids. 2015;47:p. 1675. [Google Scholar]
- 69.Sharma A., Kaur P., Kumar B., Prabhakar S., Gill K. D. Plasma lipid peroxidation and antioxidant status of Parkinson’s disease patients in the Indian population. Parkinsonism & Related Disorders. 2008;14(1):52–57. doi: 10.1016/j.parkreldis.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 70.McGrath L. T., McGleenon B. M., Brennan S., McColl D., McIlroy S., Passmore A. P. Increased oxidative stress in Alzheimer’s disease as assessed with 4-hydroxynonenal but not malondialdehyde. QJM-Monthly Journal of the Association of Physicians. 2001;94:485–490. doi: 10.1093/qjmed/94.9.485. [DOI] [PubMed] [Google Scholar]
- 71.Siddiqui M. A., Kumar V., Kashyap M. P., et al. Short-term exposure of 4-hydroxynonenal induces mitochondria-mediated apoptosis in PC12 cells. Human & Experimental Toxicology. 2012;31:336–345. doi: 10.1177/0960327111432500. [DOI] [PubMed] [Google Scholar]
- 72.Ahmed I., John A., Vijayasarathy C., Robin M. A., Raza H. Differential modulation of growth and glutathione metabolism in cultured rat astrocytes by 4-hydroxynonenal and green tea polyphenol, epigallocatechin-3-gallate. Neurotoxicology. 2002;23(3):289–300. doi: 10.1016/s0161-813x(02)00042-6. [DOI] [PubMed] [Google Scholar]
- 73.Andersen J. K. Oxidative stress in neurodegeneration: cause or consequence? Nature Reviews Neuroscience. 2004;10(7):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 74.Sesti F., Liu S., Cai S. Q. Oxidation of potassium channels by ROS: a general mechanism of aging and neurodegeneration? Trends in Cell Biology. 2010;20(1):45–51. doi: 10.1016/j.tcb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 75.Yan L.-J. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biology. 2014;2:165–169. doi: 10.1016/j.redox.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Czapski G. A., Szypula W., Kudlik M., et al. Assessment of antioxidative activity of alkaloids from Huperzia selago and Diphasiastrum complanatum using in vitro systems. Folia Neuropathologica. 2014;52(4):394–406. doi: 10.5114/fn.2014.47840. [DOI] [PubMed] [Google Scholar]
- 77.Floor E., Wetzel M. G. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. Journal of Neurochemistry. 1998;70(1):268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- 78.Mukherjee S., Kapp E. A., Lothian A., et al. Characterization and identification of dityrosine cross-linked peptides using tandem mass spectrometry. Analytical Chemistry. 2017;89(11):6137–6146. doi: 10.1021/acs.analchem.7b00941. [DOI] [PubMed] [Google Scholar]
- 79.Jammes Y., Steinberg J. G., Bregeon F., Delliaux S. The oxidative stress in response to routine incremental cycling exercise in healthy sedentary subjects. Respiratory Physiology & Neurobiology. 2004;144(1):81–90. doi: 10.1016/j.resp.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 80.Xu S., Yang X., Qian Y., Xiao Q. Parkinson disease-related DJ-1 modulates the expression of uncoupling protein 4 against oxidative stress. Journal of Neurochemistry. 2018;145(4):312–322. doi: 10.1111/jnc.14297. [DOI] [PubMed] [Google Scholar]
- 81.Sanders L. H., McCoy J., Hu X., et al. Mitochondrial DNA damage: Molecular marker of vulnerable nigral neurons in Parkinson’s disease. Neurobiology of Disease. 2014;70:214–223. doi: 10.1016/j.nbd.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alam Z. I., Jenner A., Daniel S. E., et al. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. Journal of Neurochemistry. 1997;69(3):1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 83.Bregeon D., Peignon P.-A., Sarasin A. Transcriptional Mutagenesis Induced by 8-Oxoguanine in Mammalian Cells. PLoS Genetics. 2009;5(7) doi: 10.1371/journal.pgen.1000577.e1000577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gmitterova K., Gawinecka J., Heinemann U., Valkovic P., Zerr I. DNA versus RNA oxidation in Parkinson’s disease: which is more important? Neuroscience Letters. 2018;662:22–28. doi: 10.1016/j.neulet.2017.09.048. [DOI] [PubMed] [Google Scholar]
- 85.Bender A., Krishnan K. J., Morris C. M., et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nature Genetics. 2006;38(5):515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 86.Surmeier D. J., Guzman J. N., Sanchez-Padilla J., Goldberg J. A. The origins of oxidant stress in Parkinson’s disease and therapeutic strategies. Antioxidants & Redox Signaling. 2011;14(7):1289–1301. doi: 10.1089/ars.2010.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hang L. T., Basil A. H., Lim K. L. Nutraceuticals in Parkinson’s disease. NeuroMolecular Medicine. 2016;18(3):306–321. doi: 10.1007/s12017-016-8398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gandhi S., Abramov A. Y. Mechanism of oxidative stress in neurodegeneration. Oxidative Medicine and Cellular Longevity. 2012;2012:11. doi: 10.1155/2012/428010.428010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen A. Y., Lu J.-M., Yao Q., Chen C. Entacapone is an antioxidant more potent than vitamin C and vitamin E for scavenging of hypochlorous acid and peroxynitrite, and the inhibition of oxidative stress-induced cell death. Medical Science Monitor. 2016;22:687–696. doi: 10.12659/msm.896462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dasuri K., Zhang L., Keller J. N. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radical Biology and Medicine. 2013;62:170–185. doi: 10.1016/j.freeradbiomed.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 91.Baunthiyal M., Singh V., Dwivedi S. Insights of antioxidants as molecules for drug discovery. International Journal of Pharmacology. 2017;13(7):874–889. doi: 10.3923/ijp.2017.874.889. [DOI] [Google Scholar]
- 92.Paraskevas G. P., Kapaki E., Petropoulou O., Anagnostouli M., Vagenas V., Papageorgiou C. Plasma levels of antioxidant vitamins C and E are decreased in vascular parkinsonism. Journal of the Neurological Sciences. 2003;215(1-2):51–55. doi: 10.1016/s0022-510x(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 93.Shults C. W., Oakes D., Kieburtz K., et al. Effects of coenzyme Q(10) in early Parkinson disease-evidence of slowing of the functional decline. Archives of Neurology. 2002;59(10):1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 94.Storch A. Coenzyme Q(10) in Parkinson’s disease. Der Nervenarzt. 2007;78(12):1378–1382. doi: 10.1007/s00115-007-2285-1. [DOI] [PubMed] [Google Scholar]
- 95.Kieburtz K., Ravina B., Galpern W. R., et al. A randomized clinical trial of coenzyme Q(10) and GPI-1485 in early Parkinson disease. Neurology. 2007;68(1):20–28. doi: 10.1212/01.wnl.0000250355.28474.8e. [DOI] [PubMed] [Google Scholar]
- 96.Ehrhart J., Zeevalk G. D. Cooperative interaction between ascorbate and glutathione during mitochondrial impairment in mesencephalic cultures. Journal of Neurochemistry. 2003;86(6):1487–1497. doi: 10.1046/j.1471-4159.2003.01954.x. [DOI] [PubMed] [Google Scholar]
- 97.Sun H. J., Wang Y., Hao T., Wang C.-Y., Wang Q.-Y., Jiang X.-X. Efficient GSH delivery using PAMAM-GSH into MPP-induced PC12 cellular model for Parkinson’s disease. Regenerative Biomaterials. 2016;3(5):299–307. doi: 10.1093/rb/rbw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coles L. D., Tuite P. J., Oz G., et al. Repeated-dose oral N-acetylcysteine in Parkinson’s disease: pharmacokinetics and effect on brain glutathione and oxidative stress. Journal of Clinical Pharmacology. 2018;58(2):158–167. doi: 10.1002/jcph.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Penugonda S., Mare S., Goldstein G., Banks W. A., Ercal N. Effects of N-acetylcysteine amide (NACA), a novel thiol antioxidant against glutamate-induced cytotoxicity in neuronal cell line PC12. Brain Research. 2005;1056(2):132–138. doi: 10.1016/j.brainres.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 100.Sozio P., Lannitelli A., Cerasa L. S., et al. New L-dopa codrugs as potential antiparkinson agents. Archiv Der Pharmazie. 2008;341(7):412–417. doi: 10.1002/ardp.200700228. [DOI] [PubMed] [Google Scholar]
- 101.Bender A., Koch W., Elstner M., et al. Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trial. Neurology. 2006a;67(7):1262–1264. doi: 10.1212/01.wnl.0000238518.34389.12. [DOI] [PubMed] [Google Scholar]
- 102.Loehe M., Reichmann H. Clinical neuroprotection in Parkinson’s disease-still waiting for the breakthrough. Journal of the Neurological Sciences. 2010;289(1-2):104–114. doi: 10.1016/j.jns.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 103.Ferretta A., Gaballo A., Tanzarella P., et al. Effect of resveratrol on mitochondrial function: implications in parkin-associated familiar Parkinson’s disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2014;1842(7):902–915. doi: 10.1016/j.bbadis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y., Gao J., Miao Y., et al. Pinocembrin protects SH-SY5Y cells against MPP(+)-induced neurotoxicity through the mitochondrial apoptotic pathway. Journal of Molecular Neuroscience. 2014;53(4):537–545. doi: 10.1007/s12031-013-0219-x. [DOI] [PubMed] [Google Scholar]
- 105.Esteras N., Dinkova-Kostova A. T., Abramov A. Y. Nrf2 activation in the treatment of neurodegenerative diseases: a focus on its role in mitochondrial bioenergetics and function. Biological Chemistry. 2016;397(5):382–400. doi: 10.1515/hsz-2015-0295. [DOI] [PubMed] [Google Scholar]
- 106.de Oliveira M. R., de Souza I. C. C., Fürstenau C. R. Carnosic acid induces anti-inflammatory effects in paraquat-treated SH-SY5Y cells through a mechanism involving a crosstalk between the Nrf2/HO-1 axis and NF-κB. Molecular Neurobiology. 2018;55(1):890–897. doi: 10.1007/s12035-017-0389-6. [DOI] [PubMed] [Google Scholar]
- 107.Morroni F., Sita G., Djemil A., et al. Comparison of adaptive neuroprotective mechanisms of sulforaphane and its interconversion product erucin in in vitro and in vivo models of Parkinson’s disease. Journal of Agricultural and Food Chemistry. 2018;66(4):856–865. doi: 10.1021/acs.jafc.7b04641. [DOI] [PubMed] [Google Scholar]